Abstract
Connective tissue growth factor (CTGF) is a member of the CCN super family and is reported to widely participate in bone development and regeneration. This study aimed to restore murine femoral segmental defect using CTGF-overexpressing MC3T3-E1 cells. MC3T3-E1 cells were transinfected by lenti-CTGF (LvCTGF) and lenti-negative control (LvNC) virus to obtain stably transinfected cells. Real-time PCR, Western blot, alkaline phosphatase activity assay, and alizarin red staining demonstrated that the overexpression of CTGF enhanced osteogenesis in vitro. Cell migration assay results showed that LvCTGF cells expressed higher migration ability than LvNC cells, while CCK-8 assay revealed no significant difference in cell proliferation. The LvCTGF and LvNC cells were then seeded into a chitosan/β-TCP scaffold and were used to restore a murine femoral segmental defect. Samples were harvested by the end of 2 and 5 weeks respectively. Micro-CT analysis and Masson’s trichrome staining results showed that the LvCTGF-scaffold group expressed better bone healing compared with the LvNC-scaffold and scaffold-only groups. CTGF-overexpressed cells serve as an efficient source of seeding cells for bone regeneration.
Keywords: Transinfection, osteogenesis, CTGF overexpression, bone segmental defect, bone healing
Introduction
Connective tissue growth factor (CTGF) is a member of the CCN super family, and its structure consists of four modules: 1) insulin-like growth factor-binding protein-like (IGFBP); 2) von Willebrand factor type C (vWC); 3) thrombospondin type 1 repeat (TSP1); and 4) carboxyl-terminal cysteine knot (CT) [1,2]. These CTGF sub-structures contribute to the diversity of its bioactivity.
CTGF has been reported to widely participate in bone development and regeneration [1-3]. It plays important roles in cartilage development and maturation [4], and in all procedures of endochondral ossification [3]. Nevertheless, the expression pattern of CTGF during distraction osteogenesis suggested that it may also play roles during intramembranous ossification [5]. Besides, CTGF is capable of inducing osteogenic differentiation and mineralization in various types of cells, such as mesenchymal stem cells [6,7], osteoblastic cells [8,9], vascular smooth muscle cells [10], human dental pulp cells [11], and human periodontal ligament stem cells [12]. Moreover, CTGF reportedly occurred in other osteogenic processes including vascular calcification, which was similar to the procedure of osteogenesis [13,14]. When introduced into bone regeneration, CTGF also provided favorable outcome [15,16]. These findings proved that CTGF can serve as a promising candidate for bone tissue regeneration. However, application of CTGF overexpressed cells as seeding cells for bone tissue regeneration has not occurred yet.
Chitosan and β-tricalcium phosphate (β-TCP) have been widely utilized as materials for bone tissue engineering because of their good biocompatibility and osteoinductive activities [17,18]. The combination of chitosan and β-TCP forms scaffolds that are characterized with interconnected open-pore microstructure and macropore structure, which allows cell inoculation [19]. In addition, the spongy scaffold was compressible and easy to shape [18]. Our previous studies have already demonstrated the availability of the chitosan/β-TCP (CS/β-TCP) scaffold [20,21]. Although this scaffold was not ideal for controlled-release of biofactors because of high porosity, it was sufficient for our study because the seeding cells themselves secreted CTGF. We used the hybrid of CTGF-overexpressed cells and CS/β-TCP scaffold to restore a murine femoral defect model.
Material and methods
Cell culture and lentivirus infection
MC3T3-E1 cell line (Subclone 14) was purchased from China Center for Type Culture Collection (Wuhan, China). Cells were routinely cultured in α-MEM (Hyclone, Logan, Utah) supplemented with 10% FBS (Gibco, NY) and 1% Penicillin and Streptomycin (Hyclone). The culture medium was refreshed thrice a week. For cell transinfection, cells were seeded in 24-well plates and separately infected with a GFP (green fluorescent protein)-tagged recombinant mouse CTGF lentivirus (Genepharma Corp., Shanghai, China) as the experimental group (LvCTGF), and a GFP-tagged empty lentivirus as the negative control (LvNC). The transinfected cells were then cultured as in routine medium and amplified for further experiments. For osteogenic differentiation, the transinfected cells were cultured in osteogenesis-inducing medium that consists of α-MEM, 10% FBS, 1% penicillin and streptomycin, 50 mg/ml ascorbic acid and 10 mM β-glycerophosphate.
