Abstract
Objective
To evaluate the effect of providing antenatal dietary and lifestyle advice on neonatal anthropometry, and to determine the inter-observer variability in obtaining anthropometric measurements.
Design
Randomised controlled trial
Setting
Public maternity hospitals across metropolitan Adelaide, South Australia
Population
Pregnant women with a singleton gestation between 10+0–20+0, and body mass index (BMI) ≥25kg/m2.
Methods
Women were randomised to either Lifestyle Advice (comprehensive dietary and lifestyle intervention over the course of pregnancy including dietary, exercise and behavioral strategies, delivered by a research dietician and research assistants) or continued Standard Care. Analyses were conducted using intention to treat principles.
Main Outcome Measures
Secondary outcome measures for the trial included assessment of infant body composition using body circumference and skinfold thickness measurements (SFTM), percentage body fat, and bio-impedance analysis of fat free mass.
Results
Anthropometric measurements were obtained from 970 neonates (488 Lifestyle Advice Group, and 482 Standard Care Group). In 394 of these neonates (215 Lifestyle Advice Group, and 179 Standard Care Group) bio-impedance analysis was also obtained. There were no statistically significant differences identified between those neonates born to women receiving Lifestyle Advice and those receiving Standard Care, in terms of body circumference measures, SFTM, percentage body fat, fat mass, or fat free mass. The intra-class correlation coefficient for SFTM was moderate to excellent (ICC 0.55 to 0.88).
Conclusions
Among neonates born to women who are overweight or obese, anthropometric measures of body composition were not modified by an antenatal dietary and lifestyle intervention.
Keywords: randomised trial, antenatal dietary and lifestyle intervention, neonatal anthropometry, skinfold thickness measurements
INTRODUCTION
Approximately 50% of women are overweight or obese on entering pregnancy,1,2 with many associated health risks, both for the woman and infant.3,4 Infants of women who are overweight or obese are more likely to be of high birth weight, variably defined as weight above 4.0 or 4.5kg, or above the 90th centile using growth charts.4–7 Population studies have consistently identified a significant association between maternal obesity, high infant birth weight, and early infant8 and childhood obesity,9 with some identifying increased subsequent cardiometabolic risk, including higher blood pressure.9,10
The effect of maternal BMI, particularly among women who are overweight or obese, on newborn body composition is uncertain. When compared with women of normal BMI, infants born to women who are overweight or obese have a greater proportion of body fat,11–13 although this is not universally reported.14 Similarly, the effect of maternal BMI on infant lean tissue mass is unclear, with some authors reporting a lack of association,13,14 while others have identified a negative relationship.11
A number of randomised trials evaluating antenatal dietary and lifestyle interventions have been reported, particularly among women who are overweight or obese,15–17 with the majority focusing on gestational weight gain as an outcome. There is limited reporting of clinical outcomes, and virtually none describe the effects of maternal dietary modification on infant body composition and adiposity.15–17 We have reported the findings of our randomised trial evaluating the provision of antenatal dietary and lifestyle advice to women who were overweight or obese on clinical outcomes18–20 and maternal diet and physical activity,21 where provision of lifestyle advice was associated with significant improvements in maternal diet and activity,21 and a significant reduction in infant birth weight above both 4.0kg18 and 4.5kg.19
Our current aims were to firstly report the effect of providing antenatal dietary and lifestyle advice on secondary outcome measures of neonatal anthropometry; and secondly the inter-observer variability in obtaining neonatal anthropometric measures.
METHODS
Study Design and Participants
The methods22 and clinical findings18–21 from the LIMIT randomised trial have been reported, and the trial registered on the Australian and New Zealand Clinical Trials Registry (ACTRN12607000161426). Women attending three maternity hospitals across Adelaide, South Australia, with a BMI ≥25kg/m2 and singleton pregnancy between 10+0 and 20+0 weeks gestation were eligible, while women with a multiple pregnancy, or type 1 or 2 diabetes diagnosed prior to pregnancy were excluded.
At their first antenatal appointment, all women had their height and weight measured, and BMI calculated. Eligible women were provided with written information. Approval to conduct the trial was provided by the Human Research and Ethics Committee of each participating hospital, and each woman provided written informed consent.
Randomisation, Masking and Group Allocation
Randomisation occurred by telephoning the central randomisation service, using a computer-generated schedule, with balanced variable blocks. There was stratification for parity (0 versus 1/more), BMI at antenatal booking (25–29.9kg/m2 versus ≥30kg/m2), and collaborating centre. Women were randomised to either ‘Lifestyle Advice’ or ‘Standard Care’.
Intervention
Lifestyle Advice Group
A comprehensive intervention was provided across pregnancy, consisting of a combination of dietary, exercise and behavioural strategies, which were delivered by a research dietician and trained research assistants.22 The dietary advice provided was consistent with current Australian standards,23 while physical activity advice focussed on increasing walking and incidental activity.24 The content and structure of the intervention sessions has been described in detail previously.18,21
Standard Care Group
Women in the Standard Care group received care according to local hospital guidelines. This did not include the routine provision of advice related to diet, exercise, or gestational weight gain.
Outcome Measures
Anthropometric measurements were obtained in a subset of healthy infants born at term who did not require admission to the intensive care or special care nursery, and where research staff were available to obtain measurements prior to hospital discharge. Measurements were taken within the first days after birth according to a specifically developed protocol. Measurements included biceps, triceps, abdominal, suprailiac, subscapular and thigh skinfold thicknesses, and head, chest, abdominal and right upper arm circumferences. The sum of skinfold thickness measures was obtained by adding all of the obtained skinfold thickness measurements together, and a ratio of abdominal circumference to length calculated as an indicator of central adiposity.25 Fat mass and fat free mass were calculated based on infant sex, weight, and triceps, subscapular and thigh skinfold thicknesses (as described below). Fat free mass was also determined using bio-impedance analysis (BIA). In a small subset of infants, two research assistants independently obtained the anthropometric measurements, according to the developed protocol, to assess inter-observer variability.
