ABSTRACT
Shewanella oneidensis strain MR-1 is a facultative anaerobe that thrives in redox-stratified environments due to its ability to utilize a wide array of terminal electron acceptors. Conversely, the electron donors utilized by S. oneidensis are more limited and include products of primary fermentation such as lactate, pyruvate, formate, and hydrogen. Lactate, pyruvate, and hydrogen metabolisms in S. oneidensis have been described previously, but little is known about the role of formate oxidation in the ecophysiology of these bacteria. Formate is produced by S. oneidensis through pyruvate formate lyase during anaerobic growth on carbon sources that enter metabolism at or above the level of pyruvate, and the genome contains three gene clusters predicted to encode three complete formate dehydrogenase complexes. To determine the contribution of each complex to formate metabolism, strains lacking one, two, or all three annotated formate dehydrogenase gene clusters were generated and examined for growth rates and yields on a variety of carbon sources. Here, we report that formate oxidation contributes to both the growth rate and yield of S. oneidensis through the generation of proton motive force. Exogenous formate also greatly accelerated growth on N-acetylglucosamine, a carbon source normally utilized very slowly by S. oneidensis under anaerobic conditions. Surprisingly, deletion of all three formate dehydrogenase gene clusters enabled growth of S. oneidensis using pyruvate in the absence of a terminal electron acceptor, a mode of growth never before observed in these bacteria. Our results demonstrate that formate oxidation is a fundamental strategy under anaerobic conditions for energy conservation in S. oneidensis.
IMPORTANCE Shewanella species have garnered interest in biotechnology applications for their ability to respire extracellular terminal electron acceptors, such as insoluble iron oxides and electrodes. While much effort has gone into studying the proteins for extracellular electron transport, how electrons generated through the oxidation of organic carbon sources enter this pathway remains understudied. Here, we quantify the role of formate oxidation in the anaerobic physiology of Shewanella oneidensis. Formate oxidation contributes to both the growth rate and yield on a variety of carbon sources through the generation of proton motive force. Advances in our understanding of the anaerobic metabolism of S. oneidensis are important for our ability to utilize and engineer this organism for applications in bioenergy, biocatalysis, and bioremediation.
INTRODUCTION
Shewanella oneidensis strain MR-1 (here referred to as MR-1) is a model environmental bacterium that thrives in redox-stratified environments due to its ability to couple the oxidation of organic carbon or hydrogen to a diverse array of terminal electron acceptors (1–3). Electron acceptors utilized by MR-1 range from soluble organic compounds such as fumarate to insoluble extracellular metal oxides and electrodes (1). The respiratory diversity of MR-1 has led to its use in a number of promising applications in biotechnology, and because of this, extracellular electron transfer by MR-1 has been well studied (2, 3). Electrons produced by MR-1 during anaerobic metabolism drive reduction of the quinone pool and are subsequently transferred to the inner-membrane-anchored c-type cytochrome CymA (4). CymA serves as a central hub in the inner membrane from which electrons can be transferred to multiple terminal electron acceptors such as FccA during fumarate respiration (Fig. 1A) or to a multisubunit porin-cytochrome complex, collectively termed the Mtr pathway, during extracellular respiration (5–9). This elegant system of electron transport in MR-1 is dependent on the generation of electrons from the oxidation of organic carbon sources, yet how electrons flow from organic carbon to these respiratory pathways has been understudied. Moreover, quinone cycling by the CymA redox loop not only facilitates electron transfer but should also, under some conditions, contribute to the generation of proton motive force (PMF) through the translocation of protons across the inner membrane (4, 10). A better understanding of how Shewanella gains energy from the oxidation of organic carbon is imperative for facilitating rational engineering for applications in biotechnology, bioremediation, and synthetic biology.
FIG 1.
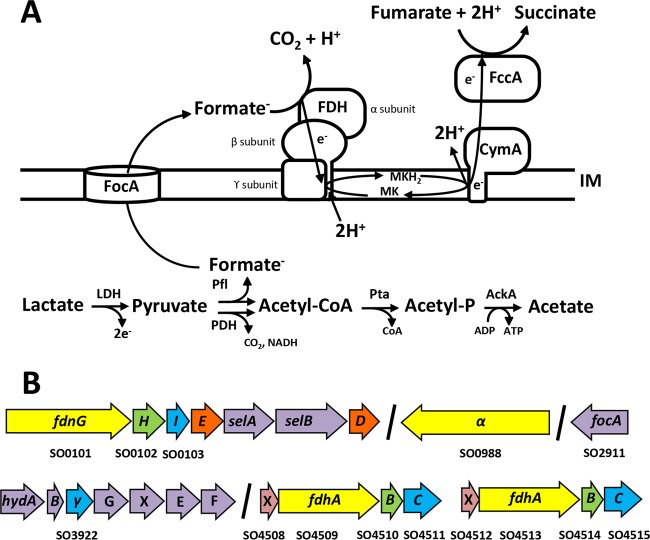
(A) Anaerobic formate metabolism in S. oneidensis MR-1. The model depicts oxidation of lactate (or pyruvate) as the sole carbon and energy source coupled to reduction of fumarate via the periplasmic fumarate reductase FccA. Two electrons are generated by lactate dehydrogenase (LDH) from the conversion of lactate into pyruvate. These electrons enter the menaquinone pool (MK) in the inner membrane (IM) directly or indirectly, depending on which of the LDH enzymes catalyze the reaction as S. oneidensis has different systems for d- and l-lactate (11, 13). (B) Genomic organization of genes directly involved in formate metabolism in MR-1. Genes encoding the α, β, and γ subunits of an FDH complex are colored yellow, green, and blue, respectively. fdhX genes, which encode small proteins of unknown function, are colored pink. The accessory genes involved in FDH complex maturation are colored orange, and the genes involved in cofactor maturation (selAB), formate transport (focA), and the hyd operon are colored purple.
In anoxic environments, MR-1 oxidizes lactate, pyruvate, formate, hydrogen, and some amino acids for anaerobic respiration, likely forming syntrophic partnerships with fermentative microorganisms (1). The underlying physiology of lactate, pyruvate, and hydrogen utilization has been described (11–13), but little is known about the role of formate in MR-1. Formate oxidation has been linked to the reduction of a variety of metals and azo dyes using in vitro assays (14–18), and formate is the only carbon compound shown to elicit a chemotactic response by MR-1 (19). Formate oxidation likely plays a significant role in energy conservation in MR-1 by providing the thermodynamic driving force required for reduction of CymA by the menaquinol pool and subsequent translocation of protons across the cytoplasmic membrane (4, 10).
Formate is critical for metabolism of many facultative anaerobic microorganisms. Formate is a prominent fermentation product of many obligate and facultative anaerobes, and secretion of formate facilitates internal redox balancing during mixed-acid fermentation. Formate secretion is pronounced in the Enterobacteriaceae, accounting for up to one-third of the carbon secreted from fermented sugar substrates (20). Due to the relatively low redox potential (−420 mV at pH 7.0), formate also serves as an important energy source for many microorganisms during aerobic or anaerobic respiration. The oxidation of formate (CHOO− at pH 7.0) results in the generation of a pair of electrons, a proton, and a molecule of carbon dioxide and is typically carried out by a formate dehydrogenase (FDH) complex in the cytoplasmic membrane (21). Oxidation occurs in the periplasm to avoid acidification of the cytoplasm, and electrons are transferred through the FDH complex to menaquinone along with protons from the cytoplasmic side of the inner membrane (21).
