Abstract
Schisandra chinensis extracts (SEs) have traditionally been used as an oriental medicine for the treatment of various human diseases, however, their further application in the biocontrol of plant disease remains poorly understood. This study was conducted to develop eco-friendly botanical pesticides from extracts of S. chinensis and assess whether they could play a key role in plant disease defense. Concentrated active fractions (SE-I, SE-II, and SE-III) were obtained from S. chinensis via specific extraction and separation. Then, lignan-like substances, such as Schisanhenol B, were detected via High-Performance Liquid Chromatography-ElectroSpray Ionization-Mass Spectrometry (HPLC-ESI-MS) analyses of the active fractions. Moreover, the results from biological tests on colony growth inhibition and spore germination indicated that SE-I, SE-II, and SE-III could inhibit hyphal growth and spore generation of three important plant pathogenic fungi (Monilinia fructicola, Fusarium oxysporum, and Botryosphaeria dothidea). The study of the mechanisms of resistant fungi revealed that the oxidation resistance system, including reactive oxygen species (ROS), malondialdehyde (MDA), catalase (CAT), and superoxide dismutase (SOD), was activated. The expression of genes related to defense, such as pathogenesis-related protein (PR4), α-farnesene synthase (AFS), polyphenol oxidase (PPO), and phenylalanine ammonia lyase (PAL) were shown to be up-regulated after treatment with SEs, which suggested an increase in apple immunity and that fruits were induced to effectively defend against the infection of pathogenic fungi (B. dothidea). This study revealed that SEs and their lignans represent promising resources for the development of safe, effective, and multi-targeted agents against pathogenic fungi.
Introduction
Bioactive substances derived from plants play important roles in environmental improvement, food production, food security, and biodiversity conservation. Increasing attention is being paid to the development and application of botanical pesticides, such as botanical insecticides, fungicides, antiseptic agents, and herbicides. An important reason for this interest is that botanical pesticides are accepted as being more environmentally friendly than synthetic organic pesticides with high residues, which can cause environmental pollution, threats to human health, and other issues [1–5].
Over the years, research regarding the application of botanical pesticides has shown that such substances are advantageous because of their high selectivity, low toxicity to humans, and biodegradability. Today they are one of the most important potential sources for the creation of new pesticides [6–9]. Several studies have shown that plant extracts contain active antibacterial ingredients, many of which have been extracted, developed, and applied as botanical fungicides [10–13]. However, identifying which species in the voluminous plant kingdom contain effective concentrations of active substances, and which of the ingredients in the 400,000 known secondary metabolites derived from plants have insecticidal activity [14], has been and will continue to be a puzzle to be solved.
In the USA, there are currently more than 245 registered biopesticide-active ingredients used in hundreds of products. In Korea, 33 biopesticides are registered, of which 19 are fungicides, 13 are insecticides, and 1 is an herbicide [15]. Industrial versions of ingredients, such as azadirachtin, matrine, rotenone, nicotine, celangulin, veratramine, ethylicin, physcion, aconitine, and eucalyptol have become the backbone of the botanical pesticide industry [10]. These substances are principally applied for their insecticidal effects. They eliminate pests through mechanisms such as acute poisoning, shutdown of the digestive system, hunger inhibition, sterilization, growth inhibition, and by acting as an attractant or repellent for the pest species. Some of these mechanisms are also active in the antiseptic and antibacterial processes of the botanical pesticides. The study of the effectiveness of botanical fungicides against crop disease remains at an early stage, and hence only a few marketable botanical fungicides have been developed thus far. To develop commercially competitive botanical fungicides, additional basic research into active antimicrobial substances is urgently needed. Thus, the immediate problem to be addressed is how to extract active ingredients from plants, develop new botanical fungicides, and improve plant disease control.
Family Schisandraceae, in the sub-class Magnoliidae, are perennial deciduous vines[16]. Plants of this family have been commonly used as medicinal and ornamental landscape plants. The family, comprising approximately 60 species, includes two genera, Schisandra Michx and Kadsura Kaempf Ex Juss. They are distributed discontinuously in Southeast Asia and North America [17]. In China, they are the most abundant plant resources and S. chinensis (Turcz.) Baill is a common species in northeastern China. The fruits, seeds, shoots, and leaves of S. chinensis (Turcz.) Baill have been used in traditional Chinese medicine since ancient times and are valued for their various curative properties [18]. The use of S. chinensis as a high-grade herbal medicine was first recorded in the ancient pharmaceutical book, “The Divine Husbandman's Herbal Foundation Canon (Shén Nóng Běn Cǎo Jīng)” [19]. Recently, S. chinensis fruit extract (SE) and its ingredients have gained substantial attention. They may play a potentially important role in the treatment of cardiovascular diseases, such as hypertension and myocardial infarction [20, 21]. A water decoction of S. chinensis is believed to act as an astringent for the lungs and kidneys. It is effective in the treatment of diarrhea, arresting excessive sweating, calming the spirit by refreshing the heart and kidneys, generating body fluid, and reducing thirst [22, 23]. Pharmacological studies on animals have shown that S. chinensis increases immunity and affords a stress-protective effect against a broad spectrum of harmful factors, including heat shock, sunburn, hypothermia, frostbite, aseptic inflammation, irradiation, and heavy metal intoxication [24, 25]. Previous studies have also proven that Schisandra contains numerous special kinds of lignans and organic acids. These include Schizandrin, deoxyschizandrin, γ-Schizandrin, isoschizandrin, Schizandrol A and B, schisantherin A-E, citricacid, malicacid, and tartaricacid, substances that could play a key role in the treatment of fatty liver, hepatitis virus infection, and chemical-induced hepatitis [26, 27]. They also help to relieve stress and anxiety [28–30], and increase insulin sensitivity [31].
However, current research on the Schisandra genus has primarily focused on species differences, medicinal ingredients, and pharmacological effects [32–35]. Less research has focused on how S. chinensis can be applied in agriculture to inhibit plant pathogens or its potential for the development of botanical fungicides. In this study, a new method of extracting active substances from S. chinensis is proposed and the effects of these extracts on Botryosphaeria dothidea, Monilinia fructicola, and Fusarium oxysporum are tested, as these are causal agents of three important diseases (apple ring rot, peach brown rot, and Fusarium wilt of strawberry, respectively) [36–38]. The tests included the detection of the effects of the extract on mycelial growth, spore germination, and disease infection. Moreover, active ingredients in the S. chinensis extracts that inhibit the three plant pathogens were determined, and the mechanism of inhibition employed by the pathogenic fungi was studied.
Therefore, this study is meaningful because it produced alternatives to chemical pesticides and thereby could reduce the negative impacts of chemical pesticide use. It highlights the potential for development of botanical pesticides from S. chinensis compounds.
Materials and Methods
Plant and microbes materials
The experimental material was annual branches and leaves of S. chinensis collected from Hong You Schisandra chinensis Farm (124.8°E; 40.8°N) (http://www.hywwz.cn; Kuandian County, Liaoning Province, China). Samples of Botryosphaeria dothidea, Monilinia fructicola, and Fusarium oxysporum were obtained from the Plant Pathology Laboratory of Beijing University of Agriculture. They were stored in a refrigerator at a constant temperature of 4°C. Apple fruits (Malus domestica ‘Fuji’/Malus prunifolia (Willd.) Borkh) were obtained from the Chang Ping District (116.23°E, 40.22°N), Beijing, China. Peach fruits (Prunus persica) were obtained from Qingzhou City (118.4° E, 36.4°N) in Shandong, China.
Ethics Statement:The study was carried out on private land called the Hong You Schisandra chinensis Farm (http://www.hywwz.cn). We confirm that the owner of the land, Guangming Lin, gave permission for his site to be sampled. No specific permissions were required for locations and field studies, as our study did not involve endangered or protected species.
Experimental design
Extraction and separation of active substances from S. chinensis
To completely remove moisture, the annual branches and leaves of S. chinensis were dried at 28°C for 10 days. Ten kilograms of air-dried materials were weighed and crushed into powder with a grinder (Huangcheng HC-1500Y2, Yongkang, China; http://shop1396630696864.cn.china.cn/supply/). Next, the powdered materials was mixed with 70% acetone at a powder:solution ratio of 1:2 at room temperature in a 70-L stainless steel container (http://www.jmxwm.com/xwm/en/index.asp; Xinweiming Stainless Steel Products Factory, Jiangmen, China). After 24 h, the solution was transferred to another container and an additional 70% acetone was added. This procedure was repeated three times, after which the soaking solution was placed in a rotary evaporator to distill at 40°C and reduced pressure (Büchi Rotavapor R-220, Essen, Germany).
The remaining water solution contained all the active substances extracted from S. chinensis. To filter impurities, the water solution was allowed to stand overnight and then filtered using a circulating water vacuum pump (http://www.gyyuhua.com; Gongyi City Yuhua Instrument Co., Gongyi, China). The filtering process was repeated three times. The filtrate was then extracted three times with pure ethyl acetate with a filtrate:ethyl acetate ratio of 1:2. The organic layer was separated from the aqueous layer and was concentrated by evaporating the organic solvent in vacuo at 40°C in a rotary vacuum evaporator (Büchi Rotavapor R-124, Essen, Germany). This process produced the primary crude extract, which was further separated along a gradient according to polarity and extraction rate. The primary crude extract was then accurately calculated. The primary extract totaled 240 g and all samples were stored in air-tight brown bottles at 4°C in a refrigerator for the duration of the subsequent experiments.
A portion of the extract (100 g) was subjected to separation using silica gel (200–300 mesh) column chromatography (CC) and sequentially eluted with a series of chloroform-acetone-methanol mixtures (1:0:0, 10:1:0, 9:1:0, 2:1:0 1:1:0, 0:1:0, and 0:0:1) to obtains seven fractions (from SE-I to SE-VII) (Table 1). For set SE-I, for example, the chromatographic column was eluted with chloroform at 20 mL min-1 for 2 h, and all the eluent was concentrated in the vacuum distillation unit (http://www.gyyuhua.com; Gongyi City Yuhua Instrument Co., Gongyi, China).
