ABSTRACT
The alternative sigma factor σH has two functions in Gram-positive bacteria: it regulates sporulation and the development of genetic competence. Listeria monocytogenes is a nonsporulating species in which competence has not yet been detected. Nevertheless, the main competence regulators and a series of orthologous genes that form the competence machinery are present in its genome; some of the competence genes play a role in optimal phagosomal escape. In this study, strains overexpressing σH and strains with a σH deletion were used to elucidate the contribution of σH to the expression of the competence machinery genes in L. monocytogenes. Gene expression analysis showed that σH is, indeed, involved in comG and comE regulation. Unexpectedly, we observed a unique regulation scheme in which σH and the transcription factor ComK were involved. Population-level analysis showed that even with the overexpression of both factors, only a fraction of the cells expressed the competence machinery genes. Although we could not detect competence, σH was crucial for phagosomal escape, which implies that this alternative sigma factor has specifically evolved to regulate the L. monocytogenes intracellular life cycle.
IMPORTANCE Listeria monocytogenes can be an intracellular pathogen capable of causing serious infections in humans and animal species. Recently, the competence machinery genes were described as being necessary for optimal phagosomal escape, in which the transcription factor ComK plays an important role. On the other hand, our previous phylogenetic analysis suggested that the alternative sigma factor σH might play a role in the regulation of competence genes. The present study shows that some of the competence genes belong to the σH regulon and, importantly, that σH is essential for intracellular growth, implying a unique physiological role of σH among Firmicutes.
INTRODUCTION
Listeria monocytogenes is a Gram-positive foodborne pathogen with a multifaceted lifestyle: it can live harmlessly as a saprophyte in a diversity of environmental locations, but it can also be an intracellular pathogen capable of causing serious infections in humans and animal species. L. monocytogenes is a resilient microorganism: it cannot form spores, but it is able to endure several environmental and host stresses, such as low pH, low temperature, exposure to bile and fatty acids, high osmolarity, competition with intestinal flora, and intracellular nutrient and iron starvation (1, 2).
Alternative sigma factors constitute the principal strategy used to control the response to specific stress conditions, growth transitions, and morphological changes by the regulation of stress response and virulence genes, other regulators, and small RNAs. L. monocytogenes has four alternative sigma factors: σB, σH, σC, and σL (3). Transcriptomic and proteomic analyses have found an overlap between the regulons and cross connections between regulators, suggesting a complex mechanism for the fine-tuning of stress responses in L. monocytogenes (4–6). σB is the most extensively characterized and is considered to be the main stress-associated sigma factor. Null mutations of the remaining sigma factors have identified limited phenotypic consequences. σL is associated with growth under osmotic and low-temperature stress (7). σC, a member of the extracytoplasmic function (ECF) family of sigma factors, is present only in lineage II strains and seems to contribute to thermal resistance (8). σH protein levels have been reported to increase at low pH (3.5) (9), and a deletion mutant showed reduced growth under alkaline conditions and in minimal medium, as well as reduced virulence in a mouse model (2).
According to a phylogenetic analysis of σ70-family sigma factors in members of the Firmicutes, L. monocytogenes σH shares a common ancestor with Bacillus σH, Streptococcus ComX, and Staphylococcus σH (10). This ComX/σH group of sigma factors is responsible for spore formation (Bacillus) or competence development (Streptococcus and Staphylococcus). Listeria σH shows a high similarity with sporulation sigma factors, including Bacillus σH, rather than with competence development factors.
L. monocytogenes is not able to form spores (11), and indeed, it lacks almost all of the sporulation-specific genes (12). On the other hand, its genome contains homologues for the competence machinery genes, although attempts to detect natural transformation have been unsuccessful (13). The main components of the competence machinery are the pseudopilus (ComG proteins), which brings the exogenous DNA to the membrane transport machinery; the DNA receptor (ComEA), which delivers the bound DNA to the channel (ComEC); and an ATP-binding protein (ComFA), which is also involved in transport across the membrane (14, 15). This machinery is required for natural genetic competence (a specific physiological state developed to undergo natural transformation), and it is broadly conserved among species, including noncompetent bacteria (15). A recent study reported a new function for the competence machinery genes during the intracellular growth of L. monocytogenes: the comG operon and comEC gene are necessary for efficient phagosomal escape (16).
Regulation of the expression of competence genes varies among species (17). In Bacillus subtilis, the main regulator is the transcription factor ComK (18), whereas Streptococcus pneumoniae (19, 20) and Staphylococcus aureus (21) use the sigma factor ComX/σH as the key regulator. In addition, staphylococcal ComK participates in the expression of competence genes (22). Thus, the ComX/σH alternative sigma factor or the transcriptional factor ComK plays a major role in the expression of competence genes in a species-specific manner.
L. monocytogenes has both comK and sigH genes in its genome. ComK was recently demonstrated to be the regulator of competence genes (16). In the present study, we aimed to clarify whether the alternative sigma factor σH is involved in the expression of competence genes in L. monocytogenes.
MATERIALS AND METHODS
Bacterial strains and growth media.
The strains and plasmids used in this study are listed in Table 1. Escherichia coli strains were grown in Luria-Bertani (LB) medium supplemented with ampicillin (50 μg/ml) or erythromycin (150 μg/ml). L. monocytogenes strains were grown in brain heart infusion (BHI) medium. BHI plates were supplemented with chloramphenicol (7.5 μg/ml), tetracycline (10 μg/ml), erythromycin (10 μg/ml), kanamycin (20 μg/ml), or X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; 100 μg/ml) when necessary.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Characteristic(s)a | Reference or source |
|---|---|---|
| Strains | ||
| L. monocytogenes | ||
| EGDe | Parent strain | 35 |
| wt | Phage-cured derivative of EGDe | This study |
| +sigH | wt carrying pRIT5H-sigH; Cmr | This study |
| ΔsigH | wt with sigH deletion | This study |
| ΔsigH+comK | Strain ΔsigH carrying pRIT5H-comK; Cmr | This study |
| ΔcomK | wt with comK deletion | This study |
| ΔcomK+sigH | Strain ΔcomK carrying pRIT5H-sigH; Cmr | This study |
| +sigH+comK | wt carrying pRIT5H-sigH and pMK3-comK; Cmr Kmr | This study |
| ΔsigHΔcomK | wt with sigH and comK deletions | This study |
| wt/pJEBan2-PcomGA | wt carrying pJEBan2-PcomGA; Ermr | This study |
| +sigH/pJEBan2-PcomGA | Strain +sigH carrying pJEBan2-PcomGA; Cmr Ermr | This study |
| +sigH+comK/pJEBan2-PcomGA | Strain +sigH+comK carrying pJEBan2-PcomGA; Cmr Kmr Ermr | This study |
| wt/pJEBan3 | wt carrying pJEBan3; Ermr | This study |
| ΔsigH/pJEBan3 | Strain ΔsigH carrying pJEBan3; Ermr | This study |
| ΔcomK/pJEBan3 | Strain ΔcomK carrying pJEBan3; Ermr | This study |
| ΔsigHΔcomK/pJEBan3 | Strain ΔsigHΔcomK carrying pJEBan3; Ermr | This study |
| wt/pMAD-tet | wt carrying pMAD-tet; Tetr | This study |
| ΔsigH/pMAD-tet | Strain ΔsigH carrying pMAD-tet; Tetr | This study |
| ΔsigH/pMAD-tet-sigH | Strain ΔsigH carrying pMAD-tet-sigH; Tetr | This study |
| c-ΔsigH | Chromosomal complementation of ΔsigH | This study |
| Staphylococcus aureus | ||
| RN | sigH translation initiation site mutant of RN4220 | This study |
| RN+sigH/pJEBan2-PcomGA | RN carrying pRIT5H-sigH and pJEBan2-PcomGA; Cmr Ermr | This study |
| Plasmids | ||
| pRIT5H | Shuttle vector; Cmr | 10 |
| pRIT5H-comK | pRIT5H derivative overexpressing comK | This study |
| pRIT5H-sigH | pRIT5H derivative overexpressing sigH | This study |
| pMK3 | Shuttle vector; Kmr | 24 |
| pMK3-comK | pMK3 derivative overexpressing comK | This study |
| pMAD-tet | pMAD derivative with temperature-sensitive replication ori; Tetr | 25 |
| pMAD-tet-ΔsigH | pMAD-tet derivative, sigH-targeting vector | This study |
| pMAD-tet-ΔcomK | pMAD-tet derivative, comK-targeting vector | This study |
| pMAD-tet-sigH | pMAD-tet derivative carrying a 3-kbp sigH locus | This study |
| pJEBan2 | Reporter plasmid; Pdlt-cfp Ermr | 29 |
| pJEBan2-PcomGA | Reporter plasmid; PcomGA-cfp Ermr | This study |
| pJEBan3 | Reporter plasmid; Pdlt-yfp Ermr | 29 |
Cmr, chloramphenicol resistant; Kmr, kanamycin resistant; Ermr, erythromycin resistant; Tetr, tetracycline resistant.
