Abstract
Background: Acute aortic dissection (AAD) patients usually show concurrent lung injury mainly featured by hyoxemia. To date, no effective treatment method has been established for the AAD complicated with acute lung injury (ALI). Matrix metalloproteinases (MMPs), especially MMP2 and MMP9, have been considered to be closely related to the onset of aortic disease including AAD. To investigate the roles of MMP in the pathogenesis of AAD complicated with ALI, we determined the expression of MMP2 and MMP9 in serum and lung tissues of AAD patients. In addition, a new rat model of AAD complicated with ALI was established to investigate the pathogenesis of such complicated conditions. Methods and results: Angiotensin II (Ang II) and MMP9 were up-regulated in the AAD complicated with ALI patients compared to those of the AAD without ALI patients, normal individuals and the patients with non-ruptured aneurysm. Besides, massive macrophages with MMP9 expression was noticed in the lung tissues in the AAD complicated with ALI patients. On this basis, AAD complicated with ALI rat model was established based on BAPN feeding and infusion of Ang II. Obvious lung injury was observed in the BAPN+Ang II group compared to that of the BAPN group, together with macrophage accumulation in lung tissues, as well as over-expression of MMP9 in lung tissues. After interference of MMP antagonist, a large number of macrophages were still accumulated in the lung tissues, but the lung injury was obviously attenuated. After the interference of AT1 receptor, the number of macrophages in the lung tissues was obviously decreased and the lung injury was obviously relieved. Conclusions: Ang II is closely related to the lung injury at the early stage of AAD through mediating the release of MMP9 in the macrophages in the lung tissues.
Keywords: Acute aortic dissection, animal model, lung injury, MMP9
Introduction
Acute aortic dissection (AAD), the most frequent and catastrophic manifestation of acute aortic syndrome, causes great threats to the public health worldwide [1-3]. Despite the recent progression of diagnosis and treatment, the mortality rate of AAD is still high. In clinical practice, AAD is frequently accompanied by acute respiratory failure such as acute respiratory distress syndrome (ARDS) and/or acute lung injury (ALI). Besides, concurrent ALI before surgery is an independent risk factor for postoperative ARDS [6-8]. Several factors have been considered as the common causes for ALI, including hypotension, pulmonary infection, as well as radiation injury and mechanical injury in lung [9-11]. However, many AAD patients show concurrent ALI despite the absence of such factors. Therefore, it is reasonable to speculate that there might a close relation between AAD and ALI. Respiratory failure in AAD patients appears to be closely correlated with the aortic injury and even the prognosis. However, the exact mechanism is far from clear. Thus, the unknown pathogenesis of AAD complicated with ALI deserves a study of establishing animal model for the clinical and experimental research.
Currently, no AAD complicated with ALI model has been established as most of the studies have focused on the induction of AAD model. According to the previous description, the induction of AAD animal models is mainly depend on the surgical methods [4,5] or the β-aminopropionitrile (BAPN) treatment. However, all these animal models are restricted to aortic dissection, and the incidence of acute lung injury (ALI) was extremely lower. In this study, a new AAD complicated ALI model was established based on the feeding of BAPN and the infusion of Ang II. On this basis, we aim to investigate the exact pathogenesis of AAD complicated with ALI in rats. Our study reveals Ang II is closely related to the lung injury at the early stage of AAD through mediating the release of MMP9 in the macrophages in the lung tissues.
Materials and methods
Human blood sample
Fifty-eight newly diagnosed AAD patients admitted in the ICU of our hospital from September 2014 to July 2015 were included in this study. Twelve matched individuals and 12 non-ruptured aortic aneurysm patients were registered. The diagnosis of AAD and aortic aneurysm was performed based on the CT scan and ultrasonic examination. Patients admitted to our hospital 7 days after the onset of disease were excluded from the study. Those with cancer, chest trauma and pulmonary infection within one month were excluded from this study. Blood sample was collected and PaO2/FiO2 was determined 1 h after admission. Twenty-one AAD patients showed combined hyoxemia before the surgery. The patient characteristics were listed in Table 1. Written informed consent was obtained from each patient. The study protocols were approved by the Ethical Committee of the Renmin Hospital of Wuhan University.
Table 1.
Clinical features of the patients
| Control (n=12) | Non-ruptured aneurysm (n=12) | AAD | ||
|---|---|---|---|---|
|
| ||||
| Complicated with lung injury AAD (n=21) | Non lung injury AAD (n=37) | |||
| Age, yr | 40±9 | 58±11 | 47±6 | 51±6 |
| Male sex, n % | 8 (66.7) | 8 (66.7) | 17 (81.0) | 30 (81.1) |
| Average duration from onset, h | N/A | N/A | 9.2 | 10.7 |
| Smoking, n (%) | 4 (33.3) | 5 (41.7) | 11 (52.4) | 19 (51.4) |
ELISA
Serum matrix metalloproteinase 2 (MMP2), MMP9, and Ang II were measured using commercial ELISA kits (category No. EK0657, EK0465 and EK0459, Biofavor Biotech Service Co., Ltd. Wuhan, China) according to the manufacture’s instructions. All tests were carried out at least in triplicate.
Histopathological examination
Pulmonary tissues of aortic dissection were obtained from 2 cadavers with AAD combined with ALI received no surgery before. Pulmonary tissues obtained from the organ donors (n=2) served as the control. The study protocols were approved by the Ethical Committee of the Renmin Hospital of Wuhan University.
Upon collection of lung tissues, the samples were fixed and embeded. Afterwards, H&E staining and immunohistostaining were performed according to the previous description [12]. The images were observed using a CKX41SF light microscope (Olypus Corporation, Tokyo, Japan) to determine the histopathological changes in lung tissues, as well as the expression of MMP9 and CD68.
