Abstract
Background
CDH13 (cadherin 13) is a special cadherin cell adhesion molecule, and the methylation of its promoter causes inactivation in a considerable number of human cancers. To explore the association between CDH13 promoter methylation and breast cancer risk and prognosis, we systematically integrated published articles to investigate the diagnostic performance of the CDH13 methylation test for breast cancer. An independent DNA methylation microarray dataset from The Cancer Genome Atlas project (TCGA) project was used to validate the results of the meta-analysis.
Methods
The relevant literature was searched using the PubMed, Cochrane Library, Web of Science and Google Scholar databases for articles published in English up to May 2015. Data were analyzed using random effect or fixed effect models. The effect sizes were estimated by measuring an odds ratio (OR) or hazard ratio (HR) with a 95% confidence interval (CI). A chi-squared based Q test and sensitivity analysis were performed to examine the between-study heterogeneity and the contribution of single studies to the final results, respectively. Funnel plots were constructed to evaluate publication bias.
Results
Seven hundred and twenty-six breast tumor samples and 422 controls were collected from 13 published studies. The data from the TCGA set include both tumor and normal samples. A significant association was observed between CDH13 promoter methylation and breast cancer, with an aggregated OR equal to 13.73 (95%CI: 8.09~23.31, z = 9.70, p<0.0001) as measured using the fixed effect model and 14.23 (95%CI: 5.06~40.05, z = 5.03, p<0.0001) as measured using a random effect model. The HR values were calculated as 0.77 (95%CI: 0.27~2.21, z = -0.49, p = 0.622) and 0.38 (95%CI: 0.09~1.69, z = -1.27, p = 0.20) for overall survival (OS) and disease-free survival (DFS), respectively, using the random effect model. This result indicated that breast cancer patients with CDH13 promoter methylation correlated non-significantly with prognosis and is therefore similar to the findings of the TCGA project.
Conclusions
The methylation status of CDH13 promoter was strongly associated with breast cancer risk. However, CDH13 promoter methylation was not significantly related to the OS and DFS of breast cancer and may have limited prognostic value for breast cancer patients.
Introduction
Breast cancer is the most commonly diagnosed cancer and the leading cause of cancer death in women worldwide. Globally, there were an estimated 1.7 million new cases (25% of all cancers in women) and 0.5 million cancer deaths (15% of all cancer deaths in women) in 2012 [1]. More than 25% of breast cancer patients develop metastatic disease, which is largely incurable and often limited to palliative therapeutic options [2]. The bottleneck in improving survival is therefore early detection [3]. Because DNA hypermethylation is an important mechanism for tumor suppressor gene inactivation in cancer, the measurement of such methylation could act as a powerful biomarker for the early detection of breast cancer. Therefore, we believe that the measurement of DNA methylation could become a powerful means of breast cancer diagnosis.
The cadherin 13 (CDH13) gene, a new member of the cadherin superfamily, was isolated recently and has been mapped to 16q24 [4]. Cadherins are transmembrane glycoproteins expressed on the epithelial cell surface that mediate intercellular Ca2+-dependent adhesion, which is important for maintaining normal tissue structure. Abnormalities in the CDH13 gene have been identified in human malignancies [5, 6]. Moreover, an association between the abnormal expression of CDH13 and its promoter methylation in lung cancer has been demonstrated [7–9]. However, the diagnostic role of the methylation status of the CDH13 gene in breast cancer lacks quantitative assessment.
Although CDH13 methylation in breast cancer has been reported as an effective biomarker for diagnosis [10, 11], the results differ dramatically between different research studies. Here, we conducted a meta-analysis of the risk and prognosis of CDH13 methylation in relation to breast cancer diagnosis. As we all know, The Cancer Genome Atlas project (TCGA) includes comprehensive clinical and demographic information and has collected hundreds of whole genome DNA methylation microarray datasets of breast cancer samples. Therefore, it provides an additional resource that might be without publication bias. Considering the above factors, we integrated the data from published articles with the data from the TCGA project to evaluate the relationship between CDH13 promoter methylation and breast cancer. Therefore, an integrated analysis with unbiased conclusions was conducted to examine the relationship between CDH13 methylation and breast cancer.
Methods
Search strategy, selection of studies and data extraction
This pooled study involved searching a range of digital databases, including PubMed, Cochrane Library, Web of Science and Google Scholar, for articles published in English up to May 2015. The study used a subject and text word strategy: ‘breast cancer or breast neoplasms or breast carcinoma or mammary cancer’, ‘CDH13 or cadherin 13 or H-cadherin’, ‘methylation or hypermethylation or epigenetic’. The included articles satisfy the following criteria: (1) original study, and the diagnosis of breast cancer was based on histopathology; table) the subjects in every study included breast cancer patients and normal controls; (3) the articles were focused on methylation of the CDH13 promoter; (4) the papers were written in English. Exclusion criteria were as follows: (1) the methylation of CDH13 promoter was conducted only in the cell lines or raw; (2) review papers, commentaries, letters, and studies that examined other associations.
Data were extracted from each study by three independent reviewers (Jingyu Yang and Heng Niu) using pre-specified selection standards. Decisions were made and any disagreements about study selection were resolved by discussion. The following information was extracted from the studies: the first author’s last name, the year of publication, the original country of patients, racial descent (ethnicity), the number of CDH13 methylations in individual cases and controls in individuals, and more. Racial descent was grouped as Caucasian, Asian, or mixed when ethnic origin was unclear.
