Abstract
Pseudomonas fluorescens isolates Clinto 1R, Wayne 1R, and Wood 1R, which produce the antibiotic 2,4-diacetylphloroglucinol (DAPG), can suppress soilborne diseases and promote plant growth. Consequently, these beneficial bacterial isolates were tested on watermelon plants for suppression of Meloidogyne incognita (root-knot nematode: RKN) and Fusarium oxysporum f. sp. niveum (Fon). In a greenhouse trial, Wayne 1R root dip suppressed numbers of RKN eggs per gram root on ‘Charleston Gray’ watermelon by 28.9%. However, in studies focused on ‘Sugar Baby’ watermelon, which is commercially grown in Maryland, a Wayne 1R root dip did not inhibit RKN reproduction or plant death caused by Fon. When all three isolates were applied as seed coats, plant stand in the greenhouse was reduced up to 60% in treatments that included Fon ± P. fluorescens, and eggs per gram root did not differ among treatments. In a microplot trial with Clinto 1R and Wayne 1R root dips, inoculation with P. fluorescens and/or Fon resulted in shorter vine lengths than treatment with either P. fluorescens isolate plus RKN. Root weights, galling indices, eggs per gram root, and second-stage juvenile (J2) numbers in soil were similar among all RKN-inoculated treatments, and fruit production was not affected by treatment. Plant death was high in all treatments. These studies demonstrated that the tested P. fluorescens isolates resulted in some inhibition of vine growth in the field, and were not effective for enhancing plant vigor or suppressing RKN or Fon on watermelon.
Keywords: beneficial bacteria, biological control, Citrullus lanatus var. lanatus, DAPG, Fusarium oxysporum, Fusarium wilt, management, Meloidogyne incognita, Pseudomonas fluorescens, root-knot nematode, watermelon
Watermelon is an economically important vegetable crop in Maryland, and Fusarium wilt caused by Fon is the major disease of this crop (Zhou and Everts, 2006). Host resistance is the primary approach for managing the pathogen, but watermelon cultivars generally lack resistance to all races of the fungus, creating a need for supplementary management practices. In addition, RKN infestation causes crop losses on watermelon, and can also increase damage caused by Fusarium wilt (Thies et al., 2010).
A potential method for suppressing these pathogens is to apply, or encourage growth of, plant growth–promoting rhizobacteria. Many isolates of P. fluorescens fall into this group, and are efficacious for improving plant growth and yields and for suppressing soilborne plant pathogens (McSpadden Gardener, 2007; Weller, 2007; Lim et al., 2012). The beneficial effects of these bacteria can be caused by multiple factors, including production of secondary metabolites. The antibiotic DAPG is one of these metabolites, and can affect plant vigor, including root weights and lengths and root branching (De Leij et al., 2002; Brazelton et al., 2008); exhibit antagonistic activity against bacteria, fungi, nematodes, oomycetes, plants, and viruses (Delany et al., 2001; De Souza et al., 2003; Weller et al., 2007; Zhou et al., 2014); and trigger induced systemic resistance (ISR) (Iavicoli et al., 2003; Siddiqui and Shaukat, 2003a; Bakker et al., 2007; Weller et al., 2012).
Fusarium wilt can be suppressed by DAPG-producing strains of P. fluorescens. Pseudomonas fluorescens CHA0 (genotype A; McSpadden Gardener et al., 2000) was active against F. oxysporum f. sp. cucumerinum on cucumber, with enhanced protection from the antibiotic-overproducing strain CHA0/pME3090 (Maurhofer et al., 1995). Strain CHA0 and the complemented mutant CHA625/pME3128 were more effective than the DAPG-deficient strain CHA625 for inhibiting growth of F. oxysporum on agar, although CHA625 could still produce hydrogen cyanide (HCN) and pyoluteorin (Keel et al., 1992). The same study also demonstrated that synthetic DAPG was suppressive to F. oxysporum f. sp. lycopersici and f. sp. lini in vitro. Isolation of DAPG-producing P. fluorescens from roots of pea plants growing in soils suppressive to Fusarium wilt (F. oxysporum f. sp. pisi) indicated that these bacteria were involved in suppression of the pathogen, with the D genotype predominating in the rhizosphere (Landa et al., 2002). Pseudomonas fluorescens Pf4-92 reduced F. oxysporum f. sp. ciceri on chickpea (Saikia et al., 2009), and P. fluorescens Pf-1 was active against F. oxysporum f. sp. lycopersici on tomato and F. oxysporum f. sp. cubense on banana (Manikandan et al., 2010; Selvaraj et al., 2014). Pseudomonas fluorescens strain J2 and the DAPG-overproducing mutant J2-phlF- were both inhibitory to growth of F. oxysporum in vitro (Zhou et al., 2014). In contrast, Mazurier et al. (2009) found that DAPG production was not a primary cause for suppression of Fusarium wilt on flax; the activity was attributed to production of phenazine by Pseudomonas spp. combined with carbon uptake by nonpathogenic F. oxysporum (Mazurier et al., 2009).
In vitro exposure of nematodes to DAPG indicated that effects range from lethal to stimulatory, depending on the nematode taxon and life stage. Synthetic DAPG was toxic to adults of the plant-parasitic nematode Xiphinema americanum, but with RKN, hatch of J2 from eggs was suppressed, whereas J2 activity was not (Meyer et al., 2009). The antibiotic 2,4-diacetylphloroglucinol did not reduce viability of Heterodera glycines or Pratylenchus scribneri, or of the bacterial-feeding nematodes Pristionchus pacificus and Rhabditis rainai. Caenorhabditis elegans hatch was stimulated by the compound, while first-stage juvenile (J1) viability was not affected (Meyer et al., 2009). In vitro assays with Globodera rostochiensis demonstrated that synthetic DAPG increased hatch of the potato cyst nematode, but decreased mobility of hatched J2 (Cronin et al., 1997).
Application of DAPG-producing P. fluorescens to soil and roots can also be inhibitory to soilborne plant-parasitic nematodes, with other compounds such as AprA protease, HCN, and pyoluteorin involved in nematode suppression (Siddiqui et al., 2005, 2006; Neidig et al., 2011). Strain CHA0 was active against different species of Meloidogyne on several host crops (Siddiqui and Shaukat, 2002, 2003a, 2003b). The authors concluded that activity of CHA0 against nematodes was in part due to production of the secondary metabolites DAPG, HCN, and pyoluteorin (Siddiqui and Shaukat, 2004). Pseudomonas fluorescens Pf-1 suppressed Helicotylenchus multicinctus on banana (Selvaraj et al., 2014). The DAPG-producing isolate Wood 1R (D genotype) had varied effects on nematode suppression, depending on the nematode genus and the host crop (Timper et al., 2009). Although measurements of in situ DAPG production had been reported (Bonsall et al., 1997), it is unclear whether strains with the genes to produce this secondary metabolite do so in the rhizosphere of all crops to which they are applied.