Development of AAD complicated lung injury model in rats
Patients with AAD complicated with lung injury showed elevation of serum Ang II. On this basis, AAD complicated with lung injury rat model was established using BAPN treatment combined with pumping of Ang II. The male SD rats (3-week-old) were provided by the Animal Center of Wuhan University and were divided into three groups: control group, wild type male SD rats fed by normal diet for 4 weeks; BAPN group, wild type male SD rats fed by normal diet for 4 weeks, combined with treatment of BAPN (0.1 g/kg/d, Sigma-Aldrich, St. Louis, MO) for 4 weeks; BAPN+Ang II group, wild type male SD rats fed by normal diet, combined with treatment of BAPN (0.1 g/kg/d) for 4 weeks, and pumping of Ang II (1 μg/kg/min, Sigma-Aldrich, St. Louis, MO) for 24 hours. Only the animals with formation of aortic dissection were used for the subsequent study. All animal handling was performed in accordance with the Wuhan Directive for Animal Research and the current Guidelines for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication no. 85-23, revised 1996). Rats were sacrificed after successfully anesthetized with 2% phenobarbital (40 mg/Kg). Extensive measures were taken to lower the sufferings of the animals. The animal study was approved by the Ethical Committee of the Renmin Hospital of Wuhan University.
Verification of AAD complicated lung injury model in rats
To confirm whether the Ang II involves in the recruitment of macrophages in this study, we determined the accumulation of macrophages in the lung tissues after interference of valsartan (10 mg/kg per day, Norvatis, Beijing, China). For pharmacological depletion of MMP9, BAPN-fed rats were administered by gastric lavage with a broad-spectrum MMP inhibitor, ONO-4817 (300 mg/kg per day, Bludgebio Co., Ltd, Shanghai, China, category No. 2628), daily for 2 days before Ang II administration until sacrifice.
Reverse transcription PCR
Total RNA was extracted from the lung tissues using Trizol reagent (Invitrogen, Shanghai, China) according to the manufacturer’s instructions. Reverse transcription PCR was carried out using the specific primers targeting MMP9 gene as previously described [13]. PCR primers were synthesized by the Biofavor Biotech Service Co., Ltd. (Wuhan, China). GAPDH served as internal standard. The primer sequences for MMP9 were as follows: forward, 5’-CTTTGTAGGGTCGGTTCTG-3’; reverse, 5’-CCTGTGAGTGGGTTGGATT-3’. The primer sequences for GAPDH were as follows: forward, 5’-CAGTGCCAGCCTCGTCTCAT-3’; reverse, 5’-AGGGGCCATCCACAGTCTTC-3’.
Immunohistological examination
Sections (4 μm thick) were cut from the paraffin-embedded lung specimens obtained from the cadavars. MMP9 was labeled using rat monoclonal antibody purchased from Proteintech (category No. 10375-2-AP, Wuhan, China). The CD68 of macrophages were labeled using the nonoclonal antibody purchased from Biolegend (category No. 333801, San Diego, CA, China). MMP9 and CD68 were labeled with Cy3 (category No. BA1032, Boster Co., Ltd. Wuhan, China) and fluorescein isothiocyanate (FITC, category No. BA11.0, Boster Co., Ltd. Wuhan, China). The images were observed under the Eclipse 80i fluorescence microscope (Nikon, Tokyo, Japan). Immunohistochemistry image analysis was performed using IPP6.0 software.
Additional methods
For the additional methods, the expression of malondialdehyde (MDA) content and the weight-to-dry weight ratios (W/D ratios) and superoxide dismutase (SOD) in lung were determined. All the tests were carried out at least in triplicate.
Statistical analysis
All statistical analysis were performed with SPSS 20.0 for windows. The data were presented as mean ± standard deviation. Human blood sample data were analyzed with 2-sample t-tests, and the occurrence rate of rats AAD complicated lung injury was analyzed with Fisher’s exact test. P value less than 0.05 was regarded as significant.
Results
Roles of MMP9 and Ang II in the onset of AAD plus ALI
AAD patients showed remarkable decrease of PO2 and SpO2
No statistical difference was noticed in the age and gender in the AAD patients, normal individuals and the non-ruptured aneurysm patients. Compared with the normal individuals and non-ruptured aneurysm patients, the levels of PO2 and SpO2 were remarkably decreased in the AAD patients (Figure 1). Among the 58 AAD patients, 21 (36.2%) showed hyoxemia with a PaO2/FiO2 of ≤ 300 mmHg. This revealed patients with AAD showed lung injury mainly featured by hyoxemia.
Figure 1.
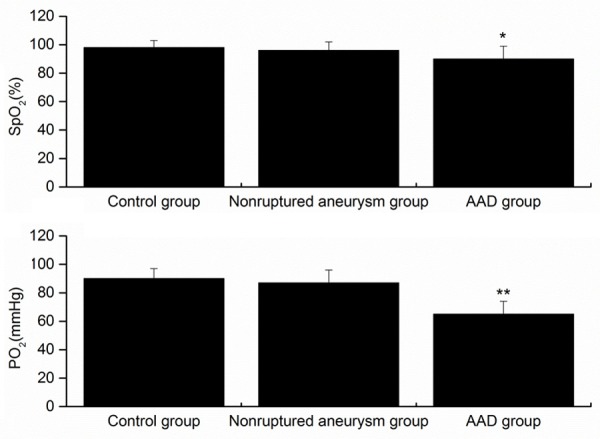
Levels of PO2 and SpO2 in each group under oxygen-free conditions within 1 h after admission. control volunteers (n=12); patients with nonruptured, chronic aortic aneurysm (n=12); AAD patients (n=58). *P<0.05 versus control, **P<0.01 versus control. AAD, acute aortic dissection.
Elevation of MMP9 and Ang II in blood samples from AAD complicated with ALI patients
The concentration of Ang II and MMP9 in the patients with AAD complicated with ALI was remarkably elevated compared to the normal individuals, the non-ruptured aneurysm patients, and the AAD patients without ALI (Figure 2), which implied Ang II and MMP9 may be associated with the onset of AAD complicated with ALI. Whereas, the expression of MMP2 in the AAD complicated with ALI patients showed no difference compared with the other groups.
Figure 2.
