Abstract
There is accumulating evidence that breast cancer 1 (BRCA1), sirtuin 1 (SIRT1), and epidermal growth factor receptor (EGFR) help to modulate cisplatin cytotoxicity. The role of dynamic crosstalk among BRCA1, SIRT1, and EGFR in cisplatin sensitivity remains largely unknown. We found that BRCA1, SIRT1, and EGFR levels were increased in cisplatin-resistant ovarian cancers compared with those in cisplatin-sensitive ovarian cancers. Hypomethylation in the BRCA1 promoter was associated with BRCA1 activation, significantly elevated SIRT1 levels, decreased nicotinamide adenine dinucleotide (NAD)-mediated SIRT1 activity, and decreased EGFR levels. Treatment with 5 and 10 μg/ml cisplatin induced a gradual increase in BRCA1 and SIRT1 levels and a gradual decrease in NAD levels and NAD-mediated SIRT1 activity, whereas EGFR levels were increased or decreased by treatment with 5 or 10 μg/ml cisplatin, respectively. The overexpression of SIRT1 or the enhancement of SIRT1 activity synergistically enhanced the BRCA1-mediated effects on EGFR transcription. In contrast, the knockdown of SIRT1 or the inhibition of SIRT1 activity inhibited the BRCA1-mediated effects on EGFR transcription. BRCA1 regulates EGFR through a BRCA1-mediated balance between SIRT1 expression and activity. Those results improve our understanding of the basic molecular mechanism underlying BRCA1-related cisplatin resistance in ovarian cancer.
Keywords: BRCA1, SIRT1, EGFR, cisplatin, ovarian cancer
Introduction
Ovarian cancer is the most common gynecological malignancy and is a leading cause of mortality among women worldwide [1]. Although the primary cause of ovarian cancer remains elusive, breast cancer 1 (BRCA1) mutations are major hereditary risk factors [2]. Accumulating evidence demonstrates that BRCA1 and BRCA1-IRIS, the protein product of the BRCA1 locus, play a critical role in determining cisplatin sensitivity [3,4], presumably through effects on homologous recombination-dependent DNA repair [5]. Little is known, however, about the other mechanisms of involving BRCA1-related cisplatin resistance.
BRCA1 is a tumor-suppressor gene and is widely involved in transcriptional regulation [6,7], epigenetic modification [8], and cellular metabolism [9]. Previously, we demonstrated a novel interaction between BRCA1 and sirtuin 1 (SIRT1), which might be beneficial for the dynamic balance between BRCA1-related biological processes and SIRT1-related energy metabolism and stress response [10]; and BRCA1 has been implicated as a key transcriptional regulator of epidermal growth factor receptor (EGFR) in ovarian cancer progression [11,12]. Furthermore, a growing body of evidence suggests that BRCA1, SIRT1, and EGFR play direct or indirect roles in the modulation of cisplatin cytotoxicity [3,4,13,14]. Therefore, we investigated the potential crosstalk among BRCA1, SIRT1, and EGFR in cisplatin-sensitive and cisplatin-resistant ovarian cancer. Our results provide novel insight into the mechanisms involved in BRCA1-related cisplatin resistance.
Materials and methods
Ethical statements
This investigation was conducted in accordance with ethical standards of the Helsinki Declaration of 1975.
Patients and tissue collection
This study was approved by the Institutional Review Board at China Medical University. Patients with serous ovarian cancer (15 chemosensitive and 12 chemoresistant) were enrolled between 2011 and 2014. All enrolled patients gave informed consent. Fresh tumor samples were obtained at the time of primary surgery before any chemotherapy or radiotherapy. Three staff pathologists performed hematoxylin and eosin staining of the samples for histopathological diagnosis and grading using the World Health Organization criteria.