The genome of MR-1 contains gene clusters predicted to encode three complete FDH complexes (Fig. 1B), raising a number of questions regarding the role of formate in the ecophysiology of the genus Shewanella. Here, we demonstrate that formate, of endogenous and/or exogenous origin, contributes to the growth rate and yield of MR-1 through the generation of PMF. Notably, formate greatly accelerates the anaerobic growth of MR-1 on N-acetylglucosamine (NAG), a carbon source typically utilized slowly under anaerobic conditions. The deletion of all three FDH operons also enables the anaerobic growth of S. oneidensis on pyruvate without the addition of a terminal electron acceptor, suggesting the existence of a feedback mechanism or an inability to turn over reducing equivalents, which prevents MR-1 from growing when the menaquinone pool is fully reduced.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The strains and plasmids used in this study are listed in Table S1 in the supplemental material. The Escherichia coli strains used for cloning and conjugal transfer were maintained on lysogeny broth (LB) agar plates supplemented with 50 μg/ml kanamycin and/or 250 μM 2,6-diaminopimelic acid as necessary. During routine propagation, S. oneidensis was maintained on LB agar containing 50 μg/ml kanamycin as necessary. For growth assays, strains were grown in Shewanella basal medium containing 5 ml/liter vitamin mix, 5 ml/liter mineral mix (2), and 0.05% Casamino Acids (here referred to as SBM) supplemented with lactate, pyruvate, NAG, formate, and/or fumarate when indicated. The growth assays were performed as follows. Strains stored in 15% glycerol at −80°C were freshly streaked onto LB agar plates and incubated for ∼16 h at 30°C. Single colonies were used to inoculate LB medium shaken at 250 rpm at 30°C for 6 to 8 h and were then subcultured in aerobic SBM shaken for ∼16 h at 30°C. Cells were then washed twice and added to Balch tubes stoppered with butyl rubber containing an argon headspace to a final optical density at 600 nm (OD600) of ∼0.02 (22).
Reagents and materials.
Enzymes were purchased from New England BioLabs (Ipswich, MA). Kits for gel purification and plasmid mini preps were purchased from Invitrogen (Carlsbad, CA). All related reactions were carried out according to the manufacturer's instructions. Medium components were purchased from Becton, Dickinson and Company (Sparks, MD), and chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Generation of deletion mutants.
The oligonucleotide primers used to amplify portions of the MR-1 chromosome for deletion constructs are listed in Table S2 in the supplemental material. PCR products were cloned using standard laboratory molecular biology protocols. Regions flanking the deletion targets were amplified using PCR and cloned into the pSMV3 suicide vector. In-frame gene deletions were generated using homologous recombination as previously described (23). Deletion constructs were moved into S. oneidensis by conjugal transfer from E. coli donor strain WM3064. All plasmid constructs and gene deletions were verified by sequencing (University of Minnesota Genomics Center).
High-performance liquid chromatography.
Metabolites were quantified by high-performance liquid chromatography (HPLC) using Shimadzu Scientific equipment, including an SCL-10A system controller, an LC-10AT pump, an SIL-10AF autoinjector, a CTO-10A column oven, an RID-10A refractive index detector, and an SPD-10A UV-visible (UV-Vis) detector. The mobile phase consisted of 15 mN H2SO4 set at a flow rate of 0.400 ml min−1. Injection volumes of 50 μl were separated on an Aminex HPX-87H column maintained at 30°C.
Membrane potential measurements.
Membrane potential measurements for MR-1 and the Δfdh mutant were conducted using the carbocyanine dye 3,3-diethyloxacarbocyanine iodide (DiOC2) and flow cytometry. The cells grown anaerobically in SBM containing 20 mM lactate and 40 mM fumarate were washed twice, resuspended in SBM without lactate or fumarate to an OD600 of 0.5, and incubated at 30°C. After 72 h, the starved cells were diluted to a final cell density of 106 cells/ml in anaerobic SBM (lacking Casamino Acids) containing 20 mM fumarate and either no carbon source, 10 mM lactate, or 10 mM formate. The cells were incubated for 48 h at 30°C and were then exposed to the DiOC2 dye (30 μM final concentration) for 15 min in the dark. Fluorescence intensities at wavelengths greater than 640 nm were measured for 10,000 cells using a FACSCalibur flow cytometer (Becton Dickinson). As a depolarized control, the protonophore carbonyl cyanide 3-chlorophenylhydrazone (CCCP) was added at a final concentration of 5 μM where indicated.
Sequence analysis.
Homologs of the FDH alpha subunits SO4509 and SO4513 were identified for other members of the Shewanellaceae via BLAST (http://blast.ncbi.nlm.nih.gov) and a Top IMG Homolog Hit search using the JGI database (http://img.jgi.doe.gov). The resulting coding regions were aligned via MUSCLE (24), and evolutionary analyses were conducted in MEGA6 (25). From this alignment, a bootstrapped maximum likelihood (300 replicates) tree based on the general time-reversible model was constructed using MEGA6 (26, 27). The initial trees for the heuristic search were obtained automatically by applying the neighbor-joining and BioNJ algorithms to a matrix of pairwise distances estimated using the maximum composite likelihood (MCL) approach and then selecting the topology with superior log-likelihood value. A discrete gamma distribution was used to model the evolutionary rate differences among the sites (5 categories [+G, parameter = 0.77]). The rate variation model allowed for some sites to be evolutionarily invariable ([+I], 30.02% sites). The analysis involved 88 nucleotide sequences. Codon positions included were 1st plus 2nd plus 3rd plus noncoding. All positions with less than 95% site coverage were eliminated. That is, fewer than 5% alignment gaps, missing data, and ambiguous bases were allowed at any position. There were a total of 2,844 positions in the final data set.
RESULTS
The chromosome of MR-1 contains multiple gene regions predicted to encode proteins involved in formate metabolism, including three putative FDH complexes. Region SO0101-0103 is annotated as fdnGHI, encoding a predicted selenocysteine-containing, nitrate-inducible FDH similar to the canonical Fdh-N in E. coli (Fig. 1B). Gene regions SO4508-4511 and SO4512-4515 are annotated as fdhXABC and are predicted to encode two individual, but related, FDH complexes similar to Fdh-O of E. coli (Fig. 1B). FdhX is a member of an uncharacterized protein family and may be a small subunit or accessory protein of FDH, while the fdhABC genes encode the α, β, and γ subunits of FDH, respectively. An additional FDH γ subunit (SO3922) is annotated as part of the hyd locus (SO3920-3926), which encodes an [Fe-Fe] hydrogenase (12). SO3920-3926 is proposed to encode a formate hydrogen lyase (FHL), as the region is expressed as a polycistronic unit under anaerobic conditions, and formate addition was linked to hydrogen production in MR-1 (12). The MR-1 genome also contains SO0988, which encodes an orphan FDH α subunit (Fig. 1B). SO0988 does not encode a twin-arginine translocation pathway signal sequence and is therefore predicted to be cytoplasmic. The MR-1 genome contains SO2911, which encodes a predicted bidirectional formate transporter, FocA, and which is directly upstream of the regions encoding pyruvate formate lyase (PflB) and its activating enzyme PflA (Fig. 1B). The preponderance of FDH gene clusters in the MR-1 genome is unusual and warranted additional investigation regarding their phylogeny and dispersal across other sequenced Shewanella species.