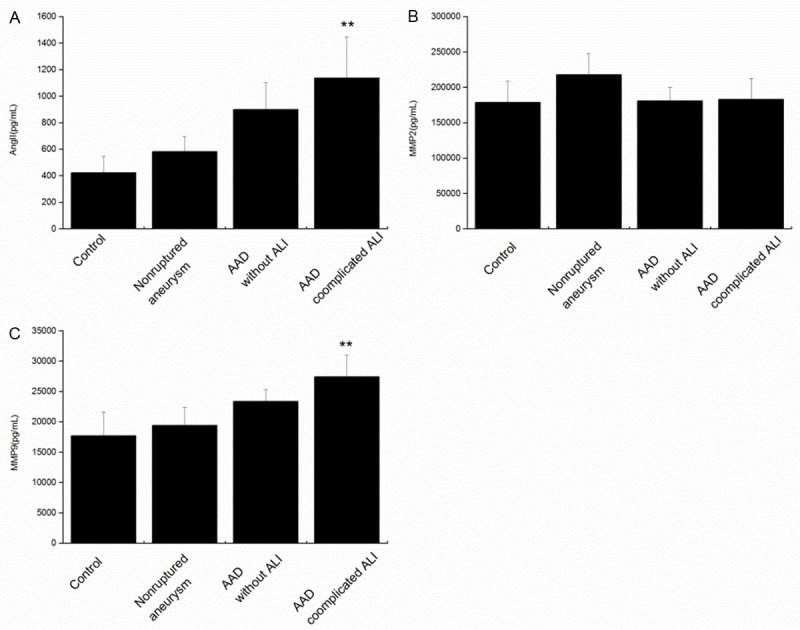
Circulating Ang II (A), MMP2 (B) and MMP9 (C) are elevated in the blood samples from AAD complicated with lung injury patients. Ang II, MMP9 and MMP2 were assayed by the systems for each marker in the human peripheral blood samples from healthy control volunteers (n=12); patients with nonruptured, chronic aortic aneurysm (n=12); AAD patients without ALI (n=37) or AAD complicated with ALI patients (n=21). **P<0.01 versus control. Ang II, angiotensin II; AAD, acute aortic dissection; MMP9 indicates matrix metalloproteinase 9.
MMP9 in lung tissues from AAD complicated with ALI patients was mainly released from macrophage
Abundant MMP9 positive cells were located mainly in the lung tissues in the AAD complicated with ALI patients as revealed by immunolocalization, while rare expression of MMP9 was identified in the normal tissues. In addition, a large number of neutrophils and macrophages were noticed in the lung tissues from the AAD complicated with ALI patients. This indicated the MMP9 in the lung tissues of AAD complicated with ALI patients may derive from the neutrophils or macrophages. To confirm this, double immunofluorescence labeling was performed to the MMP9/MPO (neutrophils) and MMP9/CD68 (macrophages), and the results revealed high colocalization of MMP9 and CD68 in the cells, while rare colocalization of MMP9 and MPO was observed in the cells (Figure 3).
Figure 3.
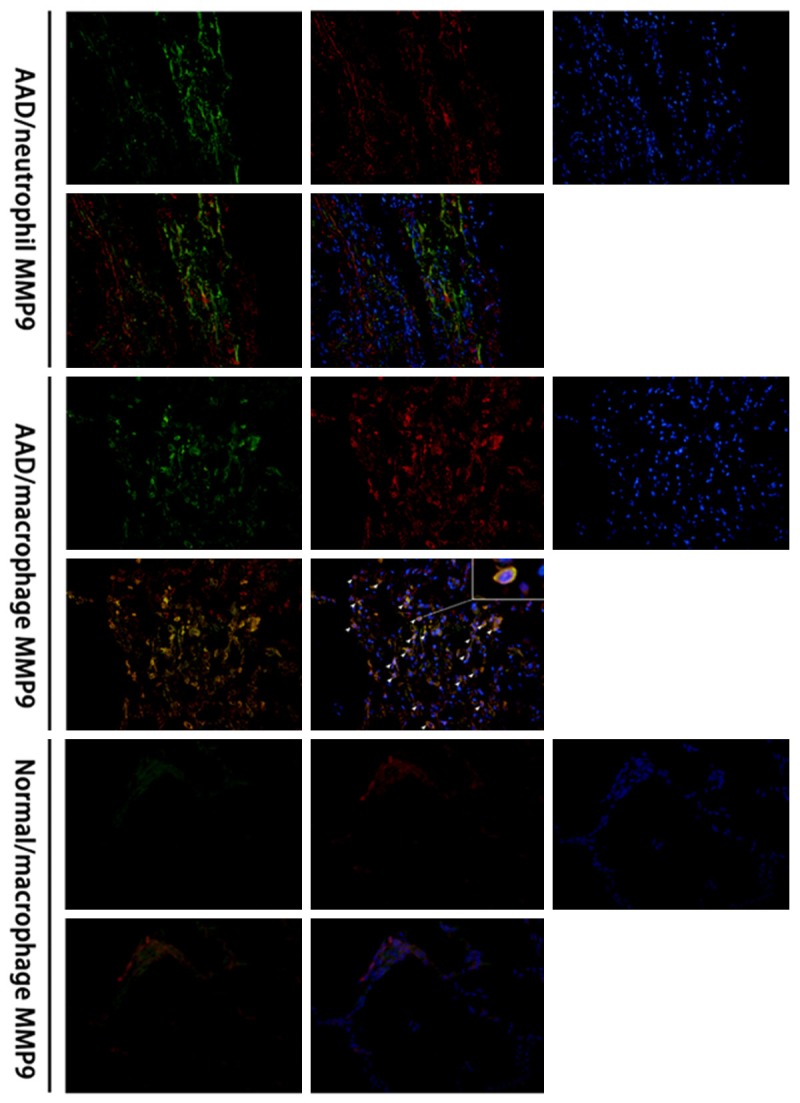
MMP9 expression and neutrophils and macrophages in the lung tissues of cadavers with AAD complicated with lung injury using immunofluorescence labeling. Colocalization of MMP9 and CD68 was observed in the lung tissues in AAD patients, but rare colocalization of MMP9 and MPO was observed, which indicated the MMP9 was mainly derived from the macrophages in lung tissues. Green color: MPO (neutrophil) and CD68 (monocyte/macrophage), red color: MMP9, blue color: nucleus.