Total RNA extraction and quantitative real-time PCR analysis
For quantitative real-time PCR, cells were seeded in 12-well plates (Corning, Albany, NY). Samples were washed with PBS for 3 times, total RNA was extracted using EZNA total RNA kit(Omega, Norcross, GA) according to the manufacturer’s protocol, the RNA templates (1 µg) were then reverse-transcribed into cDNA using First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA). Quantitative RT-PCR was performed on Bio-Rad CFX 96 real-time PCR machine using the All-in-One™ qPCR Mix (GeneCopoeia, Rockville, MD) following the manufacturer’s instructions. Primers were also purchased from GeneCopoeia. Expression of osteogenic differentiation markers were examined, including alkaline phosphatase (ALP), Osterix (Osx), Osteopontin (OPN) and Runt-related transcription factor 2 (Runx2). All values were normalized to GAPDH and analyzed using ΔΔCt method.
Total protein extraction and western blot
For Western blot, cells were seeded in 3.5 cm flask (Corning) and grew to confluence. Total cellular protein was prepared by lysing cells with RIPA (Thermo Fisher Scientific) supplemented with 1% PMSF (Roche, Basel, Switzerland). The concentration of the total protein was assessed using Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific). Then 20 µg protein samples were separated by SDS-PAGE, and transferred to a polyvinylidene difluoride (PVDF) membrane (Roche) by electro blotting. After 1 hour blocking in 5% degreased milk powder-TBST solution at room temperature, membranes were incubated overnight on a shaker at 4°C with one of the following antibodies: Anti-ALP (Abcam, Cambridge, MA), anti-Runx2 (Abcam), anti-Osterix (Abcam), and anti-Osteopontin (Abcam). The membranes were then incubated with a secondary antibody, including HRP-conjugated goat-anti-rabbit IgG (Abbkine, Redlands, California) or goat-anti-mouse IgG (Abbkine). β-actin was used as the internal reference.
ALP activity
For ALP activity assay, cells were seeded in 6-well plates (Corning) and treated after reaching sub-confluence. Total protein was then isolated on Day 7. ALP activity was measured using an ALP assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer’s instructions. Optic density at 520 nm (OD520nm) was used to represent the ALP activity, and OD520nm normalized to total protein amount was compared among groups.
Alizarin red assay
Cells were seeded into 12-well plates and cultured to sub confluence in complete medium which was then replaced by osteogenic medium. After 21 days culture with the medium refreshed thrice a week, cells were fixed with 95% ethanol, washed twice with tri-distilled water, and incubated with 1% Alizarin Red S/Tris-HCl solution at pH 4.2 for 30 min to stain the mineralized nodules. After that the solution was discarded, cells were gently washed twice with tri-distilled water and air- dried. Microscopic images were obtained using the Nikon inverted microscope. The pigmented mineralized nodules were subsequently dissolved with Cetylpyridinium Chloride (Sigma-Aldrich) separately and optical density at 562 nm (OD520nm) was measured.
Cell proliferation assay
For the measurement of cell proliferation, the cells were cultured in 96-well plates. After the cells reached sub-confluence, FBS concentration was reduced to 1%. Cell numbers were measured using a CCK-8 kit (Dojindo, Mashikimachi, Japan) following the manufacturer’s instructions on Days 1, 2, and 3.
Cell migration assay
For the measurement of cell migration, the cells were seeded into the upper chamber of the Costar Transwell inserts (#3422, pore size, 8 μm) (Corning) at a density of 1.0 × 104 cells/well. After incubation for 12 h at 37°C, the cells in the upper chamber were carefully removed using cotton swab. The cells went through the permeable supports and were fixed in 4% paraformaldehyde (PFA) and stained with DAPI. Then, cells were photographed with a fluorescence microscope (Leica, Wetzlar, Germany) and quantified using Image Pro Plus 6.0 software (Media Cybernetics, Rockville, MD).
Hybrid of cells and scaffold
The chitosan/beta-tricalcium phosphate (CS/β-TCP) scaffold with the porosity of 87.5% was fabricated as previously described [19,20]. The scaffold was shaped into 2 mm*1 mm* 1 mm sized cuboid sticks and disinfected in 75% alcohol for 24 h, and then washed thoroughly with sterilized PBS and air-dried under UV light sanitization. Transinfected cells were cultured in 100 mm petri dish to confluence and harvested, and then centrifuged into a pellet. After resuspension, the concentration of cells was measured and adjusted to 1*107/ml. Then, cells were inoculated into the scaffold by centrifugal force following a previously described protocol [22]. The cell-scaffold hybrids were then incubated for 4 h in an incubator and subsequently used for bone defect restoration.
