Abstract
Multiple myeloma (MM) remains an incurable disease in most patients. Homoharringtonine (HHT) is a natural alkaloid produced by various Cephalotaxus species, and is approved by the United States of America Food and Drug Administration to treat patients with acute and chronic myeloid lymphoma. The aim of this study was to develop the high proportion polyethyleneglycol (PEG) of long-circulating HHT liposomes (LCL-HHT-H-PEG) and investigate its therapeutic applicability in vitro and in vivo against RPMI8226 MM. The optimized formulation of LCL-HHT-H-PEG showed a higher association with cytotoxicity against MM RPMI8226 cells than those of low proportion PEG of long-circulating HHT liposomes, liposome-encapsulated-HHT, micelle-HHT, and HHT in vitro. Therapeutic experiments in severe combined immunodeficient mice implanted with MM RPMI8226 cells by the subcutaeous route showed the significant inhibition of tumor growth in LCL-HHT-H-PEG group compared with the HHT group, and other control groups. The analysis of flow cytometry and transmission electron microscopy indicated that LCL-HHT-H-PEG exerted the cytotoxicity against MM by inducing the MM apoptosis in vitro and in vivo. This study suggests that our developed LCL-HHT-H-PEG may be regarded as a promising nano-device to deliver anti-MM drug HHT for treatment of MM patients.
Keywords: Multiple myeloma, homoharringtonine, long-circulating liposomes, polyethyleneglycol, cytotoxicity
Introduction
Multiple myeloma (MM) is a plasma cell neoplasm which constitutes about 10% of all hematologic malignancies, characterized by aberrant expansion of plasma cells within bone marrow and extramedullary sites. The treatment landscape for patients with MM is constantly evolving. Over the last decades, the paradigm of MM therapy has changed dramatically, from the conventional combination of oral melphatan plus prednisone, high-dose chemotherapy with stem cell support to the novel agents such proteasome inhibitors (bortezomib and carfilzomib) and immunomodulatory drugs (thalidomide, lenalidomide, and pomalidomide), which has led to an elongation of patient survival, yet MM runs an aggressive and incurable course in the vast majority of patients [1-3]. Drug resistance remains a major clinical challenge for MM treatment. The main cause of resistance in MM is the minimal residual disease cells that are resistant to the original therapy such as bortezomib and high dose melphalan in stem cell transplant. As such, there is an unmet need for new therapies to increase survival for MM patients [1,4].
Homoharringtonine (HHT) was originally isolated from the Cephalotaxus evergreen tree and has been widely used in traditional Chinese medicine for treatment of a variety of hematologic malignancies since the 1970s. Also, HHT was approved by the United States of America Food and Drug Administration for treating patients with in acute and chronic myeloid lymphoma [5-7]. However, HHT may induce unanticipated side effects in the gastrointestinal tract, such as diarrhea and nausea/vomiting as well as cardiac toxicity, which limits its wide clinical utility, but the mechanisms behind these severe side effects has not been clarified [5].
Accumulating evidence has showed that therapeutic approaches to cancer focus on developing novel delivery systems to reinforce the therapeutic efficacy of anticancer agents by targeted drugs to diseased cells and away from normal tissues [8,9]. Liposome encapsulation of anticancer drugs can alter their pharmacokinetics and in vivo distribution, resulting in reduced toxicity and enhanced efficacy [10-12]. Researchers have developed the long-circulating dose-independent liposome formulations containing polyethyleneglycol (PEG) on the surface of liposomes [9,13-15], which has been approved for treatment of varied tumors [16-19].
In the present study, liposomes with long-circulating properties were used as delivery system for HHT. We developed conventional and PEGylated long circulating liposomes containing HHT to investigate the inhibitory effect of this novel reagent on MM RPMI8226 cells by in vitro and in vivo experiments. Here, we showed that among the diverse formulations, the high proportion PEG of long-circulating HHT liposomes (LCL-HHT-H-PEG) exhibited encouraging results of inhibitory MM cells growth in vitro and in vivo RPMI8226 MM bearing non-obese-diabetic/severe-combined-immunodeficiency (NOD/SCID) mice, compared with low proportion PEG of long-circulating HHT liposomes (LCL-HHT-L-PEG), and other control formulations including general liposome-encapsulated-HHT (L-Enc-HHT), and micelle-HHT (MI-HHT). The further animal experiments demonstrated that our developed LCL-HHT-H-PEG appeared as a new and attractive approach to promote chemotherapy drug delivery for further clinic trials in treatment of MM patients.
Materials and methods
Cells and mice
Human MM RPMI 8226 cells were ordered from the Cell Institute in Beijing, People’s Republic of China. Cells were cultured in complete medium consisting of RPMI 1640, 2 mM L-glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin, and 10% fetal bovine serum at 37ºC in a humidified incubator containing 5% CO2. NOD/SCID mice at 5-6 weeks of age with 16-18 grams in weight were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., China. All the mice were maintained in a pathogen-free facility that has a 12-hour light/dark cycle and relative humidity ranged from 40% to 50% at 22ºC. All the animal experiments were performed in compliance with the Guidelines of the Animal Research Ethics Board of Southeast University. Full details of approval of the study can be found in the approval ID: 20080925.
Main materials
HHT was purchased from Dalian Meilun Biotech CO., Ltd of China. Matrigel was purchased from BD Biosciences (USA). The annexin V-PE/7-AAD apoptosis assay kit (KGA1017) was purchased from Nanjing KeyGen Biotech. Co., Ltd. 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N- [methoxy (polyethylene glycol)- 2000]-biotin (DSPE-PEG 2000-Biotin), and 25-NBD-cholesterol were purchased from Avanti Polar Lipids, Inc. All other chemicals were commercially available and of analytical grade.