Table 1. Method of separating active substances extracted from S. chinensis.
| S. chinensis extract | Organic solvent ratio | Elution time (h) | Velocity of flow(mL min-1) |
|---|---|---|---|
| SE-I | Chloroform | 2 | 20 |
| SE-II | Chloroform:Acetone = 10:1 | 2 | 20 |
| SE-III | Chloroform:Acetone = 9:1 | 2.5 | 20 |
| SE-IV | Chloroform:Acetone = 2:1 | 1.5 | 20 |
| SE-V | Chloroform:Acetone = 1:1 | 1 | 20 |
| SE-VI | Acetone | 0.8 | 20 |
| SE-VII | Methanol | 0.8 | 20 |
The elution time and velocity of flow to obtain all the factions are listed in Table 1. The seven fractions were concentrated in vacuo at 35°C in the vacuum rotary evaporator (Büchi Rotavapor R-124, Essen, Germany). The yield of the seven fractions from SE-Ito SE-VII was 5.1 g, 6.6 g, 8.2 g, 7.1 g, 10.2 g, 6.9 g, and 13.1 g, respectively.
Preparation of spore suspensions of the three fungi strains
The three test strains were inoculated on potato dextrose agar medium (PDA) and cultivated in a biochemical incubator (Fuma SPX-160B-2, Shanghai, China) at 28°C with alternating light and dark every 12 h for 4 d. Following this, sterile water was continually added to the medium to wash off and collect the conidia of the fungi. To remove fragments of mycelium and medium, the solution was filtered through gauze and condensed in a centrifuge (Sigma 3K-15, Osterode am Harz, Germany) at 1,000 r min-1 for 1 min. The concentration of spores was then determined under a microscope (Leica DM750, Wetzlar, Germany) and adjusted to 1 × 103 spores mL-1.
Analysis of the mycelial growth rate of the three pathogenic fungi
Fractions from SE-Ito SE-VII (1 g) were dissolved in 1 mL dimethyl sulfoxide (DMSO) and the mother liquor (1g mL-1) for each fraction was obtained. Sterile liquid PDA (3 mL) was placed in each well of the six-well culture plate and different volumes (1, 10, or 20 μL) of mother liquor of extracts were added. The final concentrations of the medium-containing extracts were 0.3, 3, and 6 mg mL-1, respectively. Control wells contained the same volumes of dimethyl sulfoxide (DMSO). All concentrations were run in triplicate. All solutions were allowed to solidify for 4 h and a 10-μL suspension of different pathogen spores (1× 103 spores mL-1) was added to the center of each SE-containing medium. After 3 h, the water in the spore suspension had completely evaporated and all six wells in the culture plates were placed in an incubator (Fuma SPX-160B-2, Shanghai, China) at 28°C for 48 h. The colony diameter was measured three times by the crossing method, and inhibition rates were calculated for each pathogens and extracts using the following equation1:
E1, The inhibition rate of mycelial growth
CC, Colony diameter of control group
CT, Colony diameter of treatment group
Observing mycelial morphology with scanning electron microscopy (SEM)
Scanning electron microscopy (SEM) was performed to determine morphological changes in the mycelia on the treated media. First, the growing mycelia (3mm × 5mm) on medium treated with SE-III were sampled with a double-sided knife, and samples for SEM were fixed in 2.5% glutaraldehyde stationary liquid for 12 h. Next, samples were irrigation three times with 0.1 mol L-1 phosphate buffer. The samples were dehydrated with a gradient concentration of alcohol systematically at 30%, 50%, 70%, 80%, 90%, 95%, and 100% with 15 min at each gradient. Anhydrous acetone was then applied three times for dehydration and each bout lasted 15 min. A critical point drying apparatus (EMS 850, Pennsylvania, USA) was used to dry the sample completely. The dried sample was then pasted onto the sample stage to be sputtered with gold (Hitachi ion sputter E-1010, Tokyo, Japan) and observed via SEM (Tescan VEGA TS 5136XM, Brno, Czech) under 2,500× magnification.
Analysis of the spore generation rate of the pathogenic fungi
The method for preparation of the mother liquor (1 g mL-1) of every fraction was the same as described above. Spore suspensions (0.5 mL) of the three fungi (B. dothidea, M. fructicola, and F. oxysporum) were mixed with 0.1 mL of 1 g mL -1 SE-I, SE-II, and SE-III, respectively, and the mixtures were then cultured on a concave slide to retain moisture at 28°C for 24–48 h. The same volume of DMSO was mixed with spore suspensions as a control. Each treatment was replicated three times. Spore germination was observed under a microscope (40×, Leica-DM750, Wetzlar, Germany) after 24 h and 48 h. Inhibition rate of spore germination was calculated by the following equation2:
E2, The inhibition rate of spore germination
TC, Total number of checked spores
NG, Number of germinated spores
The minimum number of spores examined was 200. Germination was defined as occurring when the germ tube of the conidia was longer than the short radius of the spore.
Analysis of the infection status of the fruit pericarp by pathogenic fungi
The extracts SE-I, SE-II, and SE-III in an amount of 5 μL (0.1 g mL-1) were sprayed evenly on the pericarp of apple and peach fruits in a 3-cm diameter circle. The control group was treated with a corresponding volume of DMSO and all sprayed fruits were placed in a biochemical incubator (Fuma SPX-160B-2, Shanghai, China) at 28°C. Each treatment was replicated a total of 12 times for each of the four processing periods (0 h, 24 h, 48 h, and 72 h). After the processing period elapsed, a 0.1-cm sample of the apple pericarp was taken from the sprayed area and all samples were frozen in-80°C liquid nitrogen for the next analysis of ROS, SOD, CAT, and MDA.
Pathogenic fungi was activated and cultivated for 4 d and 0.5-cm diameter colonies were transplanted by pasting them on the center of the sprayed area. This process was repeated for pathogenic fungi corresponding to extracts of SE-I, SE-II, and SE-III, as described above. The apples and peaches were wrapped with cling film to retain moisture and then placed in a biochemical incubator (Fuma SPX-160B-2, Shanghai, China) at 28°C for 96 h. Observations took place 3 d later. To test the protective effect of the SEs in preventing M. fructicola and B. dothidea from infecting peach and apple fruits, the average diameters of lesions were measured.
Antioxidant analysis
Measurement of intracellular reactive oxygen species (ROS)
A Reactive Oxygen Species Assay Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) was used to measure the level of intracellular reactive oxygen species (ROS) following the manufacturer’s instructions. The probe 2',7'-dichlorofluorescin diacetate (DCFH-DA) is oxidized by ROS to 2′, 7′-dichlorofluorescein (DCF), which is highly fluorescent at 530 nm. Briefly, a homogenate of apple pericarp was mixed with 1 mol L-1 DCFH-DA (V/V = 19:1). The relative levels of fluorescence were quantified by a multi-detection microplate reader with 485 nm excitation and 535 nm emission (Thermo Scientific, Varioskan Flash).
Thiobarbituric acid (TBA) test for lipid peroxidation
A Malondialdehyde (MDA) Assay Kit (Nanjing Jiancheng Bioengineering Institute) was used to measure the level of lipid peroxidation following the manufacturer’s instructions. MDA, the product of lipid peroxide degradation, can condense with thiobarbituric acid (TBA) to form red substances that are highly fluorescent at 532 nm.
Measurement of Superoxide Dismutase (SOD)
A Superoxide Dismutase (SOD) Assay Kit (Nanjing Jiancheng Bioengineering Institute) was used to measure the level of intracellular ROS following the manufacturer’s instructions.
Measurement of Catalase (CAT)
A Measurement of Catalase (CAT) Assay Kit (Nanjing Jiancheng Bioengineering Institute) was applied to measure the level of intracellular ROS following the manufacturer’s instructions.
Total RNA extraction and real-time PCR analysis
Total RNA was extracted from the pericarp of apples treated by the SEs for 0 h, 24 h, 48 h, and 72 h using a RN42-EASYspin Plus Plant Total RNA Kit (Aidlab, Beijing, China) according to the manufacturer’s instructions and then treated with DNase I (TaKaRa, Tokyo, Japan) to remove the residual DNA. The total RNA was then subjected to a reverse transcription reaction to generate first-strand cDNA using oligo (dT) primers (TaKaRa, Tokyo, Japan).
Expression analysis of genes was conducted by real-time PCR with SYBR Green qPCR Mix (TaKaRa, Tokyo, Japan) and a Bio-Rad CFX96 Real-Time PCR System (BIO-RAD, Hercules, USA). Actin (GenBank accession number: DQ341382) was selected as a ribosomal reference gene. Sequences of genes MdPR4, MdAFS, MdPPO, and MdPAL special primers used in this study are listed in S1 Table. The PCR mixture (20 μL total volume) for the reaction contained 10 μL SYBR Green Mix, 2 μL cDNA (diluted 10 times), 1 μL of each primer (10 μM), and 6 μL RNase-free water. Samples were amplified for 40 cycles at 95°C for 15 s and at 59°C for 20 s, and the reactions were all run with three biological replicates and two technical replicates. The real-time PCR data were calculated using the 2^ (-ΔΔCt) analysis method with error bars representing the standard deviation (SD).
HPLC-ESI (±)-MS2 analysis and identification of lignans
High-Performance Liquid Chromatography-ElectroSpray Ionization-Mass Spectrometry (HPLC-ESI-MS) analyses of the lignans were performed using an Agilent-1100 HPLC system equipped with a UV detector coupled to a LC-MSD Trap VL ion-trap mass spectrometer with an ESI source (Agilent Technologies, Palo Alto, CA, USA). The HPLC separation conditions were identical to those used for HPLC-DAD analysis. Negative-ion (PI) mode was used for the MS analysis of lignans.
ESI was performed using the following conditions: capillary voltage, 5.0 kV; nebulization pressure, 241.3 kPa; gas (N2) temperature, 350°C; and flow rate, 8.0 L min-1. The capillary offset and exit voltage were 68.4 V and 100.6 V, respectively. The skim 1 voltage was 29.5 V, the skim 2 voltage was 6.0 V, and the MS and MS2 spectra were recorded over an m/z range of 150 to 2000 NI. The MS2 scan of the target ions used normalized collision energy of 20%–45%. The experimental standards of Schizandrol A, Schizandrol B, Schizandrin B, Schizandrin A, and isoschizandrin were purchased from Sigma-Aldrich (Steinheim, Germany); data obtained for experimental standards and information regarding the identified compounds were combined to identify the lignans.