Phage excision.
A prophage-cured derivative of the L. monocytogenes EGDe strain was isolated by bacteriophage induction using UV light (23). Cells (10 ml) were collected by centrifugation at 6,000 × g for 10 min, resuspended in 10 ml of phosphate-buffered saline (PBS), and transferred to a sterile petri dish. Next, they were exposed to UV light irradiation for 60, 90, or 120 s. After irradiation, serial dilutions were made, plated, and incubated at 37°C. Colonies from which phage was excised were detected by PCR using primers for the bacterial comK gene and phage int gene (Table 2).
TABLE 2.
Oligonucleotides used in this study
| Name | Sequence (5′–3′) | Target | Reference or source |
|---|---|---|---|
| sigH-F | CTGAATTCGGAGGAATACAGGCAGTTATGGATAATTCAGTGA | sigH | This study |
| sigH-R | CTTAGGTCGACAATTGTTTTTTCATTCTAGC | sigH | This study |
| V05 | GGATCGAATTCTCAAACATAGAGGAGTTG | comK | This study |
| V06 | CTACTCTGCAGTAGCTGGCTTTTATTTA | comK | This study |
| V07 | GTCATGAATTCAATTATATCCTC | comG promoter | This study |
| V09 | CGACCTCAATCCGGTAGTTTTGAAAAAA | comGA | This study |
| V10 | AGGGGATGGCATTTTTCTCCGCAGAAGG | comGA | This study |
| V11 | CATGGATCCAAGAACCTCCTTACAA | comG promoter | This study |
| V12 | ATGAAGGCAGCTATTTATATACGCG | int | This study |
| V13 | GTAATTGTTTTGCTTCTTCTTC | int | This study |
| V14 | CGGCGAATTCTTCTGCAAGCAATCTC | comK upstream region | This study |
| V15 | TTCATTCCCGGGCTCTATGTTTGTAT | comK upstream region | This study |
| V28 | ATCCCGGGCAGCTACGTAATAGTAGCTGGC | comK downstream region | This study |
| V29 | TTAGATCTAGGTGGAGTAGCATCTGTTGG | comK downstream region | This study |
| V38 | GGATACCAGATGGTAAAACAGATACAG | comEA | This study |
| V39 | CTACTGTTTTTTCGCCAATACCTGAAACG | comEA | This study |
| V40 | CGGGGATCCCAAGTTCAATTTGTACCTAAA | sigH upstream region | This study |
| V43 | TAGCTGCAGCGGCTCCTTTACGACAGGAT | sigH upstream region | This study |
| V44 | AGAGGATCCGATGGAACAAATTCTGCTTG | sigH upstream region | This study |
| V45 | AAACTGCAGTGACTAGCTAAGAGCCAAATG | sigH downstream region | This study |
| V46 | TCTGTCGACTCGGCTTCTCGTTTAAACTC | sigH downstream region | This study |
| V47 | TTTGTCGACCCGGAGTAAGTATGAACTAC | sigH downstream region | This study |
| V53 | GCCGTCGACTTCCTGAATAAATCTTTCA | Pspa (pRIT5H) | This study |
| V63 | ATCTTCCCGGGAAGACTGTATCAAGAA | comFA | This study |
| V64 | CACTTTTTCTCTGGTGTTGCTGTGATTA | comFA | This study |
| V67 | CGAATTTTACAAGCTCTATCGTTCCCAT | comEC | This study |
| V68 | TGTAGCAGCTCGTATGACTGGCGGA | comEC | This study |
| OP133 | CACCTGGAGTAAACCAATTAGTACG | rpoB | 28 |
| OP134 | TAGTGGGTTAAGCATGATATCAACA | rpoB | 28 |
Construction of overexpression strains.
Overexpression strains were constructed using the pRIT5H high-copy-number plasmid (10). The sigH gene (lmo0243) was amplified using primers sigH-F and sigH-R. The forward primer included 18 bp of the rpoD gene (lmo1454) and contained its Shine-Dalgarno sequence and starting codon; the starting codon of sigH was thereby changed from GTG to ATG. The amplified fragment was inserted into the EcoRI-SalI sites of the pRIT5H plasmid. The resulting vector (pRIT5H-sigH) could not be cloned into E. coli JM109, probably due to the toxic effect of σH in E. coli. Therefore, it was directly cloned into L. monocytogenes EGDe.
The comK gene was amplified with primers V05 and V06 and introduced into the EcoRI-PstI sites of pRIT5H, generating the comK expression vector pRIT5H-comK. Another comK expression vector (pMK3-comK) was constructed by transferring the Pspa-comK fragment, amplified from pRIT5H-comK with primers V53 and V06, into the SalI-PstI site of pMK3 (24).
Construction of deletion mutant and complementation strains.
Deletion strains were constructed by double homologous recombination using the pMAD-tet plasmid (25). A pair of fragments encompassing the sigH gene was amplified by PCR with primers V44-V43 and V45-V47. These were digested with the primer-attached PstI sites, ligated, and amplified by PCR with primers V44 and V47. The resultant fragment was introduced into the BamHI-SalI site of the pMAD-tet vector. Fragments surrounding the comK gene (amplified with primers V14-V15 and V28-V29) were sequentially inserted into the EcoRI-SmaI and SmaI-BglII sites of pMAD-tet.
Mutants were isolated using the protocol of Boneca et al. (26) with slight modifications. After electroporation, bacterial cells were plated onto BHI plates supplemented with tetracycline and X-Gal and cultured at 30°C for at least 48 h. Blue colonies were selected, transferred into BHI broth supplemented with tetracycline, and cultured at 42°C for 48 h. Dilutions were plated onto BHI–tetracycline–X-Gal plates and cultured overnight at 42°C. Light blue colonies were selected and subjected to at least three cycles of growth in drug-free BHI broth at 30°C for 12 h and at 42°C for 12 h. Serial dilutions were made, plated onto BHI–X-Gal agar, and incubated at 42°C. White colonies were selected, and successful gene deletion was checked by PCR.
The sigH-complemented strain ΔsigH/pMAD-tet-sigH was generated as follows. A 3-kbp fragment containing the sigH gene was amplified by PCR using primers V40 and V46 from the genomic DNA of the wild-type (wt) strain. The fragment was ligated into the BamHI-SalI site of the pMAD-tet vector to generate pMAD-tet-sigH. The ligation was directly introduced into the ΔsigH strain by electroporation. The existence of the sigH locus in strain ΔsigH/pMAD-tet-sigH was confirmed by PCR. As a vector control, pMAD-tet was introduced into the ΔsigH strain (ΔsigH/pMAD-tet). Cells carrying pMAD-tet derivatives were grown at 30°C.
The sigH-cured strain (strain c-ΔsigH) was constructed from strain ΔsigH/pMAD-tet-sigH by the same protocol described above. The precise recovery of the sigH locus was confirmed by PCR.
Growth curves.
Overnight cultures were diluted 1:200, and 200 μl was transferred to a 96-well sterile polystyrene microtiter plate (Corning). The optical density at 600 nm was measured, using a multimode plate reader (EnSpire; PerkinElmer), every 30 min for 24 h with shaking (180 rpm) at 37°C.
Semiquantitative reverse transcription-PCR (RT-PCR).