Meta-analysis
The data we acquired were analyzed and visualized mainly using R (R version 3.2.0) software. The strength of the relationship between CDH13 promoter methylation and the risk/prognosis of breast cancer was measured by a pooled odds ratio (OR)/hazard ratio (HR) with a 95% confidence interval (CI). The significance of the pooled OR was determined by the Z test with a threshold of p<0.05. The I2 statistic was used to test the heterogeneity. I2 values over 50% and Chi-squared test values of p ≤ 0.1 showed strong heterogeneity between the studies[12]. To explain the heterogeneity of the subgroup differences, we used Tau-squared (τ2). The data were pooled using a random effects model (DerSimonian and Laird method) (I2>50%, p≤0.1) or fixed effects model (Mantel-Haenszel) (I2<50%) according to the heterogeneity statistic I2 [13]. When heterogeneity was shown among the included studies, the pooled OR estimates were calculated by the random effects model [14]. Otherwise, the fixed effects model was used [13]. To assess the contributions of single studies to the final results, sensitivity analyses were performed. Generally, the funnel plot symmetry can be used to evaluate the publication bias and whether the results of a meta-analysis were affected by it. Therefore, Begg’s test and Egger’s test were used to examine publication bias [15]. When bias exists, we use a conventional meta-trim method to re-estimate the effect size.
The extraction and analysis of TCGA data
DNA methylation information for breast cancer was collected from the TCGA project using methylation 450 K datasets [http://cancergenome.nih.gov/]. We calculated the methylation of each CG probe according to the Illumina Infinium Human Methylation 27/450 Bead Array platform instruction: Beta-value = intensity value from the methylated bead type/(intensity values from the methylated + intensity value from unmethylated bead types + 100).
The methylation signals of the 25,978 shared CpG sites in the 450 K datasets were extracted, and the methylation status of each probe was defined according to the beta-value. Any beta value equal to or greater than 0.6 was considered fully methylated. Any beta value equal to or less than 0.2 was considered to be fully unmethylated. Beta values between 0.2 and 0.6 were considered to be partially methylated. The CpG site will be considered methylated when the beta-value is greater than the empirical threshold of 0.3 for tissue/blood data [16].
Results
Study characteristics
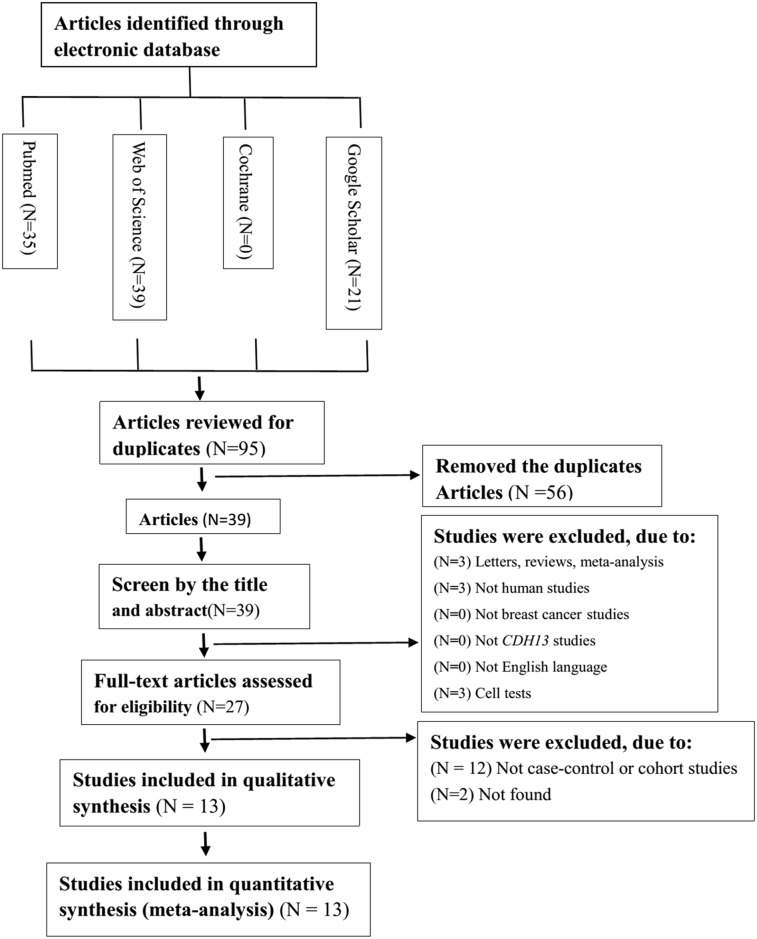
Based on the above electronic search strategy, we identified 13 potentially relevant articles (Fig 1) upon further screening for inclusion on the basis of their titles, abstracts and full texts. Finally, 13 articles were selected covering two factors, the risk and prognosis of breast cancer. The characteristics of the 13 articles and the data on risk and prognosis in breast cancer are shown in Tables 1 and 2 [10, 17–29]. All of these articles were written in English. In total, 726 breast cancer tissues/serum samples and 422 normal counterpart tissues/serum samples were collected. The main aim of each study differed; 11 articles were focused predominantly on the risk of breast cancer, whereas the others were focused on prognosis. Among the studies examining the risk of breast cancer, 2 focused on Asian subjects, 6 focused on Caucasian subjects, and 3 examined the risk in mixed-race subjects. We also obtained 3 articles that examined serum and 9 articles that examined tissue. Regarding the experimental methods used to explore CDH13 promoter methylation status, 5 of 13 inclusions used methylation-specific polymerase chain reaction (MSP), while the others used quantitative MSP (qMSP, such as Methylight, Prosequencing, and so on).