Cell culture, cisplatin treatment, shRNAs, and cell proliferation assay
The human ovarian cancer cell-line A2780 was maintained in RPMI 1640 with 2 mM glutamine and 10% fetal bovine serum (Invitrogen, CA, USA). Cis-Diamineplatinum (II) dichloride was purchased from Sigma (St. Louis, MO, USA). SRT1720 and EX 527 were purchased from Selleck Chemicals (Houston, TX, USA). Lentiviral vectors (Table 1) expressing short hairpin RNAs (shRNAs) against BRCA1 (NM_007299) were obtained from GeneChem Co., Ltd (Shanghai, China). A non-silencing shRNA sequence was used as a negative control. Lentiviral vectors expressing shRNAs for SIRT1 (sc-40986-V) were purchased from Santa Cruz Biotechnology (CA, USA). For the overexpression of SIRT1, the open reading frames of SIRT1 (NM_012238) were cloned into the lentiviral vector GV287 (Ubi-MCS-3FLAG-SV40-EGFP; GeneChem Co., Ltd). Transfections were performed using polybrene and enhanced infection solution (GeneChem Co., Ltd) according to the manufacturer’s protocol. The efficiency of BRCA1 and SIRT1 transfection was as previously reported [10,15]. A2780 cells were treated with 5 or 10 μg/ml cisplatin for 48 h. After 48 h cisplatin treatment, cell proliferation was determined using the Cell-Light™ EdU Apollo®643 In Vitro Imaging Kit (Ribobio, Guangzhou, China) following the manufacturer’s instructions.
Table 1.
Primers used in this study
| Gene | Primers | Description |
|---|---|---|
| BRCA1-SH-F | 5’-CcggaaCCTGTCTCCACAAAGTGTGCTCGAGCACACTTTGTGGAGACAGGTTTTTTTg | Lentiviral packaging sequences for BRCA1 knockdown |
| BRCA1-SH-R | 5’-aattcaaaaaaaCCTGTCTCCACAAAGTGTGCTCGAGCACACTTTGTGGAGACAGGTT | |
| BRCA1-CON-F | 5’-ccggTTCTCCGAACGTGTCACGTctcgagACGTGACACGTTCGGAGAAtttttg | Lentiviral packaging sequences for negative control |
| BRCA1-CON-R | 5’-aattcaaaaaTTCTCCGAACGTGTCACGTctcgagACGTGACACGTTCGGAGAA | |
| BRCA1-RTP-F | 5’-GGCTATCCTCTCAGAGTGACATTT | Real-time PCR for BRCA1 |
| BRCA1-RTP-R | 5’-GCTTTATCAGGTTATGTTGCATGG | |
| SIRT1-RTP-F | 5’-GCGATTGGGTACCGAGATAAC | Real-time PCR for SIRT1 |
| SIRT1-RTP-R | 5’-GGCCTTGGAGTCCAGTCACTA | |
| EGFR-RTP-F | 5’-GCGAATTCCTTTGGAAAACC | Real-time PCR for EGFR |
| EGFR-RTP-R | 5’-AAGGCATAGGAATTTTCGTAGTACA | |
| GAPDH-RTP-F | 5’-AGGTGAAGGTCGGAGTCA | Real-time PCR for GAPDH |
| GAPDH-RTP-R | 5’-GGTCATTGATGGCAACAA | |
| BRCA1-P1-F | GGGGGTGGAGGGAAATAA | Methylation analysis for BRCA1 |
| BRCA1-P1-R-bio | AAACATAACACTCCAATCCATAACT | |
| BRCA1-P1-S | GTTTTATGGAGAGGAATATTTAA |
Abbreviations: CON, control; SH, shRNAs; RTP, real-time PCR; F, forward primer; R, reverse primer.
DNA methylation analysis by pyrosequencing
Genomic DNA was bisulfite modified using the EpiTect Plus DNA Bisulfite Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. The bisulfite-modified DNA was amplified using the EPIK™ Amplification Kit (Bioline) according to the manufacturer’s protocol. The specific primer sequences are listed in Table 1. The conditions were as follows: 2 min at 95°C, followed by 40 cycles of 15 s at 95°C, 15 s at 56°C, and 30 s at 72°C. Following amplification, the biotinylated PCR products were purified and incubated with the sequencing primer AAACATAACACTCCAATCCATAACT, which was designed to bind adjacent to the CpG site of interest. Pyrosequencing was conducted using PyroMark™ Gold Q96 reagents (Qiagen) with subsequent quantification of methylation levels using the PyroMark Q24 1.010 software. The relative differences among peak heights were used to calculate the percentage of methylated cytosines.
Real-time quantitative PCR
Total RNA was extracted using Trizol reagent (Invitrogen) according to the manufacturer’s protocol. DNA contamination was removed by adding DNase I (Invitrogen) according to the manufacturer’s protocol. Total RNA was then reverse-transcribed from 2 μg RNA using the PrimeScript RT Master Mix kit (TaKaRa, Dalian, China) and amplified using SYBR Premix Ex TaqTM II (TaKaRa) in a Roche LightCycler 2.0 (Roche Diagnostics, Mannheim, Germany). The specific primer sequences are listed in Table 1. GAPDH mRNA was amplified as an internal control for the normalization of each sample. All samples were analyzed using the 2-∆∆CT method.