Weight and Length
Within the first two hours of birth, the attending midwife measured both infant weight and length. Length was measured using a length board with the infant laid supine, the head held against the top of the board and a sliding foot plate moved until it rested flat against the infant’s foot with the leg fully extended, and read to the nearest 0.1 cm. Weight was measured with the infant undressed using a calibrated electronic scale to the nearest 1 gram.
Body Circumference Measurements
Research staff obtained skinfold thickness and body circumference measurements with the infant undressed, prior to discharge from hospital, according to a standardised protocol. All circumference measurements were taken with the infant in the supine position using a fibreglass measuring tape, and recorded to the nearest 0.1 cm.
Head circumference was measured at the widest circumference immediately above the eyebrows anteriorly (glabella) and the most prominent point of the occiput posteriorly (inion).
Arm circumference was measured using the right arm at the midpoint between the tip of the shoulder (acromion) and the tip of the elbow (olecranon).
Chest circumference was measured with the tape placed around the infant’s chest at the level of the nipples in a plane at right angles to the spine, at the end of a normal expiration.
Abdominal circumference was measured with the tape placed at or (if umbilical clip was in place) minimally above the level of the umbilicus in a plane at right angles to the spine, at the end of a normal expiration.
Skinfold Thickness Measurements
All SFTM were taken on the right side of the infant’s body using Harpenden Skinfold Callipers. The skinfold site was identified, grasped and lifted so that a double fold of skin and the underlying subcutaneous adipose tissue were held between the left thumb and index finger with care not to incorporate underlying muscle tissue.26 The jaws of the callipers were placed perpendicular to the length of the skinfold and the measurement recorded two seconds after the full pressure of the calliper was applied. Measurements were duplicated for each site and if the second value differed from the first by more than 1.0mm, a third measurement was taken. The final value for a site was the average of two measurements or the median of three.26
Biceps SFTM was obtained with the infant supine and arm by his/her side, and identified as the midpoint between the tip of the shoulder (acromion) and the tip of the elbow (olecranon). The skinfold was measured over the biceps muscle, parallel to the long axis of the arm.
Thigh SFTM was identified as the midpoint of the front of the thigh midway between the inguinal fold and the top of the patella and measured parallel to the long axis of the thigh.
Lateral abdominal SFTM was identified 2cm to the right of the umbilicus and measured perpendicular to the long axis of the abdomen.
Suprailiac SFTM was identified with the infant on his/her left side. The superior anterior edge of the iliac crest was located, intersecting an imaginary line from the mid-axilla down the midline of the body and the edge of the iliac crest, with the skinfold angled downward anteriorly.
Triceps SFTM was identified with the infant prone, as the midpoint between the acromion and the olecranon and measured parallel to the long axis of the arm.
Subscapular SFTM was identified by locating the lower tip of the scapula. The observer’s thumb was placed below this laterally and the skinfold pinched, and measured.
Percentage body fat
Fat mass was calculated from anthropometric measurements according to the formula reported by Deirelein,27 using the sum of triceps, subscapular and thigh SFTM, together with infant gender, ethnicity, age and birth weight.
Fat mass (kg) = −0.012 − 0.064 × gender + 0.024× postnatal age (days) − 0.150 × weight (kg) + 0.055 × weight (kg)2 + 0.046 × ethnicity + 0.020 × ∑3 skinfolds (mm)
Where gender is boys = 1, girls = 0, ethnicity is non-Hispanic=0, and skinfolds = triceps, subscapular and thigh.
Fat free mass was calculated by subtracting fat mass from birth weight. Percentage body fat and fat free mass were calculated by dividing fat mass and fat free mass respectively by birth weight and multiplying by 100.
Bio-impedance Analysis
Multi-frequency bio-impedance analysis (MFBIA) using the ImpSFB7 was performed at the bedside before hospital discharge.28 With the infant in the supine position, adhesive voltage (sense) electrodes were attached to the infant’s right wrist (dorsal surface adjacent to the head of the ulna) and right ankle (at the level of the medial and lateral malleoli). Current source electrodes were placed on the palmar surface of the right hand (distal to the palmer crease), and the plantar surface of the right foot (across metatarsal bones). A current of 200µA RMS was delivered and impedance and phase measured at 256 frequencies ranging from 4 to 1000kHz. Up to 5 readings were taken in succession and repeated if required.
We calculated fat free mass using resistance at frequency of 0kHz (R0), as the best estimate of extra cellular fluid, using the formula below.29
Fat free mass = 0·779 + 0·655W − 0·072S + 0·037L/R0
Where W = birth weight, S = sex (boy = 1, girl = 2), L= birth length, R0 = resistance at frequency 0kHz.
Statistical Analyses
Analyses comparing measures of neonatal body composition between the two treatment groups were performed on an intention to treat basis, according to the assignment at randomisation. Live born infants were included in the analysis if their mother consented to anthropometric measurements being taken, and did not withdraw consent to use their data. Analyses were performed using linear regression models to determine the effect of treatment group, adjusting for centre, parity, BMI category, age, socioeconomic status and smoking status. Exploratory analyses were also conducted to assess whether the effect of treatment varied by maternal BMI category (overweight vs obese), by including an interaction between treatment group and BMI category in the regression model. Based on the results of these analyses, post hoc analyses were performed to investigate (i) whether the previously reported effect of the intervention on birth weight >4kg18 was attenuated in the subgroup of infants with anthropometric measurements using log binomial regression; and (ii) whether the intervention influenced the distribution of body composition in the tails, rather than the mean, using the Kolmogorov-Smirnov and Kuiper tests, and quantile regression on the 80th, 85th, 90th and 95th percentiles. Statistical significance was assessed at the 2-sided p<0.05 level and no adjustment was made for multiple comparisons. As these were secondary outcomes, any significant findings should be interpreted with caution. All analyses were performed using SAS v9.3 (Cary, NC, USA).