Preparation and characterization of diverse HHT formulations
In preparation of the diverse HHT formulations, 20.42 mg of HHT, 206.24 mg of natural phospholipids, 10.15 mg of cholesterol and 100 mg of DSPE-PEG2000 were synchronously dissolved in 1 ml of ethanol with ultrasound. Then this solution was added in 20 ml pure water slowly drop by drop with vortex to get the mixture. After removing the organic solvent from the mixture, liposome suspension was obtained. The suspension was concentrated to reduce the size of liposomes with probe ultrasound. After determination of content, the suspension was freeze dried with 1.2 ml liposome and 1.2 ml (100 mg/ml) sucrose solution in each vial. Finally, we got the high proportion PEG of long-circulating HHT liposomes (1.006 mg/vial), and named it for LCL-HHT-H-PEG.
The LCL-HHT-L-PEG and L-Enc-HHT were prepared by the same method with different proportion of ingredients. The MI-HHT were prepared as follows: 21.01 mg of HHT and 208.07 mg of Solutol®HS15 were dissolved in 2 ml of ethanol with ultrasound. The following steps were performed according to LCL-HHT-H-PEG’s protocol. After determination of content, the suspension was freeze dried with 1.5 ml suspension and 1.5 ml (100 mg/ml) sucrose solution in each vial [20-22].
The morphology of the diverse HHT formulations was observed by using transmission electron microscopy (TEM, JEM-1011, Japan). The mean size, size distribution and zeta potential of HHT were analyzed by using a Malvern mastersizer 2000 (Malvern, UK).
Clonal assay of MM RPMI 8226 cells
For colony formation, the assay protocol followed was previously described in references 23 and 24. Briefly, 2×102 MM RPMI 8226 cell suspensions or tumor tissue suspensions were resuspended in 0.8 mL growth media containing 0.3% low melting temperature agarose (Promega, Madison, WI, USA) and a different HHT formulations (40 ng/0.8 ml), then were plated in triplicate on 24-well plate over a base layer of 0.8 mL growth media containing 0.6% low melting temperature agarose. The plates were incubated for two weeks until colonies were formed. Colony diameters larger than 75 μm or colony cells more than 50 cells were then counted as 1 positive colony visualized by light a microscope (10 10), according to the previous report [23]. Colony efficiency was calculated as (number of colony/number of cells inoculated) ×100%.
Apoptosis assay
1×106 MM RPMI 8226 cell suspensions or tumor tissue suspensions were seeded into a 6-well plate containing with the different concentration of HHT formulations each well (50 ng/ml) at 37ºC in 5% CO2 for 72 hours, and cells were stained with FITC-conjugated Annexin V and Propidium Iodide (PI), and resuspended in PBS. The suspended cells at 100 μL were incubated with 5 μL Annexin V-FITC (KeyGen Biotech. Co. Ltd) and 10 μL of 50 μg/mL PI for 15 mins at room temperature in the dark. The rate of cell apoptosis was analyzed within 1 hour by flow cytometry using FACS Caliber (BD, U.S.A) [25,26].
MM xenograft model and therapeutic experiments
In primal animal experiments, 5×106 MM RPMI 8226 cells were mixed with matrigel (100 μL in volume), and injected subcutaneously into groin side in NOD/SCID mice. The tumors palpable at the injection sites were examined after around 21 days, and these MM-bearing mice were randomly divided into three groups of equal size (3 mice). Group 1 was respectively treated with 100 mL PBS (left groin side, control) and HHT (right groin side , 150 mg/kg HHT); Group 2 was respectively treated with L-Enc-HHT (left groin side, 150 mg/kg L-Enc-HHT) and LCL-HHT-L-PEG (right groin side, 150 mg/kg LCL-HHT-L-PEG); Group 3 was respectively treated with LCL-HHT-H-PEG (left groin side, 150 mg/kg LCL-HHT-H-PEG) and MI-HHT (right groin side, 150 mg/kg MI-HHT). Each mouse tumor was locally treated with the above different reagents (100 μL in volume) 8 days after tumor formation, once three days, in total of 8 times. In further animal experiments, 5×106 MM RPMI 8226 cells were injected subcutaneously into dorsal side in NOD/SCID mice, and the tumor treatment was respectively treated with PBS, HHT, LCL-HHT-H-PEG, and LCL-H-PEG. The administration times and dosage were same as the primal animal experiment. The tumour growth in mice was monitored once three days for tumor volume, the general health indicators, such as overall behavior, feeding, body weight and appearance of fur after treatment. The endpoint for this study was the diameter of tumor ≥15 mm, at which point mice were euthanized. Mouse tumour tissues and bones were collected for further analysis. The mouse bone mineral density of the humerus, femurs and vertebra was measured by in vivo Micro-CT imaging with MCT-1108 (China) at a setting of voltage 45 kV and electric current 80 μA [27].
Observation of ultra-structure by electron microscope
Tumor tissue suspensions were collected and fixed with 2% paraformaldehyde/2.5% electron microscope grade glutaraldehyde in 0.1 mol/L sodium cacodylate buffer (pH 7.4) at 37°C. Ultrathin sections were cut and placed on formvar-coated slot copper grids. Sections were then counterstained with uranyl acetate and lead citrate and viewed with TEM. Digital images were acquired with a camra(ORIUS SC200, Gatan, U.S.A) [25].
Statistical analysis
Data are expressed in the mean and standard deviations. The Student’s t-test was used to evaluate the differences between the different experimental groups. Bonferroni correction was used where multiple comparisons were made. P values < 0.05 were taken as statistically significant.
Results
Characterization of the diverse HHT formulations
The focus of this assay lies on the proportion of DSPE-PEG2000 to liposomes’ characteristic. The diverse HHT formulations, including high and low proportion PEG of long-circulating HHT liposomes, general liposomes and micelles, were shown in Figure 1. It is known that lipids make the most part of carries, and cholesterol enhances the intensity of the carry structure. DSPE-PEG2000 makes carries more hydrophilic to circulate long time in vivo so that HHT in lipids releases slowly with circulation. Micelles were made with surfactant Solutol®HS15 that has hydrophilic-hydrophobic property to make a drug capsule.
Figure 1.