Data Analysis
Three replicates were performed for each experiment. Data are presented as the mean ± standard deviation (SD). Experimental data processing and analysis were completed using SPSS 20.0 (SPSS Inc., Chicago IL, USA). Analysis of variance of all values was used to assess differences in the means among samples (P < 0.05). The virulence regression equation was also calculated using SPSS 20.0. Inhibition ratio was converted to the probability value (y) and concentration of the SEs was converted to logarithm (x). A virulence regression equation is then calculated as y = a + bx, where “a” is the intercept and “b” is slope. According to the equation, the EC50 value was calculated. Graphs were prepared in OriginPro 8.0 software (Origin Lab Corporation, Northampton, MA, USA).
Results
The inhibiting effect of SE-I to SE-VII on the mycelial growth of three pathogenic fungi
In the study, according to gradient elution, seven fractions were obtained from crude S. chinensis extract and were named SE-I, SE-II, SE-III, SE-IV, SE-V, SE-VI, and SE-VII (SE series, SEs) according to polarity sequence.
In order to investigate the inhibiting effect of SE-I to SE-VII on the mycelial growth of M. fructicola, F. oxysporum, and B. dothidea, antifungal tests with different concentrations (0.3 mg L-1, 3 mg L-1, and 6 mg L-1) of the SE series using the plate cultivation method were conducted. The results indicated that SE-I to SE-III had inhibitive effects against M. fructicola, F. oxysporum, and B. dothidea, but their activities varied among the different pathogenic fungi, with the inhibitive ability of SE-III being best of the three candidate fractions based on their EC50 values. However, SE-IV to SE-VII had no critical impact on the growth of pathogenic fungi (Table 2).
Table 2. Percentage of inhibitive effect for seven SEs on the mycelial growth of three pathogenic fungi.
| Schisandra extract | Strains | Concentration | Toxicity regression equation | r | EC50(mg/mL) | EC50 ( ± SD /(mg/mL) | ||
|---|---|---|---|---|---|---|---|---|
| 0.3mg mL-1 3 mg mL-1 6mg mL-1 | ||||||||
| M. fructicola | 23.21% | 47.27% | 50.94% | y = 4.59 + 0.60x | 0.99 | 4.96 | ||
| SE-I | F. oxysporum | 26.90% | 44.44% | 51.39 | y = 4.65 + 0.48x | 1.00 | 5.54 | 4.67 ± 1.05 (P = 0.01) |
| B. dothidea | 29.25% | 39.63% | 63.55% | y = 4.70 + 0.59x | 0.87 | 3.51 | ||
| M. fructicola | 25.00% | 41.82% | 58.49% | y = 4.62 + 0.67x | 0.99 | 3.82 | ||
| SE-II | F. oxysporum | 21.72% | 61.81% | 66.67% | y = 4.48 + 0.97x | 0.99 | 1.91 | 2.95 ± 0.97 (P = 0.04) |
| B. dothidea | 23.58% | 52.99% | 54.19% | y = 4.67 + 0.71x | 1.00 | 3.13 | ||
| M. fructicola | 55.36% | 70.91% | 77.36% | y = 5.36 + 0.46x | 1.00 | 0.18 | ||
| SE-III | F. oxysporum | 47.93% | 65.28% | 77.78% | y = 5.27 + 0.52x | 0.95 | 0.31 | 0.34 ± 0.18 (P = 0.08) |
| B. dothidea | 37.26% | 65.44% | 100% | y = 5.92 + 3.27x | 0.78 | 0.53 | ||
| M. fructicola | — | — | — | y = 0.61 + 1.78x | 0.66 | 305.02 | ||
| SE-IV | F. oxysporum | — | — | — | y = 3.24 + 0.10x | 0.98 | — | — |
| B. dothidea | — | — | — | — | — | — | ||
| M. fructicola | — | — | — | y = 3.26 + 0.24x | 0.73 | — | ||
| SE-V | F. oxysporum | — | — | — | — | — | — | — |
| B. dothidea | — | — | — | — | — | — | ||
| M. fructicola | — | — | — | y = 1.48 + 2.78x | 1 | 19.75 | ||
| SE-VI | F. oxysporum | — | — | — | — | — | — | — |
| B. dothidea | — | — | — | — | — | — | ||
| M. fructicola | — | — | — | — | — | — | ||
| SE-VII | F. oxysporum | — | — | — | — | — | — | — |
| B. dothidea | — | — | — | — | — | — | ||
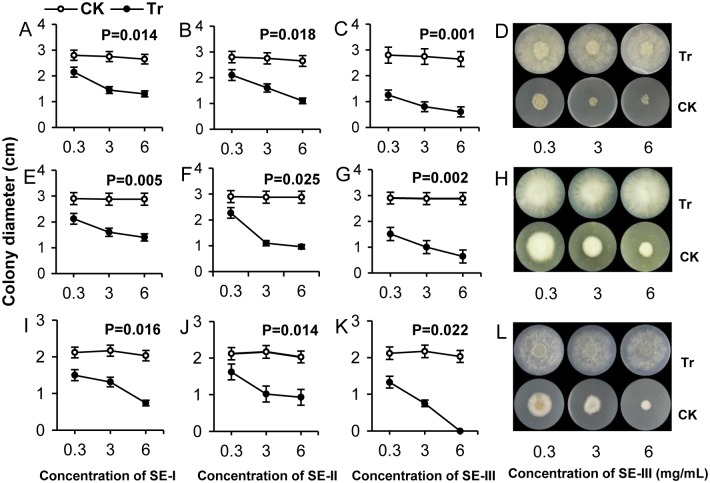
For the SE-I, the EC50 values were 4.96, 5.54, and 3.51 mg mL-1, respectively, for M. fructicola, F. oxysporum, and B. dothidea (Table 2), showing that the inhibitive effect of SE-I was best on B. dothidea. For SE-III, the EC50 values were 0.18, 0.31, and 0.53 mg mL-1, respectively, and the colony diameters of the three pathogenic fungi cultured on the SE-III medium were the smallest (Fig 1: C (P = 0.001), G (P = 0.002), K (P = 0.022), D, H, L).
Fig 1. Inhibiting effects of S. chinensis extracts SE-I, SE-II, and SE-III on mycelial growth of pathogenic fungi.
Inhibiting effects of S. chinensis extracts SE-I (A, E, and I), SE-II (B, F, and J), and SE-III (C, G, and K) on colony diameter of M. fructicola (A, B, C, and D), F. oxysporum (E, F, G, and H), and B. dothidea (I, J, K, and L). The data represent the mean ± SD and P values indicate a significant difference at P < 0.05. D, H, and L corresponded to C, G, and K, respectively.
The inhibition rates for SE-I, SE-II, and SE-III at three different treatment concentrations (0.3, 3, and 6 mg mL-1) were significantly different and inhibition rate increased with larger concentration of SEs. Thus, the higher the concentration of SE-I, SE-II, and SE-III in the medium, the higher the inhibition rate impacting the growth of the pathogenic fungi. The inhibition rate of SE-III at the three treatment concentrations was higher than that of SE-I and SE-II, and reached 100% when the treatment concentration was 6 mg mL-1 on B. dothidea (Fig 1K and 1L).
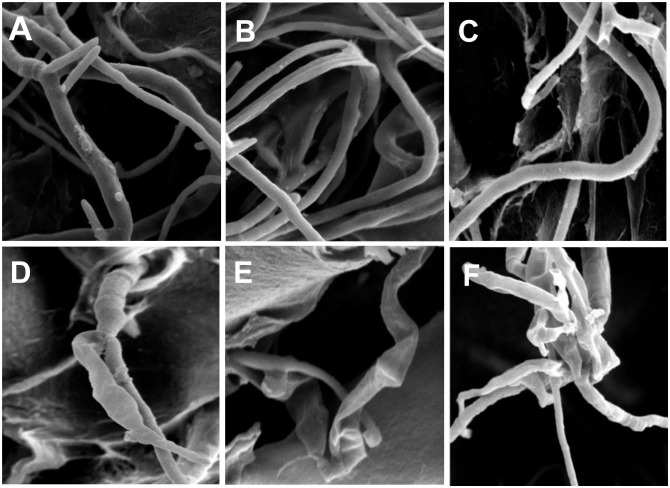
The effect of SE-III on the mycelial morphology of the three tested pathogenic fungi
When the treatment concentration of SE-III was 6 mg mL-1, the physiological condition of the three tested pathogenic fungi changed. Under the electron microscope (Fig 2), hyphae on the control medium were smooth and plump, having normal morphology (Fig 2A, 2B and 2C), whereas the hyphae from the SE-III-treated medium was shriveled and dehydrated, indicating that the mycelia of the pathogenic fungi presented abnormal morphological changes, such as deformation, rupture, and dissolution of the hyphae (Fig 2D, 2E and 2F). Based these results, there is direct evidence that the SEs displayed broad inhibition activity against pathogenic fungi infecting plants.
Fig 2. Mycelial morphology of the three tested pathogenic fungi under the electron microscope.
Mycelial morphology of the three tested pathogenic fungi from SE-III-treated medium under the electron microscope. A, B, and C represent the normal condition of M. fructicola, F. oxysporum, and B. dothidea, respectively. D, E, and F represent the hypha of M. fructicola (D), F. oxysporum (E), and B. dothidea (F) from the treated medium.
