Abstract
Micro-beam radiation therapy (MRT) uses parallel planes of high dose narrow (10–100 um in width) radiation beams separated by a fraction of a millimeter to treat cancerous tumors. This experimental therapy method based on synchrotron radiation has been shown to spare normal tissue at up to 1000Gy of entrance dose while still being effective in tumor eradication and extending the lifetime of tumor-bearing small animal models. Motion during the treatment can result in significant movement of micro beam positions resulting in broader beam width and lower peak to valley dose ratio (PVDR), and thus can reduce the effectiveness of the MRT. Recently we have developed the first bench-top image guided MRT system for small animal treatment using a high powered carbon nanotube (CNT) x-ray source array. The CNT field emission x-ray source can be electronically synchronized to an external triggering signal to enable physiologically gated firing of x-ray radiation to minimize motion blurring. Here we report the results of phantom study of respiratory gated MRT. A simulation of mouse breathing was performed using a servo motor. Preliminary results show that without gating the micro beam full width at tenth maximum (FWTM) can increase by 70% and PVDR can decrease up to 50%. But with proper gating, both the beam width and PVDR changes can be negligible. Future experiments will involve irradiation of mouse models and comparing histology stains between the controls and the gated irradiation.
Keywords: Micro-beam Radiation Therapy, MRT, Physiological Gating, Field Emission, CNT Cathodes
1. INTRODUCTION AND BACKGROUND
1.1. Microbeam radiation therapy
Microbeam radiation therapy (MRT) is an experimental therapy method that uses arrays of microscopically thin fan-beam X-radiation for the treatment of various radio-resistant and deep-seated tumors1. Experimentally discovered, it was shown in small animal tumor models that the absorbed dose threshold for tissue damage using micron-width beams was on the order of several hundred Gy, orders of magnitude higher than that for broad beam radiation2. Dose profiles consisting of micro-planar beams that are <100um in width and spaced by up to 500um3 have been shown to ablate brain tumors in rats while sparing normal tissue in a variety of animals.
Although the concept has been around for decades, it has only been successfully implemented in recent years at a few synchrotron light sources3. This is due to the ultra-high dose rates (>100 Gy/s) available at synchrotron facilities allowing for the instantaneous delivery of the high dose in tens of milliseconds, thus maintaining the micro-beam profile despite the physiological motion which is on the order of hundreds of milliseconds. Another advantage of using a synchrotron is that the near parallel beam and high dose rate make it easier to collimate the beam into parallel micro-planar beams.
However, being limited to a synchrotron facility for beam generation poses problems for clinical studies. To counter this problem, research was done with thicker beams, mini-beams, and it was shown that the tolerances still remain high. An experimental irradiation of the spinal cords, using 680um wide beams at 400Gy with 4mm spacing and 170 Gy at 1.36mm spacing, showed that 7 of the 8 mice tested had no paralysis or behavioral changes4.
One of the factors in determining the effectiveness of MRT treatment is the measurement of the valley dose – the dose between the microbeams. Though the actual biology behind the treatment is not fully understood, one of the possibilities being that the valley areas harbor tissue-reparative cells and thus must be kept at a low enough dose level to restore the damaged healthy tissue. All current theories on why MRT work are based on a low valley dose compared to the microbeam dose5. The PVDR in synchrotron studies ranges from 10 to 100 depending on the procedure.
1.2. Compact image guided MRT system for small animal treatment
Taking advantage of the unique capabilities of the carbon nanotube (CNT) x-ray source array technology5,6 we have recently developed a compact image-guided MRT system for treatment of small animal tumor models7.8. The device uses a spatially distributed CNT x-ray source array to generate a linear focused x-ray beam which is then collimated to microbeam radiation. The microbeam irradiator is integrated with a home-made micro-CT scanner for registration and image guidance. To increase the throughput, two mice can be irradiated at the same time with independent anesthesia and physiological monitoring capacity. Preliminary results have demonstrated the capability of targeted delivery of 100Gy of 160kVp microbeam radiation to the brain tumor sites in mouse models.
