Abstract
Accumulating evidence showed that microRNA-132 (miR-132) are involved in development and progression of several types of cancers, however, the function and underlying molecular mechanism of miR-132 in ovarian cancer remains unclear. In this study we investigated the biological roles and molecular mechanism of miR-132 in ovarian cancer. Here, we found that that the expression levels of miR-132 were dramatically decreased in ovarian cancer cell lines and clinical ovarian cancer tissue samples. Then, we found that introduction of miR-132 significantly suppressed the proliferation, colony formation, migration and invasion of ovarian cancer cells. Mechanism investigation revealed that miR-132 inhibited the expression of transcription factor E2F5 by specifically targeting its mRNA 3’UTR. Moreover, the expression level of E2F5 was significantly increased in ovarian cancer tissues than in the adjacent normal tissues, and its expression was inversely correlated with miR-132 expression in clinical ovarian cancer tissues. Additionally, silencing E2F5 was able to inhibit the proliferation, colony formation, migration and invasion of ovarian cancer cells, parallel to the effect of miR-132 overexpression on the ovarian cancer cells. Meanwhile, overexpression of E2F5 reversed the inhibition effect mediated by miR-132 overexpression. These results indicate that miR-132 suppresses the cell proliferation, invasion, migration in ovarian cancer cells by targeting E2F5.
Keywords: Ovarian cancer, E2F5, proliferation, migration, invasion
Introduction
Ovarian cancer (OC) is the fifth leading cause of cancer related deaths among women in the world, and is one of the most common gynecological malignancies, causing over 140,000 deaths annually [1]. Although great progress in surgical technique, radiotherapy, diagnostic method, and new chemotherapy regimens, and the overall survival rate of ovarian cancer has not changed in the past 50 years because of late diagnosis and chemoresistance [2]. Therefore, the identification of new molecular biomarkers and the development of individualized treatment regimens are important for improving the clinical outcome of patients suffering from ovarian cancer.
MicroRNAs (miRNAs) are a class of endogenous, single-stranded, short (18-24 nucleotides in length), highly conserved noncoding RNAs that regulate gene expression at the post-transcriptional level via complete or incomplete complementarity with biding sites in the 3’-untranslated region (3’-UTR) of the target messenger RNA [3]. It has been showed that miRNAs involved in various biological procession, such as cell differentiation, proliferation, apoptosis, and metastasis [4]. Accumulating evidences demonstrate that miRNAs have essential roles in tumor initiation and progression, function as oncogenes or tumor suppressor [5,6]. In ovarian cancer, multiple miRNAs have been reported to regulate tumor cell proliferation, apoptosis, migration, and invasion [7,8].
miR-132, located in the intron of a non-coding gene on chromosome 17 in humans [9], has been reported to involve in tumor initiation and development by regulating cancer cell proliferation, cycle, apoptosis, migration and invasion and angiogenesis [10-15]. Recently, a report has demonstrated that miR-132 expression was downregulated in serum of patients with ovarian cancer [16], however, the biological roles and potential molecular mechanism of miR-132 in ovarian cancer remains largely unclear. Therefore, the aim of the present study was to determine the role and potential mechanisms of miR-132 in ovarian cancer.
Materials and methods
Patients and tissue samples
Ovarian cancer tissue samples and the corresponding adjacent normal tissue taken from the 32 patients with primary ovarian cancer who underwent surgery at Guangzhou Women and Children’s Medical Center of Guangzhou Medical University, (Guangzhou, China) from August 2014 to August 2015. All tissue samples were immediately frozen in liquid nitrogen, and stored at -80°C until RNA extraction. None of the patients recruited in this study had undergone preoperative chemotherapy, radiotherapy or other therapy. Informed consent was obtained from all patients before surgery. This study was approved by the ethics committee of Guangzhou Women and Children’s Medical Center of Guangzhou Medical University, (Guangzhou, China).
Cell culture
A human ovarian surface epithelial cell line (HOSEpiC) and three human ovarian cancer cell lines (SKOV3, OVCAR3 and A2780) were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China), and were cultured in Roswell Park Memorial Institute (RPMI) 1640 (Gibco, Grand Island, NY, USA) medium supplemented with 10% (v/v) fetal bovine serum (FBS; HyClone, USA), 100 units/ml penicillin and 100 mg/ml streptomycin. All cells were cultured in a humidified atmosphere consisting of 5% CO2 and 90% humidity at 37°C.
