Abstract
Although blockade of the renin-angiotensin-system (RAS) has become standard therapy for diabetic nephropathy (DN), decline in kidney function towards end-stage renal disease is seen in many patients. Elevated plasma aldosterone often accompanies RAS blockade by a phenomenon known as “aldosterone escape” and activates the mineralocorticoid receptor (MR). We therefore examined whether addition of the MR antagonist eplerenone to an ACEI would enhance the efficacy in slowing the progression of DN. Untreated uninephrectomized diabetic db/db mice developed progressive albuminuria and glomerulosclerosis between weeks 18 and 22, associated with decreased number of podocytes and increased renal expression of fibrotic markers. The therapeutic effect of eplerenone at 100 mg/kg BW/d on albuminuria, podocyte injury and renal fibrosis was similar to that of enalapril given alone at maximally effective doses. Adding eplerenone to enalapril resulted in further reduction in these measurements. Renal expressions of TNF-α, MCP-1, Nox2 and p47phox and renal TBARS levels, markers of inflammation and oxidative stress, were increased during disease progression in diabetic mice, which were reduced by eplerenone or enalapril given alone and further reduced by the two drugs given in combination. However, there were no treatment related effects on plasma K+. Our results suggest that eplerenone is effective in slowing the progression of DN in db/db mice and that the effect is additive to an ACEI. The addition of an MR antagonist void of effects on plasma K+ to an ACEI may offer additional renoprotection in progressive DN via blocking the effects of aldosterone due to escape or diabetes-induction.
Keywords: Aldosterone, ACEI, albuminuria, podocyte, renal fibrosis
Introduction
Activation of the renin-angiotensin system (RAS) and generation of angiotensin II (Ang II) play a crucial role in diabetic nephropathy (DN) through both pressure-dependent and pressure-independent mechanisms [1]. Ang II blockade has been a great therapeutic breakthrough for slowing DN in the past two decades. However, accumulating evidence from both clinical and animal research has suggested that halting renal fibrosis cannot be achieved by angiotensin converting enzyme inhibitors (ACEI) or Ang II receptor blockers (ARB) alone or in combination given at maximal dosages and correct frequency [2-5]. One reason could be the effects of aldosterone. Ang II is the main stimulus for aldosterone secretion and blocking Ang II’s generation and action with an ACEI and/or ARB or a direct renin inhibitor (DRI) should theoretically inhibit the secretion of aldosterone. However, elevated plasma aldosterone levels are frequently observed in patients chronically treated with ACEI, a phenomenon known as “aldosterone escape” or “aldosterone breakthrough” [6,7]. The elevated aldosterone level is seen not only in plasma but also in local tissue due to alternative pathways of Ang II generation including chymase and chathepsin G [8]. Although it was once thought that aldosterone acts primarily as a circulating hormone involved in the regulation of sodium excretion through mineralocorticoid receptor (MR)-dependent mechanisms, evidence has been mounting to suggest that aldosterone also promotes fibrosis, inflammation, and generation of reactive oxygen species (ROS), along with endothelial dysfunction, cell growth, and proliferation that were previously attributed solely to Ang II [9,10]. In fact, MR antagonists have been shown to decrease mortality in patients with congestive heart failure, to improve endothelial function, to reduce circulating markers of collagen turnover and to decrease microalbuminuria [11-14]. Addition of the MR antagonist, spironolactone, to ACEI markedly reduced proteinuria in patients with renal failure and in patients with diabetes [15,16]. In a randomized trial of 268 type 2 diabetics, the addition of eplerenone to an ACEI also reduced albuminuria, at the same time elevating plasma K+ [17]. In rats, studies have consistently demonstrated that aldosterone infusion causes glomerular injury, interstitial inflammation and fibrosis in hypertensive rat kidneys [18]. MR antagonists markedly ameliorated glomerular and/or tubulointerstitial injury in several models of nephropathy including spontaneously hypertensive stroke-prone rats, diabetic rats, nephritic rats and chronic cyclosporine-induced nephrotoxic rats [19-24]. Moreover, MR antagonism not only reduced the development of glomerulosclerosis but also induced regression of existing glomerulosclerosis in rats after 5/6 nephrectomy [25]. These studies together not only emphasized the beneficial effect of MR antagonism in progressive renal diseases but also suggested that the profibrotic effects of aldosterone might be independent of other components of the RAS. Thus the aldosterone escape phenomenon occurring during long-term use of Ang II blockade may, in fact, contribute to the limited effectiveness of this therapy, which provides a rational and methodology for further prevention and treatment of diabetic nephropathy by adding MR antagonist to an ACEI or an ARB. However, whether such therapeutic combination leads a further attenuation in the progression of renal disease therefore maximizing renoprotection in diabetic patients has yet to be defined.
We thus proposed to use a well-established experimental diabetes model to investigate whether adding eplerenone, a selective mineralocorticoid receptor antagonist, to the standard treatment with an ACEI could further slow the progression of glomerulosclerosis in diabetic nephropathy and explore the underlying mechanisms of the combination therapy.
Materials and methods
Reagents
Eplerenone was provided by AstraZeneca (Molndal, Sweden). Unless specified, all other reagents were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Animals
Diabetic male db/db mice (BKS.Cg-Dock7m +/+ Leprdb/J homozygotes) and their littermate male db/m mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) and housed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The experimental protocols were approved by the Animal Care Committee at the University of Utah. The db/db mice were determined to be diabetic by the vendor on the basis of obesity at approximately 5 weeks of age and were further demonstrated to be hyperglycemic in our laboratory at week 7. Mice were subjected to right uninephrectomy under anesthesia at week 8 to hasten the development of diabetic nephropathy as described previously [26]. Db/m mice received uninephrectomy at week 8 served as the operation control.
Experimental design
Groups of uninephrectomized mice were assigned and treated at 18 weeks of age as follows: (i) untreated non-diabetic db/m mice (n=9) as healthy controls; (ii) untreated diabetic db/db mice (n=8) as a diseased control at 18 weeks of age; (iii) untreated diabetic db/db mice at 22 weeks of age (n=8) as another disease control; (iv) diabetic db/db mice treated with enalapril (n=9), 200 mg/liter enalapril in drinking water; (v) diabetic db/db mice treated with elperenone (n=9), 100 mg/kg BW/d mixed with food, and (vi) diabetic db/db mice treated with eplerenone plus enalapril (n=8) for 4 weeks from week 18 to week 22. The maximally renoprotective dose of enalapril was determined previously [5,27]. Low dosage of eplerenone at 50 mg/kg BW/d has been tested in type 2 diabetic rats with a limited therapeutic effect in kidney injury [28]. However, high dosage of eplerenone (such as 200 mg/kg BW/d) may be associated with an increased risk for hyperkalemia in patients [17]. Therefore, our study investigated whether eplerenone at the dosage of 100 mg/kg BW/d added to the ACE inhibitor enalapril results in a substantive reduction in albuminuria and the progression of diabetic nephropathy with a lesser risk for hyperkalemia. This dosage of eplerenone has been shown the desired benefits in several animal models [29,30]. The water consumption was monitored daily. Food consumption was measured weekly to confirm correct dosing of eplerenone.
Systolic blood pressure was measured before and at the end of treatment in conscious and trained mice at room temperature by the tail-cuff method (MC4000 multi channel blood pressure analysis system, Hatteras Instrument, Inc., Cary, NC, USA). The blood glucose level and glycosylated hemoglobin (HbA1C) level were monitored in tail blood samples using a blood glucose meter (Glucometer Elite XL, Bayer Healthcare, Elkhart, IN, USA) and the DC2000+ HbA1C kit (Bayer Healthcare) respectively before treatment and at the time of sacrifice. Twenty-four-hour urine was obtained from each mouse after placement in metabolic cages before treatment and at the time of sacrifice. Urine albumin was measured using the DC2000+ microalbumin reagent kit (Bayer Healthcare). As for the reproducibility of this assay, the coefficients of variance (CV) were less than 3% when the same sample was measured three times consecutively. Plasma and urine aldosterone was measured using the commercial available ELISA kit (Alpha Diagnostic Intl. Inc., San Antonio, TX, USA).