Because of the demonstrated activity of DAPG-producing pseudomonads and DAPG against F. oxysporum and RKN, this study was conducted to determine efficacy of several genetically diverse isolates of P. fluorescens against these pathogens on watermelon. There are 22 known genotypes of P. fluorescens that produce DAPG (Weller et al., 2012), and three were selected for comparison in our study. The strains used in this investigation were Clinto 1R (genotype S, isolated from corn/soybeans), Wayne 1R (genotype A, from corn/soybeans), and Wood 1R (genotype D, from corn) (McSpadden Gardener et al., 2005). All of these strains were isolated from corn and soybean fields in Ohio and had demonstrated some activity against multiple plant pathogens (McSpadden Gardener et al., 2005). Wood 1R had also been shown to have some activity against pathogenic nematodes on corn (Timper et al., 2009). Each isolate was therefore tested for suppression of Fon and RKN applied as individual pathogens on watermelon plants. In addition, each of the three isolates was investigated for activity against combined infection with both pathogens on watermelon, thus determining whether any suppressive effects were altered by the increased disease pressure.
Materials and Methods
Meloidogyne, Fusarium, and Pseudomonas cultures and watermelon cultivars:
Cultures of M. incognita Race 1, originally collected in Maryland, were maintained on greenhouse-grown pepper (Capsicum annuum) ‘PA-136.’ Eggs were collected as described in Meyer et al. (2008). Watermelon (Citrullus lanatus var. lanatus) cv. Sugar Baby was grown for most experiments; ‘Charleston Gray’ was used for one greenhouse trial with P. fluorescens Wayne 1R. For culture maintenance and all greenhouse experiments, the greenhouse was maintained at 24°C to 29°C, with natural and supplemental lighting combined for a 16-hr daylength.
For greenhouse experiments, Fon race 1 isolate F-030-1 was grown 10 to 14 d in nutrient broth (NB; Difco, Becton, Dickinson and Company, Sparks, MD) or liquid mineral salt medium (Netzer, 1976) at 150 rpm at room temperature, and the cultures then filtered through four layers of cheesecloth. For the microplot trial, Fon isolate F-030-1 was grown on potato dextrose agar (PDA; Difco, Becton, Dickinson and Company) for 1 wk, a 1-cm disc was removed and placed in a 750 ml sterile mixture of 1% soy hull fiber in distilled water, and the culture then placed on a shaker at 150 rpm for 11 d. Inoculum was harvested by homogenizing the soy hull fiber culture and filtering through three layers of cheesecloth.
Pseudomonas fluorescens Clinto 1R, Wayne 1R, and Wood 1R (Ohio State University) were cultured on 1/10 tryptic soy broth (TSB) or 1/10 tryptic soy agar (TSA) for 24 to 48 hr. For the greenhouse trial with ‘Charleston Gray’ watermelon, Wayne 1R was cultured on the Pseudomonas-selective medium ⅓ × King’s medium B+++ (⅓ × KMB+++) (McSpadden Gardener et al., 2001) for 24 hr.
To isolate P. fluorescens from roots and soil, roots were rinsed and cut into pieces, and 1 to 3 g of each root system, or 1.0 g soil per pot, was placed into 15 ml sterile deionized water (SDW) in scintillation vials, sonicated 1 min and vortexed 1 min. Serial dilutions and subsequent colony counts on Pseudomonas-selective agar were conducted with procedures similar to those described in McSpadden Gardener et al. (2001). Genotyping of P. fluorescens cultures was performed by PCR amplification of the gene phlD using primers B2BF and BPR4 followed by digestion of the amplicon with restriction enzyme HaeIII as described previously (McSpadden-Gardener et al., 2001).
Greenhouse: Wayne 1R applied as a root dip:
Several greenhouse trials were conducted focusing on one isolate, Wayne 1R. For an initial experiment with ‘Charleston Gray’ watermelon, Wayne 1R was scraped from culture plates, suspended in SDW and diluted to 1 × 107 CFU/ml. Roots of 10-d-old seedlings (originally planted in sand) were dipped in Wayne 1R suspension or in water as a control, and then transplanted into Cone-tainers (Ray Leach Cone-tainers, RLC4, Stuewe & Sons Inc., Tangent, OR; 2.5 cm diam., 16 cm deep) containing moist, steam-pasteurized sand. Root-knot nematode-inoculated Cone-tainers each received 5,000 RKN eggs in 1 ml water (pipetted into the sand). The treatment combinations were Wayne 1R + RKN and Water + RKN. There were 10 replicate plants per treatment, and the experiment was not repeated. The plants were harvested 1 mon after transplant. Root fresh weights and numbers of galls on roots were recorded. Nematode eggs were collected from roots and counted following procedures described in Meyer et al. (2008).
Subsequent greenhouse trials with Wayne 1R were conducted with the commercially grown watermelon cv. Sugar Baby. ‘Sugar Baby’ watermelon seeds were planted into steamed starter mix (Premier Promix; Premier Horticulture, Quakertown, PA), and seedlings were transplanted 1 mon later into 10-cm-diam. pots; one seedling per pot, with each pot containing ca. 400 g of steamed, air-dried, enriched greenhouse soil mixture (16 sand:9 compost, v/v; 83.1% sand, 6.4% silt, 10.5% clay, pH 6.9; 0.8% organic matter).
At transplant, seedlings that received P. fluorescens were rinsed in water and then dipped into Wayne 1R that had been cultured for 2 d on 1/10 TSA, scraped from the plates, and prepared at ca. 5 × 108 CFU/ml SDW. Plants without Wayne 1R were dipped in water only. Fon was cultured for 10 to 11 d in mineral salts broth, and 5 ml Fon (1.6 × 106 mixed microspores and macrospores/ml water) or 5 ml water control, was then distributed among three holes around the base of each plant. For RKN treatments, 5,000 eggs suspended in 1 ml SDW were added to one hole per pot; control pots received water. The eight treatments were (i) untreated control, (ii) Fon, (iii) RKN, (iv) Fon + RKN, (v) Wayne 1R, (vi) Wayne 1R + Fon, (vii) Wayne 1R + RKN, and (viii) Wayne 1R + Fon + RKN. Ten replicate plants were used per treatment, and the pots were arranged in a randomized complete block design. The experiment was conducted twice. Plants were fertilized as needed with Osmocote Plus 15N–9P–12K fertilizer (Scotts-Sierra Horticultural Products Co., Marysville, OH).