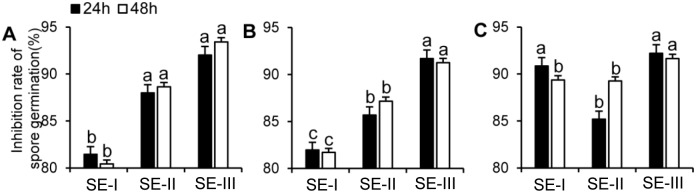
The inhibiting effect of SEs on the spore germination and infection of pathogenic fungi
To further investigate the inhibitive effect of the SEs on the pathogenic fungi, spore germination rates of the three pathogenic fungi were calculated after the spores were treated with SEs for 24 h and 48 h. The results indicated that the SE-III treatment significantly suppressed the germination rate of the three kinds of spores after inoculation for 24 h and 48 h, and the inhibition rate reached 90% for the germination of all three kinds of spores (Fig 3C). Extract SE-I only inhibited spore germination of B. dothidea by more than 90%, whereas its inhibitive effects on spore germination of M. fructicola and F. oxysporum were not apparent (Fig 3A). The average inhibition rate of SE-II on spore germination of the three kinds of pathogenic fungi was higher than that of SE-I, but lower than that of SE-III during both treatment periods (Fig 3B). This indicated that SEs might prevent fruit from damage caused by pathogenic fungi through inhibiting spore generation of the fungi.
Fig 3. Inhibiting effect of S. chinensis extracts SE-I, SE-II, and SE-III on the spore germination of pathogenic fungi.
Inhibiting effect of S. chinensis extracts SE-I, SE-II, and SE-III on the spore germination of M. fructicola (A), F. oxysporum (B) and B. dothidea (C). The black bar represents spores cultivated in the SE-containing medium for 24 h and the white for 48 h. The data represent the mean ± SD and letters indicate a significant difference (P < 0.05).
The preventive effect of SEs on fruit diseases
The SEs were sprayed on the pericarp of apple fruits and the fruits treated with SEs tended to be more resistant to B. dothidea infection. The results indicated that the diameter of the disease spots on apples treated with SEs was smaller than those on the controls (Fig 4A and 4C). Disease spots on apples without treatment with the SEs expanded to more than 2 cm. However, the disease spots on the SE-treated apples were significantly restricted with withered fungal hyphae.
Fig 4. The preventative effect of S. chinensis extracts SE-I, SE-II, and SE-III on apple ring rot and peach brown rot.
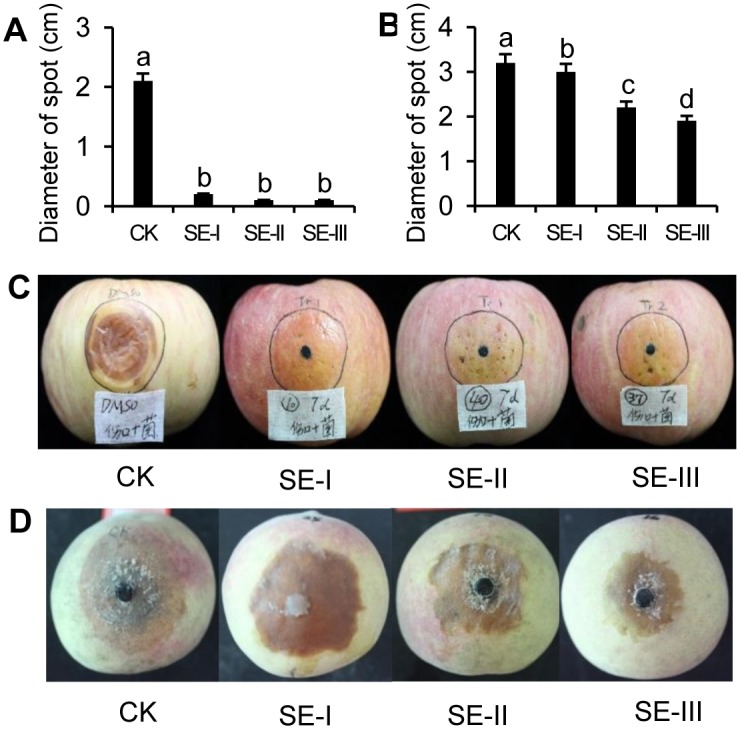
The preventive effect of S. chinensis extracts SE-I, SE-II, and SE-III on apple ring rot (C) and peach brown rot (D). A and B, respectively, correspond to C and D indicating the scab diameter with treatment with S. chinensis extracts. The data represent the mean ± SD and letters indicate a significant difference (P < 0.05).
The SEs were sprayed on the pericarp of peach fruits, and the fruits treated with SEs tended to be more resistant to M. fructicola infection. Compared with the control disease spot diameter (3.2 cm), peaches treated with SEs displayed smaller disease spot diameters, which were 3 cm, 2.2 cm, and 1.9 cm for SE-I, SE-II, and SE-III, respectively, to pathogen infection (Fig 4B and 4D). Results showed that among the three treatments, SE-III performed best, with the size of disease spots on the peach fruits treated with SE-III only 50% of that on the controls. These results indicated that SEs have substantial potential for direct application to prevent fruits from infection by pathogenic fungi.
Analysis of compounds derived from fractions of S. chinensis extract by LC-UV-ESI-MS2
In the study, SE-I, SE-II, and SE-III exhibited outstanding performance among the seven fractions in the preliminary toxicity experiment. Furthermore, the HPLC-ESI (±)-MS2 was employed to identify the compounds included in these three fractions (SE-I, SE-II, and SE-III) by standards.
From the results shown in Table 3, Schisanhenol B, Schizandrin A, Schizarin D, Schizandrin B, and isomeride Schizandrin B were found in the SE-I fraction. Schisanhenol B, Gomisin L, Schizandrin A, and Schizandrin B were in the SE-II fraction, and Schizarin D, Schizandrol A, Schizandrol B, Isoschizandrin, Schisanlignone A, Kadsulignan M, and isomeride Schizandrol B were in SE-III.
Table 3. Main compound types identified by LC-UV-ESI-MS2 in stems and leaves of Schisandra chinensis.
| NO. | MW | [M+H]-a | MS2[M-H]-(m/z)a | Putative identifier | SE-I | SE-II | SE-III |
|---|---|---|---|---|---|---|---|
| 1 | 386 | 387 | [387] | Schisanhenol B | √ | √ | ND |
| 2 | 386 | 387 | [387]:409 | Gomisin L | ND | √ | ND |
| 3 | 416 | 417 | [417]:439 | Schizandrin A | √ | √ | ND |
| 4 | 484 | 485 | [387]:507, 467, 436 | Schizarin D | √ | ND | √ |
| 5 | 400 | 401 | [401]:439, 386 | Schizandrin B | √ | ND | ND |
| 6 | 400 | 401 | [401]:423, 386, 371 | Schizandrin B | √ | √ | ND |
| 7 | 432 | 433 | [433]:415, 400, 384 | Schizandrol A | ND | ND | √ |
| 8 | 416 | 417 | [417]:399, 369 | Schizandrol B | ND | ND | √ |
| 9 | 432 | 433 | [433]:455, 415 | Isoschizandrin | ND | ND | √ |
| 10 | 430 | 431 | [431]:399, 387 | Schisanlignone A | ND | ND | √ |
| 11 | 430 | 431 | [431]:453, 387, 413 | Kadsulignan M | ND | ND | √ |
| 12 | 416 | 417 | [417]:369, 399, 384 | Schizandrol B | ND | ND | √ |
ND, none detected.
√, detected.
a, obtained by ion trap mass spectrometry.
Differences in antioxidant content and profile of the related genes expression between SEs treated fruits and control fruits
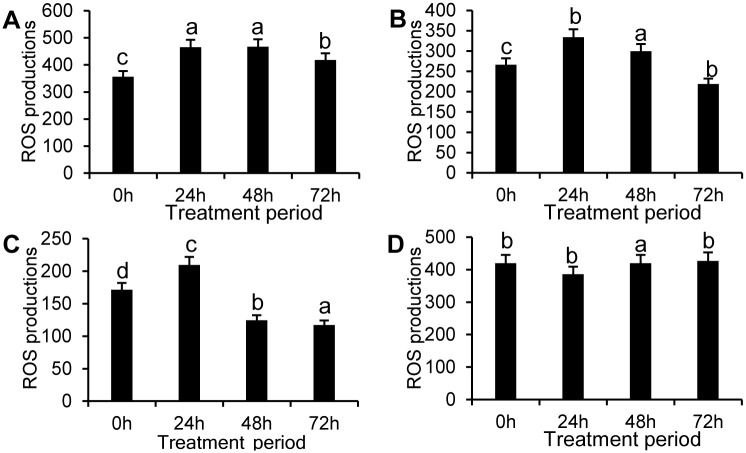
The results showed that in the apples treated with SEs, ROS production rose in the first stage and then decreased in both the control and treatment groups, except for SE-III. However, production in treated pericarps was significantly lower than that in the control, especially for SE-III. In the SE-III treated group, ROS production reached a minimum level at 72 h. ROS production in the control group was 3.65 times that of the SE-III treated group (Fig 5).
Fig 5. Measurement of ROS production in apple pericarps treated by the SEs.
Measurement of ROS production in apple pericarps treated by SE-I(A), SE-II (B), SE-III(C), and control (D) for four different treatment periods. The data represent the mean ± SE and letters indicate a significant difference (P < 0.05).
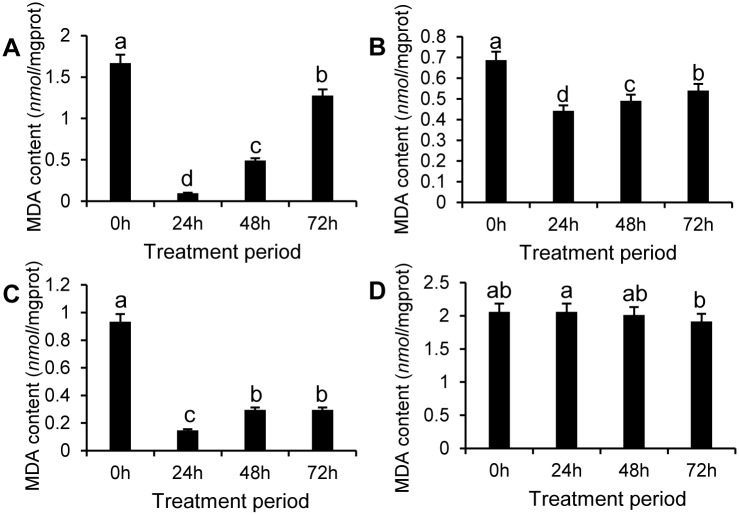
MDA (a product of lipid peroxidation in organisms) content in the apple pericarp first decreased, and then increased in the SE-treated groups during the treatment period, although the content was significantly lower in the treated apple pericarp at all test stages compared with that of the control (Fig 6). MDA content was lowest at 24 h in the SE-III treated group and was 6.7% percent of that of the control (Fig 6).