Mice were sacrificed under isoflurane anesthesia. Blood samples were obtained by cardiac puncture for the measurements of plasma K+, creatinine and aldosterone concentration. Plasma K+ levels were determined on a Radiometer ABL 700 blood gas analyser (Radiometer, Copnchagen DK). Urine creatinine was measured by the Jaffe method (Creatinine FS, REF117119910021, DiaSys). Plasma creatinine was analyzed on a LC-MS/MS system consisting of a Waters Quattro Premiere mass spectrometer and an Agilent 1100 HPLC. Column was a Phenomenex Kinetex HILIC 2.6 µ, 2.1 × 100 mm. Mobile phase A was acetonitrile and mobile phase B was 40 mM Ammonium formate. Gradient was from 5% B to 50% B in 10 min with 5 min equilibration time. As internal standard, 0.2 µm D3-Creatinine in CAN:H2O, 9:1 was used. The creatinine clearance (Ccr) was calculated as the following formula, (Urine creatinine levels/plasma creatinine levels) × 24 h-urine volume (mL)/(24 h × 60 min). Kidneys were perfused through the heart with cold PBS and then excised. Renal cortex was harvested by dissection and saved for further analysis as described previously [26]. Briefly, two pieces of cortex were either snap-frozen in 2-methylbutane at -80°C or fixed in 10% neutralized formalin for immunohistochemistric staining. Other pieces of cortex were stored in liquid nitrogen for western blot or treated with Tri Reagent for isolation of RNA or treated with 100 mM NaCl and 20 mM HEPES to be sonicated for 30 seconds, 3 times on ice and centrifuged at a 12,000 rpm for 15 min at 4°C. The supernatant was then collected and stored at -80°C for TGFβ1 ELISA [31,32] and protein measurement by a bicinchoninic acid (BCA) protein assay kit (PIERCE, Rockford IL, USA).
Measurement of urinary TNF-α, MCP-1 and renal TBARS levels
Twenty-four hours-urine collected from diabetic mice at the end of experiment was analyzed for the levels of TNF-α, and MCP-1. Urine TNF-α and MCP-1 levels were measured using a commercial available ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA). Their excretion was expressed as the total amount excreted in 24 h.
Malondialdehyde (MDA) is also named as thiobarbituric acid-reactive substances (TBARS), which is derived from lipid hydroperoxides produced by oxidative stress and reacts with thiobarbituric acid (TBA), using a colorimetric assay (Cayman Chemical Company, Ann Arbor MI, USA). Renal TBARS concentration was obtained from a standard curve produced by serially diluting MDA and corrected by the total protein levels in renal cortex tissue.
Histological analyses
Formalin-fixed renal cortex tissues were subsequently embedded in paraffin. Three-micrometer sections were cut from the tissue blocks and stained with periodic acid-Schiff (PAS). The PAS-positive glomerular matrix was quantitated in a blinded fashion by a computer-assisted method as previously described [26]. At least 20 glomeruli from each individual mouse were assessed. The PAS-positive material area in the mesangium was normalized by that of the total glomerular tuft where the percentage of mesangial matrix occupying each glomerulus area was rated. Glomerular sclerosis index was graded from 0 to 4+ as follows: 0 represents no lesion, 1+ represents sclerosis of <25% of the glomerulus, while 2=, 3+ and 4+ represent sclerosis of 25% to 50%, >50% to 75%, and >75% of the glomerulus. A whole kidney average sclerosis index was obtained by averaging scores from all glomeruli on one section.
Immunofluorescent staining for fibronectin (FN) and type IV collagen (Col IV) was performed on frozen sections as described [26]. Immunofluorescent staining for podocin and Wilms tumor protein 1 (WT-1) was also performed on frozen sections. The polyclonal goat anti-podocin IgG and the polyclonal rabbit anti-WT1 IgG (C-19) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) were used as the primary antibodies. The Rhodamin (TRITC)-conjugated donkey anti-goat IgG (H+L) (Jackson ImmunoResearch, Laboratories Inc.) and Rhodamin redTM-X-conjugated donkey anti-rabbit F(ab’)2 (Jackson ImmunoResearch, Laboratories Inc.) were used as the secondary antibodies. Intraglomerular positive staining of FN, type IV collagen and podocin was quantified separately in a blinded fashion by a computer-assisted method as described previously [33]. The number of WT-1-positive podocyte per glomerulus was counted in 20 glomeruli selected randomly per section.
Western blot analysis
Equal amounts of renal cortex tissue (15 mg) from each mouse of each group were homogenized in lysis buffer (Cell Signaling Technology, Inc., Beverly, MA, USA) with 1% NP40, 1 mM PMSF and 1 tablet/5 ml protease inhibitor mix (Complete, Mini; Roche Diagnostics Corporation, Indianapolis, IN). Protein sample from 10 mice of each group was pooled for further examination. For western blot analysis, protein samples (20 µg each) were subjected to SDS-PAGE in 4-12% gradient gel (Invitrogen) and immunblotting on immobilon-P transfer membranes (Millipore Corporation, Bedford, MA, USA). Desmin, p-smad2/3, total smad2/3, PAI-1, TNF-α, p47phox and Nox2 were assessed on the Western blots using mouse monoclonal anti-desmin IgG2a (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), rabbit anti-pSmad2/3 (sc-11769-R, Santa Cruz Biotechnology, Inc.), goat anti-total smad2/3 IgG (Santa Cruz Biotechnology, Inc.), mouse anti-PAI-1 (BD Transduction LaboratoriesTM, USA), mouse monoclonal anti-TNF-α IgG (sc57469, Santa Cruz Biotechnology, Inc.), mouse anti-gp91 [phox] (Nox2) IgG and mouse anti-p47 [phox] IgG (BD Biosciences San Jose, CA, USA). The immunostaining band was visualized and quantified as described previously [26]. Briefly, bound antibodies were detected by developing the blot in enhanced chemiluminescence (ECL)TM western blotting detection reagents (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, UK). Quantitation of the bands on autoradiograms was performed using a Bio-Rad GS-700 imaging densitometer (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Each protein level was corrected for the densitometric intensity of the β-actin (using mouse monoclonal anti-β-actin IgG). For comparison, this ratio was set at unity for normal control samples and other lanes on the same gel were expressed as fold-change over this value. All blots were run at least three times.
RNA preparation and real-time RT-PCR
Total RNA was extracted from renal cortex tissue using Tri Reagent according to the manufacturer’s instructions. Two micrograms of total RNA were reverse-transcribed using the superscript III first-strand synthesis system for RT-PCR kit (Invitrogen). Real-time RT-PCR was performed using the SYBR green dye I (Applied Biosystems, Foster City, CA, USA) and the ABI 7900 Sequence Detection System (Applied Biosystems) as described previously [26]. Samples were run as triplicates in separate tubes to permit quantification of the target gene normalized to β-actin. Sequences of primers used for fibrotic markers and sequences of new primers used for podocyte markers were described previously [26,33]. The specificity of the PCR products was confirmed on a 1.5% agarose gel by showing a specific single band with the expected size.
Statistical analysis
Data are expressed as mean ± S.D. with n representing the number of animals. The urinary albumin excretion (UAE) values were log10-transformed prior to comparison to account for the skewed distribution. Groups were analyzed by one-way ANOVA and subsequent Dunnett testing for multiple comparisons. P<0.05 was considered statistically significant.
Results
Systemic parameters
There was no significant difference in survival rate between the non-diabetic db/m mouse and diabetic db/db mouse with or without treatment. The baseline and final characteristics of the six groups of mice are presented in Table 1. At baseline, 18-week body weights of db/db mice were consistently greater than the corresponding values in db/m controls. The non-diabetic db/m mice gained approximately 2.3 g in body weight in 4 weeks. In contrast, the diabetic db/db mice lost ~ 4.6 g in body weight during the experimental period. Diabetic mice remained hyperglycemic throughout the experimental period as indicated by blood glucose and glycosylated hemoglobin (HbA1c) levels and HbA1c levels were further increased by 1.09%. Body weight and hyperglycemia were not affected by either treatment. We also found that diabetic uninephrectomized db/db mice at 18 weeks of age still had normal blood pressure and treatment with enalapril or eplerenone or the combination of enalapril and eplerenone for 4 weeks from week 18 to week 22 had no effect on systolic blood pressure (SBP). Plasma K+ levels in db/db mice at 18 weeks of age were similar to those in non-diabetic db/m mice but were higher in db/db mice at 22 weeks of age regardless of treatment. As seen in other rodent studies and contrary to observations in man [34], there were no treatment related effects on plasma K+ levels.
Table 1.