Both trials were harvested 7 wk after transplant. At harvest, shoot height, shoot fresh weight, Fusarium wilt, and plant stand were recorded. Roots and soil were removed from pots, the roots were rinsed, and root fresh weights and root galling indices (Daulton and Nusbaum, 1961) were recorded. Root galling index values were 0 = no galls, 1 = less than 5 galls, 5 = 5 to 25 galls, 10 = 26 to 100 galls, and 25 = more than 100 galls. Roots were stored at 4°C until determination of RKN egg population numbers. Presence or absence of Fon was determined by excising three 0.2-cm–diam. pieces of plant tissue from a 2-cm-long piece removed from the bottom of each stem. Tissue was surface sterilized for 3 min in 20% bleach solution, and plated onto PDA. After 7 d, plates were assessed for fungal growth and the presence of Fon was determined based on morphology. To check for the presence of Wayne 1R, root washes were made from root systems of five Pseudomonas-treated plants and five untreated plants, as described above.
Greenhouse: Clinto 1R, Wayne 1R, and Wood 1R applied as seed treatments:
Procedures were similar to those in the greenhouse trials described above, except that three P. fluorescens isolates were used, and all were applied as seed treatments. Pseudomonas fluorescens Clinto 1R, Wayne 1R, and Wood 1R were scraped from 1/10 TSA plates and suspended in SDW. For each P. fluorescens isolate, 100 seeds of ‘Sugar Baby’ watermelon were treated with 1 ml bacterial suspension of 1 × 107 CFU/ml in SDW (seeds and bacterial suspension were shaken together in a plastic bag for 5 min) to obtain ca. 6 × 105 CFU/seed. The Pseudomonas-treated seeds and untreated seeds were planted into the steamed starter mix. One month later, watermelon seedlings were transplanted into 10-cm-diam. pots. There were 20 treatments: (i) Untreated control (no Fon, RKN or P. fluorescens), (ii) Fon in NB, (iii) RKN, (iv) Fon + RKN, (v) Clinto 1R, (vi) Clinto 1R + Fon, (vii) Clinto 1R + RKN, (viii) Clinto 1R + Fon + RKN, (ix) Wayne 1R, (x) Wayne 1R + Fon, (xi) Wayne 1R + RKN, (xii) Wayne 1R + Fon + RKN, (xiii) Wood 1R, (xiv) Wood 1R + Fon, (xv) Wood 1R + RKN, (xvi) Wood 1R + Fon + RKN, (xvii) NB, (xviii) Clinto 1R + NB, (xix) Wayne 1R + NB, and (xx) Wood 1R + NB. For treatments with one or both pathogens, RKN and Fon were pipetted into holes made in the soil near the plants on the day of transplant. Soil inoculated with RKN received 5,000 eggs in 1 ml water/pot; control pots received water. Soil inoculated with Fon received 10 ml/pot (1.5 × 106 mixed microspores and macrospores/ml suspended in NB); control pots received 10 ml NB or water. Ten replicate plants were used per treatment, and the pots were arranged in a randomized complete block design. The experiment was conducted once. Plants were fertilized as needed with Osmocote Plus 15N–9P–12K fertilizer.
Plants were periodically rated for Fusarium wilt on a 0 to 5 scale, where 0 = all foliage healthy, 1 = 20% of foliage wilted (one or two leaves with wilting only at the edges), 2 = 40% wilted (one or two leaves completely wilted and not turgid), 3 = 60% wilted (most of the plant exhibiting wilt and some leaves had lost turgor pressure), 4 = 80% wilted (most leaves had lost all turgor but the stem was still green and upright), and 5 = dead. At harvest (6 wk after transplant), plant stand, shoot lengths, and shoot fresh weights were recorded. A 2-cm-long piece was then cut from the base of each shoot and analyzed for the presence of Fon, as described above. Roots and soil were removed from pots, the roots were rinsed, and root fresh weights and number of galls per root system were recorded. RKN eggs were also counted, as described above.
To check for the presence of P. fluorescens, root washes were made from one plant per treatment for RKN-inoculated plants, and from two plants per treatment for plants without RKN. Soil dilutions were made from an additional three pots per treatment, for a total of four to five pots per treatment.
Microplots: Clinto 1R and Wayne 1R applied as root dips:
The experiment was conducted in 2008 at the Lower Eastern Shore Research and Education Center in Salisbury, MD, in 56-cm-diam. polystyrene microplots that had been placed on a Fort Mott loamy sand with a pH of 7.4 and 1.2% organic matter. The microplots designated as RKN treatments had been inoculated with RKN in previous years, and galling was observed on plant roots in 2007. Microplots not inoculated with RKN were used as controls. The experiment was designed with nine treatments and five blocks. Treatments were (i) untreated control, (ii) RKN, (iii) Fon + RKN, (iv) Clinto 1R, (v) Clinto 1R + RKN, (vi) Clinto 1R + Fon + RKN, (vii) Wayne 1R, (viii) Wayne 1R + RKN, and (ix) Wayne 1R + Fon + RKN. On the day of transplant (June 25), soil samples were taken for RKN; six soil cores (each 2.5 cm diam., 15-cm deep) were randomly removed from each microplot, the six samples combined and mixed in a bucket, a 150 cm3 subsample removed from each sample and refrigerated until processing, and the remaining soil returned to the microplot. A subset of each soil sample (50 cm3) was placed onto a Baermann funnel for 3 d; 10 ml of nematodes suspended in water was then taken from the tube attached to each funnel, and RKN J2 were counted.
Fon was adjusted to deliver 1.5 × 104 mixed microspores and macrospores per gram soil, with inoculum incorporated into the upper 19 to 20 cm of soil. Watermelon ‘Sugar Baby’ was seeded in the greenhouse in 72-cell trays and grown for 3 wk. Seedlings were placed outside for 10 d prior to June 25, gently removed from the trays, and dipped into P. fluorescens isolate Clinto 1R or Wayne 1R (1 × 107 CFU/ml), or into water. Four watermelon seedlings were transplanted into each of the 45 microplots. There were five microplots per treatment, with four seedlings per microplot, for a total of 20 plants per treatment.
On July 14 (19 d after transplant; DAT), the numbers of live and dead plants and of wilted plants in each microplot were counted and vine lengths were measured on all live plants. In each microplot, where plants had wilted or died, up to two plants were excised, and presence or absence of Fon was determined by excising three 0.2-cm-diam. pieces of stem from the plants; the pieces were surface sterilized for 3 min in 20% bleach solution, and plated onto PDA. Plates were assessed for growth and presence of F. oxysporum, based on morphology, after 7 d.