Taken together, we concluded that AAD complicated with ALI patients showed remarkable elevation of MMP9 and Ang II. MMP9 was mainly released from the macrophages. Ang II may play a crucial role in the occurrence of AAD complicated with ALI. Ang II, one of the representative vasopressors, is considered to be closely related to in the pathogenesis of several vascular diseases including AAD. Also, it is known to induce MMP9 expression in macrophage [14,15]. Besides, it involves in inflammation through recruiting inflammatory cells in local tissues [16]. On this basis, we hypothesize that Ang II may modulate the release of MMP9 from macrophage and involve in recruitment of macrophages in lung tissues. In this study, animal model was used to investigate the roles of Ang II in the modulation of macrophage in the subsequent section.
Confirmation of Ang II roles in animal models
Ang II infusion contributes to the establishing of AAD complicated with ALI rat model
Based on the remarkable elevation of Ang II and MMP9 in AAD complicated with ALI patients, the rat model was established through feeding of BAPN and subcutaneous infusion of Ang II. Compared with the rats received BAPN feeding, a higher incidence of lung injury was observed in the rats subject to BAPN feeding and infusion of Ang II (85.7% vs. 28.6%). Procedures were performed to verify whether the rat model was successfully induced. For the HE staining, massive infiltration of inflammatory cells were noticed in the lung tissues in the BAPN+Ang II group, together with injury of pulmonary alveoli. Besides, red blood cells, inflammatory cells and exudate were noticed in the alveolar space (Figure 4). Further, the malondialdehyde (MDA) content and the weight-to-dry weight ratios (W/D ratios) in lung was remarkably elevated together with obvious decrease of superoxide dismutase (SOD) activities (Table 2). Taken together, it is reasonable to conclude that obvious lung injury was observed in the BAPN+Ang II group. On this basis, Ang II may be related to the establishing of AAD complicated with ALI rat model.
Figure 4.
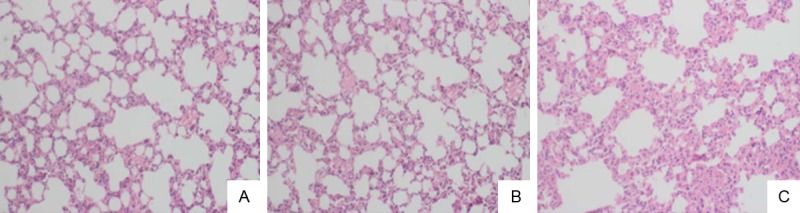
HE staining of the lung tissues in rats. A: In the normal group, slight infiltration of inflammation was observed in the alveolar mesenchyma. B: In the BAPN group, slight oedema was observed in the alveolar space and infiltration of inflammatory cells in the alveolar mesenchyma. C: In the BAPN+Ang II group, obvious oedema and infiltration of inflammatory cells alveolar atrophy and collapse was observed together with compensatory emphysema in part of the pulmonary alveolus. The images were observed with a magnification of 200×.
Table 2.
Determination of MDA, SOD and W/D in each group
| Group | MDA (nmol/mgprot) | SOD (U/mgprot) | W/D |
|---|---|---|---|
| Normal control | 1.34±0.31 | 48.19±4.24 | 3.25±0.37 |
| BAPN group | 1.77±0.26 | 41.74±2.83 | 3.48±0.54 |
| BAPN+Ang II group | 4.84±0.49* | 20.70±3.17* | 4.57±0.66* |
P<0.05, compared with normal control.
Up-regulation of MMP9 expression in the rats model with AAD complicated with ALI
MMP9 expression was determined using reverse transcription polymerase chain reaction in the lung tissues of rats 24 hours after Ang II infusion. The expression of MMP9 mRNA was remarkably elevated in the BAPN+Ang II group (Figure 5A) compared to that of the BAPN group and control group, respectively. Immunohistochemistry results indicated massive oedema in the lung tissues, infiltration of inflammatory cells in alveolar mesenchyma, as well as obvious elevation of MMP9 in the BAPN+Ang II group (Figure 5B).
Figure 5.
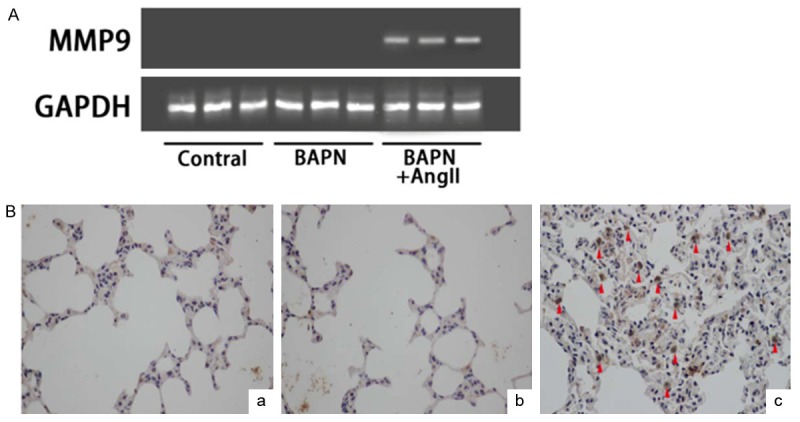
MMP9 expression in the lung tissues in BAPN+Ang II group. A: MMP9 mRNA expression in each group using RT PCR. B: Immunohistochemistry analysis of MMP9 in each group (a: control; b: BAPN group; c: BAPN+Ang II group). The images were observed under a magnification of 400×.