Fig 6. Measurement of MDA content in apple pericarps treated by SEs.
Measurement of MDA content in apple pericarps treated by SE-I (A), SE-II (B), SE-III (C), and control (D) for four different treatment periods. The data represent the mean ± SE.
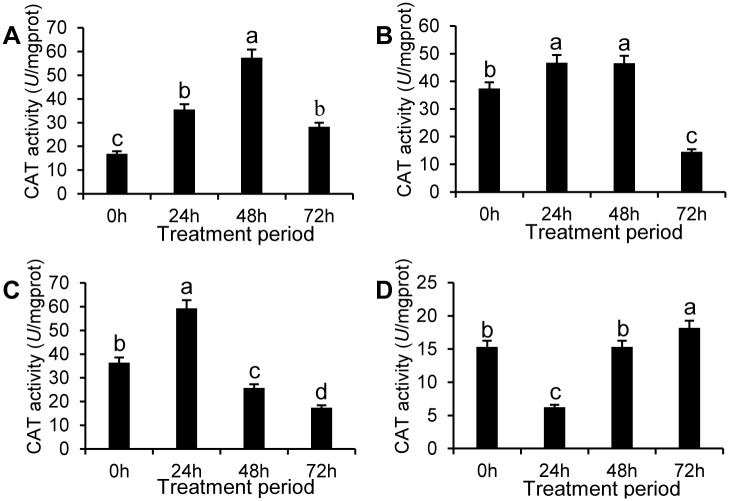
CAT activity was markedly upgraded by the SEs and initially exhibited an increase followed by a decreasing tendency with processing time for all the treated groups. CAT activity in pericarps treated with SE-III was 2.37, 9.51, and 1.68 times that of the control during the first three sampling times, respectively, whereas there was no apparent difference between treatment and control groups at 72 h (Fig 7).
Fig 7. CAT activity in apple pericarps treated by SEs.
CAT activity in the apple pericarp treated by SE-I (A), SE-II(B), SE-III(C), and control (D) for four different treatment periods. The data represent the mean ± SD and letters indicate a significant difference (P < 0.05).
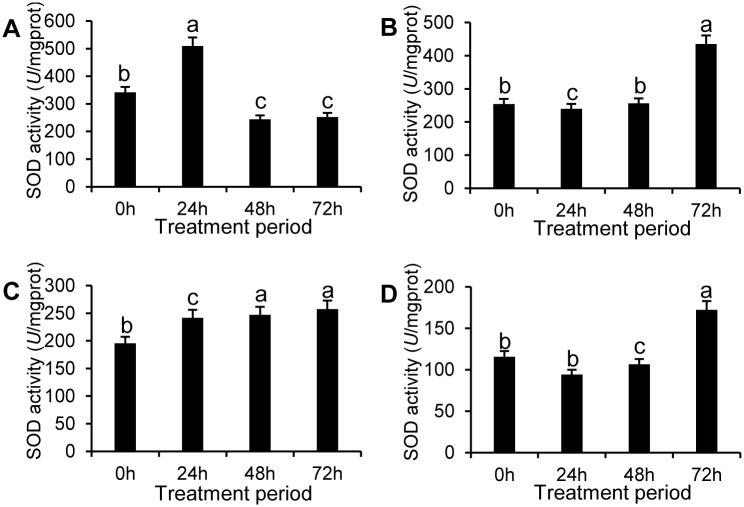
SOD activity of apple pericarps treated with SEs was improved and positively correlated with time except for SE-I. In the SE-I treated group, SOD activity reached a peak and was 2.13 times that of the control group at 24 h (Fig 8). In the SE-II and SE-III treated groups, SOD activity peaked at 72 h and was 152% and 49% higher than that of the control, respectively.
Fig 8. SOD activity in apple pericarps treated by SEs.
SOD activity in the apple pericarps treated by SE-I (A), SE-II(B), and SE-III(C), and control group (D) for four different treatment periods. The data represent the mean ± SD and letters indicate a significant difference (P < 0.05).
The results indicated that peroxidation caused by ROS in the infected tissues rose gradually and led to MDA accumulation caused by lipid peroxidation. Furthermore, as the pathogenic fungi infected the fruit, SOD and CAT activity in treated apples rose and then, changing trend, dropped, with the activity of the two enzymes in the treated fruit displaying high performance levels compared to those of control fruits at all test stages. This indicated that SE-III possibly induced plant tissues to promote activities of preventative enzymes, inhibiting the accumulation of ROS, and then downgraded lipid peroxidation in apple pericarp tissue.
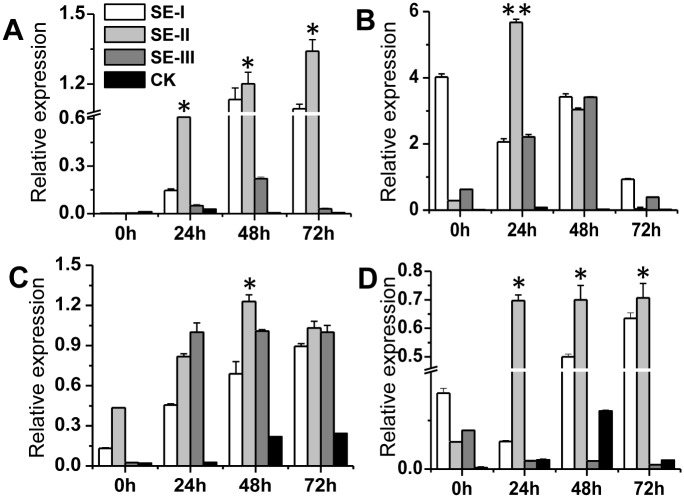
In this study, the genes related to pathogenic fungi infection and plant infection prevention were selected, and we investigated their profile in SE-III treated apple fruits. Relative gene expressions for both treated and control groups were determined by qRT-PCR, in an attempt to identify differential expression. Four genes were found to be differentially expressed between the parents, including MdPR4(A), MdAFS (B), MdPAL (C), and MdPPO (D). All were more highly expressed in the SEs treated group than in the control group and expression was also positively correlated with treatment period.
Compared with that in control groups, MdPR4 expression in apples was significantly up-regulated by SE-I, SE-II, and SE-III during the treatment period and nearly all expression increased with time, especially for SE-II. In the SE-II treated group, MdPR4 expression was 30.5, 5.45 and 44.67 times that of the control group at 24 h, 48 h, and 72 h, respectively (Fig 9A), although MdPR4 expression decreased when treated by SE-III after 48 h.
Fig 9. The relative expression of the four genes under treatment of three SEs.
The relative expression of the four genes (A: MdPR4; B: MdAFS; C: MdPAL; D: MdPPO) under the treatment of three SEs for 4 different treatment period (0 h, 24 h, 48 h, and 72 h). The data represent the mean ± SD and asterisks indicate a significant difference (P < 0.05).
Compared with that of control groups, expression of MdAFS was evidently up-regulated by the SEs. For example, in the SE-I treated group, MdAFS expression was 402, 29.43, 171, and 93 times of the control, respectively with time (Fig 9B). In SE-II and SE-III treated group, MdAFS expression showed a downward trend, after an initial first rise, with processing time. MdPAL expression increased with processing time in the SE-I, SE-II, and SE-III treated groups(Fig 9C). MdPPO expression was evidently up-regulated by SE-I and SE-II, whereas expression in the SE-I treated group initially increased before decreasing slightly with time. The expression of MdPPO in the SE-II treated group increased with processing time, and SE-III did not play a key role (Fig 9D).
Discussion
The clinical effects of S. chinensis on pathogenic bacteria, fungi, and viruses has been widely demonstrated. However, active substances extracted from the stems and leaves of S. chinensis have been rarely reported. Whether the active substances have the potential to effectively inhibit the growth of pathogenic fungi and the potential to be a new broad-spectrum fungicide has also not yet been determined. Fruits of S. chinensis are typically chosen as experimental materials [39–42]. However, Yu (1998) reported there was no significant difference in the lignin content of the fruits, stems, and leaves [43]. Therefore, the relatively low cost and rarely studied stems and leaves of S. chinensis were chosen as our experimental material.
This study proposed a new and modern method to make full use of the previously worthless stems and leaves of S. chinensis. They not only greatly reduced extraction costs and saved resources, but also provided a new method for the future development of botanical fungicides. To obtain active substances with different polarities, different ratios of the polar organic solvent were applied to separate the primary crude extract. According to the antifungal experiment, SE-I, SE-II, and SE-III were effective, although SE-IV to SE-VII had almost no antifungal activity.
When investigating the inhibitory effects of SE-I, SE-II, and SE-III on the mycelial growth of M. fructicola, F. oxysporum and B. dothidea, the SEs show inhibitive effects against the fungi, and the effect of SE-III was the highest according to the EC50 values. At the same time, the inhibition rate is positively correlated to the concentration of SE applications. The inhibition rate of SE-III reached 100% against B. dothidea growth. These results were also verified by the fact that SE-III remarkably changed the growth status of test fungal hypha cultivated on the medium, causing the hypha to shrivel and dry up.
To varying degrees, the SE fractions largely suppressed the germination rates of the three kinds of spores after inoculation for 24 h and 48 h. Consistent with previous results, SE-III had the greatest effect in the experiments on spore germination. As the development and use of biological fungicide, S. chinensis extract should be further applied to postharvest disease control on fruit. Thus, this work further explored the preventive effect of SEs against pathogen invasion. As we expected, SEs can indeed prevent the invasion of pathogenic fungi to a certain degree. After spraying the SEs on the pericarp of apples and peaches, the fungal scab area of apple ring rot and peach brown rot visibly decreased. All of these factors provided evidence that the SEs can inhibit pathogenic fungi in both the in vitro and in vivo experiments. However, there was a slight injury on the surface of peach fruit. Application for pathogen defense needs to be further improved. After comprehensive analysis of its effect in fungistasis and disease prevention, our results indicated that the optimal active substance was obtained by chloroform and acetone at the ratio of 9:1.
