Abstract
Background: Tacrolimus inhibits hepatitis B virus entry into hepatocytes through targeting the HBV receptor, sodium taurocholate cotransporting polypeptide. This study was performed to evaluate the efficacy and safety of Tacrolimus combined with entecavir antiviral therapy for HBV-associated glomerulonephritis patients with biopsy-proven membranous nephropathy. Method: A cohort of 42 patients was enrolled in this retrospective study. Twenty-three patients received Tacrolimus (0.05 mg/kg/day) in combination entecavir over 24 weeks, whereas the other 19 patients only received entecavir monotherapy. Results: The probability of proteinuria remission in the Tacrolimus+entecavir group was 69 and 87% after 12 and 24 weeks, whereas was only 26 and 42%, respectively, in the entecavir group. The mean time to partial or complete remission was 18.6 weeks in the Tacrolimus+entecavir group and 34.3 weeks in the entecavir group (P<0.001). A decrease in the HBV DNA titer was observed in all patients with active HBV replication. None of the HBV carriers in the Tacrolimus+entecavir group showed evidence of HBV reactivation. The serum creatinine and alanine aminotransferase levels remained stable in both groups. The Tacrolimus target trough concentration was 5-10 ng/mL. Conclusion: Tacrolimus combined with entecavir rapidly and effectively induced remission of HBV-GN in Chinese adults. Furthermore, Tacrolimus may have a synergistic antiviral effect with entecavir.
Keywords: Glomerulonephritis, tacrolimus, membranous nephropathy, hepatitis B virus
Introduction
Hepatitis B virus (HBV)-associated glomerulonephritis (HBV-GN) was first described by Combes et al. in 1971 [1]. Various pathological types of HBV-GN, including membranous nephropathy (MN), mesangiocapillary glomerulonephritis (MPGN), and mesangial proliferative glomerulo-nephritis (MSPGN), have been reported [2-4]. To date, chronic HBV infection remains an important cause of MN in endemic areas. Moreover, the natural history of HBV-GN is not well defined. Spontaneous remission of HBV-associated membranous nephropathy (HBV-MN) is common in children, but rare in adults [4-7]. HBV-MN progresses slowly; however, approximately 30% of adult patients progress to renal failure, and 10% of these patients will require renal replacement therapy [8,9]. The poor prognosis of adults with HBV-GN has led to the development of various therapeutic approaches, including corticosteroids, lamivudine and interferon-alpha (IFN) [4,10-12].
In clinical practice, the Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommend that patients with HBV-GN receive antiviral treatment with IFN or nucleoside analogues. Some previous studies have shown that antiviral treatment can effectively attenuate proteinuria and lamivudine therapy is significantly better than IFN therapy [12,13]. However, a lamivudine-resistant HBV mutation could decrease the efficacy and increase recurrence [11]. In addition, the duration of lamivudine therapy for HBV-GN remains an unresolved issue, especially in Asian patients, as emerging data have demonstrated that disease progression can continue after anti-HBe seroconversion in this population [14]. These limits of antiviral-only treatment have stimulated the search for other specific and effective treatments for HBV-GN.
The pathogenesis of HBV-GN involves the deposition of circulating immune complexes (CICs) or in situ immune complexes formed by the HBV antigen and antibody in renal tissue [15], providing the rationale for treatment with immunosuppressive agents. Accordingly, some researchers have attempted to treat HBV-GN patients with a regimen of antiviral agents combined with corticosteroids or cytotoxic agents. This treatment approach do reduce proteinuria, but it is harmful through enhancing viral replication and precipitating in hepatitic flares [16].
There is currently no consensus on the optimal choice of treatment for HBV-GN. Thus, there is an urgent need to identify and validate novel treatment modules that not only attenuate proteinuria but also inhibit HBV replication. It has been shown that tacrolimus (TAC) inhibits HBV entry into hepatocytes through targeting the candidate HBV receptor, sodium taurocholate cotransporting polypeptide (NTCP) [17,18]. Furthermore, many studies have demonstrated that TAC monotherapy or in combination with low-dose steroids, are effective against idiopathic MN and minimal change nephrotic syndrome [19-21]. However, the effect of TAC in conjunction with antiviral agents for HBV-GN in adults remains to be determined.
In the present study, we have assessed the efficacy and safety of TAC in combination with entecavir for HBV-GN patients in adults.