Animal models and operational treatments
All animals in this study were treated according to internationally recognized guidelines on animal welfare and with the approval of the Medical Ethics Committee of Hospital of Stomatology, Wuhan University. Twenty-three mice were purchased from Hubei Provincial Laboratory Animal Center and bred at the SPF Animal Lab of Wuhan University Stomatology School. One mouse died during anesthetic treatment, 22 underwent bilateral surgery and 4 of the ones that underwent surgery died postoperatively. Mouse femoral segmental defects (1.2 mm) were established according to our previously published protocols [23]. Then, the defects were separately filled with CS/β-TCP scaffold (S group), CS/β-TCP scaffold loaded with LvNC cells (S-N group) and CS/β-TCP scaffold loaded with LvCTGF cells (S-C group), according to the grouping protocol listed in Table 1. After 2 and 5 postoperative weeks, the mice were euthanized, and femora were dissected in full length as samples and fixed in 4% PFA for 48 h. Samples were further treated for micro-CT scanning and histological analysis.
Table 1.
Grouping protocol

|
Micro-computed tomography (μ-CT) analysis
Femora were flushed using running water for 24 h after 4% PFA fixation, then embedded in paraffin. The fixation devices were carefully removed. The paraffin-embedded samples were scanned using a μ-CT 50 imaging system (Scanco Medical AG, Bassersdorf, Switherland). The X-ray tube was set at 70 kV, 85 mA with the resolution was 20 μm (Integration time = 400 ms) paralleling the axis of the femora. Analysis and three-dimensional (3D) reconstruction were performed using the software provided by the manufacturer.
Histology
Following fixation, the samples underwent decalcification, dehydration, waxing and embedding. The fixation devices were then carefully removed from the paraffin-embedded samples, and the voids left were resealed with paraffin. Samples were sliced into 5 μm-thick longitudinal serial sections and stained with Masson’s trichrome.
Data analysis
For all quantitative data, analysis of variance (ANOVA) was employed to evaluate the effect of one variable on two or more independent groups. In the event of a significant group effect, individual pairs of means were compared using the Bonferroni post-hoc test. Data were calculated as mean ± standard deviation, and in some cases, converted to percent of control. A value of p < 0.05 was used to determine whether differences were statistically significant.
Results
Lenti-virus mediated overexpression of CTGF in MC3T3-E1 cells
We transinfected MC3T3-E1 cells with lenti-NC and lenti-CTGF viruses separately to obtain stably transinfected cell lines. Then the cells were routinely cultured in full medium, and total RNA and protein were extracted after 3 days. When compared with the LvNC group, the LvCTGF group showed more than 7-fold higher expression of CTGF mRNA, as shown by quantitative realtime PCR (Figure 1A), and 4.3-fold higher expression of CTGF protein, as shown by Western blot (Figure 1B). These results confirmed that the LvCTGF cells expressed higher levels of Ctgf mRNA and CTGF protein than LvNC cells.
Figure 1.

CTGF was overexpressed via lentivirus transinfection: A. Realtime PCR of Ctgf mRNA expression in LvCTGF cells compared with MC3T3-E1 and LvNC cells (***P < 0.001; One way ANOVA with GraphPad Prism5.0). B. Western blot of CTGF expression in LvCTGF cells compared with MC3T3-E1 and LvNC cells.
CTGF overexpression enhanced osteogenic differentiation of MC3T3-E1 cells in vitro
To determine the osteogenic effect of CTGF overexpression, LvNC and LvCTGF cells were cultured in osteogenesis-inducing medium. Medium was replaced every 3 days. For realtime PCR, cells were cultured for 3 days and total mRNA was extracted. For Western blot and ALP activity quantitative assay, cells were cultured for 7 days, and total protein was extracted. For ALP activity qualitative staining, cells were cultured for 14 days and fixed with 4% PFA. For alizarin red, cells were cultured for 21 days and fixed with 4% PFA. Realtime PCR and western blot results showed that LvCTGF cells expressed significantly higher osteogenic markers, including OPN, Runx2, and Osterix, than LvNC cells (Figure 2A-D). ALP activity quantitative assay showed that ALP activity of LvCTGF cells was as 1.59-fold high as that of LvNC cells, in accordance with the ALP activity qualitative staining (Figure 2E). Alizarin red assay showed that more mineralized nodules formed in LvCTGF cells than in LvNC cells (Figure 2F).
Figure 2.

CTGF overexpression enhanced osteogenic differentiation in MC3T3-E1 cells in vitro. A-C. Realtime PCR of Opn, Osx, and Runx2 mRNA expression in LvCTGF cells versus LvNC cells (**P < 0.01, ***P < 0.001; Two-tailed t test with GraphPad Prism5.0). D. Western blot of OPN, Osx, and Runx2 expression in LvCTGF cells versus LvNC cells. E. ALP activity of LvCTGF cells versus LvNC cells (***P < 0.001; Two-tailed t test). F. Alizarin red assay of LvCTGF cells versus LvNC cells (***P < 0.001; Two-tailed t test).