A stationary-phase culture (10 ml) was centrifuged at 5,000 rpm for 10 min at 4°C. Pellets were frozen with liquid nitrogen and stored at −80°C until RNA purification. RNA was purified according to a previously published method (27) with some modifications. Cells were resuspended in 460 μl of cold resuspension solution (10% glucose, 12.5 mM Tris, pH 7.6, 65 mM EDTA) and transferred into a 2 ml screw-cap microtube containing 500 μl of acid phenol solution, pH 4.5 (Sigma), and 0.4 g of glass beads (acid washed; diameter, ≤106 μm; Sigma). Cells were disrupted by use of a FastPrep-24 instrument (MP Biomedicals) for 30 s at 6.0 m/s twice with a 1-min interval between disruptions. The tubes were then centrifuged at 4°C for 5 min at 13,000 rpm, and the supernatant was transferred to a new tube. One milliliter of TRI Reagent (Sigma) and 100 μl of chloroform-isoamyl alcohol (24:1) were added, followed by vortexing and centrifugation at 13,000 rpm for 5 min at 4°C. The aqueous phase was transferred to a new tube and subjected to a second chloroform-isoamyl alcohol extraction. RNA was precipitated by the addition of 500 μl of isopropanol, incubation for 15 min at −30°C, and centrifugation for 15 min at 13,000 rpm and 4°C. The pellet was washed with 1 ml of 70% ethanol, dried, and resuspended in 100 μl of Milli-Q water. The purified RNA was DNase treated using a Turbo DNA-free kit (Invitrogen) following the manufacturer's rigorous DNase treatment protocol.
For cDNA synthesis, 1 μg of total RNA was mixed with 5× first-strand buffer (final concentration, 1×; Clontech) and random primers (0.03 μg/μl; Invitrogen). This mixture was exposed to 65°C for 5 min and to ice for 5 min. Then, dithiothreitol (10 mM), deoxynucleoside triphosphate mix (1.7 mM), RNaseOUT (0.4 U/μl; Invitrogen), and PowerScript reverse transcriptase (2 U/μl; Clontech) were added. The mixture (30 μl) was incubated at 42°C for 70 min and 95°C for 10 min. The absence of genomic DNA in the RNA preparations was verified by adding control tubes with all the components except the reverse transcriptase. PCRs were performed using 2 μl of cDNA for comK, sigH, comGA, comFA, comEA, comEC, and rpoB (the primers are listed in Table 2).
Cyan fluorescent protein (CFP) reporter assay.
A reporter plasmid was constructed using pJEBan2 (29). The comG promoter region was amplified (with primers V07 and V11) and cloned between the EcoRI and BamHI sites of the pJEBan2 plasmid (directly upstream of the cfp gene). Overnight cultures of strains carrying the reporter vector were diluted in drug-free medium and incubated for 8 h. Fluorescence microscopy was carried out with an Olympus FSX100 microscope. The bacteria in five distinct fields were counted in each of three independent experiments.
Site-directed mutagenesis of sigH in S. aureus.
Potential translation initiation codons (TTG) of sigH in S. aureus RN4220 were changed to Ochre stop codons (TAA) by using pMADt492 as described previously (21).
Intracellular survival.
Intracellular survival in cells of the mouse macrophage cell line RAW 264.7 (American Type Culture Collection) and the human epithelial cell line HeLa was tested. Cells were cultured in RPMI 1640 supplemented with penicillin (100 U/ml), streptomycin (100 U/ml), l-glutamine (2 mM), and 10% heat-inactivated fetal bovine serum (FBS; Equitech-Bio) at 37°C with 5% CO2.
Cells were seeded into 24-well plates (1 × 106 cells/ml, 1 ml per well). Following overnight incubation, the medium was replaced with 1 ml of RPMI 1640 supplemented with l-glutamine (2 mM) and 5% FBS and the cells were incubated for 30 min. Macrophages were infected with RPMI 1640 suspensions of L. monocytogenes at a multiplicity of infection (MOI) of 1. At 30 min postinfection, the macrophages were washed twice with PBS, and fresh medium containing gentamicin (5 μg/ml) was added. HeLa cells were infected at an MOI of 50 and were washed at 1 h postinfection (hpi). At each time point, the cells were washed twice with PBS and lysed with cold PBS with Triton X-100 (0.05%). Cell lysates were diluted with BHI, plated, and cultured for 24 h. Data were normalized using an intracellular growth coefficient (IGC), which was calculated as follows: (IBt = n − IBt = 0)/IBt = 0, where IBt = n is intracellular bacterial numbers (numbers of CFU per well) at a specific time point (t = n), and IBt = 0 is the intracellular bacterial numbers (numbers of CFU per well) at 30 min postinfection (for RAW 264.7 cells) or 1 h postinfection (for HeLa cells) (t = 0) (30).
Phagosomal escape.
For phagosomal escape assays, L. monocytogenes strains were tagged with the reporter plasmid pJEBan3 (29). RAW 264.7 cells were plated onto 18-mm glass coverslips in 12-well plates (2 × 106 cells/ml, 1 ml per well) and were infected at an MOI of 1 as described above. At 3 hpi, the cells were washed twice with PBS and fixed with paraformaldehyde (4%) for 10 min at room temperature. They were then washed twice with PBS, permeabilized with 0.1% Triton X-100 for 5 min at room temperature, and washed twice with PBS, and actin was labeled with Alexa Fluor 594-phalloidin (5 U/ml; Molecular Probes, Invitrogen) with 1% bovine serum albumin for 20 min at room temperature. After labeling, the coverslips were washed twice with PBS and mounted with ProLong Diamond antifade reagent with DAPI (4′,6-diamidino-2-phenylindole; Molecular Probes, Invitrogen). Fluorescence microscopy was carried out with an Olympus FSX100 microscope, and 150 to 160 intracellular bacterial cells were examined in each experiment.
TEM.
For transmission electron microscopy (TEM), RAW 264.7 macrophages (2 × 106 cells/ml) were infected at an MOI of 10 as described above. At 3 hpi they were fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer, harvested by scraping, and stored at 4°C until use. Samples were then processed by the electron microscopy facility at Tsukuba University according to the facility's protocols. Thin sections were observed with a JEOL JEM-1400 electron microscope.
RESULTS
Canonical σH recognition sequences are present upstream of the comG and comE operons.
Among Gram-positive bacteria, two master regulators control the expression of competence-related genes: the transcription factor ComK (in B. subtilis) (18) and the alternative sigma factor σH (in S. pneumoniae [19, 20], S. aureus [21], and Lactobacillus sakei [31]). Rabinovich et al. reported the presence of a ComK recognition site (the ComK box) upstream of the competence machinery operons (comG, comE, and comF) in L. monocytogenes (16). However, the involvement of σH in the expression of these genes has not been explored. Using the L. monocytogenes and B. subtilis σH promoter consensus sequences (4, 32), a recognition site for σH was identified in the upstream region of the comG and comE operons but not in that of the comF operon (Fig. 1). Although transcriptome analysis did not detect any competence genes in its regulon (4, 6), this observation implies that σH may be involved in the expression of comG and comE.
FIG 1.
Consensus sequences recognized by ComK and σH in L. monocytogenes. (A) comG operon; (B) comE operon; (C) comF operon. Arrows, open reading frame; underlines, ComK box (16); boxes, σH promoter consensus sequence (in B. subtilis, AGGAWWT-12 to 14 residues-RGAAT, where W is A or T and R is A or G [32]; in L. monocytogenes, AGG … GAA [4]); dashed line, suggested σA (rpoD) promoter consensus sequence conserved among Listeria spp. (16). We could not find a conserved region similar to the σA-type consensus sequence in comE promoter regions.
σH can express comE and comG operons.
The L. monocytogenes EGDe strain has a prophage-disrupted comK gene. This prophage has been reported to induce the expression of competence machinery genes by its excision from comK during intracellular growth (16). To eliminate the influence of the comK-disrupting phage, we cured the EGDe strain and used this as the wild-type (wt) strain for subsequent strain constructions and comparisons. To clarify the contribution of σH, strains overexpressing σH and strains with a σH deletion were constructed. No significant difference in bacterial growth was observed between the strains (Fig. 2).