Patients and methods
Patients
A cohort of 42 patients who were hospitalized at Guangdong General Hospital from 2012 to 2014 was enrolled in this study. The study protocol was reviewed and approved by Guangdong General Hospital’s Ethic Committee, and all participants provided written informed consents. The entry criteria were the following: 1) all HBV-GN cases with biopsy-proven MN; 2) age between 18 and 70 years; 3) evidence of chronic HBV infection based on the presence of HBsAg, HBeAg or HBV DNA in the serum; 4) an estimated glomerular filtration rate (eGFR) >50 ml/min/1.73 m2 according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula; and 5) no immunosuppressive treatment within the previous 2 weeks. The exclusion criteria included a diagnosis of idiopathic MN, systemic lupus erythematosus, malignancy, diabetes mellitus, severe infections or any other systemic disease known to be associated with secondary MN.
From 2012 to 2014, twenty-three HBV-MN patients with nephrotic syndrome were given TAC (0.05 mg/kg/day) combined with entecavir. TAC was divided into two daily doses at 12-hour intervals. Subsequent doses were adjusted to achieve a whole blood 12-hour trough level between 5 and 10 ng/ml. The participants’ baseline demographic and clinical data were compared with 19 historic control subjects who had biopsy-proven HBV-MN and received entecavir-only therapy for proteinuria less than 4.0 g/d. All of the patients were instructed to maintain the same doses of angiotensin converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB) as they were taking before enrollment. The patients were treated with or without additional antihypertensive agents to achieve a blood pressure target of below 130/85 mmHg.
Clinical data were collected from all patients, including serum creatinine (SCr), serum HBV DNA, alanine aminotransferase (ALT), electrolyte, glucose, total protein, albumin, total cholesterol, triglycerides, 24-h excretion of urine protein, and trough levels of TAC.
Methods
Serum DNA levels were determined using polymerase chain reaction (PCR). Other serum HBV markers, including HBsAg, HBeAg, HBsAb, HBeAb, and HBcAb, were detected using enzyme-linked immunosorbent assays (ELISA).
The current diagnostic criteria for HBV-GN are as follows: 1) the presence of a serum HBV antigen (inactive carriers were included); 2) the diagnosis of glomerulonephritis with the exclusion of other types of secondary nephritis; and 3) the presence of renal HBV antigen, which is required for the diagnosis of HBV-GN.
Histologic diagnosis of HBV-associated MN was based on studies with light, immunofluorescence and electron microscopy. Renal tissue samples were routinely processed and stained with hematoxylin-eosin, periodic acid-Schiff, Masson’s trichrome, Jones’s silver, and Congo red stains. For direct immunofluorescence, frozen sections of the fresh tissue samples were stained with antisera against human C3, C4, C1q, IgA, IgM, IgG, and fibrinogen. HBV-specific antigens were observed by indirect immunofluorescence using a rabbit polyclonal antibody against HBcAg and monoclonal antibodies against HBeAg and HBsAg, according to the instructions as previously described [22]. Two renal pathologists, who did not have knowledge of the patients’ clinical status, performed histologic grading of MN.
Outcome variables and definitions
The primary outcome variables were the number of patients who reached complete or partial remission (CR or PR). CR was defined as <0.5 g/24 h proteinuria or lower plus stable renal function (eGFR >50 ml/min/1.73 m2) and PR as proteinuria <3.5 g/24 h and 50% lower than baseline proteinuria plus stable renal function. Secondary outcome variables included the SCr levels, serum HBV DNA, ALT and TAC dosing and serum trough levels.
Statistical analysis
Continuous data are expressed as the mean ± sd. The quantitative data were analyzed using a t-test or the Wilcoxon rank sum test for two independent group comparison. A paired t-test or the Wilcoxon singed rank was used for two-paired group. Repeated measures analysis of variance was used for comparison of Scr in each time point. The categorical variables were analyzed using a chi-squared test and Fisher exact test. The cumulative percentage of patients who went into proteinuria remission was calculated with the Kaplan-Meier method, and comparisons between the two groups were made with the log-rank test. P<0.05 was considered statistically significant. All of the statistical analyses were performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA).
Results
TAC combined with entecavir significantly reduces proteinuria and shortens the mean time of partial or complete remission
A total of 42 patients with biopsy-proven HBV-MN were enrolled in this study. Their baseline demographic, clinical, histological and laboratory characteristics are listed in Table 1. Most patients in both groups were less than 40 years old. The 24-h excretion of urine protein was significantly higher in the TAC combined with entecavir group than in the entecavir-only group at baseline. There were no significant differences in both active HBV replication and HBV carriers between the two groups.
