Abstract
Interleukin-1β (IL-1β) plays an important role in brain injury after focal ischemia, and bone marrow-derived mesenchymal stem cells (BMSCs) are capable of reducing the expression of IL-1β, we investigated the effects of BMSCs transplantation on brain edema and cerebral infarction as well as the underlying mechanisms via IL-1β. Male Sprague-Dawley rats were randomly divided into five groups: Normal + phosphate-buffered saline (PBS), middle cerebral artery occlusion (MCAO) + PBS, Normal + BMSCs, MCAO + BMSCs and MCAO + IL-1ra (an antagonist of IL-1β). BMSCs were transplanted 24 hours after MCAO, and brain edema was evaluated by Magnetic Resonance Imaging (MRI) and brain water content method after BMSCs transplantation. The expression of NeuN and AQP4 was analyzed by immunofluorescence staining. Protein level of AQP4 and IL-1β was detected by western blot analysis 48 hours after transplantation. The results showed that BMSCs transplantation reduced brain edema by measurement of brain water content and ADC Value of MRI, as well as the expression of AQP4 and IL-1β. It was also found that BMSCs transplantation could alleviate the cerebral infarction volume and neuronal damage. Both the brain edema and the cerebral infarction were associated with IL-1β expression. In conclusion, BMSCs transplantation was capable of alleviating brain edema as well as reducing cerebral infarction via down-regulation of IL-1β expression, thus repair the injured brain in focal cerebral ischemic rats.
Keywords: Bone marrow-derived mesenchymal stem cells, brain edema, cerebral infarction, interleukin-1β, cerebral ischemia, rats
Introduction
Stroke is the second most common cause of mortality and the leading cause of adult neurological disability worldwide [1,2]. In China, stroke has been the leading cause of death since 2010 [3]. Thus, it is of great importance to find effective therapies to reduce stroke and prevent its subsequent death and disability. Bone marrow-derived mesenchymal stem cells (BMSCs) are multipotent adult stem cells with the capacity to differentiate into neural cells. Because BMSCs are easily obtainable from bone marrow and rapidly expandable for transplantation, they are regarded as potential treatment agents after stroke [4]. But the mechanisms underlying the BMSCs therapy is still unclear.
There is growing evidence that inflammation plays an important role in the pathophysiology of a wide range of transient focal cerebral ischemia [5], which included not only the initial brain edema but also the subsequent permanent brain injury. Among all the inflammatory cytokines, Interleukin-1β (IL-1β) is a key mediator of inflammation in cerebral ischemia [6,7], while it had been demonstrated that the reduction of the expression of IL-1β could offer the neuroprotection against focal cerebral ischemia in rats [8,9]. Thus, it is very important to inhibit the IL-1β at the initial period to prevent brain injury. Recent studies have showed that BMSCs expressed various neurotrophic factors [10] that could inhibit the expression of IL-1β [11,12], which is known to play a role in the formation of brain edema after ischemia, but the underlying neuroprotective mechanisms were still unclear. Water channel protein 4 (Aquaporin-4, AQP4) is widely distributed in the brain and plays important roles in regulating water [13]. Some studies have shown that IL-1β stimulates the expression of AQP4 via the nuclear factor-κB pathway [14]. So we hypothesized that BMSCs transplantation might inhibit the immune inflammatory reaction via IL-1β, thus decreasing the expression of AQP4 and relieving brain edema and neuron damage.
Therefore, in this study, we would explore the effects of BMSCs transplantation on IL-1β, AQP4 expression, and brain edema followed by blocking or increasing the expression of IL-1β, and then observe the changes in AQP4 expression, edema and cerebral infarction volume by tissue staining, western blots analysis, brain water content measurement and Magnetic resonance imaging (MRI) techniques, especially diffusion-weighted images (DWI) after BMSCs transplantation in rats subjected to transient focal cerebral ischemia.
Materials and methods
Experimental animals and study design
Adult male Sprague-Dawley rats (weight 260 ~ 280 g from the Shandong Traditional Medicine University) were randomly divided into five groups: (1) normal control + PBS group [N + PBS, normal rats receiving PBS]; (2) Middle cerebral artery occlusion (MCAO) + PBS group (MCAO rats receiving PBS); (3) normal control + BMSC group [N + BMSCs, normal rats receiving BMSCs]; (4) MCAO + BMSC group (MCAO rats receiving BMSCs); and (5) MCAO + IL-1ra (MCAO rats receiving an equal volume of IL-1ra, an antagonist of IL-1β). The protocol was approved by the institutional animal care and use committee of Weifang Medical University.
