Abstract
Background: Diaphragm is a primary inspiratory muscle and often receives off-target dose in patients with thoracic radiotherapy, and whether acute effect of low dose irradiation would cause contractile dysfunction of the diaphragm remains unclear. We use a rat model to investigate the effect of low-dose irradiation on diaphragm contractile function in the current study. Methods: The radiation dose distributions in patients with esophageal cancer receiving radiotherapy were calculated to determine the dose received by the off-target diaphragm area. Rats were randomly assigned to an irradiated or a non-irradiated control group (n = 10 per group). A single-fraction of 5 Gy radiation was then delivered to the diaphragms of Sprague-Dawley rats in the irradiated group. The control group received sham irradiation (0 Gy). Rats were sacrificed 24 hours after the irradiation procedures and diaphragms were removed en bloc for contractile function assessment, oxidative injury and DNA damage analysis. Oxidative injury was determined by analyzing concentration of protein carbonyls and DNA damage was determined by analyzing retention of γH2AX foci in nuclei of diaphragmatic tissue. Results: At 24 hours after delivery of a single dose of 5 Gy radiation, specific twitch (p = 0.03) and tetanus tension (p = 0.02) were significantly lower in the irradiated group than in the control group. The relative force-frequency curves showed a significant downward shift in the irradiated group. Protein carbonyl level (p < 0.01) and percentage of γH2AX-positive diaphragm muscle cells were significantly higher in the irradiated group than in the control group 24 hours after irradiation (58% vs. 30%, p = 0.01). Conclusions: Off-target low dose irradiation could induce acute contractile dysfunction of the diaphragm which was related to radiation-induced direct DNA and indirect oxidative damage.
Keywords: Irradiation, respiratory muscles, oxidative stress, respiratory measurement
Introduction
Radiotherapy is a common treatment option for patients with advanced thoracic cancer [1]. With advanced radiotherapy techniques, optimal radiation doses could be delivered to the targeted areas while sparing critical organs. Surrounding areas of the treatment target are exposed to significant low doses of radiation simultaneously during radiotherapy, thereby increasing the risk for the development of normal tissue toxicity [2]. Coppes et al found that the out-of-field effects of radiation on vascular damage were very similar to the in-field effects in an irradiated rat lung model [3]. Furthermore, studies have shown that low-dose irradiation, as an off-target dose, significantly modulates the systemic pharmacokinetics of anticancer drugs [4,5]. These data suggest that low-dose irradiation can produce unexpected or unwanted biological effects.
Skeletal muscle is generally considered to be radiation resistant due to its post-mitotic state [6]. Irradiation not only damages deoxyribonucleic acid (DNA) directly but also increases oxidative stress, which can result in oxidative damage to proteins, lipids, and DNA [7]. Muscle lesion repair and reconstruction depends on the ability of satellite cells to activate proliferative myoblasts [8]. However, satellite cell and myoblast proliferation has been shown to be reduced in tissue exposed to low-dose irradiation (range, 2 to 6 Gy) due to oxidative stress and cell death [9-11]. Low-dose irradiation has also been shown to impair skeletal muscle development and remodeling by impairing satellite cell function [9,12].
The diaphragm often receives off-target irradiation during radiotherapy in patients with thoracic cancers mainly due to its anatomical position and movement during tidal breathing. Diaphragmatic dysfunction increases the risk of developing atelectasis and subsequent chronic respiratory failure that results in a decline in physical activity and thus impaired quality of life [13]. Although there is evidence to suggest that low-dose radiation might influence peripheral skeletal muscle remodeling [12], the effect of low-dose irradiation on contractile function of the diaphragm has yet to be determined. Thus, the purpose of this study was to examine the acute effects of a clinically relevant off-target irradiation dose on diaphragm contractile function in a rat model.
Methods and materials
Animals and sample preparation
A total of 20 adult male Sprague-Dawley (SD) rats weighing between 300 and 350 g were utilized in this study (Bio-LASCO Taiwan CO., Taiwan). Animals were maintained in an environmentally controlled room (25 ± 1°C; 12 h light/dark cycle) and fed a standard rat chow and water ad libitum in the Laboratory Animal Center at the Mackay Memorial Hospital (Taipei, Taiwan). The study was approved by the Institutional Animal Care and Use Committee at the Mackay Memorial Hospital, Taiwan (MMH-A-S-100-36) and was carried out in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD).