Clinical parameters of the experimental groups of mice
| db/m -22 wk (n=9) | db/db -18 wk (n=8) | db/db -22 wk (n=8) | db/d+Ena -22 wk (n=9) | db/db+Epl -22 wk (n=9) | db/db+Ena +Epl-22 wk (n=8) | |
|---|---|---|---|---|---|---|
| Body wt. initial, g | 26.4±0.9 | 43.8±7.7* | 45.2±7.7* | 45.5±6.0* | 46.4±6.9* | 44.6±5.4* |
| Final, g | 28.7±2.4 | 40.6±8.1* | 38.5±7.7* | 39.6±7.4* | 40.2±8.0* | |
| SBP, mmHg | 92.2±0.9 | 95.2±7.7 | 92.2±21.6 | 90.2±7.3 | 105.7±17.6 | 99.2±23.5 |
| Plasma glucose, initial, mg/dl | 127±11.7 | 583±31.3* | 600±0* | 537±50* | 575±41.2* | 592±20.8* |
| Final, mg/dl | 108±30.1 | 600±0.0* | 600±0.0* | 578±39.5* | 600±0.0* | |
| HbA1c (%) | ||||||
| Initial | 3.73±0.1 | 11.9±0.8* | 12.33±0.7* | 11.57 ±0.8* | 11.78 ±0.6* | 12.20 ±0.7* |
| Final | 3.98±0.0 | 13.42±0.3* | 13.36±0.4* | 13.01±0.3* | 13.88±0.1* | |
| UAE (µg/24 h), | ||||||
| Initial | 9.26±6.9 | 296.05±115.1 | 374.03±327.8 | 356.11±185.0 | 341.13±176.2 | 354.9±210.6 |
| Final | 3.18±0.5 | 475.3±355.5 | 188.37±123.2 | 202.2±160.8 | 100.27±63.4 | |
| logUAE, | ||||||
| Initial | 0.77±0.5 | 2.44±0.1* | 2.48±0.2* | 2.49±0.2* | 2.47±0.2* | 2.48±0.2* |
| Final | 0.49±0.1 | 2.58±0.3* | 2.20±0.3*,#,§ | 2.16±0.4*,#,§ | 1.90±0.3*,#,§ | |
| Aldosterone levels | ||||||
| In plasma (pg/ml) | 591.6±69.6 | 757.0±53.4* | 1065.3±116*,# | 1366.1±158.5*,# | 1166.4±68.5*,# | 1067±46.1*,# |
| In urine (ng/24 h) | 2.31±0.1 | 31.75±15.8* | 32.73±11.4* | 28.39±9.6* | 19.65±8.2*,#,§ | 18.05±7.7*,#,§ |
| Plasma Cr (µM) | 3.4±0.3 | 4.2±0.4 | 3.8±0.2 | 3.6±0.2 | 3.5±0.2 | 3.5±0.2 |
| Ccr (ml/min) | 0.81±0.2 | 1.83±0.5* | 2.43±0.7*,# | 2.67±0.7*,# | 2.27±0.6* | 2.38±0.9* |
| Plasma K+ (mM) | 3.3±0.1 | 3.4±0.1 | 4.7±0.2*,# | 4.6±0.2*,# | 5.0±0.2*,# | 4.7±0.2*,# |
Diabetic db/db mice were treated with enalapril (Ena), eplerenone (Epl), or combination of enalapril plus eplerenone (Ena+Epl) for 4 weeks between 18 and 22 weeks of age. Unless specified, parameters were recorded at the end of the experimental period (22 weeks of age). wt, body weight. SBP: systolic blood pressure measured at week 18 (for group db/db-18 wk) or week 22 (for other groups). UAE: urinary albumin excretion. Cr: creatinine. Ccr: clearance of creatinine.
P<0.05 vs. db/m;
P<0.05 vs. db/db at 18 wk.
P<0.05 vs. db/db at 22 wk.
Plasma and urinary aldosterone levels were markedly increased in diabetic db/db mice compared with non-diabetic db/m control mice. Enalapril treatment had no significant effect on the elevated plasma and urinary aldosterone levels. Interestingly, urinary aldosterone levels were decreased in eplerenone alone or in combination with enalapril treated db/db mice.
Plasma creatinine concentration of diabetic db/db mice was identical to those of the non-diabetic db/m mice. Treatment with enalapril and eplerenone either alone or in combination had no effect on plasma creatinine levels. However, the calculated Ccr in db/db mice at 18 weeks of age was significantly higher than in db/m mice and was further increased in db/db mice at 22 weeks of age. These results may reflect hyperfiltration, an early symptom of diabetic nephropathy. The Ccr was not affected by treatment with enalapril, eplerenone or enalapril plus eplerenone.
Effect of combination therapy with ACEI and eplerenone on albuminuria in the db/db mice
At the beginning of intervention (18 weeks of age), UAE in db/db mice was similar among the various groups and higher than in db/m mice (Table 1). In vehicles treated db/db mice at week 22, a statistically non-significant elevation of UAE by 27% vs. week 18 was observed. All treatments reduced the UAE to lower than baseline values seen in db/db mice at the beginning of intervention (18 weeks of age). Treatment with enalapril or eplerenone alone reduced UAE by 47% and 41% compared to baseline, respectively. When enalapril was combined with eplerenone, an enhanced anti-albuminuric effect was achieved, leading to a total reduction of 72% in UAE when compared with vehicles treated db/db mice at week 22.
Effect of combination therapy with ACEI and eplerenone on podocyte injury in the db/db mice
As shown in Figure 1A and 1B by immunofluorescent staining, non-diabetic db/m mice showed intense and linear staining for podocin along the capillaries in glomeruli. In contrast, an attenuation of staining by 21% for podocin was observed in db/db mice at 18 weeks of age, which was further decreased by another 21% at 22 weeks of age. The loss of podocyte specific podocin seen in diabetes between weeks 18 and 22 was largely and similarly prevented by enalapril or eplerenone alone. Combining enalapril and eplerenone was more effective in maintaining glomerular levels of podocin than either treatment alone (Figure 1A and 1B).
Figure 1.
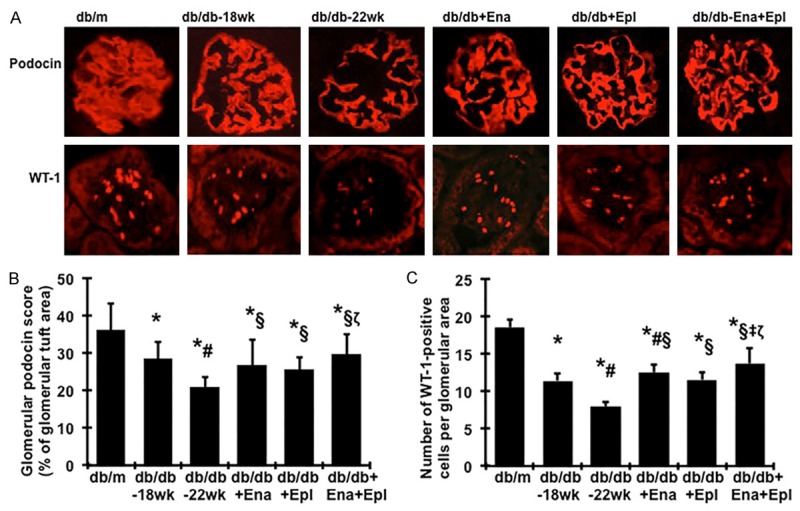
Effect of enalapril, elperenone and combination therapy on glomerular immunofluorescent staining for podocin and WT-1 in diabetic db/db mice from weeks 18 to 22. (A) Representative photomicrographs (at original magnification × 400) of glomeruli from normal control mice (db/m), diabetic db/db mice without treatment at week 18 (db/db-18 wk) or at week 22 (db/db-22 wk) and diabetic db/db mice treated with enalapril (Ena), eplerenone (Epl), combination of enalapril and eplerenone (Ena+Epl). Graphic representation of glomerular podocin (B) staining scores or glomerular number of WT-1 positive podocytes (C) was shown below. *P<0.05, vs. db/m. #P<0.05, vs. db/db-18 wk. §P<0.05, vs. db/db-22 wk. ‡P<0.05, vs. db/db+Ena. ζP<0.05, vs. db/db+Epl.
WT-1 is a nuclear protein specific for podocyte and parietal glomerular epithelial cell in the adult kidney [35,36]. WT-1-positive cells within the glomerular tuft excluding the parietal epithelium were counted in diabetic mice and the cell number per glomerulus was calculated as described in the method section. Consistent with the staining of podocin, diabetic glomeruli from disease-control db/db mice at 18 weeks of age contained fewer podocytes than did glomeruli from normal-control db/m mice, as illustrated by immunofluorescence staining in Figure 1A, 1C. The average number of podocytes per glomerular cross section was further decreased by 19% in db/db mice at 22 weeks of age. Treatment of diabetic db/db mice with enalapril or eplerenone alone reversed the decreases in glomerular WT-1 positive cells between weeks 18 and 22. Mice treated with the enalapril and eplerenone combination had increased podocyte numbers compared to mice treated with enalapril or eplerenone alone (Figure 1A, 1C).
By western blot, we observed that desmin levels in renal cortex tissues, a conventional marker for podocyte injury, were 66% higher in diabetic db/db at week 18 and 81% higher in diabetic db/db at week 22 vs. db/m mice, as shown in Figure 2A and 2B. Although we could not confirm whether the elevated production of desmin was from podocytes, the altered desmin levels were consistent with the changes of the slit-diaphragm-associated protein podocin and glomerular podocyte numbers shown in Figure 1, which suggests that increased renal desmin production is related to podocyte injury in diabetic db/db mice. Consistent with the results on podocyte numbers and podocin production, treatment with enalapril or eplerenone prevented the disease-induced increase of desmin protein. Combination treatment with eplerenone and enalapril further reduced disease-induced renal desmin production to levels lower than non-diabetic db/m controls (Figure 2).