Phylogenetic analysis of FdhA across the Shewanellaceae.
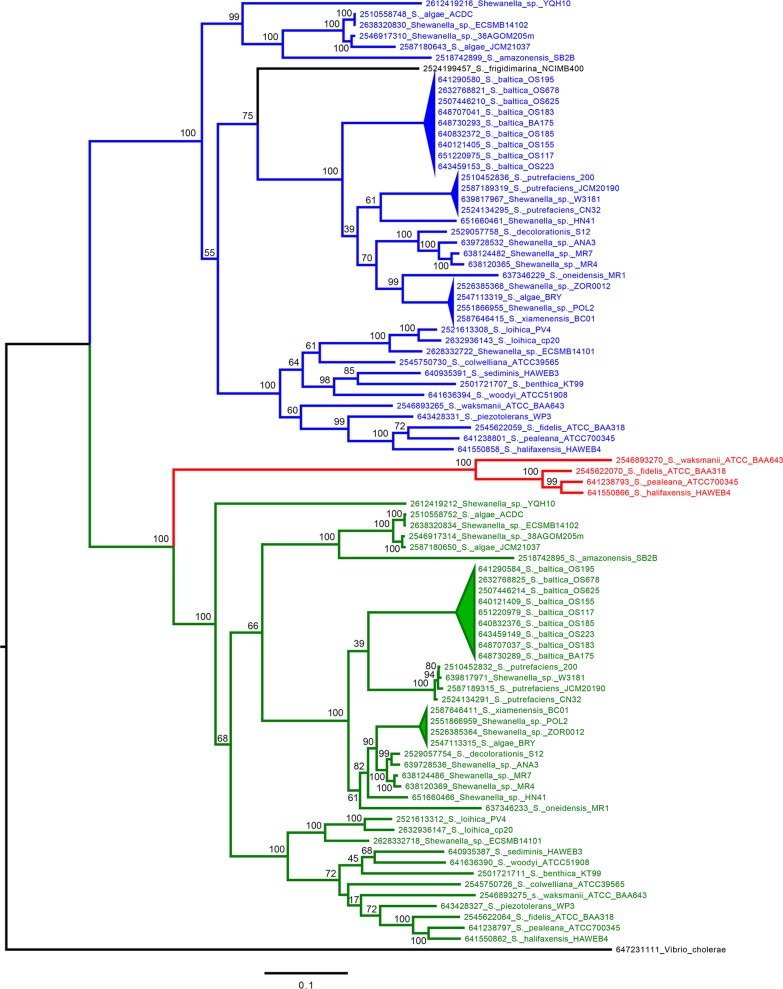
The tandem fdhXABC gene clusters (SO4508-4511 and SO4512-4513) in MR-1 have arisen from a gene duplication event, sharing 68% identity at the nucleotide level over the entire length. At the amino acid level, the FdhX pairs are 46% identical (57% similar), the FdhA pairs are 74% identical (85% similar), the FdhB pairs are 88% identical (94% similar), and the FdhC pairs are 54% identical (70% similar). To look further into the history of the duplicated FDH region, a phylogenetic tree was created using the fdhA nucleotide sequences (aligned by codons) for all sequenced Shewanella species available in the JGI database (http://img.jgi.doe.gov). fdhA was chosen for the phylogenetic analysis because it encodes the catalytic subunit of the FDH complex, and it is also the largest gene in the operon (∼3 kb), providing the most information on sequence divergence. The tree outlining the phylogenetic lineage of the fdhA sequences results in three main clades (highlighted in blue, green, and red text in Fig. 2). The clades highlighted in blue and green represent the initial duplication of the FDH region, which appears to have arisen in a common ancestor predating the current identification of species in the Shewanella genus (Fig. 2). In all species containing the duplication, the fdhA sequences associated with the upstream FDH operon comprise a single clade (blue), while the fdhA sequences associated with the downstream FDH operon comprise another (green), but the branching pattern cannot resolve which gene cluster came first. Unlike the majority of Shewanella species, S. frigidimarina contains a single FDH operon. The placement of S. frigidimarina within a clade subsequent to the original duplication suggests that its FDH region was once duplicated and has since been lost (Fig. 2). The phylogenetic analysis also highlighted a small subset of species, which includes S. halifaxensis, S. pealeana, S. waksmanii, and S. fidelis (highlighted in red text), indicating a second duplication event resulting in three copies of the FDH gene cluster in these species (Fig. 2). Overall, the phylogenetic analysis highlights the selection for maintenance of multiple gene regions involved in formate oxidation in the Shewanella genus.
FIG 2.
Molecular phylogenetic analysis of FdhA across the Shewanellaceae. Duplications of FDH regions are separated by clades in either blue or green text. Species containing a triplication of the FDH region are highlighted in red text. S. frigidimarina, which appears to have lost the duplicated region, is in black text. Numbers preceding the species names represent the gene identification number from the JGI database (http://img.jgi.doe.gov). The evolutionary history was inferred using the maximum-likelihood method (300 bootstrap replications) based on the general time-reversible model. The tree is drawn to scale with the scale bar representing substitutions per site. The analysis involved 88 nucleotide sequences, and there were a total of 2,844 positions in the final data set. The evolutionary analyses were conducted in MEGA6 (25).
Oxidation of endogenously generated formate increases the growth rate and yield of MR-1 during anaerobic respiration.
MR-1 produces formate when growing anaerobically on substrates that enter central metabolism at or above the level of pyruvate through the action of pyruvate formate lyase (Pfl) (Fig. 1A), an enzyme required for growth under anaerobic conditions with lactate and fumarate (28–30). Despite obligatory generation, formate does not accumulate in MR-1 culture supernatants, suggesting oxidation by one or more FDH complexes (28, 29). To determine the contribution of each FDH complex to formate oxidation, we generated strains lacking one, two, or all three regions encoding the α, β, and γ subunits of each of the three annotated FDH clusters (SO0101-0103, SO4509-4511, and SO4513-4515) (Fig. 1B).