On August 6 (42 DAT), stand counts were again recorded. If there were four live plants in a microplot, one plant per RKN-inoculated microplot was collected to determine root fresh weights, root galling indices, RKN egg numbers, Fusarium colonization, and presence of P. fluorescens. Roots from treatments 1, 3, 4, 5, and 7 were tested for the presence of P. fluorescens. Because initial spring J2 soil counts from Baermann funnel collection were low and variable in microplots, plots with RKN were inoculated with an additional 1,000 eggs (from greenhouse cultures) in 1 ml water per plant, pipetted into a hole near the plant roots.
Fusarium wilt ratings (as described above) were recorded 10 d before harvest. On August 25 (61 DAT), fruit were harvested and counted, and all fruit were individually weighed. Brix (sugar content) was assessed from 1 or 2 fruit per microplot with a hand-held refractometer. Nine days after fruit harvest (September 3; 70 DAT), stand counts were made, and soil samples and roots were collected for determining end-of-season root fresh weights, RKN egg and J2 numbers, root galling indices, and P. fluorescens recovery.
Statistical methods:
Data from most of these laboratory, greenhouse, and field studies were analyzed with the statistical package JMP 11.2.0 (SAS Institute, Cary, NC). Unless otherwise indicated, differences among treatments were determined by ANOVA, and means were compared using Tukey–Kramer’s honest significant difference test for multiple comparisons (P ≤ 0.05). For the greenhouse experiment with P. fluorescens isolate Wayne 1R on ‘Charleston Gray’ watermelon, data for the root fresh weights, galls per gram root, and eggs per gram root were analyzed with a Student’s t-test (P = 0.80, 0.11, and 0.09, respectively).
For the two greenhouse trials with Wayne 1R on ‘Sugar Baby’ watermelon, shoot heights and shoot and root fresh weights were each analyzed as two-factor linear models using Proc Mixed (SAS Institute, 2015), with trial and treatment as the factors. The variance grouping technique was used to correct for variance heterogeneity in all of the variables except shoot fresh weight (where it was not needed). Means comparisons were done with Sidak adjusted P values so that the experiment-wise error was P ≤ 0.05. Data for the galling indices and eggs per gram root from these trials were analyzed with a Student’s t-test (P ≤ 0.05; JMP).
Fruit weight and J2 in 50-cm3 soil from the microplots, and eggs per gram root from the greenhouse experiment with P. fluorescens Clinto 1R, Wayne 1R, and Wood 1R, were log10 (x + 1) transformed for analysis (P ≤ 0.05); nontransformed means are presented. Only microplots that had been inoculated with RKN were included in analyses of nematode population data. A Kruskal–Wallis test with Wilcoxon each pair nonparametric multiple comparisons was used to determine differences (P ≤ 0.05) among the numbers of live and of wilted plants per microplot on July 14.
Results
Greenhouse: Wayne 1R applied as a root dip:
On ‘Charleston Gray’ watermelon seedlings inoculated with RKN, root fresh weights did not vary between the treatments (data not shown). The number of galls per gram root was 17.8% lower on the plants treated with Wayne 1R (mean = 249 galls/g root) than on the control plants (303 galls/g root), but the difference was not significant. However, the number of eggs per gram root was suppressed (P = 0.09) by 28.9% with Wayne 1R inoculation: mean = 333,113 eggs/g root on control plants vs. 23,557 on plants inoculated with Wayne 1R.
On ‘Sugar Baby’ watermelon, the Wayne 1R root dip did not inhibit Fusarium wilt and ensuing plant death caused by Fon. All plants inoculated with Fon died in Trial 1, and only one plant in each of two Fon treatments lived to harvest in Trial 2. Because of this plant death, no measurements were recorded at harvest from plants inoculated with Fon.
The means for plant data for the two trials were statistically different, but there was no statistically significant trial-by-treatment interaction. Inoculation with RKN generally resulted in shorter shoots with lower fresh weights than in plants without RKN, whereas root fresh weights tended to be greater with RKN inoculation (Table 1). Neither the galling indices (mean = 20.5 for RKN alone, and 22.8 for Wayne 1R + RKN), nor eggs per gram root (mean = 2,633 eggs/g root with RKN alone, and 2,106 egg/g root with Wayne 1R + RKN) were influenced by the Wayne 1R root dip. Standard serial dilutions plated to determine CFU resulted in either colonies too numerous to count or no P. fluorescens.
Table 1.
‘Sugar Baby’ watermelon plant vigor in greenhouse trials with Meloidogyne incognita (RKN) and with Pseudomonas fluorescens isolate Wayne 1R, applied as a root dip at transplant.
Greenhouse: Clinto 1R, Wayne 1R, and Wood 1R applied as seed treatments:
Inoculation with RKN alone, a P. fluorescens isolate alone, or combinations of RKN and a P. fluorescens isolate without Fon all resulted in 100% plant viability, as did water (untreated), NB control, and any P. fluorescens isolate + NB (Table 2). Compared with these treatments, plant stand was significantly reduced by treatment with Fon alone combined with any P. fluorescens isolate. The lowest plant stand (60% lower than the controls) was recorded after treatment with Wood 1R + Fon + RKN.
Table 2.
‘Sugar Baby’ watermelon plant viability and vigor, Meloidogyne incognita (RKN) egg populations, and root galling in a greenhouse trial with Pseudomonas fluorescens isolates Clinto 1R, Wayne 1R, and Wood 1R, applied individually as seed coats, and the Fusarium wilt pathogen Fusarium oxysporum f. sp. niveum (Fon).
Shoot lengths and fresh weights were greatest in plants that were inoculated with RKN (Table 2), and lowest in plants treated with Clinto 1R + Fon + RKN. Fresh weight was also low in the untreated control plants. Except for the RKN-inoculated plants, shoot lengths did not differ from the untreated controls. No treatment was significantly different from all other treatments in shoot length or weight. There were no significant differences among Fon treatments in Fusarium Wilt ratings (Table 2). Root fresh weights tended to be highest in plants inoculated with RKN alone or with RKN and any P. fluorescens isolate. Numbers of galls and eggs (per gram root fresh weight) did not differ among treatments (Table 2).
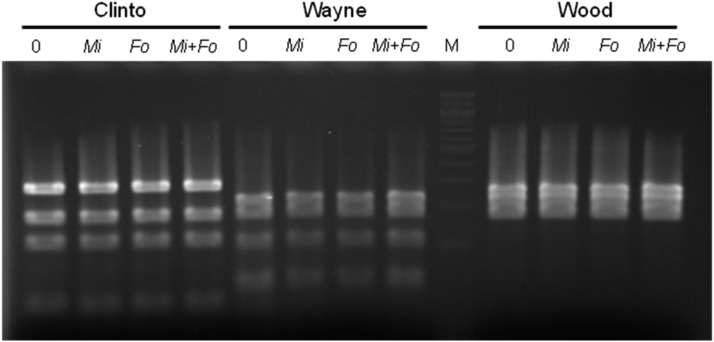
The three isolates of P. fluorescens were detected in the pooled root and soil root washes at the end of the experiment, and the patterns matched the expected genotype patterns for each of the bacterial strains (Fig. 1). Serial dilutions plated to determine CFU generally resulted in either colonies too numerous to count or no P. fluorescens. There were two CFU counts from soil: 1.0 × 105 CFU/g soil from Wood 1R + Fon + RKN, and 9.8 × 105 CFU/g soil from Wayne 1R + Fon. Fon was consistently recovered from inoculated and symptomatic plants, but no Fon was isolated from noninoculated plants.
