ABSTRACT
Methylobacterium extorquens AM1 has two distinct types of methanol dehydrogenase (MeDH) enzymes that catalyze the oxidation of methanol to formaldehyde. MxaFI-MeDH requires pyrroloquinoline quinone (PQQ) and Ca in its active site, while XoxF-MeDH requires PQQ and lanthanides, such as Ce and La. Using MeDH mutant strains to conduct growth analysis and MeDH activity assays, we demonstrate that M. extorquens AM1 has at least one additional lanthanide-dependent methanol oxidation system contributing to methanol growth. Additionally, the abilities of different lanthanides to support growth were tested and strongly suggest that both XoxF and the unknown methanol oxidation system are able to use La, Ce, Pr, Nd, and, to some extent, Sm. Further, growth analysis using increasing La concentrations showed that maximum growth rate and yield were achieved at and above 1 μM La, while concentrations as low as 2.5 nM allowed growth at a reduced rate. Contrary to published data, we show that addition of exogenous lanthanides results in differential expression from the xox1 and mxa promoters, upregulating genes in the xox1 operon and repressing genes in the mxa operon. Using transcriptional reporter fusions, intermediate expression from both the mxa and xox1 promoters was detected when 50 to 100 nM La was added to the growth medium, suggesting that a condition may exist under which M. extorquens AM1 is able to utilize both enzymes simultaneously. Together, these results suggest that M. extorquens AM1 actively senses and responds to lanthanide availability, preferentially utilizing the lanthanide-dependent MeDHs when possible.
IMPORTANCE The biological role of lanthanides is a nascent field of study with tremendous potential to impact many areas in biology. Our studies demonstrate that there is at least one additional lanthanide-dependent methanol oxidation system, distinct from the MxaFI and XoxF MeDHs, that may aid in classifying additional environmental organisms as methylotrophs. Further, our data suggest that M. extorquens AM1 has a mechanism to regulate which MeDH is transcribed, depending on the presence or absence of lanthanides. While the mechanism controlling differential regulation is not yet understood, further research into how methylotrophs obtain and use lanthanides will facilitate their cultivation in the laboratory and their use as a biomining and biorecycling strategy for recovery of these commercially valuable rare-earth elements.
INTRODUCTION
Methylotrophs have gained worldwide interest as platforms for the production of value-added chemicals from single-carbon compounds, turning atmospheric pollutants like methane and methanol into green chemicals, including biofuels and biodegradable plastics (1–5). A key step in this process is the oxidation of methanol to formaldehyde, which is carried out by different enzymes, including methanol dehydrogenase (MeDH) and alcohol oxidase, depending on the specific methylotroph (6, 7). Recently, it was discovered that some types of MeDHs require rare-earth elements, specifically lanthanides, as cofactors (8–11).
Rare-earth elements include 15 lanthanides (lanthanum [La], cerium [Ce], praseodymium [Pr], neodymium [Nd], promethium [Pm], samarium [Sm], europium [Eu], gadolinium [Gd], terbium [Tb], dysprosium [Dy], holmium [Ho], erbium [Er], thulium [Tm], ytterbium [Yb], and lutetium [Lu]) and two chemically similar elements (scandium [Sc] and yttrium [Y]). The term “rare earth” is deceptive, as these lanthanide elements are relatively abundant in the Earth's crust, found at levels similar to those seen for copper and zinc (Ce, 66 ppm; La 39 ppm; Cu, 60 ppm, Zn, 70 ppm) (12). However, lanthanides are highly insoluble and are rarely found in pure form (13). Because of their spectroscopic, superconductive, and magnetic properties, lanthanides are used in lasers, wind turbines, hybrid car batteries, and military weaponry, along with many of our “everyday use” items, such as smart phones and computers (14, 15). While commercially important, the low bioavailability of lanthanides prompted the belief that biology had evolved to effectively ignore these elements (16). However, in some environments, such as volcanic mud pots, concentrations of soluble lanthanides can reach the low micromolar range (8). Studies have shown that plants can effectively concentrate lanthanides like La from the soil, with measurements ranging from 0.18 to 3.1 μg La/g dry leaf mass (11). These studies have had profound impacts on the study of methylotrophic organisms, as different methylotrophic bacteria have now been shown to use lanthanides directly for MeDH activity and addition of lanthanides to growth media has facilitated the growth of organisms that had been difficult or impossible to culture before (8, 11, 17).
Until recently, it was believed that in Methylobacterium extorquens AM1, a model organism for understanding methylotrophic growth, methanol oxidation was catalyzed solely by the extensively studied Ca- and pyrroloquinoline quinone (PQQ)-dependent MeDH encoded by the mxaFI genes (18). Each large subunit (MxaF) contains Ca and PQQ, both of which are essential for the oxidation of methanol to formaldehyde in the periplasmic space. The xoxF1 gene was first identified in M. extorquens AM1 over a decade ago as a predicted PQQ-dependent periplasmic alcohol dehydrogenase, though its role in methylotrophy was unclear at the time (19, 20). When completed, the genome sequence of M. extorquens AM1 revealed a second xoxF gene, xoxF2, which, like xoxF1, encodes a protein that shares 50% amino acid identity with the MxaF subunit of the Ca-dependent MeDH (21, 22).
After the initial discovery of xoxF1, the physiological role of the XoxF proteins remained unknown for ∼15 years. In 2011, XoxF1 was purified and demonstrated a low rate of methanol oxidation in vitro (Vmax = 0.015 U/mg) (23), yet overexpression of xoxF was unable to functionally complement an mxaF mutant in vivo (22), complicating the interpretation of these findings. Intriguingly, the xoxF genes were also shown to be required for expression of the mxaFI genes, suggesting that XoxF has a regulatory role, in addition to a catalytic role, in M. extorquens AM1 (22). A breakthrough as to the enzymatic role of XoxF came when Hibi et al. grew Methylobacterium radiodurans in medium containing La and purified MeDH by following its activity (24). N-terminal sequencing revealed that XoxF and not MxaFI was responsible for this MeDH activity (24). This suggested that XoxF might function as an La-dependent MeDH, but there was still a lack of direct proof. It was formally possible that XoxF-MeDH required Ca for the oxidation of methanol but was upregulated in the presence of La. In 2012, this was resolved when Nakagawa et al. demonstrated that MeDH, purified from M. extorquens AM1 cells grown in medium containing La, consisted of a XoxF dimer containing 1.24 atoms of La and lacked Ca (11). Since these findings, XoxF has been purified from a variety of methylotrophs, displaying differences in subunit composition, optimal enzyme assay parameters, and oxidation products depending on the organism studied (9, 10, 25). In 2014, Pol et al. addressed the biochemical promiscuity of XoxF MeDHs by determining that a variety of lanthanides, including La, Ce, Pr, and Nd, could support methanol-dependent growth of Methylacidiphilum fumariolicum SolV (8).
Our current understanding of mxa and xox gene regulation is incomplete. In M. extorquens AM1, two two-component systems (MxcQE and MxbDM) and an additional response regulator (MxaB) are required for expression of the mxa operon, though it is not known if the requirement for these regulators is direct or indirect or what is being sensed by the systems (22, 26, 27). Additionally, the MxbDM two-component system is required for repression of the xox1 operon in the absence of lanthanides, while nothing is known about the regulation of xoxF2 expression (22). Among methylotrophic bacteria that contain both mxaF and xoxF homologs, the presence and copy numbers of these regulator genes vary, making it difficult to define a general requirement and role for the regulators in methylotrophy (25). The studies addressing how methylotrophic bacteria sense lanthanides and differentially regulate expression of the mxa and xox genes are limited and conflicting. In M. extorquens AM1, it was initially reported that La does not affect expression of the mxa or xox1 operon genes (11), which conflicted with the subsequent findings described by Skovran and Martinez-Gomez (17). Following these reports, Farhan et al. showed that in the methanotrophic bacterium Methylosinus trichosporium OB3b, exogenous Ce upregulates expression of both xoxF copies in its genome and represses expression of the mxaFI genes (28). Interestingly, significant repression of mxaF and mxaI was seen only in the absence of copper, which controls differential expression of the soluble and particulate methane monooxygenases that oxidize methane to methanol (29). M. trichosporium OB3b does not contain obvious homologs of mxbD and mxbM but does contain a sensor kinase gene (mxaY) and a response regulator gene (mxaB) upstream of the mxa genes whose products share 34% and 36% identity with MxcQ and MxaB in M. extorquens AM1, respectively. Whether these regulators are required for differential expression of the xox and mxa genes is not yet known.