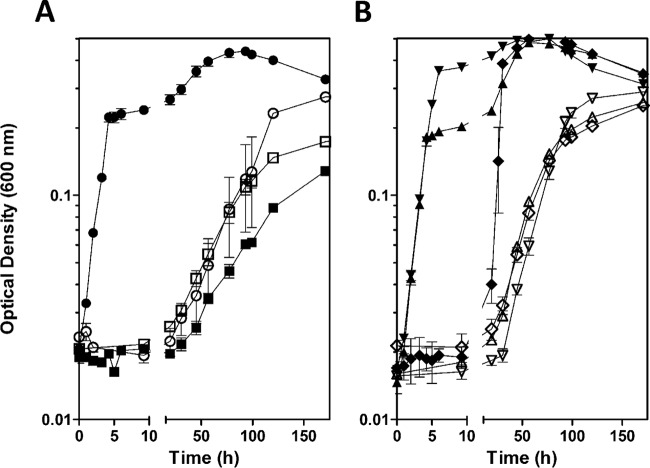
To determine the physiological significance of formate oxidation, we examined the growth rates and yields of MR-1 and a strain with all three FDH gene clusters deleted (Δfdh) in liquid culture. The strains were grown in SBM (see Materials and Methods) containing either 20 mM lactate or 20 mM pyruvate under an argon atmosphere supplemented with 40 mM fumarate. Growth of the mutant in the presence of oxygen, where formate is not generated, was indistinguishable from that of MR-1 (data not shown). In contrast, when grown anaerobically with fumarate, the Δfdh mutant grew slightly slower and generated less biomass than MR-1 both on lactate and pyruvate (Fig. 3). Under anaerobic conditions using fumarate as the electron acceptor, the generation times and final culture densities of MR-1 were comparable when grown on pyruvate (1.41 ± 0.04 h and 0.37 ± 0.00 OD600) or lactate (1.34 ± 0.07 h and 0.40 ± 0.01 OD600), indicating that the oxidation of lactate to pyruvate during carbon metabolism in MR-1 does not appreciably contribute to the growth rate or yield (Fig. 3). The oxidation of lactate to pyruvate by MR-1 occurs by way of two lactate dehydrogenases specific for either the d- or l-isomer (11, 13, 31). While a reduced menaquinol is likely generated, the difference in the redox potential between the lactate/pyruvate redox couple and the fumarate/succinate redox couple is small (−190 mV and 30 mV, respectively) and likely does not support energy conservation.
FIG 3.
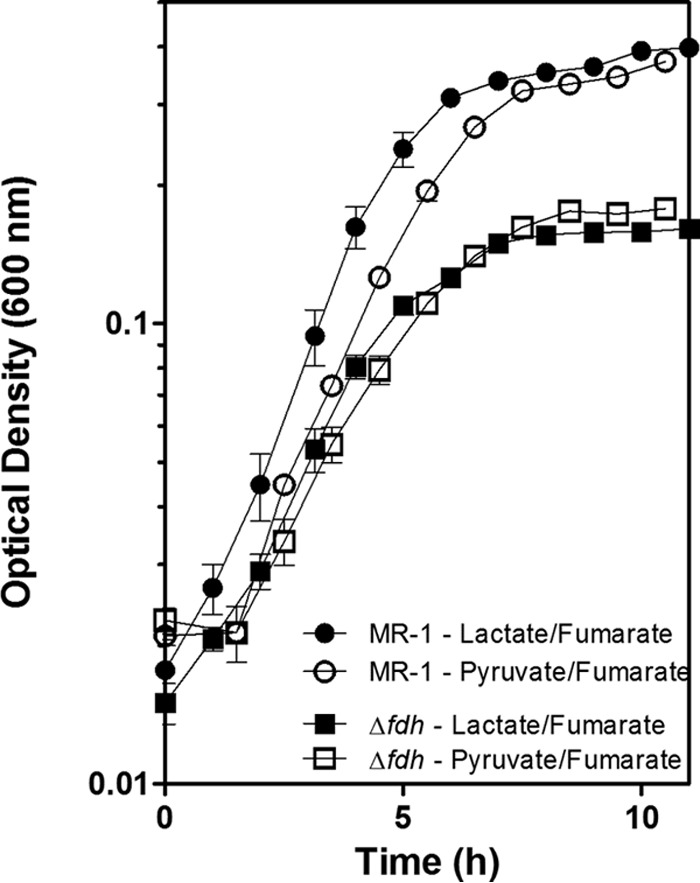
The ability to oxidize formate contributes to the growth rate and yield of MR-1. The growth curves of MR-1 and the Δfdh triple mutant were determined in anoxic SBM with 20 mM lactate or 20 mM pyruvate and 40 mM fumarate. The reported values are the averages from at least three independent experiments, and the error bars represent the standard errors of the mean (SEM).
During anaerobic growth on lactate and fumarate (Fig. 1A), MR-1 catalyzed nearly stoichiometric conversion of lactate to acetate and coupled oxidation of each lactate molecule to the reduction of two molecules of fumarate (Table 1). MR-1 utilizes the majority of the carbon for energy generation with very little being diverted to produce biomass. As had been observed previously (29), formate did not accumulate in MR-1 cultures (Table 1). Conversely, the Δfdh strain was predicted to couple lactate oxidation to fumarate reduction in a 1:1 ratio, as two reducing equivalents remain with nonoxidized formate (Fig. 1A). The Δfdh strain converted approximately 90% of the lactate linked to acetate formation to the generation of pyruvate and formate; however, the ratio of fumarate reduced was slightly higher than the stoichiometric ratio expected (Table 1). To determine if oxidation of pyruvate by the enzyme pyruvate dehydrogenase (PDH) (Fig. 1A) and subsequent electron flow to fumarate from NADH oxidation were occurring, the gene for the E1 decarboxylase component of the PDH complex, aceE, was deleted in the Δfdh strain background (referred to as Δfdh Δpdh). The Δfdh Δpdh strain coupled lactate utilization to fumarate reduction at ratios nearer to the 1:1 ratio expected (Table 1). No difference was observed in the anaerobic growth rate or yield of the Δfdh Δpdh strain compared with that of the Δfdh mutant (data not shown).
TABLE 1.
Carbon source utilization/production following anaerobic growth in SBM containing 20 mM lactate or pyruvate and 40 mM fumaratea
| Carbon source | Carbon source utilization/production (mM) by the indicated strain with: |
|||||
|---|---|---|---|---|---|---|
| 20 mM lactate-40 mM fumarate |
20 mM pyruvate-40 mM fumarate |
|||||
| MR-1 | Δfdh mutant | Δfdh Δpdh mutant | MR-1 | Δfdh mutant | Δfdh Δpdh mutant | |
| Lactate | −20.2 ± 0.0 | −14.7 ± 0.2 | −12.2 ± 0.2 | ND | ND | ND |
| Pyruvate | 0.8 ± 0.0 | 4.0 ± 0.1 | 4.0 ± 0.1 | −22.2 ± 0.0 | −13.0 ± 0.7 | −11.4 ± 0.2 |
| Acetate | 18.0 ± 0.3 | 12.0 ± 0.3 | 9.1 ± 0.1 | 19.7 ± 0.2 | 11.8 ± 0.3 | 8.1 ± 0.1 |
| Formate | ND | 7.1 ± 0.2 | 6.4 ± 0.1 | ND | 5.7 ± 0.6 | 5.8 ± 0.0 |
| Fumarate | −39.2 ± 0.0 | −18.3 ± 0.2 | −14.2 ± 0.2 | −20.4 ± 0.8 | −6.4 ± 2.2 | −2.0 ± 0.0 |
| Succinate | 38.2 ± 0.5 | 18.8 ± 0.6 | 13.6 ± 0.2 | 21.3 ± 0.4 | 4.9 ± 0.6 | 2.5 ± 0.1 |
Reported concentrations are averages ± SEM from three independent experiments. ND, not detected.