To measure inter-observer variability, the mean and standard deviation of the skinfold thickness measurements and percentage body fat were calculated across all research assistants and all infants with duplicate measures. The intra-class correlation coefficient (ICC) was calculated as a measure of reliability. As the two research assistants who took the skinfold thickness measurements differed for each infant, the ICC was determined from a one-way random effects model.30 An ICC of 0 indicates a completely unreliable measurement, while an ICC of 1 represents perfect reliability.31 The standard error of the measurement (SEM), or the standard deviation of repeated measurements taken on the same infant, was calculated as a measure of agreement for skinfold thickness measurements, also from a one-way random effects model.31 For each infant, the absolute difference in measurements between research assistants was calculated. The number and percentage of infants where this difference did not exceed 1mm (for skinfold thickness measurements) or 1% (for percent body fat) was determined as an additional measure of agreement.
Our predetermined sample size of 2,180 women was based on our primary trial outcome, the incidence of large for gestational age infants, as previously reported.18
RESULTS
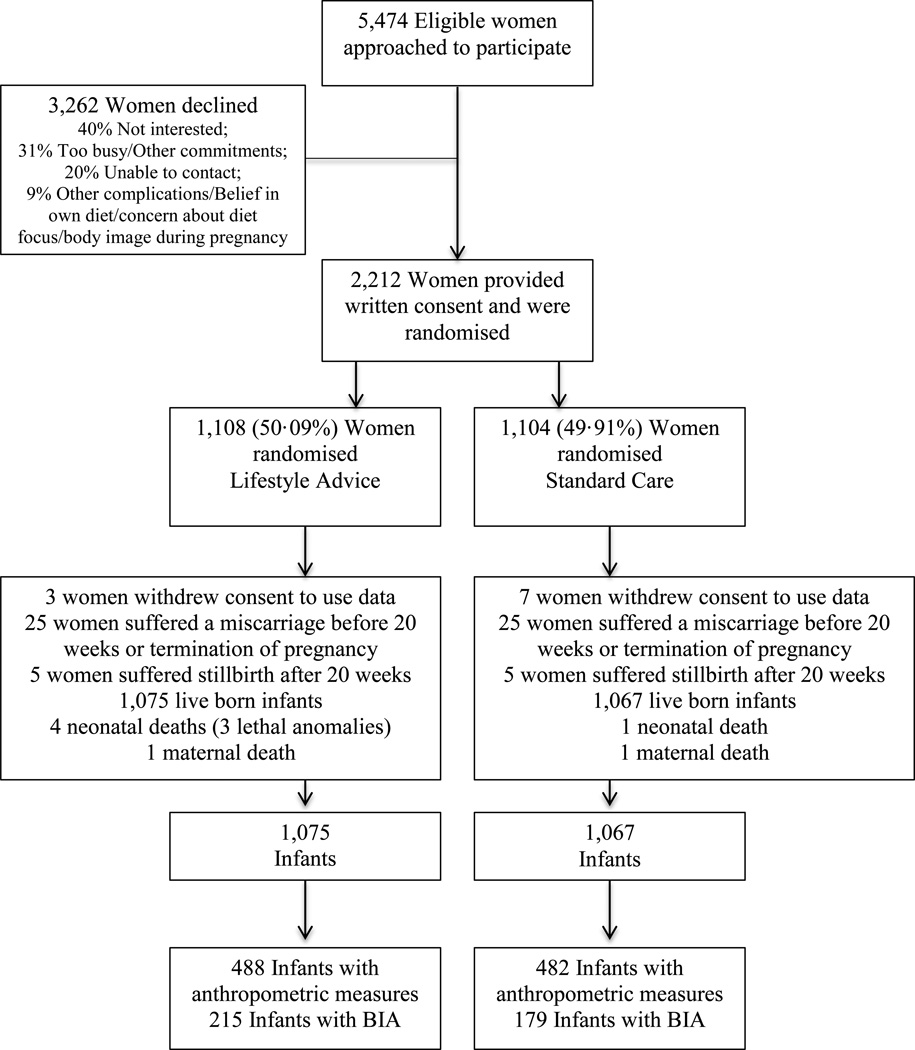
Between June 2008 and December 2011, we recruited and randomised 2,212 women, with 1,108 allocated to receive Lifestyle Advice, and 1,104 Standard Care. There were a total of 2,142 live-born infants (1,075 Lifestyle Advice; 1,067 Standard Care). Detailed anthropometric measurements were obtained in 970 neonates (488 Lifestyle Advice; 482 Standard Care) (Figure 1). The baseline characteristics of mothers of these neonates were similar between treatment groups (Table 1), and similar to the full randomised groups (Table S1).18 Bio-impedance analysis was performed on 394 infants (215 Lifestyle Advice; 179 Standard Care).
FIGURE 1.
Flow of participants through the trial
TABLE 1.