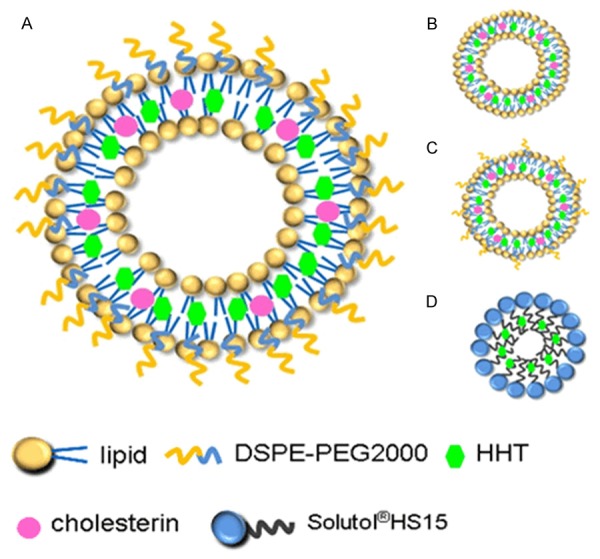
Schematic illustration of the diverse HHT formulations. A. High proportion PEG of long-circulating HHT liposomes (LCL-HHT-H-PEG). B. General liposomes. C. Low proportion PEG of long-circulating HHT liposomes (LCL-HHT-L-PEG). D. Micelles.
As shown in Figure 2, the characterizations of LCL-HHT-H-PEG was performed by using TEM. The morphology of LCL-HHT-H-PEG showed that it was maintained in the spherical shape, and that there were some floccules in the hollow carries. The carries became well-demarcated, and no rupture of the capsule wall was found, strongly suggesting that a typical vesicle structure had been formed. The resulting liposomes exhibited a narrow size distribution, with a diameter of approximate 73.63 nm. The zeta potential of the liposomes was -31 mV that means the suspension of the LCL-HHT-H-PEG has good stability as is shown in Figure 2. Compared to the LCL-HHT-H-PEG, the size of other carries are bigger and the diameter distribution are wider. The size of LCL-HHT-L-PEG are 82.90 nm with the protein dispersibility index (PDI) of 0.179 and the general liposomes are 74.71 nm with PDI of 0.290, and the micelles are 97.34 nm with PDI of 0.199. For zeta potential, other three carries are less than -30 mV that means the carries are not stable sometimes. From the above data, we concluded that the LCL-HHT-H-PEG have the smallest size, most narrow diameter distribution and best stability among all diverse HHT formulations.
Figure 2.
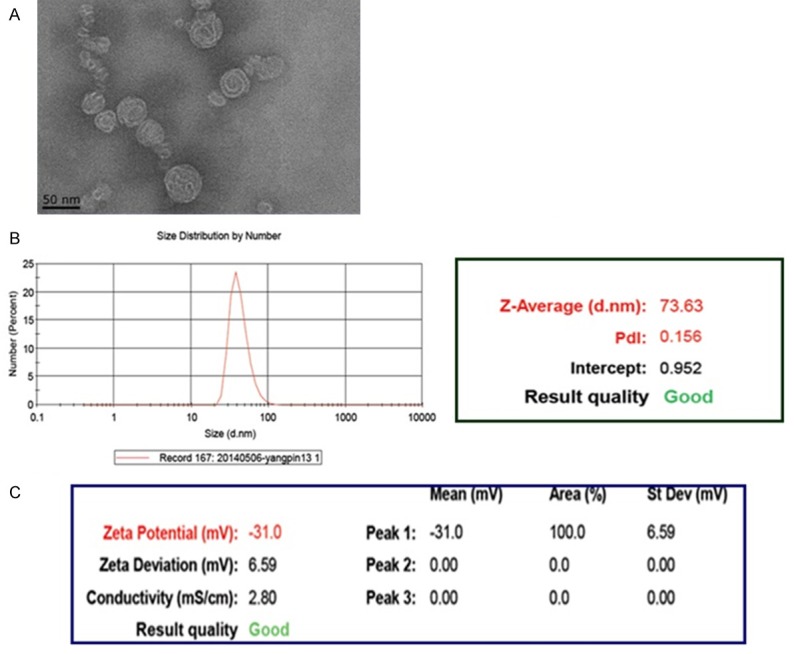
Characterization of the LCL-HHT-H-PEG. A. The TEM image of the LCL-HHT-H-PEG. B. Diameter distribution of the LCL-HHT-H-PEG determined by dynamic light scattering measurements. C. Zeta potential of the LCL-HHT-H-PEG.
Effect of the LCL-HHT-H-PEG on clone formation and apoptosis of MM RPMI 8226 cells in vitro
To test the inhibitory efficacy of the diverse HHT formulations on human MM RPMI 8226 cells, we first observed the ability of cell clone formation when MM RPMI 8226 cells were cultured with the diverse HHT formulations, respectively. Figure 3A, 3B showed that clone formation ratio in LCL-HHT-H-PEG group was a significant lower than that of control group after 14 day culture (2.3±0.81% versus 23±1.92%, P < 0.001) or than that of HHT group (2.3±0.81% versus 7.3±1.15%, P < 0.05). The clone formation ratio among LCL-HHT-H-PEG, LCL-HHT-L-PEG, L-Enc-HHT, and MI-HHT groups were accounting for 2.3±0.81%, 2.8±0.95%, 3.8±2.29%, and 3.5±2.21% in order, which exhibited a little bit difference, however, there is no significant difference among these groups (P < 0.05).
Figure 3.