Table 1.
Demographic, clinical, histological and laboratory characteristics of patients at baseline
| TAC+entecavir | entecavir | P value | |
|---|---|---|---|
| Age | 40.8±17.0 | 38.5±13.9 | 0.89 |
| Distribution of ages by tertiles (<40/40-50/>50 years) | 13/1/9 | 12/3/4 | 0.76 |
| Gender (Male/Female) | 8/15 | 12/7 | 0.19 |
| Scr (µmol/l) | 78.8±26.1 | 65.9±18.3 | 0.08 |
| eGFR (ml/min/1.73 m2) | 101.4±29.2 | 105.5±16.8 | 0.63 |
| Distribution of eGFR (>90/60-89/50-59 ml/min/1.73 m2) | 16/4/3 | 16/3/0 | 0.13 |
| Proteinuria (g/24 h) | 5.2±3.1 | 2.3±2.4 | <0.001 |
| Serum albumin (g/l) | 16.4±3.8 | 27.2±6.9 | <0.001 |
| Percentage of glomerular sclerosis | 7.1±8.5 | 4.3±5.5 | 0.33 |
| Percentage of renal interstitial fibrosis | 11.3±10.8 | 12.7±15.9 | 0.79 |
| Serum HBV DNA (log) | 1.6±1.9 | 3.2±2.8 | 0.06 |
| Active HBV replication (n) | 6 | 12 | 0.19 |
| HBV carriers (n) | 17 | 7 | 0.09 |
| Renal tissue HBsAg(+) (n) | 21 | 17 | 0.48 |
| Renal tissue HBcAg(+) (n) | 2 | 2 | 0.73 |
| 2 times the upper limit of normal | 2 | 2 | 0.84 |
| Hypocomplementemia (n) | 8 | 6 | 0.83 |
| SBP (mmHg) | 131±19 | 128±20 | 0.59 |
| DBP (mmHg) | 82±15 | 80±13 | 0.64 |
Remission of proteinuria is shown in Table 2. A significant reduction in proteinuria was observed in patients treated with the combination of TAC and entecavir within at least 12 weeks of therapy and beyond. The percentages of proteinuria remission (PR+CR) in the TAC combined with entecavir group vs. entecavir alone group were 69% vs. 26%, respectively, at 12 weeks (P=0.013), 87% vs. 42% at 24 weeks (P=0.008), and 92% vs. 47% at 52 weeks (P<0.001). The mean time to PR or CR was 18.6 weeks (95% confidence interval (CI): 12.8-24.4) in the TAC combined with entecavir group and 34.3 weeks (95% CI: 26.2-42.4) in the entecavir group (P=0.001). The mean time for achieving PR and CR was 21.7 weeks (95% CI: 14.6-28.8) and 34.8 weeks (95% CI: 28.5-41.0) in the TAC combined with entecavir group, respectively. The mean time for achieving PR and CR was 38.4 weeks (95% CI: 30.3-46.6) and 42.9 weeks (95% CI: 36.7-49.2) in the entecavir group (P=0.001).
Table 2.
Proteinuria remission of patients in TAC combined with entecavir group and entecavir monotherapy group post-treatment
| Time | Remission | TAC+entecavir n=23 (%) | entecavir n=19 (%) | P value |
|---|---|---|---|---|
| 8 week | No | 19 (82.6) | 17 (89.5) | 1.000 |
| PR | 3 (13.0) | 2 (10.5) | ||
| CR | 1 (4.4) | 0 (0.0) | ||
| 12 week | No | 7 (30.4)* | 14 (73.7) | 0.013 |
| PR | 14 (60.9) | 4 (21.0) | ||
| CR | 2 (8.7) | 1 (5.3) | ||
| 24 week | No | 3 (13.0)* | 11 (57.9)* | 0.008 |
| PR | 8 (34.8) | 4 (21.1) | ||
| CR | 12 (52.2) | 4 (21.1) | ||
| 36 week | No | 3 (13.0)* | 10 (52.6)* | 0.028 |
| PR | 6 (26.1) | 2 (10.5) | ||
| CR | 14 (60.9) | 7 (36.8) | ||
| 52 week | No | 0 (0.0)* | 10 (52.6)* | <0.001 |
| PR | 5 (21.7) | 1 (5.3) | ||
| CR | 18 (78.3) | 8 (42.1) |
No, without remission; PR, partial remission; CR, complete remission;
compared with 8 week P<0.05.