In this study, we describe the range of lanthanides that allow lanthanide-dependent methanol growth in M. extorquens AM1 and describe the contribution by each known MeDH to growth using MeDH mutant strains. Further, we report for the first time the existence of an additional, unidentified lanthanide-dependent methanol oxidation system. We also refute the conclusions of Nakagawa et al. regarding expression of the mxa and xox1 operon genes (11), as we show that differential expression from the mxa and xox1 promoters is very sensitive to the presence of La, Ce, Pr, and Nd. While it is clear that lanthanides differentially control expression of the xoxF1 and mxaFI genes, the regulatory network mediating this differential expression is complex and poorly understood and has been investigated only in the absence of lanthanides (22). Once it is understood how methylotrophs are able to sense and acquire lanthanides, they may be better engineered as alternative biomining and biorecycling strategies for recovery of these highly valued elements from lanthanide ores and discarded items, such as computer magnets and hybrid car batteries.
MATERIALS AND METHODS
Chemicals.
Chemicals were purchased through Fisher Scientific (Pittsburgh, PA) unless otherwise stated. All lanthanides were used in their chloride salt forms for the preparation of stock solutions and were in the 3+ oxidation state.
Strain construction.
The strains and plasmids used in this study are listed in Table 1. Plasmids were conjugated into M. extorquens AM1 via biparental matings using the Escherichia coli S17-1 helper strain (30) or via triparental matings using E. coli TOP10 (Invitrogen, Carlsbad, CA) and the E. coli helper strain containing the conjugative plasmid pRK2013 (31), as previously described (32), except that conjugation took place directly on the surface of nutrient agar instead of on a 45-μm membrane filter.
TABLE 1.
Strains and plasmids used in the study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| M. extorquensa | ||
| AM1 | Rifr derivative (wild type) | 43 |
| CM194.1 | ΔmxaF | 44 |
| ES890 | xoxF1::Km | 22 |
| ES970 | ΔxoxF2 | 22 |
| ES1022 | ΔxoxF2 xoxF1::Km | 22 |
| ES1017 | ΔmxaF xoxF1::Km | This study |
| ES2288 | ΔmxaF ΔxoxF2 | This study |
| ES1100 | ΔmxaF ΔxoxF2 xoxF1::Km | 22 |
| E. coli | ||
| TOP10 | Competent cells for cloning (Smr) | Invitrogen |
| S17-1 | Helper strain for conjugation (Tpr Smr) | 30 |
| Plasmids | ||
| pAYC61 | Allelic exchange suicide vector (Tcr Apr) | 33 |
| pAP5 | Promoterless venus fusion vector constructed from pCM62 (Tcr) | 22 |
| pCM157 | cre expression vector (Tcr) | 34 |
| pCM184 | Allelic exchange suicide vector (Kmr Tcr Apr) | 34 |
| pES134-135 | pCM184 with xoxF2 upstream and downstream flanks (Kmr Tcr Apr) | 22 |
| pES502 | pAP5 containing the mxa promoter region (Tcr) | 22 |
| pES503 | pAP5 containing the xox1 promoter region (Tcr) | 22 |
| pHV-F2 | pAP5 containing the xoxF2 promoter region (Tcr) | This study |
| pLC6168 | pAYC61 with xoxF1 upstream and downstream flanks (Kmr Tcr Apr) | 20 |
| pRK2013 | Conjugative helper plasmid (Kmr) | 31 |
All M. extorquens AM1 strains used in the study are isogenic.
All M. extorquens AM1 strains used in this study (including those previously published) were created from identical wild-type frozen stocks. Null mutations in xoxF1 and xoxF2 were constructed in the wild type and mxaF deletion strains using the allelic marker exchange suicide vectors pAYC61 (20, 33) and pCM184 (34), respectively. The kanamycin (Km) resistance cassette in the mxaF xoxF2::Km mutant strain was deleted using the cre expression plasmid pCM157, as previously described (34). The Km insertion in xoxF1 and the Km deletion in xoxF2 were verified by diagnostic colony PCR. The expected presence or absence of mxaF, xoxF1, and xoxF2 was verified in all the strains. Complementation of the xoxF and mxaF single mutants and the xoxF1 xoxF2 double-mutant strains was previously described (22, 35).
Transcriptional reporter fusion vectors were constructed using the multicopy plasmid pAP5 containing a modified version of yellow fluorescent protein, Venus, as previously described (22). The promoter regions of the mxa and xox1 operons were amplified and cloned into the AclI restriction site using the following primer pairs to create pES502 and pES503, respectively: paF-4538BegAclI, TGAACGTTAGTTGTGCGCGTTCAGCATCACG, and paF-4538EndAclI, TGAACGTTCGAGGAGCCGATGATGACCTTGT for mxa and pF1-1740BegAclI, TGAACGTTCGGCATCGTCGCTCAGTAGAATC, and pF1-1740EndAclI, TGAACGTTTTCTTGCCGGACTTCAGGTCGTAG, for xox1. Correct orientation of the promoter was confirmed by diagnostic PCR and sequencing. The primers pF2_2757BegAclI, TGAACGTTAGATGGCGGTGCAGGACCACGAA, and pF2_2757EndEcoRI, TCGAATTCGACCAGTTGACCTTGCCGGTCTT, were used to amplify the promoter region of xoxF2, followed by digestion with AclI and EcoRI and ligation into pAP5 to create pHV-F2.
Growth conditions.
M. extorquens AM1 strains were grown at 29°C in Methylobacterium PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)] (MP) medium (36), except that FeSO4 was omitted from the C7 metal mixture (citrate-chelated trace element solution containing 7 metals) and prepared separately. Succinate (0.4%) and/or methanol (0.5%) was added as a carbon source(s) to media containing calcium chloride (20 μM) and/or lanthanide chlorides (2.5 nM to 20 μM). Difco nutrient agar was used for bi- and triparental matings. E. coli strains were cultured at 37°C on Luria-Bertani (LB) agar. When appropriate, media were supplemented with antibiotics at the following concentrations: tetracycline (Tc) at 5 μg/ml for MP medium and 10 μg/ml for LB medium, Km at 50 μg/ml, and rifamycin (Rif) at 50 μg/ml.
Preparation of glassware.
Even after washing with dilute acid, trace lanthanides can contaminate glassware at concentrations that vary by batch (8). In our studies, this resulted in variation in the levels of expression from the mxa and xox1 promoters and variation in the growth of the MeDH mutant strains. Trace lanthanides were removed from the glassware by pregrowing wild-type M. extorquens AM1 to full density in MP methanol medium lacking lanthanides. The glassware was then washed with deionized water and autoclaved for use in subsequent experiments. When the glassware was prepared in this manner, experimental results were reproducible and variation between replicates was minimal.
Growth curve analysis.
Cells were grown at 29°C in borosilicate glass tubes (2-ml cultures in 16- by 125-mm tubes or 6-ml cultures in 20- by 150-mm tubes) placed in custom-built culture tube rack holders angled at 60° to achieve maximum aeration. The cultures were shaken at 180 rpm in an Excella E25 shaking incubator (New Brunswick Scientific, Edison, NJ). M. extorquens AM1 strains were grown to late exponential phase in 2 ml of MP succinate medium without exogenous Ca or lanthanides and then subcultured (200 μl) into 6 ml MP methanol medium containing or lacking exogenous Ca or lanthanides as indicated in the text. Optical density measurements were recorded at 600 nm (OD600) using a Spectronic 20D spectrophotometer (Milton Roy Company, Warminster, PA) adapted to read absorbance in 20- by 150-mm tubes.