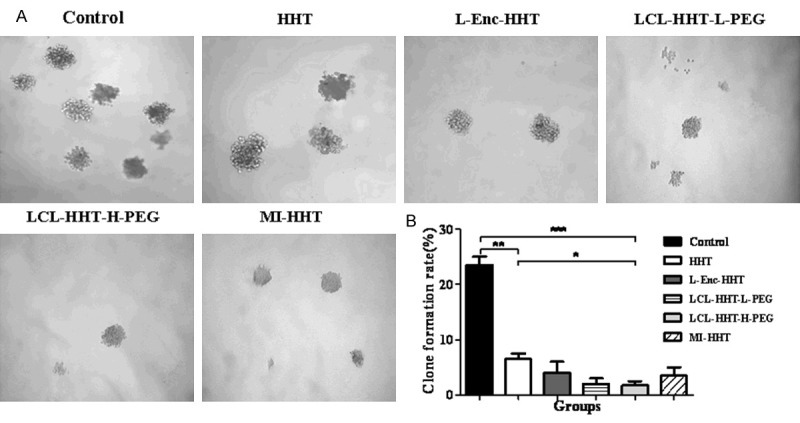
In vitro MM RPMI 8226 cell clone formation assay. A. Clone formation ratio of MM RPMI 8226 cells in vitro as described in the section of Materials and methods. B. Quantification of clone formation ratio. *p < 0.05, **p < 0.01, and ***p < 0.005.
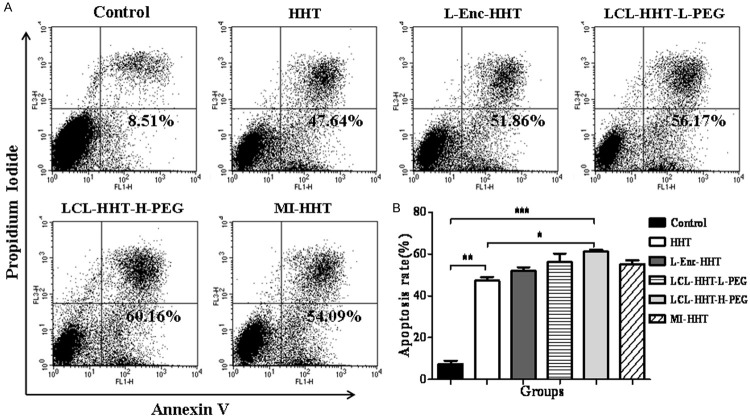
Subsequently, we analyzed these diverse HHT formulations that induced the apoptotic efficacy on MM RPMI 8226 cells. The apoptotic ratio was the highest (60±1.10%) in LCL-HHT-H-PEG group among all experiment groups when cells were incubated with the different reagents for 72 hours. It was found that other apoptotic ratio was around 47±1.36% for the HHT group, 52±1.09% for the L-Enc-HHT group, 56±4.07% for the LCL-HHT-L-PEG group, and 54±1.10% for the MI-HHT group, respectively, which was statistically significant (P < 0.01) compared with control group (Figure 4A, 4B). These results suggested that the diverse HHT formulations had obvious inhibitive effect on MM RPMI 8226 cell growth by inhibition of clone formation and induction of cell apoptosis in vitro.
Figure 4.
In vitro MM RPMI 8226 cell apoptosis assay. A. Apoptosis ratio of MM RPMI 8226 cells in vitro as described in the section of Materials and methods. B. Quantification of apoptosis ratio. *p < 0.05, **p < 0.01, and ***p < 0.005.
LCL-HHT-H-PEG inhibits the xenograft growth in MM bearing mice
Next, we wanted to know whether our in vitro observations would translate into anti-RPMI 8226 MM efficacy in vivo. To this end, we established the murine xenograft model by injection of 5×106 human MM RPMI 8226 cell to the NOD/SCID mice. All the injected mice developed tumors that were felt by touch in around 21 days. We evaluated the anti-tumor efficacy by treatment of MM beraing NOD/SCID mice with the LCL-HHT-H-PEG 8 days after tumor formation. Figure 5A gives a treatment protocol in mice, and Figure 5B shows the images of tumor sizes on day 27 after the mice were initially treated with the diverse HHT formulations in total 8 times, once 3 days. All the treated mice had smaller tumors than that of control group mice, but the smallest tumor sizes was seen in the mice treated with LCL-HHT-H-PEG. The difference was statistically significant between the LCL-HHT-H-PEG and the control groups (p < 0.01), between the LCL-HHT-H-PEG and the LCL-HHT-H-PEG groups, and between the LCL-HHT-H-PEG and the HHT groups (p < 0.03); however, there was no significant difference in the tumor sizes between the LCL-HHT-H-PEG and the LCL-HHT-L-PEG groups, between the LCL-HHT-H-PEG and the L-Enc-HHT or MI-HHT groups (p > 0.05) although a larger decrease of tumor sizes was found in the LCL-HHT-H-PEG group, which were shown in Figure 5B and 5C. From the above results, we concluded that the LCL-HHT-H-PEG have a significant inhibition of tumor growth compared with other HHT formulation’s treatments. However, there were some confounding factors in this model due to two reagents for treatment of tumors of two sides in one mouse concurrently. It is a little of difficult to objectively evaluate the anti-RPMI 8226 MM efficacy in the model. For this reason, we focused the effect of LCL-HHT-H-PEG on the xenograft growth in MM bearing mice in further animal experiments as described in section of Materials and Methods.
Figure 5.
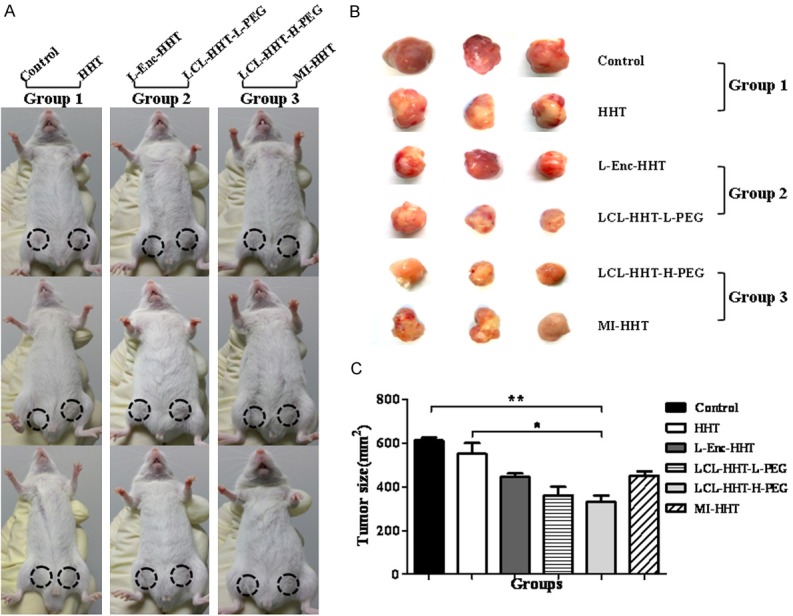
Significant increase of therapeutic efficacy by the LCL-HHT-H-PEG in MM mice. A. Images of NOD/SCID mice indicate the treatment protocol of the diverse HHT formulations. B. Images of tumor sizes after the mice were initially treated with the diverse HHT formulations for 8 times as described in the section of Materials and methods. C. Quantification of tumor sizes. *p < 0.05 and **p < 0.01.