Fig 1. Flow chart of study identification.
Table 1. Characteristics of eligible studies considered in the report.
| Author | Year | Country | Method | Sample | Mean age (year) | TNM stage | Male/Female | Control | Case | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| U | M | U | M | ||||||||
| Hafez et al. | 2015 | Kingdom of Saudi Arabia | MSP | Tissue | 48.00 | I-III | 0/180 | 81 | 19 | 53 | 27 |
| Pang et al. | 2014 | Australia | MS-HRM | Tissue | 54.00 | NA | 0/80 | 17 | 1 | 37 | 43 |
| Twelves et al. | 2013 | UK | MSP | Blood | 52.00 | I-II | 0/54 | 46 | 0 | 42 | 12 |
| Twelves et al. | 2013 | UK | MSP | Tissue | 52.00 | I-II | 0/36 | 46 | 0 | 1 | 35 |
| Jung et al. | 2013 | Korea | MS-MLPA | Tissue | 50.50 | I-IV | 0/60 | 57 | 3 | 46 | 14 |
| Wang et al. | 2012 | USA | BSP | Tissue | 54.00 | I-III | 0/65 | 65 | 0 | 59 | 6 |
| Verschuur-Maes et al. | 2012 | Netherlands | MS-MLPA | Tissue | 54.00 | NA | 0/56 | 9 | 1 | 40 | 16 |
| Kornegoor et al. | 2012 | Netherlands | MS-MLPA | Tissue | 66.00 | NA | 108/0 | 10 | 1 | 25 | 83 |
| Moelans et al. | 2011 | Japan | MS-MLPA | Tissue | NA | NA | NA | 7 | 2 | 27 | 8 |
| Chen et al. | 2011 | USA | MS-MLPA | Blood | NA | NA | NA | 12 | 3 | 10 | 7 |
| Feng et al. | 2010 | Italy | BSP | Tissue | 59.00 | I-IV | 0/76 | 22 | 1 | 10 | 66 |
| Riener et al. | 2008 | Germany | Microarray | Blood | 55.60 | NA | NA | 2 | 0 | 3 | 18 |
| Lewis et al. | 2005 | USA | MSP | Tissue | 50.00 | 0-II | 0/38 | 16 | 1 | 24 | 14 |
MSP, methylation-specific polymerase chain reaction; MS-HRM, Methylation-sensitive high-resolution melting; MS-MLPA, methylation-specific multiplex ligation-dependent probe amplification; BSP, bisulfite sequencing polymerase chain reaction; M, number of patients with methylation; U, number of patients with unmethylation; NA, not available.
Table 2. Baseline characteristics of eligible studies evaluating CDH13 methylation and OS or DFS in breast cancer patients.
| Author | Year | Country | Method | Sample | TNM. stage | N | DFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | Lowera | Uppera | p | HR | Lowera | Uppera | p | |||||||
| Kong et al. | 2015 | China | IHC | Protein | I-III | 142 | 0.163 | 0.051 | 0.52 | 0.002 | 0.374 | 0.11 | 1.276 | 0.067 |
| Xu et al. | 2012 | USA | BSP | DNA | I-II | 193 | 0.75 | 0.42 | 1.33 | 0.32 | 1.15 | 0.77 | 1.7 | 0.5 |
N, number of patients; HR: hazard ratios; OS: overall survival; DFS: disease-free survival; BSP, bisulfite sequencing polymerase chain reaction; IHC, immunohistochemistry;
a the upper/lower limit of the 95% confidence interval.
According to the previous study [20], we analyzed 13 different probes located in or near the CDH13 promoter region and chose three of the 13 probes (cg01492639, cg06341397, cg02263260) containing the transcription start site of the CDH13 gene. A total of 699 breast cancer tissue/blood samples and 96 normal samples were obtained from the TCGA project (S1 Table). Of the 699 patients, 18.60% had CDH13 methylation, while there was no methylation of CDH13 in the normal sample. Among the 699 patients, there were 677/7 female/male. The patients’ ages ranged from 26 to 90, and the AJCC pathologic tumor stage ranged from I-X.
Meta-analysis of risk and prognosis in breast cancer
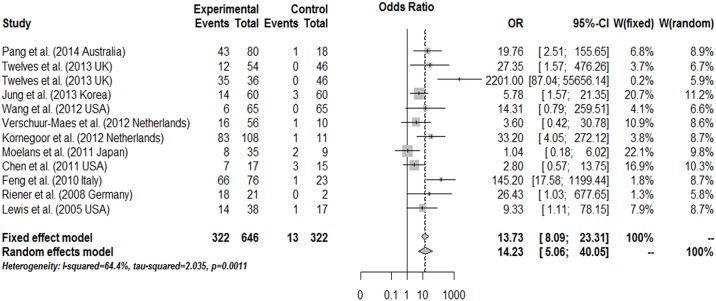
The results of the association between the promoter methylation of CDH13 and breast cancer are shown in Fig 2. For the CDH13 gene association with breast cancer risk, our findings demonstrate that the frequency of CDH13 promoter methylation in cancer was OR = 13.73, 95%CI: 8.09~23.31, z = 9.70, p<0.0001 by the fixed effect model and OR = 14.23, 95%CI: 5.06~40.05, z = 5.031, p<0.0001 by the random effect model, which suggests a statistically significant increase in the likelihood of CDH13 promoter methylation in breast cancer compared to the controls. Upon analyzing the prognosis data for CDH13 promoter methylation in breast cancer patients, we found that the HR was 0.77 (95%CI: 0.27~2.21, z = -0.49, p = 0.622) and 0.38 (95%CI: 0.09~1.69, z = -1.27, p = 0.20) for overall survival (OS) and disease-free survival (DFS), respectively, as assessed by the random effect model with any heterogeneity. This result indicates that breast cancer patients with CDH13 promoter methylation have limited prognostic value for clinical diagnosis.