Assays of nicotinamide adenine dinucleotide (NAD) levels and SIRT1 activity
For the NAD assay, 20 mg frozen ovarian tissue or 20 μl packed, cultured cells was homogenized in 400 μl BioVsion NAD/NADH Extraction Buffer (BioVsion, CA, USA). The homogenate was ultrafiltered using BioVsion 10-kD cut-off filters (14000 g, 30 min, 4°C). Assays were performed using NAD/NADH Quantification Kits according to the manufacturer’s instructions (BioVsion). SIRT1 deacetylase activity was evaluated using a commercially available CycLex SIRT1/Sir2 Deacetylase Fluorometric Assay Kit (Cyclex Co., Ltd., Nagano, Japan) according to the manufacturer’s protocols [10].
Statistical analysis
The data are presented as mean ± standard deviation (SD) or standard error (SE). Statistical differences in the data were evaluated by Student’s t test or one-way analysis of variance (ANOVA) as appropriate, and were considered significant at when P < 0.05.
Results
BRCA1, SIRT1, and EGFR levels were elevated in cisplatin-resistant cancers
BRCA1 is a potential trigger in the transcriptional regulation of SIRT1 and EGFR in ovarian cancer [10,12]. The intracellular BRCA1, EGFR, and SIRT1 levels and SIRT1 activity were measured in cisplatin-sensitive and cisplatin-resistant ovarian cancer tissues, respectively. The BRCA1, SIRT1, and EGFR levels were elevated in the cisplatin-resistant cancers compared with those in cisplatin-sensitive cancers (Figure 1A, 1B and 1E). There was no significant difference between the cisplatin-sensitive and cisplatin-resistant cancers, however, in the NAD level or the SIRT1 activity (Figure 1C and 1D).
Figure 1.
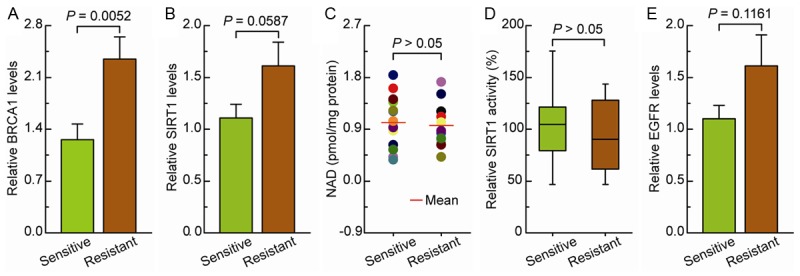
Intracellular BRCA1, SIRT1, and EGFR levels and NAD-dependent SIRT1 activity in cisplatin-sensitive and cisplatin-resistant ovarian cancer. A-E. BRCA1 levels, SIRT1 levels, NAD levels, SIRT1 activity, and EGFR levels were measured in cisplatin-sensitive and cisplatin-resistant ovarian cancer tissues. Bar graphs show mean ± SE (n = 15 for the sensitive group, n = 12 for the resistant group).
Cisplatin treatment induced differential BRCA1, SIRT1, EGFR levels and NAD-dependent SIRT1 activity
The effects of cisplatin on the regulation of BRCA1, EGFR, and SIRT1 expression and SIRT1 activity were evaluated in the ovarian cancer-cell line A2780. The proliferation of the A2780 cells was gradually inhibited as the concentration of cisplatin in the growth medium was increased from 5 to 10 μg/ml (Figure 2A and 2B). Furthermore, 5 and 10 μg/ml cisplatin induced a gradual increase in BRCA1 and SIRT1 levels (Figure 2C and 2D) and a gradual decrease in NAD levels and NAD-dependent SIRT1 activity (Figure 2E and 2F). EGFR levels were increased by 5 μg/ml cisplatin and decreased by 10 μg/ml cisplatin (Figure 2G). Those results indicate that cisplatin could be responsible for the regulation of BRCA1, EGFR, and SIRT1 expression and NAD-dependent SIRT1 activity.
Figure 2.