Baseline characteristics of women whose infants had anthropometric measurements taken
| Characteristic | Lifestyle Advice (N=488) |
Standard Care (N=482) |
|---|---|---|
| Maternal Age (Years)* | 29.8 (5.6) | 29.8 (5.2) |
| Gestational Age at Entry (Weeks)+ | 13.9 (11.6–16.6) | 14.0 (11.7–16.9) |
| Body Mass Index (kg/m2)+ | 31.3 (28.2–35.9) | 31.2 (27.8–36.3) |
| Body Mass Index Category# | ||
| . BMI 25.0–29.9 | 187 (38.3) | 200 (41.5) |
| . BMI 30.0–34.9 | 156 (32.0) | 136 (28.2) |
| . BMI 35.0–39.9 | 91 (18.6) | 76 (15.8) |
| . BMI >=40.0 | 54 (11.1) | 70 (14.5) |
| Public Patient# | 479 (98.2) | 471 (97.7) |
| Weight (kg)* | 89.2 (18.3) | 89.2 (18.6) |
| Height (cm)* | 164.6 (6.4) | 164.6 (6.5) |
| Race# | ||
| . Caucasian | 439 (90.0) | 440 (91.3) |
| . Asian | 12 (2.5) | 17 (3.5) |
| . Indian | 21 (4.3) | 15 (3.1) |
| . Other | 16 (3.3) | 10 (2.1) |
| Smoker# | 65 (13.3) | 53 (11.0) |
| Nulliparous# | 206 (42.2) | 208 (43.2) |
| Previous Preterm Birth# | 21 (4.3) | 23 (4.8) |
| Previous Pre-eclampsia# | 19 (3.9) | 25 (5.2) |
| Previous Stillbirth# | 5 (1.0) | 2 (0.4) |
| Previous Neonatal Death# | 6 (1.2) | 3 (0.6) |
| Previous Caesarean Section# | 102 (20.9) | 114 (23.7) |
| Family History of Diabetes# | 130 (26.6) | 135 (28.0) |
| Family History of Hypertension# | 183 (37.5) | 174 (36.1) |
| Family History of Heart Disease# | 97 (19.9) | 87 (18.0) |
| Index of Socio-economic Disadvantage^ | ||
| . Unknown | 1 (0.2) | 0 (0.0) |
| . Quintile 1 (Most Disadvantaged) | 162 (33.2) | 151 (31.3) |
| . Quintile 2 | 100 (20.5) | 121 (25.1) |
| . Quintile 3 | 74 (15.2) | 66 (13.7) |
| . Quintile 4 | 80 (16.4) | 78 (16.2) |
| . Quintile 5 (Least Disadvantaged) | 71 (14.5) | 66 (13.7) |
= mean and standard deviation
= median and interquartile range
= number and %
= Socioeconomic index as measured by SEIFA
Average body circumferences, skinfold thicknesses and calculated body fat measures were similar between the treatment groups, with no statistically significant differences identified (Table 2). There were also no statistically significant differences identified between the two groups, with regards fat free mass (R0) and percentage fat free mass (R0) obtained using bio-impedance analysis (Table 2). There was no evidence to suggest that the intervention effect was modified by maternal BMI category (data not shown). Post hoc analyses indicated that the intervention effect on birth weight >4kg in the subgroup of infants with body composition measures was similar to that reported previously in the overall cohort,18 with an estimated RR of 0.78 (95% CI 0.59 to 1.02). There was some suggestion that the tails of the body composition distributions in the Lifestyle Advice group differed from those of the Standard Care group, however none of these differences were statistically significant (data not shown).
TABLE 2.
Neonatal anthropometric measurements by treatment group
| Outcome | Lifestyle Advice (N=488*) |
Standard Care (N=482*) |
Unadjusted Treatment Effect (95% CI) |
Unadjusted P- value |
Adjusted Treatment Effect (95% CI) |
Adjusted P- value |
|---|---|---|---|---|---|---|
| Chest Circumference (cm) | 34.24 (1.92) | 34.27 (2.08) | −0.03 (−0.28, 0.23) | 0.83 | −0.01 (−0.26, 0.25) | 0.94 |
| Arm Circumference (cm) | 11.23 (1.01) | 11.18 (1.12) | 0.05 (−0.09, 0.19) | 0.48 | 0.04 (−0.10, 0.17) | 0.60 |
| Abdominal Circumference (cm) | 32.75 (2.06) | 32.76 (2.31) | −0.01 (−0.28, 0.27) | 0.96 | −0.02 (−0.30, 0.25) | 0.86 |
| Biceps SFTM (mm) | 4.37 (1.12) | 4.31 (1.13) | 0.06 (−0.08, 0.21) | 0.39 | 0.06 (−0.09, 0.20) | 0.45 |
| Triceps SFTM (mm) | 5.45 (1.30) | 5.41 (1.44) | 0.03 (−0.14, 0.21) | 0.70 | 0.02 (−0.16, 0.19) | 0.85 |
| Subscapular SFTM (mm) | 5.15 (1.30) | 5.11 (1.21) | 0.04 (−0.12, 0.20) | 0.64 | 0.01 (−0.15, 0.18) | 0.90 |
| Suprailiac SFTM (mm) | 5.76 (1.83) | 5.75 (1.92) | 0.01 (−0.24, 0.26) | 0.95 | −0.01 (−0.25, 0.24) | 0.97 |
| Abdominal SFTM (mm) | 3.85 (1.02) | 3.82 (1.06) | 0.03 (−0.11, 0.16) | 0.71 | 0.01 (−0.12, 0.15) | 0.85 |
| Thigh SFTM (mm) | 6.99 (1.85) | 7.02 (1.90) | −0.03 (−0.27, 0.21) | 0.82 | −0.04 (−0.28, 0.20) | 0.74 |
| Sum of SFTM | 31.38 (6.90) | 31.35 (7.22) | 0.03 (−0.93, 0.98) | 0.96 | −0.08 (−1.02, 0.86) | 0.86 |
| Abdominal Circumference to Length Ratio |
0.65 (0.04) | 0.65 (0.04) | 0.00 (−0.00, 0.01) | 0.86 | −0.00 (−0.01, 0.00) | 0.90 |
| Fat Mass (g) | 522.72 (180.70) | 523.48 (189.05) | −0.77 (−25.11, 23.57) | 0.95 | −0.86 (−25.30, 23.58) | 0.94 |
| Fat Free Mass (g) | 3026.64 (339.96) | 3030.07 (362.54) | −3.43 (−49.68, 42.83) | 0.88 | 0.90 (−44.72, 46.52) | 0.97 |
| Percentage Body Fat | 14.41 (3.39) | 14.37 (3.44) | 0.04 (−0.41, 0.49) | 0.85 | 0.03 (−0.43, 0.48) | 0.91 |
| Percentage Fat Free Mass | 85.59 (3.39) | 85.63 (3.44) | −0.04 (−0.49, 0.41) | 0.85 | −0.03 (−0.48, 0.43) | 0.91 |
| Fat Free Mass R0 (g)^ | 3096.62 (320.97) | 3133.15 (348.92) | −16.54 (−82.59, 49.52) | 0.62 | −19.68 (−85.27, 45.91) | 0.56 |
| Percentage Fat Free Mass R0^ | 88.98 (2.98) | 89.10 (3.40) | −0.13 (−0.76, 0.51) | 0.69 | −0.09 (−0.71, 0.45) | 0.79 |
Values are mean (SD) and treatment effects are differences in means.