Rat transient MCAO model and neurological scoring
A 4-0 monofilament nylon suture, with its tip rounded by heating, was advanced from the external carotid artery into the lumen until it blocked the origin of the middle cerebral artery. Two hours after MCAO, reperfusion was achieved by withdrawal of the suture. The body temperature of the animals was monitored and maintained at 37 ± 0.5°C during the entire surgical procedure.
The neurological function of MCAO rats was evaluated according to the ZeaLonga scoring method [15]. Neurological scores were assessed, and rats that scored 2~3 points were included in the experiment.
Isolation, culture, and characterization of BMSCs
BMSCs from Sprague-Dawley rats were purchased from Cyagen Biosciences and cultured in low-glucose Dulbecco’s modified Eagle’s Medium with 10% fetal bovine serum (FBS, Gibco, USA). The surface antigens expression of the third-passage BMSCs were examined by Cyagen Bioscience via flow cytometry. These cells were identified as CD90, CD29 and CD44 positive, CD34 and CD45 negative.
BMSCs transplantation
Twenty-four hours after MCAO procedure, rats in the MCAO + BMSCs and N + BMSCs groups were transferred to a stereotaxic apparatus, and 3×106 living cells/2 μL were transplanted via lateral ventricle with a 5-μL Hamilton microsyringe. The MCAO + IL-1ra group was injected with IL-1ra at the same time. The N + PBS and MCAO + PBS groups were injected with 2 μL PBS at the same time.
MRI T2-weighted imaging and DWI detection and MRI analysis
Animals were anesthetized and placed in the MRI apparatus, an Achieva 3.0 T horizontal-bore magnet (Philips, Best, The Netherlands) equipped with a dedicated solenoid rat coil at 6, 24, and 48 hours after transplantation. Two sequences were used: a T2-weighted imaging sequence (T2WI) and a DWI with the following parameters: repetition time (TR) 2000 ms, echo time (TE) 101 ms, Matrix 320×640, field of view (FOV) 48 × 48 mm, slice thickness 1.0 mm; for DWI, TR 1800 ms, TE 55 ms, Matrix 112×176, FOV = 50 × 50 mm. The following diffusion-sensitive coefficient was taken: b = 800 s/mm2 and b = 0 s/mm2. The ADC images were acquired and their mean relative ADC values were measured using Functool software.
Measurement of brain water content
After deep anesthesia, the injured hemispheres were removed and immediately weighed to obtain the wet weight. Then the samples were dried in an oven at 100°C for 24 h and reweighed to obtain the dry weight. The brain water content (BW) was then calculated with the following formula: BW = [(wet weight - dry weight)/wet weight] × 100%.
2,3,5 - triphenyltetrazolium chloride (TTC) staining
Rats were deeply anesthetized 48 hours after transplantation; brains were removed immediately, stored at -20°C for about 20 minutes, and then cut into 2-mm slices. The slices were incubated with a 1% TTC solution at 37°C for 30 minutes, and then fixed with 4% paraformaldehyde for 24 hours. The stained slices were photographed and the size of the infarct determined using cellscanning 16.0 software. Infarct volume was calculated as a volume percentage of the lesion compared with the contralateral hemisphere, 6 rats in each group. Infarct percentage = Infarct volume (contralateral hemisphere region - noninfarcted region in the ipsilateral hemisphere)/volume of the contralateral hemisphere × 100%.
Tissue preparation and neuron-specific nuclear protein (NeuN)/DAPI and AQP4/DAPI immunofluorescence staining
After deep anesthesia, the brains were removed 48 hours after transplantation, embedded in paraffin. 3-µm coronal sections were cut, and placed on polylysine-coated slides. Sections were deparaffinized, and antigen was retrieved by microwave and blocked in serum albumin for 1 hour at 37°C. Sections were subsequently incubated with the primary antibodies (NeuN 1:100 dilution, chemicon and AQP4 1:75 dilution, Santa Cruz Biotechnology) at 4°C overnight. After washing, slices were incubated with appropriate second antibodies for 1 hour at 37°C in the dark and then washed. The slices were rinsed and covered with fluorescence DAPI mounting medium (F6057, Sigma, USA). Staining was visualized and analyzed by fluorescence microscope and cellscanning software 1.6 (BX-51, Olympus, Japan).