Cell transfection
The miR-132 mimic and corresponding negative control (miR-NC), siRNA against E2F5 (si-E2F5), siRNA against negative scramble control (si-NC) plasmid were obtained from GenePharma (Shanghai, China). The gene encoding E2F5 was amplified from human genomic DNA by PCR, and cloned into the pcDNA3 vector (Invitrogen, Carlsbad, CA, USA), termed as pCDNA3-E2F5. These molecular productions were transfected SKOV3 cells using Lipofectamine 2000 (Invitrogen) according to manufacturer’s instructions. Transfection efficiencies were determined in every experiment at 48 h after transfection.
Quantitative real-time PCR
Total RNA was isolated from cultured cells or tissues using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. To quantify miR-132, the RNA including miRNAs was reversely transcribed into cDNA using One Step Prime script miRNA cDNA Synthesis Kit (Qiagen, Valencia, CA) following the manufacturer’s instructions. The expression levels of miR-132 were quantified by the TaqMan miRNA assay kits (Applied Biosystems, Foster City, CA, USA) using miR-132 and U6 primers(Applied Biosystems) under ABI 7900 Fast system (Applied Biosystems). To quantify E2F5, cDNA were synthesized using PrimeScript RT reagent Kit (Takara, Dalian, China) according to the manufacturer’s instructions. The PCR assay was performed using the SYBR Premix Ex Taq system (TaKaRa) under ABI 7900 Fast system. The primers of E2F5 and β-actin were used as described previously [17]. U6 and β-actin were used as internal standard to normalize the miR-132 and E2F5 expression level, respectively, using 2-ΔΔCt method.
Western blot
Protein was extracted from the resected specimens or cultured cells with RIPA lysis buffer (1% NP40, 0.1% sodium dodecyl sulfate (SDS), 100 μg/ml phenylmethylsulfonyl fluoride, 0.5% sodiumdeoxycholate, in PBS) containing proteinase inhibitor (Sigma, USA) on ice for 30 min. The supernatants were collected by centrifugation at 12,000×g at 4°C for 20 min. Concentrations of protein were determined using a BCA assay kit (Pierce, Rockford, IL). Twenty micrograms of protein mixed with 2× SDS loading buffer (125 mmol/l Tris-HCl, 4% SDS, 20% glycerol, 100 mmol/l dithiothreitol (DTT), and 0.2% bromophenol blue) was loaded per lane, separated by 10% sodium dodecylsulfate-polyacrylamide gels (SDS-PAGE), and then transferred onto the polyvinylidene difluoride membranes (PVDF, Millipore, Bedford, MA, USA). The membranes were blocked with 5% non-fat dry milk in TBST (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% Tween-20) for 2 hour at room temperature. Then membranes were incubated with antibody against E2F5 (1:500, Santa Cruz, CA, USA) or anti-β-actin (1:5000, Santa Cruz) at 4°C overnight. Finally, the membrane was washed and incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (1:5000, Santa Cruz) at room temperature for 2 h. Protein bland were detected using the enhanced chemiluminescence (ECL) luminol reagent (PerkinElmer Inc.). β-actin was used as a loading control.
Cell proliferation and colony formation assays
For the cell proliferation assay, transfected cells were plated into 96-well plates and cultured for 24 h-72 h. In indicated times (24 h, 48 h and 72 h), 10 μl CCK-8 (DoJinDo, Japan) was added to each well, and cultured for an additional 2 h, and the absorbance was then detected at a wavelength of 450 nm.
For the colony formation assay, transfected cells were seeded onto six-well plates at a density of 500 cells per well, and cultured for 10 days. Then the colonies were fixed with methanol and stained with 1% crystal violet (Sigma), and the number of colonies was then counted under a light microscope (Olympus, Tokyo, Japan).