Because the majority of the anaerobic pyruvate disproportionation by MR-1 occurs via a lyase reaction catalyzed by Pfl, the only unbalanced redox reaction associated with pyruvate metabolism is the oxidation of formate (Fig. 1A). Therefore, during anaerobic growth with pyruvate and fumarate, MR-1 couples each molecule of pyruvate converted to acetate to one molecule of fumarate reduced to succinate (Table 1). Since the Δfdh strain cannot oxidize formate, it was predicted to convert pyruvate to formate and acetate with no subsequent reduction of fumarate (Fig. 1A). Contrary to predictions, the amount of formate measured in Δfdh culture supernatants was only half of that of acetate produced, and a significant amount of fumarate was still reduced to succinate (Table 1). In the Δfdh Δpdh strain, the ratios of formate and acetate produced were closer to the 1:1 ratio expected, and reduction of fumarate to succinate was also minimized (Table 1). To determine if the reduced growth rate and yield of the Δfdh mutant were due to the accumulation of formate in the culture medium, we repeated the experiment with the addition of 20 mM formate. The Δfdh strain showed an initial lag and grew at a slightly lower rate but to a similar final yield in the presence of exogenous formate (see Fig. S1 in the supplemental material). MR-1 was unaffected and grew similarly in both the presence and absence of added formate (see Fig. S1).
Oxidation of exogenous formate accelerates growth on suboptimal carbon sources.
Because the oxidation of formate clearly contributes to growth on lactate and pyruvate, we hypothesized that formate would also enhance growth on suboptimal carbon sources. Shewanella spp. readily utilize NAG as the sole carbon and energy source in the presence of oxygen (32); however, under anaerobic conditions, NAG is utilized very slowly with generation times of 24.50 ± 0.11 h and 37.47 ± 1.31 h for MR-1 and the Δfdh mutant, respectively (Fig. 4A) (28). Augmentation of NAG-grown anaerobic cultures with 20 mM formate resulted in a dramatically higher growth rate, with a generation time of 1.23 ± 0.08 h, comparable to that of lactate-grown cultures (Fig. 4A). Exogenous formate did not enhance the growth rate of the Δfdh strain on NAG but rather arrested growth prematurely (Fig. 4A).
FIG 4.
Oxidation of formate increases the growth rate of MR-1 on NAG. The growth curves were determined in the absence (open symbols) or presence (closed symbols) of 20 mM formate. (A) The growth curves of MR-1 (circles) and the Δfdh triple mutant (squares) were determined in anoxic SBM with 10 mM NAG and 60 mM fumarate. Note the prolonged time scale compared to that for growth on lactate or pyruvate in Fig. 2. (B) The growth curves of ΔSO0101-0103 ΔSO4509-4511 (downward facing triangles), ΔSO0101-0103 ΔSO4513-4515 (upward facing triangles), and ΔSO4509-4511 ΔSO4513-4515 (diamonds) mutants were determined as for panel A. The reported values are the averages from at least three independent experiments, and the error bars represent the SEM.
The enhanced growth rate of MR-1 using NAG and fumarate supplemented with formate provides a convenient assay for physiologically relevant formate oxidation by each FDH complex. The generation times of the ΔSO0101-0103 ΔSO4509-4511 and ΔSO0101-0103 ΔSO4513-4515 mutants on NAG decreased dramatically upon addition of 20 mM formate to 1.28 ± 0.11 h and 1.23 ± 0.01 h, respectively, mirroring the enhanced growth rate observed in MR-1 (Fig. 4B). The ΔSO4509-4511 ΔSO4513-4515 strain also responded to formate, but the growth rate was only partially enhanced with a generation time of 3.87 ± 0.84 h (Fig. 4B). Our results suggest that the complex encoded by SO0101-0103 does not contribute appreciably to the oxidation of formate during anaerobic growth on fumarate. The amino acid sequence encoded by SO0101, which is predicted to encode the catalytic subunit of the FDH complex, shares considerable similarity with that of FdnG (76%), the α subunit of the nitrate-inducible FDH of E. coli (Fdh-N). Proteins encoded by SO0101-0103 may play a larger role in formate oxidation in the presence of nitrate as transcription of SO0101-0103 was found to be significantly upregulated under nitrate-reducing conditions (33).
Elimination of formate oxidation enables growth on pyruvate without a terminal electron acceptor.
Bacteria from the genus Shewanella are generally thought to be respiratory organisms, requiring an electron acceptor for growth (1, 2, 28). It has also been reported previously that MR-1 is not capable of fermentative growth on pyruvate (12). Because formate oxidation is the only unbalanced redox step associated with pyruvate metabolism, we hypothesized that deletion of the FDHs would enable anaerobic growth by Shewanella solely on pyruvate (Fig. 1A). To test this, we cultured both MR-1 and the Δfdh mutant in anaerobic SBM supplemented with 20 mM pyruvate but lacking a terminal electron acceptor. We do not refer to this mode of growth as fermentation because pyruvate disproportionation occurs via a lyase reaction; hence, if the resulting formate is not oxidized, there is no need to cycle reducing equivalents. The Δfdh strain was able to grow under anaerobic conditions solely on pyruvate, while the MR-1 culture completed less than one doubling over 24 h (Fig. 5). Analysis of the culture supernatants by HPLC indicates that the Δfdh strain catalyzed a nearly stoichiometric conversion of pyruvate to acetate and formate, while only a small amount of pyruvate was converted to acetate by MR-1 with no terminal electron acceptor present (Table 2). The Δfdh strain consumed less than half of the available pyruvate before growth ceased, which was not due to low levels of pyruvate oxidation by PDH, as the Δfdh Δpdh strain grew similarly to the Δfdh strain (Fig. 5) and consumed similar amounts of pyruvate (Table 2). An inability to consume all of the available pyruvate may indicate inhibition of growth by the accumulated formate or an inability to cycle reducing equivalents in the other metabolic pathways required for growth.
FIG 5.
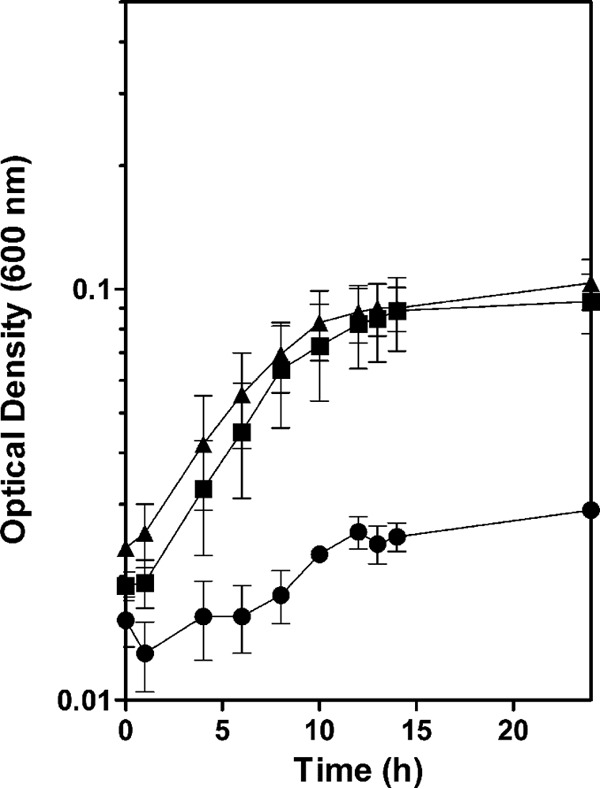
Growth of the Δfdh triple mutant on pyruvate without a terminal electron acceptor. The growth curves of MR-1 (circles), the Δfdh triple mutant (squares), and the Δfdh Δpdh triple mutant (triangles) were determined in anoxic SBM supplemented with 20 mM pyruvate. The reported values are the averages from at least three independent experiments, and the error bars represent the SEM.