Fig 2. Combined estimates for the association between CDH13 promoter methylation and breast cancer with forest plot.
Author, year, and country of the studies, methylated (M) and total number of samples (T) in case and control, and combined odds ratio (OR) with 95% confidence region were labeled in the right column of the figure. The DerSimonian-Laird estimator and Mantel-Haenszel method were selected to conduct combination estimation for the random effects model and fixed effects model, respectively.
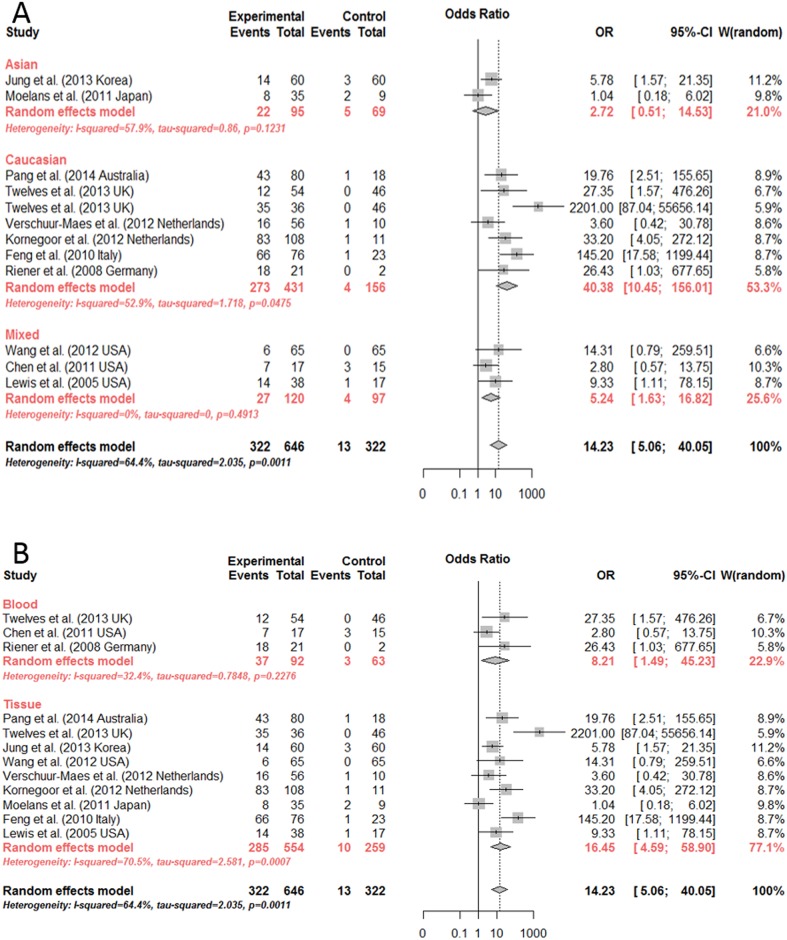
Significant differences were found among the mixed-race (OR = 5.24, 95%CI: 1.63~16.82, by random effects model), Caucasian (OR = 40.38, 95%CI: 10.45~156.01, by random effects model) and Asian (OR = 2.72, 95%CI: 0.51~14.53, by fixed effect model) subgroups (p<0.0001) (Fig 3A). As we have inferred, studies of both tissue and serum showed a significant association between CDH13 methylation and breast cancer (OR = 16.45, 8.21 by random effects model, respectively) (Fig 3B). Hence, the methylation status of CDH13 could be used as a potential biomarker for breast cancer diagnosis for either tissue or serum samples.
Fig 3. Subgroup meta-analysis for the relationship between CDH13 promoter methylation and breast cancer.
(A, B). Subgroup meta-analysis based on race and sample by random effects model and fixed effects model, respectively.
Bias analysis and sensitivity analysis
To assess the publication bias of the articles, we used Begg’s test and Egger’s test. The assessment of Egger’s test revealed some evidence of obvious asymmetry in the ensemble analysis (t = -1.11, p = 0.29), and Begg’s test indicated an absence of publication bias (t = 2.43, p = 0.035) (Fig 4A).
Fig 4. Funnel plot for publication bias test and sensitivity analysis of the summary odds ratio coefficients on the relationships between CDH13 methylation and breast cancer patients.
To our knowledge, the modification of the inclusive criteria of the meta-analysis may affect the final results. Therefore, a sensitivity analysis was conducted. The OR of the sensitivity analysis ranged from 10.21 (95% CI, 5.82~17.91) to 15.95 (95% CI, 8.94~28.44) upon omitting a single study with the random effect model (Fig 4B). Hence, as indicated by the sensitivity analysis, no single study would affect the pooled OR.