P-value of comparison between TAC combined with entecavir group and entecavir group.
The probability of proteinuria remission was estimated by the Kaplan-Meier method. The CR of proteinuria remission in TAC combined with entecavir group was significantly higher than that of entecavir group (Figure 1A). Moreover, the probability of the combined CR and PR of proteinuria remission between TAC combined with entecavir group and entecavir group were 69.6% vs. 26.3%, 87% vs. 47.4%, and 87% vs. 52.6% after 12, 24 and 36 weeks, respectively (Figure 1B, P<0.001 by log-rank test). In consistent, proteinuria was significantly decreased in the TAC combined with entecavir group at weeks 12 and 24 after treatment. In the TAC combined with entecavir group, proteinuria decreased from 5.2 g/24 h (95% CI: 3.8-6.5) at baseline to 2.6 g/24 h (95% CI: 1.2-3.9) (P<0.0001) at week 12 and to 2.2 g/24 h (95% CI: 0.6-3.6) at week 24 (P<0.005). Proteinuria also decreased in the entecavir group from 2.3 g/24 h (95% CI: 1.1-3.4) at baseline to 2.2 g/24 h (95% CI: 1.4-2.9) by week 12 (P>0.05) and to 1.7 g/24 h by week 24 (95% CI: 0.7-2.6) (P>0.05), but the decrease was no significant different at either week 12 and 24. The results showed that treatment with TAC combined with entecavir significantly increased proteinuria remission.
Figure 1.
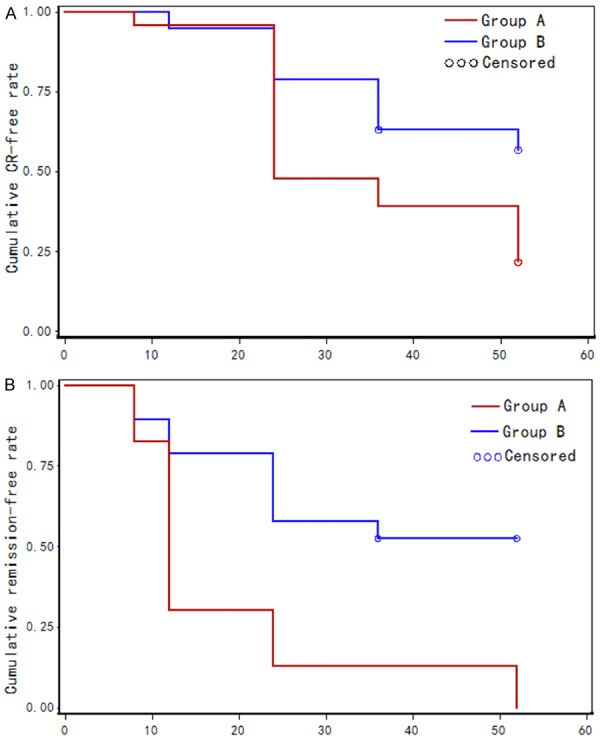
Survival curves of proteinuria remission as estimated by the Kaplan-Meier method. A. Survival curve of CR in patients in group A (TAC combined with entecavir) compared with curve of patients in group B (entecavir), P<0.01; B. Survival curve of CR+PR of patients in group A (TAC combined with entecavir) compared with curve of patients in group B (entecavir), P<0.01.
Changes in renal function between the two groups
During therapy, a decrease in the median serum creatinine (SCr) level was observed in both groups (Figure 2). In the TAC combined with entecavir group, the Scr values changed from 78.8±26.1 µmol/L at baseline to 68.9±19.4 µmol/L by week 52. Compared to week 0, the Scr values were significantly downregulated from week 24 to 52 (P<0.05). In the entecavir monotherapy group, the Scr values changed from 65.9±18.3 µmol/L at baseline to 61.7±18.1 µmol/L by week 52, but there was no significantly change during the course of therapy (P>0.05). One patient in each group progressed to twice their baseline SCr levels at 4, 8, and 12 weeks, and one patient in the TAC combined with entecavir group presented with a 50% SCr increase at 36 weeks; these Scr levels recovered to the baseline level after the TAC treatment were reduced. By the end of treatment, none of the patients had progressed to end-stage renal disease. The results demonstrated that treatment of HBV-MN with TAC in combination with entecavir significantly decreased SCr level at late stage of therapy.