Differences in the principle composition of the substances could be the key reason for their different efficacy. In the present study, to determine the differences in active ingredient composition among SE-I, SE-II, and SE-III, analyses of the lignans were conducted with an evaluation of HPLC-MS. According to our results, there were significant differences in lignan composition among the extracts and SE-I, SE-II, and SE-III were found to contain 5, 4, and 7 kinds of lignans, respectively. The SE-III fraction had the highest lignan abundance, and this may explain its stronger biological activity. In recent experiments on the preventative effects of lignans on fungal infection, results indicated that lignans from S. chinensis exhibited obvious inhibition and regulation of fungal infection. For example, some lignans, such as gomisin A, gomisin G, Schizandrin, and schisanhenol were recently reported to possess valuable anti-tumor promoting bioactivities by inhibiting Epstein-Barr virus early antigen (EBV-EA) activation[44], reversing P-glycoprotein-mediated multidrug resistance (Pgp-MDR) in cancer cells [45], and enhancing doxorubicin-induced apoptosis in human hepatic cancer cells, as well as through the inhibition of platelet aggregation and anti-HIV (human immunodeficiency virus) effects [46, 47]. In our study, a variety of lignans were also detected; therefore, we speculated that lignans may play a key role in suppressing pathogens. In the next experiment, we further explored physiological and molecular antifungal mechanisms.
Previous studies indicated that lignan compounds can effectively improve an organism's antioxidant levels. Research concerning SB (Schisandrin B), a major lignan isolated from S. chinensis, showed it has a high antioxidant potential in both in vitro and in vivo experiments [48–51]. Schisandrin B has been shown to prevent ischemia-reperfusion injuries in isolated perfused rat hearts by enhancing the glutathione antioxidant response and mitochondrial ATP generation capacity, or by increasing the expression levels of cardiac protective proteins, such as heat shock proteins 25 (HSP25) and HSP70. Moreover, recent studies have shown that gomisin N has anti-apoptotic activity in H9c2 cells through suppressing mitochondrial permeability transition [52, 53]. In a study on rats, You (2006) showed that SCE (S. chinensis extract) attenuates doxorubicin-induced cardiomyopathy by elevating myocardial glutathione peroxidase and superoxide dismutase (SOD) activities and reducing lipid peroxidation [54]. Extracted solutions of S. chinensis can significantly scavenge free radicals and suppress the growth of different kinds of pathogenic bacteria, such as Staphylococcus aureus, Pneumobacillus, and Enteritis bavoil [55]. In addition, SCE suppressed doxorubicin-induced toxicity by reducing ROS production and lipid peroxidation and also regulated the expression levels of glutathione metabolism and detoxification-related genes [56]. Our results were consistent with those detailed above. The study revealed that the antioxidant system of fruits was pre-induced and activated by active substances of Schisandra. It may be the most likely mechanism for postharvest fruit to effectively defend against disease pathogen invasion. Experimental results suggested that with pathogenic fungi (B. dothidea) infection, ROS production was lower in the control, especially in the SE-III treated group. MDA content was significantly lower in the SE-treated apple pericarp when compared with that of the control. SOD and CAT were widely present in the plant cells. Within a cell, SOD constitutes the first line of defense against ROS. The typical catalase reaction is the dismutation of two molecules of H2O2 to water and O2 [57]. SOD and CAT enzymes directly remove ROS to regulate its level and convert it into less cytotoxic substances. SODs are seen as a sensor of intracellular ROS [58–60]. Furthermore, SOD and CAT activity in induced apple pericarp presented higher levels compared to those recorded in the control. This indicated that the SEs might induce plant tissues to promote activities of preventative enzymes, inhibiting the accumulation of ROS and then downgrading lipid peroxidation in the infected apple pericarp.
The study found that tested genes (MdPR4, MdAFS, MdPPO, and MdPAL) related to the defense reaction were significantly up-regulated by the SEs. Previous studies have shown that the pathogenesis-related protein gene PR, as the terminal gene in the plant defense pathway, produces a variety of ROS and toxic secondary metabolites that cooperatively kill pathogens, and some PR gene over expression can significantly improve plant disease resistance [61]. There are other functions of PR genes, such as those involved in senescence [62], osmotic stress and mechanical injury [63]. MdPPO (a polyphenol oxidase gene) is considered to be the plant defense protein [64–67] and plays an important role in plant defense responses. MdPAL (phenylalanine ammonia lyase) plays a catalytic role in flavonoid biosynthesis, and flavonoids have antibacterial, antiviral, anti-allergic, anti-inflammatory, vasodilatation inhibiting, and other biological functions[68]. Moreover, flavonoids can also inhibit lipid peroxidation and the enzyme systems [69], as α-farnesene synthase (AFS) is the ultimate limiting enzyme in the synthesis of α-farnesene plant mevalonate pathway [70].
The present study indicated that spraying with SEs up-regulated the expression of the four genes to different degrees during the treatment period. We speculated that the active ingredients in the fractions from S. chinensis plants not only induced the up-regulation of the four genes, but also activated the antioxidant system in fruits to prevent infection, although the response of the plant itself occurs in case of infection.
Fruit quality characteristics in the process of maturity directly affect the economic value of the commercial crop. However, during this period several physiological disorders may occur. Fruit need to be induced to be resistant to infection through promoting preventative enzyme activities and regulating other scavengers or protectors, decreasing ROS production and reducing lipid peroxidation. This mechanism may extend storage time of fresh apples. According to this study, fractions from S. chinensis plants could improve antioxidant levels and promote the capacity for self-regulation while directly inhibiting disease and infection. This indicates that compounds of S. chinensis can be used as preservative or protective agents for future commercial development.
Herbal ingredients and their chemically modified derivatives have been invaluable resources for the development of therapeutic substances [71]. Technological advances in lead-generation strategies have drawn notable attention to the chemical complexity and diversity of herbal ingredients [71]. Accordingly, bioactive lignans may be a promising source of new-lead compounds for future drug development. On the other hand, lignans could be exploited as chemical tools for the starting point of new scientific discoveries—assisting in the dissection of complex phenomena into their constituent parts and the identification of the hidden molecular targets and mechanisms underlying pathophysiological phenotypes. Extracts of S. chinensis as promising resources for the development of safe, effective and multi-targeted agents against pathogenic fungi, will offer us a novel insight into future challenges and perspectives on S. chinensis research and future clinical investigations and strategies.
Conclusions
The results described in this work showed that active ingredients were extracted with 70% acetone in S. chinensis and were separated with different organic solvents. The fraction SE-III-separated by a ratio of chloroform to acetone of 9: 1 was the most potent and antifungal active substance extracted. S. chinensis extraction was verified to successfully suppress the growth of three tested pathogens, inhibit the germination of fungal spores, and protect apple fruit from infection by pathogenic fungi. A possible mechanism to explain the results may be that SEs induced and activated the antioxidant system and also up-regulated defense-related genes. Thus, active ingredients in plants could play a major role in the development and exploitation of botanical fungicides.
Supporting Information
(XLSX)
Acknowledgments
We thank the Fruit Tree Key Laboratory at the Beijing University of Agriculture and the Beijing Nursery Engineering Research Center for Fruit Crops for providing basic experimental facilities.
Financial support was provided by scientific research projects “Extraction and utilization of active ingredients in Schisandra chinensis (Turcz.) Baill and the study of their antifungal effect” (KM201510020004) of Beijing municipal education commission in 2015. Financial support was also provided by the project of construction of innovative teams and teacher career development for universities and colleges under Beijing municipality (IDHT20140509; IDHT20150503), and by the NSFC for Outstanding Young Scholar (81422046). Beijing Collaborative innovation center for eco-environmental improvement with forestry and fruit trees’ (CEFF-PXM2016-014207-000038) also provide financial support.
Abbreviations
- SE
Schisandra chinensis Extract
- CAT
Catalase
- SOD
Superoxide dismutase
- MDA
Malondialdehyde
- ROS
Reactive oxygen species
- PAL
Phenylalanine ammonia lyase
- PPO
Polyphenol oxidase
- AFS
α-Farnesene synthase
Data Availability
All data necessary to replicate the findings of our study is available on Dryad (doi:10.5061/dryad.21533).
Funding Statement
The authors thank the Fruit Tree Key Laboratory at the Beijing University of Agriculture and the Beijing Nursery Engineering Research Center for Fruit Crops for providing basic experimental facilities. Financial support was provided by scientific research projects “Extraction and utilization of active ingredients in Schisandra chinensis (Turcz.) Baill and the study of their antifungal effect” (KM201510020004) of Beijing municipal education commission in 2015. Financial support was also provided by the project of construction of innovative teams and teacher career development for universities and colleges under Beijing municipality (IDHT20140509;IDHT20150503), and by the NSFC for Outstanding Young Scholar (81422046). Beijing Collaborative innovation center for eco-environmental improvement with forestry and fruit trees’ (CEFF-PXM2016-014207-000038) also provide financial support.