Experimental protocol
Rats were randomly assigned to an irradiated or a non-irradiated control group (n = 10 per group). Rats in the irradiated group received a single radiation dose of 5 Gy while rats in the control group received sham irradiation. Rats in both groups were sacrificed 24 hours after irradiation (or sham irradiation), and the diaphragms were removed en bloc with their rib cage origin intact as previously described [14]. Diaphragm strips measuring approximately 2 mm in width were dissected from the anterolateral portion of the diaphragm parallel to the long axis of the muscle fibers, with its attachments to the central tendon and rib cage being left intact. The remaining diaphragms were fixed in formalin for subsequent immunohistochemistry or frozen in liquid nitrogen and stored at -80°C for subsequent biochemical analysis.
Diaphragm irradiation
To establish radiation exposure of the diaphragm in the rat model, the radiation dose distributions in patients with esophageal cancer who received radiotherapy were used to calculate the appropriate off-target dose for rat diaphragms. In 6 patients with lower-third esophageal carcinoma, the mean dose received by the right side of the diaphragm was 4.3 Gy and that received by the left side of the diaphragm was 7.8 Gy. The mean dose received by the whole diaphragm was 5.9 Gy during the treatment course (Figure 1). Based on the clinical data and evidence provided in the study by Caiozzo et al which showed that a radiation dose of 5 Gy causes significant damage to myogenic stem cells in skeletal muscle [11], we chose 5 Gy as a feasible dose to examine the acute effect of off-target irradiation on the diaphragm in the rat model.
Figure 1.
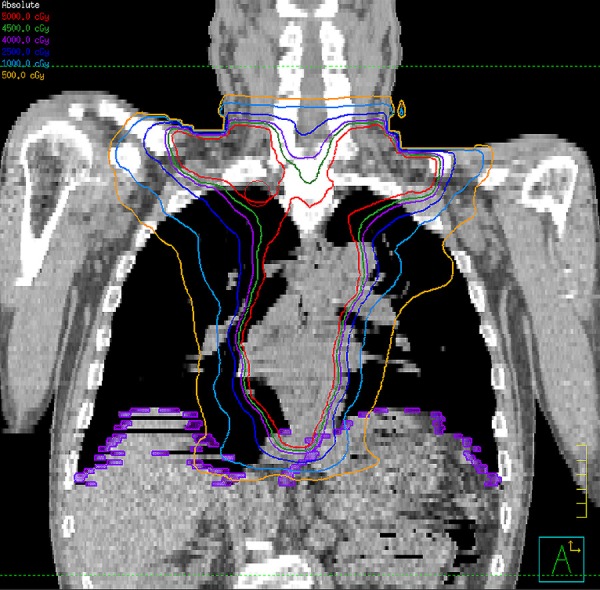
Radiotherapy planning for a patient with lower-third esophageal cancer in coronal view. The orange and light blue colored isodose lines represent 5 Gy and 10 Gy radiation doses, respectively.
Rats in the irradiated group were anesthetized and immobilized on a board before undergoing computed tomography. Considering the respiratory motion, the craniocaudal margin of irradiation was set at 1.5 cm above and below the dome of the diaphragm. The width of irradiation field opened to the right and left thoracic cage with 5 mm expansion bilaterally. Radiation was delivered using a conventional radiotherapy technique to the anterior-posterior (AP) and posterior-anterior (PA) fields in which 6-MV X-ray beams were delivered at a dose rate of 600 MU/min for a total of 5 Gy using a Varian 600CD linear accelerator (Varian Medical Systems, Palo Alto, CA).
Assessment of diaphragm strip contractility
Contractile function of the diaphragm was assessed as previously reported [15]. Briefly, intact diaphragm strips were dissected from the left costal diaphragm and mounted vertically in water-jacketed organ baths (37°C, bubble 95% O2/5% CO2) containing Tyrode solution (137 mM NaCl, 4 mM KCl, 0.5 mM MgCl2, 0.5 mM NaH2PO4, 11.9 mM NaHCO3, 5.6 mM glucose, and 2.7 mM CaCl2). The rib end of the strips was attached to the bottom of the baths by silk ties, and the central tendon end was tied to a force transducer (XDFT200, Diagnostic & Research Instruments Co., Taiwan). Platinum field electrodes were placed around the strips and connected to a Grass S88 stimulator (Grass Technologies, Warwick, RI, USA). All data were recorded and analyzed using a XctionViewII Data Acquisition System recorder (Diagnostic & Research Instruments Co., Taiwan).