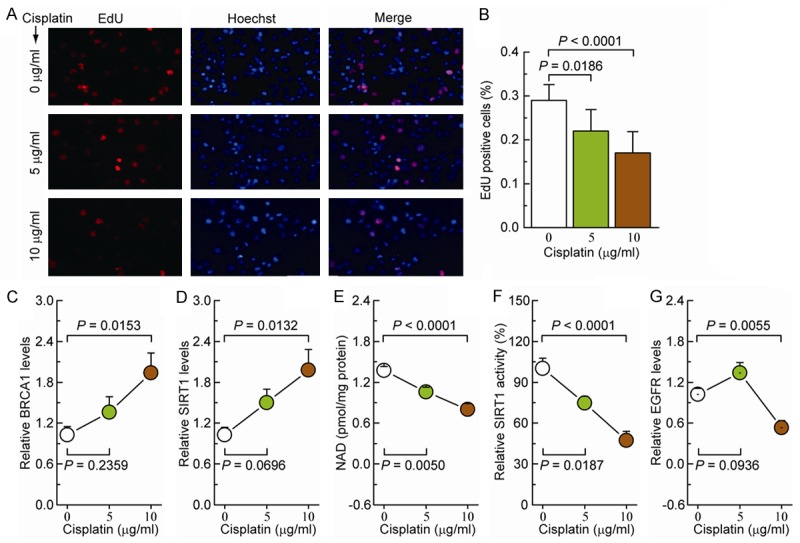
Effects of cisplatin on BRCA1, SIRT1, and EGFR levels and NAD-dependent SIRT1 activity in ovarian cancer cells. A. EdU labeling showing the proliferation of A2780 cells after 5 or 10 μg/ml cisplatin treatment for 48 h. Red: EdU labeling of nuclei of proliferative cells; Blue: Hoechst 33342 labeling of cell nuclei. B. The EdU incorporation rate was expressed as the ratio of EdU-positive cells to total Hoechst 33342-positive cells. C-G. BRCA1 levels, SIRT1 levels, NAD levels, SIRT1 activity, and EGFR levels in A2780 cells after incubation with 5 or 10 μg/ml cisplatin (repeated six times). Bar graphs show mean ± SD.
BRCA1 regulates EGFR expression via SIRT1 in ovarian cancer cells
To further clarify the role of SIRT1 in the regulation of BRCA1-mediated EGFR expression, the effects of BRCA1 knockdown, combined with SIRT1 overexpression or knockdown, and the enhancement or inhibition of SIRT1 activity were evaluated. BRCA1 knockdown effectively increased EGFR levels in the ovarian cancer cells. SIRT1 overexpression or the enhancement of SIRT1 activity synergistically enhanced the BRCA1-mediated effects on EGFR transcription. In contrast, SIRT1 knockdown or the inhibition SIRT1 activity effectively abolished the BRCA1-mediated effects on EGFR transcription. Those results indicate that SIRT1 might be a key factor in the BRCA1-mediated regulation of EGFR expression (Figure 3).
Figure 3.
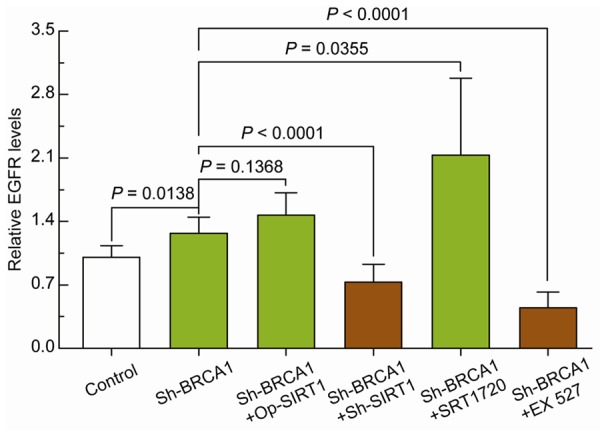
Effects of BRCA1 and/or SIRT1 on EGFR expression in ovarian cancer cells. Relative EGFR mRNA levels in A2780 cells before and after BRCA1 knockdown or BRCA1 knockdown together with SIRT1 overexpression, SIRT1 knockdown, SIRT1 activity enhancement by SRT1720, or SIRT1 activity inhibition by EX 527. Bar graphs show mean ± SD. Sh: short hairpin RNAs; Op: overexpression.