References
- 1.Isman MB. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annual Review of Entomology. 2006; 51: 45–66. 10.1146/annurev.ento.51.110104.151146 . [DOI] [PubMed] [Google Scholar]
- 2.Isman MB. Perspective botanical insecticides: For richer, for poorer. Pest Management Science. 2008; 64: 8–11. [DOI] [PubMed] [Google Scholar]
- 3.Kudom AA, Mensah BA, Botchey MA. Aqueous neem extract versus neem powder on Culex quinquefasciatus: Implications for control in anthropogenic habitats. Journal of Insect Science. 2011; 11(142). 10.1673/031.011.14201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lv C, Zhong B, Zhong G, Weng Q, Chen S, Hu M, et al. Four botanical extracts are toxic to the hispine beetle, Brontispa longissima, in laboratory and semi–field trials. Journal of Insect Science. 2012; 12(58). 10.1673/031.012.5801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ladhari A, Laarif A, Omezzine F, Haouala R. Effect of the extracts of the spiderflower, Cleome arabica, on feeding and survival of larvae of the cotton leafworm, Spodoptera littoralis. Journal of Insect Science. 2013; 13(61). 10.1673/031.013.6101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Copping LG, Duke SO. Natural products that have been used commercially as crop protection agents. Pest Manage Science. 2007; 63: 524−554. [DOI] [PubMed] [Google Scholar]
- 7.Dayan FE, Cantrell CL, Duke SO. Natural products in crop protection. Bioorganic & Medicinal Chemistry. 2009; 17: 4022−4034. [DOI] [PubMed] [Google Scholar]
- 8.Duke SO, Cantrell CL, Meepagala KM, Wedge DE. Tabanca N, Schrader KK. Natural toxins for use in pest management. Toxins (Basel). 2010; 2:1943−1962. 10.3390/toxins2081943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantrell CL, Dayan FE, Duke SO. Natural products as sources for new pesticides. Journal of Natural Products. 2012; 75: 1231−1242. 10.1021/np300024u . [DOI] [PubMed] [Google Scholar]
- 10.Tomar A, Singh Rathore H, Singh K. Scope and limitations of neem products and other botanicals in plant protection Handbook of Pesticides: CRC Press: 2009; 67–89. [Google Scholar]
- 11.Nabavi SM, Marchese A, Izadi M, Curti V, Daglia M, Nabavi SF. Plants belonging to the genus Thymus as antibacterial agents: from farm to pharmacy. Food Chemistry. 2015; 173: 339–47. 10.1016/j.foodchem.2014.10.042 . [DOI] [PubMed] [Google Scholar]
- 12.Zabka M, Pavela R, Prokinova E. Antifungal activity and chemical composition of twenty essential oils against significant indoor and outdoor toxigenic and aeroallergenic fungi. Chemosphere. 2014; 112: 443–8. 10.1016/j.chemosphere.2014.05.014 . [DOI] [PubMed] [Google Scholar]
- 13.Jing Li, Lei Zhentian, Li Ligai, Xie Rangjin, Xi Wanpeng, Guan Yu, et al. Antifungal Activity of Citrus Essential Oils. Journal of Agricultural and Food Chemistry. 2014; 62 (14): 3011–33. 10.1021/jf5006148 . [DOI] [PubMed] [Google Scholar]
- 14.Swain T. Secondary compouds as protective agents. Annual Review of Plant Physiology. 1977; 28: 479–501. [Google Scholar]
- 15.Yoon MY, Cha B, Kim JC. Recent trends in studies on botanical fungicides in agriculture. Plant Pathology Journal. 2013. 29(1):1–9. 10.5423/PPJ.RW.05.2012.0072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society. 2009; 161(2): 105–121. 10.1111/j.1095-8339.2009.00996.x [DOI] [Google Scholar]
- 17.Hancke JL, Burgos RA, Ahumada F. Schisandra chinensis (Turcz.) Baill. Fitoterapia. 1999; 70: 451–71. [Google Scholar]
- 18.Chun JN, Cho M, So I, Jeon JH. The protective effects of Schisandra chinensis fruit extract and its lignans against cardiovascular disease: A review of the molecular mechanisms. Fitoterapia. 2014; 97: 224–33. 10.1016/j.fitote.2014.06.014 [DOI] [PubMed] [Google Scholar]
- 19.Lee KH, Morris-Natschke S, Qian K, Dong Y, Yang X, Zhou T, et al. Recent progress of research on herbal products used in traditional Chinese medicine: the herbs belonging to The Divine Husbandman's Herbal Foundation Canon (Shén Nóng Běn Cǎo Jīng). Journal of Traditional Complementary and Medicine. 2012; 2(1): 6–26. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexander JS, Wang Y. Therapeutic potential of Schisandra chinensis extracts for treatment of hypertension. Introduction to: ‘antihypertensive effect of gomisin A from Schisandra chinensis on angiotensin II induced hypertension via preservation of nitric oxide bioavailability’ by Park et al. Hypertension Research. 2012; 35: 892–3. 10.1038/hr.2012.101 . [DOI] [PubMed] [Google Scholar]
- 21.Park EJ, Chun JN, Kim SH, Kim CY, Lee HJ, Kim HK, et al. Schisandrin B suppresses TGFbeta1 signaling by inhibiting Smad 2/3 and MAPK pathways. Biochemical Pharmacology 2012; 83(3): 378–84. 10.1016/j.bcp.2011.11.002 . [DOI] [PubMed] [Google Scholar]
- 22.Panossian A, Wikman G. Pharmacology of Schisandra chinensis Bail: an overview of Russian research and uses in medicine. Journal of Ethnopharmacology. 2008; 118(2): 183–212. 10.1016/j.jep.2008.04.020 . [DOI] [PubMed] [Google Scholar]
- 23.Jiang Y, Fan X, Wang Y, Tan H, Chen P, Zeng H, et al. Hepato-protective effects of six Schisandra lignans on acetaminophen-induced liver injury are partially associated with the inhibition of CYP-mediated bioactivation. Chemico-Biological Interactions. 2015; 231: 83–9. 10.1016/j.cbi.2015.02.022 . [DOI] [PubMed] [Google Scholar]
- 24.Zhong S, Nie YC, Gan ZY, Liu XD, Fang ZF, Zhong BN, et al. Effects of Schisandra chinensis extracts on cough and pulmonary inflammation in a cough hypersensitivity guinea pig model induced by cigarette smoke exposure. Journal of Ethnopharmacology. 2015; 165:73–82. 10.1016/j.jep.2015.02.009 . [DOI] [PubMed] [Google Scholar]
- 25.Kim EY, Baek IH, Rhyu MR. Cardioprotective effects of aqueous Schizandra chinensis fruit extract on ovariectomized and balloon-induced carotid artery injury rat models: effects on serum lipid profiles and blood pressure. Journal of Ethnopharmacology. 2011; 134(3):668–75. 10.1016/j.jep.2011.01.019 [DOI] [PubMed] [Google Scholar]
- 26.Cyong JC, Ki SM, Iijima K, Kobayashi T, Furuya M. Clinical and pharmacological studies on liver disease treated with Kampo herbal medicine. American Journal of Chinese Medicine. 2000; 28: 351–60. 10.1142/S0192415X00000416 . [DOI] [PubMed] [Google Scholar]
- 27.Pan SY, Yu Q, Zhang Y, Wang XY, Sun N, Yu ZL, et al. Dietary Fructus Schisandrae extracts and fenofibrate regulate the serum /hepatic lipid-profile in normal and hypercholesterolemic mice, with attention to hepatotoxicity. Lipids in Health and Disease. 2012; 11: 1186–94. 10.1186/1476-511X-11-120 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S, Kim DH, Jung JW, Oh JH, Park HJ, Park C, et al. Schizandra chinensis and Scutellaria baicalensis counter stress behaviors in mice. Phytotherapy Research. 2007; 21(12): 1187–1192. 10.1002/ptr.2233 . [DOI] [PubMed] [Google Scholar]
- 29.Panossian A, Hambardzumyan M, Hovhanissyan A, Wikman G. The adaptogens rhodiola and Schizandra modify the response to immobilization stress in rabbits by suppressing the increase of phosphorylated stress-activated protein kinase, nitric oxide and cortisol. Drug Target Insights. 2007; 2: 39–54. Available: http://www.la-press.com/copyright.htm. . [PMC free article] [PubMed] [Google Scholar]
- 30.Chen WW, He RR, Li YF, Li SB, Tsoi B, Kurihara H. Pharmacological studies on the anxiolytic effect of standardized Schisandra lignans extract on restraint-stressed mice. Phytomedicine. 2011; 18(13): 1144–47. 10.1016/j.phymed.2011.06.004 . [DOI] [PubMed] [Google Scholar]
- 31.Kwon DY, Kim DS, Yang HJ, Park S. The lignan-rich fractions of Fructus Schisandrae improve insulin sensitivity via the PPAR-γ pathways in in vitro and in vivo studies. Journal of Ethnopharmacology, 2011; 135(2): 455–62. 10.1016/j.jep.2011.03.037 . [DOI] [PubMed] [Google Scholar]
- 32.Opletal L, Sovová H, Bártlová M. Dibenzo[a,c] cyclooctadiene lignans of the genus Schisandra: importance, isolation and determination. Journal of Chromatography B. 2004; 812(1–2): 357–71. 10.1016/j.jchromb.2004.07.040 . [DOI] [PubMed] [Google Scholar]
- 33.Huang TH, Shen PN, Shen YG. Preparative separation and purification of deoxyschisandrin and γ-schisandrin from Schisandra chinensis (Turcz.) Baill by high-speed counter-current chromatography. Journal of Chromatography A. 2005; 1066(1–2): 239–42. . [DOI] [PubMed] [Google Scholar]
- 34.Tian K, Qi SD, Cheng YQ, Chen XG, Hu Z. Separation and determination of lignans from seeds of Schisandra species by micellar electrokinetic capillary chromatography using ionic liquid as modifier. Journal of Chromatography A. 2005; 1078(1–2): 181–7. . [DOI] [PubMed] [Google Scholar]
- 35.He F, Li XY, Yang GY, Li XN, Luo X, Zou J, et al. Nortriterpene constituents from Schisandra sphenanthera. Tetrahedron. 2012; 68(2): 440–6. [Google Scholar]
- 36.Tang W, Ding Z, Zhou ZQ, Wang YZ, Guo LY. Phylogenetic and pathogenic analyses show that the causal agent of apple ring rot in China is Botryosphaeria dothidea. Plant Disease. 2012; 96:486–96. [DOI] [PubMed] [Google Scholar]