After determining the optimal length (Lo) of the diaphragm strips, they were allowed to thermoequilibrate to 37°C for 15 minutes. Twitch tension (Pt) was obtained using 1-ms supramaximal square wave pulses, and tetanic tension (Po) was obtained by applying a train of supramaxial stimuli for 400 ms at optimal length. A force-frequency curve was constructed by stimulating the strips with trains of supramaximal stimuli at 1, 15, 30, 50, 80, 100 and 120 Hz with a 1-min rest period between adjacent stimulus trains. Finally, the fatigue characteristic was measured by giving the muscle a series of 300 ms tetanic stimulations every 3 seconds at a frequency adjusted to produce 50% of Po for 10 minutes. The tetanic force measured after 2 minutes of stimulation was then used to calculate the fatigue index as follows:
Fatigue index = tension measured at 2 minutes of fatigue test/Po
Measurement of protein carbonyls
The concentration of protein carbonyls in the diaphragm was assessed using an enzyme-linked immunoassay (ELISA) and a protein carbonyl content assay kit (Abcam®, Cambridge, UK) according the manufacturer’s instructions. The absorbance was determined at 375 nm and the results were expressed as nmol carboynl per mg protein.
γH2AX-immunohistochemistry
Retention of γH2AX foci 24 hours after irradiation was analyzed and used as an indicator of DNA damage. Cross sections of diaphragm bundles were cut on a cryostat (5 μm) and stored at -80°C. For immunohistochemical analysis, diaphragm tissue was fixed in formalin and embedded in paraffin. Antigen was retrieved in a decloaking chamber (Biocare Medical) using Target Retrieval Solution (pH 9.0) (DAKO, K3468) and a magnetic laboratory hot plate stirrer (PC-420, Corning, MA, USA). Tissues were incubated with primary antibody Phospho-histone H2AX (ser139) (Cell Signaling Technology, Danvers, MA, USA) and antibody (Ab) was detected using SS Multilink HK340-9K (BioGenex Laboratories, San Ramon, CA) and SS HRP Label HK330-9K (BioGenex Laboratories, San Ramon, CA). Slides were viewed with a Nikon E600 microscope (Melville, NY, USA) and an Axionplan 2 fluorescence microscope (Carl Zeiss Inc, Thornwood, NY, USA). ImageJ software (National Institute of Health, USA) was used to calculate the number of γH2AX foci. Images were captured with a Spot RT digital camera (Diagnostic Instruments, Sterling Heights, MI, USA) MicroFire (Optronics Inc., OK, USA) and a PowerMac G4 computer (Apple, Cupertino, CA, USA) XH61V (Shuttle Inc., Taipei, Taiwan) equipped with Spot RT software, version 4.0 (Diagnostic Instruments) PictureFrame (Optronics Inc., OK, USA). For quantitative analysis, foci were counted visually using an objective magnification of x40. Foci counting was done until at least 40 foci (tissues) were registered for each data point.
Data and statistical analyses
Cross-sectional area (CSA) of the diaphragm was calculated as follows:
CSA = muscle weight (g)/(length (cm) × specific gravity)
Where specific gravity equals 1.06 g/cm2 [16]. Specific twitch and tetanus of the diaphragm strips were calculated as follows:
T (N/cm 2) = tension (kg) × 9.8 (m/s 2) × length (cm) × 1.06 (g/cm 3)/muscle weight (g)
A twitch tension curve was used to determine time-to-peak tension (TPT) and time-to-half relaxation duration (½RT).
The results are presented as means ± standard error (SE) of the mean. Differences in means of continuous measurements were tested by the Student’s t Test. To compare differences in force-frequency curves between the two groups, we used a generalized estimating equation (GEE) regression model with an exchangeable correlation matrix to take into account the repeated measurements of tension. A p value of < 0.05 was considered to indicate statistical significance. All statistical analyses were performed with the statistical package SPSS for Windows (Version 17.0, SPSS, Chicago, IL, USA).
Results
There were no significant differences in age (p = 0.97), body weight (p = 0.34), average diaphragm strip length (p = 0.64) or strip weight (p = 0.33) between the two groups. The average CSA area was 4.8 ±0.6 in the control group and 4.6 ±0.5 mm2 in the irradiated group (p = 0.82).
The effects of low-dose radiation on diaphragm contractile properties are shown in Figure 2. A single dose of 5 Gy radiation significantly decreased the peak twitch (radiation: 6.3± 0.4 N/cm2 vs. control: 8.6±0.8, p = 0.02) and tetanus tension (irradiated: 16.7±3.2 N/cm2 vs. control: 27.6±2.7, p < 0.001). The Pt/Po ratio was similar between the two groups (p = 0.2). Although there was no significant difference in TPT between the two groups (p = 0.2), the ½RT was significantly longer in the irradiated group than in the control group (p < 0.001) (Figure 3). In addition, the mean Pt/TPT ratio was significantly lower in the irradiated group than in the control (p < 0.001).
Figure 2.