Transcriptional reporter fusion assays.
Cells were grown in culture tubes as described above with addition of Tc to the MP medium. When the cells reached mid-exponential phase (OD600, ∼0.6), a 200-μl aliquot was removed, and the relative fluorescence units (RFU) were measured in optical-bottom black 96-well plates using a Spectramax M2 plate reader (Molecular Devices, Sunnyvale, CA). The excitation and emission spectra used were 490 nm and 540 nm, respectively. The OD600 measurements from the Spectramax M2 plate reader were used to normalize fluorescence to culture density (RFU/OD600).
Methanol dehydrogenase assays.
To prepare cell extracts, 2 ml of biological duplicates grown to mid-exponential phase was used to inoculate 50 ml of MP methanol medium (36) containing or lacking 2 μM La. Cells were harvested at an OD600 of 0.8 to 1.2 by centrifugation at 4,729 × g at 4°C for 10 min. The cells were then washed with 100 mM Tris-HCl buffer at pH 9 and lysed with a French press. Cell debris was removed by centrifugation for 2 min at 28,078 × g and 4°C. The protein concentration was determined by the bicinchoninic acid (BCA) method (Sigma-Aldrich, St. Louis, MO).
MeDH activity was measured in cell extracts according to the method of Anthony and Zatman (37) with minor modifications: the reaction mixture (final volume, 200 μl) was prepared in 100 mM Tris-HCl at pH 9 and contained 15 mM NH4Cl, 200 μM KCN, 500 μM phenazine methosulfate (PMS), 300 μM 2,6-dichlorophenol-indolphenol (DCPIP), and cell extract (2.4 to 8.4 μg/μl protein). Autobleaching of the reaction mixture has been reported (38), and to minimize this, the reaction mixtures were incubated at 30°C for 10 min before 190 μl of the reaction mixture was added to the microplate well containing 10 μl of 250 mM methanol or double-distilled H2O (ddH2O). Absorbance of the reaction mixture was followed at 600 nm in an Epoch2 microplate spectrophotometer (Biotek, Winooski, VT). Using this method, background activity in the absence of methanol was not detectible. The specific activity (SA) was calculated using a molar extinction coefficient at 600 nm (ε600) for DCPIP of 21 mM−1 cm−1 and a conversion factor of 0.667 to convert absorbance readings to a 1-cm path length.
RESULTS
XoxF and an unknown methanol oxidation system contribute to lanthanide-dependent methanol growth.
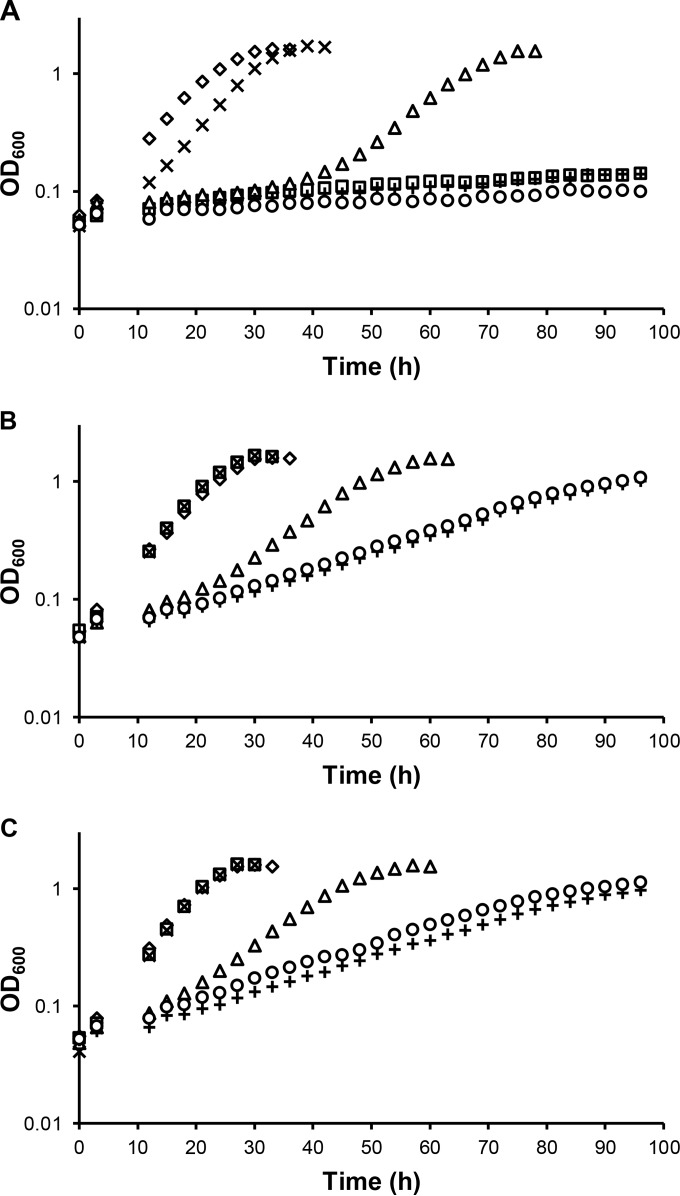
To determine the requirements for the MxaFI, XoxF1, and XoxF2 MeDH enzymes during Ca- and lanthanide-dependent methanol growth, null mutations were constructed to generate mxaF, xoxF1, xoxF2, xoxF1 xoxF2, and mxaF xoxF1 xoxF2 mutant strains. These strains were grown in methanol medium containing 20 μM Ca, 20 μM La, or both 20 μM Ca and 2 μM La (Fig. 1). The growth of these mutant strains in the absence of La was consistent with published data (22) that used hypho minimal medium (39) instead of MP medium (Fig. 1A). Strains lacking mxaF were unable to grow because the Ca-dependent MeDH had been eliminated, while strains lacking both xoxF1 and xoxF2 were unable to grow because XoxF is required for expression of mxaF (22). As reported by Nakagawa et al. (11), addition of La allowed wild-type growth of an mxaF mutant strain in methanol medium (Fig. 1B). Loss of xoxF1 resulted in an increased lag of ∼30 h and an increase in the doubling time (TD) (TD = 5.5 ± 0.2 h [wild type]; TD = 8.6 ± 0.9 h [xoxF1]). Loss of xoxF2 in addition to xoxF1 significantly further increased the doubling time (TD = 19.1 ± 0.8 h), but surprisingly, the xoxF1 xoxF2 mutant strain was able to grow. Growth was not eliminated in the mxaF xoxF1 xoxF2 triple-mutant strain, suggesting that there is at least one additional, undiscovered enzyme or pathway that can oxidize methanol when La is present in the growth medium. When Ca and La were both added to the growth medium with the concentration of La 10-fold lower than that of Ca (Fig. 1C), the mutant phenotypes were identical to those when Ca was not added to the medium, indicating that La is dominant over Ca.
FIG 1.
Growth of wild-type (♢), mxaF (□), xoxF1 (△), xoxF2 (×), xoxF1 xoxF2 (+), and mxaF xoxF1 xoxF2 (○) strains in MP methanol medium containing 20 μM Ca (A), 20 μM La (B), or both 20 μM Ca and 2 μM La (C). The graphs show representative data obtained from three biological replicates. Variation in growth rates for all replicates was within 2%.
Methanol dehydrogenase activity in cell extracts is consistent with growth.