High BRCA1 levels mediated by promoter hypomethylation are accompanied by increased SIRT1 levels, and decreased of NAD-dependent SIRT1 activity and EGFR expression
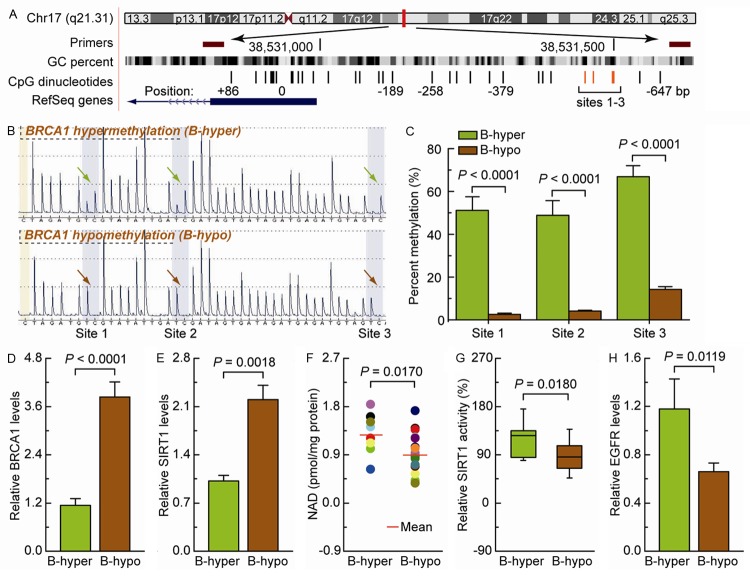
In mammals, promoter methylation is an epigenetic modification involved in regulating gene expression [7,8]. Our previous study suggests that the methylation levels of sites 1-3 accurately represent the methylation levels of the BRCA1 core promoter [10]. Ovarian cancer tissues with a hypomethylated sites 1-3 (Figure 4A-C) displayed increased levels of BRCA1 compared with ovarian cancer tissues with a hypermethylated BRCA1 promoter (Figure 4D). Therefore, the differential levels of BRCA1 mediated by promoter methylation were an appropriate model for investigating the physiological relationships among BRCA1, SIRT1, and EGFR. The high BRCA1 levels mediated by hypomethylation of the BRCA1 promoter were accompanied by a marked increase in SIRT1 levels (Figure 4E) and a significant decrease in NAD-dependent SIRT1 activity (Figure 4F and 4G) and EGFR expression (Figure 4H).
Figure 4.
Intracellular BRCA1, SIRT1, and EGFR levels and NAD-dependent SIRT1 activity in ovarian cancer with hypomethylated promoter-mediated BRCA1 activation. (A) The location of CpG sites in the core promoter region of BRCA1. Genomic coordinates are shown, along with the primer-amplified fragments, GC percentage, location of individual CpG dinucleotides (dashes), and BRCA1 RefSeq gene (exon 1 is shown as a blue box, and the intron is shown as an arrowed line). The arrow indicates the direction of transcription. (B) Comparative analysis of methylation patterns at sites 1-3 of the BRCA1 core promoter in ovarian cancer tissues. The yellow regions indicate control regions for the automatic assessment of bisulfide conversion (unmethylated C should be fully converted to T), and the blue regions indicate the percentage of CpG methylation. (C) Summary of the methylation levels of the BRCA1 gene from the measurements shown in (B). (D-H) BRCA1 levels, SIRT1 levels, NAD levels, SIRT1 activity, and EGFR levels were measured in ovarian cancer tissues with an identified hypermethylated BRCA1 promoter and compared with those in ovarian cancer tissues with a hypomethylated BRCA1 promoter. Bar graphs show mean ± SE (n = 8 for the hypermethylated group, n = 19 for the hypomethylated group).
Discussion
The results demonstrate a novel mechanism of BRCA1-mediated, SIRT1-related transcriptional regulation of EGFR, which could play a significant role in cisplatin cytotoxicity. There is mounting evidence suggesting that BRCA1 plays a role in cisplatin resistance. POU class 1 homeobox 1 (Pit-1) can specifically inhibit BRCA1 expression, sensitizing breast cancer cells to cisplatin therapy [16]. Altered BRCA1 expression can modulate cisplatin sensitivity through the mitochondrial fission program [3]. Some microRNAs, miR-9 [17] and miR-638 [18], affect DNA repair and cisplatin sensitivity via BRCA1 deregulation. BRCA1-IRIS inactivation sensitizes ovarian cancer cells to cisplatin [4]. CDK12 inactivation reduces BRCA1 levels, disrupts homologous recombination-dependent DNA repair, and sensitizes ovarian cancer cells to cisplatin [19].