FIG 2.
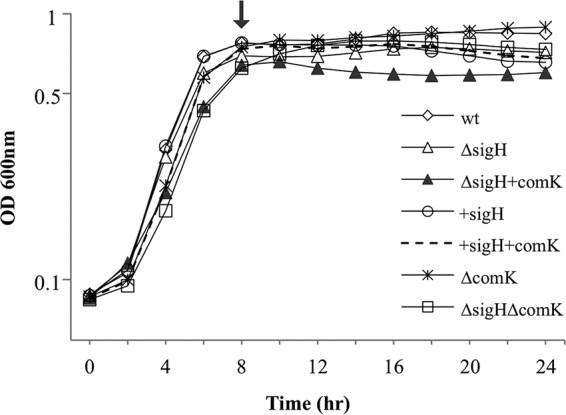
Growth of the L. monocytogenes strains (in BHI at 180 rpm and 37°C) used in this study. Arrow, sampling point; wt, phage-cured EGDe strain; ΔcomK, a comK deletion mutant; ΔsigH, a sigH deletion mutant; ΔsigH+comK, a strain overexpressing comK in the sigH deletion background, +sigH, the wt strain overexpressing sigH; +sigH+comK, the wt strain overexpressing sigH and comK; ΔsigHΔcomK, a sigH and comK double deletion mutant. OD 600nm, optical density at 600 nm.
The expression of the regulators (sigH and comK) and the competence machinery genes in cells grown to the stationary phase was determined by semiquantitative RT-PCR (Fig. 3). The first gene in each operon (comGA, comEA, and comFA) was used as the representative gene.
FIG 3.
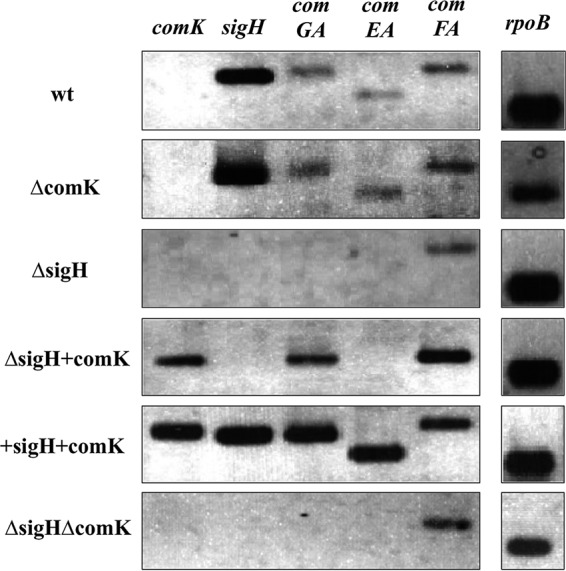
Semiquantitative RT-PCR of the comK, sigH, comGA, comEA, comFA, and rpoB genes. wt, phage-cured strain EGDe; ΔcomK, a comK deletion strain; ΔsigH, a sigH deletion strain; ΔsigH+comK, a strain overexpressing comK in the sigH deletion background; +sigH+comK, the wt strain overexpressing sigH and comK; ΔsigHΔcomK; a sigH and comK double deletion mutant. The sampling point is shown in Fig. 2. A representative result from 3 independent experiments is shown.
The wt strain (phage-cured EGDe) expressed detectable levels of mRNAs from comGA, comEA, and comFA; this strain also expressed sigH mRNA, but comK expression was undetectable. The comK deletion mutant (strain ΔcomK) showed the same pattern as the wt strain. The deletion of the sigH gene (in strain ΔsigH) reduced the expression of comGA and comEA to undetectable levels, while it had no effect on comFA expression. Double deletion mutant ΔsigHΔcomK did not express comGA or comEA, but comFA could still be detected in this mutant. To test whether comK expression could induce the expression of competence genes in the absence of sigH, a plasmid (pRIT5H-comK) was introduced into strain ΔsigH, resulting in strain ΔsigH+comK. comK expression induced comGA expression but not comEA expression. In contrast, when both regulators were overexpressed (resulting in strain +sigH+comK), mRNAs of both comGA and comEA were induced. Complementation of the ΔsigH strain restored the mRNA signal for the comGA and comEA genes (see Fig. S1A in the supplemental material).
These observations indicate the following: (i) σH and ComK are involved in comGA transcription, but σH is not essential for ComK-dependent induction; (ii) σH is essential for the expression of comEA, even when comK is overexpressed; and (iii) σH has no effect on comFA expression, while ComK has a positive effect. Thus, the alternative sigma factor σH can induce the transcription of the competence machinery operons, but its essentiality is different between comG and comE.
Competence genes are transcribed in a fraction of the population.
We observed that σH can activate the transcription of competence genes in L. monocytogenes. In other Gram-positive species, the percentage of the population that expresses these genes and develops competence varies greatly. In B. subtilis and S. aureus, only a fraction of the cells can express competence genes (10 to 20% and 2%, respectively) (21, 33). In contrast, in S. pneumoniae, all the cells can become competent (34). To evaluate the activation of com genes at a population level, a reporter assay was performed. The promoter region of comGA was cloned upstream of the CFP-coding sequence in a reporter plasmid. The wt strain (with endogenous expression of the regulators) did not produce any fluorescent signal during growth (data obtained at 8 h are shown in Fig. 4; data obtained at 12, 16, and 20 h are not shown); it is likely that the mRNA levels detected in the semiquantitative RT-PCR (Fig. 3) were below the detection limit of this assay. Overexpression of σH showed that 6% of the cells expressed the comG operon; comK expression was not detected in this strain (data not shown). When ComK was also overexpressed, this percentage increased to 20%. Thus, the comGA promoter was active in a minor proportion of the population (Fig. 4). These observations suggest that the competence genes in L. monocytogenes are tightly regulated, resulting in subpopulation-specific expression, similar to the findings for B. subtilis and S. aureus.
FIG 4.
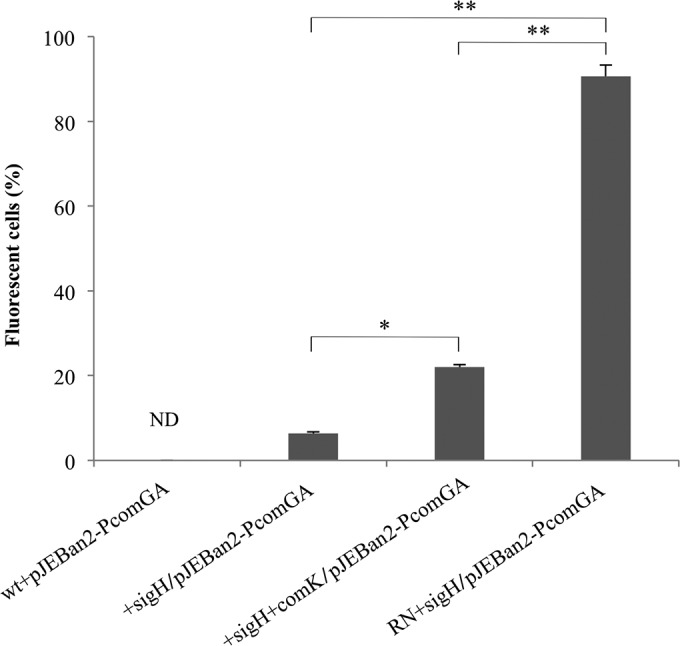
Cells expressing CFP under the control of the comGA promoter. wt, phage-cured strain EGDe; +sigH, the wt strain overexpressing sigH; +sigH+comK, the wt strain overexpressing sigH and comK; RN+sigH, S. aureus RN overexpressing L. monocytogenes sigH. Data represent the averages with SDs from 3 independent experiments. Significant differences are indicated. *, P = 0.002; **, P = 0.00001. ND, none detected. The sampling point is shown in Fig. 2.