Migration and invasion assays
Migration and invasion assays were performed using Transwell chamber inserts (Millipore, USA) without (for migration) or with Matrigel (for invasion) according to the manufacturer’s protocol. In briefly, the transfected cells were seeded into the upper chamber of the insert at a density 2 × 105 in serum-free medium. The bottom of the insert was incubated in a RPMI1640 medium containing 10% FBS to serve as chemoattractant. After 48 h incubation, cells on the surface of upper chamber were removed by scraping with a cotton swab, and the cells that had migrated or invaded were fixed with 4% paraformaldehyde, stained with 0.1% crystal violet for 10 min. Photographs of five randomly selected fields of the fixed cells were taken and counted using a light microscope (Olympus).
Luciferase reporter assay
The wide-type E2F5 3’UTR, including the binding site for miR-132, was amplified by RT-PCR, and the PCR product was cloned into the reporter plasmid pGL3 (Promega, Madison, WI, USA) downstream of the luciferase reporter gene. Mutant-type E2F5 3’UTR were constructed using QuickChange Site-Directed Mutagenesis kits (Stratagene, La Jolla, CA, USA), and inserted into reporter plasmid pGL3 (Promega, Madison, WI, USA). For luciferase assay, SKOV3 cells were plated in a 96-well plate at a density of 5 × 103 cells per well. At 24 h after plating, cells were co- transfected with 50 nM miR-132 mimic or miR-NC mimic and 500-ng E2F5 3’-UTR wide-type or E2F5 3’UTR Mutant-type plasmid per well using Lipofectamine 2000 (Invitrogen), according to the manufacturer’s protocol. After 48 h of transfection, luciferase activity was calculated using the dual-luciferase assay system (Promega, Madison, USA) according to the manufacturer’s instructions. Renilla-luciferase was used for normalization.
Statistical analysis
The data are expressed as the means ± standard deviation (SD) from at least three separate experiments. All statistical analyses were performed using the SPSS 19.0 statistical software package (Chicago, IL, USA). The differences between groups were analyzed using Student’s t-test or one-way ANOVA. The relationship between miR-132 and E2F5 expressions was tested using two-tailed Pearson’s correlation assay. A P value of less than 0.05 was considered significant.
Results
miR-132 expression was down-regulated in ovarian cancer cells and tissues
To assess the expression of miR-132 level, qRT-PCR was conducted on 32 pairs of ovarian cancer samples and the corresponding adjacent normal tissues. As shown in Figure 1A, miR-132 expression is down-regulated significantly in ovarian cancer tissues compared with the corresponding adjacent normal tissues (P < 0.01). We then determined the expression levels of miR-132 in a panel of human ovarian cell lines as wells as normal ovarian surface epithelial cell line (HOSEpiC). Our results indicate a significant down-regulation in expression of miR-132 in ovarian cancer cell lines as compared with I normal ovarian surface epithelial cell line (HOSEpiC) (Figure 1B). SKOV3 cells exhibited the lowest expression of miR-132 in among ovarian cancer cell lines (Figure 1B), and were selected for further studies.
Figure 1.

miR-132 expression was down-regulated in ovarian cancer cells and tissues. A. Relative miRNA levels of miR-132 was determined by qRT-PCR in 32 ovarian cancer tissues and corresponding adjacent normal tissues samples. **P < 0.01 compared to Normal samples. B. Relative miRNA levels of miR-132 in human three ovarian cell lines (OVCAR3, SKOV3, and A2780), as compared to those in human ovarian surface epithelial (HOSEpic cells). U6 snRNA was used as the internal control. **P < 0.01 compared to HOSEpic.
miR-132 inhibited cell growth of ovarian cancer cells
To determine whether miR-132 could affect cell growth, SKOV3 cells were transiently transfected with miR-132 mimic or miR-NC, and then cell proliferation, colony formation were measured by CCK8 and colony formation assays. Compared with the miR-NC control, the miR-132 level was increased nearly 6 times in SKOV3 cells transfected with miR-132 mimic (P < 0.01) (Figure 2A). CCK8 assay showed that the overexpression of miR-132 significantly inhibited cell proliferation (P < 0.01, Figure 2B). Clone formation assay demonstrated that overexpression of miR-132 inhibited the number of colonies, compared with miR-NC (P < 0.01) (Figure 2C).
Figure 2.