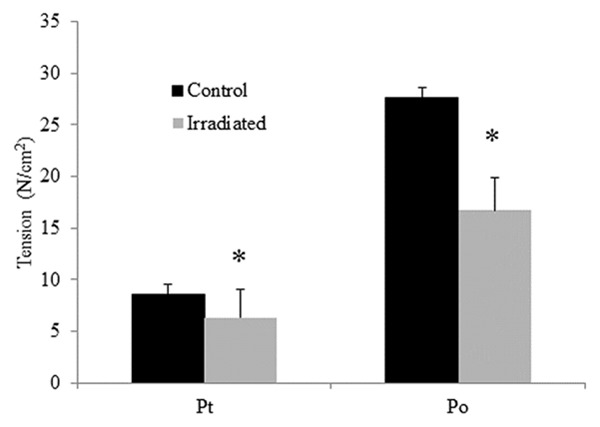
Specific twitch and tentanic tension (N/cm2) of diaphragm strips in the control group (black bars) and irradiated group (white bars). Data are means ± SE. *Indicates significant difference between the two groups (p < 0.05).
Figure 3.
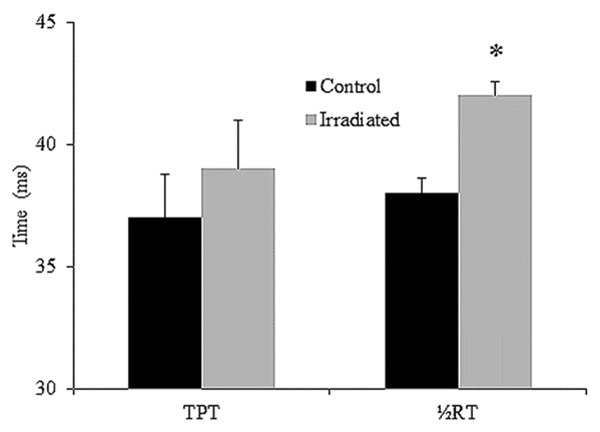
Time-to-peak tension (TPT) and half relaxation time (½RT) of diaphragm strips in the control group (black bars) and irradiated group (white bars). Data are means ± SE. *Indicates significant difference between the two groups (p < 0.05).
The force-frequency curves are presented in Figure 4. The force-frequency curve for the irradiated group was significantly downward shifted, i.e., there was less diaphragmatic force with increased frequency in the irradiated group than in the control group. At stimulation frequencies of 50 Hz and above (all p < 0.05), specific tensions in the irradiation group were significantly lower than those in the control group (Figure 4).
Figure 4.
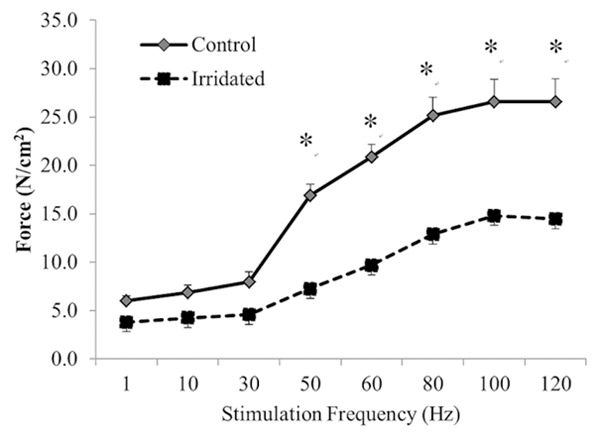
Diaphragmatic force (N/cm2)-frequency (Hz) curve for the control (-♦-) and irradiated (--■--) groups. Values are mean ± SE. *Indicates significant difference between the two groups (p < 0.05).
Relative force (force as a percentage of its initial value) over time curves for repetitive fatiguing electrical stimulation trials performed on diaphragm strips for both groups are displayed in Figure 5. Diaphragm strips in the irradiated group showed significantly greater fatigue than those in the control group after 2 mins of repetitive stimulation. The fatigue index in the irradiated group (57.2±5.6) was significantly lower than that in the control group (66.2±6.5) (p < 0.01).
Figure 5.
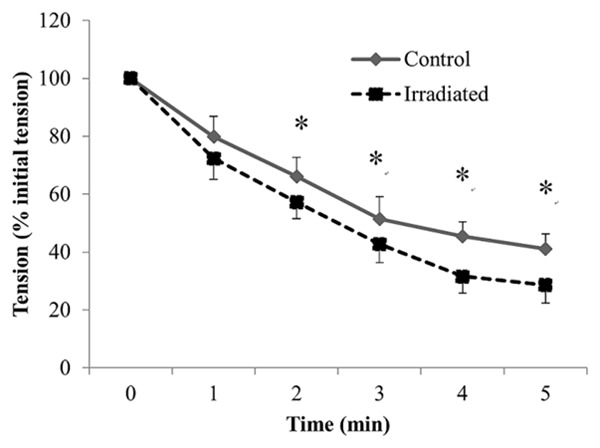
Mean changes in peak tetanic tension during repetitive stimulation of diaphragmatic stripes in the control (-♦-) and irradiated (--■--) groups. Peak tetanic tension is plotted as a percentage of the initial tetanic tension, and time is from the onset of stimulation. Values are mean ± SE. *Indicates significant difference between the two groups (p < 0.05).