The mechanisms regulating ComK and ComX/σH are different between species, and we wanted to test whether the subpopulation-specific expression of the comG promoter by Listeria σH could be observed in a different background. We were not able to use E. coli due to the toxic effect of σH. We overexpressed L. monocytogenes sigH in a S. aureus background (strain RN+sigH). The S. aureus strain employed (strain RN) carries mutations in the translation initiation codon of its own sigH. In the S. aureus background, we observed that 90% of the population expressed CFP (Fig. 4); this observation shows that σH can function as the sigma factor for the comGA promoter in almost all the population and implies the existence of a Listeria-specific regulation of σH.
σH is essential for intracellular growth in phagocytic and nonphagocytic cells.
Attempts to detect natural transformation in L. monocytogenes have failed so far (13). Since L. monocytogenes competence genes are homologous to those of B. subtilis (35), all these efforts have been focused only on ComK regulation. Here, we tested the strain overexpressing both regulators (strain +sigH+comK) for transformation. The S. aureus protocol (21) was used, but we could not detect any transformants (data not shown).
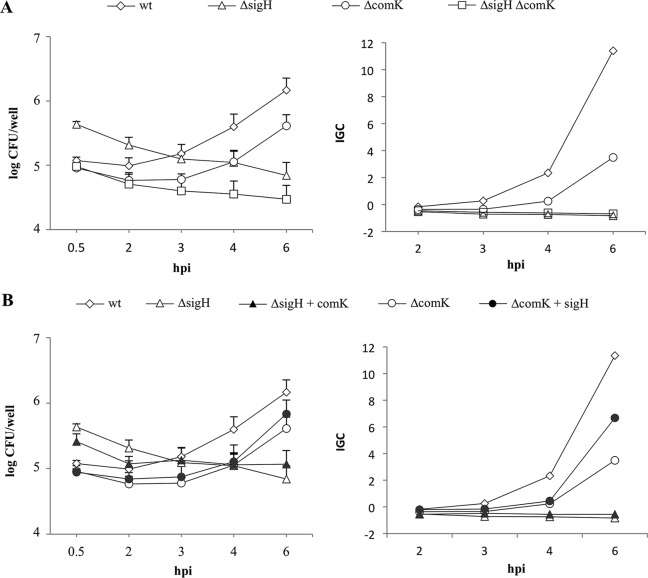
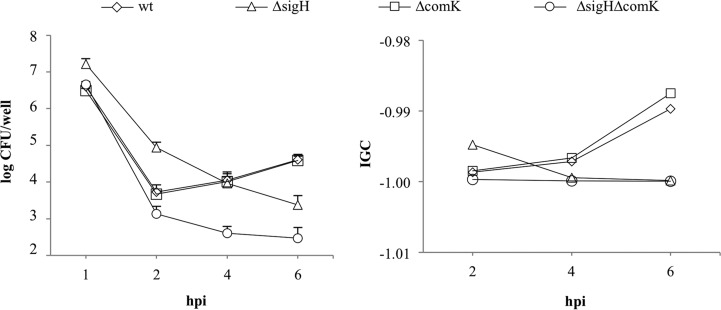
Another role for the competence genes in L. monocytogenes is to facilitate optimal phagosomal escape (16). To test the role of σH during intracellular growth, RAW 264.7 macrophages were infected with sigH and comK single and double deletion mutant strains at a multiplicity of infection of 1. Strain ΔsigH was phagocytosed at significantly higher levels than the wt strain (P = 0.0005), but its intracellular growth was impaired; the number of CFU of strain ΔsigH did not increase throughout the time course tested. In accordance with findings described in a previous report (16), we observed an intracellular growth defect in strain ΔcomK as a delayed increase in its number of CFU. The double mutant could not grow; like strain ΔsigH, the numbers of CFU decreased over time (Fig. 5A). The overexpression of one regulator in the absence of the other could not restore normal intracellular growth (Fig. 5B). Intracellular growth was also tested in HeLa cells at a multiplicity of infection of 50. Strains ΔsigH and ΔsigHΔcomK showed impaired growth, while strain ΔcomK showed normal growth (Fig. 6). sigH complementation restored intracellular growth in RAW 264.7 and HeLa cells (see Fig. S1B to D in the supplemental material). Taken together, these observations lead us to believe that σH and ComK play different roles during intracellular growth.
FIG 5.
Intracellular growth in RAW 264.7 macrophages. The numbers of CFU (left) were normalized using an IGC (right). (A) wt, phage-cured strain EGDe; ΔsigH, a sigH deletion strain; ΔcomK, a comK deletion strain; ΔsigHΔcomK, a sigH and comK double deletion mutant; (B) wt, phage-cured strain EGDe; ΔsigH, a sigH deletion strain; ΔsigH+comK, a strain overexpressing comK in the sigH deletion background; ΔcomK, a comK deletion strain; ΔcomK+sigH, a strain overexpressing sigH in the comK deletion background. The means from 3 independent experiments are shown.
FIG 6.
Intracellular growth in HeLa cells. The numbers of CFU (left) were normalized using an IGC (right). wt, phage-cured strain EGDe; ΔsigH, a sigH deletion strain; ΔcomK, a comK deletion strain; ΔsigHΔcomK, a sigH and comK double deletion mutant. The means from 3 independent experiments are shown.
It was reported that the comG operon and the comEC gene (but not comEA or comEB) are required for optimal intracellular growth in macrophages (16). According to our results, comG operon expression can be induced by σH or ComK, while comE operon expression seems to be dependent on σH. To test whether the observed intracellular growth deficiency in strain ΔsigH is caused by the diminished transcription of comEC, we checked its transcription by semiquantitative RT-PCR. Unexpectedly, our results showed that comEC can be expressed in the absence of σH and can be activated by ComK (Fig. 7). Thus, the impaired intracellular growth of strain ΔsigH was not simply attributable to the lack of the essential comEC expression.
FIG 7.
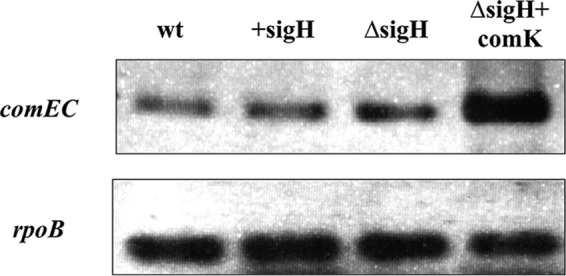
Semiquantitative RT-PCR of the comEC gene. wt, phage-cured strain EGDe; +sigH, the wt strain overexpressing sigH; ΔsigH, a sigH deletion strain; ΔsigH+comK, a strain overexpressing comK in the sigH deletion background. The sampling point is shown in Fig. 2.
In summary, σH has an essential role in intracellular growth in phagocytic and nonphagocytic cells. The lack of σH cannot be compensated for by ComK (which can induce the essential comG operon and the comEC gene) during macrophage infection. Importantly, the intracellular growth ability in both macrophages and HeLa cells was completely diminished in strain ΔsigH, while the intracellular growth of strain ΔcomK was merely delayed in macrophages and was normal in HeLa cells. These data suggest that σH is responsible for certain essential processes during the L. monocytogenes intracellular life cycle.
σH is required for phagosomal escape.