Western blot analysis
Fresh brain tissue was obtained 48 hours after transplantation. Samples, ground into fine powder and homogenized in a tissue-lysis buffer, were electrophoresed on 12% polyacrylamide gels together with prestained low-molecular-weight markers (BioRad, USA) and transferred to nitrocellulose membrane filters with Tris-glycine-methanol buffer (pH 8.3). The membrane was blocked in 5% powdered milk for 1 h, then incubated with AQP4 (1:500 dilution, Santa Cruz Biotechnology), IL-1β (1:400 dilution, Santa Cruz Biotechnology) or GAPDH (1:2000, proteintech Group) at 4°C overnight. The membrane was washed with 10% Tween PBS and then incubated with the appropriate HRP-conjugated second antibodies at room temperature for 2 h. After thorough washing, the positive band was revealed using ECL detection reagents and autoradiography film. The relative concentration of AQP4 or IL-1β protein is expressed as the ratio of the optical density (OD) of AQP4 or IL-1β to that of GAPDH.
Statistical analysis
Data are presented as mean ± standard deviation (SD). Analysis of variance (ANOVA) was used to compare mean values among the groups. P < 0.05 was considered significant. Statistical differences between groups were examined by the Newman-Keuls method.
Results
Decreased infarct volume
The effects of BMSCs transplantation on infarct volume were assessed by TTC staining. There was no infarct area observed and all the brain slices were stained red in the N + PBS and N + BMSCs groups. The infarct volume was significantly reduced in the MCAO + BMSCs group as compared with the MCAO + PBS group (P < 0.01). The infarct volume was lowered in the MCAO + IL-1ra group as compared to the MCAO + PBS (P < 0.01) and the MCAO + BMSCs groups (P < 0.01) (Figure 1).
Figure 1.
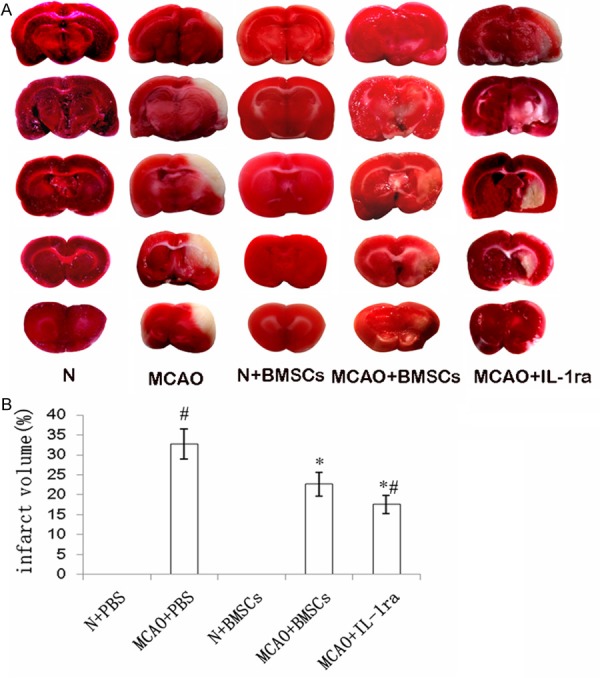
Effects of BMSCs transplantation on infarct volume. A. The infarct areas were visualized by TTC staining. Representative coronal sections indicate the following groups: N + PBS, MCAO + PBS, N + BMSCs, MCAO + BMSCs, and MCAO + IL-1ra groups. No infarction areas were observed and all the brain tissues were stained red in the N + PBS and N + BMSCs groups. The infarction areas were stained white and observed in the MCAO + PBS, N + BMSCs, MCAO + BMSCs, and MCAO + IL-1ra groups. B. The bar chart showed the percentage of infarct volume of different groups. Error bars show standard deviation, *P < 0.01 versus the MCAO + PBS group, #P < 0.01 versus the MCAO + BMSCs group.
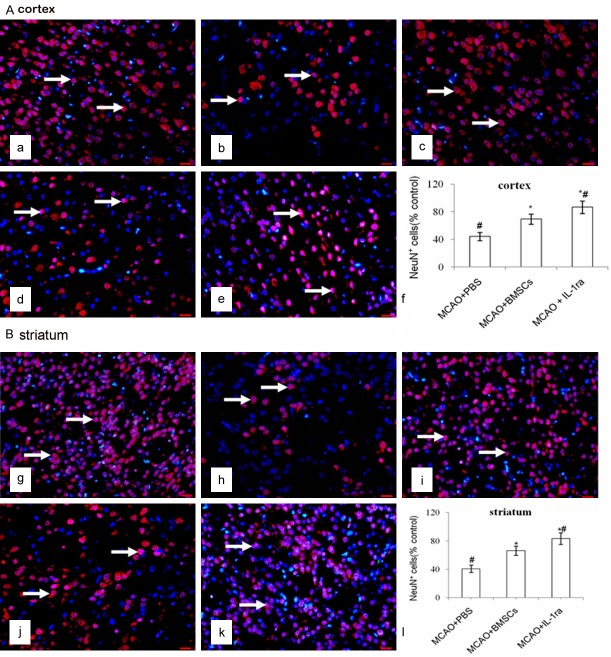
Qantification of NeuN+DAPI+ cells in the cortex and striatum
Fewer NeuN+DAPI+ cells were identified in the injured cortex and striatum in the MCAO + PBS group compared with the N + PBS group (P < 0.01). There were more NeuN+DAPI+ cells in the cortex and striatum in the MCAO + BMSCs (P < 0.01) and MCAO + IL-1ra (P < 0.01) groups than in the MCAO + PBS group. There was no difference in the NeuN+DAPI+ cells in both the cortex (P > 0.05) and striatum (P > 0.05) between the N + PBS and the N + BMSCs groups. Furthermore, there were more NeuN+DAPI+ cells in the MCAO + IL-1ra group compared with the MCAO + BMSCs group in both the cortex (P < 0.01) and striatum (P < 0.01) (Figure 2).