CTGF overexpression promoted the migration of MC3T3-E1 cells but not proliferation
To assess the influences of CTGF overexpression on migration of MC3T3-E1 cells, transwell assay was performed. As shown in Figure 3A and 3B, LvCTGF cells expressed higher migration behavior than LvNC cells, indicating that CTGF overexpression promoted the migration of MC3T3-E1 cells. However, CCK-8 assay showed that the number of LvCTGF cell was not significantly altered when compared with LvNC cells on Day 0, 1, 3 and 7 (Figure 3C).
Figure 3.
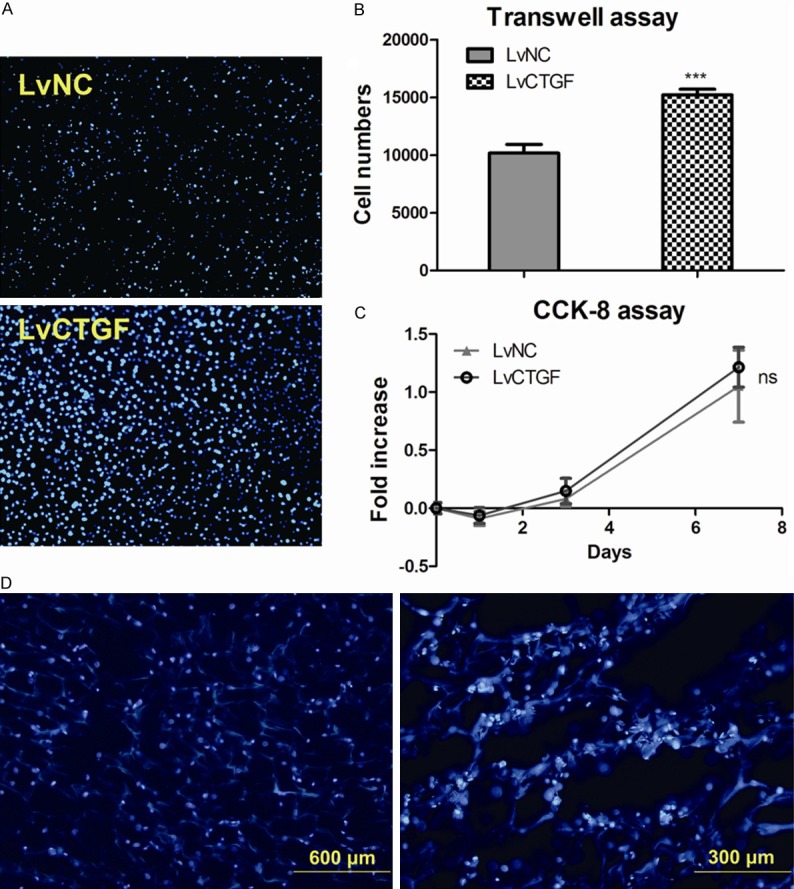
CTGF overexpression promoted the migration but did not alter the proliferation of MC3T3-E1 cells. Cells integrated to the scaffold. A. Fluorescent images (Magnification 200 X) of DAPI stained transwell upper chamber, LvNC cells versus LvCTGF cells. Bright blue spots showed the nucleuses. B. Quantification data of transwell assay images, nucleuses were counted using Image Pro Plus6.0 software (***P < 0.001; Two-tailed t test with GraphPad Prism5.0). C. CCK-8 assay on Day 0, 1, 3, and 7. Data points showed the relative fold increase to values of Day 0. (ns, P > 0.001; two-tailed t test). D. Fluorescent images of DAPI stained frozen section of cell-scaffold hybrid, bright blue spots showed the nucleuses, and dark blue structure showed the scaffold, scale bars were stamped.
Hybrid of transinfected cells and TCP/Chitosan scaffold
We produced cell-scaffold hybrids as described in the Material and Methods. Some of the hybrids were fixed in 4% PFA for overnight, and then went through the procedures of frozen section. The frozen section slices were then stained with DAPI and observed using a Leica fluorescence microscope. Images were taken by a Nikon camera. Fluorescent images showed that the cells were well permeated into the scaffold, and most of cells were located on the wall of pores in the scaffold (Figure 3D).