Figure 2.
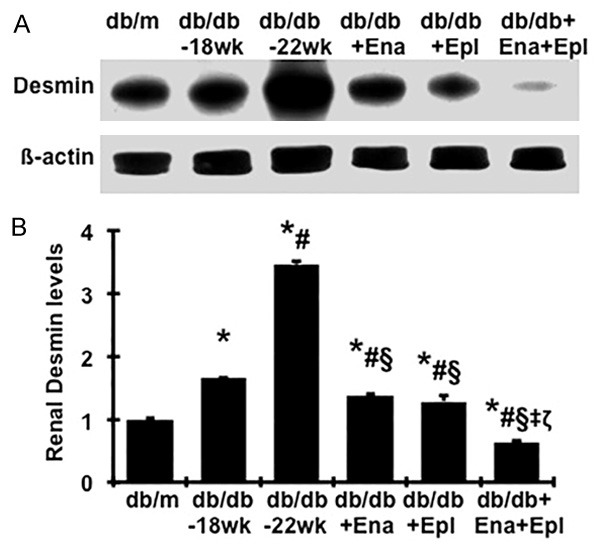
Effect of enalapril, elperenone and combination therapy on renal desmin protein levels in diabetic db/db mice from weeks 18 to 22. A. The representative western blots illustrate desmin protein expression. B. The respective graphs summarize the results of band density measurements. Changes in desmin protein expression were determined by normalizing against the densitometric intensity of its β-actin for each sample. For comparison, this ratio was set at unity for normal control samples and other groups were expressed as fold-increase over this value. *P<0.05, vs. db/m. #P<0.05, vs. db/db-18 wk. §P<0.05, vs. db/db-22 wk. ‡P<0.05, vs. db/db+Ena. ζP<0.05, vs. db/db+Epl.
In agreement with the protein levels, gene expression levels of podocin and WT-1 were lower in diabetic db/db mice at week 18 when compared to those of db/m controls, and a further reduced expression was seen at week 22 (Figure 3A, 3B). Diabetes induced decreases in mRNA expression of these podocyte markers were significantly ameliorated by treatment with enalapril or eplerenone alone. Addition of eplerenone to enalapril further increased podocin and WT-1 mRNA expression by 50% and 31% to the total increases of 75% and 100% respectively when compared to vehicles treated db/db mice at week 22. mRNA expression of desmin was markedly increased in diabetic renal tissue, particularly at week 22 by 2.15-fold, compared to that found in normal renal tissue, indicating that podocyte injury progressed in diabetic nephropathy from weeks 18 to 22. Addition of eplerenone to enalapril resulted in further reduced desmin mRNA expression to the levels close to that of non-diabetic normal controls (Figure 3C).
Figure 3.
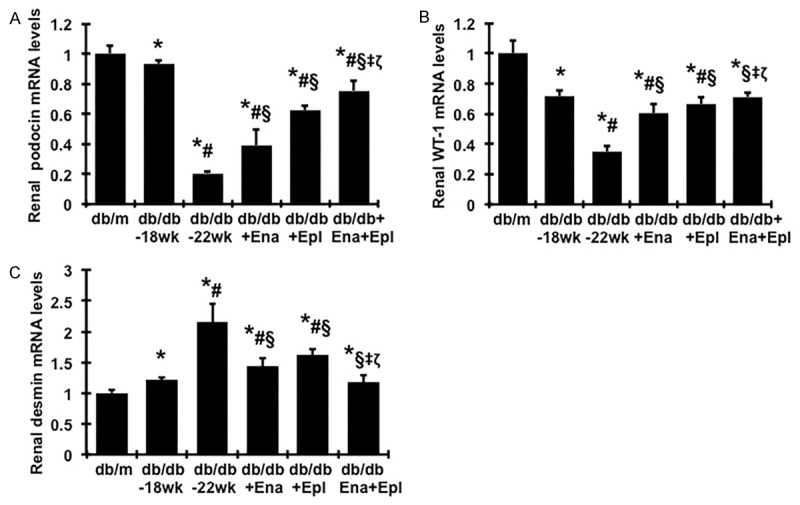
Effect of enalapril, elperenone and combination therapy on renal mRNA expression of podocin (A), WT-1 (B), and desmin (C) in diabetic db/db mice from weeks 18 to 22. Expression of mRNA was determined by real-time RT/PCR. Changes in mRNA levels were determined by first correcting the amplification of β-actin for each sample. For comparison, this ratio was set at unity for normal control samples and other groups were expressed as fold-increase over this value. *P<0.05, vs. db/m. #P<0.05, vs. db/db-18 wk. §P<0.05, vs. db/db-22 wk. ‡P<0.05, vs. db/db+Ena. ζP<0.05, vs. db/db+Epl.
Collectively, these data suggest that the ACE inhibitor enalapril or the MR antagonist eplerenone at the doses used here are equally effective in arresting the progression of markers of glomerular podocyte injury in the db/db mice from weeks 18 to 22. The combination of enalapril and eplerenone was more effective in reducing podocyte injury than either treatment alone. These results are consistent with their effects on the remission of albuminuria, indicating that the combined therapy of enalapril and eplerenone provides a more protective effect on diabetes induced podocyte injury than monotherapy with either drug.
Effect of combination therapy with ACEI and eplerenone on renal fibrosis in the db/db mice
Renal histology
At the end of the study (22 weeks), the glomeruli of db/db mice demonstrated increased glomerulosclerosis with evident accumulation of PAS-positive extracellular matrix (ECM) proteins including fibronectin and collagen IV in the mesangium compared with glomeruli of db/m mice (Figure 4). Treatment with either enalapril or eplerenone prevented increases in glomerular matrix protein deposition in diabetic db/db mice between 18 and 22 weeks. Of note, addition of eplerenone to enalapril resulted in additional 28%, 61% and 33% reduction in glomerular ECM accumulation, FN and collagen IV deposition, respectively, compared to enalapril used alone.
Figure 4.
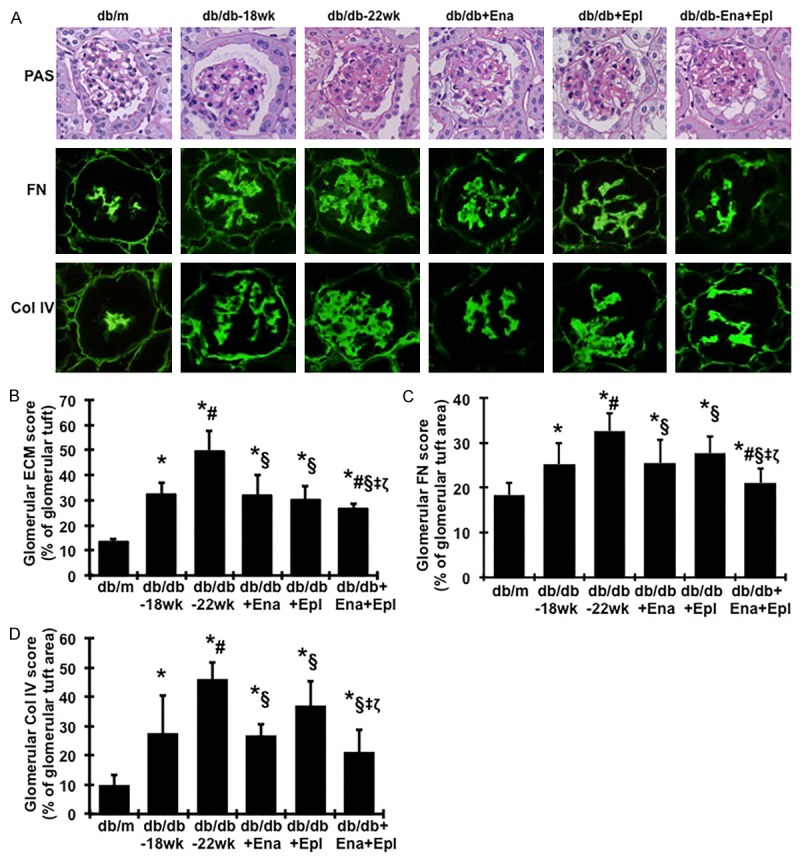
Effect of enalapril, elperenone and combination therapy on glomerular matrix protein accumulation in diabetic db/db mice from weeks 18 to 22. (A) The histological sections stained with PAS and glomerular immunofluorescent staining for fibronectin (FN) and type IV collagen (Col IV) are presented at 400 × magnification. Representative photomicrographs of glomeruli from normal control mice (db/m), diabetic db/db mice without treatment at week 18 (db/db-18 wk) or at week 22 (db/db-22 wk) and diabetic db/db mice treated with enalapril (Ena), eplerenone (Epl), combination of enalapril and eplerenone (Ena+Epl). Graphic representations of glomerular matrix score (B), FN staining score (C) and Col IV staining score (D) are shown below. *P<0.05, vs. db/m. #P<0.05, vs. db/db-18 wk. §P<0.05, vs. db/db-22 wk. ‡P<0.05, vs. db/db+Ena. ζP<0.05, vs. db/db+Epl.