Figure 2.
Effects of BMSCs transplantation on neurons in the cortex and striatum. A. NeuN/DAPI immunofluorescence staining in the cortex. NeuN+DAPI+ cells were stained both red and blue in the nucleus (white arrows). a-e indicate the N + PBS, MCAO + PBS, N + BMSCs, MCAO + BMSCs, and MCAO + IL-1ra groups in the cortex. The scale bar is 20 μm and is labeled in each figure. f indicates the bar chart, which shows the percentage of NeuN+DAPI+ cells to the contralateral side in the cortex. Error bars show standard deviation, *P < 0.01 versus the MCAO + PBS group, #P < 0.01 versus the MCAO + BMSCs group. B. NeuN/DAPI immunofluorescence staining in the striatum. g-k indicate the N + PBS, MCAO + PBS, N + BMSCs, MCAO + BMSCs, and MCAO + IL-1ra groups respectively in the striatum. The scale bar is 20 μm and is labeled in each figure. l indicates the bar chart, which shows the percentage of NeuN+DAPI+ cells to the contralateral side in the striatum. Error bars show standard deviation, *P < 0.01 versus the MCAO + PBS group, #P < 0.01 versus the MCAO + BMSCs group.
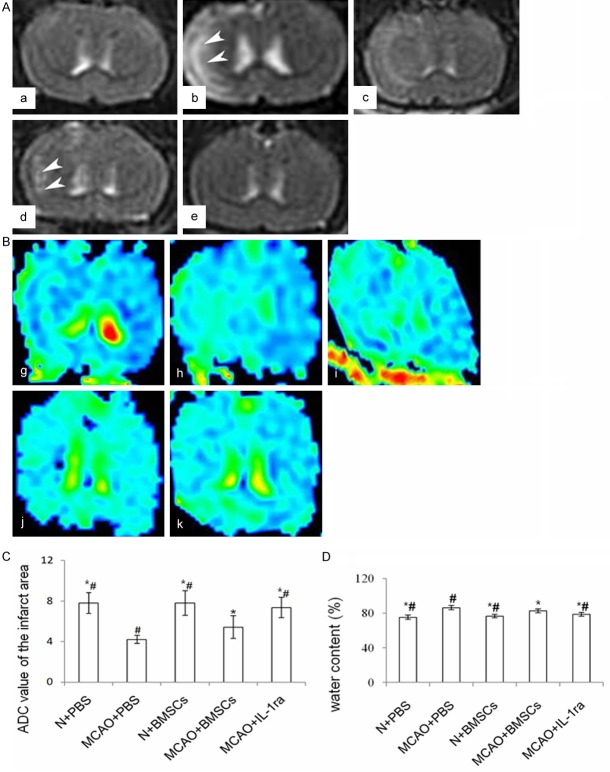
Magnetic resonance imaging
T2-weighted imaging
There were no abnormal signals in either cerebral hemisphere of the N + PBS or N + BMSCs groups. Six hours after transplantation, abnormal signals were observed in the MCAO + PBS and MCAO + BMSCs. Infarct regions were also identified in the MCAO + PBS, MCAO + BMSCs, and MCAO + IL-1ra groups both 24 and 48 hours after transplantation.
Diffusion-weighted imaging and ADC analysis
The ADC values of the ischemic area were lower in the MCAO + PBS group than in the MCAO + BMSCs (P < 0.05) or N + PBS groups (P < 0.01) 6 hours after transplantation. The ADC values 24 hours and 48 hours after transplantation were higher in the MCAO + BMSCs group than in the MCAO + PBS group (P < 0.01). There existed no difference in the ADC values between the N + PBS and the N + BMSCs groups 24 hours (P > 0.05) and 48 hours (P > 0.05) after transplantation. Forty-eight hours after transplantation, the ADC values were higher in the MCAO + IL-1ra group compared with the MCAO + BMSCs group (P < 0.05) (Figure 3).