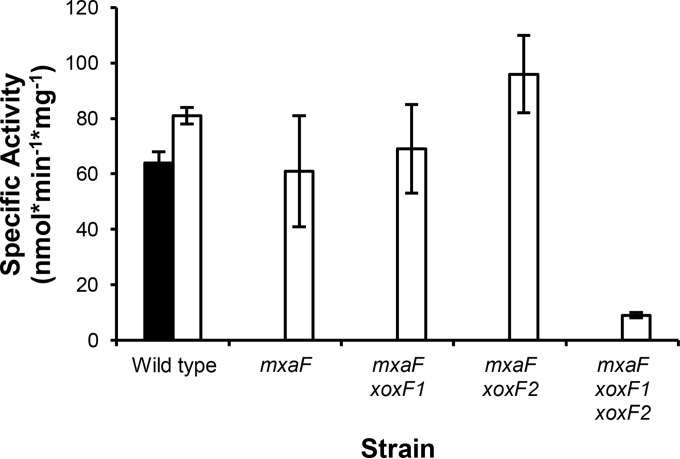
To determine if MeDH activity correlates with growth, MeDH activity was measured in cell extracts prepared from strains grown in methanol medium containing 20 μM Ca with or without 2 μM La (Fig. 2). While a small increase in MeDH activity for the wild type was observed when cells were grown with La (SACa = 64 ± 4 nmol · min−1 · mg−1; SALa = 81 ± 3 nmol · min−1 · mg−1), the ∼10-fold increase in MeDH activity, as reported by Nakagawa et al., was not seen (11). Loss of mxaF alone or of xoxF1 or xoxF2 in addition to mxaF did not significantly decrease MeDH activity in La-grown cells, but loss of all known MeDHs together resulted in a 9-fold reduction in activity (SAmxaF xoxF1 xoxF2 = 9 ± 1 nmol · min−1 · mg−1). This low level of MeDH activity is consistent with the significantly increased doubling time for the MeDH triple-mutant strain in methanol medium containing La (Fig. 1C).
FIG 2.
Methanol dehydrogenase activities in wild-type, mxaF, mxaF xoxF1, mxaF xoxF2, and mxaF xoxF1 xoxF2 strains in MP methanol medium containing 20 μM Ca (black bar; wild type only) and both 20 μM Ca and 2 μM La (white bars). Average specific activities (nmol · min−1 · mg−1) were determined from two biological and six technical replicates. The error bars represent standard deviations from the means.
One micromolar La supports the maximum growth rate and optical density of an mxaF mutant strain during growth in methanol medium.
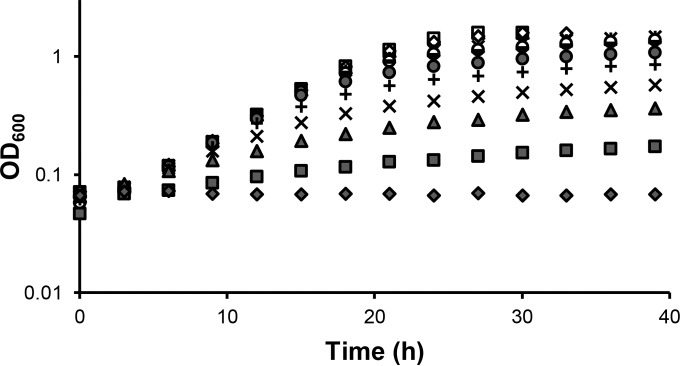
To assess the concentrations of exogenous La that would support La-dependent methanol growth, the mxaF mutant strain was grown in methanol medium containing 20 μM Ca supplemented with La concentrations ranging from 0 to 20 μM (Fig. 3). The mxaF mutant strain was used to eliminate any contribution by the Ca-dependent MxaFI-MeDH. La-dependent methanol growth, albeit slow, was observed using La concentrations as low as 2.5 nM, while 1 μM La resulted in maximal growth and culture density.
FIG 3.
Growth of the mxaF mutant strain in MP methanol medium containing 20 μM Ca (◆) and 20 μM Ca with increasing concentrations of La: 2.5 nM (■), 5 nM (▲), 10 nM (×), 25 nM (+), 50 nM (●), 100 nM (-), 250 nM (○), 500 nM (×), 1 μM (♢), 2 μM (□), and 20 μM (△). The growth curve shows representative data from three biological replicates. Variation in growth rates between biological triplicates was within 1%.
Multiple lanthanides support growth of the mxaF and mxaF xoxF1 xoxF2 mutant strains.
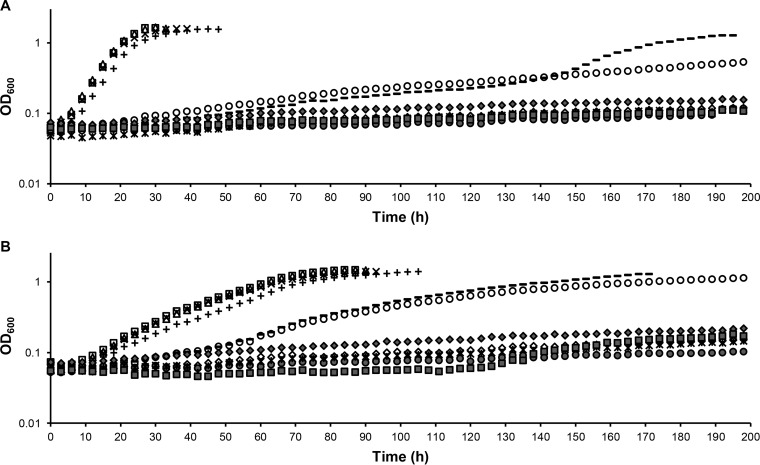
To investigate the abilities of different lanthanides to support methanol growth, 2 μM (each) La, Ce, Pr, Nd, Sm, Dy, Ho, Er, and Yb was added to methanol medium, and growth of the mxaF and mxaF xoxF1 xoxF2 mutant strains was assessed (Fig. 4). Growth occurred with the first four lanthanides, La, Ce, Pr, and Nd, while 2 μM Sm allowed growth of the mxaF mutant strain at a significantly reduced rate (TD = 67.2 ± 1.5 h) (Fig. 4A). To determine if increased Sm concentrations would improve growth of the mxaF mutant strain, 20 μM Sm was also tested. In all three mxaF biological replicates, an increase in the growth rate occurred after 150 h, resulting in a doubling time of 18.3 ± 0.9 h. To ensure cultures were not contaminated, all the strains were streaked onto methanol media lacking or containing La. Growth of the mxaF mutant strains from Sm cultures did not occur on medium lacking La, but they grew like the wild type on medium containing La. While this confirmed that mxaF was still nonfunctional and contamination did not occur, we were not able to confirm or refute whether a second site suppressor mutation arose that specifically allowed increased Sm-dependent growth. Similarly, growth of the MeDH triple-mutant strain occurred with La, Ce, Pr, and Nd and occurred at a reduced rate with Sm. Intriguingly, Sm-dependent growth of the MeDH triple mutant is faster than with the mxaF mutant alone when 2 μM Sm is present (TD = 67.2 ± 1.5 h [mxaF]; TD = 18.0 ± 0.9 h [mxaF xoxF1 xoxF2]).
FIG 4.
Growth of mxaF (A) and mxaF xoxF1 xoxF2 (B) mutant strains in MP methanol medium containing 20 μM Ca (♢) and 20 μM Ca with 2 μM different lanthanides—La (□), Ce (△), Pr (×), Nd (+), Dy (●), Ho (×), Er (◆), and Yb (■)—with the exception of Sm, tested at 2 μM (○) and 20 μM (-). The graphs show representative data from three biological replicates. Variation in growth rates between biological triplicates was within 2%.
Expression from the mxa and xox1 promoters is differentially regulated by La.