Protein carbonyl concentration was significantly higher in the irradiated group (2.3±0.1) than in the control group (0.8±0.1) (p < 0.01) at 24 hours after the radiation procedure. Retention of γH2AX foci 24 hours after radiation was analyzed as a proxy for radiation-induced double strand breaks (DSBs). The percentage of diaphragmatic cells with radiation-induced γH2AX foci was significantly higher in the irradiated group than in the control group (57.6±12.7 vs. 30.0±6.0%, p < 0.01) (Figure 6).
Figure 6.
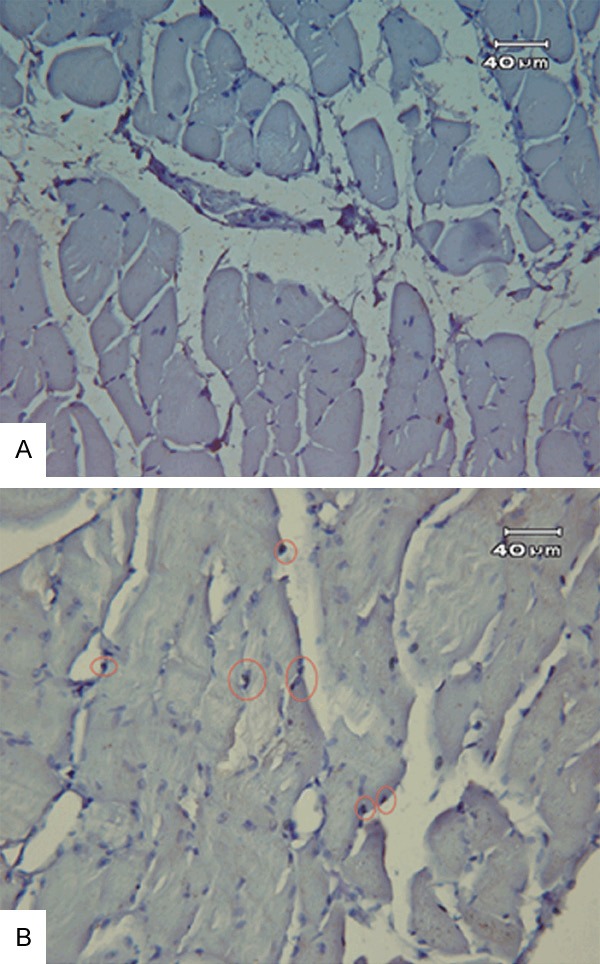
Representative immunofluorescence images used for manual foci counting. A: Control group; B: Irradiate group. The red, solid circles in the lower images were placed manually to mark individual foci in the diaphragm.
Discussion
This is the first study to show that simulating low dose off-target irradiation to the diaphragm can cause acute contractile dysfunction in rat model. The potential mechanisms for this contractile dysfunction might due to direct DNA damage or indirect damage through oxidative stress to the myofibrils.
Diaphragm contractile dysfunction is of clinical importance because it may progress to respiratory failure. Studies have shown that diaphragm muscle performance were significantly related to and predictive of exercise performance and hence influence quality of life [17,18].
Clinical studies have reported a spectrum of dose-dependent changes in skeletal muscle function after irradiation that can remain for many years [19,20]. A single dose larger than 10 Gy has been shown to result in reduced total muscle protein and RNA [12,21], and to cause interfibrillar edema, myofilament disruption and endothelial swelling [22]. However, radiation dose lower than 10 Gy on functional outcome of muscle has not been examined before. In this study, we demonstrated that the peak twitch and tetanus tension of the diaphragm decreased by 26.7% (p = 0.02) and 39.5% (p < 0.001), respectively, 24 hours after a radiation dose of 5 Gy. These results suggest that low dose irradiation to the rat diaphragm, mimicking off-target dose during radiotherapy for patients with esophageal cancer, could result in marked contractile function decline 24 hours after irradiation. Whether radiation poses similar pattern of detrimental effect on diaphragm contractile function in clinical patients receiving radiotherapy around the thoracic area warrants further studies to explore.