Fig. 1.
HaeIII restriction digest profiles of PhlD gene amplified from pooled root washes and soil in a greenhouse trial with three Pseudomonas fluorescens isolates: Clinto 1R, Wayne 1R, and Wood 1R. Mi = Meloidogyne incognita; Fo = Fusarium oxysporum f. sp. niveum.
Microplots: Clinto 1R and Wayne 1R applied as root dips:
Wilting was first observed in some microplots on July 8 (13 DAT). By 19 DAT (July 14), a third to half of the plants inoculated with Fon had begun to exhibit wilting, but numbers of live plants per microplot were not significantly different among treatments (Table 3). Vines were longest on untreated plants and plants in microplots inoculated with RKN, Clinto 1R + RKN, and Wayne 1R + RKN. Treatment with Clinto 1R and Wayne 1R resulted in shorter vine lengths than treatment with either isolate + RKN. Inoculation with Fon also tended to result in shorter vine lengths.
Table 3.
‘Sugar Baby’ watermelon plant viability and vine lengths, end-of-season root fresh weights, root galling indices, and Meloidogyne incognita (RKN) egg and second-stage juvenile (J2) populations in a 2008 microplot trial. Treatments included Pseudomonas fluorescens isolates Clinto 1R and Wayne 1R, applied individually as root dips at transplant, RKN, and the Fusarium wilt pathogen Fusarium oxysporum f. sp. niveum (Fon). Watermelon seedlings were transplanted into microplots in Salisbury, MD, on June 25.
By August 6 (42 DAT), none of the microplots inoculated with RKN and Fon contained four live plants (Table 3). To assess root galling indices and egg populations, one plant was removed from each RKN-inoculated microplot that had four live plants. The three sampled treatments were RKN (five plants collected), Clinto 1R + RKN (two plants collected), and Wayne 1R + RKN (five plants collected). Root fresh weights, root galling indices, and eggs per gram root were similar among these treatments (data not shown). Fruit were harvested 2 mon after transplant (August 25). The number of fruit per microplot, fruit weights, and percent sugar also were not affected by treatment (data not shown).
By September 3 (70 DAT), when remaining plants in RKN-inoculated plots were harvested and soil sampled for RKN populations, 70% to 95% of the plants had died in all treatments, including control microplots that had not received P. fluorescens, Fon, or RKN. In RKN-inoculated microplots, no differences were found among treatments in root fresh weights, root galling indices, eggs per gram root, or numbers of J2 in soil (Table 3). No J2 were recovered from microplots that had not been inoculated with RKN. The mean number of J2/50 cm3 soil from RKN-inoculated microplots was also recorded from treatments with only one live plant at harvest: 40 J2/50 cm3 from Fon + RKN microplots, and 17 J2/50 cm3 from Clinto 1R + Fon + RKN microplots. These numbers did not differ significantly from those reported from other RKN treatments (Table 3). Fon was isolated from sampled and symptomatic plants but not from plants in noninoculated plots, confirming that Fon was the cause of wilt.
Discussion
In these studies with watermelon and the three beneficial P. fluorescens isolates Clinto 1R (genotype S), Wayne 1R (genotype A), and Wood 1R (genotype D), seed treatments with individual strains did not suppress RKN populations on ‘Sugar Baby’ watermelon in steamed-pasteurized soil in the greenhouse. The same result was observed with Wayne 1R as a root dip in the greenhouse, and with Clinto 1R and Wayne 1R tested as root dips in microplots. None of the isolates suppressed Fusarium wilt in the same greenhouse and microplot trials. In the microplots, application of Clinto 1R or of Wayne 1R suppressed vine length, whereas application of either isolate + RKN did not. The latter indicated phytotoxicity of P. fluorescens that was reduced by some unknown mechanism in the presence of RKN. Watermelon fruit production was not affected by any treatment.
Previous research demonstrated that activity varies among these three genotypes of P. fluorescens. The D genotype was most commonly detected from corn and soybean, followed by genotype A and then genotype S on the same crops (McSpadden Gardener et al., 2005). These three genotypes were represented in this study by Wood 1R, Wayne 1R, and Clinto 1R, respectively. All three genotypes exhibited in vitro inhibition of Pythium irregular, but results varied with culture medium (McSpadden Gardener et al., 2005). The D and S genotypes were more effective than the A genotype on 1/5 × PDA, whereas the A genotype was more effective than the D and S genotypes on 1/10 × TSA and 1/5 × CMA, with less difference in activity on CMA. Wayne 1 demonstrated greater inhibition than Wood 1 on growth of eight Pythium spp. isolates, although results depended on the culture medium and the Pythium strain. When eight genotypes, including D and A, were tested for colonization of pea roots, genotype D showed greater colonization than genotype A (Landa et al., 2002). In our study, root colonization was noted but the CFU counts at harvest were not recorded from a sufficient number of samples to determine whether colonization of watermelon roots generally varied with genotype. Thus, it is possible that the levels of rhizosphere colonization attained by the applied strains were insufficient on watermelon to suppress RKN and Fon in our studies. Despite the possibility for variable activity among the genotypes, no difference in pathogen suppression was observed among the A, D, or S genotypes in our study with watermelon.
It is possible that Clinto 1R, Wayne 1R, and Wood 1R did not inhibit plant disease caused by Fon or RKN on watermelon because these P. fluorescens strains were not originally obtained from the watermelon rhizosphere. Effects of DAPG-producing P. fluorescens against pathogens vary with plant host (Bergsma-Vlami et al., 2005; De La Fuente et al., 2006). For example, inoculation with Wayne 1R resulted in increased soybean stands and yields in the field, but corn vigor and yields were not enhanced (McSpadden Gardener et al., 2005). When Wood 1R was tested for suppression of plant-parasitic nematodes, RKN populations were numerically lower on cotton and soybean than on control plants, but were only significantly reduced on corn (Timper et al., 2009). In that study, activity of DAPG-producing P. fluorescens depended on both nematode taxon and plant host. Wood 1R, tested in steam-heated soil, did not suppress Paratrichodorus minor on corn, H. glycines on soybean, or Meloidogyne arenaria on peanut (Timper et al., 2009). Plant host also affected activity of the DAPG-producing P. fluorescens strain CHA0 (genotype A). Inoculation with CHA0 resulted in ISR that suppressed Meloidogyne javanica populations on tomato, and both strain ChA0 and a derivative, antibiotic-overproducing strain (CHA0/pME3424) suppressed galling caused by M. incognita on mungbean, soybean and tomato. However, there was a lesser effect on eggplant and chili (Siddiqui and Shaukat, 2002, 2003a, 2003b). In our initial trial with watermelon ‘Charleston Gray’, the number of RKN eggs per gram root was reduced by inoculation with Wayne 1R, indicating that cultivar might also be a factor in RKN suppression. Cultivar effect has been noted before, as rhizosphere colonization by DAPG-producing strains of P. fluorescens varied with pea cultivar (De La Fuente et al., 2006). However, because of the commercial importance of the cultivar Sugar Baby, comparisons between the two watermelon cultivars were not pursued.