TGFβ1 and PAI-1 levels in renal cortex
The levels of profibrotic molecules TGFβ1 and PAI-1 in renal cortex were elevated in db/db mice compared to db/m and were further increased from week 18 to week 22 (Figure 5). Enalapril or eplerenone treatment alone completely prevented the increased TGFβ1 levels (Figure 5A) and similarly attenuated the increases of PAI-1 from week 18 to week 22 by 70% when compared with untreated db/db mice (Figure 5C, 5D). A combination of enalapril and eplerenone resulted in a further reduction in renal TGFβ1 and PAI-1 levels when compared with animals given enalapril alone. Smad proteins are important intracellular transducers of TGFβ1 signaling. We asked whether the Smad pathway is activated in the diabetic kidney. Smad2/3 activation was elevated in db/db mice at week 18 compared to db/m (Figure 5B). A further increase, which was completely reversed by enalapril treatment, was seen between week 18 and week 22. Addition of eplerenone to enalapril further lowered the levels of pSmad2/3 to values comparable to non-diabetic db/m controls. Blots for total Smad2/3 or β-actin signals confirmed that samples were equally loaded (Figure 5B).
Figure 5.
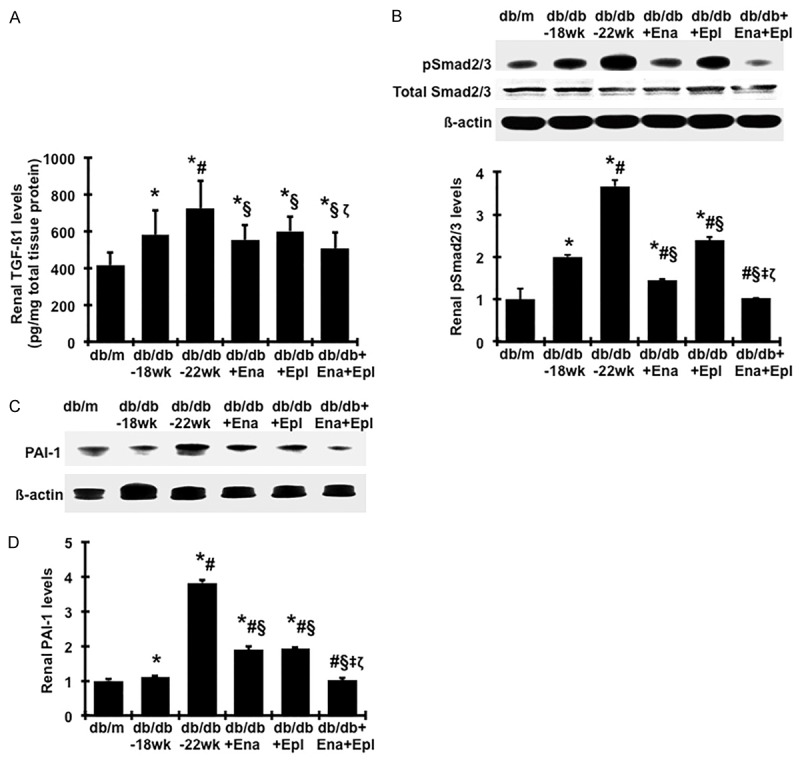
Effect of enalapril, elperenone and combination therapy on renal production of profibrotic markers in diabetic db/db mice from weeks 18 to 22. (A) Total TGFβ1 protein levels in renal cortical tissues were measured by ELISA. (B-D) Renal Smad2 signaling and protein production of PAI-1 were determined by western blot assay. The representative western blots illustrate phosphorylated Smad2/3 (p-Smad2/3), total Smad2/3 and β-actin protein expression (B) PAI-1 and β-actin protein expression (C) in renal cortical tissue tissues. The graphs summarize the results of band density measurements for PAI-1 (D). *P<0.05, vs. db/m. #P<0.05, vs. db/db-18 wk. §P<0.05, vs. db/db-22 wk. ‡P<0.05, vs. db/db+Ena. ζP<0.05, vs. db/db+Epl.
mRNA expression of TGF-β1, PAI-1 and matrix proteins in renal cortex
Renal TGFβ1, PAI-1, FN and α 1(IV) collagen mRNA levels were elevated in db/db mice at week 22 compared to week 18 (Figure 6A-D). Treatment with enalapril or eplerenone alone prevented the increased of TGFβ1, PAI-1, FN and α 1 (IV) collagen mRNA expression and the combination treatment yielded further reduced expression levels (Figure 6A-D).
Figure 6.
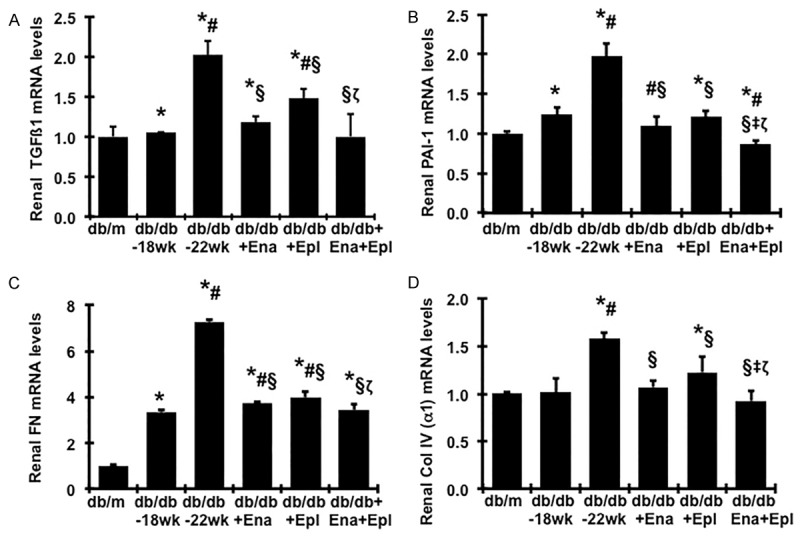
Effect of enalapril, elperenone and combination therapy on renal mRNA expression of fibrotic markers in diabetic db/db mice from weeks 18 to 22. Expression of mRNA was determined by real-time RT/PCR. Changes in mRNA levels were determined by first correcting the amplification of β-actin for each sample. For comparison, this ratio was set at unity for normal control (db/m) samples and other groups were expressed as fold-increase over this value. A. Expression of TGF-β1mRNA. B. Expression of PAI-1 mRNA. C. Expression of FN mRNA. D. Expression of type α1 (IV) collagen mRNA. *P<0.05, vs. db/m. #P<0.05, vs. db/db-18 wk. §P<0.05, vs. db/db-22 wk. ‡P<0.05, vs. db/db+Ena. ζP<0.05, vs. db/db+Epl.
Effect of combination therapy with ACEI and eplerenone on renal inflammation and oxidative stress in the db/db mice
As renal inflammation and oxidative stress contribute to the development of diabetic nephropathy [37-39], we further investigated whether the additive renoprotective effect of the combination therapy with enalapril and eplerenone is associated with a reduction of renal inflammation and oxidative stress. Renal tumor necrosis factor-α (TNF-α) levels were higher in the untreated db/db mice, compared with db/m mice (Figure 7A) was further increased during disease progression. Urinary TNF-α and monocyte-chemoattractant protein-1 (MCP-1) levels were also markedly increased in untreated diabetic mice, compared with non-diabetic db/m mice (Figure 7B, 7C). Enalapril or eplerenone treatment caused reduced renal TNF-α levels, compared to untreated mice. The combination treatment with enalapril and eplerenone yielded further reduced renal TNF-α levels as well as reduced urinary TNF-α and MCP-1 excretion.
Figure 7.