Validation by independent TCGA breast cancer dataset
To validate the results of the meta-analysis, we collected the data on the CDH13 methylation status, and clinical characteristics from the breast cancer samples of the TCGA project. We then separated the data into several subgroups based on the meta-analysis.
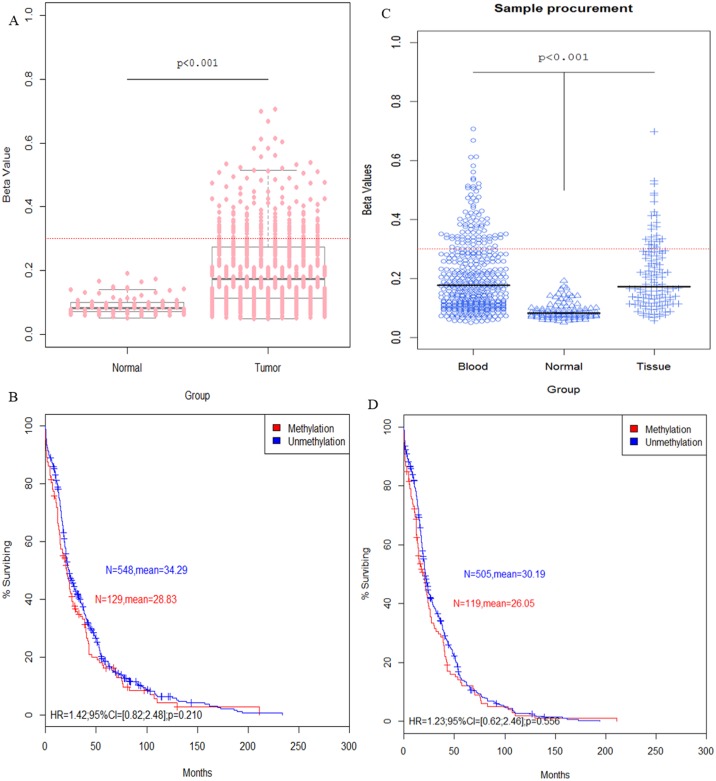
In the DNA methylation microarray dataset from the TCGA project, there was a significant difference between the cancer cases and controls (Fig 5A); the same result was also found in the race subgroup (Fig 6). According to the t-test, both the tissue resection and blood draw had greater significance than the control groups (Fig 5C).
Fig 5. The relationships of CDH13 methylation with TCGA probe, sample source and survival curve of breast cancer in the TCGA data.
(A). Different TCGA probe for 450 K datasets shows the relationship of CDH13 methylation and breast cancer risk (NTumor = 699, NNormal = 96, respectively). (C). The t-test indicates significant differences in blood (N = 444) and tissue (N = 120) samples compared to normal tissue (N = 96). (B, D). Association of patient survival and CDH13 methylation status by Kaplan-Meier method. Red dotted line indicates β = 0.3.
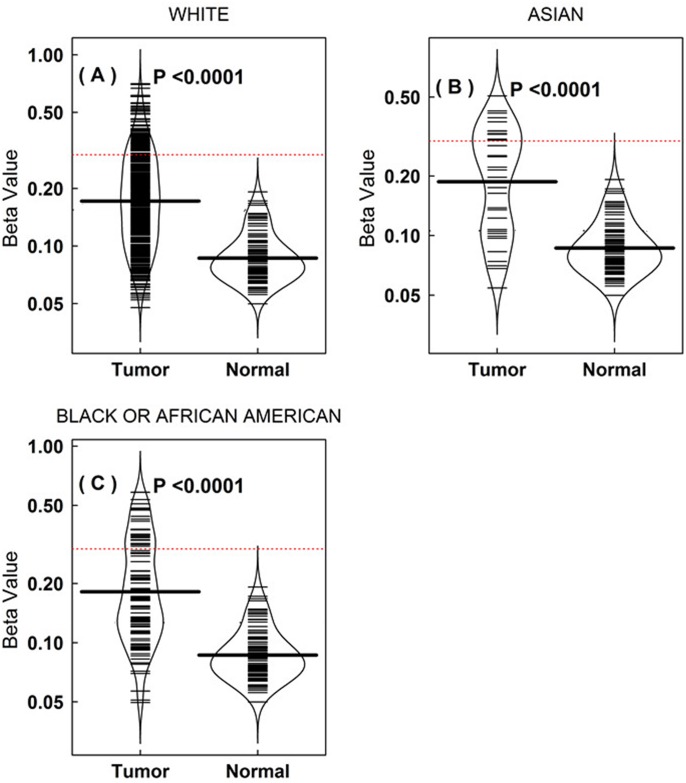
Fig 6. Different racial types affecting the OR of the CDH13 methylation to the risk of breast cancer in the TCGA data.
(A-C). The beanplot demonstrates that different racial types affect the CDH13 methylation to the risk of breast cancer in data from TCGA breast cancer datasets. The number of tumor patients is 524, 34, 101 in White, Asian and Black or African American people, respectively. Black dotted line indicates β = 0.3. The differences are significant by t-test.