Distribution of fiber types of skeletal muscle is one of the important factors affecting the contractile function of skeletal muscle [23]. In this study, ½RT was significantly longer in the irradiated group than in the control group. A lower ½RT value is often observed when muscle is injured, in fatigued state, or an alteration in the composition of fiber types occurs. The rat diaphragm contains 40%, 24% and 34% of Type I, Type IIa, and Type IIb fibers, respectively [24], and type II fibers were found to be more sensitive to radiation related injury than type I fibers [25]. Thus, the finding of a longer ½RT after a low dose of irradiation indicated that low dose would cause diaphragm injury and alter the ratio of fiber type composition, i.e., mainly due to loss of Type II fibers. Further histochemical staining is needed to confirm this exploration. Although fiber type distribution in the adult human diaphragm (55% Type I, 21% type Iia, and 24% Type IIb) is slightly different than in the rat [26], whether the same low dose irradiation would cause human diaphragm injury and result in contractile dysfunction warrants further investigation.
DSBs are the most deleterious form of radiation-induced DNA damage [27]. The γH2AX foci analysis is used to identify the number and location of DSBs and to verify the different DSB repair deficiencies [27,28]. The dynamics of radiation-induced γH2AX phosphorylation are usually starting a few minutes after irradiation and occur during the following 24 hrs [29]. In the current study, the number of nuclei in diaphragm cells that stained positive for γH2AX at 24 hours was higher in the irradiation group (58%) than in the control group (30%). In a study on reactive oxygen in skeletal muscle, Reid et al mounted diaphragm fiber bundles in chambers containing Krebs-Ringer solution and γH2AX staining became visible as early as 30 minutes after exposure to 0.5 μM H2O2 [30]. The findings could explain, at least in part, why 30% of control tissue stained positive for γH2AX in our study. Furthermore, these data suggest that DSB repair deficiencies are dominate in diaphragms after exposure to radiation.
It has been reported that when skeletal muscle undergoes atrophy, a subset of myonuclei undergo apoptosis, even though the rest of the muscle fibers themselves remain intact [31]. Fitts, et al reported that myofibrillolysis and microcirculatory endothelial cell changes were observed as early as 24 hrs after irradiation [32]. The DNA-dependent protein kinase (DNA-PK) is a nuclear serine/threonine kinase whose activity is stimulated by double-stranded DNA ends [6] and the activity of DNA-PKcs is specifically required for the induction of γH2AX [33]. It also plays an important role in triggering apoptosis in response to excessive or unrepairable DNA damage.
Inhibition of Ca2+ release from the sarcoplasmic reticulum (SR) was shown to result in changes in the time course of the twitch [34]. In a cardiac dysfunction study, Sag et al reported that targeted cardiac irradiation induced the generation of reactive oxygen species (ROS) which led to the activation of Ca2+/calmodulin-dependent protein kinase II (CaMKII) and induce a chronically low sarcoplasmic reticulum (SR) Ca2+ load [35]. Schulte et al. found that a longer relaxation time in denervated extensor digitorum longus muscle of rats was correlated with a decrease in mRNA and protein expression of the fast Ca2+ pump isoform in the SR [36]. Interestingly, in the current study, the peak twitch and tetanus tension were lower, the ½RT was longer, the Pt/TPT ratio was lower and the force-frequency curve was shifted downward in the irradiation group than in the control group. In addition, diaphragm strips in the irradiation group showed significantly greater fatigue than those in the control group. The results from the above-mentioned studies as well as this study indicate that low-dose irradiation modulates the twitch contractile parameters in the diaphragm , at least in part, may by altering Ca2+ homeostasis and muscle metabolism or muscle fiber type, which consequently has an effect on force output.
Conclusion
To the best of our knowledge this is the first study to investigate the acute effect of low-dose irradiation on diaphragm contractile function using a rat model to mimic the off-target dose to the diaphragm received during radiotherapy to the thoracic region in patients. Our findings indicate that contractile dysfunction might be related to radiation induced DNA damage that alters muscle metabolism or myofibril damage that alters fiber composition caused by radiation induced oxidative stress. Additional studies are needed to better understand the potential detrimental effects of low dose radiation to respiratory outcome in patients receiving radiotherapy to the thoracic region.
Acknowledgements
This work was supported by the Far Eastern Memorial Hospital grants (FEMH 104-2314-B-418-009- MY2).
Disclosure of conflict of interest
None.