miR-132 inhibited cell growth of ovarian cancer cells. A. Relative expression levels of miR-132 was determined in SKOV3 cells transfected with miR-132 mimic or miR-NC by qRT-PCR. B. Cell proliferation was determined in SKOV3 cells transfected with miR-132 mimic or miR-NC by CCK8 assay. C. Colony formation was determined in SKOV3 cells transfected with miR-132 mimic or miR-NC by CCK8 assay *P < 0.05, **P < 0.001 compared with miR-NC.
miR-132 inhibited cell migration and invasion of ovarian cancer cells
Next, we also investigate whether miR-132 effect on migration and invasion assays in ovarian cancer cells. Transwell assays were performed in SKOV3 cells transfected with miR-132 mimic or miR-NC. Our results demonstrated that overexpression of miR-132 could significantly suppress migration (Figure 3A) and invasion (Figure 3B) in ovarian cancer cells.
Figure 3.

miR-132 inhibited cell migration and invasion of ovarian cancer cells. A. Cell migration was determined in SKOV3 cells transfected with miR-132 mimic or miR-NC. B. Cell invasion was determined in SKOV3 cells transfected with miR-132 mimic or miR-NC. *P < 0.05, **P < 0.001 compared with miR-NC.
E2F5 is a direct target of miR-132 in ovarian cancer
To explore the underlying mechanism the growth inhibition by miR-132 in ovarian cancer cells, we used different algorithms (Targetscan 6.2, miRanda and PicTar) that predict the mRNA targets of a miR-132. The seed sequence of miR-132 was complementary to the 3’-UTR of E2F5 at position 18-25 (Figure 1A). To further confirm whether E2F5 is a direct target of miR-132, luciferase activity assay were performed. Our result showed that SKOV3 cells transfected with miR-132 mimic significantly decrease wild-type E2F5-3’UTR reporter activity (P < 0.01, Figure 4B), while transfection of miR-132 mimic had no inhibition effect on the mutant IGF1R-3’UTR reporter activity (Figure 4B), suggesting that E2F5 may be a target of miR-132 in ovarian cancer. We then sought to determine whether the overexpression of miR-132 in ovarian cancers can regulate E2F5 expression on mRNA and protein levels. qRT- PCR and western blotting confirmed that overexpression of miR-132 in SKOV3 cells significantly decrease E2F5 expression on mRNA level (Figure 4C) and protein level (Figure 4D). In addition, we also investigated E2F5 mRNA expression in 32 pair ovarian cancer tissues and corresponding adjacent normal tissues by qRT-PCR. As expected, E2F5 expression on mRNA levels were higher in ovarian cancer tissues than that of adjacent normal tissues (Figure 4E), and its expression was inversely correlated with miR-132 expression (Figure 4F; r=-0.555, P=0.001). These results suggested that E2F5 was a direct target of miR-132 in ovarian cancer.
Figure 4.

E2F5 is a direct target of miR-132 in ovarian cancer. A. The predicted binding sites for miR-132 in the 3’UTR of E2F5 (positions 18-25) and the mutations in the binding sites are shown. B. Luciferase activities in SKOV3 cells 48 h after co-transfected with wide-type E2F5 3’-UTR luciferase plasmid or Mutant-type E2F5 3’-UTR luciferase plasmid and miR-132 mimic or miR-NC. *P < 0.05 compared to miR-NC. C. Levels of E2F5 mRNA was determined by qRT-PCR in SKOV3 cells transfected with miR-132 mimic or miR-NC. **P < 0.01 compared to miR-NC. D. Levels of E2F5 protein was determined by Western blot in SKOV3 cells transfected with miR-132 mimic or miR-NC. E. Levels of E2F5 mRNA was determined by qRT-PCR in 32 ovarian cancer tissues and corresponding adjacent normal tissues samples. **P < 0.01 compared to Normal samples. F. The correlation of the expression levels of E2F5 and miR-132 in 32 ovarian cancer tissue samples.