2,4-Diacetylphloroglucinol production by Clinto 1R, Wayne 1R, and Wood 1R in the rhizosphere may not be in sufficient amounts on watermelon, or in the right locations, to trigger ISR or to result in direct toxicity to RKN and Fon. 2,4-Diacetylphloroglucinol plays an important role in both areas. Studies with CHA0 and derived mutant strains demonstrated that DAPG was needed to induce ISR on Arabidopsis thaliana against Hyaloperonospora arabidopsidis (Iavicoli et al., 2003), and DAPG production by P. fluorescens was a determinant for ISR production against P. syringae pv. tomato in A. thaliana leaves (Weller et al., 2012). The latter study also showed that ISR was not affected by genotype of the DAPG-producing P. fluorescens. The importance of DAPG has been demonstrated in other ways as well. Culture filtrates from strains CHA0 and CHA0/pME3424 were active against M. javanica eggs and J2, but CHA89 (antibiotic deficient) was not (Siddiqui and Shaukat, 2003a). Strain CHA89 also did not reduce galling on plant roots (Siddiqui and Shaukat, 2003b). The DAPG-producing strain F113 (genotype K; McSpadden Gardener et al., 2000) increased G. rostochiensis hatch and decreased J2 mobility, whereas the DAPG-negative strain F113G22 did not affect either (Cronin et al., 1997). Although immersion in synthetic DAPG did not inhibit J1 hatch or mobility of C. elegans (Meyer et al., 2009), a DAPG-overproducing mutant was more toxic to C. elegans on agar plates than the wild type strain CHA0, and DAPG production elicited the DAF-16 stress response pathway (Neidig et al., 2011). Removal of DAPG production resulted in avoidance by the nematode, but this was most likely because of increased pyoluteorin production (Neidig et al., 2011). Insufficient levels of DAPG could be due to such factors as the host plant–bacterium interaction, as indicated above, or the presence of other microbes in the soil that alter production of this metabolite, a mechanism previously implicated in take-all suppression (McSpadden Gardener and Weller, 2001). Particularly relevant to this study, an isolate of Fusarium solani suppressed nematicidal effects of P. fluorescens strain CHA0 (Siddiqui and Shaukat, 2003c). Thus, it seems likely that the failure of the strains to suppress disease in our study is due in part to interference from one or other members of the rhizosphere microbiome.
Growth of the Fon isolate used in this study was not greatly inhibited by DAPG, and might therefore not have been affected by direct DAPG toxicity in vivo. It is well known that plant-pathogenic and saprophytic isolates of F. oxysporum vary greatly in DAPG sensitivity (Schouten et al., 2004). Of 117 genotypically different strains tested, approximately 17% exhibited tolerance to DAPG, which included at least some growth at DAPG concentrations up to 400 µg/ml. Growth of F. oxysporum f. sp. lycopersici and F. oxysporum f. sp. lini was inhibited 50% at DAPG concentrations of 16 and 32 µg/ml, respectively, and completely inhibited at 128 µg/ml (Keel et al., 1992). With this level of sensitivity to DAPG, strain CHA0 was able to inhibit growth of F. oxysporum. In a small trial with Fon on 1/5 × PDA with methanol as a solvent (procedures similar to Kwak et al., 2009), Fon growth was not inhibited by 1, 2, 3, 4, 5, or 10 µg/ml DAPG, but was inhibited by 20 µg/ml DAPG by Day 4. Growth in 20 µg/ml was 72% of all other treatments (unpubl. data, Meyer et al.). This low level of inhibition may indicate that direct DAPG toxicity might not be a major factor in inhibition of this Fon strain by the tested P. fluorescens isolates.
As in our study, phytotoxicity has been recorded with DAPG-producing P. fluorescens. For example, inoculation with strain CHA0/pME3424 resulted in lower root and/or shoot weights in eggplant, chili, mungbean, and in some soybean cultivars (Siddiqui and Shaukat, 2003b). DAPG inhibited plant growth and seed germination of corn, cotton, cress, cucumber, flax, sweet corn, tobacco, tomato, and wheat, although high levels were required for inhibition of seed germination on cotton, cucumber, and wheat (Keel et al., 1992).
Our studies demonstrated that the three P. fluorescens isolates resulted in some inhibition of ‘Sugar Baby’ watermelon vine growth in the field, and were consequently not effective for enhancing plant vigor. RKN and Fon were not suppressed on ‘Sugar Baby’, although there was some indication that Wayne 1R might act against RKN on ‘Charleston Gray’. Wood 1R reduced foliar lesions and improved yields on corn in a low-pH soil (Raudales et al., 2009), and suppressed RKN on corn (Timper et al., 2009). However, results with RKN were variable in the latter study, and the authors concluded that “consistent suppression of nematodes in natural soils seems unlikely.” Based on the trials with these three isolates, the strains are also not promising candidates as biocontrol inoculants for suppression of RKN or Fon on watermelon.