To investigate the roles of MMP9 in the pathogenesis of AAD complicated with ALI, ONO-4817 (MMP antagonist) was administrated in the BAPN+Ang II group. The incidence of AAD was reduced by 28.6%. About 20.0% of the AAD rats showed ALI. The lung injury was remarkably attenuated in the AAD rats (Figure 6). Compared with the BAPN+Ang II groups, the MDA and W/D ratio were remarkably decreased in the group after interference of ONO-4187 (P<0.05). Besides, remarkable elevation was noticed in the SOD level in the group with interference of ONO-4187 compared with that of the BAPN+Ang II group (Table 3, P<0.05).
Figure 6.
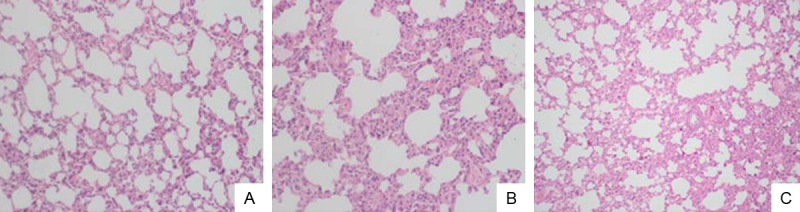
HE staining of the lung tissues in normal group (A), BAPN+Ang II group (B) and ONO-4817 group (C). In the BAPN+Ang II group, obviously oedema was observed in the alveolar space and infiltration of inflammatory cells in the alveolar mesenchyma, as well as injury of alveoli. In the ONO-4817 group, the pulmonary injuries were obviously attenuated compared with that of the BAPN+Ang II group. The images were observed under a magnification of 200×.
Table 3.
MDA, SOD and W/D changes after interference of ONO-4187
| Group | MDA (nmol/mgprot) | SOD (U/mgprot) | W/D |
|---|---|---|---|
| Normal control | 1.34±0.31 | 48.19±4.24 | 3.25±0.37 |
| BAPN+Ang II | 4.84±0.49 | 20.70±3.17 | 4.57±0.66 |
| ONO-4817 group | 2.94±0.31* | 34.74±2.83* | 3.75±0.43* |
P<0.05, compared with BAPN+Ang II group.
Ang II induced macrophages recruitment to lung injury through AT1 receptor independent from MMP9 expression
Immunohistological analysis of BAPN+Ang II group indicated adhesion and infiltration of macrophages in the lung tissues combined with obvious up-regulation of MMP9. On this basis, it is reasonable to conclude that Ang II could induced recruitment of macrophages. However, such fact could not exclude the following possibility: Ang II may induce the release of MMP9 in the lung tissues, while the latter could recruit macrophages and the consequent release of MMP9. To confirm whether the MMP9 involves in the recruitment of macrophages in this study, we determined the accumulation of macrophages in the lung tissues after interference of valsartan or ONO-4817, respectively. As shown in Figure 7, remarkable attenuation was noticed in the number of macrophages in the lung tissues subject to valsartan treatment, while no statistical difference was noticed in the macrophage count in the lung tissues subject to ONO-4817 interference. Taken together, Ang II induced the recruitment of macrophages in lung tissues through AT1 receptor independent from MMP9.
Figure 7.
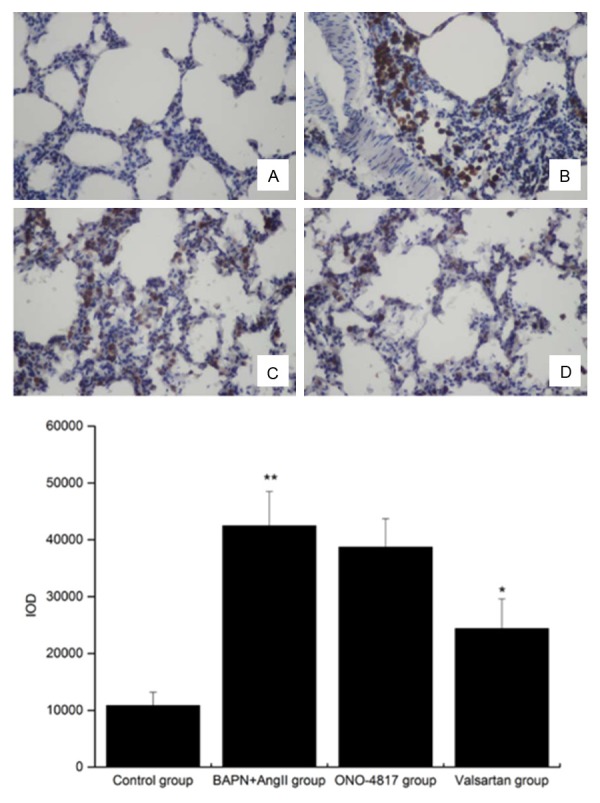
Expression of CD68+ cells in lung tissues in control group (A), BAPN+Ang II group (B), ONO-4817 group (C) and Valsartan group (D). The images were observed under a magnification of 400×. **P<0.01 versus control, *P<0.05 versus BAPN+Ang II group.
Discussion
The pathogenesis of AAD complicated lung injury is still unknown. Recent studies reveal the onset of the disease is closely related to the acute inflammatory reactions, C reactive protein, D2 dimer, body temperature and the scale of aortic dissection [17]. In this study, the level of Ang II showed remarkable increase in the patients with AAD complicated with lung injury compared to that of the normal individuals. In addition, in the lung tissues derived from the cadavers of AAD complicated with lung injury, a large number of MMP9 expressing macrophages were accumulated in the pulmonary mesenchymal. Taken together, it is reasonable to speculate that MMP9 and Ang II may involve in the onset of AAD complicated with lung injury.
Based on these results, we established an rat model of AAD complicated with lung injury based on the pumping of Ang II following the BAPN treatment. Up to now, BAPN is the most frequently drug for the induction of AAD animal models. It is a lysyl oxidase inhibitor, which could induce cystic medial degeneration in the aortic wall in rats. In the previous studies, BAPN could induce AAD in immature rats [18], however, no obvious lung injury was induced. In this study, AAD complicated with lung injury model was established using BAPN feeding combined with infusion of Ang II. Our study confirmed such treatment could induce the AAD complicated lung injury successfully.