To date, the potential of gene-specific DNA methylation has been reported as a prognostic indicator in many cancers [30–36]. In this study, we set out to explore the relationship between CDH13 promoter methylation and breast cancer survival using data extracted from the TCGA project. As predicted, patients without CDH13 methylation live almost the same time as patients with CDH13 methylation (HR = 1.41, 95% CI = [0.82; 2.48], p = 0.210) (Fig 5D), suggesting that CDH13 methylation does not decrease the survival rate in patients with breast cancer. DFS can also demonstrate a relationship to the prognosis, and the result (HR = 1.23, 95% CI = [0.62; 2.46], p = 0.556) is similar to the OS (Fig 5B). The same result was found using our meta-analysis.
Discussion
Alterations to DNA promoter methylation are the most frequent molecular changes associated with many human cancers [37–39]. Aberrant promoter methylation has been described for many different genes in various malignancies, suggesting that specific tumors might have their own distinct methylation profiles [38, 40]. The traditional diagnosis of malignant tumors uses microscopy to check for morphological changes, based on visible histopathological differences between tumor and normal tissue. Since 2000, the World Health Organization (WHO) made some major additions to the tumor classification criteria. Comprehensive information was added, such as the immune phenotype, genetic characteristics, clinical manifestation, and imaging to define tissue pathology, both sorted and graded. Compared to ordinary histopathological diagnosis, the additional information is now more beneficial, as it allows the individualized treatment of a given tumor. Hence, oncogene and tumor suppressor genes could be used as a new generation of detection markers in the clinical diagnosis of tumors. In recent years, a growing number of studies have shown that both the occurrence and development of many different tumors are linked to abnormal DNA methylation, and the abnormal methylation of tumor specific genes can be detected early, even before clinical diagnosis. Such analysis of DNA methylation offers several advantages, as it can be detected in fluids such as serum. Given that CDH13 promoter methylation is the most common genetic inactivation event found in human tumors, it would be a useful marker for the early detection of cancer.
Recent studies have reported that CDH13 is an important tumor suppressor in breast cancer patients [18, 41, 42]. Consequently, many researchers have tried to explore the role of CDH13 methylation and the epigenetics of other genes in the prognosis and early detection of breast cancer, as well as the differentiation between malignant and non-malignant lesions. Here, we conducted a meta-analysis to explore the association between CDH13 methylation and breast cancer. The systematic literature search yielded a total of 13 studies, comprising 11 studies of breast cancer risk and 2 articles on breast cancer prognosis, which were used for the final analysis. Our results revealed that the frequency of CDH13 promoter methylation in breast cancer tissue/serum was significantly higher than in normal tissue/serum. This finding was true in patients of African, Asian and Caucasian descent, suggesting a statistically significant increase in the likelihood of methylation in breast cancer compared to the controls. However, CDH13 promoter methylation was not significantly related to the OS and DFS of breast cancer. Therefore, CDH13 promoter methylation is likely to be linked to the risk of breast cancer and may have limited prognostic value for breast cancer patients.
To our knowledge, heterogeneity would affect the credibility of the meta-analysis result. As shown by the meta-analysis, there is some heterogeneity in the risk (tau2 = 2.04, I2 = 64.4%, p = 0.001) and DFS ((tau2 = 0.95, I2 = 81.2%, p = 0.021)) of breast cancer. Although sensitivity analyses found that there was no single sensitive study in this meta-analysis, Begg’s and Egger’s tests demonstrated no evidence of publication bias; we took data from the TCGA project, which would provide an additional resource that might be without publication bias, to prove the meta-analysis result. As predicted, the same result was found in the data of TCGA project of CDH13 methylation in breast cancer. Taken together, the results of this systematic review indicate a credible relationship between CDH13 methylation and breast cancer.
In conclusion, this meta-analysis of the article data provides strong evidence that the methylation status of the CDH13 promoter is strongly related to breast cancer risk. However, CDH13 methylation is not prognostic for breast cancer patients. Future studies are needed that collectively explore the possible roles of CDH13 methylation in breast cancer.
Supporting Information
(DOCX)
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1.Bernard W, Stewart CPW. World Cancer Report 2014. World Health Organization. 2014.