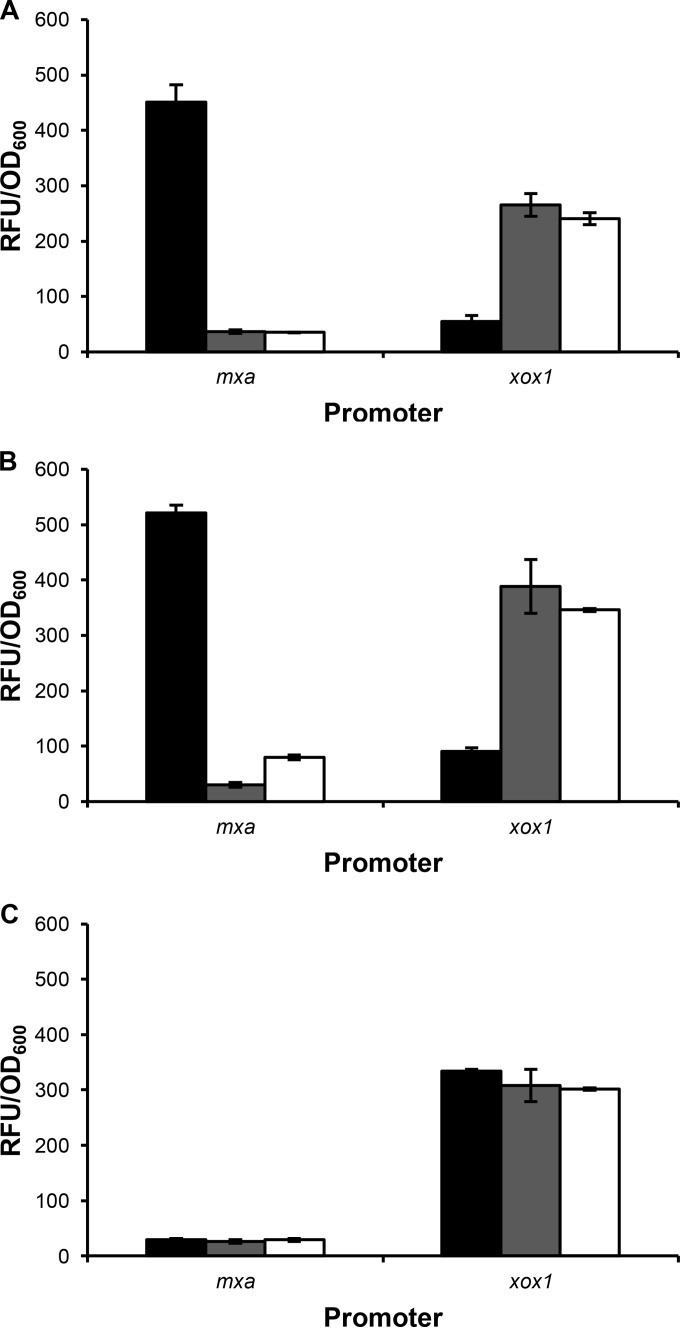
It was reported by Nakagawa et al. that exogenous La does not increase expression from the mxa and xox1 promoter regions and that regulation is likely to occur posttranslationally (11). However, when using transcriptional reporter fusions that contain significantly larger regions of DNA that extend into the first gene within the operon (1,304 bp versus 217 bp for the mxa promoter region; 1,351 bp versus 209 bp for the xox1 promoter region), differential expression was seen, with expression from the xox1 promoter upregulated in the presence of La and expression from the mxa promoter upregulated in the absence of La (Fig. 5A). It was previously shown that xoxF (but not mxaF) is required for expression of mxaF and for repression of xoxF in hypho minimal medium lacking La (22). These studies were repeated here to determine if expression from the mxa and xox1 promoters behaves identically in the more robust MP medium (36) and to determine how loss of mxaF and xoxF1 affects expression in medium containing La. As mxaF and xoxF1 are required for methanol growth in the absence of lanthanides, succinate was added as an additional carbon source. These studies confirm that loss of mxaF does not significantly affect expression from the mxa and xox1 promoter regions (Fig. 5B) but that xoxF1 is required for expression from the mxa promoter and for repression of itself (Fig. 5C). When xoxF1 is absent, expression from the xox1 promoter in medium lacking La is similar to expression in the presence of La. Transcriptional reporter fusion studies were also conducted using the xoxF2 promoter region; however, these assays were not sensitive enough to measure expression above background from the promoter, which has a low level of expression (39).
FIG 5.
Expression from the mxa and xox1 promoters using Venus as a transcriptional reporter in wild-type (A), mxaF (B), and xoxF1 xoxF2 (C) strains. The strains were grown in MP succinate-methanol medium containing 20 μM Ca (black bars), 20 μM La (gray bars), or both 20 μM Ca and 2 μM La (white bars). The expression levels are reported as RFU per OD600 unit. The background expression levels from the promoterless venus fusion vector in the wild-type, mxaF, and xoxF1 xoxF2 strains were 59 ± 6 RFU/OD600, 90 ± 6 RFU/OD600, and 82 ± 10 RFU/OD600, respectively. The data represent the average RFU/OD600 values from biological triplicates. The error bars show standard deviations from the means.
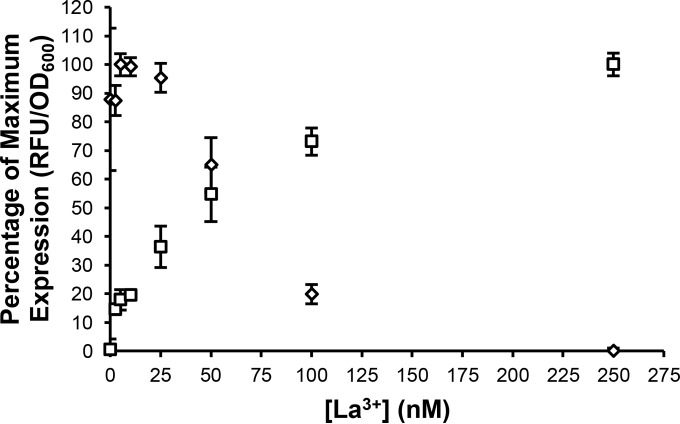
Expression from the mxa and xox1 promoter regions in the presence of increasing concentrations of La.
To determine how sensitive the mxa and xox1 transcriptional reporter fusions are to the presence of exogenous La, cells were grown in the presence of increasing concentrations of La (0 to 20 μM). An increase in expression from the xox1 promoter was seen using La concentrations as low as 2.5 nM, with expression reaching maximum at 250 nM (Fig. 6). Concentrations of La between 25 and 50 nM resulted in decreased expression from the mxa promoter, with full repression seen at 250 nM. Expression from both promoters with La concentrations above 250 nM (tested up to 20 μM) was nearly identical to the expression seen using 250 nM La (data not shown).
FIG 6.
Expression from the mxa (♢) and xox1 (□) promoters with increasing concentrations of La. Expression values are reported as the percentage of maximum expression of the mxa-venus and xox1-venus transcriptional fusions in the wild type grown in MP succinate-methanol medium containing both 20 μM Ca and various concentrations of La. The data depict the average expression from biological triplicates with background expression levels from the promoterless venus fusion vector subtracted. The error bars represent standard deviations from the means.
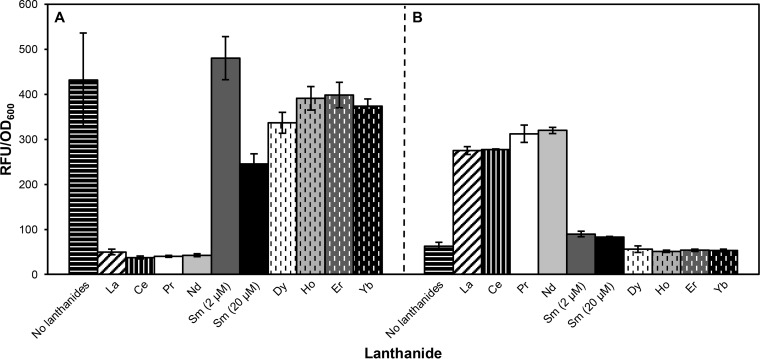
Addition of La, Ce, Pr, and Nd results in differential expression from the mxa and xox1 promoter regions.