Adjusted for centre, parity, BMI category, age, SES quintile and smoking status.
Includes all infants who had anthropometric measurements taken.
Includes 394 infants who had bio-impedance analysis undertaken (215 infants Lifestyle Advice and 179 infants Standard Care).
In 21 infants, two observers obtained anthropometric measurements. The ICC for skinfold thickness measures ranged between 0.55 and 0.88, and was 0.92 (SEM 0.75) for the calculation of percentage body fat. The SEM ranged from 0.42mm to 0.86mm for SFTM. The percentage of infants whose repeated skinfold thickness measurements were different by <1mm ranged from 52.4% (suprailiac SFTM) to 90.5% (subscapular and lateral abdominal SFTM), while calculated body fat percentage was different by less than 1.0% for 55.0% of infants (Table 3).
TABLE 3.
Inter-observer reliability and agreement for neonatal anthropometric measurements
| Outcome | Mean (SD) (N=21*) |
Intraclass Correlation Coefficient |
Standard Error of Measurement (mm or %) |
N (%) where Absolute Difference in Measurements Between Observers <1mm or 1% |
|---|---|---|---|---|
| Biceps SFTM (mm) | 4.74 (0.91) | 0.55 | 0.61 | 14 (66.67) |
| Triceps SFTM (mm) | 5.61 (0.91) | 0.74 | 0.47 | 17 (80.95) |
| Subscapular SFTM (mm) | 5.76 (1.28) | 0.88 | 0.45 | 19 (90.48) |
| Suprailiac SFTM (mm) | 5.51 (1.62) | 0.73 | 0.86 | 11 (52.38) |
| Abdominal SFTM (mm) | 3.91 (0.77) | 0.73 | 0.41 | 19 (90.48) |
| Thigh SFTM (mm) | 7.59 (1.60) | 0.72 | 0.86 | 13 (61.90) |
| Percent Body Fat | 15.44 (2.60) | 0.92 | 0.75 | 11 (55.00) |
Includes all infants who had anthropometric measurements taken by two research assistants.
DISCUSSION
Main Findings
Our findings indicate that provision of a dietary and lifestyle intervention during pregnancy for women who are overweight or obese was not associated with clinically important or statistically significant differences in anthropometric measures of newborn infant body composition. The intra-class correlation coefficient in obtaining skinfold thickness measurements was moderate to excellent (ICC 0.55 to 0.88), validating their use in a large clinical trial setting.
Strengths and Limitations
Our randomised trial is the largest reported to date evaluating the effects of an antenatal lifestyle intervention for women who are overweight or obese during pregnancy on neonatal anthropometric measures, and utilised robust methodology, both of which have been limitations in the research literature to date. Despite the sample size of this analysis of secondary outcomes involving almost 1,000 infants, this represents only 45% of all live born infants in the LIMIT trial. However, we consider the possibility of selection bias to be low, as the characteristics of both the infants for whom anthropometric measures were available, and their mothers, were similar between treatment groups, and to the full-randomised groups.18,19
While a number of techniques are available to assess neonatal body composition, no single measure is without limitations. Measurement of infant skinfold thickness32,33 and bio-impedance are considered reliable and relatively non-invasive methods of assessing fat distribution, both of which have been correlated with more invasive measures.28,32,34,35 Furthermore, percentage fat, as determined by skinfold thickness measurements has been validated against DXA calculations of fat mass in both infants and children.32,36 Our adherence to a standardised protocol, with moderate to excellent inter-observer agreement in obtaining measurements, validates their use in a large clinical trial setting, where comparisons of infant body composition between treatment groups are required.
Bio-impedance analysis provides an estimate of total body water from which the proportions of fat free mass (hydrous) and fat mass (anhydrous) can be derived.28,29,37 There are limitations however, to the use of bio-impedance analysis in newborn infants, whose adipose tissue is more vascularised, and whose adipose and lean tissue both contain a greater proportion of water, when compared with adults.28,29 Therefore, while estimates of total body water in newborn infants may be accurate, transformation to calculate fat free mass may be less reliable.28,29 Alternate methods of assessment of infant body composition may have yielded more accurate estimates in the relative proportions of adipose and lean tissue mass, however they were not feasible for use on a large scale and within the practical constraints of our trial setting.
Interpretation
Our findings of a lack of effect of dietary modification on neonatal anthropometric measures of body composition, is in contrast to our previously reported findings.18,19,21 Specifically, we have reported a significant 18% reduction in the incidence of infants with birth weight above 4.0kg,18 and a 41% reduction in incidence of birth weight above 4.5kg,19 following improvements in maternal diet and physical activity.21
We investigated whether the lack of significant intervention effects on measures of neonatal body composition could be explained by an attenuated effect of the intervention on high infant birth weight in the subgroup of infants with available body composition measures. However, we found that the estimated intervention effect on high infant birth weight in this subgroup was similar to that obtained overall, although the effect was no longer statistically significant due to the smaller sample size.