With the desktop system, a way to minimize physiological motion induced microbeam blurring is necessary because of the low dose rate as compared to what is used in synchrotron facilities. MRT research so far is mainly focused on the brain tumors. The largest intracranial motion occurs in the optic chiasm with peak displacement of 240um, with the brain stem, midbrain, and medulla displaced by 200 um. The smallest displacements occur in the occipital lobe, cerebellum, and frontal and parietal lobes (40um, 70um, 90um, and 90um respectively)9. This motion during the exposure time will broaden the width of the radiation track and potentially reduce the effectiveness of the microbeam radiation. Motion blur will become more prevalent in the abdomen due to breathing.
Unlike traditional thermionic x-ray sources, field emission cathodes can be electronically modulated and thus the delivery of the radiation can be programmed and gated to an arbitrary signal such as respiration and cardiac signals. Respiration and cardiac gating has already been applied in-vivo with small animal CT imaging with better than 100um accuracy10.
The goal of the study is to incorporate respiratory gating to our CNT x-ray source array based MRT system to minimize motion blurring and to increase the PVDR by eliminating radiation during mouse motion.
2. MATERIALS AND METHODS
2.1. Method overview
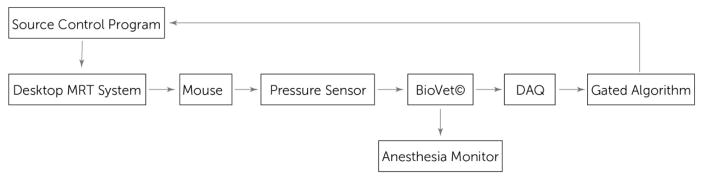
The experimental setup is summarized in a block diagram shown below in Fig. 2.
Figure 2.
Gated MRT block diagram
The system output is controlled completely using LabVIEW and its data acquisition system (DAQ). Standard operation of the tube without gating would look the same only without the gated algorithm block. The mouse is connected to a pressure sensor that monitors its breathing rate, which is read in using BioVet© and output to the LabVIEW code, which then syncs its control signal output to the breathing rate. A more detailed explanation of the algorithm is in section 2.4 below.
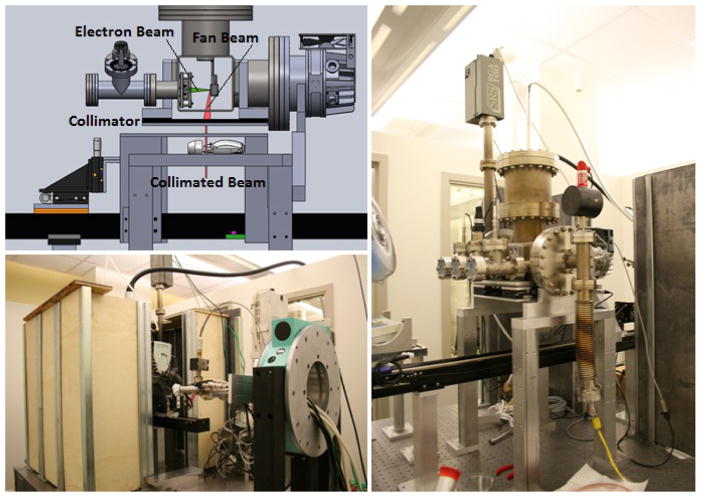
2.2. Desktop MRT System
The MRT image guided system is shown in Figure 3. The CNT microbeam irradiator is integrated with a homemade micro-CT scanner3,11, a precision mouse-positioning device, a registration and treatment planning software package (Micro-PLUNC, developed at UNC Radiation Oncology), a gas anesthesia unit, and physiological monitoring capability. To increase the throughput two mice can be irradiated at the same time with independent anesthesia and physiological monitoring capacity.
Figure 3.
Desktop MRT system with integrated micro-CT
The high-power linear CNT x-ray source array consists of a linear electron source with 5 linear CNT cathodes which emit under a bias extraction field, an electrostatic focusing lens and an opposing stationary W anode. The electron beam is focused down to a narrow focal track on the anode with approximately 200um in width and 150mm in length. The x-ray source array is operated at 160kVp anode voltage and a tube current of 30mA. The intrinsically divergent radiation is collimated into microbeam using a motorized microbeam collimator placed between the x-ray window and the object. In the present system, parallel planes of microbeams are generated by translating the object in the direction perpendicular to the microbeam plane in a step and shoot fashion. A high peak-valley-dose-ratio (PVDR) of 10 was obtained at 4:1 pitch/width ratio.