CTGF overexpressed cells promoted healing of murine femoral segmental defect
Micro-CT analysis and histological staining were performed to evaluate the healing progresses of defects at 2 and 5 weeks, respectively. The 3D reconstruction images of μ-CT showed that the healing of S-C group was dramatically better than those of the other two groups at 5 weeks postoperatively. However, no great difference was observed at 2 weeks (Figure 4A). Setting the defect area as area of interest, quantitative data of μ-CT showed that group S-C was significantly higher than the other two groups in bone mineral density (BMD), bone volume/tissue volume (BV/TV), trabecula number (Tb.N), and connectivity density (Conn.D), but lower in trabecula separation (Tb.Sp) at 5 weeks (Figure 4B). Higher BMD, BV/TV, Tb.N and Conn.D, as well as lower Tb.Sp, represented better bone healing. Meanwhile, both S-N and S-C group showed the same tendency as S-C group versus S-N group in BMD, BV/TV, Tb.N, Conn.D, and Tb.Sp compared to S group at 5 weeks, thereby indicating that cell-loaded scaffold had better restoration effects than non-cell scaffold. All the quantitative data approximated null, and showed no significant difference at 2 weeks (data not shown). However, trabecula thickness (Tb.Th) showed no significant difference among all groups.
Figure 4.

Micro-CT images and analysis showed the defect healing of group S-C was better than other groups. A. Micro-CT images of defect healing femurs of group S, S-N, and S-C at 2 and 5 weeks. B. Micro-CT quantification data of the defect area of group S, S-N, and S-C at 5 weeks (*P < 0.05, **P < 0.01, ***P < 0.001; One way ANOVA with GraphPad Prism5.0).
Masson’s trichrome staining images showed that at 2 weeks, osteoid and bone remodeling occurred in the defect of S-C group, whereas in the S-N group cells accumulated in scaffold In the S group, only scaffold structure was present (Figure 5). At 5 weeks all groups formed osteoid in defect area, in group S-C one side of the defect was sufficiently close to be bridged by newly formed bone, whereas in group S-N small amount of osteoid formed and in group S the defect remained unhealed.
Figure 5.

Masson’s trichrome staining images of defect healing femurs of group S, S-N, and S-C at 2 and 5 weeks. Red “E” showed the cortical bone edge of defect. Black “M” stood for scaffold material. “RD” stood for bone remodeling area. “NB” stood for newly formed bone. Scale bars were stamped at lower right corners.
Discussion
Many studies demonstrated that CTGF was capable of inducing osteogenesis in vitro and in vivo [9,10]. CTGF was proved to be related to several important signaling pathways that regulated osteogenesis including Wnt signaling, BMP signaling and ERK signaling [7,10,24]. Contrarily, there were also some studies reported that in some occasions the overexpression of CTGF impaired osteogenesis, these results may be explained by the ability of CTGF to bind to many bio-factors such as BMP2, IGF-1 and subsequently antagonized their effects [25,26]. In our study we successfully overexpressed CTGF in MC3T3-E1 cells by lentivirus transinfection, and results of realtime PCR and Western blot demonstrated CTGF overexpression upregulated the osteogenic markers including OPN, Osterix, Runx2 and ALP, in accordance with increased mineralized nodules in the alizarin red assay. Overexpression of CTGF enhanced osteogenesis in vitro.
Cell migration is considered important in bone healing, in some in vitro studies the migration assay was equivalent to wound healing assay [27] or fracture healing assay [28], and the promotion of cell migration was proven to facilitate bone healing [29]. Previous studies also demonstrated that CTGF participated in regulating cell migration, the addition of CTGF promotes cell migration [30-32] whereas knockdown of CTGF suppressed cell migration [33]. We performed transwell assay to assess the cell migration ability. CTGF overexpression promoted the migration behavior of MC3T3-E1 cells, representing better “wound healing” capability in vitro.
Effect of CTGF on cell proliferation remains ambiguous. Some studies showed that CTGF stimulates the proliferation of some types of cells [4,34,35], whereas others showed the contrary results or insignificant effect [36,37]. The proliferation of MC3T3-E1 was not altered statistically significantly by overexpression of CTGF. The cell numbers of all groups were slightly reduced on Day 1, and then increased normally until Day 7. This slight reduction of cell number on Day 1 may be related to the influence of cell passage procedures and culture condition changes. However, further experiments are needed to clarify the mechanism.
CS/β-TCP composites have been widely used as scaffold material in bone tissue engineering, with good biocompatibility, low cell toxicity and favorable osteoinductivity [18,19]. CS/β-TCP spongy scaffold was characterized with good plasticity and high porosity and suitable for cell delivery [20,21]. In our study, after cell inoculation and 4 hours of incubation, fluorescent images of frozen section demonstrated that the seeding cells permeated into the CS/β-TCP scaffold, homogenously distributed, and adhered onto the wall of pores, thereby indicating that the cells integrated into the scaffold and formed cell-scaffold hybrid.