Figure 3.
Effects of BMSC transplantation on infarct volume, edema and its relationship with IL-1β protein. A. Effects of BMSC transplantation on infarct volume on T2WI of MRI. a-e indicate the MRI-T2WI of the N + PBS, MCAO + PBS, N + BMSCs, MCAO + BMSCs, and MCAO + IL-1ra groups respectively. White arrows show the abnormal signals in the injured brains of all groups. B. Effects of BMSC transplantation on infarction volumes in DWI of MRI. g-k indicate ADC images of the N + PBS, MCAO + PBS, N + BMSCs, and MCAO + BMSCs, MCAO + IL-1ra groups respectively. C. Bar chart of ADC values of the infarct area in each group. D. Effects of BMSC transplantation on brain water content in each group. Error bars show standard deviation, * indicates P < 0.05 vs. the MCAO + PBS group, # indicates P < 0.05 vs. the MCAO + BMSCs group.
Effects of BMSC transplantation on water content and AQP4 protein
There was no difference in the water content between the N + PBS and N + BMSCs groups 24 hours after transplantation (P > 0.05). Water content was lower in the MCAO + BMSCs group than in the MCAO + PBS group (P < 0.01). The water content was also lower in the MCAO + IL-1ra group compared with the MCAO + BMSCs group (P < 0.05) (Figure 3).
More AQP4+DAPI+ cells were found in the MCAO + PBS group than in the N + PBS group (P < 0.01). There was no difference in the AQP4+DAPI+ cells between the N + PBS and the N + BMSCs groups 24 hours after transplantation (P > 0.05). There were fewer AQP4+DAPI+ cells in the MCAO + BMSCs group than in the MCAO + PBS group (P < 0.01). There were fewer AQP4+DAPI+ cells in the MCAO + IL-1ra group compared with the MCAO + BMSCs (P < 0.05) and MCAO + PBS groups (P < 0.01) (Figure 4).
Figure 4.
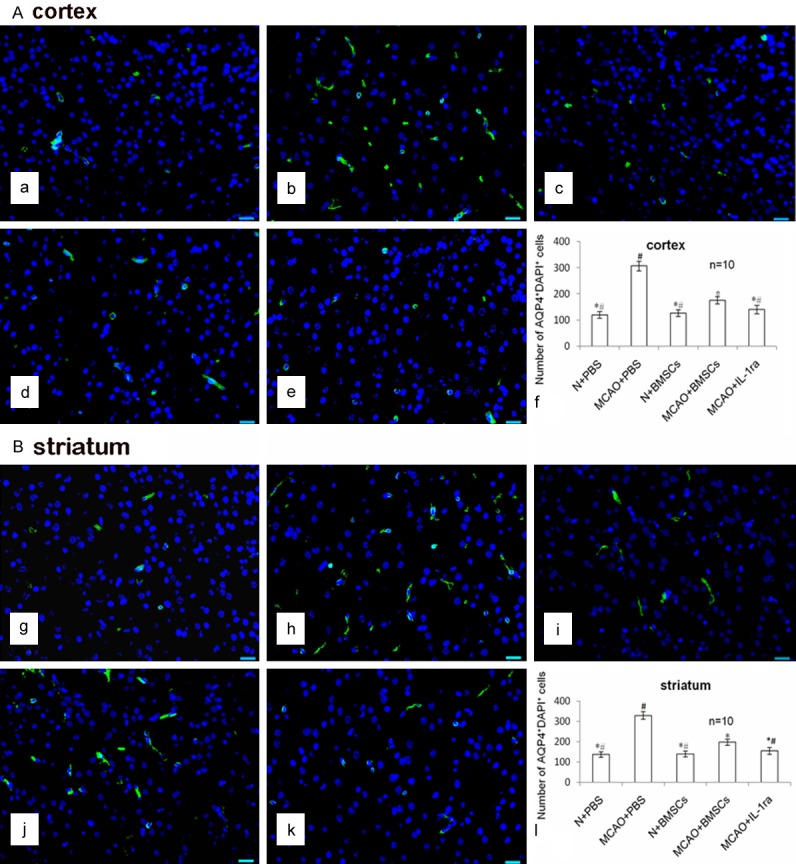
Effects of BMSC transplantation on AQP4 protein in the cortex and striatum. A. AQP4/DAPI immunofluorescence staining in the cortex. The AQP4+DAPI+ cells were stained green in the cytoplasm and blue in the nucleus (white arrows). The scale bar is 20 μm and is labeled in each figure. a-e indicate AQP4/DAPI immunofluorescence staining of the N + PBS, MCAO + PBS, N + BMSCs, MCAO + BMSCs, and MCAO + IL-1ra groups in the cortex. The scale bar is 20 μm and is labeled in each figure. f indicates the bar chart of the number of AQP4+DAPI+ cells in the cortex. Error bars show standard deviation, * indicates P < 0.05 vs. the MCAO + PBS group, # indicates P < 0.05 vs. the MCAO + BMSCs group. B. AQP4/DAPI immunofluorescence staining in the striatum. g-k indicate the AQP4/DAPI staining of the N + PBS, MCAO + PBS, N + BMSCs, MCAO + BMSCs, and MCAO + IL-1ra groups in the striatum. The scale bar is 20 μm and is labeled in each figure. l indicates the bar chart of the number of AQP4+DAPI+ cells in the striatum. Error bars show standard deviation, * indicates P < 0.05 vs. the MCAO + PBS group, # indicates P < 0.05 vs. the MCAO + BMSCs group.