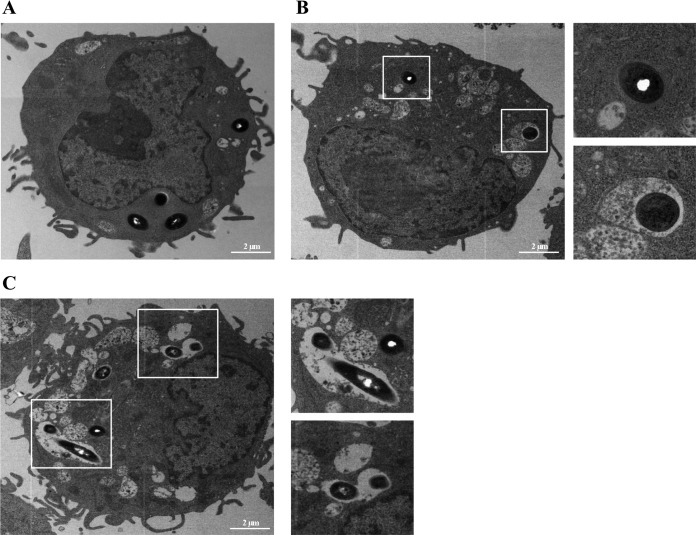
L. monocytogenes can infect phagocytic and nonphagocytic cells, where its successful intracellular life cycle relies on a crucial first step, i.e., phagosomal/vacuole escape (36, 37). In order to survive, bacteria need to escape vacuole acidification and fusion with lysosomes; phagosome/vacuole lysis occurs quickly after invasion. After escaping to the cytosol, L. monocytogenes grows and eventually infects the next cell by using actin-based motility. To determine the stage in which strain ΔsigH is impaired, we performed a phagosomal escape assay in RAW 264.7 macrophages and observed cytosolic bacteria by their association with actin filaments. At 3 hpi, most of the wt strain had escaped the phagosome (69%), whereas only 6% of strain ΔsigH was associated with actin. As expected, strain ΔcomK showed reduced phagosomal escape (28%) (Fig. 8). This result suggests that the intracellular growth deficiency of strain ΔsigH is due to a failure of phagosomal escape. However, this observation might be caused by a downregulation of the actA gene (responsible for actin polymerization [38]) and not by a deficiency in phagosomal escape. To test this, infected macrophages were observed using transmission electron microscopy (TEM) at 3 hpi; 50 to 70 bacterial cells were counted. While 66% of the cells of the wt strains and 45% of the cells of strain ΔcomK had escaped the phagosome, only 6% of the cells of strain ΔsigH were in the cytoplasm (Fig. 9), and thus, σH is a factor essential for phagosomal escape.
FIG 8.
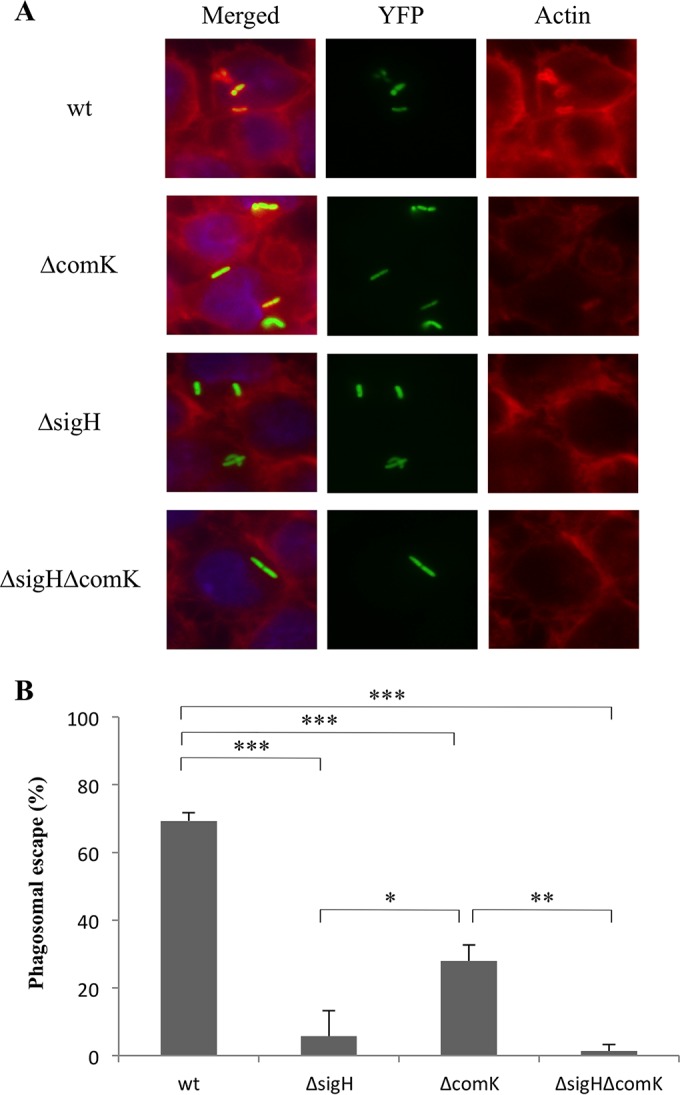
Phagosomal escape at 3 hpi in RAW 264.7 macrophages. (A) Representative images from fluorescence microscopy observations. YFP, yellow fluorescent protein. (B) Average values of phagosomal escape percentages with SDs from 4 independent experiments. Significant differences are indicated. *, P = 0.01; **, P < 0.0001; ***, P < 0.00001. wt, phage-cured strain EGDe; ΔsigH, a sigH deletion strain; ΔcomK, a comK deletion strain; ΔsigHΔcomK, a sigH and comK double deletion mutant.
FIG 9.
Representative TEM images of L. monocytogenes in RAW 264.7 macrophages. Macrophages were infected at an MOI of 10 and fixed at 3 hpi. (A) wt (phage-cured strain EGDe); (B) strain ΔcomK (a comK deletion mutant); (C) strain ΔsigH (a sigH deletion mutant). In panels B and C, the boxed areas in the panels on the left are enlarged in the two panels on the right.
DISCUSSION
Unique regulation of expression of competence genes in L. monocytogenes.
We report, for the first time, the involvement of the alternative sigma factor σH in the expression of competence genes in L. monocytogenes. Together with the previous finding that the transcriptional factor ComK induces their expression (16), the regulation of competence machinery expression in L. monocytogenes has unique characteristics.
The transcription of the comE operon is shown to be complex. comEA transcription requires σH, while ComK is dispensable. However, the situation is the opposite in the case of comEC: σH is unnecessary and ComK induces its transcription. These observations suggest that the comE genes are not simply under the control of the comEA promoter activity alone. The comE operon has a ComK box upstream of the comEA gene (16), but we could not find any box close to comEC. In B. subtilis, ComK binds to the promoter region of comG, thus causing the upstream DNA to bend around it and allowing ComK to interact with the holoenzyme and stabilizing the RNA polymerase-promoter complex (39). The σH-independent expression of comEC might involve a similar mechanism; ComK binds upstream of comEA and bends to induce comEC expression with an RNA polymerase holoenzyme that contains another sigma factor.
With respect to the comGA promoter, the percentage of active cells increased when σH and ComK were overexpressed (Fig. 4), possibly integrating two distinct signaling inputs. The additive effect of ComK and σH was reported in a transcriptome analysis of S. aureus, but the regulons of σH and ComK in this species overlap completely (22).
comF transcription does not require σH but seems to be enhanced by ComK; comFA transcription could be detected even in the absence of both regulators. This observation indicates that the RNA polymerase holoenzyme that transcribes the comF operon contains another sigma factor, most likely the housekeeping σA (rpoD), because consensus sequences for σB (40) and σL (4) could not be found (σC controls few genes [4, 6], and there is no reported consensus sequence). Therefore, comF expression in L. monocytogenes resembles B. subtilis regulation, in which comF expression is regulated by σA and enhanced by ComK (18, 41).
Thus, the expression of the competence machinery genes in L. monocytogenes involves both regulators, σH and ComK, and their roles are different even within an operon depending on each com gene.
σH negative regulation.
In L. monocytogenes, overexpression of σH and ComK could induce the expression of PcomGA-cfp in only 20% of the population. However, we also observed that σH could induce its expression in the entire population when it was expressed in a different species (S. aureus). The low percentage of activation observed in the L. monocytogenes background (even with the overexpression of both regulators) suggests the existence of a strong negative regulation in the majority of the population. One candidate is the negative competence regulator MecA, which negatively regulates ComK in B. subtilis (42) and σH in Streptococcus mutans and Streptococcus thermophilus (43, 44). Further studies on σH negative regulation are necessary to gain insight into the specific conditions that trigger the expression of competence genes.
Competence/transformation development.
We succeeded in activating the transcription of competence machinery genes in a fraction of the population by overexpressing ComK and σH, but our attempt to detect transformants did not succeed. This does not exclude the possibility that L. monocytogenes has the ability to develop natural competence for DNA transformation. The competence state is a time-limited response to a specific environmental condition and to organism-specific processes (45). In S. aureus, for example, the transformation of σH-expressing cells further requires specific growth conditions (21). In the present study, we bypassed some signals by overexpressing ComK and SigH, but whether further additional layers of signaling or internal regulation are present remains to be evaluated.