To determine if lanthanide-dependent expression correlates with lanthanide-dependent methanol growth, different lanthanides were tested for the ability to repress expression from the mxa promoter and upregulate expression from the xox1 promoter. Similar to growth, differential expression was observed when 2 μM La, Ce, Pr, and Nd was present, and a small effect on expression was observed when Sm was present in the growth medium (Fig. 7).
FIG 7.
Expression from the mxa (A) and xox1 (B) promoters in the wild type grown in MP succinate-methanol medium containing 20 μM Ca without (control) and with 2 μM different lanthanides, with the exception of Sm (tested at 2 μM and 20 μM). The background expression level from the promoterless venus fusion vector was 58 ± 5 RFU/OD600. The data were obtained from three biological replicates, and the error bars show standard deviations from the means.
DISCUSSION
Using MeDH mutant strains, we show that methanol-dependent growth of an mxaF mutant strain can occur if La, Ce, Pr, Nd, and, to a lesser extent, Sm are added to the growth medium and that M. extorquens AM1 preferentially uses lanthanides over Ca even when lanthanides are present at a 10-fold-lower concentration. It has been suggested that lanthanides are more efficient Lewis acids than Ca and may enhance the catalytic properties of MeDH (25). Additionally, initial characterization of two XoxF-type MeDHs demonstrated superior oxidation capacities, so that in one case, oxidation of methanol leads to direct production of formate instead of formaldehyde (8). Surprisingly, our growth studies reveal the existence of at least one additional, unknown lanthanide-dependent enzyme or pathway that can oxidize methanol. Once this new methanol oxidation system is revealed and biochemically characterized, it may become clearer why M. extorquens AM1 has multiple enzymes that carry out the same function. Enzyme redundancy in M. extorquens AM1 is a common occurrence. For example, there are at least four distinct formate dehydrogenases that oxidize formate to CO2 (40, 41), four copies of the zwf gene encoding glucose-6-phosphate dehydrogenase, and five copies of a putative oxalate formate antiporter (21). The discovery of a novel non-XoxF lanthanide-dependent methanol oxidation system may have a significant impact on the field of environmental microbiology, helping to ascertain whether organisms previously classified as nonmethylotrophs are, in fact, methylotrophic after all. It may be that some organisms contain only this unknown methanol oxidation system to oxidize methanol for energy, if not for carbon.
The ability of only La, Ce, Pr, and Nd, and Sm to a lesser extent, to facilitate methylotrophic growth and differential expression of the Ca- and lanthanide-dependent MeDH genes could be explained by several distinct possibilities. It may be that the cofactor range for XoxF-MeDH and the unknown methanol oxidation system is limited by the ionic radius of the lanthanide. If the ionic radius is too large, it may not be inserted into XoxF. It is also possible that larger lanthanides could support XoxF function but are unable either to be transported into the cell or to be sensed by the regulatory network controlling mxa and xox1 expression. Detailed biochemical and molecular analyses are needed to distinguish between these possibilities.
Each of the above-mentioned possibilities could explain why increasing the Sm concentration 10-fold in the medium allows increased growth (Fig. 4). The ∼150-h lag seen for the mxaF mutant strain in the presence of Sm is intriguing. One possible explanation for this lag could be that Sm is poorly sensed and transported into the cell but allows high activity of the lanthanide-dependent MeDHs once inside. If this is true, then xoxF1 would be expressed at a very low level, consistent with the expression data shown in Fig. 7, and it would take significant time to produce enough active XoxF to allow rapid growth. A second explanation for the increase in the growth rate of the mxaF mutant strain after 150 h is that a second site suppressor mutation arose that resulted in, for example, upregulation of the unknown lanthanide-dependent MeDH or increased transport of Sm.
Curiously, growth of the mxaF xoxF1 xoxF2 triple-mutant strain is faster than that of the mxaF single-mutant strain in the presence of Sm (Fig. 4). It is intriguing to speculate that the unknown methanol oxidation system may be upregulated in the absence of xoxF1. XoxF has been shown to be required for expression of the mxa genes and for repression of the xox1 genes (22) (Fig. 5) and may also be involved in regulation of the unknown system.
The MeDH activity and MeDH gene expression data presented here conflict with previously published data (11). Nakagawa et al. reported that exogenous La resulted in a 10-fold increase in MeDH activity. Additionally, the specific activities for MeDH in cell extracts were significantly different. This could be due in part to differences in strain backgrounds, how the cells were grown (the medium used and the growth phase of the cells isolated), and how the assays were conducted. When conducting MeDH assays using the protocol reported by Nakagawa et al., MeDH activity from the wild type grown in methanol medium with La was higher (138 nmol · min−1 · mg−1 after subtracting background oxidation) than when activity was measured using our assay modifications (81 nmol · min−1 · mg−1); however, the rate of methanol-independent oxidation, or autobleaching, was 283 nmol · min−1 · mg−1 when methanol was added to the assay mixture. This high background activity was observed for all the strains. Additionally, MeDH activity in the mxaF xoxF1 xoxF2 triple-mutant strain grown in methanol medium containing La was not detectible when using the assay. By preincubating the reaction mixture for 10 min before substrate addition, background oxidation was eliminated and activity for the unknown methanol oxidation system could be detected. The activities reported here for Ca-dependent methanol growth are identical to the activities reported by Smejkalová et al. (42). Smejkalová et al. noted that wide discrepancies in MeDH activity have been published for years, with specific activities for the Ca-dependent MeDH ranging from 64 to 540 nmol · min−1 · mg protein−1, depending on the protocol used to measure MeDH activity.
The question remains as to how cells sense, acquire, and transport lanthanides so that the lanthanide-dependent MeDH enzymes, but not the Ca-dependent MeDH, are used when lanthanides are present. In the methanotrophic bacterium M. trichosporium OB3b, it was shown that the presence of lanthanides upregulates expression of the xoxF-MeDH genes and represses expression of the mxaFI-MeDH genes. However, in M. extorquens AM1, it was reported that lanthanides do not affect expression of the mxa or xox1 genes and that regulation is likely to occur posttranslationally (11). We show that in M. extorquens AM1, differential expression from the mxa and xox1 promoters is very sensitive to the presence of La, Ce, Pr, and Nd. Titration experiments with La showed that induction of expression from the xox1 promoter occurs with concentrations as low as 2.5 nM and reaches maximum expression with 250 nM La. Repression from the mxa promoter is seen when concentrations of >25 nM La are present, with full repression occurring with 250 nM La. As these reporter fusions are contained on a multicopy plasmid, these data likely do not reflect the exact concentrations required for chromosomal expression and repression from these promoters, but they do suggest that there may be a lanthanide concentration range in which both MxaFI and XoxF enzymes are expressed and used equally.
Our expression data refute the conclusions of Nakagawa et al. and provide a likely explanation for the discrepancy in the results due to the different lengths of the promoter regions used in these comparative studies (11). The promoter regions used in our studies are significantly larger and extend well into the xoxF1 and mxaF genes to include any possible repressor binding sites, while the Nakagawa et al. study used much shorter promoter regions that end at the start of the xoxF1 gene and 114 bp before the start of the mxaF gene. In the absence of lanthanides, it is known that the response regulator MxbM is required for repression of the xox1 operon (22). The lack of repression of the xox1 genes seen by Nakagawa et al. may be due to the lack of the repressor binding site on the region of DNA chosen for use in their study. Additionally, the level of expression seen from their mxa promoter region in the absence of lanthanides was low, even though the genes encoding the Ca-dependent MeDH are among the most highly expressed in the cell (39). It is possible that the mxa promoter region used in this study lacks the binding site for a transcriptional activator. As direct binding by regulators required for mxa expression has never been demonstrated, it is difficult to determine if this explanation is correct or if these differences are due to differences in the media used and the strain backgrounds.