Figure 2.
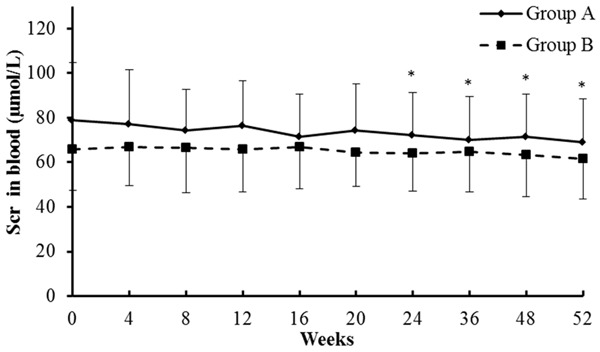
Serum creatinine (µmol/L) levels in the blood of patients post-treatment. Data are expressed as mean±sd. *compared with 0 week of group A, P<0.05. Group A: TAC combined with entecavir; Group B: Entecavir monotherapy.
Replication of serum HBV DNA and changes in ALT between the two groups
In the TAC combined with entecavir therapy group, 6 patients showed evidence of active HBV replication, and 17 patients were not active HBV carriers. The serum levels of HBV DNA in all 6 patients with active HBV replication decreased after treatment. Circulating HBV DNA levels even fell below the detection threshold in one of these six patients during the 24 weeks of treatment (Figure 2). Moreover, during therapy, HBV reactivation was not observed in any of the patients. In the entecavir monotherapy group, 12 patients showed evidence of active HBV replication, and 7 patients were not active HBV carriers. The serum levels of HBV DNA in all 12 patients with active replication decreased after treatment; one of these patients became HBV DNA negative within 24 weeks after starting therapy (Figure 3).
Figure 3.
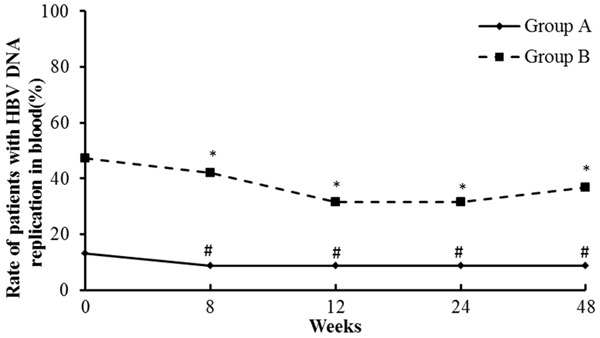
Rate of patients with HBV DNA replication in the blood post-treatment. *compared with 0 week of group B, P>0.05. #compared with 0 week of group A, P>0.05. Group A: TAC combined with entecavir; Group B: Entecavir monotherapy.
Seroconversion from HBeAg and HBsAg to HBeAb and HBsAb was not present after 24 weeks of treatment in either group. The serum ALT levels decreased in all 42 patients during therapy. The mean serum ALT level decreased from 85.5 U/L at baseline to 21.1 U/L at the end of therapy for TAC combined with entecavir and from 26.5 U/L at baseline to 19.9 U/L at the end of therapy for entecavir. There was no significant difference in the change in the serum ALT and AST levels at any time point between the two groups (Table 3).
Table 3.
Frequency of patients with abnormal ALT and AST pre- and post-treatment
| Variables | Pre-treatment | Post-treatment | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Group A | Group B | ||||||
|
| |||||||
| Normal | Abnormal | Total | Normal | Abnormal | Total | ||
| ALT | Normal | 20 | 1 | 21 | 17 | 0 | 17 |
| Abnormal | 2 | 0 | 2 | 2 | 0 | 2 | |
| Total | 22 | 1 | 23 | 19 | 0 | 19 | |
| AST | Normal | 20 | 0 | 20 | 17 | 0 | 17 |
| Abnormal | 3 | 0 | 3 | 2 | 0 | 2 | |
| Total | 19 | 0 | 23 | 19 | 0 | 19 | |
TAC trough concentration
During the first 36-week period of treatment, the mean doses of TAC were 0.05-0.06 mg/kg/d. The mean trough blood level of TAC during therapy was shown in Figure 4. TAC was well tolerated, and there were no serious side effects from TAC treatment.
Figure 4.