- 37.Hu M-J, Cox KD, Schnabel G, Luo C-X. Monilinia species causing brown rot of peach in China. PLoS ONE. 2011; 6(9): e24990 10.1371/journal.pone.0024990 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koike ST, Kirkpatrick SK, Gordon TR. Fusarium wilt of strawberry caused by Fusarium oxysporum in California. Plant Diseae, 2009; 93(10): 1077 10.1094/PDIS-93-10-1077A [DOI] [PubMed] [Google Scholar]
- 39.Cheng Z, Song H, Yang Y, Zhou H, Liu Y, Liu Z. Smashing Tissue Extraction of Five Lignans From the Fruit of Schisandra chinensis. Journal of Chromatographic Science. 2016; 54(2):246–56. 10.1093/chromsci/bmv116 [DOI] [PubMed] [Google Scholar]
- 40.Lee HJ, Jo S, Ryu J, Jeong HS, Lee G, Ryu MH, et al. Effects of Schisandra chinensis Turcz. fruit on contact dermatitis induced by dinitrofluorobenzene in mice. Molecular Medicine Reports. 2015; 12(2): 2135–9. 10.3892/mmr.2015.3618 [DOI] [PubMed] [Google Scholar]
- 41.Xue Y, Li X, Du X, Li X, Wang W, Yang J, et al. Isolation and anti-hepatitis B virus activity of dibenzocyclooctadiene lignans from the fruits of Schisandra chinensis. Phytochemistry. 2015; 116: 253–61. 10.1016/j.phytochem.2015.03.009 Epub 2015 Apr 13. [DOI] [PubMed] [Google Scholar]
- 42.Cheng Z, Song H, Yang Y, Liu Y, Liu Z, Hu H, et al. Optimization of microwave-assisted enzymatic extraction of polysaccharides from the fruit of Schisandra chinensis Baill. International Journal of Biological Macromolecules. 2015. May;76:161–8. 10.1016/j.ijbiomac.2015.01.048 [DOI] [PubMed] [Google Scholar]
- 43.Yu JL, Li SH, Chen J. A comparative study of lignans among Schisandra root, stems, leaves, and fruit. Ginseng Research. 1998; (1):11–12. [Google Scholar]
- 44.Chen DF, Zhang SX, Kozuka M, Sun QZ, Feng J, Wang Q, et al. Interiotherins C and D, two new lignans from Kadsura interior and antitumor-promoting effects of related neolignans on Epstein-Barr virus activation. Journal of Natural Products. 2002; 65(9): 1242–5. . [DOI] [PubMed] [Google Scholar]
- 45.Yoo HH, Lee M, Lee MW, Lim SY, Shin J, Kim DH. Effects of Schisandra lignans on P-glycoprotein-mediated drug efflux in human intestinal Caco-2. Planta Medica. 2007; 73: 444–50. [DOI] [PubMed] [Google Scholar]
- 46.Chen M, Kilgore N, Lee KH, Chen DF. Rubrisandrins A and B, Lignans and related anti-HIV compounds from Schisandra rubriflora. Journal of Natural Products. 2006; 69(12): 1697–701. 10.1021/np060239e . [DOI] [PubMed] [Google Scholar]
- 47.Chen DF, Zhang SX, Xie L, Xie JX, Chen K, Kashiwada Y, et al. Anti-AIDS agents—XXVI. Structure-activity correlations of gomisin-G-related anti-HIV lignans from Kadsura interior and of related synthetic analogues. Bioorganic & Medicinal Chemistry. 1997; 5(8): 1715–23. . [DOI] [PubMed] [Google Scholar]
- 48.Hou W, Gao W, Wang D, Liu Q, Zheng S, Wang Y. The Protecting Effect of Deoxyschisandrin and Schisandrin B on HaCaT Cells against UVB-Induced Damage. PLoS One. 2015; 10(5):e0127177 10.1371/journal.pone.0127177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang CY, Chen YL, Yang SC, Huang GC, Tsi D, Huang CC, et al. Effect of Schisandrin B and sesamin mixture on CCl(4)-induced hepatic oxidative stress in rats. Phytotherapy Research. 2009; 23(2): 251–6. 10.1002/ptr.2602 [DOI] [PubMed] [Google Scholar]
- 50.Giridharan VV, Thandavarayan RA, Bhilwade HN, Ko KM, Watanabe K, Konishi T. Schisandrin B, attenuates cisplatin-induced oxidative stress, genotoxicity and neurotoxicity through modulating NF-κB pathway in mice. Free Radical Research. 2012; 46(1): 50–60. 10.3109/10715762.2011.638291 . [DOI] [PubMed] [Google Scholar]
- 51.Giridharan VV, Thandavarayan RA, Sato S, Ko KM, Konishi T. Prevention of scopolamine-induced memory deficits by schisandrin B, an antioxidant lignan from Schisandra chinensis in mice. Free Radical Research. 2011; 45(8): 950–8. 10.3109/10715762.2011.571682 . [DOI] [PubMed] [Google Scholar]
- 52.Chiu PY, Luk KF, Leung HY, Ng KM, Ko KM. Schisandrin B stereoisomers protect against hypoxia/reoxygenation-induced apoptosis and inhibit associated changes in Ca2+-induced mitochondrial permeability transition and mitochondrial membrane potential in H9c2cardiomyocytes. Life Sciences. 2008; 82(21–22): 1092–101. 10.1016/j.lfs.2008.03.006 . [DOI] [PubMed] [Google Scholar]
- 53.Chiu PY, Leung HY, Poon MK, Mak DH, Ko KM. (−)Schisandrin B is more potent than its enantiomer in enhancing cellular glutathione and heat shock protein production as well as protecting against oxidant injury in H9c2cardiomyocytes. Molecular and Cellular Biochemistry. 2006; 289(1–2):185–91. 10.1007/s11010-006-9163-1 . [DOI] [PubMed] [Google Scholar]
- 54.You JS, Pan TL, Hou YC. Schisandra chinensis protects against adriamycin induced cardiotoxicity in rats. Chang Gung Medical Journal. 2006; 29(1): 63–70. . [PubMed] [Google Scholar]
- 55.Li B, Meng XJ, Xue X, Wu Q, Li YS, Wang YQ. Schisandrin B from Schisandra scavenging free radical and bacteriostasis in vitro experiment. Food Science, 2011; 32 (5): 79–82. [Google Scholar]
- 56.Choi EH, Lee N, Kim HJ, Kim MK, Chi SG, Kwon DY, et al. Schisandra fructus extract ameliorates doxorubicin-induce cytotoxicity in cardiomyocytes: altered gene expression for detoxification enzymes. Genes and Nutrition. 2008; 2(4): 337–45. 10.1007/s12263-007-0073-y . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zamocky M, Furtmüller PG, Obinger C. Evolution of catalases from bacteria to humans. Antioxidants and Redox Signaling. 2008; 10(9): 1527–47. 10.1089/ars.2008.2046 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alscher RG, Erturk N, Heath LS. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. Journal of Experimental Botany. 2002; 53(372): 1331–41. 10.1093/jexbot/53.372.1331 . [DOI] [PubMed] [Google Scholar]
- 59.Del Río LA. Peroxisomes as a cellular source of reactive nitrogen species signal molecules. Archives of Biochemistry and Biophysics. 2011; 506(1): 1–11. 10.1016/j.abb.2010.10.022 [DOI] [PubMed] [Google Scholar]
- 60.Mhamdi A, Queval G, Chaouch S, Vanderauwera S, Breusegem FV, Noctor G. Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. Journal of Experimental Botany. 2010; 61(15): 4197–220. 10.1093/jxb/erq282 . [DOI] [PubMed] [Google Scholar]
- 61.Sarowar S, Kim YJ, Kim EN, Kim KD, Hwang BK, Islam R, et al. Over expression of a pepper basic pathogenesis-related protein 1 gene in tobacco plants enhances resistance to heavy metal and pathogen stresses. Plant Cell Reports. 2005; 24(4): 216–24. 10.1007/s00299-005-0928-x . [DOI] [PubMed] [Google Scholar]
- 62.Buchanan-Wollaston V, Earl S, Harrison E, Mathas E, Navabpour S, Page T. The molecular analysis of leaf senescence a genomics approach. Plant Biotechnology Journal. 2003; 1(1):3–22. . [DOI] [PubMed] [Google Scholar]
- 63.Slusarenko AJ, Fraser RSS, Loon LC. Mechanisms of resistance to plant diseases. 2000. (Dordrecht: Kluwer; ). [Google Scholar]
- 64.Constabel CP, Bergey DR, Ryan CA. Systemin activates synthesis of wound-inducible tomato leaf polyphenol oxidase via the octadecanoid defense signaling pathway. Proceedings of the National Academy of Sciences of the United States of America. 1995; 92(2): 407–11.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pinto MST, Siqueira FP, Oliveira AEA, Fernandes KVS. A wounding-induced PPO from cowpea (Vigna unguiculata) seedlings. Phytochemistry. 2008; 69(12): 2297–302. 10.1016/j.phytochem.2008.06.003 . [DOI] [PubMed] [Google Scholar]
- 66.Stewart RJ, Sawyer BJB, Bucheli CS, Robinson SP. Polyphenol oxidase is induced by chilling and wounding in pineapple. Australian Journal of Plant Physiology. 2001; 28(3): 181–91. 10.1071/PP00094 [DOI] [Google Scholar]
- 67.Thipyapong P, Hunt MD, Steffens JC. Antisense down-regulation of polyphenol oxidase results in enhanced disease susceptibility. Planta. 2004; 220(1): 103–17. 10.1007/s00425-004-1330-6 [DOI] [PubMed] [Google Scholar]
- 68.Freitas M, Ribeiro D, Sara TM, Artur SM, Fernandes E. Anti-inflammatory and pro-apoptotic activities of chlorinated flavonoids in human neutrophils. Free Radical Biology and Medicine. 2014, 75: S30 10.1016/j.freeradbiomed.2014.10.760 [DOI] [PubMed] [Google Scholar]
- 69.Robak J, Korbut R, Shridi F, Swies J, Rzadkowska-Bodalska H. On the mechanism of antiaggregatory effect of myricetin. Polish Journal of Pharmacy and Pharmacology. 1988; 40: 337–40. . [PubMed] [Google Scholar]
- 70.Rupasinghe HPV, Almquist KC, Paliyath G, Murr DP. Cloning of hmg1 and hmg2 cDNAs encoding 3-hydroxy-3-methylglutaryl coenzyme A reductase and their expression and activity in relation to α-farnesene synthesis in apple. Plant Physiology and Biochemistry. 2001; 39(11): 933–47. 10.1016/S0981-9428(01)01316-X [DOI] [Google Scholar]
- 71.Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nature Reviews Drug Discovery. 2005; 4(3): 206–20. 10.1038/nrd1657 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All data necessary to replicate the findings of our study is available on Dryad (doi:10.5061/dryad.21533).