We also considered whether the intervention influenced body composition, but only at the extremes of the distribution, which is consistent with our previously reported reduction in high birth weight infants, with no effect on mean infant birth weight.18 We did not find any evidence to support this, though we were likely insufficiently powered to detect effects in the extremes of the distribution curves, and thus larger studies to investigate this possibility may be warranted.
Our findings, among women who are overweight or obese, do not suggest that intervention effects on neonatal adiposity and anthropometric measures are modified by maternal BMI. However, the relationship between maternal BMI and neonatal adiposity is further complicated by maternal gestational weight gain. When considering women of normal BMI, increasing gestational weight gain has been associated with an increase in both neonatal fat mass and the percentage of body fat.38 Other studies involving women across all BMI categories similarly identify a positive association between increasing gestational weight gain and infant birth weight,14,39 fat mass,40 and percentage body fat.11,39 However, these findings are not universal, with others identifying no associations between gestational weight gain and either percentage body fat14 or fat free mass.14,40 We did not control for maternal gestational weight gain when estimating the effect of the intervention on neonatal body composition, as this was measured after the time of randomisation. However, we have previously shown that gestational weight gain was very similar between women in the lifestyle advice and standard care groups,18 and is therefore unlikely to have substantially influenced our findings.
Within the literature, there appears to be a differential effect of gestational weight gain on neonatal measures of adiposity according to maternal pre-pregnancy or early pregnancy BMI category. Waters and colleagues evaluated neonatal body composition by assessment of skinfold thickness measurements in 439 mother-infant pairs across a range of maternal BMI categories, where over 55% of women were either overweight or obese.41 In this cohort, while gestational weight gain above the Institute of Medicine recommendations among women of normal BMI was positively associated with neonatal fat mass, there was no evidence of an effect among infants born to women who were overweight or obese.41 These findings suggest firstly that among women who are overweight or obese, maternal BMI and gestational weight gain are not driving fetal growth and therefore neonatal adiposity, to the same extent as may occur among women of normal BMI. Furthermore, any potential beneficial effect in limiting gestational weight gain as a tool to modify neonatal adiposity, is therefore more likely to be observed among women with a normal pre-pregnancy or early pregnancy BMI, rather than among women who are overweight or obese.
CONCLUSION
Clinical Recommendations
Modest changes in maternal dietary intake and physical activity impact on clinically relevant infant outcomes,18,19,21 providing important opportunities for antenatal education and behaviour change, despite no demonstrable effect on neonatal adiposity and body composition.
Research Recommendations
Despite our lack of effect on neonatal adiposity, it remains important to continue to follow-up the children whose mother’s participated in this intervention trial, particularly in view of the well recognised association between high infant birth weight and subsequent childhood obesity.42 Further studies to evaluate the effect of dietary and lifestyle interventions at the extremes of the neonatal body composition distribution curves are warranted.
Supplementary Material
Acknowledgments
Financial Disclosure
This project was funded by a four-year project grant from the National Health and Medical Research Council (NHMRC), Australia (ID 519240).
JM Dodd is supported through a NHMRC Practitioner Fellowship (ID 627005).
LN Yelland is supported through a NHMRC Early Career Fellowship (ID 1052388).
RM Grivell is supported through a NHMRC Early Career Fellowship (ID 1073514).
Infrastructure support was provided by The University of Adelaide, and the Women’s and Children’s Hospital, Flinders Medical Centre, and Lyell McEwin Hospital, Adelaide.
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The following persons and institutions (except where indicated, in Adelaide, South Australia) participated in the LIMIT Trial:
Steering Group – JM Dodd (Chair), D Turnbull, A McPhee, RM Grivell, C Crowther, M Gillman (Obesity Prevention Program, Department of Population Medicine, Harvard Medical School, and Harvard Pilgrim Health Care Institute, Boston, Massachusetts, USA), G Wittert, JA Owens, JS Robinson
Co-ordinating Team – JM Dodd, A Deussen, RM Grivell, L Yelland, L Moran, C Cramp, A Newman, L Kannieappian, S Hendrijanto, M Kelsey, J Beaumont, C Danz, J Koch, A Webber, C Holst, K Robinson, S Zhang, V Ball, K Ball, H Deussen, N Salehi, R Bartley, R Stafford-Green, S Ophel, M Cooney, M Szmeja, A Short, A Melrose, S Han, I Mohamad, L Chapple
Statistical Analyses – L Yelland
Serious Adverse Events Committee – RM Grivell, J Svigos, V Bhatia, N Manton
Writing Group – JM Dodd, D Turnbull, A McPhee, A Deussen, RM Grivell, L Yelland, C Crowther, G Wittert, JA Owens, JS Robinson
Collaborating Hospitals (total number of women recruited from each site in parentheses), *indicates named associate investigator for the NHMRC grant.
Flinders Medical Centre (South Australia) (669): J McGavigan*, R Bryce, S Coppi, C Fanning, G Hannah, M Ignacio, H Pollard, F Schmidt, Y Shinners
Lyell McEwin Hospital (South Australia) (505): G Dekker*, S Kennedy-Andrews, R Beaven, J Niven, S Burgen, J Dalton, N Dewhurst, L Forst, V Mugg, C Will, H Stone
Women’s and Children’s Hospital (South Australia) (1,038): JM Dodd, JS Robinson, A Deussen, C Crowther*, C Wilkinson*, H Purcell, J Wood, D Press, K Ralph, S Donleavy, S Seager, F Gately, A Jolly, L Lahnstein, S Harding, K Daw, M Hedges, R Fraser-Trumble
We are indebted to the 2,212 women who participated in this randomised trial.