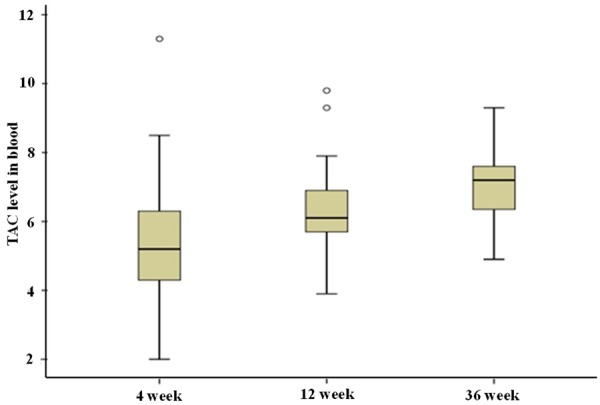
Mean trough blood levels of TAC during the 36 weeks of therapy.
Discussion
In this study, we showed that TAC combined with low-dose of entecavir antiviral therapy significantly improved the outcome of patients with HBV-GN. Of 23 HBV-GN patients who received TAC combined with entecavir, 87% showed CR or PR, and 52% achieved CR by week 24. In contrast, HBV-GN patients received only entecavir therapy demonstrated 42% CR or PR, and 16% CR. HBV reactivation was not observed in any of the patients. These results further support the therapeutic value of immunosuppressive agents in HBV-GN, as previously demonstrated for cyclosporine A [23].
It is widely accepted that the immune complex plays a critical role in the pathogenesis of HBV-GN. At least one of the three major HBV antigens, namely HBsAg, HBeAg, and HBcAg, are present in the glomerulus capillary wall, suggesting a mechanism involving HBV-related immune complexes formed by passive trapping or in situ formation [24]. Notably, in Asian patients, emerging data have shown that disease progression can continue even after anti-HBe seroconversion occurs [14]. Therefore, it is highly possible to treat HBV-GN patients with immunosuppressive agents. Indeed, TAC has been increasingly used in patients with idiopathic MN. Moreover, a recent study suggests that TAC may inhibit HBV entry into hepatocytes through targeting the candidate HBV receptor, NTCP [17]. Entecavir monotherapy has recently been reported to be effective in treating HBV-MN [25,26]. However, antiviral-alone treatments have some limitations, such as HBV variants, a relatively low remission rate and a longer mean time of remission.
Our study applied TAC combined with entecavir in adults with HBV-MN. Previous studies have shown that steroids and cytotoxic agents may reduce proteinuria but enhance HBV DNA replication. Similarly, the use of azathioprine in some patients results in reactivation of HBV and fatal acute hepatitis [4,11]. We found that TAC, combined with entecavir, induced a rapid reduction of proteinuria without enhancing HBV DNA replication in HBV-MN. Regardless of TAC administration, the serum levels of HBV DNA decreased in all active HBV infected patients, and HBV reactivation was not observed in any HBV carriers. There was no difference in the circulating HBV DNA level, which fell below the detection threshold by 24 weeks of treatment, between the two groups. Moreover, the one patient with higher initial aminotransferase levels did not progress to fatal acute hepatitis after TAC combined with entecavir treatment. Our findings suggest that TAC combined with entecavir therapy is effective and safe compared to other immunosuppressive agents for patients with HBV-GN.
The pharmacology of TAC in treating patients with HBV-GN has not been reported. Our results suggest that TAC may directly exert immunosuppressive and HBV DNA replication inhibitory effects. It has recently been reported that NTCP is essential for HBV-specific entry [18], and inhibition of NTCP by calcineurin can inhibit HBV infection [17]. Further study using cyclosporine A may identify more potent anti-HBV compounds [17]. Our results demonstrated TAC not only reduced proteinuria but also inhibited HBV DNA replication. However, the number of patients with active HBV DNA replication was too small to compare the degree of HBV DNA titer decrease and the mean time for HBV DNA to fall below the detection threshold between the two groups. Therefore, further studies with a large cohort of patients are needed to validate the inhibitory effect of TAC on HBV DNA replication.
Importantly, our study suggests that TAC does not increase the risk of acute renal dysfunction. No side effects, such as serious infection or glucose intolerance, were observed throughout the study. Moreover, TAC was well tolerated due to the relatively low doses and serum trough concentration of TAC.
Nevertheless, this study was limited by its observational analysis, relatively small size and short follow-up period. Furthermore, we did not compare the decrease in HBV DNA at any time point between the two groups. Therefore, a prospective, multi-center, randomized control trial is needed to evaluate the potential role of TAC in combination with antiviral agents in HBV-GN.