Literature Cited
- Bakker PAHM, Pieterse CMJ, van Loon LC. Induced systemic resistance by fluorescent Pseudomonas spp. Phytopathology. 2007;97:239–243. doi: 10.1094/PHYTO-97-2-0239. [DOI] [PubMed] [Google Scholar]
- Bergsma-Vlami M, Prins ME, Raaijmakers JM. Influence of plant species on population dynamics, genotypic diversity and antibiotic production in the rhizosphere by indigenous Pseudomonas spp. FEMS Microbiology Ecology. 2005;52:59–69. doi: 10.1016/j.femsec.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Bonsall RF, Weller DM, Thomashow LS. Quantification of 2,4-diacetylphloroglucinol produced by fluorescent Pseudomonas spp. in vitro and in the rhizosphere of wheat. Applied and Environmental Microbiology. 1997;63:951–955. doi: 10.1128/aem.63.3.951-955.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazelton JN, Pfeufer EE, Sweat TA, McSpadden Gardener BB, Coenen C. 2,4-Diacetylphloroglucinol alters plant root development. Molecular Plant-Microbe Interactions. 2008;21:1349–1358. doi: 10.1094/MPMI-21-10-1349. [DOI] [PubMed] [Google Scholar]
- Cronin D, Moënne-Loccoz Y, Fenton A, Dunne C, Dowling DN, O’Gara F. Role of 2,4-diacetylphloroglucinol in the interactions of the biocontrol pseudomonad strain F113 with the potato cyst nematode Globodera rostochiensis. Applied and Environmental Microbiology. 1997;63:1357–1361. doi: 10.1128/aem.63.4.1357-1361.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daulton RAC, Nusbaum CJ. The effect of soil temperature on the survival of the root-knot nematodes Meloidogyne javanica and M. hapla. Nematologica. 1961;6:280–294. [Google Scholar]
- De La Fuente L, Landa BB, Weller DM. Host crop affects rhizosphere colonization and competitiveness of 2,4-diacetylphloroglucinol-producing Pseudomonas fluorescens. Phytopathology. 2006;96:751–762. doi: 10.1094/PHYTO-96-0751. [DOI] [PubMed] [Google Scholar]
- Delany IR, Walsh UF, Ross I, Fenton AM, Corkery DM, O’Gara F. Enhancing the biocontrol efficacy of Pseudomonas fluorescens F113 by altering the regulation and production of 2,4-diacetylphloroglucinol. Plant and Soil. 2001;232:195–205. [Google Scholar]
- De Leij FAAM, Dixon-Hardy JE, Lynch JM. Effect of 2,4-diacetylphloroglucinol-producing and non-producing strains of Pseudomonas fluorescens on root development of pea seedlings in three different soil types and its effect on nodulation by Rhizobium. Biology and Fertility of Soils. 2002;35:114–121. [Google Scholar]
- De Souza JT, Arnould C, Deulvot C, Lemanceau P, Gianinazzi-Pearson V, Raaijmakers JM. Effect of 2,4-diacetylphloroglucinol on Pythium: Cellular responses and variation in sensitivity among propagules and species. Phytopathology. 2003;93:966–975. doi: 10.1094/PHYTO.2003.93.8.966. [DOI] [PubMed] [Google Scholar]
- Iavicoli A, Boutet E, Buchala A, Métraux JP. Induced systemic resistance in Arabidopsis thaliana in response to root inoculation with Pseudomonas fluorescens CHA0. Molecular Plant-Microbe Interactions. 2003;16:851–858. doi: 10.1094/MPMI.2003.16.10.851. [DOI] [PubMed] [Google Scholar]
- Keel C, Schnider U, Maurhofer M, Voisard C, Laville J, Burger U, Wirthner P, Haas D, Défago G. Suppression of root diseases by Pseudomons fluorescens CHA0: Importance of the bacterial secondary metabolite 2,4-diacetylphloroglucinol. Molecular Plant-Microbe Interactions. 1992;5:4–13. [Google Scholar]
- Kwak Y-S, Bakker PAHM, Glandorf DCM, Rice JT, Paulitz TC, Weller DM. Diversity, virulence and 2,4-diacetylphloroglucinol sensitivity of Gaeumannomyces graminis var. tritici isolates from Washington State. Phytopathology. 2009;99:472–479. doi: 10.1094/PHYTO-99-5-0472. [DOI] [PubMed] [Google Scholar]
- Landa BB, Mavrodi OV, Raaijmakers JM, McSpadden Gardener BB, Thomashow LS, Weller DM. Differential ability of genotypes of 2,4-diacetylphloroglucinol-producing Pseudomonas fluorescens strains to colonize the roots of pea plants. Applied and Environmental Microbiology. 2002;68:3226–3237. doi: 10.1128/AEM.68.7.3226-3237.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CK, Hassan KA, Tetu SG, Loper JE, Paulsen IT. The effect of iron limitation on the transcriptome and proteome of Pseudomonas fluorescens Pf-5. PLoS One. 2012;7:1–18. doi: 10.1371/journal.pone.0039139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikandan R, Saravanakumar D, Rajendran L, Raguchander T, Samiyappan R. Standardization of liquid formulation of Pseudomonas fluorescens Pf1 for its efficacy against Fusarium wilt of tomato. Biological Control. 2010;54:83–89. [Google Scholar]
- Maurhofer M, Keel C, Haas D, Défago G. Influence of plant species on disease suppression by Pseudomonas fluorescens strain CHA0 with enhanced antibiotic production. Plant Pathology. 1995;44:40–50. [Google Scholar]
- Mazurier S, Corberand T, Lemanceau P, Raaijmakers JM. Phenazine antibiotics produced by fluorescent pseudomonads contribute to natural soil suppressiveness to Fusarium wilt. The ISME Journal. 2009;3:977–991. doi: 10.1038/ismej.2009.33. [DOI] [PubMed] [Google Scholar]
- McSpadden Gardener BB. Diversity and ecology of biocontrol Pseudomonas in agricultural systems. Phytopathology. 2007;97:221–226. doi: 10.1094/PHYTO-97-2-0221. [DOI] [PubMed] [Google Scholar]
- McSpadden Gardener BB, Gutierrez LJ, Joshi R, Edema R, Lutton E. Distribution and biocontrol potential of phlD+ pseudomonads in corn and soybean fields. Phytopathology. 2005;95:715–724. doi: 10.1094/PHYTO-95-0715. [DOI] [PubMed] [Google Scholar]
- McSpadden Gardener BB, Mavrodi DV, Thomashow LS, Weller DM. A rapid polymerase chain reaction-based assay characterizing rhizosphere populations of 2,4-diacetylphloroglucinol-producing bacteria. Phytopathology. 2001;91:44–54. doi: 10.1094/PHYTO.2001.91.1.44. [DOI] [PubMed] [Google Scholar]
- McSpadden Gardener BB, Schroeder KL, Kalloger SE, Raaijmakers JM, Thomashow LS, Weller DM. Genotypic and phenotypic diversity of phlD-containing Pseudomonas strains isolated from the rhizosphere of wheat. Applied and Environmental Microbiology. 2000;66:1939–1946. doi: 10.1128/aem.66.5.1939-1946.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSpadden Gardener BB, Weller DM. Changes in populations of rhizosphere bacteria associated with take-all disease of wheat. Applied and Environmental Microbiology. 2001;67:4414–4425. doi: 10.1128/AEM.67.10.4414-4425.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer SLF, Halbrendt JM, Carta LK, Skantar AM, Liu T, Abdelnabby HME, Vinyard BT. Toxicity of 2,4-diacetylphloroglucinol (DAPG) to plant-parasitic and bacterial-feeding nematodes. Journal of Nematology. 2009;41:274–280. [PMC free article] [PubMed] [Google Scholar]
- Meyer SLF, Lakshman DK, Zasada IA, Vinyard BT, Chitwood DJ. Phytotoxicity of clove oil to vegetable crop seedlings and nematotoxicity to root-knot nematodes. HortTechnology. 2008;18:631–638. [Google Scholar]
- Neidig N, Paul RJ, Scheu S, Jousset A. Secondary metabolites of Pseudomonas fluorescens CHA0 drive complex non-trophic interactions with bacterivorous nematodes. Microbial Ecology. 2011;61:853–859. doi: 10.1007/s00248-011-9821-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netzer D. Physiological races and soil population level of Fusarium wilt of watermelon. Phytoparasitica. 1976;4:131–136. [Google Scholar]
- Raudales RE, Stone E, McSpadden Gardener BB. Seed treatment with 2,4-diacetylphloroglucinol-producing pseudomonads improves crop health in low-pH soils by altering patterns of nutrient uptake. Phytopathology. 2009;99:506–511. doi: 10.1094/PHYTO-99-5-0506. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc 2015. SAS/STAT 14.1 user’s guide. Cary, NC: SAS Institute.