- 2.Barinoff J, Hils R, Bender A, Gross J, Kurz C, Tauchert S, et al. Clinicopathological differences between breast cancer in patients with primary metastatic disease and those without: a multicentre study. Eur J Cancer. 2013;49(2):305–11. 10.1016/j.ejca.2012.07.027 . [DOI] [PubMed] [Google Scholar]
- 3.van Hoesel AQ, Sato Y, Elashoff DA, Turner RR, Giuliano AE, Shamonki JM, et al. Assessment of DNA methylation status in early stages of breast cancer development. Br J Cancer. 2013;108(10):2033–8. Epub 2013/05/09. 10.1038/bjc.2013.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeuchi T, Ohtsuki Y. Recent progress in T-cadherin (CDH13, H-cadherin) research. Histol Histopathol. 2001;16(4):1287–93. . [DOI] [PubMed] [Google Scholar]
- 5.Sato M, Mori Y, Sakurada A, Fujimura S, Horii A. The H-cadherin (CDH13) gene is inactivated in human lung cancer. Hum Genet. 1998;103(1):96–101. 10.1007/s004390050790 WOS:000075402400017. [DOI] [PubMed] [Google Scholar]
- 6.Zhong YH, Peng H, Cheng HZ, Wang P. Quantitative Assessment of the Diagnostic Role of CDH13 Promoter Methylation in Lung Cancer. Asian Pac J Cancer Prev. 2015;16(3):1139–43. . [DOI] [PubMed] [Google Scholar]
- 7.Kim H, Kwon YM, Kim JS, Lee H, Park JH, Shim YM, et al. Tumor-specific methylation in bronchial lavage for the early detection of non-small-cell lung cancer. J Clin Oncol. 2004;22(12):2363–70. Epub 2004/06/16. 10.1200/jco.2004.10.077 . [DOI] [PubMed] [Google Scholar]
- 8.Hanabata T, Tsukuda K, Toyooka S, Yano M, Aoe M, Nagahiro I, et al. DNA methylation of multiple genes and clinicopathological relationship of non-small cell lung cancers. Oncol Rep. 2004;12(1):177–80. Epub 2004/06/18. . [PubMed] [Google Scholar]
- 9.Toyooka KO, Toyooka S, Virmani AK, Sathyanarayana UG, Euhus DM, Gilcrease M, et al. Loss of expression and aberrant methylation of the CDH13 (H-cadherin) gene in breast and lung carcinomas. Cancer Res. 2001;61(11):4556–60. Epub 2001/06/05. . [PubMed] [Google Scholar]
- 10.Kong DD, Yang J, Li L, Wang W, Chen YN, Wang SB, et al. T-cadherin association with clinicopathological features and prognosis in axillary lymph node-positive breast cancer. Breast Cancer Res Treat. 2015;150(1):119–26. Epub 2015/02/14. 10.1007/s10549-015-3302-x . [DOI] [PubMed] [Google Scholar]
- 11.Xu J, Shetty PB, Feng W, Chenault C, Bast RC Jr., Issa JP, et al. Methylation of HIN-1, RASSF1A, RIL and CDH13 in breast cancer is associated with clinical characteristics, but only RASSF1A methylation is associated with outcome. BMC Cancer. 2012;12:243 Epub 2012/06/15. 10.1186/1471-2407-12-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. . [DOI] [PubMed] [Google Scholar]
- 14.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–48. . [PubMed] [Google Scholar]
- 15.Egger M, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ open. 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sproul D, Nestor C, Culley J, Dickson JH, Dixon JM, Harrison DJ, et al. Transcriptionally repressed genes become aberrantly methylated and distinguish tumors of different lineages in breast cancer. Proc Natl Acad Sci U S A. 2011;108(11):4364–9. Epub 2011/03/04. 10.1073/pnas.1013224108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pang JM, Deb S, Takano EA, Byrne DJ, Jene N, Boulghourjian A, et al. Methylation profiling of ductal carcinoma in situ and its relationship to histopathological features. Breast Cancer Res. 2014;16(5):423 Epub 2014/10/22. 10.1186/s13058-014-0423-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Twelves D, Nerurkar A, Osin P, Dexter T, Ward A, Gui GP, et al. DNA promoter hypermethylation profiles in breast duct fluid. Breast Cancer Res Treat. 2013;139(2):341–50. Epub 2013/05/16. 10.1007/s10549-013-2544-8 . [DOI] [PubMed] [Google Scholar]
- 19.Jung EJ, Kim IS, Lee EY, Kang JE, Lee SM, Kim DC, et al. Comparison of methylation profiling in cancerous and their corresponding normal tissues from korean patients with breast cancer. Ann Lab Med. 2013;33(6):431–40. Epub 2013/11/10. 10.3343/alm.2013.33.6.431 ; PubMed Central PMCID: PMCPmc3819443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Dorsey TH, Terunuma A, Kittles RA, Ambs S, Kwabi-Addo B. Relationship between tumor DNA methylation status and patient characteristics in African-American and European-American women with breast cancer. PLoS One. 2012;7(5):e37928 Epub 2012/06/16. 10.1371/journal.pone.0037928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verschuur-Maes AH, de Bruin PC, van Diest PJ. Epigenetic progression of columnar cell lesions of the breast to invasive breast cancer. Breast Cancer Res Treat. 2012;136(3):705–15. Epub 2012/10/30. 10.1007/s10549-012-2301-4 . [DOI] [PubMed] [Google Scholar]
- 22.Kornegoor R, Moelans CB, Verschuur-Maes AH, Hogenes M, de Bruin PC, Oudejans JJ, et al. Promoter hypermethylation in male breast cancer: analysis by multiplex ligation-dependent probe amplification. Breast Cancer Res. 2012;14(4):R101 Epub 2012/07/07. 10.1186/bcr3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moelans CB, Verschuur-Maes AH, van Diest PJ. Frequent promoter hypermethylation of BRCA2, CDH13, MSH6, PAX5, PAX6 and WT1 in ductal carcinoma in situ and invasive breast cancer. J Pathol. 2011;225(2):222–31. Epub 2011/06/29. 10.1002/path.2930 . [DOI] [PubMed] [Google Scholar]