Images of Figure 6A, 6B show the tumor bearing mice and the tumor sizes on day 27 after the mice were treated with the LCL-HHT-H-PEG and other control reagents. Compared with other three control groups, the LCL-HHT-H-PEG formulation has shown to be a significant inhibition of tumor growth in RPMI 8226 MM mouse model. The difference was statistically significant (Figure 6C).
Figure 6.
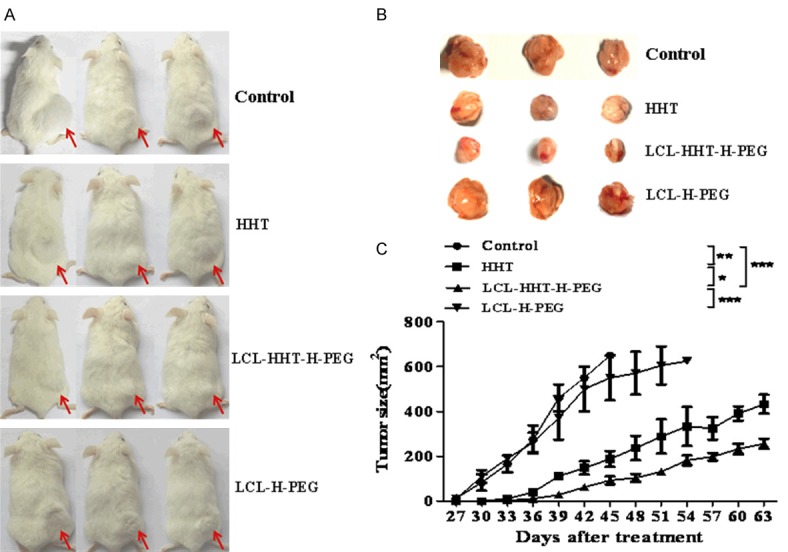
LCL-HHT-H-PEG inhibits RPMI8226 MM in further animal experiments. A. Images of RPMI8226 MM bearing NOD/SCID mice. B. Images of tumor sizes stripped from the mice treated with the LCL-HHT-H-PEG and other control reagents as described in the section of Materials and methods. C. Quantification of tumor sizes. *p <0.05, **p < 0.01, and ***p < 0.005.
Lytic bone lesions in the MM RPMI 8226 cell-engrafted mice
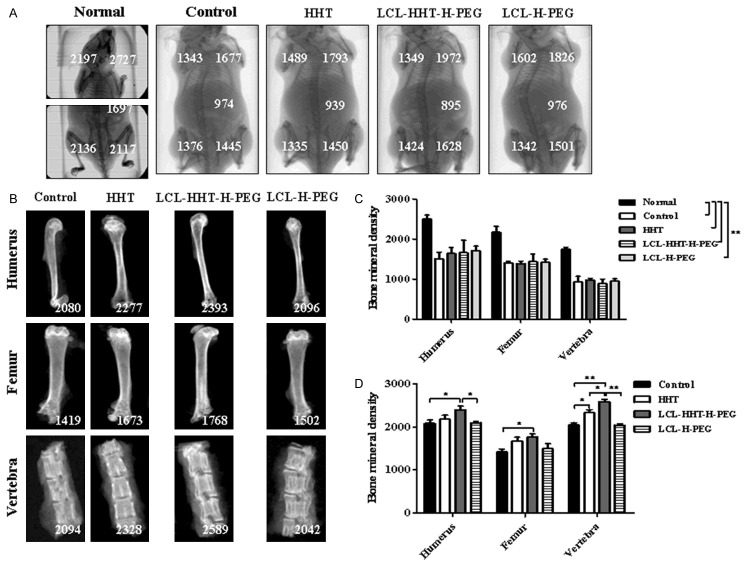
Having found that the therapeutic efficacy of LCL-HHT-H-PEG formulation was efficient in the RPMI 8226 MM-bearing murine model, we next wanted to determine whether this effect had an influence on the improvement of lytic bone lesions in mice. Figure 7A shows the representative clinical pictures taken on day 27, scaned by in vivo Micro-CT imaging after mice were treated with PBS, HHT, LCL-HHT-H-PEG, and LCL-H-PEG respectively, and the left picture of mouse (Figure 7A) was used in normal control, in where the numbers represent the mouse bone mineral density. The difference was statistically significant (p < 0.01) as is shown in Figure 7C. After treatment, mice were sacrificed, and their bones (femurs, humerus and vertebra) were collected, and the bone mineral density was analyzed (Figure 7B). The treatment of LCL-HHT-H-PEG formulation significantly increased of the bone mineral density and ameliorated lytic bone lesions compared with the HHT and other control in the MM RPMI 8226 cell xenograft mice, which was statistically significant shown in Figure 7D. The results showed that the application of LCL-HHT-H-PEG to RPMI 8226 MM bearing NOD-SCID mice increased significantly mouse bone mineral density compared with other control mice.
Figure 7.
LCL-HHT-H-PEG increases significantly bone mineral density in MM RPMI 8226 cell xenograft mice. (A) A representative clinical images of different NOD/SCID mice scaned by in vivo Micro-CT imaging. (B) Images of mouse bone mineral density (the numbers) after mice were treated with the LCL-HHT-H-PEG and other control reagents. (C) Quantification of whole mouse bone mineral density in (A). (D) Quantification of mouse bone mineral density of femurs, humerus and vertebra in (B). *p < 0.05 and **p < 0.01.