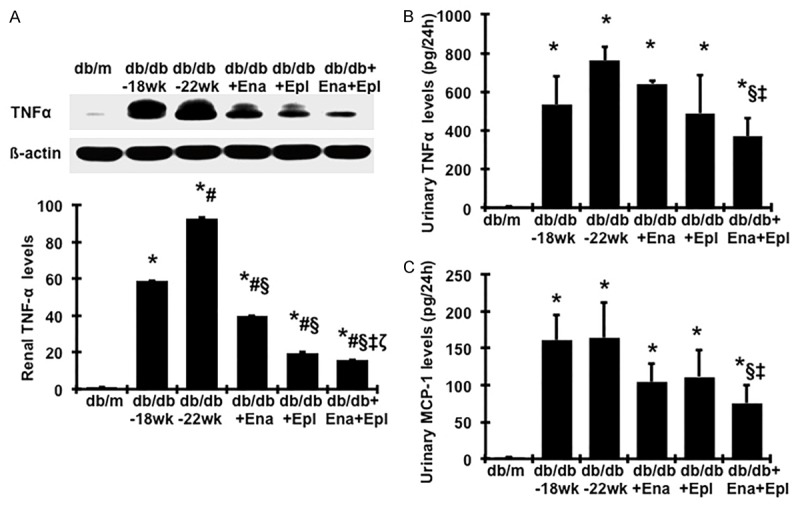
Effect of enalapril, elperenone and combination therapy on protein production of TNFα in renal cortical tissue and urine TNFα and MCP-1 levels. (A) Representative western blots illustrating TNFα and β-actin protein expression. The lower respective graph summarizes the results of band density measurements. Urinary TNFα (B) MCP-1 (C) levels were measured by ELISA. *P<0.05, vs. db/m. #P<0.05, vs. db/db-18 wk. §P<0.05, vs. db/db-22 wk. ‡P<0.05, vs. db/db+Ena. ζP<0.05, vs. db/db+Epl. Diabetic db/db mice were treated with enalapril (Ena), eplerenone (Epl), combination of enalapril and eplerenone (Ena+Epl) from weeks 18 to 22.
Renal Nox2 and p47phox protein levels, the family members of the NADPH oxidase, were elevated in untreated db/db mice, compared with db/m (Figure 8A, 8B), indicating significant activation of Nox2 in db/db mice, particularly at 22 weeks of age. Elevated levels of Nox2 and its regulatory subunit p47phox from week 18 to week 22 in db/db mice were prevented by enalapril treatment and partially prevented by eplerenone treatment. Combination treatment with enalapril and eplerenone normalized Nox2 and p47phox proteins to the levels observed in normal db/m mice (Figure 8A, 8B).
Figure 8.
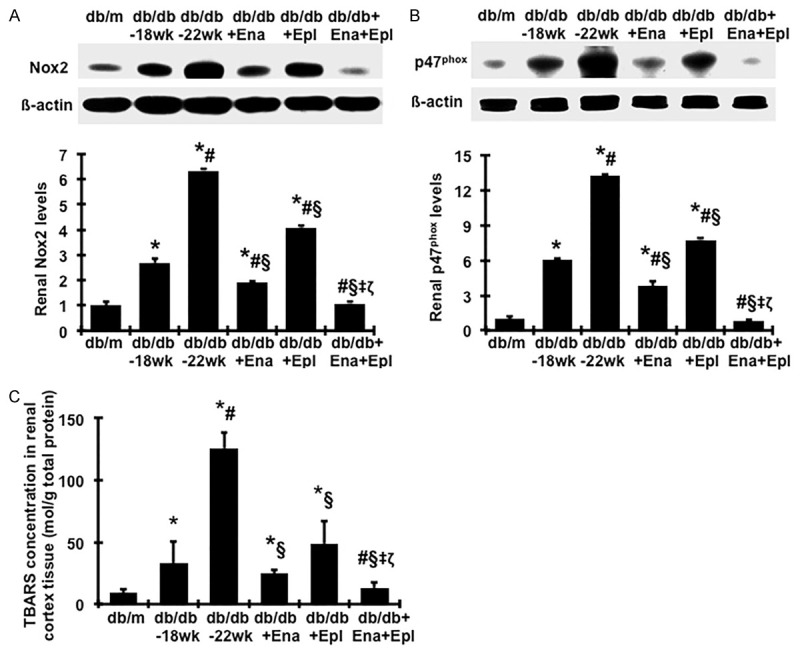
Effect of enalapril, elperenone and combination therapy on protein production of renal NAPDH oxidases and the TBARS levels in renal cortex tissue. Representative western blots illustrate Nox2, p47phox and β-actin protein expression and graphic representation of the mean band intensity of Nox2 (A) and p47phox (B) normalized against the band intensity of β-actin. (C) Renal TBARS levels were detected by a colorimetric assay. *P<0.05, vs. db/m. #P<0.05, vs. db/db-18 wk. §P<0.05, vs. db/db-22 wk. ‡P<0.05, vs. db/db+Ena. ζP<0.05, vs. db/db+Epl. Diabetic db/db mice were treated with enalapril (Ena), eplerenone (Epl), combination of enalapril and eplerenone (Ena+Epl) from weeks 18 to 22.
Lipid peroxidation, a marker of oxidative stress, was measured by TBARS levels. As seen in Figure 8C, renal TBARS levels were consistently and dramatically increased in diabetic db/db mice at 18 weeks of age compared to nondiabetic db/m mice, and were further increased at 22 weeks of age. Treatment with enalapril or eplerenone alone significantly lowered the elevated renal TBASRS levels. Moreover, diabetic db/db mice treated with enalapril and eplerenone in combination had less renal TBARS production than those treated with either drug alone. These data suggest that the combined therapy of enalapril and eplerenone attenuates renal inflammation and NAPDH oxidase-mediated oxidative stress in diabetic db/db mice to a greater extent than either enalapril or eplerenone alone.
Discussion
In the present study, we demonstrated the protective effect of eplerenone on the progression of diabetic nephropathy in a mouse model reflective of type 2 diabetic nephropathy in humans. The uninephrectomized diabetic db/db mice developed more severe glomerular mesangial expansion and significant albuminuria by 18 weeks of age. Disease progression was remarkable between 18 and 22 weeks of age, which was associated with increased renal oxidative stress, inflammation, TGFβ and PAI-1 expression and production of fibronectin and other matrix proteins, and podocyte injury, features that are reminiscent of human diabetic nephropathy. However, the increase in albuminuria was halted and the progression of podocyte injury and glomerulosclerosis was ameliorated by eplerenone. It has been well demonstrated that MR antagonist reduced albuminuria and histopathological evidence of renal damage in the course of development of the disease in animal models of both type 1 and 2 diabetes [21,22,40,41]. The present study extends these findings to the model of progressive diabetic nephropathy, i.e. damage is present before initiating MR antagonist therapy, which is likely occurring in humans. Furthermore, the significantly anti-albuminuric, anti-renal TGFβ1 synthesis and its signaling, anti-renal NAPDH oxidase-mediated oxidative stress and inflammation properties of eplerenone observed in the present study may contribute to its therapeutic effect in slowing the progression of diabetic nephropathy.
Given that local renal aldosterone levels could not be directly reflected by circulating plasma aldosterone l levels [42], urinary aldosterone levels may reflect aldosterone production in kidney more closely. In consistent with previous reports [43], aldosterone concentration is markedly up-regulated in the diabetic kidney (Table 1). Thus, pharmacologic interruption of the effects of aldosterone at the tissue level by agents that reduce aldosterone generation and/or action could be especially useful in slowing the progression of diabetic nephropathy. The present study specially compared the renoprotective properties of eplerenone that selectively inhibits the binding of aldosterone to its receptor with those of enalapril that should reduce aldosterone generation used alone on the regression of kidney disease in diabetes. The similar beneficial effects of both drugs suggest that blockade of the renin-angiotensin-aldosterone system at the levels of MR is as effective as Ang II blockade in reducing renal injury in diabetes.
Of importance is that adding eplerenone treatment to ACE inhibition even for a short period, 4 weeks still caused a further decreased in urinary albumin excretion, podocyte injury and glomerulosclerosis but had no treatment related effect on plasma K+, suggesting that the inhibition of the aldosterone system has additional beneficial effects independent of Ang II blockade on progressive diabetic nephropathy. These results are consistent with the previous report that long-term treatment with eplerenone at large dose and enalapril for 6 months ameliorated progression of diabetic renal disease in OLETF rats, a type II diabetic rat model [30]. Although we were unable to provide direct evidence if diabetic db/db mice developed aldosterone escape during ACEI therapy as observed in humans, diabetic mice treated with enalapril alone or in combination with ARB or DRI still had higher levels of aldosterone as observed in the present and previous studies [43]. In addition, increasing data indicate that aldosterone production can be driven by non-angiotensin II stimuli [7,44,45]. It has been shown that aldosterone promotes fibrosis, inflammation, and generation of reactive oxygen species independent of Ang II [46]. We have previously shown that aldosterone-induced PAI-1 overexpression by both renal mesangial cells and tubular cells in vitro is partially mediated by TGF-β1 [47]. Increased PAI-1 leads to decreased ECM degradation. While aldosterone alone induces TGF-β1 weakly, aldosterone and TGF-β1 added together produce dramatic synergistic effects on PAI-1 production and subsequent ECM accumulation. Apparently, the elevated aldosterone due to ACEI escape or induced by diabetes itself in vivo, may amplify its fibrotic actions through a synergistic interaction of aldosterone and aldosterone-induced or disease-induced TGF-β1. It is, thus, not surprising that adding eplerenone at an optimal dose to enalapril when maximal effective dose of enalapril was applied not only further reduced albuminuria, but also further suppressed active TGF-β1 signaling, renal inflammation and oxidative stress and yielded further beneficial effect on renal outcomes when compared with enalapril treated alone. Our study further clarified the mechanisms that underlie the renoprotective actions of this combination therapy in diabetes.