Besides scaffold, seeding cells and bio-factors were considered indispensable for bone regeneration [38]. By using genetic modified cells, researchers could combine the two elements together. The transinfected seeding cells may affect the osteogenic differentiation of endogenous osteoprogenitor cells orchestrating bone repair and regeneration. In our study, micro-CT showed higher BMD, BV/TV, Tb.N, Conn.D, and lower Tb.Sp in S-C group compared with other groups, suggesting that bone healing of S-C group was superior to other groups. However, there was no significant difference in Tb.Th, showing no observable change in bone microstructure. Therefore, micro-CT and histological results demonstrated that bone healing of LvCTGF cell-scaffold hybrid was better than LvNC cell-scaffold hybrid and scaffold alone at 2 and 5 postoperative weeks, thereby proving that CTGF overexpression cells facilitate cell accumulation and bone regeneration.
In conclusion, we demonstrated that the overexpression of CTGF enhanced osteogenesis in vitro. We also showed that, CTGF overexpression cells serve as efficient seeding cell source for bone regeneration.
Acknowledgements
This work was supported by Grants 81271107 and 81470718 from the National Natural Science Foundation of China.
References
- 1.Takigawa M, Nakanishi T, Kubota S, Nishida T. Role of CTGF/HCS24/ecogenin in skeletal growth control. J Cell Physiol. 2003;194:256–266. doi: 10.1002/jcp.10206. [DOI] [PubMed] [Google Scholar]
- 2.Kubota S, Takigawa M. Role of CCN2/CTGF/Hcs24 in bone growth. Int Rev Cytol. 2007;257:1–41. doi: 10.1016/S0074-7696(07)57001-4. [DOI] [PubMed] [Google Scholar]
- 3.Takigawa M. CCN2: a master regulator of the genesis of bone and cartilage. J Cell Commun Signal. 2013;7:191–201. doi: 10.1007/s12079-013-0204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishida T, Kubota S, Nakanishi T, Kuboki T, Yosimichi G, Kondo S, Takigawa M. CTGF/Hcs24, a hypertrophic chondrocyte-specific gene product, stimulates proliferation and differentiation, but not hypertrophy of cultured articular chondrocytes. J Cell Physiol. 2002;192:55–63. doi: 10.1002/jcp.10113. [DOI] [PubMed] [Google Scholar]
- 5.Kadota H, Nakanishi T, Asaumi K, Yamaai T, Nakata E, Mitani S, Aoki K, Aiga A, Inoue H, Takigawa M. Expression of connective tissue growth factor/hypertrophic chondrocyte-specific gene product 24 (CTGF/Hcs24/CCN2) during distraction osteogenesis. J Bone Miner Metab. 2004;22:293–302. doi: 10.1007/s00774-004-0486-2. [DOI] [PubMed] [Google Scholar]
- 6.Wang JJ, Ye F, Cheng LJ, Shi YJ, Bao J, Sun HQ, Wang W, Zhang P, Bu H. Osteogenic differentiation of mesenchymal stem cells promoted by overexpression of connective tissue growth factor. J Zhejiang Univ Sci B. 2009;10:355–367. doi: 10.1631/jzus.B0820252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo Q, Kang Q, Si W, Jiang W, Park JK, Peng Y, Li X, Luu HH, Luo J, Montag AG, Haydon RC, He TC. Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279:55958–55968. doi: 10.1074/jbc.M407810200. [DOI] [PubMed] [Google Scholar]
- 8.Nishida T, Nakanishi T, Asano M, Shimo T, Takigawa M. Effects of CTGF/Hcs24, a hypertrophic chondrocyte-specific gene product, on the proliferation and differentiation of osteoblastic cells in vitro. J Cell Physiol. 2000;184:197–206. doi: 10.1002/1097-4652(200008)184:2<197::AID-JCP7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 9.Safadi FF, Xu J, Smock SL, Kanaan RA, Selim AH, Odgren PR, Marks SC Jr, Owen TA, Popoff SN. Expression of connective tissue growth factor in bone: its role in osteoblast proliferation and differentiation in vitro and bone formation in vivo. J Cell Physiol. 2003;196:51–62. doi: 10.1002/jcp.10319. [DOI] [PubMed] [Google Scholar]
- 10.Huang J, Huang H, Wu M, Li J, Xie H, Zhou H, Liao E, Peng Y. Connective tissue growth factor induces osteogenic differentiation of vascular smooth muscle cells through ERK signaling. Int J Mol Med. 2013;32:423–429. doi: 10.3892/ijmm.2013.1398. [DOI] [PubMed] [Google Scholar]