A pulse generator is used to produce x-rays at scheduled intervals for specific amounts of time. In other experiments where gating is not used, the tube is operated at an 8% duty cycle using 500 um width pulses. The beam delivers a dose a 0.9Gy/minute. The setup is shown in Fig. 3.
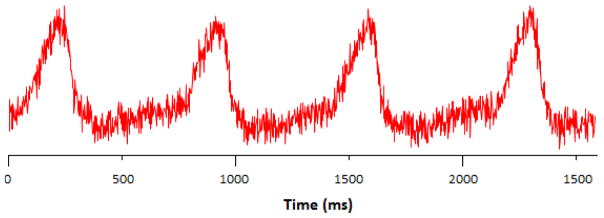
2.3. Physiological Motion Monitor
Actual respiratory motion signal of the mouse (Fig. 5) was obtained using a physiological monitoring and triggering system (BioVet©, Spin Systems, South Brisbane, Australia). The respiration signal is obtained from a respiration sensor pad that would be placed on the surface of the animal under the abdomen to monitor chest cavity motion10,12,13. The period is 800ms and the duty cycle measured at full width-half max is approximately 25%, or 200ms. This means that between each breath, there is a ~600ms range when the body is motionless.
Figure 5.
BioVet© respiratory signal from an anesthetized adult mouse
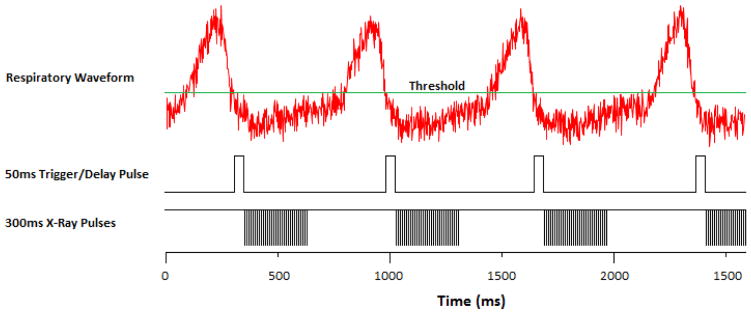
2.4. Gated MRT Algorithm
As the field emission x-ray beam is electronically controlled, gating is possible. This is especially useful in MRT due to motion blur resulting in a higher valley dose and destroying the main principle behind micro-beam therapy. A pressure sensor will be attached to a mouse’s chest and its respiration will be monitored. A sedated mouse breathes at ~70 bpm with 25% of the period taken up by the breathing motion, as shown in Fig. 5. BioVet© comes with a threshold trigger output which will be set to trigger at the beginning of the 75% motionless state. To make sure the mouse is completely motionless, the trigger will be set to 50ms to act as a delay pulse as well. The x-ray exposure has a negative edge trigger and pulses at an 8% duty cycle of 500us wide pulses for 300ms. The pulse diagram is shown below in Fig. 6.
Figure 6.
X-ray exposure pulse diagram
2.5. Feasibility Study
We ran a simulation of a mouse breathing using a servo motor pushing on a pressure sensor for 800ms at a 25% duty cycle. The setup in shown in Fig. 7 and 8. The 50mm long arm was set to move 1.5 degrees, equating to a motion of about 1mm which realistically reflects actual mouse breathing motion of the abdomen and would make a significant blur on the 300um thick beams separated by 900um.
Figure 7.