LCL-HHT-H-PEG reduces the clone formation and increases the apoptosis of MM cells ex vivo
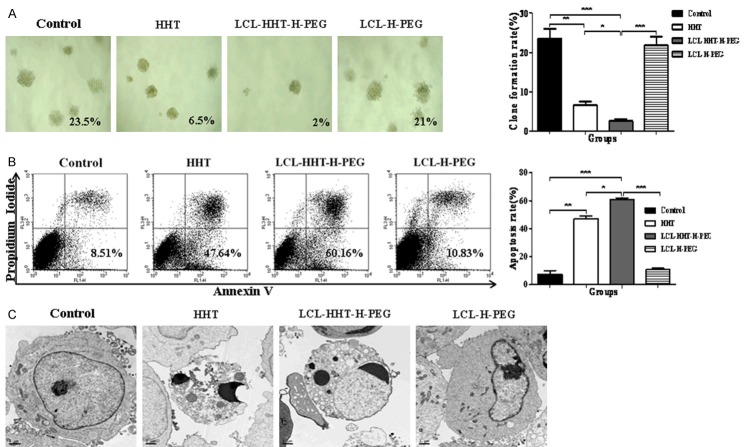
Further, we sought to know the possible mechanisms of this inhibitory efficacy. The tumor cells were isolated from the tumor tissues dissected from the RPMI8226 MM bearing mice, and incubated with the LCL-HHT-H-PEG, HHT and other control formulations, respectively, whose concentration was same as the in vitro assays. The results of the clone formation assay ex vivo showed that the clone formation ratio was dramatically inhibited in LCL-HHT-H-PEG group compared to HHT and control groups (Figure 8A), and refer to the statistically significant differences as indicated in histogram of Figure 8A. Consistently, the quantitative analysis by FCM showed that, upon incubation of MM cell-derived from the RPMI8226 MM bearing mice with LCL-HHT-H-PEG for 72 hours, cells underwent to significant apoptosis ex vivo in contrast to HHT and control groups. The apoptosis ratio was shown in histogram of Figure 8B.
Figure 8.
Obvious inhibition of clone formation and induction of MM apoptosis. A. Clone formation ratio of MM cells derived from treated MM mice as described in the section of Methods. B. Apoptosis of MM cells derived from treated MM mice was analyzed by FCM. C. Ultrastructural morphology of MM cells derived from treated MM mice was analyzed by TEM. *p < 0.05, **p < 0.01 and ***p < 0.005.
Finally, the ultrastructural morphology of the MM cells was further analyzed by TEM. TEM imaging from the Figure 8C indicates that the ultrastructure of MM cell-derived from the RPMI8226 MM bearing mice treated with PBS, HHT, LCL-HHT-H-PEG, and LCL-H-PEG respectively, in where the LCL-HHT-H-PEG group exhibited the apoptotic morphologic characteristics and showed a chromatin condensation and margination to a crescent shape. These were not found in the PBS control or LCL-H-PEG treated MM cells. However, the HHT treated cells showed some degrees of chromatin condensation too.
Discussion
From the present study, we have shown that the LCL-HHT-H-PEG formulation exhibited more obvious inhibitory effects on clone formation and more apoptosis of MM RPMI8226 cells than any other formulation controls did in vitro. In vivo primal animal experiments, human MM cell (the RPMI8226 cell line) xenografts established in SCID mice showed that treatment with LCL-HHT-H-PEG significantly inhibited the tumor growth compared with that of mice treated with low dose of LCL-HHT-L-PEG, L-Enc-HHT, MI-HHT and free drug, respectively. Although the LCL-HHT-L-PEG also exhibited a good inhibitory effects on RPMI8226 MM cells in vitro and in vivo compared with L-Enc-HHT or MI-HHT, this effect was not so strong as LCL-HHT-H-PEG did. This is because that long-circulating, high dose of PEGylated, liposome-encapsulated HHT may have the efficacy of a steric hindrance and a hydrophilicity of membrane surface, which protects liposome-encapsulated HHT from the degradation by enzyme digestion or other biological activity materials. Therefore, the LCL-HHT-H-PEG formulation generally outperforms the conventional LCL-HHT-L-PEG, suggesting the importance of LCL-HHT-H-PEG caused by enhanced permeability, delivered high amount of HHT, and reserved therapeutic compound in MM, which leads to reinforced antitumor efficacy and decreased side effects compared to LCL-HHT-L-PEG and other control reagents [28]. Furthermore, the high inhibitory efficacy of LCL-HHT-L-PEG on the RPMI8226 MM in vivo was further confirmed by the following animal experiments, especially in the marked induction of MM cell apoptosis that was demonstrated by TEM analysis.
MM is the most common cancer type in haematological system. In most cases, no curative treatment options are available for refractory/relapsed MM as the tumors are highly resistant to chemotherapy. Targeted drug delivery by using liposomal drug delivery system is an attractive approach to enhance the efficacy of anticancer drugs and reduce side effects, thereby potentially promoting the therapeutic index, particularly in the increasing survival of elderly MM patients who are generally more susceptible to adverse events [1-3]. Indeed, reduced adverse effects would be a key attribute for new agents to facilitate their use in a greater proportion of MM patients. In most preclinical models, PEGylated long circulating liposomes containing anticancer drugs were shown to decrease disease burden with reduced drug toxicity in B-cell lymphoma, colon, breast, lung cancers etc [8,9,14,15,17,28].