Footnotes
Clinical Trial Registration: Australian and New Zealand Clinical Trials Registry (ACTRN12607000161426)
Author Contributions
Each author fulfils the requirements for authorship. All authors have been involved equally in the study concept and design of the trial, supervision of conduct of the trial, the acquisition of data, the analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and provides approval of the final submitted version. J Dodd, S Rifas-Shiman, L Yelland, and J Louise were responsible for conducting the statistical analyses. J Dodd drafted the manuscript, had full access to all of the study data, and takes responsibility for the integrity of the data, and the accuracy of the data analysis.
Competing Interests
The authors have no competing interests to declare.
REFERENCES
- 1.Chu SY, Kim SY, Bish CL. Prepregnancy obesity prevalence in the United States, 2004–2005. Maternal Child Health J. 2008 doi: 10.1007/s10995-008-0388-3. (July 10 (Epub)) [DOI] [PubMed] [Google Scholar]
- 2.Scheil W, Scott J, Catcheside B, Sage L. Pregnancy Outcome Unit SA Health. Adelaide: Government of South Australia; 2014. Pregnancy outcome in South Australia 2012. [Google Scholar]
- 3.Callaway LK, Prins JB, Chang AM, McIntyre HD. The prevalence and impact of overweight and obesity in an Australian obstetric population. MJA. 2006;184(2):56–59. doi: 10.5694/j.1326-5377.2006.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 4.Dodd JM, Grivell RM, Nguyen A-M, Chan A, Robinson JS. Maternal and perinatal health outcomes by body mass index category. ANZJOG. 2011;51(2):136–140. doi: 10.1111/j.1479-828X.2010.01272.x. [DOI] [PubMed] [Google Scholar]
- 5.Abenhaim HA, Kinch RA, Morin L, Benjamin A, Usher R. Effect of prepregnancy body mass index categories on obstetrical and neonatal outcomes. Arch Gynecol Obstet. 2007;275:39–43. doi: 10.1007/s00404-006-0219-y. [DOI] [PubMed] [Google Scholar]
- 6.Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103:219–224. doi: 10.1097/01.AOG.0000107291.46159.00. [DOI] [PubMed] [Google Scholar]
- 7.Cnattingius S, Bergstrom R, Lipworth L, Kramer MS. Prepregnancy weight and the risk of adverse pregnancy outcomes. New England Journal of Medicine. 1998;338:147–152. doi: 10.1056/NEJM199801153380302. [DOI] [PubMed] [Google Scholar]
- 8.Tikellis G, Ponsonby AL, Wells JC, Pezic A, Cochrane J, Dwyer T. Maternal and infant factors associated with neonatal adiposity: Results from the Tasmanian Infant Health Survey (TIHS) Int J Obes. 2012;36(4):496–504. doi: 10.1038/ijo.2011.261. [DOI] [PubMed] [Google Scholar]
- 9.Fraser A, Tilling K, Macdonald-Wallis C, et al. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation. 2010;121:2557–2564. doi: 10.1161/CIRCULATIONAHA.109.906081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mamun AA, Kinarivala M, O'Callaghan MJ, Williams GM, Najman JM, Callaway LK. Associations of excess weight gain during pregnancy with long-term maternal overweight and obesity: evidence from 21y postpartum follow-up. Am J Clin Nutr. 2010;91:1336–1341. doi: 10.3945/ajcn.2009.28950. [DOI] [PubMed] [Google Scholar]
- 11.Hull HR, Dinger MK, Knehans AW, Thompson DM, Fields DA. Impact of maternal body mass index on neonate birthweight and body composition. American Journal of Obstetrics and Gynecology. 2008;198(4):416 e1–416 e6. doi: 10.1016/j.ajog.2007.10.796. [DOI] [PubMed] [Google Scholar]
- 12.Modi N, Murgasova D, Ruager-Martin R, et al. The influence of maternal body mass index on infant adiposity and hepatic lipid content. Pediatr Res. 2011;70(3):287–291. doi: 10.1203/PDR.0b013e318225f9b1. [DOI] [PubMed] [Google Scholar]
- 13.Sewell MF, Huston-Presley L, Super DM, Catalano PM. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. American Journal of Obstetrics and Gynecology. 2006;195(4):1100-003. doi: 10.1016/j.ajog.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson B, Löf M, Forsum E. Body composition in full-term healthy infants measured with air displacement plethysmography at 1 and 12 weeks of age. Acta Paediatr. 2010;99(4):563–568. doi: 10.1111/j.1651-2227.2009.01665.x. [DOI] [PubMed] [Google Scholar]
- 15.Dodd JM, Grivell RM, Crowther CA, Robinson JS. Antenatal interventions for overweight or obese pregnant women: a systematic review of randomised trials. British Journal of Obstetrics and Gynaecology. 2010;117(11):1316–1326. doi: 10.1111/j.1471-0528.2010.02540.x. [DOI] [PubMed] [Google Scholar]
- 16.Oteng-Ntim E, Varma R, Croker H, Poston L, Doyle P. Lifestyle interventions for overweight and obese pregnant women to improve pregnancy outcome: systematic review and meta-analysis. BMC Med. 2012 May 10;10:47. doi: 10.1186/1741-7015-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thangaratinam S, Rogozinska E, Jolly K, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ. 2012 May 16;344:e2088. doi: 10.1136/bmj.e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dodd JM, Turnbull DA, McPhee AJ, et al. Antenatal lifestyle advice for women who are overweight or obese: the LIMIT randomised trial. BMJ. 2014;348:g1285. doi: 10.1136/bmj.g1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dodd JM, McPhee AJ, Turnbull DA, et al. The effect of antenatal lifestyle advice for women who are overweight or obese on neonatal health: the LIMIT randomised trial. BMC Med. 2014;12:163. doi: 10.1186/s12916-014-0163-9. http://www.biomedcentral.com/1741-7015/12/163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dodd JM, Kannieappan LM, Grivell RM, et al. Effects of an antenatal dietary intervention on maternal anthropometric measures in pregnant women with obesity. Obesity (Silver Spring) 2015;23(8):1555–1562. doi: 10.1002/oby.21145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dodd JM, Cramp CS, Sui Z, et al. Effects of antenatal lifestyle advice for women who are overweight or obese on maternal diet and physical activity: the LIMIT randomised trial. BMC Med. 2014;12:161. doi: 10.1186/s12916-014-0161-y. http://www.biomedcentral.com/1741-7015/12/161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dodd JM, Turnbull DA, McPhee AJ, Wittert G, Crowther CA, Robinson JS. Limiting weight gain in overweight and obese women during pregnancy to improve health outcomes: the LIMIT randomised controlled trial. BMC Pregnancy and Childbirth. 2011 Oct 26;11:79. doi: 10.1186/1471-2393-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Australian Guide to Health Eating. http://www.health.gov.au/internet/main/publishing.nsf/content/health-pubhlth-publicat-document-fdcons-cnt.htm.