E2F5 deletion inhibited cell proliferation, colony formation, migration, and invasion in ovarian cancer cells
To determine the role of E2F5 in ovarian cancer cells lines, SKVO3 cells were transfected with si-E2F5 or the si-NC. As shown in Figure 5A and 5B, the E2F5 mRNA and protein expression levels were decreased in cells transfected with si-E2F5 compared with cells transfected with si-NC. In addition, we also found that downregulation of E2F5 in SKOV3 cells significantly cell proliferation and colony formation, migration and invasion (Figure 5C-F), which mimicked the effect of miR-132 overexpression on ovarian cancer cells.
Figure 5.

E2F5 deletion inhibited cell proliferation, colony formation, migration, and invasion in ovarian cancer cells. (A and B) Levels of E2F5 mRNA (A) and protein (B) were determined in SKVO3 cells transfected with si-E2F5 or miR-NC by qRT-PCR and western blot, respectively. (C-F) Cell proliferation (C), colony formation (D), migration (E) and invasion (F) were determined in SKVO3 cells transfected with si-E2F5 or miR-NC. *P < 0.05, **P < 0.001 compared with si-NC.
MiR-132 inhibited cell proliferation, colony formation migration, and invasion via targeting E2F5 in ovarian cancer cells.
To confirm that miR-132 exerted its functions on ovarian cancer cells via targeting E2F5, a rescue experiment was performed. SKOV3 cells were transfected with miR-132 mimic or miR-NC, together with either vector control (pcDNA3) or overexpression E2F5 plasmids (pcDNA3-E2F5). qRT-PCR and western blot showed that the expression level of E2F5 was restored by cotransfection with pcDNA3-E2F5 and miR-132 when compared with the control vectors combination with miR-132 (Figure 6A and 6B). In addition, we found that transfection of E2F5 overexpression plasmid was able to counteract the inhibitory effect of miR-132 on cell proliferation (Figure 6C), colony formation (Figure 6D), cell migration (Figure 6E), and cell invasion (Figure 6F) in ovarian cancer cells. Taken together, these data suggested that miR-132 inhibited cell proliferation, colony formation migration, and invasion via targeting E2F5 in ovarian cancer cells.
Figure 6.
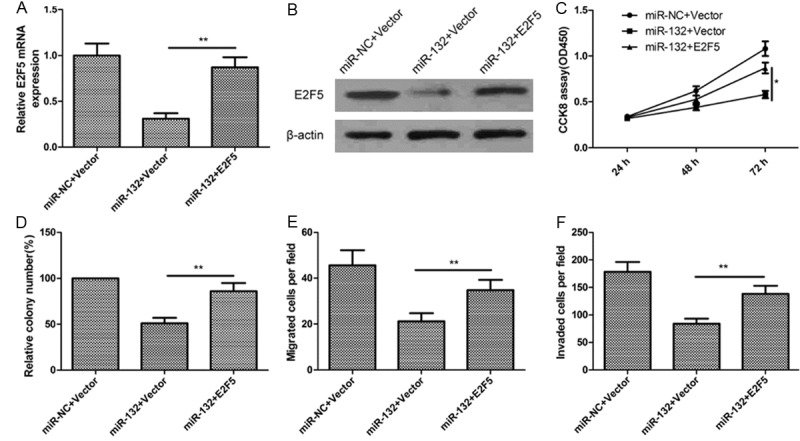
miR-132 inhibits cell proliferation, colony formation, migration, and invasion via targeting E2F5 expression. (A and B) Levels of E2F5 mRNA (A) and protein (B) were determined in SKVO3 cells transfected with miR-132 mimic or NC, together with either vector control (pcDNA3) or overexpression E2F5 plasmids (pcDNA3-E2F5). Cell proliferation (C), colony formation (D), migration (E) and invasion (F) were determined in SKVO3 cells transfected with miR-132 mimic or NC, together with either vector control (pcDNA3) or overexpression E2F5 plasmids (pcDNA3-E2F5). *P < 0.05, **P < 0.01 compared with cells transfected with miR-132 plus control vector.