In this study, the level of Ang II in the AAD complicated with lung injury was obviously higher than that of the normal control, the non-ruptured aneurysm group and AAD without ALI patients. Unlike the previous animal models based on BAPN [19], our study showed the lung injury was severe in the rat models subject to infusion of Ang II combined with BAPN feeding. Besides, the expression of MMP9 was higher in the lung tissues. This indicated that Ang II may play a crucial role in the onset of AAD complicated lung injury.
Ang II has been acknowledged as an important hormone for the renin-angiotensin system (RAS). Ang II could bind the receptor types AT1R and AT2R. It plays a crucial role in the recruitment of inflammatory cells and induction of monocytes and macrophages. Moreover, it could stimulate the release of IL-1, IL-6, IL-8, tumor necrosis factor- α (TNF-α), and macrophage chemoattractant protein-1 (MCP-1), which finally results in ALI [20-22]. In a previous study, Kaliappan et al. revealed Ang II played a crucial role in the pathogenesis of aortic aneurysm through recruiting macrophages in the aortic wall [23]. In addition, monocytes are important target cells of Ang II and express both AT1 receptor and AT2 receptor. These cells have substantial roles in promoting inflammation, foam cell formation and MMP secretion [24,25]. In our study, compared with the BAPN group, the cell aggregation of macrophages was more obvious in the BAPN+Ang II group, and such phenomenon was obvious attenuated after administration of AT1 antagonist. This indicated the induction of macrophages recruiting mediated by Ang II was depend on the AT1 receptor. Moreover, Ang II contributed to the expression of EMMPRIN and MMP9 in macrophages through protein kinase C and Rho kinase inflammatory signaling pathway. Such biological process was also depend on the AT1 receptor rather than the AT2 receptor [26,27]. In a previous study, up-regulation of EMMPRIN could contribute to the autocrine and paracrine secretion of MMPs, which subsequently enhanced the migration of monocytes and macrophages into the lung mesenchyma [28]. Recently, extensive studies reveal Ang II play crucial roles in the pathogenesis of AAD [18,29-32]. For example, Kurihara et al. reported Ang II mediated MMP9 release in the neutrophils in the aortic wall, and was considered as the major cause for the pathogenesis of AAD [18]. This implied Ang II contributed significantly to the onset of inflammation through modulating the recruiting of inflammatory cells in various tissues.
ALI/ARDS is a complex condition of acute, progressive respiratory failure [33] caused by inflammatory reactions in the lung mediated by inflammatory cells especially the macrophages and the dysfunction of blood gas barrier induced by inflammatory disorder [34,35]. MMP9 is a proteinase involved in the degradation and denaturation of type III-V collagen protein and elastin [36], which were the major components of the extracellular matrix and basilar membrane of blood vessels. Therefore, MMP9 could degrade the extracellular matrix and basilar membrane in the pulmonary capillary. In addition, it could destroy the integrity of basilar membrane, and contribute to the migration of inflammatory cells in the inflammation. Moreover, it could affect the alveolocapillary barrier and result in increase of capillary permeability, infiltration of inflammatory cells and formation of oedema in lung [37-42]. In this study, patients with AAD complicated with ALI showed massive accumulation of MMP9 expressing macrophages in lung tissues, and such phenomenon was obviously attenuated after interference of MMPs antagonist without affecting the accumulation of macrophages.
There are still limitations for our study. Firstly, the animal based model could not mimic the pathogenesis of AAD complicated with ALI in human completely. Secondly, we can not exclude the involvement of other inflammatory cells in the induction of ALI.
In conclusion, AAD complicated with ALI rat model was established based on BAPN feeding and infusion of Ang II. Ang II and MMP9 are considered as the potential diagnostic indices of AAD complicated with ALI. Early-stage administration of AT1R blocking agent and MMPs antagonist may prevent or attenuate the pathogenesis and development of ALI. Besides, Ang II is closely related to the lung injury at the early stage of AAD through mediating the release of MMP9 in the macrophages in the lung tissues.
Disclosure of conflict of interest
None.
References
- 1.Kamberi LS, Gorani DR, Karabulut AM, Beqiri AI, Mustafai AI. Follow-up of acute aortic dissection. Med Arh. 2011;65:89–90. [PubMed] [Google Scholar]
- 2.Bonser RS, Ranasinghe AM, Loubani M, Evans JD, Thalji NMA, Bachet JE, Carrel TP, Czerny M, Di Bartolomeo R, Grabenwöger M, Lonn L, Mestres CA, Schepens MA, Weigang E. Evidence, lack of evidence, controversy, and debate in the provision and performance of the surgery of acute type A aortic dissection. J Am Coll Cardiol. 2011;58:2455–2474. doi: 10.1016/j.jacc.2011.06.067. [DOI] [PubMed] [Google Scholar]
- 3.LeMaire SA, Russell L. Epidemiology of thoracic aortic dissection. Nat Rev Cardiol. 2011;8:103–113. doi: 10.1038/nrcardio.2010.187. [DOI] [PubMed] [Google Scholar]