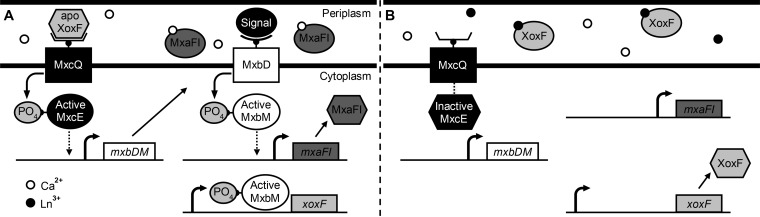
Taken together, these data expand on the regulatory hypothesis proposed by Skovran et al., where apo-XoxF may function as a cellular sensor of lanthanide presence in the periplasm, interacting with one or both of the sensor kinases MxcQ and MxbD (Fig. 8). When lanthanides are absent, MxcQ and/or MxbD could signal to the MxcE and/or MxbM response regulators to activate mxa expression and repress xox1 expression (22). When lanthanides are present, apo-XoxF may be converted to active XoxF, thereby decreasing the signal to MxcQ/MxbD, which would result in upregulation from the xox1 promoter and repression from the mxa promoter. As more is learned about how expression of the various MeDH genes is controlled, a more complete and accurate model can be described.
FIG 8.
Hypothesis for how XoxF and lanthanides (Ln) may differentially regulate expression of the mxa and xox1 genes in the absence (A) and presence (B) of lanthanides. MxcQE and MxbDM are two-component systems required for expression of the mxa genes, though whether this requirement is direct or indirect has not been demonstrated. In this model, apo-XoxF acts as a sensor for lanthanide presence. (A) When lanthanides are absent, apo-XoxF activates expression of the mxa genes and represses expression of the xox1 genes as mediated through the two-component systems MxcQE and MxbDM. (B) When lanthanides are present, XoxF resumes its catalytic role as a lanthanide-dependent MeDH and is unavailable or not in the correct conformation to interact with the two-component systems, resulting in the repression of the mxa genes and activation of the xox1 genes.
The discovery of a non-XoxF type of lanthanide-dependent MeDH is intriguing. This finding could greatly impact the environmental study of methylotrophic communities, as it is possible that organisms that have only this third, unknown system are disregarded as nonmethylotrophs. While it is clear that lanthanides differentially control the expression of the XoxF- and MxaFI-MeDHs, the regulatory network controlling expression of these MeDH genes is complex and poorly understood and has been investigated only in the absence of lanthanides (22). Once it is better understood how methylotrophs are able to sense and acquire lanthanides, it may be possible to engineer these bacteria as biorecycling and biomining agents to enhance yields from lanthanide mining and to recover these highly valued rare-earth elements from end-of-life products. As lanthanide-mining deposits continue to decrease while lanthanide demands continue to rise, the development of alternative mining and recovery strategies is an attractive prospect.
ACKNOWLEDGMENTS
We thank Marco Parent and Librado Veliz for designing and constructing the angled culture tube rack holders used in these studies. We also thank Richard Ngo, Fauna Yarza, Ralph Crisostomo, Mayra Resnik, Dorothy Chung, Charumathi Raghuraman, and Karen Ngo for their help in conducting growth curve analysis.
Funding Statement
Funding to support the contribution by Nathan M. Good and N. Cecilia Martinez-Gomez came from Michigan State University startup funds.
REFERENCES
- 1.Puri AW, Owen S, Chu F, Chavkin T, Beck DAC, Kalyuzhnaya MG, Lidstrom ME. 2015. Genetic tools for the industrially promising methanotroph Methylomicrobium buryatense. Appl Environ Microbiol 81:1775–1781. doi: 10.1128/AEM.03795-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ochsner AM, Sonntag F, Buchhaupt M, Schrader J, Vorholt JA. 2015. Methylobacterium extorquens: methylotrophy and biotechnological applications. Appl Microbiol Biotechnol 99:517–534. doi: 10.1007/s00253-014-6240-3. [DOI] [PubMed] [Google Scholar]
- 3.Hwang IY, Lee SH, Choi YS, Park SJ, Na JG, Chang IS, Kim C, Kim HC, Kim YH, Lee JW, Lee EY. 2014. Biocatalytic conversion of methane to methanol as a key step for development of methane-based biorefineries. J Microbiol Biotechnol 24:1597–1605. doi: 10.4014/jmb.1407.07070. [DOI] [PubMed] [Google Scholar]
- 4.Kalyuzhnaya MG, Yang S, Rozova ON, Smalley NE, Clubb J, Lamb A, Gowda GAN, Raftery D, Fu Y, Bringel F, Vuilleumier S, Beck DAC, Trotsenko YA, Khmelenina VN, Lidstrom ME. 2013. Highly efficient methane biocatalysis revealed in a methanotrophic bacterium. Nat Commun 4:2785. doi: 10.1038/ncomms3785. [DOI] [PubMed] [Google Scholar]
- 5.Höfer P, Vermette P, Groleau D. 2011. Production and characterization of polyhydroxyalkanoates by recombinant Methylobacterium extorquens: combining desirable thermal properties with functionality. Biochem Eng J 54:26–33. doi: 10.1016/j.bej.2011.01.003. [DOI] [Google Scholar]
- 6.Chistoserdova L. 2011. Modularity of methylotrophy, revisited. Environ Microbiol 13:2603–2622. doi: 10.1111/j.1462-2920.2011.02464.x. [DOI] [PubMed] [Google Scholar]
- 7.Reid MF, Fewson CA. 1994. Molecular characterization of microbial alcohol dehydrogenases. Crit Rev Microbiol 20:13–56. doi: 10.3109/10408419409113545. [DOI] [PubMed] [Google Scholar]
- 8.Pol A, Barends TRM, Dietl A, Khadem AF, Eygensteyn J, Jetten MSM, Op den Camp HJM. 2014. Rare earth metals are essential for methanotrophic life in volcanic mudpots. Environ Microbiol 16:255–264. doi: 10.1111/1462-2920.12249. [DOI] [PubMed] [Google Scholar]
- 9.Wu ML, Wessels HJCT, Pol A, Op den Camp HJM, Jetten MSM, van Niftrik L, Keltjens JT. 2015. XoxF-type methanol dehydrogenase from the anaerobic methanotroph “Candidatus Methylomirabilis oxyfera”. Appl Environ Microbiol 81:1442–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogart JA., Lewis AJ, Schelter EJ. 2015. DFT study of the active site of the XoxF-type natural, cerium-dependent methanol dehydrogenase enzyme. Chemistry 21:1743–1748. doi: 10.1002/chem.201405159. [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa T, Mitsui R, Tani A, Sasa K, Tashiro S, Iwama T, Hayakawa T, Kawai K. 2012. A catalytic role of XoxF1 as La3+-dependent methanol dehydrogenase in Methylobacterium extorquens strain AM1. PLoS One 7:e50480. doi: 10.1371/journal.pone.0050480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnamurthy N, Gupta CK. 2004. Extractive metallurgy of rare earths. CRC Press, Boca Raton, FL. [Google Scholar]
- 13.Hu Z, Richter H, Sparovek G, Schnug E. 2004. Physiological and biochemical effects of rare earth elements on plants and their agricultural significance: a review. J Plant Nutr 27:183–220. doi: 10.1081/PLN-120027555. [DOI] [Google Scholar]
- 14.Jain D, Jung Y, Barua P, Alam S, Sahu JK. 2015. Demonstration of ultra-low NA rare-earth doped step index fiber for applications in high power fiber lasers. Opt Express 23:7407–7415. doi: 10.1364/OE.23.007407. [DOI] [PubMed] [Google Scholar]
- 15.Rademaker JH, Kleijn R, Yang Y. 2013. Recycling as a strategy against rare earth element criticality: a systemic evaluation of the potential yield of NdFeB magnet recycling. Environ Sci Technol 47:10129–10136. doi: 10.1021/es305007w. [DOI] [PubMed] [Google Scholar]