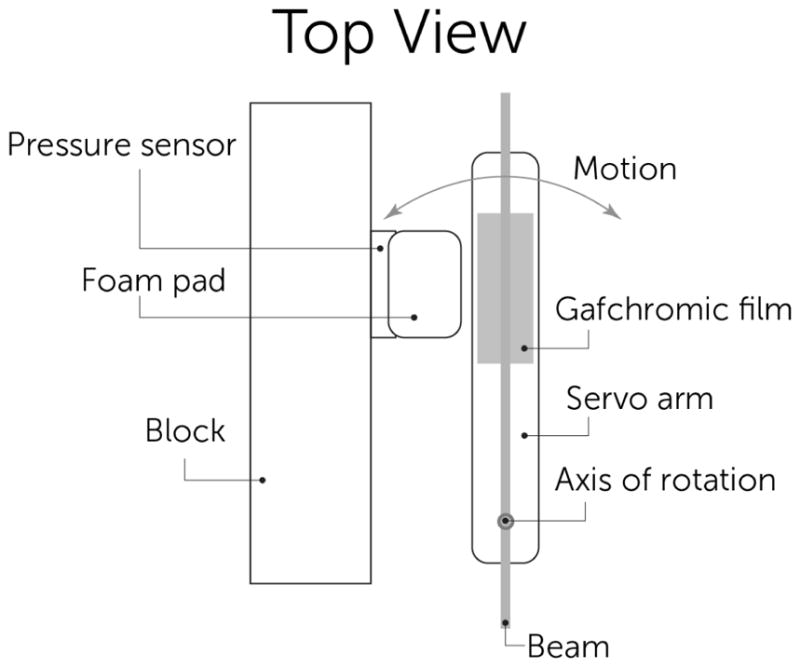
Schematic of mouse motion phantom using servo motor arm
Figure 8.
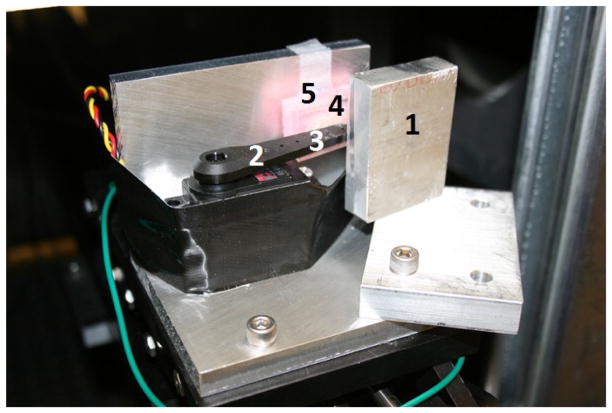
Servo motor phantom setup simulating mouse abdonimal motion during breathing. 1) Aluminum block to prevent arm vibration. 2) Servo arm. 3) Gafchromic film location. 4) Foam pad to simulate abdomen. 5) Pressure sensor behind pad
Two types of experiments were run. The first was a single line irradiation at 160 kVp delivering 0.9 Gy/min for 5 minutes. The second was a three line irradiation, separated by 900um with the same irradiation conditions for each line. We recorded the dose profile by irradiating EBT2 Gafchromic© dosimetry film and scanning and processing the resulting lines.
3. RESULTS
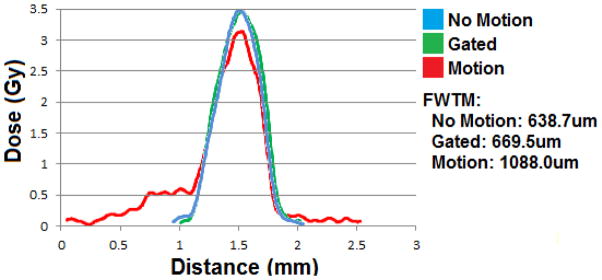
In the single beam case, the film was scanned and analyzed using Film QA Pro©. The program determines the shape of the dose pattern along with the dose delivered. Figure 9 shows an example of dose profiles for a single microbeam in three cases: no motion, motion without gating, motion with gating. When there is no motion, the FWHM of the dose profile is approximately 440um, which is expected from geometrical consideration. When the phantom motion is turned on, the broadening of the dose profile due to motion is clearly seen in the motion control as a side tail. The FWHM of all curves is the same because the motion was not sinusoidal. Had it been, the peak would have truly ‘broadened’ but since the motion was driven by a 25% duty cycle pulse, the movement contribution is seen as the 0.5Gy shelf to the left. A more accurate measurement would be to look at the full width at tenth maximum (FWTM). FWTM increases from 638 to 1088um when the motion is turned on. By applying gating the FWTM is reduced back to 669um, almost the same as that without motion.