σH function.
In the present study, we have shown that the σH factor is required for phagosomal escape during intracellular infection. The comK mutant just delays its growth, suggesting that competence genes are not essential for phagosomal escape. In contrast, SigH mutant growth was completely abolished (Fig. 5), even though it had the ability to express ComG and ComEC factors when ComK was expressed (Fig. 3 and 7, strain ΔsigH+comK). Therefore, we suppose that the mechanism of phagosomal escape controlled by σH is due to not merely the expression of the competence genes but also certain essential factors broadly required for the intracellular life cycle.
Leaving aside the main stress sigma factor (σB), σH positively regulates the largest number of genes and overlaps with other regulons, particularly σB (4, 6). It is expected that the function of σH in L. monocytogenes is complex and not directly linked to one specific process. The reported σH regulon does not include any of the genes involved in phagosome/vacuole escape (prfA [46], hly [47, 48], and plcA and plcB [49]), and indeed, we could not find a σH promoter consensus sequence in these genes. A recent report showed that a σH in-frame deletion mutant is affected during growth in minimal medium and shows a low but significant reduction in virulence in a mouse model, leading to the conclusion that σH has a role in the acquisition or utilization of nutrients (2). The impaired intracellular growth of our sigH mutant might be partly attributed to this broad physiological role. Alternatively, σH might have an indirect effect on the expression of the master regulator, prfA.
Among Gram-positive bacteria, σH has evolved to regulate two different stress-related processes: sporulation and competence development. Here, we showed that L. monocytogenes σH can induce the transcription of competence genes. In addition, σH was crucial for intracellular growth in phagocytic and nonphagocytic cells. Thus, the role of σH has further diverged in L. monocytogenes along with its intracellular life cycle.
Supplementary Material
ACKNOWLEDGMENTS
We thank Tine R. Licht (National Food Institute, Technical University of Denmark) for providing the plasmids pJEBan2 and pJEBan3. We also thank Yuko Jinsenji for transmission electron microscopy.
This work was partly supported by Pfizer Academic Contributions.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00718-15.
REFERENCES
- 1.Gray MJ, Freitag NE, Boor KJ. 2006. How the bacterial pathogen Listeria monocytogenes mediates the switch from environmental Dr. Jekyll to pathogenic Mr. Hyde. Infect Immun 74:2505–2512. doi: 10.1128/IAI.74.5.2505-2512.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rea RB, Gahan CGM, Hill C. 2004. Disruption of putative regulatory loci in Listeria monocytogenes demonstrates a significant role for Fur and PerR in virulence. Infect Immun 72:717–727. doi: 10.1128/IAI.72.2.717-727.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaturongakul S, Raengpradub S, Wiedmann M, Boor KJ. 2008. Modulation of stress and virulence in Listeria monocytogenes. Trends Microbiol 16:388–396. doi: 10.1016/j.tim.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaturongakul S, Raengpradub S, Palmer ME, Bergholz TM, Orsi RH, Hu Y, Ollinger J, Wiedmann M, Boor KJ. 2011. Transcriptomic and phenotypic analyses identify coregulated, overlapping regulons among PrfA, CtsR, HrcA, and the alternative sigma factors σB, σC, σH and σL in Listeria monocytogenes. Appl Environ Microbiol 77:187–200. doi: 10.1128/AEM.00952-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garmyn D, Augagneur Y, Gal L, Vivant AL, Piveteau P. 2012. Listeria monocytogenes differential transcriptome analysis reveals temperature-dependent Agr regulation and suggests overlaps with other regulons. PLoS One 7:e43154. doi: 10.1371/journal.pone.0043154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mujahid S, Orsi RH, Boor KJ, Wiedmann M. 2013. Protein level identification of the Listeria monocytogenes sigma H, sigma L, and sigma C regulons. BMC Microbiol 13:156. doi: 10.1186/1471-2180-13-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raimann E, Schmid B, Stephan R, Tasara T. 2009. The alternative sigma factor σL of L. monocytogenes promotes growth under diverse environmental stresses. Foodborne Pathog Dis 6:583–591. doi: 10.1089/fpd.2008.0248. [DOI] [PubMed] [Google Scholar]
- 8.Zhang C, Nietfeldt J, Zhang M, Benson AK. 2005. Functional consequences of genome evolution in Listeria monocytogenes: the lmo0423 and lmo0422 genes encode σC and LstR, a lineage II-specific heat shock system. J Bacteriol 187:7243–7253. doi: 10.1128/JB.187.21.7243-7253.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phan-Thanh L, Mahouin F. 1999. A proteomic approach to study the acid response in Listeria monocytogenes. Electrophoresis 20:2214–2224. doi:. [DOI] [PubMed] [Google Scholar]
- 10.Morikawa K, Inose Y, Okamura H, Maruyama A, Hayashi H, Takeyasu K, Ohta T. 2003. A new staphylococcal sigma factor in the conserved gene cassette: functional significance and implication for the evolutionary processes. Genes Cells 8:699–712. doi: 10.1046/j.1365-2443.2003.00668.x. [DOI] [PubMed] [Google Scholar]
- 11.Dortet L, Veiga-Chacon V, Cossart P. 2009. Listeria monocytogenes, p 182–198. In Schaechter M. (ed), Encyclopedia of microbiology, 3rd ed Elsevier, Amsterdam, Netherlands. [Google Scholar]
- 12.Stragier P. 2002. A gene odyssey: exploring the genomes of endospore-forming bacteria, p 519–526. In Sonenshein AL, Hoch JA, Losick R (ed), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC. [Google Scholar]
- 13.Borezee E, Msadek T, Durant L, Berche P. 2000. Identification in Listeria monocytogenes of MecA, a homologue of the Bacillus subtilis competence regulatory protein. J Bacteriol 182:5931–5934. doi: 10.1128/JB.182.20.5931-5934.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen I, Dubnau D. 2004. DNA uptake during bacterial transformation. Nat Rev Microbiol 2:241–249. doi: 10.1038/nrmicro844. [DOI] [PubMed] [Google Scholar]
- 15.Claverys J-P, Martin B. 2003. Bacterial ‘competence’ genes: signatures of active transformation, or only remnants? Trends Microbiol 11:161–165. doi: 10.1016/S0966-842X(03)00064-7. [DOI] [PubMed] [Google Scholar]
- 16.Rabinovich L, Sigal N, Borovok I, Nir-Paz R, Herskovits AA. 2012. Prophage excision activates Listeria competence genes that promote phagosomal escape and virulence. Cell 150:792–802. doi: 10.1016/j.cell.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 17.Johnsborg O, Eldholm V, Håvarstein LS. 2007. Natural genetic transformation: prevalence, mechanisms and function. Res Microbiol 158:767–778. doi: 10.1016/j.resmic.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Van Sinderen D, Luttinger A, Kong L, Dubnau D, Venema G, Hamoen L. 1995. comK encodes the competence transcription factor, the key regulatory protein for competence development in Bacillus subtilis. Mol Microbiol 15:455–462. doi: 10.1111/j.1365-2958.1995.tb02259.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee M, Morrison D. 1999. Identification of a new regulator in Streptococcus pneumoniae linking quorum sensing to competence for genetic transformation. J Bacteriol 181:5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo P, Morrison DA. 2003. Transient association of an alternative sigma factor, ComX, with RNA polymerase during the period of competence for genetic transformation in Streptococcus pneumoniae. J Bacteriol 185:349–358. doi: 10.1128/JB.185.1.349-358.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morikawa K, Takemura AJ, Inose Y, Tsai M, Nguyen Thi LT, Ohta T, Msadek T. 2012. Expression of a cryptic secondary sigma factor gene unveils natural competence for DNA transformation in Staphylococcus aureus. PLoS Pathog 8:e1003003. doi: 10.1371/journal.ppat.1003003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fagerlund A, Granum PE, Håvarstein LS. 2014. Staphylococcus aureus competence genes: mapping of the SigH, ComK1 and ComK2 regulons by transcriptome sequencing. Mol Microbiol 94:557–579. doi: 10.1111/mmi.12767. [DOI] [PubMed] [Google Scholar]