Interestingly, in attempts to prevent the progression of diabetic nephropathy by optimal RAS inhibitors, we have compared a number of therapies in the same model in our laboratory using consistent methods for evaluation. We have shown that either of enalapril (ACEI) or valsartan (ARB) or aliskiren (DRI) was clearly effective in slowing the progression of renal fibrosis in the db/db mice, but the kidney disease was only partially ameliorated [43]. We further showed that the addition of the maximally effective dose of enalapril or valsartan to that of aliskiren yielded no additional reduction of disease measures, in accordance with clinical trial results [2,48,49] and the notion that these drugs act through the same Ang II-dependent pathway. The current data from the present study imply that aldosterone inhibition based on Ang II blockade provides enhanced renal protective effects in diabetes, indicating that alternative combination strategies that include Ang II inhibitors and other agents such as MR antagonists, which act through different mechanisms, should be pursued to maximize renoprection in diabetes.
However, hyperkalemia is a mechanism-based and serious adverse effect for the use of MR antagonists in patients [17,29] although the risk of hyperkalemia has not been approved in rodent models including in the present study. A novel type of MR antagonist with a low potential risk of hyperkalemia is desired for patients with diabetes.
In conclusion, the present study demonstrated that eplerenone and enalapril identically slowed the progression of diabetic nephropathy in type 2 diabetic db/db mice. The addition of eplerenone to enalapril is more effective in decreasing proteinuria, podocyte injury, renal inflammation and oxidative stress and confers extra renoprotection over the use of either drug alone. Taken together, the addition of MR antagonist to ACEI may further block the profibrotic effects of aldosterone due to escape or diabetes-induction and thus potentially improve clinical outcomes in progressive diabetic nephropathy than ACEI alone if the effects of treatment on plasma K+ can be avoided in humans.
Acknowledgements
We thank Beux Dmitrich for his excellent assistant with animal studies. This work was supported by a grant from AstraZeneca R&D (Y.H.) and National Institutes of Health grants K01DK077955 (Y.H.) and R21DK081815 (Y.H.). Dr. Guangyu Zhou is the recipient of postdoctoral fellowship grant from Shengjing Hospital Foundation affiliated to China Medical University.
Disclosure of conflict of interest
None.
References
- 1.Kagami S, Border WA, Miller DE, Noble NA. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J Clin Invest. 1994;93:2431–2437. doi: 10.1172/JCI117251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan T, Anderson A, Bertram D, MacInnis RJ. Effect of candesartan and lisinopril alone and in combination on blood pressure and microalbuminuria. J Renin Angiotensin Aldosterone Syst. 2004;5:64–71. doi: 10.3317/jraas.2004.012. [DOI] [PubMed] [Google Scholar]
- 3.Forclaz A, Maillard M, Nussberger J, Brunner HR, Burnier M. Angiotensin II receptor blockade: is there truly a benefit of adding an ACE inhibitor? Hypertension. 2003;41:31–36. doi: 10.1161/01.hyp.0000047512.58862.a9. [DOI] [PubMed] [Google Scholar]
- 4.Doulton TW. ACE inhibitor-angiotensin receptor blocker combinations: a clinician’s perspective. Mini Rev Med Chem. 2006;6:491–497. doi: 10.2174/138955706776876168. [DOI] [PubMed] [Google Scholar]
- 5.Peters H, Border WA, Noble NA. Targeting TGFbeta overexpression in renal disease: maximizing the antifibrotic action of angiotensin II blockade. Kidney Int. 1998;54:1570–1580. doi: 10.1046/j.1523-1755.1998.00164.x. [DOI] [PubMed] [Google Scholar]
- 6.Schjoedt KJ, Andersen S, Rossing P, Tarnow L, Parving HH. Aldosterone escape during blockade of the renin-angiotensin-aldosterone system in diabetic nephropathy is associated with enhanced decline in glomerular filtration rate. Diabetologia. 2004;47:1936–1939. doi: 10.1007/s00125-004-1542-0. [DOI] [PubMed] [Google Scholar]
- 7.Bomback AS, Rekhtman Y, Klemmer PJ, Canetta PA, Radhakrishnan J, Appel GB. Aldosterone breakthrough during aliskiren, valsartan, and combination (aliskiren + valsartan) therapy. J Am Soc Hypertens. 2012;6:338–345. doi: 10.1016/j.jash.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Li S, Wu P, Zhong S, Guo Z, Lai W, Zhang Y, Liang X, Xiu J, Li J, Liu Y. Effects of long-term enalapril and losartan therapy of hypertension on cardiovascular aldosterone. Horm Res. 2001;55:293–297. doi: 10.1159/000050016. [DOI] [PubMed] [Google Scholar]
- 9.Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001;345:1689–1697. doi: 10.1056/NEJMra000050. [DOI] [PubMed] [Google Scholar]
- 10.Epstein M. Aldosterone as a determinant of cardiovascular and renal dysfunction. J R Soc Med. 2001;94:378–383. doi: 10.1177/014107680109400803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 12.Farquharson CA, Struthers AD. Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation. 2000;101:594–597. doi: 10.1161/01.cir.101.6.594. [DOI] [PubMed] [Google Scholar]
- 13.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 14.Schjoedt KJ, Rossing K, Juhl TR, Boomsma F, Tarnow L, Rossing P, Parving HH. Beneficial impact of spironolactone on nephrotic range albuminuria in diabetic nephropathy. Kidney Int. 2006;70:536–542. doi: 10.1038/sj.ki.5001580. [DOI] [PubMed] [Google Scholar]
- 15.Chrysostomou A, Becker G. Spironolactone in addition to ACE inhibition to reduce proteinuria in patients with chronic renal disease. N Engl J Med. 2001;345:925–926. doi: 10.1056/NEJM200109203451215. [DOI] [PubMed] [Google Scholar]
- 16.Rachmani R, Slavachevsky I, Amit M, Levi Z, Kedar Y, Berla M, Ravid M. The effect of spironolactone, cilazapril and their combination on albuminuria in patients with hypertension and diabetic nephropathy is independent of blood pressure reduction: a randomized controlled study. Diabet Med. 2004;21:471–475. doi: 10.1111/j.1464-5491.2004.01194.x. [DOI] [PubMed] [Google Scholar]
- 17.Epstein M, Williams GH, Weinberger M, Lewin A, Krause S, Mukherjee R, Patni R, Beckerman B. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2006;1:940–951. doi: 10.2215/CJN.00240106. [DOI] [PubMed] [Google Scholar]
- 18.Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003;63:1791–1800. doi: 10.1046/j.1523-1755.2003.00929.x. [DOI] [PubMed] [Google Scholar]
- 19.Rocha R, Chander PN, Khanna K, Zuckerman A, Stier CT Jr. Mineralocorticoid blockade reduces vascular injury in stroke-prone hypertensive rats. Hypertension. 1998;31:451–458. doi: 10.1161/01.hyp.31.1.451. [DOI] [PubMed] [Google Scholar]
- 20.Nagase M, Shibata S, Yoshida S, Nagase T, Gotoda T, Fujita T. Podocyte injury underlies the glomerulopathy of Dahl salt-hypertensive rats and is reversed by aldosterone blocker. Hypertension. 2006;47:1084–1093. doi: 10.1161/01.HYP.0000222003.28517.99. [DOI] [PubMed] [Google Scholar]
- 21.Fujisawa G, Okada K, Muto S, Fujita N, Itabashi N, Kusano E, Ishibashi S. Spironolactone prevents early renal injury in streptozotocin-induced diabetic rats. Kidney Int. 2004;66:1493–1502. doi: 10.1111/j.1523-1755.2004.00913.x. [DOI] [PubMed] [Google Scholar]
- 22.Han KH, Kang YS, Han SY, Jee YH, Lee MH, Han JY, Kim HK, Kim YS, Cha DR. Spironolactone ameliorates renal injury and connective tissue growth factor expression in type II diabetic rats. Kidney Int. 2006;70:111–120. doi: 10.1038/sj.ki.5000438. [DOI] [PubMed] [Google Scholar]