Using conventional HHT or PEGylated long-circulating liposome-engrafted HHT for acute and chronic myeloid leukemia has been reported in the last decades [5-7,29-33], but has never been studied in RPMI8226 MM. For this reason, we investigated the therapeutic effect of LCL-HHT-H-PEG on RPMI8226 MM. To really evaluate the function of LCL-HHT-H-PEG, we used MI-HHT as control formulation. It is known that lipid micelles serve as versatile and effective drug carriers for cancer therapy and have shown to overcome several limitations of poorly soluble drugs such as non-specific biodistribution and targeting, lack of water solubility and poor oral bioavailability [34,35]. In the primal study, MI-HHT indeed exhibited stronger therapeutic effect in reduction of clone formation and induction of cell apoptosis in vitro as well as inhibition of tumor growth in vivo than HHT and L-Enc-HHT did, but the LCL-HHT-H-PEG formulation resulted in greater intratumoral drug accumulation over time and reduce tumor growth than that of MI-HHT in the primal study or than that of HHT in the further study. Nevertheless, the different carriers provide specific advantages, which should be considered when formulating optimal combination therapies.
In conclusion, the data presented here indicates the LCL-HHT-H-PEG are effective for treatment of RPMI8226 MM in vitro and in vivo MM bearing NOD/SCID mice. The findings suggests LCL-HHT-H-PEG may be as a potential anti-MM drug for enhancing MM treatment outcome with decrease of chemotherapeutic side effects.
Acknowledgements
The study has been supported by the National Natural Science Foundation of China (No. 81572887, No. 81473160), and partly supported by the Fundamental Research Funds for the Central Universities, Southeast University (3290005416) as well as partly supported by the Collaborative Innovation Center of Suzhou NanoScience and Technology.
Disclosure of conflict of interest
None.
References
- 1.Lonial S, Durie B, Palumbo A, San-Miguel J. Monoclonal antibodies in the treatment of multiple myeloma: current status and future perspectives. Leukemia. 2016;30:526–35. doi: 10.1038/leu.2015.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayed AO, Chang LJ, Moreb JS. Immunotherapy for multiple myeloma: Current status and future directions. Crit Rev Oncol Hematol. 2015;15:S1040–8428. doi: 10.1016/j.critrevonc.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Spicka I. Advances in multiple myeloma therapy during two past decades. Comput Struct Biotechnol J. 2014;10:38–40. doi: 10.1016/j.csbj.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maiso P, Huynh D, Moschetta M, Sacco A, Aljawai Y, Mishima Y, Asara JM, Roccaro AM, Kimmelman AC, Ghobrial IM. Metabolic signature identifies novel targets for drug resistance in Multiple Myeloma. Cancer Res. 2015;75:2071–2082. doi: 10.1158/0008-5472.CAN-14-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al Ustwani O, Griffiths EA, Wang ES, Wetzler M. Omacetaxine mepesuccinate in chronic myeloid leukemia. Expert Opin Pharmacother. 2014;15:2397–2405. doi: 10.1517/14656566.2014.964642. [DOI] [PubMed] [Google Scholar]
- 6.Philipp S, Sosna J, Plenge J, Kalthoff H, Adam D. Homoharringtonine, a clinically approved anti-leukemia drug, sensitizes tumor cells for TRAIL-induced necroptosis. Cell Commun Signal. 2015;13:25. doi: 10.1186/s12964-015-0103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watari A, Hashegawa M, Yagi K, Kondoh M. Homoharringtonine increases intestinal epithelial permeability by modulating specific claudin isoforms in Caco-2 cell monolayers. Eur J Pharm Biopharm. 2015;89:232–238. doi: 10.1016/j.ejpb.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Lopes de Menezes DE, Pilarski M, Allen TM. In vitro and in vivo targeting of immuno-liposomal doxorubicin to human B-cell lymphoma. Cancer Res. 1998;58:3320–3330. [PubMed] [Google Scholar]
- 9.Reddy MS, Raghavendra R, Nayak UY, Kumar AR, Deshpande PB, Udupa N, Behl G, Dave V, Kushwaha K. PEGylated liposomes of anastrozole for long-term treatment of breast cancer: in vitro and in vivo evaluation. J Liposome Res. 2015;8:1–19. doi: 10.3109/08982104.2015.1029493. [DOI] [PubMed] [Google Scholar]
- 10.Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, Huang SK, Lee KD, Woodle MC, Lasic DD, Redemann C, et al. Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Nati Acad Sci U S A. 1991;88:1460–1464. doi: 10.1073/pnas.88.24.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen TM, Mehra T, Hansen C, Chin YC. Stealth liposomes: animproved sustained release system for l-b-D-arabinofuranosylcytosine. Cancer Res. 1992;52:2431–2439. [PubMed] [Google Scholar]
- 12.Allen TM, Hansen CB, Lopes de Menezes DE. Pharmacokinetics of long circulating liposomes. Adv Drug Del Rev. 1995;16:267–284. [Google Scholar]
- 13.Allen TM, Hansen CB, Martin F, Redemann C, Yau-Young A. Liposomes containing synthetic lipid derivatives of polytethylene glycol) show prolonged circulation half-lives in vivo. Biochim Biophys Acta. 1991;7066:29–36. doi: 10.1016/0005-2736(91)90246-5. [DOI] [PubMed] [Google Scholar]
- 14.Shi C, Cao H, He W, Gao F, Liu Y, Yin L. Novel drug delivery liposomes targeted with a fully human anti-VEGF165 monoclonal antibody show superior antitumor efficacy in vivo. Biomed Pharmacother. 2015;73:48–57. doi: 10.1016/j.biopha.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Woodle MC, Matthay KK, Newman MS, Hidayat JE, Collins LR, Redemann C, Martin FJ, Papahadjopoulos D. Versatility in lipid compositions showing prolonged circulation with Sterically stabilized liposomes. Biochim Biophys Acta. 1992;1105:193–200. doi: 10.1016/0005-2736(92)90194-q. [DOI] [PubMed] [Google Scholar]