References
- 1.Gebski V, Burmeister B, Smithers BM, Foo K, Zalcberg J, Simes J. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a metaanalysis. Lancet Oncol. 2007;8:226–234. doi: 10.1016/S1470-2045(07)70039-6. [DOI] [PubMed] [Google Scholar]
- 2.Shueng PW, Lin SC, Chang HT, Chong NS, Chen YJ, Wang LY, Hsieh YP, Hsieh CH. Toxicity risk of non-target organs at risk receiving lowdose radiation: case report. Radiat Oncol. 2009;4:71. doi: 10.1186/1748-717X-4-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coppes RP, Muijs CT, Faber H, Gross S, Schippers JM, Brandenburg S, Langendijk JA, van Luijk P. Volume-dependent expression of in-field and out-of-field effects in the protonirradiated rat lung. Int J Radiat Oncol Biol Phys. 2011;81:262–269. doi: 10.1016/j.ijrobp.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh CH, Hsieh YJ, Liu CY, Tai HC, Huang YC, Shueng PW, Wu LJ, Wang LY, Tsai TH, Chen YJ. Abdominal irradiation modulates 5-Fluorouracil pharmacokinetics. J Transl Med. 2010;8:29. doi: 10.1186/1479-5876-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai TH, Chen YJ, Hou ML, Wang LY, Tai HC, Hsieh CH. Pelvic irradiation modulates the pharmacokinetics of cisplatin in the plasma and lymphatic system. Am J Transl Res. 2015;7:375–384. [PMC free article] [PubMed] [Google Scholar]
- 6.Burma S, Chen DJ. Role of DNA-PK in the cellular response to DNA double-strand breaks. DNA Repair. 2004;3:909–918. doi: 10.1016/j.dnarep.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Azimzadeh O, Scherthan H, Sarioglu H, Barjaktarovic Z, Conrad M, Vogt A, Calzada-Wack J, Neff F, Aubele M, Buske C, Atkinson MJ, Tapio S. Rapid proteomic remodeling of cardiac tissue caused by total body ionizing radiation. Proteomics. 2011;11:3299–3311. doi: 10.1002/pmic.201100178. [DOI] [PubMed] [Google Scholar]
- 8.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 9.Olive M, Blanco R, Rivera R, Cinos C, Ferrer I. Cell death induced by gamma irradiation of developing skeletal muscle. J Anat. 1995;187:127–132. [PMC free article] [PubMed] [Google Scholar]
- 10.Jurdana M, Cemazar M, Pegan K, Mars T. Effect of ionizing radiation on human skeletal muscle precursor cells. Radiol Oncol. 2013;47:376–381. doi: 10.2478/raon-2013-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caiozzo VJ, Giedzinski E, Baker M, Suarez T, Izadi A, Lan M, Cho-Lim J, Tseng BP, Limoli CL. The radiosensitivity of satellite cells: cell cycle regulation, apoptosis and oxidative stress. Radiat Res. 2010;174:582–589. doi: 10.1667/RR2190.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardee JP, Puppa MJ, Fix DK, Gao S, Hetzler KL, Bateman TA, Carson JA. The effect of radiation dose on mouse skeletal muscle remodeling. Radiol Oncol. 2014;48:247–256. doi: 10.2478/raon-2014-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laroche CM, Carroll N, Moxham J, Green M. Clinical significance of severe isolated diaphragm weakness. Am Rev Respir Dis. 1988;138:862–866. doi: 10.1164/ajrccm/138.4.862. [DOI] [PubMed] [Google Scholar]
- 14.Tarasiuk A, Scharf SM, Miller MJ. Effect of chronic resistive loading on inspiratory muscles in rats. J Appl Physiol (1985) 1991;70:216–222. doi: 10.1152/jappl.1991.70.1.216. [DOI] [PubMed] [Google Scholar]
- 15.Oishi PE, Cholsiripunlert S, Gong W, Baker AJ, Bernstein HS. Myo-mechanical analysis of isolated skeletal muscle. J Vis Exp. 2011 doi: 10.3791/2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Close RI. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972;52:129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- 17.England R, Maddocks M, Manderson C, Wilcock A. Factors influencing exercise performance in thoracic cancer. Respir Med. 2012;106:294–299. doi: 10.1016/j.rmed.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Mohan A, Singh P, Singh S, Goyal A, Pathak A, Mohan C, Guleria R. Quality of life in lung cancer patients: impact of baseline clinical profile and respiratory status. Eur J Cancer Care (Engl) 2007;16:268–276. doi: 10.1111/j.1365-2354.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- 19.Lazarus C, Logemann JA, Pauloski BR, Rademaker AW, Helenowski IB, Vonesh EF, Maccracken E, Mittal BB, Vokes EE, Haraf DJ. Effects of radiotherapy with or without chemotherapy on tongue strength and swallowing in patients with oral cancer. Head Neck. 2007;29:632–637. doi: 10.1002/hed.20577. [DOI] [PubMed] [Google Scholar]