In summary, this study has demonstrated the beneficial effects of TAC combined with entecavir therapy, such as significant proteinuria remission, in Chinese adult patients with HBV-GN. This combinatorial treatment does not enhance HBV DNA replication or acute renal function worsening. Our results suggest that TAC, as a first-line immunosuppressive agent, could be used for adult patients with HBV-GN.
Disclosure of conflict of interest
None.
References
- 1.Combes B, Shorey J, Barrera A, Stastny P, Eigenbrodt EH, Hull AR, Carter NW. Glomerulonephritis with deposition of Australia antigen-antibody complexes in glomerular basement membrane. Lancet. 1971;2:234–237. doi: 10.1016/s0140-6736(71)92572-4. [DOI] [PubMed] [Google Scholar]
- 2.Takekoshi Y, Tanaka M, Shida N, Satake Y, Saheki Y, Matsumoto S. Strong association between membranous nephropathy and hepatitis-B surface antigenaemia in Japanese children. Lancet. 1978;2:1065–1068. doi: 10.1016/s0140-6736(78)91801-9. [DOI] [PubMed] [Google Scholar]
- 3.Hsu HC, Lin GH, Chang MH, Chen CH. Association of hepatitis B surface (HBs) antigenemia and membranous nephropathy in children in Taiwan. Clin Nephrol. 1983;20:121–129. [PubMed] [Google Scholar]
- 4.Lai KN, Li PK, Lui SF, Au TC, Tam JS, Tong KL, Lai FM. Membranous nephropathy related to hepatitis B virus in adults. N Engl J Med. 1991;324:1457–1463. doi: 10.1056/NEJM199105233242103. [DOI] [PubMed] [Google Scholar]
- 5.Kleinknecht C, Levy M, Peix A, Broyer M, Courtecuisse V. Membranous glomerulonephritis and hepatitis B surface antigen in children. J Pediatr. 1979;95:946–952. doi: 10.1016/s0022-3476(79)80281-4. [DOI] [PubMed] [Google Scholar]
- 6.Hsu HC, Wu CY, Lin CY, Lin GJ, Chen CH, Huang FY. Membranous nephropathy in 52 hepatitis B surface antigen (HBsAg) carrier children in Taiwan. Kidney Int. 1989;36:1103–1107. doi: 10.1038/ki.1989.307. [DOI] [PubMed] [Google Scholar]
- 7.Hepatitis B surface antigenemia in North American children with membranous glomerulonephropathy. Southwest Pediatric Nephrology Study Group. J Pediatr. 1985;106:571–578. doi: 10.1016/s0022-3476(85)80074-3. [DOI] [PubMed] [Google Scholar]
- 8.Ayodele OE, Salako BL, Kadiri S, Arije A, Alebiosu CO. Hepatitis B virus infection: implications in chronic kidney disease, dialysis and transplantation. Afr J Med Med Sci. 2006;35:111–119. [PubMed] [Google Scholar]
- 9.Bhimma R, Coovadia HM. Hepatitis B virusassociated nephropathy. Am J Nephrol. 2004;24:198–211. doi: 10.1159/000077065. [DOI] [PubMed] [Google Scholar]
- 10.Lin CY, Lo SC. Treatment of hepatitis B virus-associated membranous nephropathy with adenine arabinoside and thymic extract. Kidney Int. 1991;39:301–306. doi: 10.1038/ki.1991.37. [DOI] [PubMed] [Google Scholar]
- 11.Moon JY, Lee SH. Treatment of hepatitis B virus-associated membranous nephropathy: lamivudine era versus post-lamivudine era. Korean J Intern Med. 2012;27:394–396. doi: 10.3904/kjim.2012.27.4.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng XY, Wei RB, Tang L, Li P, Zheng XD. Meta-analysis of combined therapy for adult hepatitis B virus-associated glomerulonephritis. World J Gastroenterol. 2012;18:821–832. doi: 10.3748/wjg.v18.i8.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yi Z, Jie YW, Nan Z. The efficacy of anti-viral therapy on hepatitis B virus-associated glomerulonephritis: A systematic review and meta-analysis. Ann Hepatol. 2011;10:165–173. [PubMed] [Google Scholar]