- 11.Muromachi K, Kamio N, Matsumoto T, Matsushima K. Role of CTGF/CCN2 in reparative dentinogenesis in human dental pulp. J Oral Sci. 2012;54:47–54. doi: 10.2334/josnusd.54.47. [DOI] [PubMed] [Google Scholar]
- 12.Yuda A, Maeda H, Fujii S, Monnouchi S, Yamamoto N, Wada N, Koori K, Tomokiyo A, Hamano S, Hasegawa D, Akamine A. Effect of CTGF/CCN2 on osteo/cementoblastic and fibroblastic differentiation of a human periodontal ligament stem/progenitor cell line. J Cell Physiol. 2015;230:150–159. doi: 10.1002/jcp.24693. [DOI] [PubMed] [Google Scholar]
- 13.Shioi A. [Molecular mechanisms of vascular calcification] . Clin Calcium. 2010;20:1611–1619. [PubMed] [Google Scholar]
- 14.Sinha S, Eddington H, Kalra PA. Vascular calcification: lessons from scientific models. J Ren Care. 2009;35(Suppl 1):51–56. doi: 10.1111/j.1755-6686.2009.00065.x. [DOI] [PubMed] [Google Scholar]
- 15.Kikuchi T, Kubota S, Asaumi K, Kawaki H, Nishida T, Kawata K, Mitani S, Tabata Y, Ozaki T, Takigawa M. Promotion of bone regeneration by CCN2 incorporated into gelatin hydrogel. Tissue Eng Part A. 2008;14:1089–1098. doi: 10.1089/ten.tea.2007.0167. [DOI] [PubMed] [Google Scholar]
- 16.Ono M, Kubota S, Fujisawa T, Sonoyama W, Kawaki H, Akiyama K, Shimono K, Oshima M, Nishida T, Yoshida Y, Suzuki K, Takigawa M, Kuboki T. Promotion of hydroxyapatite-associated, stem cell-based bone regeneration by CCN2. Cell Transplant. 2008;17:231–240. doi: 10.3727/000000008783907143. [DOI] [PubMed] [Google Scholar]
- 17.Algul D, Sipahi H, Aydin A, Kelleci F, Ozdatli S, Yener FG. Biocompatibility of biomimetic multilayered alginate-chitosan/beta-TCP scaffold for osteochondral tissue. Int J Biol Macromol. 2015;79:363–369. doi: 10.1016/j.ijbiomac.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Yin Y, Ye F, Cui J, Zhang F, Li X, Yao K. Preparation and characterization of macroporous chitosan-gelatin/beta-tricalcium phosphate composite scaffolds for bone tissue engineering. J Biomed Mater Res A. 2003;67:844–855. doi: 10.1002/jbm.a.10153. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Zhang M. Synthesis and characterization of macroporous chitosan/calcium phosphate composite scaffolds for tissue engineering. J Biomed Mater Res. 2001;55:304–312. doi: 10.1002/1097-4636(20010605)55:3<304::aid-jbm1018>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 20.Cheng G, Chen X, Li Z, Lu H, Davide O. Comparison of three inoculation methods for bone tissue engineering. Int J Oral Maxillofac Implants. 2012;27:1340–1350. [PubMed] [Google Scholar]
- 21.Cheng G, Li Z, Wan Q, Lv K, Li D, Xing X. A novel animal model treated with tooth extraction to repair the full-thickness defects in the mandible of rabbits. J Surg Res. 2015;194:706–716. doi: 10.1016/j.jss.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Beloti MM, Tambasco De Oliveira P, Perri De Carvalho PS, Rosa AL. Seeding osteoblastic cells into a macroporous biodegradable CaP/PLGA scaffold by a centrifugal force. J Biomater Appl. 2009;23:481–495. doi: 10.1177/0885328208094082. [DOI] [PubMed] [Google Scholar]
- 23.Liu K, Li D, Huang X, Lv K, Ongodia D, Zhu L, Zhou L, Li Z. A murine femoral segmental defect model for bone tissue engineering using a novel rigid internal fixation system. J Surg Res. 2013;183:493–502. doi: 10.1016/j.jss.2013.02.041. [DOI] [PubMed] [Google Scholar]
- 24.Mercurio S, Latinkic B, Itasaki N, Krumlauf R, Smith JC. Connective-tissue growth factor modulates WNT signalling and interacts with the WNT receptor complex. Development. 2004;131:2137–2147. doi: 10.1242/dev.01045. [DOI] [PubMed] [Google Scholar]