Figure 9.
Single beam dose curves for three 5 minute irradiations at 160kVp.
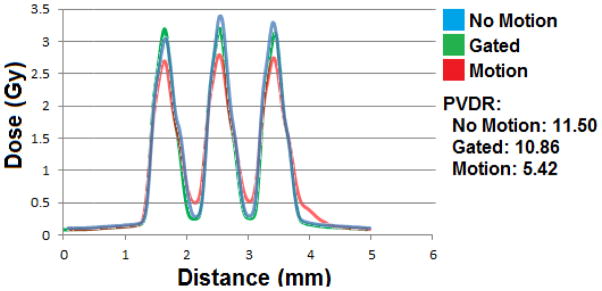
A more important quantity for the effectiveness of the MRT is the PVDR for the multiple line case. Figure 10 shows the beam profiles for three-line irradiations. Here we see how that shelf in the single line affects the PVDR of the multi-line set. The motion cause a 50% drop in PVDR from 11.5 to 5.4 but by effective gating, one can almost recover the PVDR value. There is only 5.5% decrease in PVDR in the case of motion with gating compared to the no motion case. There is also small drop in the peak dose from 3.4Gy to 3.2Gy.
Figure 10.
Three beam dose curves curves for three 5 minute/line irradiations at 160kVp.
4. DISCUSSION AND CONCLUSION
Gated MRT has been shown to reduce valley dose and enhance PVDR between the lines during long-time irradiation of a moving object. The technique allows for more precise MRT treatment and can be expanded to other treatment and imaging modalities as well. This gating is not limited to the breathing motion of the abdomen and can easily be expanded to cardiac synced motion in the brain with cardiac gating.
Field emission CNT MRT has also been shown to be the only system currently able to safely gate to any physiological motion. Synchrotron facilities use physical shutters to control the beam with little safety features and are limited by their motor speed and accuracy. Electronic control allows for many types of integration, including physiological gating, with the irradiation system.
In a future study, a healthy mouse will be used to verify the phantoms study results. We will use a control and experiment group and deliver 3 MRT beams to the liver and verify the PVDR difference between normal and gated MRT with histology.
Figure 1.
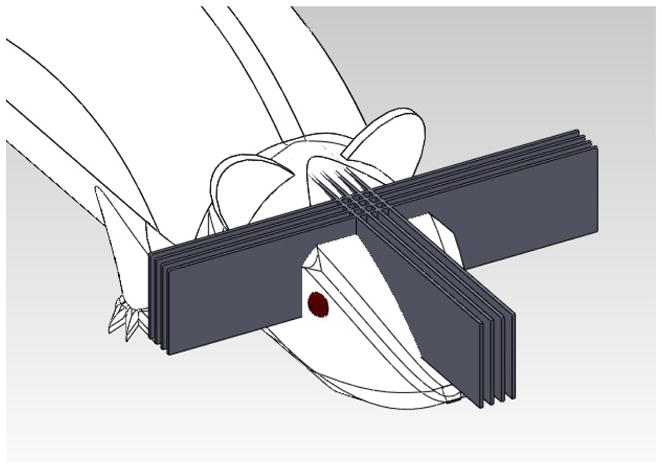
MRT beam visualization using a crosshatched irradiation pattern
Figure 4.
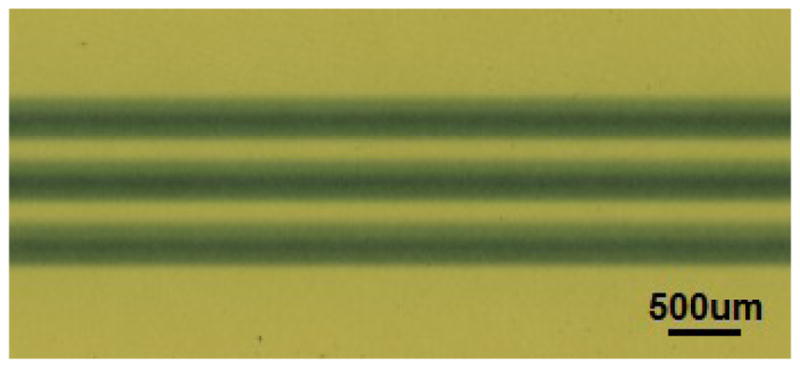
Stationary micro-beam lines shown on EBT2 Gafchromic© film from desktop MRT source.