TABLE 2.
Carbon source utilization/production following anaerobic growth in SBM containing 20 pyruvate with no terminal electron acceptora
| Carbon source | Carbon source utilization/production (mM) with 20 mM pyruvate by: |
||
|---|---|---|---|
| MR-1 | Δfdh mutant | Δfdh Δpdh mutant | |
| Pyruvate | −1.8 ± 0.4 | −6.6 ± 0.3 | −6.7 ± 0.6 |
| Lactate | ND | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Formate | ND | 5.0 ± 0.0 | 5.2 ± 0.1 |
| Acetate | 1.4 ± 0.1 | 4.1 ± 1.3 | 5.4 ± 0.2 |
The reported concentrations are averages ± SEM from three independent experiments. ND, not detected.
Formate oxidation by MR-1 generates PMF under anaerobic conditions.
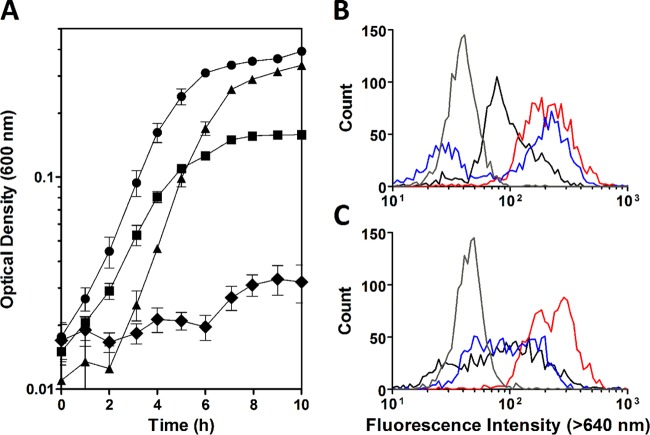
Under anaerobic conditions, MR-1 generates ATP primarily through substrate-level phosphorylation, and some of the ATP pool is utilized to generate PMF through proton pumping by the F-type ATPase (28). Formate oxidation linked to reduction of a terminal electron acceptor should also contribute to PMF as the FDHs are predicted to reduce menaquinone in a redox loop mechanism that transports protons across the cytoplasmic membrane (Fig. 1A) (10). To test this, we compared the growth of MR-1, the Δfdh strain, an Δatp mutant (a strain with a deletion of the entire ATP synthase operon SO4746-4754) (28), and a Δfdh Δatp mutant in SBM supplemented with 20 mM lactate and 40 mM fumarate. The Δatp strain exhibited an initial lag phase but otherwise grew similarly to MR-1 while the Δfdh strain grew at a lower rate and to a lower OD than MR-1 (Fig. 6A). Elimination of all three FDHs and the F-type ATPase severely inhibited growth, with strains completing less than one doubling over 13 h (Fig. 6A). The lower yield of the Δfdh strain and severely inhibited growth of the Δfdh Δatp strain with lactate and fumarate indicate that formate oxidation and proton pumping through the reversal of the F-type ATPase are the main contributors to the generation of PMF by MR-1 under anaerobic conditions.
FIG 6.
PMF is generated by the anaerobic oxidation of formate in MR-1. (A) The growth curves of MR-1 (circles), the Δfdh triple mutant (squares), the Δatp mutant (triangles), and the Δfdh Δatp mutant (diamonds) were performed in anoxic SBM with 20 mM lactate and 40 mM fumarate. The reported values are the averages from at least three independent experiments, and the error bars represent the SEM. (B and C) Histograms from flow cytometry measurement of red fluorescence intensity using the dye DiOC2 for MR-1 (B) and the Δfdh mutant (C) in anaerobic SBM containing 20 mM fumarate supplemented with no carbon source (black lines), 10 mM formate (blue lines), 10 mM lactate (red lines), or 10 mM lactate followed by addition of 5 μM CCCP (gray lines). Flow cytometry was performed on three independent cultures for each condition, and representative data are shown.
To directly measure the generation of the membrane potential resulting from formate oxidation, the fluorescence intensities of cells exposed to DiOC2 were monitored using flow cytometry. Cultures of MR-1 and the Δfdh mutant were incubated in anaerobic minimal medium lacking a carbon source to deenergize the membrane potential. Following starvation, cells incubated in anaerobic medium with fumarate in the presence or absence of an electron donor were stained with DiOC2, and fluorescence intensities were assayed. The positively charged DiOC2 exhibits green fluorescence that shifts toward red as the cytosolic concentration of the dye increases and aggregates in response to an interior net-negative membrane potential. In the presence of lactate, both the MR-1 and Δfdh cultures produced a population of energized cells (higher fluorescence intensity) compared with starved cells (lower fluorescence intensity) or cells depolarized by the addition of the protonophore CCCP (Fig. 6B and C). Conversely, in the presence of formate as the electron donor, MR-1 cultures produced a population of energized cells, whereas the c strain remained deenergized (Fig. 6B and C). Because cells are growing in the presence of lactate and fumarate, the initial population of starved cells is diluted out, while both populations of starved and charged cells can be quantified when MR-1 is given both formate and fumarate (Fig. 6B), a condition where MR-1 is metabolically active but unable to grow (data not shown). Regardless, the presence of an energized cell population in MR-1 but not Δfdh cultures incubated with formate indicates that PMF is generated through formate oxidation by FDH under the conditions tested.
DISCUSSION
The development and rational engineering of MR-1 for applications in biotechnology require a clear understanding of its carbon source utilization and energy metabolism. Although formate is obligately produced by MR-1 under anaerobic conditions during the disproportionation of pyruvate by Pfl, little is known about the role formate plays in the ecophysiology of MR-1 or other members of the genus Shewanella. Beyond formate production, the presence of multiple genetic loci linked to its metabolism serves to emphasize the importance of formate in the core carbon metabolism of MR-1 and other members of the genus Shewanella. To further address the role of formate oxidation, we generated mutants lacking one, two, or all three regions encoding the α, β, and γ subunits of each of the three annotated FDH clusters (SO0101-0103, SO4509-4511, and SO4513-4515) (Fig. 1B). We then monitored the growth phenotypes of these strains during the oxidation of various carbon sources.