- 4.Li M, Luo N, Bai Z, Wang S, Shi Y. A canine model of multiple organ dysfunction following acute type-A aortic dissection. Surg Today. 2012;42:876–883. doi: 10.1007/s00595-011-0073-9. [DOI] [PubMed] [Google Scholar]
- 5.Blanton FS Jr, Muller WH Jr, Warren WD. Experimental production of dissecting aneurysms of the aorta. Surgery. 1959;45:81–90. [PubMed] [Google Scholar]
- 6.Olsson C, Franco-Cereceda A. Impact of organ failure and major complications on outcome in acute Type A aortic dissection. Scand Cardiovasc J. 2013;47:352–358. doi: 10.3109/14017431.2013.845307. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Xue S, Zhu H. Risk factors for postoperative hypoxemia in patients undergoing Stanford A aortic dissection surgery. J Cardiothorac Surg. 2013;8:118. doi: 10.1186/1749-8090-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurabayashi M, Okishige K, Azegami K, Ueshima D, Sugiyama K, Shimura T, Maeda M, Aoyagi H, Isobe M. Reduction of the PaO2/FiO2 ratio in acute aortic dissection-relationship between the extent of dissection and inflammation. Circ J. 2010;74:2066–2073. doi: 10.1253/circj.cj-10-0336. [DOI] [PubMed] [Google Scholar]
- 9.Toy P, Popovsky MA, Abraham E, Ambruso DR, Holness LG, Kopko PM, McFarland JG, Nathens AB, Silliman CC, Stroncek D National Heart, Lung and Blood Institute Working Group on TRALI. Transfusion-related acute lung injury: definition and review. Crit Care Med. 2005;33:721–726. doi: 10.1097/01.ccm.0000159849.94750.51. [DOI] [PubMed] [Google Scholar]
- 10.Randolph AG. Management of acute lung injury and acute respiratory distress syndrome in children. Crit Care Med. 2009;37:2448–2454. doi: 10.1097/CCM.0b013e3181aee5dd. [DOI] [PubMed] [Google Scholar]
- 11.Marks LB, Yu X, Vujaskovic Z, Small W, Folz R, Anscher MS. Radiation-induced lung injury. Semin Radiat Oncol. 2003;13:333–45. doi: 10.1016/S1053-4296(03)00034-1. [DOI] [PubMed] [Google Scholar]
- 12.Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao Q, Meijer MJW, Kubben FJGM, Sier CFM, Kruidenier L, van Duijn W, van den Berg M, van Hogezand RA, Lamers CB, Verspaget HW. Expression of matrix metalloproteinases-2 and-9 in intestinal tissue of patients with inflammatory bowel diseases. Dig Liver Dis. 2005;37:584–592. doi: 10.1016/j.dld.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Yu X, Lin SG, Huang XR, Bacher M, Leng L, Bucala R, Lan HY. Macrophage migration inhibitory factor induces MMP-9 expression in macrophages via the MEK-ERK MAP kinase pathway. J Interferon Cytokine Res. 2007;27:103–109. doi: 10.1089/jir.2006.0054. [DOI] [PubMed] [Google Scholar]
- 15.Kong YZ, Yu X, Tang JJ, Ouyang X, Huang XR, Fingerle-Rowson G, Bacher M, Scher LA, Bucala R, Lan HY. Macrophage migration inhibitory factor induces MMP-9 expression: implications for destabilization of human atherosclerotic plaques. Atherosclerosis. 2005;178:207–215. doi: 10.1016/j.atherosclerosis.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 16.Inoue N, Muramatsu M, Jin D, Takai S, Hayashi T, Katayama H, Kitaura Y, Tamai H, Miyazaki M. Effects of chymase inhibitor on angiotensin II-induced abdominal aortic aneurysm development in apolipoprotein E-deficient mice. Atherosclerosis. 2009;204:359–364. doi: 10.1016/j.atherosclerosis.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 17.Sugano Y, Anzai T, Yoshikawa T, Satoh T, Iwanaga S, Hayashi T, Maekawa Y, Shimizu H, Yozu R, Ogawa S. Serum C-reactive protein elevation predicts poor clinical outcome in patients with distal type acute aortic dissection: association with the occurrence of oxygenation impairment. Int J Cardiol. 2005;102:39–45. doi: 10.1016/j.ijcard.2004.03.076. [DOI] [PubMed] [Google Scholar]
- 18.Kurihara T, Shimizu-Hirota R, Shimoda M, Adachi T, Shimizu H, Weiss SJ, Itoh H, Hori S, Aikawa N, Okada Y. Neutrophil-derived matrix metalloproteinase 9 triggers acute aortic dissection. Circulation. 2012;126:3070–3080. doi: 10.1161/CIRCULATIONAHA.112.097097. [DOI] [PubMed] [Google Scholar]
- 19.Van Dorp DR, Malleis JM, Sullivan BP, Klein MD. Teratogens inducing congenital abdominal wall defects in animal models. Pediatr Surg Int. 2010;26:127–139. doi: 10.1007/s00383-009-2482-z. [DOI] [PubMed] [Google Scholar]
- 20.Wösten-van Asperen RM, Lutter R, Haitsma JJ, Merkus MP, van Woensel JB, Van Der Loos CM, Florquin S, Lachmann B, Bos AP. ACE mediates ventilator-induced lung injury in rats via angiotensin II but not bradykinin. Eur Respir J. 2008;31:363–371. doi: 10.1183/09031936.00060207. [DOI] [PubMed] [Google Scholar]
- 21.Ji Y, Gao F, Sun B, Hao J, Liu Z. Angiotensin-Converting Enzyme 2 Inhibits Apoptosis of Pulmonary Endothelial Cells During Acute Lung Injury Through Suppressing SMAD2 Phosphorylation. Cell Physiol Biochem. 2015;35:2203–2212. doi: 10.1159/000374025. [DOI] [PubMed] [Google Scholar]
- 22.Imai Y, Kuba K, Penninger JM. The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice. Exp Physiol. 2008;93:543–548. doi: 10.1113/expphysiol.2007.040048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gopal K, Nagarajan P, Jedy J, Raj AT, Gnanaselvi SK, Jahan P, Sharma Y, Shankar EM, Kumar JM. β-carotene attenuates angiotensin II-induced aortic aneurysm by alleviating macrophage recruitment in Apoe-/-mice. PLoS One. 2013;8:e67098. doi: 10.1371/journal.pone.0067098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim MP, Zhou M, Wahl LM. Angiotensin II increases human monocyte matrix metalloproteinase-1 through the AT2 receptor and prostaglandin E2: implications for atherosclerotic plaque rupture. J Leukoc Biol. 2005;78:195–201. doi: 10.1189/jlb.1204715. [DOI] [PubMed] [Google Scholar]