- 20.Levendag PC, Teguh DN, Voet P, van der Est H, Noever I, de Kruijf WJ, Kolkman-Deurloo IK, Prevost JB, Poll J, Schmitz PI, Heijmen BJ. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007;85:64–73. doi: 10.1016/j.radonc.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Schwenen M, Altman KI, Schroder W. Radiation-induced increase in the release of amino acids by isolated, perfused skeletal muscle. Int J Radiat Biol. 1989;55:257–69. doi: 10.1080/09553008914550291. [DOI] [PubMed] [Google Scholar]
- 22.Khan MY. Radiation-induced changes in skeletal muscle. An electron microscopic study. J Neuropathol Exp Neurol. 1974;33:42–57. doi: 10.1097/00005072-197401000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Bottinelli R, Canepari M, Pellegrino MA, Reggiani C. Force-velocity properties of human skeletal muscle fibres: myosin heavy chain isoform and temperature dependence. J Physiol. 1996;495:573–586. doi: 10.1113/jphysiol.1996.sp021617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilarski W, Sjostrom M. Systematic distribution of muscle fibre types in the rat and rabbit diaphragm: a morphometric and ultrastructural analysis. J Anat. 1990;168:13–30. [PMC free article] [PubMed] [Google Scholar]
- 25.Khan MY. Radiation-induced changes in skeletal muscle. An electron microscopic study. J Neuropathol Exp Neurol. 1974;33:42–57. doi: 10.1097/00005072-197401000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Polla B, D’Antona G, Bottinelli R, Reggiani C. Respiratory muscle fibres: specialisation and plasticity. Thorax. 2004;59:808–817. doi: 10.1136/thx.2003.009894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rube CE, Grudzenski S, Kuhne M, Dong X, Rief N, Lobrich M, Rube C. DNA double-strand break repair of blood lymphocytes and normal tissues analysed in a preclinical mouse model: implications for radiosensitivity testing. Clin Cancer Res. 2008;14:6546–6555. doi: 10.1158/1078-0432.CCR-07-5147. [DOI] [PubMed] [Google Scholar]
- 28.Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, Pommier Y. GammaH2AX and cancer. Nature reviews. Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paris L, Cordelli E, Eleuteri P, Grollino MG, Pasquali E, Ranaldi R, Meschini R, Pacchierotti F. Kinetics of gamma-H2AX induction and removal in bone marrow and testicular cells of mice after X-ray irradiation. Mutagenesis. 2011;26:563–572. doi: 10.1093/mutage/ger017. [DOI] [PubMed] [Google Scholar]
- 30.Reid MB, Haack KE, Franchek KM, Valberg PA, Kobzik L, West MS. Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J Appl Physiol (1985) 1992;73:1797–1804. doi: 10.1152/jappl.1992.73.5.1797. [DOI] [PubMed] [Google Scholar]
- 31.Liu CC, Ahearn JM. Apoptosis of skeletal muscle cells and the pathogenesis of myositis: a perspective. Curr Rheumatol Rep. 2001;3:325–333. doi: 10.1007/s11926-001-0037-y. [DOI] [PubMed] [Google Scholar]
- 32.Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- 33.Mukherjee B, Kessinger C, Kobayashi J, Chen BP, Chen DJ, Chatterjee A, Burma S. DNAPK phosphorylates histone H2AX during apoptotic DNA fragmentation in mammalian cells. DNA Repair (Amst) 2006;5:575–590. doi: 10.1016/j.dnarep.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Rassier DE, Tubman LA, MacIntosh BR. Inhibition of Ca2+ release in rat atrophied gastrocnemius muscle. Exp Physiol. 1997;82:665–676. doi: 10.1113/expphysiol.1997.sp004055. [DOI] [PubMed] [Google Scholar]
- 35.Sag CM, Wolff HA, Neumann K, Opiela MK, Zhang J, Steuer F, Sowa T, Gupta S, Schirmer M, Hunlich M, Rave-Frank M, Hess CF, Anderson ME, Shah AM, Christiansen H, Maier LS. Ionizing radiation regulates cardiac Ca handling via increased ROS and activated CaMKII. Basic Res Cardiol. 2013;108:385. doi: 10.1007/s00395-013-0385-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulte L, Peters D, Taylor J, Navarro J, Kandarian S. Sarcoplasmic reticulum Ca2+ pump expression in denervated skeletal muscle. Am J Physiol. 1994;267:C617–622. doi: 10.1152/ajpcell.1994.267.2.C617. [DOI] [PubMed] [Google Scholar]