- 16.Northfelt DW, Martin FJ, Working P, Volberding PA, Russell J, Newman M. Doxorubicin encapsulated in liposomes containing surface-bound polyethylene glycol: pharmacokinetics. tumour localization, and safety in patients with AIDSrelated Kaposi’s sarcoma. J Clin Pharmacol. 1996;50:55–63. doi: 10.1002/j.1552-4604.1996.tb04152.x. [DOI] [PubMed] [Google Scholar]
- 17.Peng PC, Hong RL, Tsai YJ, Li PT, Tsai T, Chen CT. Dual-effect liposomes encapsulated with doxorubicin and chlorin e6 augment the therapeutic effect of tumor treatment. Lasers Surg Med. 2015;47:77–87. doi: 10.1002/lsm.22312. [DOI] [PubMed] [Google Scholar]
- 18.Seo JW, Mahakian LM, Tam S, Qin S, Ingham ES, Meares CF. The pharmacokinetics of Zr-89 labeled liposomes over extended periods in a murine tumor model. Nucl Med Biol. 2015;42:155–163. doi: 10.1016/j.nucmedbio.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andriyanov AV, Koren E, Barenholz Y, Goldberg SN. Therapeutic efficacy of combining pegylated liposomal doxorubicin and radiofrequency (RF) ablation: comparison between slow-drugreleasing, non-thermosensitive and fast-drugreleasing, thermosensitive nano-liposomes. PLoS One. 2014;9:e92555. doi: 10.1371/journal.pone.0092555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shibata H, Yoshida H, Izutsu KI, Haishima Y, Kawanishi T, Okuda H, Goda Y. Interaction kinetics of serum proteins with liposomes and their effect on phospholipase-induced liposomal drug release. Int J Pharm. 2015;495:827–839. doi: 10.1016/j.ijpharm.2015.09.053. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y, Du D, Lin Y. Glucose encapsulating liposome for signal amplification for quantitative detection of biomarkers with glucometer readout. Biosens Bioelectron. 2015;72:348–354. doi: 10.1016/j.bios.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 22.Yang Q, Moulder K R, Cohen MS, Cai S, Forrest LM. Cabozantinib Loaded DSPE- PEG2000 Micelles as Delivery System: Formulation, Characterization and Cytotoxicity Evaluation. BAOJ Pharm Sci. 2015;1 pii: 001. [PMC free article] [PubMed] [Google Scholar]
- 23.Dou J, Li Y, Zhao F, Hu W, Wen P, Tang Q, Chu L, Wang Y, Cao M, Jiang C, Gu N. Identification of tumor stem-like cells in a mouse myeloma cell line. Cell Mol Biol (Noisy-le-grand) 2009;55(Suppl):OL1151–1160. [PubMed] [Google Scholar]
- 24.Dou J, Pan M, Wen P, Li Y, Tang Q, Chu L. Isolation and identification of cancer stem-like cells from murine melanoma cell lines. Cell Mol Immunol. 2007;4:467–472. [PubMed] [Google Scholar]
- 25.Yang C, Xiong F, Wang J, Dou J, Chen J, Chen D, Zhang Y, Luo S, Gu N. Anti-ABCG2 monoclonal antibody in combination with paclitaxel nanoparticles against cancer stem-like cell activity in multiple myeloma. Nanomedicine (Lond) 2014;9:45–60. doi: 10.2217/nnm.12.216. [DOI] [PubMed] [Google Scholar]
- 26.Sun WL, Chen J, Wang YP, Zhang H. Autophagy protects breast cancer cells from epirubicininduced apoptosis and facilitates epirubicinresistance development. Autophagy. 2011;7:1035–1044. doi: 10.4161/auto.7.9.16521. [DOI] [PubMed] [Google Scholar]
- 27.Bonnin P, Sabaa N, Flamant M, Debbabi H, Tharaux PL. Ultrasound imaging of renal vasoocclusive events in transgenic sickle mice exposed to hypoxic stress. Ultrasound Med Biol. 2008;34:1076–1084. doi: 10.1016/j.ultrasmedbio.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Kroon J, Metselaar JM, Storm G, van der Pluijm G. Liposomal nanomedicines in the treatment of prostate cancer. Cancer Treat Rev. 2014;40:578–584. doi: 10.1016/j.ctrv.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Wolff NC, Pavía-Jiménez A, Tcheuyap VT, Alexander S, Vishwanath M, Christie A. Highthroughput simultaneous screen and counterscreen identifies homoharringtonine as synthetic lethal with von Hippel-Lindau loss in renal cell carcinoma. Oncotarget. 2015;6:16951–16962. doi: 10.18632/oncotarget.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao W, Liu Y, Zhang R, Zhang B, Wang T, Zhu X. Homoharringtonine induces apoptosis and inhibits STAT3 via IL-6/JAK1/STAT3 signal pathway in Gefitinib-resistant lung cancer cells. Sci Rep. 2015;5:8477. doi: 10.1038/srep08477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng Z, Li Y, Li YF. Immunological status of chronic myelogenous leukemia patients with complete cytogenetic response after treatment. Tumori. 2015;101:323–327. doi: 10.5301/tj.5000287. [DOI] [PubMed] [Google Scholar]
- 32.Komoto N, Nakane T, Matsumoto S, Hashimoto S, Shirota O, Sekita S, Kuroyanagi M. Acyl flavonoids, biflavones, and flavonoids from Cephalotaxus harringtonia var. nana. J Nat Med. 2015;69:479–486. doi: 10.1007/s11418-015-0912-x. [DOI] [PubMed] [Google Scholar]
- 33.Tong Y, Niu N, Jenkins G, Batzler A, Li L, Kalari KR, Wang L. Identification of genetic variants or genes that are associated withHomoharringtonine (HHT) response through a genomewide association study in human lymphoblastoid cell lines (LCLs) Front Genet. 2015;5:465. doi: 10.3389/fgene.2014.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gill KK, Kaddoumi A, Nazzal S. PEG-lipid micelles as drug carriers: physiochemical attributes, formulation principles and biological implication. J Drug Target. 2015;23:222–231. doi: 10.3109/1061186X.2014.997735. [DOI] [PubMed] [Google Scholar]
- 35.Hayama A, Yamamoto T, Yokoyama M, Kawano K, Hattori Y, Maitani Y. Polymeric micelles modified by folate-PEG-lipid for targeted drug delivery to cancer cells in vitro. J Nanosci Nanotechnol. 2008;8:3085–3090. doi: 10.1166/jnn.2008.093. [DOI] [PubMed] [Google Scholar]