- 24.Chen KM, Stephen JK, Raju U, Worsham MJ. Delineating an epigenetic continuum for initiation, transformation and progression to breast cancer. Cancers (Basel). 2011;3(2):1580–92. Epub 2011/07/22. 10.3390/cancers3021580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng W, Orlandi R, Zhao N, Carcangiu ML, Tagliabue E, Xu J, et al. Tumor suppressor genes are frequently methylated in lymph node metastases of breast cancers. BMC Cancer. 2010;10:378 Epub 2010/07/21. 10.1186/1471-2407-10-378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riener MO, Nikolopoulos E, Herr A, Wild PJ, Hausmann M, Wiech T, et al. Microarray comparative genomic hybridization analysis of tubular breast carcinoma shows recurrent loss of the CDH13 locus on 16q. Hum Pathol. 2008;39(11):1621–9. Epub 2008/07/29. 10.1016/j.humpath.2008.02.021 . [DOI] [PubMed] [Google Scholar]
- 27.Lewis CM, Cler LR, Bu DW, Zochbauer-Muller S, Milchgrub S, Naftalis EZ, et al. Promoter hypermethylation in benign breast epithelium in relation to predicted breast cancer risk. Clin Cancer Res. 2005;11(1):166–72. Epub 2005/01/27. . [PubMed] [Google Scholar]
- 28.Hafez MM, Al-Shabanah OA, Al-Rejaie SS, Al-Harbi NO, Hassan ZK, Alsheikh A, et al. Increased hypermethylation of glutathione S-transferase P1, DNA-binding protein inhibitor, death associated protein kinase and paired box protein-5 genes in triple-negative breast cancer Saudi females. Asian Pac J Cancer Prev. 2015;16(2):541–9. Epub 2015/02/17. . [DOI] [PubMed] [Google Scholar]
- 29.Pang JM, Deb S, Takano EA, Byrne DJ, Jene N, Boulghourjian A, et al. Methylation profiling of ductal carcinoma in situ and its relationship to histopathological features. Breast Cancer Res. 2014;16(5):423 Epub 2014/10/22. 10.1186/s13058-014-0423-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho YH, Shen J, Gammon MD, Zhang YJ, Wang Q, Gonzalez K, et al. Prognostic significance of gene-specific promoter hypermethylation in breast cancer patients. Breast Cancer Res Treat. 2012;131(1):197–205. 10.1007/s10549-011-1712-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martins AT, Monteiro P, Ramalho-Carvalho J, Costa VL, Dinis-Ribeiro M, Leal C, et al. High RASSF1A promoter methylation levels are predictive of poor prognosis in fine-needle aspirate washings of breast cancer lesions. Breast Cancer Res Treat. 2011;129(1):1–9. 10.1007/s10549-010-1160-0 . [DOI] [PubMed] [Google Scholar]
- 32.Tanemura A, Terando AM, Sim MS, van Hoesel AQ, de Maat MF, Morton DL, et al. CpG island methylator phenotype predicts progression of malignant melanoma. Clin Cancer Res. 2009;15(5):1801–7. Epub 2009/02/19. 10.1158/1078-0432.ccr-08-1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Misawa A, Tanaka S, Yagyu S, Tsuchiya K, Iehara T, Sugimoto T, et al. RASSF1A hypermethylation in pretreatment serum DNA of neuroblastoma patients: a prognostic marker. Br J Cancer. 2009;100(2):399–404. Epub 2009/01/24. 10.1038/sj.bjc.6604887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kioulafa M, Kaklamanis L, Mavroudis D, Georgoulias V, Lianidou ES. Prognostic significance of RASSF1A promoter methylation in operable breast cancer. Clin Biochem. 2009;42(10–11):970–5. Epub 2009/04/21. 10.1016/j.clinbiochem.2009.04.003 . [DOI] [PubMed] [Google Scholar]
- 35.Jo H, Kim JW, Kang GH, Park NH, Song YS, Kang SB, et al. Association of promoter hypermethylation of the RASSF1A gene with prognostic parameters in endometrial cancer. Oncol Res. 2006;16(4):205–9. Epub 2006/11/24. . [DOI] [PubMed] [Google Scholar]
- 36.Kawai Y, Sakano S, Suehiro Y, Okada T, Korenaga Y, Hara T, et al. Methylation level of the RASSF1A promoter is an independent prognostic factor for clear-cell renal cell carcinoma. Ann Oncol. 2010;21(8):1612–7. Epub 2009/12/30. 10.1093/annonc/mdp577 . [DOI] [PubMed] [Google Scholar]
- 37.Sidransky D. Emerging molecular markers of cancer. Nature reviews Cancer. 2002;2(3):210–9. 10.1038/nrc755 . [DOI] [PubMed] [Google Scholar]
- 38.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3(6):415–28. 10.1038/nrg816 . [DOI] [PubMed] [Google Scholar]
- 39.Rountree MR, Bachman KE, Herman JG, Baylin SB. DNA methylation, chromatin inheritance, and cancer. Oncogene. 2001;20(24):3156–65. 10.1038/sj.onc.1204339 . [DOI] [PubMed] [Google Scholar]
- 40.Esteller M. CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene. 2002;21(35):5427–40. Epub 2002/08/03. 10.1038/sj.onc.1205600 . [DOI] [PubMed] [Google Scholar]
- 41.Hebbard LW, Garlatti M, Young LJ, Cardiff RD, Oshima RG, Ranscht B. T-cadherin supports angiogenesis and adiponectin association with the vasculature in a mouse mammary tumor model. Cancer Res. 2008;68(5):1407–16. Epub 2008/03/05. 10.1158/0008-5472.can-07-2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riou P, Saffroy R, Chenailler C, Franc B, Gentile C, Rubinstein E, et al. Expression of T-cadherin in tumor cells influences invasive potential of human hepatocellular carcinoma. FASEB J. 2006;20(13):2291–301. 10.1096/fj.06-6085com . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.