Discussion
Increasing evidence suggested that numerous miRNAs involved in ovarian cancer development, progression, and metastasis by targeting a large number of critical protein-coding genes [7,8]. For example, miR-494 drastically inhibited ovarian cancer cell proliferation, colony formation, migration and invasion, induced cell apoptosis and cell cycle arrest at G1 stage in vitro, as well as reduced tumor growth in vivo by targeting insulin-like growth factor 1 receptor (IGF1R) [18]. miR-338-3p significantly inhibited ovarian cancer cell proliferation, colony formation, migration and invasion, as well as induced cell apoptosis and enhanced caspase-3, -8, and -9 activities by targeting Runx2 [19]. miR-214 inhibited ovarian cell proliferation and promoted apoptosis by repressing Sema 4D [20]. Here, we showed that miR-132 expression was obviously decreased in ovarian cancers tissue and cell lines, which is consistent with previous study [16]. Of note, to our knowledge, we first showed that the miR-132 inhibited ovarian cancer cell growth, migration and invasion. These results suggested that miR-132 might be involved in ovarian cancer initiation and progression.
miR-132, arising from the miR-212/132 cluster, has been reported to involved in various biological procession, such as inflammation, cell transformation, the vascular smooth muscle dysfunction mediated as well as tumourigenesis [10-16,21-23]. For cancer, miR-132 has been found to be downregulated, and function as a tumor suppressor in a series of cancers, such as breast cancer [10], colorectal cancer [11], osteosarcoma [12], non-small lung cancer [13], and hepatocellular carcinoma [14]. On the contrary, in glioma and gastric cancer, miR-132 expression was upregulated, and served as oncogene [24,25]. Although recently a report demonstrated that the miR-132 is downregulated in serum of patients with ovarian cancer [16], the detail function and underlying molecular mechanism of miR-132 in ovarian cancer remains largely unclear. In current study, we determined that miR-132 levels were markedly decreased in ovarian cancer tissues and cell lines, Moreover, miR-132 overexpression in ovarian cancer cells reduced cell proliferation, colony formation, migration and invasion in vitro. All the results from our study suggested that miR-132 was a tumor suppressor in ovarian cancer progression.
It was well known that miRNAs exerted its biological function by regulating target genes [26]. Therefore, identification and characterization of the targets of altered miRNAs may contribute to elucidate the molecular mechanisms involved in carcinogenesis. E2F5 were selected as the potential target of miR-132 for further validation by several biological soft (Targetscan6.2, miRanda and PicTar). E2F5 is an important member of the E2F family that binds to the promoters of the target genes involved in cell cycle control and that consequently regulates the expression of these target genes [27]. It has been showed that The E2F family involved in cell growth and proliferation through regulating the genes involved in cell cycle progression [28]. It has been reported that E2F5 expression was upregulated in various solid tumors including ovarian cancer [29], suggest E2F5 is oncogene gene. In addition, E2F5 has been identified as a target gene of several miRNAs, including miR-106 [30], miR-181a [31], miR-34a [32] and miR-128-2 [33]. In this study, we characterized E2F5 as a functional target of miR-132 by luciferase reporter gene assays, qRT-PCR and Western blot analysis, respectively. We also showed that the expression level of E2F5 was significantly increased in ovarian cancer tissues and its expression was inversely correlated with miR-132 expression in clinical ovarian cancer tissues. Of note, we found that downregulation of E2F5 have similar inhibition effect of miR-132 overexpression on the ovarian cancer cells, and that overexpression of E2F5 reversed the inhibition effected mediated by miR-132 overexpression. These results suggested that miR-132 exerted tumor suppressor role in ovarian cancer by targeting E2F5.
In summary, the results presented here demonstrate that miR-132 expression level was decreased in ovarian cancer tissues and ovarian cell lines, and that miR-132 overexpression in ovarian cancer cells drastically inhibited cell proliferation, colony formation, migration and invasion by repressing E2F5. These results suggested that miR-132 functioned as tumor suppressor in ovarian cancer by targeting E2F5, and that miR-132 may serve as a potential therapeutic candidate in the treatment of ovarian cancer.
Disclosure of conflict of interest
None.