We have shown that oxidation of formate contributes to both the growth rate and yield of MR-1 on a variety of carbon sources under anaerobic conditions with fumarate as the terminal electron acceptor. During anaerobic growth with fumarate and either lactate or pyruvate, the strains unable to oxidize formate (Δfdh mutants) grew at a slightly lower rate and to an overall lower yield than MR-1 (Fig. 3). HPLC analysis indicated that, unlike MR-1, the strains lacking FDH complexes accumulated formate in the supernatant, and the ratio of formate secreted was closer to the predicted 1:1 ratio with acetate when the gene for the E1 decarboxylase component of the PDH complex, aceE, was also deleted (in the Δfdh Δpdh strain) (Table 1). Previous work reported no measurable activity by PDH in MR-1 extracts under anaerobic conditions (29); however, our HPLC analyses indicate that while the majority of pyruvate is routed through Pfl, PDH remains active, albeit at low levels or perhaps slightly induced in the absence of Pfl, under anaerobic conditions (Table 1). The low levels of PDH activity may be in response to the Δfdh strain's inability to oxidize formate. It is also interesting that the amount of formate “missing” (∼2 mM) for the Δfdh Δpdh strain grown anaerobically on pyruvate or lactate (based on the predicted 1:1 ratio of formate and acetate secreted) is equivalent to the concentration of “extra” fumarate reduced to succinate (∼2 mM) (Table 1). Taken together, these results indicate that either formate is oxidized by an unidentified FDH or formate is converted to another product capable of being oxidized by the cell. It is also possible that once secreted formate reaches a maximal concentration, it is no longer effectively trafficked out of the cytoplasm. In E. coli, when the medium pH drops below 6.8, the transporter FocA switches from passive formate export to active formate import (34). The imported formate is then converted to dihydrogen via FHL to control both cellular pH and excess reducing power (34).
Formate transport and oxidation appear to be central metabolic processes in MR-1, and the inability of the Δfdh strain to oxidize formate helped to answer another main question related to the metabolism of MR-1. Pyruvate has been shown to enhance the survival of MR-1 in stationary phase, and MR-1 contains lactate dehydrogenases capable of pyruvate reduction (13, 29). Previous work has also shown that the majority of ATP is produced through substrate-level phosphorylation by MR-1 under anaerobic conditions (28). Therefore, it has remained unclear why pyruvate cannot support fermentative growth of MR-1. Our data show that pyruvate sustains growth of the Δfdh strain in anaerobic medium without a terminal electron acceptor (Fig. 5). Growth solely on pyruvate by the Δfdh strain suggests that, in the absence of exogenous electron acceptors, formate oxidation by MR-1 leads to an irreversible reduction of the menaquinone pool and subsequent cessation of growth. It is curious that MR-1 cultures do not simply secrete formate in the absence of a terminal electron acceptor similar to the Δfdh strain (Table 2). The inability of MR-1 to secrete formate, which would enable growth, highlights the critical importance of formate oxidation in the energy metabolism of MR-1. Consistent with this observation, we also demonstrated that formate oxidation directly contributes to generation of a proton gradient in MR-1 (Fig. 6).
Exogenous formate also greatly accelerated the growth rate, likely due to enhanced PMF, of MR-1 on the suboptimal carbon source NAG under anaerobic conditions with fumarate (Fig. 4A). Experiments utilizing double mutants indicate that this increase in the growth rate was mainly due to the activity by the FDHs encoded by SO4509-4511 and SO4513-4515 (Fig. 4B). While the final OD values were similar for mutants with SO4509-4511 or SO4513-4515 present, strains containing SO4509-4511 reached the maximum OD within the first 6 h of growth, which may indicate a difference in efficiency or affinity for formate by each complex (Fig. 4B). It is interesting that the two FDH complexes that appear to have the largest effect on growth are the complexes that arose from a gene duplication event preceding the current identification to the species level of the Shewanella genus (Fig. 2). A previous analysis of 106 bacterial genomes found that gene duplications tend to be retained when they are important for adapting to constantly changing environments (35). Indeed, a hallmark of the Shewanella genus is the ability to thrive in redox-stratified environments where conditions are in a constant state of flux, and this may explain the occurrence of multiple paralogs enabling modularity in respiratory pathways (36) and now formate metabolism. Presumably, the preservation of multiple FDH operons confers an evolutionary advantage to members of the Shewanella genus, perhaps through an affinity for formate across a range of available concentrations or through the efficiency of formate oxidation with different terminal electron acceptors. Maintenance of the duplication or triplication of this FDH region in the vast majority of Shewanella species serves to highlight the centrality of formate in energy conservation by this genus.
Supplementary Material
ACKNOWLEDGMENTS
We thank Antony Dean (University of Minnesota) for help with phylogenetic analysis and Jeffrey Flynn (University of Minnesota) for generating some of the FDH mutant strains. We also thank Daniel Bond (University of Minnesota) and Clive Butler (University of Exeter) for helpful discussions and for critical review of the manuscript.
This work was supported by the Office of Naval Research (awards N000141210309 and N000141310552 to J.A.G.). R.M. and N.J.K. were supported in part by the University of Minnesota Biotechnology Training Grant Program through the National Institutes of Health.
Funding Statement
R.M. and N.J.K. were supported in part by the University of Minnesota Biotechnology Training Grant Program through the National Institutes of Health.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00927-15.
REFERENCES
- 1.Nealson KH, Scott J. 2006. Ecophysiology of the genus Shewanella, p 1133–1151. In Falkow S, Schleifer K-H, Stacebrandt E, Dworkin M (ed). The prokaryotes: a handbook on the biology of bacteria, 3rd ed, vol 6 Springer, New York, NY. [Google Scholar]
- 2.Hau HH, Gralnick JA. 2007. Ecology and biotechnology of the genus Shewanella. Annu Rev Microbiol 61:237–258. doi: 10.1146/annurev.micro.61.080706.093257. [DOI] [PubMed] [Google Scholar]
- 3.Fredrickson JK, Romine MF, Beliaev AS, Auchtung JM, Driscoll ME, Gardner TS, Nealson KH, Osterman AL, Pinchuk G, Reed JL, Rodionov DA, Rodrigues JLM, Saffarini DA, Serres MH, Spormann AM, Zhulin IB, Tiedje JM. 2008. Towards environmental systems biology of Shewanella. Nat Rev Microbiol 6:592–603. doi: 10.1038/nrmicro1947. [DOI] [PubMed] [Google Scholar]
- 4.Marritt SJ, Lowe TG, Bye J, McMillan DGG, Shi L, Fredrickson J, Zachara J, Richardson DJ, Cheesman MR, Jeuken LJC, Butt JN. 2012. A functional description of CymA, an electron-transfer hub supporting anaerobic respiratory flexibility in Shewanella. Biochem J 444:465–474. doi: 10.1042/BJ20120197. [DOI] [PubMed] [Google Scholar]
- 5.Myers J, Myers C. 2000. Role of the tetraheme cytochrome CymA in anaerobic electron transport in cells of Shewanella putrefaciens MR-1 with normal levels of menaquinone. J Bacteriol 182:67–75. doi: 10.1128/JB.182.1.67-75.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marritt SJ, McMillan DGG, Shi L, Fredrickson JK, Zachara JM, Richardson DJ, Jeuken LJC, Butt JN. 2012. The roles of CymA in support of the respiratory flexibility of Shewanella oneidensis MR-1. Biochem Soc. Trans. 40:1217–1221. doi: 10.1042/BST20120150. [DOI] [PubMed] [Google Scholar]