- 24.Royal College of Obstetricians and Gynaecologists RCOG. Recreational exercise and pregnancy: information for you. RCOG Press; 2006. [Google Scholar]
- 25.Horan MK, McGowan CA, Gibney ER, Donnelly JM, McAuliffe FM. Maternal low glycaemic index diet, fat intake and postprandial glucose influences neonatal adiposity--secondary analysis from the ROLO study. Nutr J. 2014 Aug 1;13:78. doi: 10.1186/1475-2891-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marfell-Jones M, Olds T, Stewart A, Carter L. International standards for anthropometric assessment. The International Society for the Advancement of Kinanthropometry. 2006 [Google Scholar]
- 27.Deierlein A, Thornton J, Hull H, Gallagher D. An anthropomettric model to estimate neonatal fat mass using air displacement pelthysmography. Nutrition & Metabolism. 2012;9:21. doi: 10.1186/1743-7075-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lingwood BE, Dodrill P, Davies PS, Callaway L, Colditz P. Measurement of infant body composition using the peapod and bioimpedance analysis. J Paed Child Health. 2008;44(Suppl 1):A99. [Google Scholar]
- 29.Lingwood BE, Storm van Leeuwen AM, Carberry AE, et al. Prediction of fat-free mass and percentage of body fat in neonates using bioelectrical impedance analysis and anthropometric measures: validation against the PEA POD. Br J Nutr. 2012;107(10):1545–1552. doi: 10.1017/S0007114511004624. [DOI] [PubMed] [Google Scholar]
- 30.Shrout PE. Intraclass correlation: uses in assessing rater reliability. Psychological Bulletin. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 31.de Vet HCW, Knol DL, Bouter LM. When to use agreement versus reliability measures. Journal of Clinical Epidemiology and Community Health. 2006;59:1033–1039. doi: 10.1016/j.jclinepi.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 32.Schmelzle HR, Fusch C. Body fat in neonates and young infants: validation of skinfold thickness versus dual-energy X-ray absorptiometry. Am J Clin Nutr. 2002;76:1096–1100. doi: 10.1093/ajcn/76.5.1096. [DOI] [PubMed] [Google Scholar]
- 33.Gillman MW, Rich-Edwards JW, Huh S, et al. Maternal corticotrophin-releasing hormone levels during pregnancy and offspring adiposity. Obesity. 2006;14:1647–1653. doi: 10.1038/oby.2006.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomson R, Brinkworth GD, Buckley JD, Noakes M, Clifton PM. Good agreement between bioelectrical impedance and dual energy X-ray absorptiometry for estimating changes in body composition during weight loss in overweight young women. Clin Nutr. 2007;26(6):771–777. doi: 10.1016/j.clnu.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Volgyi E, Tylavsky FA, Lyytikainen A, Suominen H, Alen M, Cheng S. Assessing body composition with DXA and bioimpedance: effects of obesity, physical activity and age. Obesity. 2008;16(3):700–705. doi: 10.1038/oby.2007.94. [DOI] [PubMed] [Google Scholar]
- 36.Godang K, Qvigstad E, Voldner N, et al. Assessing body composition in healthy newborn infants: reliability of dual-energy x-ray absorptiometry. J Clin Densitom. 2010;13(2):151–160. doi: 10.1016/j.jocd.2010.01.121. [DOI] [PubMed] [Google Scholar]
- 37.Koletzko B, Brands B, Poston L, Godfrey K, Demmelmair H for the Early Nutrition Project. Early nutrition programming of long-term health. Proc Nutr Soc. 2012;71(3):371–378. doi: 10.1017/S0029665112000596. [DOI] [PubMed] [Google Scholar]
- 38.Josefson JL, Hoffmann JA, Metzger BE. Excessive weight gain in women with a normal pre-pregnancy BMI is associated with increased neonatal adiposity. Pediatr Obes. 2013;8(2):e33–e36. doi: 10.1111/j.2047-6310.2012.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Au CP, Raynes-Greenow CH, Turner RM, Carberry AE, Jeffery H. Fetal and maternal factors associated with neonatal adiposity as measured by air displacement plethysmography: a large cross-sectional study. Early Hum Dev. 2013;89(10):839–843. doi: 10.1016/j.earlhumdev.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 40.Crozier SR, Inskip HM, Godfrey KM, et al. Weight gain in pregnancy and childhood body composition: findings from the Southampton Women's Survey. Am J Clin Nutr. 2010;91(6):1745–1751. doi: 10.3945/ajcn.2009.29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waters TP, Huston-Presley L, Catalano PM. Neonatal body composition according to the revised institute of medicine recommendations for maternal weight gain. J Clin Endocrinol Metab. 2012;97(10):3648–3654. doi: 10.1210/jc.2012-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cunningham SA, Kramer MR, Narayan KM. Incidence of childhood obesity in the United States. N Engl J Med. 2014;370(5):403–411. doi: 10.1056/NEJMoa1309753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.