References
- 1.Maldonado L, Hoque MO. Epigenomics and ovarian carcinoma. Biomark Med. 2010;4:543–570. doi: 10.2217/bmm.10.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coleman RL, Monk BJ, Sood AK, Herzog TJ. Latest research and treatment of advancedstage epithelial ovarian cancer. Nat Rev Clin Oncol. 2013;10:211–224. doi: 10.1038/nrclinonc.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 4.Mendell JT. MicroRNAs: critical regulators of development, cellular physiology and malignancy. Cell Cycle. 2005;4:1179–1184. doi: 10.4161/cc.4.9.2032. [DOI] [PubMed] [Google Scholar]
- 5.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tie J, Fan D. Big roles of microRNAs in tumorigenesis and tumor development. Histol Histopathol. 2011;26:1353–1361. doi: 10.14670/HH-26.1353. [DOI] [PubMed] [Google Scholar]
- 7.Kinose Y, Sawada K, Nakamura K, Kimura T. The role of microRNAs in ovarian cancer. Biomed Res Int. 2014;2014:249393. doi: 10.1155/2014/249393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Kim S, Kim IM. Regulation of Metastasis by microRNAs in Ovarian Cancer. Front Oncol. 2014;4:143. doi: 10.3389/fonc.2014.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nudelman AS, DiRocco DP, Lambert TJ, Garelick MG, Le J, Nathanson NM, Storm DR. Neuronal activity rapidly induces transcription of the CREB-regulated microRNA-132, in vivo. Hippocampus. 2010;20:492–498. doi: 10.1002/hipo.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang ZG, Chen WX, Wu YH, Liang HF, Zhang BX. MiR-132 prohibits proliferation, invasion, migration, and metastasis in breast cancer by targeting HN1. Biochem Biophys Res Commun. 2014;454:109–114. doi: 10.1016/j.bbrc.2014.10.049. [DOI] [PubMed] [Google Scholar]
- 11.Zheng YB, Luo HP, Shi Q, Hao ZN, Ding Y, Wang QS, Li SB, Xiao GC, Tong SL. miR-132 inhibits colorectal cancer invasion and metastasis via directly targeting ZEB2. World J Gastroenterol. 2014;20:6515–6522. doi: 10.3748/wjg.v20.i21.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Xu G, Shen F, Kang Y. miR-132 targeting cyclin E1 suppresses cell proliferation in osteosarcoma cells. Tumour Biol. 2014;35:4859–4865. doi: 10.1007/s13277-014-1637-2. [DOI] [PubMed] [Google Scholar]
- 13.Zhang B, Lu L, Zhang X, Ye W, Wu J, Xi Q, Zhang X. Hsa-miR-132 regulates apoptosis in non-small cell lung cancer independent of acetylcholinesterase. J Mol Neurosci. 2014;53:335–344. doi: 10.1007/s12031-013-0136-z. [DOI] [PubMed] [Google Scholar]
- 14.Wei X, Tan C, Tang C, Ren G, Xiang T, Qiu Z, Liu R, Wu Z. Epigenetic repression of miR-132 expression by the hepatitis B virus x protein in hepatitis B virus-related hepatocellular carcinoma. Cell Signal. 2013;25:1037–1043. doi: 10.1016/j.cellsig.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Hao J, Xie F, Hu X, Liu C, Tong J, Zhou J, Wu J, Shao C. Downregulation of miR-132 by promoter methylation contributes to pancreatic cancer development. Carcinogenesis. 2011;32:1183–1189. doi: 10.1093/carcin/bgr105. [DOI] [PubMed] [Google Scholar]
- 16.Chung YW, Bae HS, Song JY, Lee JK, Lee NW, Kim T, Lee KW. Detection of microRNA as novel biomarkers of epithelial ovarian cancer from the serum of ovarian cancer patients. Int J Gynecol Cancer. 2013;23:673–679. doi: 10.1097/IGC.0b013e31828c166d. [DOI] [PubMed] [Google Scholar]