- 14.Lai CL, Ratziu V, Yuen MF, Poynard T. Viral hepatitis B. Lancet. 2003;362:2089–2094. doi: 10.1016/S0140-6736(03)15108-2. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Meng H, Han X, Han C, Sun C, Ye F, Jin X. The relationship between HBV serum markers and the clinicopathological characteristics of hepatitis B virus-associated glomerulonephritis (HBV-GN) in the northeastern chinese population. Virol J. 2012;9:200. doi: 10.1186/1743-422X-9-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai KN, Tam JS, Lin HJ, Lai FM. The therapeutic dilemma of the usage of corticosteroid in patients with membranous nephropathy and persistent hepatitis B virus surface antigenaemia. Nephron. 1990;54:12–17. doi: 10.1159/000185802. [DOI] [PubMed] [Google Scholar]
- 17.Watashi K, Sluder A, Daito T, Matsunaga S, Ryo A, Nagamori S, Iwamoto M, Nakajima S, Tsukuda S, Borroto-Esoda K, Sugiyama M, Tanaka Y, Kanai Y, Kusuhara H, Mizokami M, Wakita T. Cyclosporin A and its analogs inhibit hepatitis B virus entry into cultured hepatocytes through targeting a membrane transporter, sodium taurocholate cotransporting polypeptide (NTCP) Hepatology. 2014;59:1726–1737. doi: 10.1002/hep.26982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, Fu L, Song M, Chen P, Gao W, Ren B, Sun Y, Cai T, Feng X, Sui J, Li W. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Praga M, Barrio V, Juárez GF, Luño J Grupo Español de Estudio de la Nefropatía Membranosa. Tacrolimus monotherapy in membranous nephropathy: a randomized controlled trial. Kidney Int. 2007;71:924–930. doi: 10.1038/sj.ki.5002215. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Li H, Chen J, He Q, Lv R, Lin W, Li Q, He X, Qu L, Suya W. Tacrolimus as a steroid-sparing agent for adults with steroid-dependent minimal change nephrotic syndrome. Nephrol Dial Transplant. 2008;23:1919–1925. doi: 10.1093/ndt/gfm637. [DOI] [PubMed] [Google Scholar]
- 21.Choudhry S, Bagga A, Hari P, Sharma S, Kalaivani M, Dinda A. Efficacy and safety of tacrolimus versus cyclosporine in children with steroid-resistant nephrotic syndrome: a randomized controlled trial. Am J Kidney Dis. 2009;53:760–769. doi: 10.1053/j.ajkd.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 22.Lai KN, Lai FM, Tam JS. Comparison of polyclonal and monoclonal antibodies in determination of glomerular deposits of hepatitis B virus antigens in hepatitis B virus-associated glomerulonephritides. Am J Clin Pathol. 1989;92:159–165. doi: 10.1093/ajcp/92.2.159. [DOI] [PubMed] [Google Scholar]
- 23.Ochi A, Ishimura E, Ichii M, Ohno Y, Nakatani S, Kobayashi I, Shima H, Tsuda A, Shidara K, Mori K, Tamori A, Inaba M. Successful Treatment of Hepatitis B Virus-associated Membranous Nephropathy with Entecavir and Immunosuppressive Agents. Nephrology (Carlton) 2014;19:595–596. doi: 10.1111/nep.12292. [DOI] [PubMed] [Google Scholar]
- 24.Tang S, Lai FM, Lui YH, Tang CS, Kung NN, Ho YW, Chan KW, Leung JC, Lai KN. Lamivudine in hepatitis B-associated membranous nephropathy. Kidney Int. 2005;68:1750–1758. doi: 10.1111/j.1523-1755.2005.00591.x. [DOI] [PubMed] [Google Scholar]
- 25.Igarashi T, Shimizu A, Hanaoka K, Yoshizaki K, Shigemori T, Shimizu S, Komeichi H, Itoh Y. Seroconversion of hepatitis B envelope antigen by entecavir in a child with hepatitis B virus-related membranous nephropathy. J Nippon Med Sch. 2013;80:387–395. doi: 10.1272/jnms.80.387. [DOI] [PubMed] [Google Scholar]
- 26.Yang YF, Xiong QF, Zhao W, Zhong YD. Complete remission of hepatitis B virus-related membranous nephropathy after entecavir monotherapy. Clin Res Hepatol Gastroenterol. 2012;36:e89–92. doi: 10.1016/j.clinre.2012.03.033. [DOI] [PubMed] [Google Scholar]