Both IL-1β and AQP4 protein expression were higher in the MCAO + PBS group than in the N + PBS group (P < 0.01). The MCAO + BMSCs group showed lower IL-1β (P < 0.01) and AQP4 (P < 0.05) protein expression than that in the MCAO + PBS group. There was no difference in both IL-1β and AQP4 protein expression between the N + PBS and the N + BMSCs groups (P > 0.05). AQP4 protein expression was lower in the MCAO + IL-1ra group than in the MCAO + BMSCs group (P < 0.05) (Figure 5).
Figure 5.
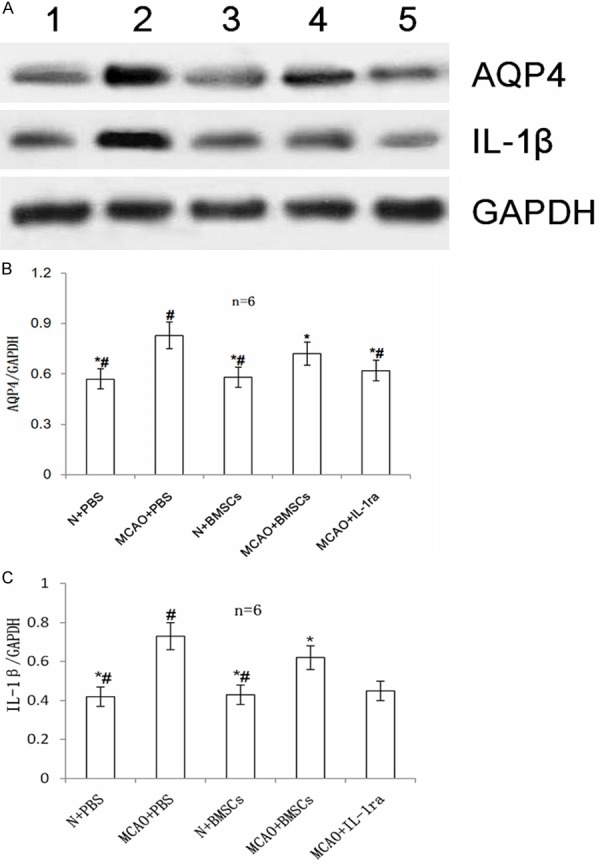
Effects of BMSC transplantation on AQP4 and IL-1β proteins in the injured cerebral hemisphere. A. Western blot analysis of the expression of AQP4 and IL-1β proteins in all groups. Lanes 1-5 denote the N + PBS, MCAO + PBS, N + BMSCs, MCAO + BMSCs, and MCAO + IL-1ra groups, respectively. B. The bar chart of AQP4/GAPDH from each group. C. The bar chart of IL-1β/GAPDH from each group. Error bars show standard deviation. * indicates P < 0.05 vs. the MCAO + PBS group, # indicates P < 0.05 vs. the MCAO + BMSCs group.
Discussion
In the present study, we investigated the effects of BMSCs transplantation on the transient focal brain injury in a MCAO rat model. Our results showed that an enlargement of infarct volume consistent with the increasing abnormal signal in the MR-T2-Weighted imaging and reduction of NeuN-positive cells in the cortex and striatum of the cerebral hemisphere in MCAO rat models, which indicated that we have set up the MCAO model successfully. And less infarct volume and abnormal signal were observed, so were less NeuN positive cells in MCAO rats after BMSCs transplantation, which indicated that BMSCs transplantation was neuroprotective and could alleviate cerebral infarction, thus repair the injured brain.