- 23.Gullulu M, Akdag I, Kahvecioglu S, Filiz G, Savci V. Aldosterone blockage in proliferative glomerulonephritis prevents not only fibrosis, but proliferation as well. Ren Fail. 2006;8:509–514. doi: 10.1080/08860220600779033. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Rojas J, Blanco JA, Cruz C, Trujillo J, Vaidya VS, Uribe N, Bonventre JV, Gamba G, Bobadilla NA. Mineralocorticoid receptor blockade confers renoprotection in preexisting chronic cyclosporine nephrotoxicity. Am J Physiol Renal Physiol. 2007;292:F131–139. doi: 10.1152/ajprenal.00147.2006. [DOI] [PubMed] [Google Scholar]
- 25.Aldigier JC, Kanjanbuch T, Ma LJ, Brown NJ, Fogo AB. Regression of existing glomerulosclerosis by inhibition of aldosterone. J Am Soc Nephrol. 2005;16:3306–3314. doi: 10.1681/ASN.2004090804. [DOI] [PubMed] [Google Scholar]
- 26.Huang Y, Border WA, Yu L, Zhang J, Lawrence DA, Noble NA. A PAI-1 Mutant, PAI-1R, Slows Progression of Diabetic Nephropathy. J Am Soc Nephrol. 2008;19:329–338. doi: 10.1681/ASN.2007040510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu C, Zhou G, Noble NA, Border WA, Cheung AK, Huang Y. Targeting reduction of proteinuria in glomerulonephritis: Maximizing the antifibrotic effect of valsartan by protecting podocytes. J Renin Angiotensin Aldosterone Syst. 2014;15:177–189. doi: 10.1177/1470320312466127. [DOI] [PubMed] [Google Scholar]
- 28.Kang YS, Ko GJ, Lee MH, Song HK, Han SY, Han KH, Kim HK, Han JY, Cha DR. Effect of eplerenone, enalapril and their combination treatment on diabetic nephropathy in type II diabetic rats. Nephrol Dial Transplant. 2009;24:73–84. doi: 10.1093/ndt/gfn448. [DOI] [PubMed] [Google Scholar]
- 29.Mavrakanas TA, Gariani K, Martin PY. Mineralocorticoid receptor blockade in addition to angiotensin converting enzyme inhibitor or angiotensin II receptor blocker treatment: an emerging paradigm in diabetic nephropathy: a systematic review. Eur J Intern Med. 2014;25:173–176. doi: 10.1016/j.ejim.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Lian M, Hewitson TD, Wigg B, Samuel CS, Chow F, Becker GJ. Long-term mineralocorticoid receptor blockade ameliorates progression of experimental diabetic renal disease. Nephrol Dial Transplant. 2012;27:906–912. doi: 10.1093/ndt/gfr495. [DOI] [PubMed] [Google Scholar]
- 31.Rennard SI, Berg R, Martin GR, Foidart JM, Robey PG. Enzyme-linked immunoassay (ELISA) for connective tissue components. Anal Biochem. 1980;104:205–214. doi: 10.1016/0003-2697(80)90300-0. [DOI] [PubMed] [Google Scholar]
- 32.Yu L, Border WA, Anderson I, McCourt M, Huang Y, Noble NA. Combining TGF-β inhibition and Angiotensin II blockade results in enhanced anti-fibrotic effect. Kidney Int. 2004;66:1774–1784. doi: 10.1111/j.1523-1755.2004.00901.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhou G, Cheung AK, Liu X, Huang Y. Valsartan slows the progression of diabetic nephropathy in db/db mice via a reduction in podocyte injury, and renal oxidative stress and inflammation. Clin Sci (Lond) 2014;126:D707–720. doi: 10.1042/CS20130223. [DOI] [PubMed] [Google Scholar]
- 34.Ando K, Ohtsu H, Uchida S, Kaname S, Arakawa Y, Fujita T. Anti-albuminuric effect of the aldosterone blocker eplerenone in non-diabetic hypertensive patients with albuminuria: a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2:944–953. doi: 10.1016/S2213-8587(14)70194-9. [DOI] [PubMed] [Google Scholar]
- 35.Sanden SK, Wiggins JE, Goyal M, Riggs LK, Wiggins RC. Evaluation of a thick and thin section method for estimation of podocyte number, glomerular volume, and glomerular volume per podocyte in rat kidney with Wilms’ tumor-1 protein used as a podocyte nuclear marker. J Am Soc Nephrol. 2003;14:2484–2493. doi: 10.1097/01.asn.0000089829.45296.7c. [DOI] [PubMed] [Google Scholar]
- 36.Guo JK, Menke AL, Gubler MC, Clarke AR, Harrison D, Hammes A, Hastie ND, Schedl A. WT1 is a key regulator of podocyte function: reduced expression levels cause crescentic glomerulonephritis and mesangial sclerosis. Hum Mol Genet. 2002;11:651–659. doi: 10.1093/hmg/11.6.651. [DOI] [PubMed] [Google Scholar]
- 37.Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond) 2013;124:139–152. doi: 10.1042/CS20120198. [DOI] [PubMed] [Google Scholar]
- 38.Tojo A, Asaba K, Onozato ML. Suppressing renal NADPH oxidase to treat diabetic nephropathy. Expert Opin Ther Targets. 2007;11:1011–1018. doi: 10.1517/14728222.11.8.1011. [DOI] [PubMed] [Google Scholar]
- 39.Xu M, Dai DZ, Dai Y. Normalizing NADPH oxidase contributes to attenuating diabetic nephropathy by the dual endothelin receptor antagonist CPU0213 in rats. Am J Nephrol. 2009;29:252–256. doi: 10.1159/000157628. [DOI] [PubMed] [Google Scholar]
- 40.Taira M, Toba H, Murakami M, Iga I, Serizawa R, Murata S, Kobara M, Nakata T. Spironolactone exhibits direct renoprotective effects and inhibits renal renin-angiotensin-aldosterone system in diabetic rats. Eur J Pharmacol. 2008;589:264–271. doi: 10.1016/j.ejphar.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 41.Guo C, Martinez-Vasquez D, Mendez GP, Toniolo MF, Yao TM, Oestreicher EM, Kikuchi T, Lapointe N, Pojoga L, Williams GH, Ricchiuti V, Adler GK. Mineralocorticoid receptor antagonist reduces renal injury in rodent models of types 1 and 2 diabetes mellitus. Endocrinology. 2006;147:5363–5373. doi: 10.1210/en.2006-0944. [DOI] [PubMed] [Google Scholar]
- 42.Luik PT, Kerstens MN, Hoogenberg K, Navis GJ, Dullaart RP. Low plasma aldosterone despite normal plasma renin activity in uncomplicated type 1 diabetes mellitus: effects of RAAS stimulation. Eur J Clin Invest. 2003;233:787–793. doi: 10.1046/j.1365-2362.2003.01215.x. [DOI] [PubMed] [Google Scholar]
- 43.Zhou G, Liu X, Cheung AK, Huang Y. Efficacy of aliskiren, compared with angiotensin II blockade, in slowing the progression of diabetic nephropathy in db/db mice: should the combination therapy be a focus? Am J Transl Res. 2015;7:825–840. [PMC free article] [PubMed] [Google Scholar]
- 44.Sun QL, Li M, Rui HL, Chen YP. Inhibition of local aldosterone by eplerenone reduces renal structural damage in a novel model of chronic cyclosporine A nephrotoxicity. J Renin Angiotensin Aldosterone Syst. 2015;16:301–310. doi: 10.1177/1470320314561248. [DOI] [PubMed] [Google Scholar]
- 45.Siragy HM, Xue C. Local renal aldosterone production induces inflammation and matrix formation in kidneys of diabetic rats. Exp Physiol. 2018;93:817–824. doi: 10.1113/expphysiol.2008.042085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Namsolleck P, Unger T. Aldosterone synthase inhibitors in cardiovascular and renal diseases. Nephrol Dial Transplant. 2014;29(Suppl 1):i62–i68. doi: 10.1093/ndt/gft402. [DOI] [PubMed] [Google Scholar]
- 47.Huang W, Xu C, Kahng KW, Noble NA, Border WA, Huang Y. Aldosterone and TGF-beta1 synergistically increase PAI-1 and decrease matrix degradation in rat renal mesangial and fibroblast cells. Am J Physiol Renal Physiol. 2008;294:F1287–1295. doi: 10.1152/ajprenal.00017.2008. [DOI] [PubMed] [Google Scholar]
- 48.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsarinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, doubleblind, controlled trial. Lancet. 2008;372:547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 49.Harel Z, Gilbert C, Wald R, Bell C, Perl J, Juurlink D, Beyene J, Shah PS. The effect of combination treatment with aliskiren and blockers of the renin-angiotensin system on hyperkalaemia and acute kidney injury: systematic review and meta-analysis. BMJ. 2012;344:e42. doi: 10.1136/bmj.e42. [DOI] [PMC free article] [PubMed] [Google Scholar]