- 25.Smerdel-Ramoya A, Zanotti S, Stadmeyer L, Durant D, Canalis E. Skeletal overexpression of connective tissue growth factor impairs bone formation and causes osteopenia. Endocrinology. 2008;149:4374–4381. doi: 10.1210/en.2008-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mundy C, Gannon M, Popoff SN. Connective tissue growth factor (CTGF/CCN2) negatively regulates BMP-2 induced osteoblast differentiation and signaling. J Cell Physiol. 2014;229:672–681. doi: 10.1002/jcp.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latifi-Pupovci H, Kuci Z, Wehner S, Bonig H, Lieberz R, Klingebiel T, Bader P, Kuci S. In vitro migration and proliferation (“wound healing”) potential of mesenchymal stromal cells generated from human CD271(+) bone marrow mononuclear cells. J Transl Med. 2015;13:315. doi: 10.1186/s12967-015-0676-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Q, Dong H, Li Y, Zhu Y, Zeng L, Gao H, Yuan B, Chen X, Mao C. Microgrooved Polymer Substrates Promote Collective Cell Migration To Accelerate Fracture Healing in an in Vitro Model. ACS Appl Mater Interfaces. 2015;7:23336–23345. doi: 10.1021/acsami.5b07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang W, Kim R, Park SI, Jung YJ, Ham O, Lee J, Kim JH, Oh S, Lee MY, Kim J, Park MS, Chung YA, Hwang KC, Maeng LS. Enhanced Healing of Rat Calvarial Bone Defects with Hypoxic Conditioned Medium from Mesenchymal Stem Cells through Increased Endogenous Stem Cell Migration via Regulation of ICAM-1 Targeted-microRNA-221. Mol Cells. 2015;38:643–650. doi: 10.14348/molcells.2015.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan WH, Pech M, Karnovsky MJ. Connective tissue growth factor (CTGF) stimulates vascular smooth muscle cell growth and migration in vitro. Eur J Cell Biol. 2000;79:915–923. doi: 10.1078/0171-9335-00122. [DOI] [PubMed] [Google Scholar]
- 31.Crean JK, Finlay D, Murphy M, Moss C, Godson C, Martin F, Brady HR. The role of p42/44 MAPK and protein kinase B in connective tissue growth factor induced extracellular matrix protein production, cell migration, and actin cytoskeletal rearrangement in human mesangial cells. J Biol Chem. 2002;277:44187–44194. doi: 10.1074/jbc.M203715200. [DOI] [PubMed] [Google Scholar]
- 32.Tan TW, Lai CH, Huang CY, Yang WH, Chen HT, Hsu HC, Fong YC, Tang CH. CTGF enhances migration and MMP-13 up-regulation via alphavbeta3 integrin, FAK, ERK, and NF-kappaBdependent pathway in human chondrosarcoma cells. J Cell Biochem. 2009;107:345–356. doi: 10.1002/jcb.22132. [DOI] [PubMed] [Google Scholar]
- 33.Huang Y, Zhao S, Zhang C, Li X. Downregulation of connective tissue growth factor reduces migration and invasiveness of osteosarcoma cells. Mol Med Rep. 2016;13:1888–94. doi: 10.3892/mmr.2015.4701. [DOI] [PubMed] [Google Scholar]
- 34.Shimo T, Nakanishi T, Nishida T, Asano M, Kanyama M, Kuboki T, Tamatani T, Tezuka K, Takemura M, Matsumura T, Takigawa M. Connective tissue growth factor induces the proliferation, migration, and tube formation of vascular endothelial cells in vitro, and angiogenesis in vivo. J Biochem. 1999;126:137–145. doi: 10.1093/oxfordjournals.jbchem.a022414. [DOI] [PubMed] [Google Scholar]
- 35.Shimo T, Nakanishi T, Kimura Y, Nishida T, Ishizeki K, Matsumura T, Takigawa M. Inhibition of endogenous expression of connective tissue growth factor by its antisense oligonucleotide and antisense RNA suppresses proliferation and migration of vascular endothelial cells. J Biochem. 1998;124:130–140. doi: 10.1093/oxfordjournals.jbchem.a022071. [DOI] [PubMed] [Google Scholar]
- 36.Chang HM, Pan HH, Cheng JC, Zhu YM, Leung PC. Growth differentiation factor 8 suppresses cell proliferation by up-regulating CTGF expression in human granulosa cells. Mol Cell Endocrinol. 2016;422:9–17. doi: 10.1016/j.mce.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Zhen Y, Ye Y, Yu X, Mai C, Zhou Y, Chen Y, Yang H, Lyu X, Song Y, Wu Q, Fu Q, Zhao M, Hua S, Wang H, Liu Z, Zhang Y, Fang W. Reduced CTGF expression promotes cell growth, migration, and invasion in nasopharyngeal carcinoma. PLoS One. 2014;8:e64976. doi: 10.1371/journal.pone.0064976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: current concepts and future directions. BMC Med. 2011;9:66. doi: 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]