Brain ischemia could result in not only cerebral infarction, but also the brain edema, especially at the early period. In this study, the changes of brain edema were observed by MRI - DWI, brain water content and AQP4 staining. Results showed that lower ADC value, higher brain water content and more AQP4 positive cells were observed, which proved that focal brain ischemia could result in brain edema, and it was in accordance with previous findings [18]. After BMSCs transplantation, higher ADC value, lower brain water content and less AQP4 positive cells were observed, which all indicated that BMSCs could alleviate brain edema. All the results above indicated that BMSCs transplantation could reduce brain injury in focal cerebral ischemic rats. However, the mechanism underlying the BMSCs treatment is still uncertain. It is of great importance to clarify the underlying mechanism.
As the inflammatory response contributes to the pathogenesis of ischemic brain damage, anti-inflammatory treatment becomes one possible key treatment for cerebral ischemia [16]. IL-1β, an important inflammatory factor, is closely relevant to brain edema and damage [17], but it was unknown whether the neuroprotective effects of BMSCs transplantation were correlated to IL-1β. In this study, the expression of IL-1β was elevated in MCAO rats compared with normal control rats, which was in accordance with previous findings [18]. After BMSCs transplantation, expression of IL-1β were reduced in the MCAO + BMSCs group than in the MCAO + PBS group, in accordance with the alleviation of brain edema and cerebral infarction volume, which indicated that the neuroprotective effects of BMSCs might be associated with the down-regulation of IL-1β in focal cerebral ischemic rats. To prove our hypothesis, the antagonist of IL-1β, IL-1ra protein, was injected, both brain edema and cerebral infarction was greatly alleviated. This implied that the brain injury after transient focal cerebral ischemia was associated with the increasing of IL-1β in the injured brain hemisphere, and the blockade of IL-1β or decline of IL-1β had the neuroprotective effect on focal cerebral ischemia. And as the BMSCs transplantation has the same effects as the IL-1ra, we conferred that neuroprotective effect of BMSCs transplantation was correlated with down-regulation of IL-1β.
In conclusion, the data presented here indicate that BMSCs transplantation was neuroprotective, alleviated the expression of AQP4 protein and brain edema, and reduced brain damage by down-regulation of IL-1β, thus repaired the injured brain in MCAO rats.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81000268), the Natural Science Foundation of Shandong Province (ZR2014JL049), the Natural Science Foundation of Shandong Province (ZR2013HL067), the Bill & Melinda Gates Foundation (BMGF: 01075000191), the Muscular Dystrophy Association, the ALS Therapy Alliance, and the Shandong Province Taishan scholar project.
Disclosure of conflict of interest
None.
Abbreviations
- ADC
apparent diffusion coefficient
- AQP4
aquaporin 4
- BMSCs
Bone marrow-derived mesenchymal stem cells
- DWI
diffusion-weighted images
- IL-1β
Interleukin-1β
- MCAO
Middle cerebral artery occlusion
- MRI
Magnetic resonance Imaging
- PBS
phosphate-buffered saline
- T2WI
T2-weighted images
- TTC
2,3,5 - triphenyltetrazolium chloride
References
- 1.Meairs S, Wahlgren N, Dirnagl U, Lindvall O, Rothwell P, Baron JC, Hossmann K, Engelhardt B, Ferro J, McCulloch J, Kaste M, Endres M, Koistinaho J, Planas A, Vivien D, Dijkhuizen R, Czlonkowska A, Hagen A, Evans A, De Libero G, Nagy Z, Rastenyte D, Reess J, Davalos A, Lenzi GL, Amarenco P, Hennerici M. Stroke research priorities for the next decade-A representative view of the European scientific community. Cerebrovasc Dis. 2006;22:75–82. doi: 10.1159/000093098. [DOI] [PubMed] [Google Scholar]
- 2.Zhang F, Li N, Jiang L, Chen L, Huang M. Neuroprotective Effects of (-)-Epigallocatechin-3-Gallate Against Focal Cerebral Ischemia/Reperfusion Injury in Rats Through Attenuation of Inflammation. Neurochem Res. 2015;40:1691–1698. doi: 10.1007/s11064-015-1647-5. [DOI] [PubMed] [Google Scholar]