- Saikia R, Varghese S, Singh BP, Arora DK. Influence of mineral amendment on disease suppressive activity of Pseudomonas fluorescens to Fusarium wilt of chickpea. Microbiological Research. 2009;164:365–373. doi: 10.1016/j.micres.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Schouten A, van de Berg G, Edel-Hermann V, Steinberg C, Gautheron N, Alabouvette C, de Vos CHR, Lemanceau P, Raaijmakers JM. Defense responses of Fusarium oxysporum to 2,4-diacetylphloroglucinol, a broad-spectrum antibiotic produced by Pseudomonas fluorescens. Molecular Plant-Microbe Interactions. 2004;17:1201–1211. doi: 10.1094/MPMI.2004.17.11.1201. [DOI] [PubMed] [Google Scholar]
- Selvaraj S, Ganeshamoorthi P, Anand T, Raguchander T, Seenivasan N, Samiyappan R. Evaluation of a liquid formulation of Pseudomonas fluorescens against Fusarium oxysporum f. sp. cubense and Helicotylenchus multicinctus in banana plantation. BioControl. 2014;59:345–355. [Google Scholar]
- Siddiqui IA, Haas D, Heeb S. Extracellular protease of Pseudomonas fluorescens CHA0, a biocontrol factor with activity against the root-knot nematode Meloidogyne incognita. Applied and Environmental Microbiology. 2005;71:5646–5649. doi: 10.1128/AEM.71.9.5646-5649.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui IA, Shaukat SS. Rhizobacteria-mediated induction of systemic resistance (ISR) in tomato against Meloidogyne javanica. Journal of Phytopathology. 2002;150:469–473. [Google Scholar]
- Siddiqui IA, Shaukat SS. Suppression of root-knot disease by Pseudomonas fluorescens CHA0 in tomato: Importance of bacterial secondary metabolite, 2,4-diacetylphloroglucinol. Soil Biology and Biochemistry. 2003a;35:1615–1623. [Google Scholar]
- Siddiqui IA, Shaukat SS. Plant species, host age and host genotype effects on Meloidogyne incognita biocontrol by Pseudomonas fluorescens strain CHA0 and its genetically-modified derivatives. Journal of Phytopathology. 2003b;151:231–238. [Google Scholar]
- Siddiqui IA, Shaukat SS. Non-pathogenic Fusarium solani represses the biosynthesis of nematicidal compounds in vitro and reduces the biocontrol of Meloidogyne javanica by Pseudomonas fluorescens in tomato. Letters in Applied Microbiology. 2003c;37:109–114. doi: 10.1046/j.1472-765x.2003.01349.x. [DOI] [PubMed] [Google Scholar]
- Siddiqui IA, Shaukat SS. Systemic resistance in tomato induced by biocontrol bacteria against the root-knot nematode, Meloidogyne javanica is independent of salicylic acid production. Journal of Phytopathology. 2004;152:48–54. [Google Scholar]
- Siddiqui IA, Shaukat SS, Sheikh IH, Khan A. Role of cyanide production by Pseudomonas fluorescens CHA0 in the suppression of root-knot nematode, Meloidogyne javanica in tomato. World Journal of Microbiology and Biotechnology. 2006;22:641–650. [Google Scholar]
- Thies JA, Ariss JJ, Hassell RL, Olson S, Kousik CS, Levi A. Grafting for management of southern root-knot nematode, Meloidogyne incognita, in watermelon. Plant Disease. 2010;94:1195–1199. doi: 10.1094/PDIS-09-09-0640. [DOI] [PubMed] [Google Scholar]
- Timper P, Koné D, Yin J, Ji P, McSpadden Gardener BB. Evaluation of an antibiotic-producing strain of Pseudomonas fluorescens for suppression of plant-parasitic nematodes. Journal of Nematology. 2009;41:234–240. [PMC free article] [PubMed] [Google Scholar]
- Weller DM. Pseudomonas biocontrol agents of soilborne pathogens: Looking back over 30 years. Phytopathology. 2007;97:250–256. doi: 10.1094/PHYTO-97-2-0250. [DOI] [PubMed] [Google Scholar]
- Weller DM, Landa BB, Mavrodi OV, Schroeder KL, De La Fuente L, Blouin Bankhead S, Allende Molar R, Bonsall RF, Mavrodi DV, Thomashow LS. Role of 2,4-diacetylphloroglucinol-producing fluorescent Pseudomonas spp. in the defense of plant roots. Plant Biology. 2007;9:4–20. doi: 10.1055/s-2006-924473. [DOI] [PubMed] [Google Scholar]
- Weller DM, Mavrodi DV, Van Pelt JA, Pieterse CMJ, Van Loon LC, Bakker PAHM. Induced systemic resistance in Arabidopsis thaliana against Pseudomonas syringae pv. tomato by 2,4-diacetylphloroglucinol-producing Pseudomonas fluorescens. Phytopathology. 2012;102:403–412. doi: 10.1094/PHYTO-08-11-0222. [DOI] [PubMed] [Google Scholar]
- Zhou T-T, Li C-Y, Chen D, Wu K, Shen Q-R, Shen B. phlF- mutant of Pseudomonas fluorescens J2 improved 2,4-DAPG biosynthesis and biocontrol efficacy against tomato bacterial wilt. Biological Control. 2014;78:1–8. [Google Scholar]
- Zhou XG, Everts KL. 2006 Suppression of Fusarium wilt of watermelon enhanced by hairy vetch green manure and partial cultivar resistance. Plant Health Progress doi:10.1094/PHP-2006-0405-01-RS. [Google Scholar]