- 25.Kanome T, Watanabe T, Nishio K, Takahashi K, Hongo S, Miyazaki A. Angiotensin II upregulates acyl-CoA: cholesterol acyltransferase-1 via the angiotensin II type 1 receptor in human monocyte-macrophages. Hypertens Res. 2008;31:1801–1810. doi: 10.1291/hypres.31.1801. [DOI] [PubMed] [Google Scholar]
- 26.Yang LX, Liu H, Guo RW, Ye J, Wang XM, Qi F, Guo CM, Liang X. Angiotensin II induces EMMPRIN expression in THP-1 macrophages via the NF-κB pathway. Regul Pept. 2010;163:88–95. doi: 10.1016/j.regpep.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Yaghooti H, Firoozrai M, Fallah S, Khorramizadeh MR. Angiotensin II induces NF-κB, JNK and p38 MAPK activation in monocytic cells and increases matrix metalloproteinase-9 expression in a PKC-andRho kinase-dependent manner. Braz J Med Biol Res. 2011;44:193–199. doi: 10.1590/s0100-879x2011007500008. [DOI] [PubMed] [Google Scholar]
- 28.Vérollet C, Charrière GM, Labrousse A, Cougoule C, Le Cabec V, Maridonneau-Parini I. Extracellular proteolysis in macrophage migration: losing grip for a breakthrough. Eur J Immunol. 2011;41:2805–2813. doi: 10.1002/eji.201141538. [DOI] [PubMed] [Google Scholar]
- 29.Hu Z, Wang Z, Wu H, Yang Z, Jiang W, Li L, Hu X. Ang II Enhances Noradrenaline Release from Sympathetic Nerve Endings Thus Contributing to the Up-Regulation of Metalloprotease-2 in Aortic Dissection Patients’ Aorta Wall. PLoS One. 2013;8:e76922. doi: 10.1371/journal.pone.0076922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Ren Z, Hu Z, Hu X, Zhang H, Wu H, Zhang M. Angiotensin-II induces phosphorylation of ERK1/2 and promotes aortic adventitial fibroblasts differentiating into myofibroblasts during aortic dissection formation. J Mol Histol. 2014;45:401–412. doi: 10.1007/s10735-013-9558-8. [DOI] [PubMed] [Google Scholar]
- 31.Anzai A, Shimoda M, Endo J, Kohno T, Katsumata Y, Matsuhashi T, Yamamoto T, Ito K, Yan X, Shirakawa K, Shimizu-Hirota R, Yamada Y, Ueha S, Shinmura K, Okada Y, Fukuda K, Sano M. Adventitial CXCL1/G-CSF Expression in Response to Acute Aortic Dissection Triggers Local Neutrophil Recruitment and Activation Leading to Aortic Rupture. Circ Res. 2015;116:612–623. doi: 10.1161/CIRCRESAHA.116.304918. [DOI] [PubMed] [Google Scholar]
- 32.Ito S, Ozawa K, Zhao J, Kyotani Y, Nagayama K, Yoshizumi M. Olmesartan inhibits cultured rat aortic smooth muscle cell death induced by cyclic mechanical stretch through the inhibition of the c-Jun N-terminal kinase and p38 signaling pathways. J Pharmacol Sci. 2015;127:69–74. doi: 10.1016/j.jphs.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Koh Y. Update in acute respiratory distress syndrome. J Intensive Care. 2014;2:1–6. doi: 10.1186/2052-0492-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herold S, Gabrielli NM, Vadász I. Novel concepts of acute lung injury and alveolar-capillary barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2013;305:L665–L681. doi: 10.1152/ajplung.00232.2013. [DOI] [PubMed] [Google Scholar]
- 35.Pelosi P, Rocco PR, Negrini D, Passi A. The extracellular matrix of the lung and its role in edema formation. An Acad Bras Cienc. 2007;79:285–297. doi: 10.1590/s0001-37652007000200010. [DOI] [PubMed] [Google Scholar]
- 36.Ergul A, Portik-Dobos V, Hutchinson J, Franco J, Anstadt MP. Downregulation of vascular matrix metalloproteinase inducer and activator proteins in hypertensive patients. Am J Hypertens. 2004;17:775–782. doi: 10.1016/j.amjhyper.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 37.Hillman GG. Tumor Models in Cancer Research. Springer; 2011. Experimental animal models for investigating renal cell carcinoma pathogenesis and preclinical therapeutic approaches; pp. 287–305. [Google Scholar]
- 38.Wu NC, Wang JJ. Curcumin Attenuates Liver Warm Ischemia and Reperfusion-Induced Combined Restrictive and Obstructive Lung Disease by Reducing Matrix Metalloprotease 9 Activity. Transplant Proc. 2014;46:1135–1138. doi: 10.1016/j.transproceed.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 39.Ma B, Zhou PY, Ni W, Wei W, Ben DF, Lu W, Xia ZF. Inhibition of activin receptor-like kinase 5 induces matrix metallopeptidase 9 expression and aggravates lipopolysaccharide-induced pulmonary injury in mice. Eur Rev Med Pharmacol Sci. 2013;17:1051–1059. [PubMed] [Google Scholar]
- 40.Trocme C, Deffert C, Cachat J, Donati Y, Tissot C, Papacatzis S, Braunersreuther V, Pache JC, Krause KH, Holmdahl R, Barazzone-Argiroffo C, Carnesecchi S. Macrophage-specific NOX2 contributes to the development of lung emphysema through modulation of SIRT1/MMP-9 pathways. J Pathol. 2015;235:65–78. doi: 10.1002/path.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villalta PC, Rocic P, Townsley MI. Role of MMP2 and MMP9 in TRPV4-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;307:L652–L9. doi: 10.1152/ajplung.00113.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim JH, Suk MH, Yoon DW, Lee SH, Hur GY, Jung KH, Jeong HC, Lee SY, Lee SY, Suh IB, Shin C, Shim JJ, In KH, Yoo SH, Kang KH. Inhibition of matrix metalloproteinase-9 prevents neutrophilic inflammation in ventilatorinduced lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291:L580–L7. doi: 10.1152/ajplung.00270.2005. [DOI] [PubMed] [Google Scholar]