- 3.Yang G, Wang Y, Zeng Y, Gao GF, Liang X, Zhou M, Wan X, Yu S, Jiang Y, Naghavi M, Vos T, Wang H, Lopez AD, Murray CJ. Rapid health transition in China, 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2013;381:1987–2015. doi: 10.1016/S0140-6736(13)61097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin G, Qiu G, Wu D, Hu Y, Qiao P, Fan C, Gao F. Allogeneic bone marrow-derived mesenchymal stem cells attenuate hepatic ischemiareperfusion injury by suppressing oxidative stress and inhibiting apoptosis in rats. Int J Mol Med. 2013;31:1395–1401. doi: 10.3892/ijmm.2013.1340. [DOI] [PubMed] [Google Scholar]
- 5.Sun BZ, Chen L, Wu Q, Wang HL, Wei XB, Xiang YX, Zhang XM. Suppression of inflammatory response by flurbiprofen following focal cerebral ischemia involves the NF-kappa B signaling pathway. Int J Clin Exp Med. 2014;7:3087–3095. [PMC free article] [PubMed] [Google Scholar]
- 6.Denes A, Wilkinson F, Bigger B, Chu M, Rothwell NJ, Allan SM. Central and haematopoietic interleukin-1 both contribute to ischaemic brain injury in mice. Dis Model Mech. 2013;6:1043–1048. doi: 10.1242/dmm.011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McColl BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci. 2008;28:9451–9462. doi: 10.1523/JNEUROSCI.2674-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Zhang X, Wang Y, Lei H, Su H, Zeng J, Pei Z, Huang R. Inhibition of immunoproteasome reduces infarction volume and attenuates inflammatory reaction in a rat model of ischemic stroke. Cell Death Dis. 2015;6:e1626. doi: 10.1038/cddis.2014.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Yin J, Li L, Deng J, Feng C, Zuo Z. Isoflurane postconditioning reduces ischemiainduced nuclear factor-kappa B activation and interleukin 1beta production to provide neuroprotection in rats and mice. Neurobiol Dis. 2013;54:216–224. doi: 10.1016/j.nbd.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, Nagai A, Sheikh AM, Liang XY, Yano S, Mitaki S, Ishibashi Y, Kobayashi S, Kim SU, Yamaguchi S. Human mesenchymal stem cell transplantation changes proinflammatory gene expression through a nuclear factor-kappaB-dependent pathway in a rat focal cerebral ischemic model. J Neurosci Res. 2013;91:1440–1449. doi: 10.1002/jnr.23267. [DOI] [PubMed] [Google Scholar]
- 11.Mitkari B, Nitzsche F, Kerkela E, Kuptsova K, Huttunen J, Nystedt J, Korhonen M, Jolkkonen J. Human bone marrow mesenchymal stem/stromal cells produce efficient localization in the brain and enhanced angiogenesis after intra-arterial delivery in rats with cerebral ischemia, but this is not translated to behavioral recovery. Behav Brain Res. 2014;259:50–59. doi: 10.1016/j.bbr.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 12.Park HJ, Shin JY, Kim HN, Oh SH, Song SK, Lee PH. Mesenchymal stem cells stabilize the blood-brain barrier through regulation of astrocytes. Stem Cell Res Ther. 2015;6:187. doi: 10.1186/s13287-015-0180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho IA, Toh HC, Ng WH, Teo YL, Guo CM, Hui KM, Lam PY. Human bone marrow-derived mesenchymal stem cells suppress human glioma growth through inhibition of angiogenesis. Stem Cells. 2013;31:146–155. doi: 10.1002/stem.1247. [DOI] [PubMed] [Google Scholar]
- 14.Scholz CC, Cavadas MA, Tambuwala MM, Hams E, Rodriguez J, von Kriegsheim A, Cotter P, Bruning U, Fallon PG, Cheong A, Cummins EP, Taylor CT. Regulation of IL-1betainduced NF-kappaB by hydroxylases links key hypoxic and inflammatory signaling pathways. Proc Natl Acad Sci U S A. 2013;110:18490–18495. doi: 10.1073/pnas.1309718110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 16.Pandya RS, Mao L, Zhou H, Zhou S, Zeng J, Popp AJ, Wang X. Central nervous system agents for ischemic stroke: neuroprotection mechanisms. Cent Nerv Syst Agents Med Chem. 2011;11:81–97. doi: 10.2174/187152411796011321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Figueroa BE, Stavrovskaya IG, Zhang Y, Sirianni AC, Zhu S, Day AL, Kristal BS, Friedlander RM. Methazolamide and melatonin inhibit mitochondrial cytochrome C release and are neuroprotective in experimental models of ischemic injury. Stroke. 2009;40:1877–1885. doi: 10.1161/STROKEAHA.108.540765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiappetta O, Gliozzi M, Siviglia E, Amantea D, Morrone LA, Berliocchi L, Bagetta G, Corasaniti MT. Evidence to implicate early modulation of interleukin-1beta expression in the neuroprotection afforded by 17beta-estradiol in male rats undergone transient middle cerebral artery occlusion. Int Rev Neurobiol. 2007;82:357–372. doi: 10.1016/S0074-7742(07)82019-8. [DOI] [PubMed] [Google Scholar]