- 23.Raya R, Hebert E. 2009. Isolation of phage via induction of lysogens, p 23–32. In Clokie M, Kropinski A (ed), Bacteriophages: methods and protocols, vol 1 Humana Press, Totowa, NJ. [Google Scholar]
- 24.Sullivan MYR, Young F. 1984. New shuttle vectors for Bacillus subtilis and Escherichia coli which allow rapid detection of inserted fragments. Gene 29:21–26. doi: 10.1016/0378-1119(84)90161-6. [DOI] [PubMed] [Google Scholar]
- 25.Tsai M, Ohniwa RL, Kato Y, Takeshita SL, Ohta T, Saito S, Hayashi H, Morikawa K. 2011. Staphylococcus aureus requires cardiolipin for survival under conditions of high salinity. BMC Microbiol 11:13. doi: 10.1186/1471-2180-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boneca IG, Dussurget O, Cabanes D, Nahori M-A, Sousa S, Lecuit M, Psylinakis E, Bouriotis V, Hugot J-P, Giovannini M, Coyle A, Bertin J, Namane A, Rousselle J-C, Cayet N, Prévost M-C, Balloy V, Chignard M, Philpott DJ, Cossart P, Girardin SE. 2007. A critical role for peptidoglycan N-deacetylation in Listeria evasion from the host innate immune system. Proc Natl Acad Sci U S A 104:997–1002. doi: 10.1073/pnas.0609672104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Even S, Burguiere P, Auger S, Soutourina O, Danchin A, Martin-Verstraete I. 2006. Global control of cysteine metabolism by CymR in Bacillus subtilis. J Bacteriol 188:2184–2197. doi: 10.1128/JB.188.6.2184-2197.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gueriri I, Bay S, Dubrac S, Cyncynatus C, Msadek T. 2008. The Pta-AckA pathway controlling acetyl phosphate levels and the phosphorylation state of the DegU orphan response regulator both play a role in regulating Listeria monocytogenes motility and chemotaxis. Mol Microbiol 70:1342–1357. doi: 10.1111/j.1365-2958.2008.06496.x. [DOI] [PubMed] [Google Scholar]
- 29.Andersen JB, Roldgaard BB, Lindner AB, Christensen BB, Licht TR. 2006. Construction of a multiple fluorescence labelling system for use in co-invasion studies of Listeria monocytogenes. BMC Microbiol 6:86. doi: 10.1186/1471-2180-6-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deshayes C, Bielecka MK, Cain RJ, Scortti M, de las Heras A, Pietras Z, Luisi BF, Nunez Miguel R, Vazquez-Boland JA. 2012. Allosteric mutants show that PrfA activation is dispensable for vacuole escape but required for efficient spread and Listeria survival in vivo. Mol Microbiol 85:461–477. doi: 10.1111/j.1365-2958.2012.08121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmid S, Bevilacqua C, Crutz-Le Coq AM. 2012. Alternative sigma factor σH activates competence gene expression in Lactobacillus sakei. BMC Microbiol 12:32. doi: 10.1186/1471-2180-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wosten MMSM. 1998. Eubacterial sigma-factors. FEMS Microbiol Rev 22:127–150. doi: 10.1111/j.1574-6976.1998.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 33.Haijema B-J, Hahn J, Haynes J, Dubnau D. 2001. A ComGA-dependent checkpoint limits growth during the escape from competence. Mol Microbiol 40:52–64. doi: 10.1046/j.1365-2958.2001.02363.x. [DOI] [PubMed] [Google Scholar]
- 34.Tomasz A. 1965. Control of the competent state in Pneumococcus by a hormone-like cell product: an example for a new type of regulatory mechanism in bacteria. Nature 208:155–159. doi: 10.1038/208155a0. [DOI] [PubMed] [Google Scholar]
- 35.Glaser P, Frangeul L, Buchrieser C, Rusniok C, Amend A, Baquero F, Berche P, Bloecker H, Brandt P, Chakraborty T, Charbit A, Chetouani F, Couve E, de Daruvar A, Dehoux P, Domann E, Dominguez-Bernal G, Duchaud E, Durant L, Dussurget O, Entian KD, Fsihi H, Garcia-del Portillo F, Garrido P, Gautier L, Goebel W, Gomez-Lopez N, Hain T, Hauf J, Jackson D, Jones LM, Kaerst U, Kreft J, Kuhn M, Kunst F, Kurapkat G, Madueno E, Maitournam A, Vicente JM, Ng E, Nedjari H, Nordsiek G, Novella S, de Pablos B, Perez-Diaz JC, Purcell R, Remmel B, Rose M, Schlueter T, Simoes N, et al. . 2001. Comparative genomics of Listeria species. Science 294:849–852. [DOI] [PubMed] [Google Scholar]
- 36.Freitag NE, Port G, Miner M. 2009. Listeria monocytogenes—from saprophyte to intracellular pathogen. Nat Rev Microbiol 7:623–628. doi: 10.1038/nrmicro2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ray K, Marteyn B, Sansonetti PJ, Tang CM. 2009. Life on the inside: the intracellular lifestyle of cytosolic bacteria. Nat Rev Microbiol 7:333–340. doi: 10.1038/nrmicro2112. [DOI] [PubMed] [Google Scholar]
- 38.Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. 1992. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell 68:521–531. [DOI] [PubMed] [Google Scholar]
- 39.Susanna KA, van der Werff AF, den Hengst CD, Calles B, Salas M, Venema G, Hamoen LW, Kuipers OP. 2004. Mechanism of transcription activation at the comG promoter by the competence transcription factor ComK of Bacillus subtilis. J Bacteriol 186:1120–1128. doi: 10.1128/JB.186.4.1120-1128.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kazmierczak MJ, Mithoe SC, Boor KJ, Wiedmann M. 2003. Listeria monocytogenes σB regulates stress response and virulence functions. J Bacteriol 185:5722–5734. doi: 10.1128/JB.185.19.5722-5734.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Londoño-Vallejo JA, Dubnau D. 1993. comF, a Bacillus subtilis late competence locus, encodes a protein similar to ATP-dependent RNA-DNA helicases. Mol Microbiol 9:119–131. doi: 10.1111/j.1365-2958.1993.tb01674.x. [DOI] [PubMed] [Google Scholar]
- 42.Turgay K, Hamoen L, Venema G, Dubnau D. 1997. Biochemical characterization of a molecular switch involving the heat shock protein ClpC, which controls the activity of ComK, the competence transcription factor of Bacillus subtilis. Genes Dev 11:119–128. doi: 10.1101/gad.11.1.119. [DOI] [PubMed] [Google Scholar]
- 43.Boutry C, Wahl A, Delplace B, Clippe A, Fontaine L, Hols P. 2012. Adaptor protein MecA is a negative regulator of the expression of late competence genes in Streptococcus thermophilus. J Bacteriol 194:1777–1788. doi: 10.1128/JB.06800-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian XL, Dong G, Liu T, Gomez ZA, Wahl A, Hols P, Li YH. 2013. MecA protein acts as a negative regulator of genetic competence in Streptococcus mutans. J Bacteriol 195:5196–5206. doi: 10.1128/JB.00821-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas CM, Nielsen KM. 2005. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat Rev Microbiol 3:711–721. doi: 10.1038/nrmicro1234. [DOI] [PubMed] [Google Scholar]
- 46.Chakraborty T, Leimeister-Wachter M, Domann E, Hartl M, Goebel W, Nichterlein T, Notermans S. 1992. Coordinate regulation of virulence genes in Listeria monocytogenes requires the product of the prfA gene. J Bacteriol 174:568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geoffroy C, Gaillard J, Alouf J, Berche P. 1987. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysisn listeriolysin O from Listeria monocytogenes. Infect Immun 55:1641–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Portnoy DA, Jacks PS, Hinrichs DJ. 1988. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med 167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith GA, Marquis H, Jones S, Johnston NC, Portnoy DA, Goldfine H. 1995. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect Immun 63:4231–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.