- 7.Beliaev A, Saffarini D. 1998. Shewanella putrefaciens mtrB encodes an outer membrane protein required for Fe(III) and Mn(IV) reduction. J Bacteriol 180:6292–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beliaev A, Saffarini D, McLaughlin J, Hunnicutt D. 2001. MtrC, an outer membrane decahaem c cytochrome required for metal reduction in Shewanella putrefaciens MR-1. Mol Microbiol 39:722–730. doi: 10.1046/j.1365-2958.2001.02257.x. [DOI] [PubMed] [Google Scholar]
- 9.Hartshorne RS, Reardon CL, Ross D, Nuester J, Clarke TA, Gates AJ, Mills PC, Fredrickson JK, Zachara JM, Shi L, Beliaev AS, Marshall MJ, Tien M, Brantley S, Butt JN, Richardson DJ. 2009. Characterization of an electron conduit between bacteria and the extracellular environment. Proc Natl Acad Sci U S A 106:22169–22174. doi: 10.1073/pnas.0900086106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simon J, van Spanning RJM, Richardson DJ. 2008. The organisation of proton motive and non-proton motive redox loops in prokaryotic respiratory systems. Biochim Biophys Acta 1777:1480–1490. doi: 10.1016/j.bbabio.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Brutinel ED, Gralnick JA. 2012. Preferential utilization of d-lactate by Shewanella oneidensis. Appl Environ Microbiol 78:8474–8476. doi: 10.1128/AEM.02183-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meshulam-Simon G, Behrens S, Choo AD, Spormann AM. 2007. Hydrogen metabolism in Shewanella oneidensis MR-1. Appl Environ Microbiol 73:1153–1165. doi: 10.1128/AEM.01588-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinchuk GE, Rodionov DA, Yang C, Li X, Osterman AL, Dervyn E, Geydebrekht OV, Reed SB, Romine MF, Collart FR, Scott JH, Fredrickson JK, Beliaev AS. 2009. Genomic reconstruction of Shewanella oneidensis MR-1 metabolism reveals a previously uncharacterized machinery for lactate utilization. Proc Natl Acad Sci U S A 106:2874–2879. doi: 10.1073/pnas.0806798106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpentier W, Sandra K, De Smet I, Brige A, De Smet L, Van Beeumen J. 2003. Microbial reduction and precipitation of vanadium by Shewanella oneidensis. Appl Environ Microbiol 69:3636–3639. doi: 10.1128/AEM.69.6.3636-3639.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Windt W, Boon N, Van den Bulcke J, Rubberecht L, Prata F, Mast J, Hennebel T, Verstraete W. 2006. Biological control of the size and reactivity of catalytic Pd(0) produced by Shewanella oneidensis. Antonie Van Leeuwenhoek 90:377–389. doi: 10.1007/s10482-006-9088-4. [DOI] [PubMed] [Google Scholar]
- 16.Hong Y, Gu J. 2010. Physiology and biochemistry of reduction of azo compounds by Shewanella strains relevant to electron transport chain. Appl Microbiol Biotechnol 88:637–643. doi: 10.1007/s00253-010-2820-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers C, Carstens B, Antholine W, Myers J. 2000. Chromium(VI) reductase activity is associated with the cytoplasmic membrane of anaerobically grown Shewanella putrefaciens MR-1. J Appl Microbiol 88:98–106. doi: 10.1046/j.1365-2672.2000.00910.x. [DOI] [PubMed] [Google Scholar]
- 18.Ruebush S, Brantley S, Tien M. 2006. Reduction of soluble and insoluble iron forms by membrane fractions of Shewanella oneidensis grown under aerobic and anaerobic conditions. Appl Environ Microbiol 72:2925–2935. doi: 10.1128/AEM.72.4.2925-2935.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nealson K, Moser D, Saffarini D. 1995. Anaerobic electron-acceptor chemotaxis in Shewanella putrefaciens. Appl Environ Microbiol 61:1551–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leonhartsberger S, Korsa I, Bock A. 2002. The molecular biology of formate metabolism in enterobacteria. J Mol Microbiol Biotechnol 4:269–276. [PubMed] [Google Scholar]
- 21.Jormakka M, Tornroth S, Byrne B, Iwata S. 2002. Molecular basis of proton motive force generation: structure of formate dehydrogenase-N. Science 295:1863–1868. doi: 10.1126/science.1068186. [DOI] [PubMed] [Google Scholar]
- 22.Balch W, Wolfe R. 1976. New approach to cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (Hs-Com)-dependent growth of Methanobacterium ruminantium in a pressurized atmosphere. Appl Environ Microbiol 32:781–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coursolle D, Baron DB, Bond DR, Gralnick JA. 2010. The Mtr respiratory pathway is essential for reducing flavins and electrodes in Shewanella oneidensis. J Bacteriol 192:467–474. doi: 10.1128/JB.00925-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edgar R. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol. Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Felsenstein J. 1985. Confidence-limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791. doi: 10.2307/2408678. [DOI] [PubMed] [Google Scholar]
- 27.Nei M, Kumar S. 2000. Molecular evolution and phylogenetics. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 28.Hunt KA, Flynn JM, Naranjo B, Shikhare ID, Gralnick JA. 2010. Substrate-level phosphorylation is the primary source of energy conservation during anaerobic respiration of Shewanella oneidensis strain MR-1. J Bacteriol 192:3345–3351. doi: 10.1128/JB.00090-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinchuk GE, Geydebrekht OV, Hill EA, Reed JL, Konopka AE, Beliaev AS, Fredrickson JK. 2011. Pyruvate and lactate metabolism by Shewanella oneidensis MR-1 under fermentation, oxygen limitation, and fumarate respiration conditions. Appl Environ Microbiol 77:8234–8240. doi: 10.1128/AEM.05382-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flynn CM, Hunt KA, Gralnick JA, Srienc F. 2012. Construction and elementary mode analysis of a metabolic model for Shewanella oneidensis MR-1. Biosystems 107:120–128. doi: 10.1016/j.biosystems.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Chai Y, Kolter R, Losick R. 2009. A widely conserved gene cluster required for lactate utilization in Bacillus subtilis and Its involvement in biofilm formation. J Bacteriol 191:2423–2430. doi: 10.1128/JB.01464-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang C, Rodionov DA, Li X, Laikova ON, Gelfand MS, Zagnitko OP, Romine MF, Obraztsova AY, Nealson KH, Osterman AL. 2006. Comparative genomics and experimental characterization of N-acetylglucosamine utilization pathway of Shewanella oneidensis. J Biol Chem 281:29872–29885. doi: 10.1074/jbc.M605052200. [DOI] [PubMed] [Google Scholar]
- 33.Beliaev A, Klingeman D, Klappenbach J, Wu L, Romine M, Tiedje J, Nealson K, Fredrickson J, Zhou J. 2005. Global transcriptome analysis of Shewanella oneidensis MR-1 exposed to different terminal electron acceptors. J Bacteriol 187:7138–7145. doi: 10.1128/JB.187.20.7138-7145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sawers R. 2005. Formate and its role in hydrogen production in Escherichia coli. Biochem Soc Trans 33:42–46. doi: 10.1042/BST0330042. [DOI] [PubMed] [Google Scholar]
- 35.Gevers D, Vandepoele K, Simillion C, Van de Peer Y. 2004. Gene duplication and biased functional retention of paralogs in bacterial genomes. Trends Microbiol 12:148–154. doi: 10.1016/j.tim.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Coursolle D, Gralnick JA. 2010. Modularity of the Mtr respiratory pathway of Shewanella oneidensis strain MR-1. Mol Microbiol 77:995–1008. doi: 10.1111/j.1365-2958.2010.07266.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.