Acknowledgments
National Cancer Institute U54CA119343
References
- 1.Slatkin D, Spanne P, Dilmanian F, Sandborg M. Microbeam Radiation Therapy. Med Phys. 1992;19(6):1395–400. doi: 10.1118/1.596771. [DOI] [PubMed] [Google Scholar]
- 2.Laissue J, et al. Microbeam radiation therapy. Proc SPIE. 1999;38:3770. [Google Scholar]
- 3.Renier M, Brochard T, Nemoz C, Requardt H, Bräuer E, Esteve F, Balosso J, Suortti P, Baruchel J, Elleaume H, Berruyer G, Berkvens P, Bravin A. The radiotherapy clinical trials projects at the ESRF: Technical aspects. Eur J Radiol. 2008;68:147–50. doi: 10.1016/j.ejrad.2008.04.057. [DOI] [PubMed] [Google Scholar]
- 4.Dilmanian A, et al. Interlaced x-ray microplanar beams: A radiosurgery approach with clinical potential. Proc Natl Acad Sci. 2006;103(25):9709–9714. doi: 10.1073/pnas.0603567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee Y, Burk L, Wang K, Cao G, Lu J, Zhou O. Carbon nanotube based X-ray sources: Applications in pre-clinical and medical imaging. Nuclear Instruments and Methods in Physics Research. 2011;648:S281–S283. doi: 10.1016/j.nima.2010.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang S, Calderon X, Peng R, Schreiber EC, Zhou O, Chang S. A carbon nanotube field emission multipixel x-ray array source for microradiotherapy application. Applied physics letters. 2011;98(21):213701–213701. doi: 10.1063/1.3595268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang S, Zhou O. Multi-pixel electron microbeam irradiator systems and methods for selectively irradiating predetermined locations. 7,220,971. US Patent. filed December 28, 2005.
- 8.Hadsell M, Zhang J, Cao G, Schreiber E, Lu J, Chang S, Zhou O. Pilot Study for the Development of Clinical Microbeam Radiation Therapy Using a Carbon Nanotube Field Emission Micro-CT Scanner. Med Phys. 2011;38:3862. doi: 10.1118/1.4873683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong X, Meyer CH, Schlesinger DJ, Sheehan JP, Epstein FH, Larner JM, Cai J. Tracking brain motion during the cardiac cycle using spiral cine-DENSE MRI. Medical physics. 2009;36(8):3413. doi: 10.1118/1.3157109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao G, Burk L, Lee Y, Calderon-Colon X, Sultana S, Lu J, Zhou O. Prospective-gated cardiac micro-CT imaging of free-breathing mice using carbon nanotube field emission x-ray. Med Phys. 2010;37:5306–12. doi: 10.1118/1.3491806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dilmanian F, Button T, Le Duc G, et al. Response of the rat intracranial 9L gliosarcoma to Microbeam Radiation Therapy (MRT) Neuro-Oncol. 2002;4:26–38. doi: 10.1215/15228517-4-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao G, Lee YZ, Peng R, Liu Z, Rajaram R, Calderon-Colon X, et al. A dynamic micro-CT scanner based on a carbon nanotube field emission x-ray source. Phys Med Biol. 2009;54(8):2323–40. doi: 10.1088/0031-9155/54/8/005. [DOI] [PubMed] [Google Scholar]
- 13.Lee Y, Burk L, Wang K, Cao G, Volmer J, Lu J, Zhou Prospective Respiratory Gated Carbon Nanotube Micro Computed Tomography. Academic Radiology. 2011;18:588–593. doi: 10.1016/j.acra.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]