- 16.Mittal S., Pandey AK. 2014. Cerium oxide nanoparticles induced toxicity in human lung cells: role of ROS mediated DNA damage and apoptosis. Biomed Res Int 2014:891934. doi: 10.1155/2014/891934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skovran E, Martinez-Gomez NC. 2015. Just add lanthanides. Science 348:862–863. doi: 10.1126/science.aaa9091. [DOI] [PubMed] [Google Scholar]
- 18.Anthony C, Zatman LJ. 1967. The microbial oxidation of methanol. The prosthetic group of the alcohol dehydrogenase of Pseudomonas sp. M27: a new oxidoreductase prosthetic group. Biochem J 104:960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lidstrom ME, Tabita FR. 1996. Microbial growth on C1 compounds. Kluwer Academic Publishers, Dordrecht, Netherlands. [Google Scholar]
- 20.Chistoserdova L, Lidstrom ME. 1997. Molecular and mutational analysis of a DNA region separating two methylotrophy gene clusters in Methylobacterium extorquens AM1. Microbiology 143:1729–1736. doi: 10.1099/00221287-143-5-1729. [DOI] [PubMed] [Google Scholar]
- 21.Vuilleumier S, Chistoserdova L, Lee M-C, Bringel F, Lajus A, Zhou Y, Gourion B, Barbe V, Chang J, Cruveiller S, Dossat C, Gillett W, Gruffaz C, Haugen E, Hourcade E, Levy R, Mangenot S, Muller E, Nadalig T, Pagni M, Penny C, Peyraud R, Robinson DG, Roche D, Rouy Z, Saenampechek C, Salvignol G, Vallenet D, Wu Z, Marx CJ, Vorholt JA, Olson MV, Kaul R, Weissenbach J, Médigue C, Lidstrom ME. 2009. Methylobacterium genome sequences: a reference blueprint to investigate microbial metabolism of C1 compounds from natural and industrial sources. PLoS One 4:e5584. doi: 10.1371/journal.pone.0005584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skovran E, Palmer AD, Rountree AM, Good NM, Lidstrom ME. 2011. XoxF is required for expression of methanol dehydrogenase in Methylobacterium extorquens AM1. J Bacteriol 193:6032–6038. doi: 10.1128/JB.05367-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt S, Christen P, Kiefer P, Vorholt JA. 2010. Functional investigation of methanol dehydrogenase-like protein XoxF in Methylobacterium extorquens AM1. Microbiology 156:2575–2586. doi: 10.1099/mic.0.038570-0. [DOI] [PubMed] [Google Scholar]
- 24.Hibi Y, Asai K, Arafuka H, Hamajima M, Iwama T, Kawai K. 2011. Molecular structure of La3+-induced methanol dehydrogenase-like protein in Methylobacterium radiotolerans. J Biosci Bioeng 111:547–549. doi: 10.1016/j.jbiosc.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 25.Keltjens JT, Pol A, Reimann J, Op Den Camp HJM. 2014. PQQ-dependent methanol dehydrogenases: rare-earth elements make a difference. Appl Microbiol Biotechnol 98:6163–6183. doi: 10.1007/s00253-014-5766-8. [DOI] [PubMed] [Google Scholar]
- 26.Springer AL, Morris CJ, Lidstrom ME. 1997. Molecular analysis of mxbD and mxbM, a putative sensor-regulator pair required for oxidation of methanol in Methylobacterium extorquens AM1. Microbiology 143:1737–1744. doi: 10.1099/00221287-143-5-1737. [DOI] [PubMed] [Google Scholar]
- 27.Springer AL, Auman AJ, Lidstrom ME. 1998. Sequence and characterization of mxaB, a response regulator involved in regulation of methanol oxidation, and of mxaW, a methanol-regulated gene in Methylobacterium extorquens AM1. FEMS Microbiol Lett 160:119–124. doi: 10.1111/j.1574-6968.1998.tb12900.x. [DOI] [PubMed] [Google Scholar]
- 28.Farhan Ul Haque M, Kalidass B, Bandow N, Turpin EA, Dispirito AA, Semrau JD. 2015. Cerium regulates expression of alternative methanol dehydrogenases in Methylosinus trichosporium OB3b. Appl Environ Microbiol 81:7546–7552. doi: 10.1128/AEM.02542-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semrau JD, DiSpirito AA, Yoon S. 2010. Methanotrophs and copper. FEMS Microbiol Rev 34:496–531. doi: 10.1111/j.1574-6976.2010.00212.x. [DOI] [PubMed] [Google Scholar]
- 30.Simon RUPAP Priefer U, Pühler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Nat Biotechnol 1:784–791. doi: 10.1038/nbt1183-784. [DOI] [Google Scholar]
- 31.Figurski DH, Helinski DR. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A 76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fulton GL, Nunn DN, Lidstrom ME. 1984. Molecular cloning of a malyl coenzyme A lyase gene from Pseudomonas sp. strain AM1, a facultative methylotroph. J Bacteriol 160:718–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chistoserdov A. 1994. Genetic organization of the mau gene cluster in Methylobacterium extorquens AM1: complete nucleotide sequence and generation and characteristics of mau mutants. J Bacteriol 176:4052–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marx CJ, Lidstrom ME. 2002. Broad-host-range cre-lox system for antibiotic marker recycling in Gram-negative bacteria. Biotechniques 33:1062–1067. [DOI] [PubMed] [Google Scholar]
- 35.Marx CJ, Chistoserdova L, Lidstrom ME. 2003. Formaldehyde-detoxifying role of the tetrahydromethanopterin-linked pathway in Methylobacterium extorquens AM1. J Bacteriol 185:7160–7168. doi: 10.1128/JB.185.23.7160-7168.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delaney NF, Kaczmarek ME, Ward LM, Swanson PK, Lee M-C, Marx CJ. 2013. Development of an optimized medium, strain and high-throughput culturing methods for Methylobacterium extorquens. PLoS One 8:e62957. doi: 10.1371/journal.pone.0062957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anthony C, Zatman LJ. 1964. The microbial oxidation of methanol. 2. The methanol-oxidizing enzyme of Pseudomonas sp. M 27. Biochem J 92:614–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anthony C. 1982. The biochemistry of methylotrophs. Academic Press, New York, NY. [Google Scholar]
- 39.Okubo Y, Skovran E, Guo X, Sivam D, Lidstrom ME. 2007. Implementation of microarrays for Methylobacterium extorquens AM1. OMICS 11:325–340. doi: 10.1089/omi.2007.0027. [DOI] [PubMed] [Google Scholar]
- 40.Chistoserdova L, Laukel M, Vorholt JA, Lidstrom ME, Portais J. 2004. Multiple formate dehydrogenase enzymes in the facultative methylotroph Methylobacterium extorquens AM1 are dispensable for growth on methanol. J Bacteriol 186:22–28. doi: 10.1128/JB.186.1.22-28.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chistoserdova L, Crowther GJ, Vorholt JA, Skovran E, Portais J-C, Lidstrom ME. 2007. Identification of a fourth formate dehydrogenase in Methylobacterium extorquens AM1 and confirmation of the essential role of formate oxidation in methylotrophy. J Bacteriol 189:9076–9081. doi: 10.1128/JB.01229-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smejkalová H, Erb TJ, Fuchs G. 2010. Methanol assimilation in Methylobacterium extorquens AM1: demonstration of all enzymes and their regulation. PLoS One 5:e13001. doi: 10.1371/journal.pone.0013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nunn DN, Lidstrom ME. 1986. Isolation and complementation analysis of 10 methanol oxidation mutant classes and identification of the methanol dehydrogenase structural gene of Methylobacterium sp. strain AM1. J Bacteriol 166:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marx CJ, O'Brien BN, Breezee J, Lidstrom ME. 2003. Novel methylotrophy genes of Methylobacterium extorquens AM1 identified by using transposon mutagenesis including a putative dihydromethanopterin reductase. J Bacteriol 185:669–673. doi: 10.1128/JB.185.2.669-673.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]