- 17.Zou C, Li Y, Cao Y, Zhang J, Jiang J, Sheng Y, Wang S, Huang A, Tang H. Up-regulated MicroRNA-181a induces carcinogenesis in hepatitis B virus-related hepatocellular carcinoma by targeting E2F5. BMC Cancer. 2014;14:97. doi: 10.1186/1471-2407-14-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li N, Zhao X, Wang L, Zhang S, Cui M, He J. miR-494 suppresses tumor growth of epithelial ovarian carcinoma by targeting IGF1R. Tumour Biol. 2015 doi: 10.1007/s13277-015-4603-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Wen C, Liu X, Ma H, Zhang W, Li H. miR3383p suppresses tumor growth of ovarian epithelial carcinoma by targeting Runx2. Int J Oncol. 2015;46:2277–2285. doi: 10.3892/ijo.2015.2929. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Zhou H, Ma L, Hou Y, Pan J, Sun C, Yang Y, Zhang J. MiR-214 suppressed ovarian cancer and negatively regulated semaphorin 4D. Tumour Biol. 2015 doi: 10.1007/s13277-015-4708-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Magill ST, Cambronne XA, Luikart BW, Lioy DT, Leighton BH, Westbrook GL, Mandel G, Goodman RH. microRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc Natl Acad Sci U S A. 2010;107:20382–20387. doi: 10.1073/pnas.1015691107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulik S, Xu J, Reddy PB, Rajasagi NK, Gimenez F, Sharma S, Lu PY, Rouse BT. Role of miR-132 in angiogenesis after ocular infection with herpes simplex virus. Am J Pathol. 2012;181:525–534. doi: 10.1016/j.ajpath.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaked I, Meerson A, Wolf Y, Avni R, Greenberg D, Gilboa-Geffen A, Soreq H. MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity. 2009;31:965–973. doi: 10.1016/j.immuni.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 24.Liu Q, Liao F, Wu H, Cai T, Yang L, Wang ZF, Zou R. Upregulation of miR-132 expression in glioma and its clinical significance. Tumour Biol. 2014;35:12299–12304. doi: 10.1007/s13277-014-2541-5. [DOI] [PubMed] [Google Scholar]
- 25.Li W, Zhang J, Chen T, Yin P, Yang J, Cao Y. miR-132 upregulation promotes gastric cancer cell growth through suppression of FoxO1 translation. Tumour Biol. 2015 doi: 10.1007/s13277-015-3924-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Ohtsuka M, Ling H, Doki Y, Mori M, Calin GA. MicroRNA Processing and Human Cancer. J Clin Med. 2015;4:1651–1667. doi: 10.3390/jcm4081651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen HZ, Tsai SY, Leone G. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat Rev Cancer. 2009;9:785–797. doi: 10.1038/nrc2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren B, Cam H, Takahashi Y, Volkert T, Terragni J, Young RA, Dynlacht BD. E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Genes Dev. 2002;16:245–256. doi: 10.1101/gad.949802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kothandaraman N, Bajic VB, Brendan PN, Huak CY, Keow PB, Razvi K, Salto-Tellez M, Choolani M. E2F5 status significantly improves malignancy diagnosis of epithelial ovarian cancer. BMC Cancer. 2010;10:64. doi: 10.1186/1471-2407-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao YL, Wu XY, Wu JH, Gu T, Chen L, Gu JH, Liu Y, Zhang QH. Effects of microRNA-106 on proliferation of gastric cancer cell through regulating p21 and E2F5. Asian Pac J Cancer Prev. 2013;14:2839–2843. doi: 10.7314/apjcp.2013.14.5.2839. [DOI] [PubMed] [Google Scholar]
- 31.Zou C, Li Y, Cao Y, Zhang J, Jiang J, Sheng Y, Wang S, Huang A, Tang H. Up-regulated MicroRNA-181a induces carcinogenesis in hepatitis B virus-related hepatocellular carcinoma by targeting E2F5. BMC Cancer. 2014;14:97. doi: 10.1186/1471-2407-14-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu G, Sun Y, An S, Xin S, Ren X, Zhang D, Wu P, Liao W, Ding Y, Liang L. MicroRNA-34a targets FMNL2 and E2F5 and suppresses the progression of colorectal cancer. Exp Mol Pathol. 2015;99:173–179. doi: 10.1016/j.yexmp.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Donzelli S, Fontemaggi G, Fazi F, Di Agostino S, Padula F, Biagioni F, Muti P, Strano S, Blandino G. MicroRNA-128-2 targets the transcriptional repressor E2F5 enhancing mutant p53 gain of function. Cell Death Differ. 2012;19:1038–1048. doi: 10.1038/cdd.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]