The results of a number of recent studies collectively drew attention to the possibility that cisplatin sensitivity is influenced by a functional link between BRCA1-mediated SIRT1 transcription and SIRT1-related EGFR expression. SIRT1 can provoke renal fibrogenesis through the activation of EGFR signaling [20,21]. BRCA1-associated breast cancers are triple-negative, basal-like, high-grade, ductal carcinomas that frequently overexpress EGFR [11]. BRCA1 can regulate EGFR expression in ovarian cancer cells [12] and breast cancer cells [11]. High levels of SIRT1 significantly enhanced cisplatin resistance in HHUA endometrial carcinoma cell lines [22]. SIRT1 silencing can dramatically enhance cisplatin-mediated growth inhibition, G2/M phase arrest, and apoptosis [23]. EGFR overexpression is involved in resistance to cisplatin-based neoadjuvant chemotherapy [14]. Cisplatin can activate EGFR signaling, which in turn might provide a survival advantage for cancer cells [24]. Our current results provide compelling evidence that the balance between SIRT1 expression and activity is a key factor in the BRCA1-mediated regulation of EGFR expression, playing an essential role in the context of cisplatin treatment.
There is a special compensatory mechanism for SIRT1 function. For instance, BRCA1 inactivation inhibits SIRT1 expression, but it also induces a substantial increase in NAD levels and consequently enhances SIRT1 activity [10]. Consistent with those properties, cisplatin-resistant ovarian cancer tissues overexpressed BRCA1 and displayed enhanced SIRT1 function (high levels of SIRT1 with normal SIRT1 activity) and EGFR expression. In addition, 5 and 10 μg/ml cisplatin effectively induced a gradual increase in SIRT1 levels and a gradual decrease in SIRT1 activity, respectively. In that regard, EGFR levels were increased or decreased by 5 or 10 μg/ml cisplatin, respectively. Those results suggest that different doses of cisplatin might be responsible for the regulation of EGFR expression through the balance between SIRT1 expression and activity.
Our results support the hypothesis that the BRCA1-SIRT1-EGFR axis is broadly involved in the regulation of cisplatin sensitivity and emphasize the convergence of the BRCA1-mediated antitumor mechanism, the SIRT1-related energy metabolism and stress responses, and the EGFR-mediated cell proliferation pathway. Taken together, those results improve our understanding of the basic molecular mechanism underlying BRCA1-related cisplatin resistance in ovarian cancer.
Acknowledgements
This work was supported by the Clinical Capability Construction Project for Liaoning Provincial Hospitals (LNCCC-A01-2015), Fok Ying Tung Education Foundation (No. 151039), Natural Science Foundation of China (No. 81402130, 31271364), and the Doctoral Start-up Foundation of Liaoning Province (No. 20141045, 201501007).
Disclosure of conflict of interest
None.
References
- 1.Fang YY, Bi FF, Zhou YM, Sun WP, Li CY, Liu Q, Zhao Y, Li D. Nicotinamide adenine dinucleotide (NAD) may affect DNA methyltransferase 1 through regulation of BRCA1 in ovarian cancer. Am J Cancer Res. 2015;5:1199–1206. [PMC free article] [PubMed] [Google Scholar]
- 2.Pruthi S, Gostout BS, Lindor NM. Identification and Management of Women With BRCA Mutations or Hereditary Predisposition for Breast and Ovarian Cancer. Mayo Clin Proc. 2010;85:1111–1120. doi: 10.4065/mcp.2010.0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan S, Liu B, Sun L, Lv XB, Lin Z, Chen W, Chen W, Tang Q, Wang Y, Su Y, Jin S, Zhang D, Zhong J, Li Y, Wen B, Zhang Z, Yang P, Zhou B, Liang Q, Yu X, Zhu Y, Hu P, Chu J, Huang W, Feng Y, Peng H, Huang Q, Song E, Li J. Mitochondrial fission determines cisplatin sensitivity in tongue squamous cell carcinoma through the BRCA1-miR-593-5p-MFF axis. Oncotarget. 2015;6:14885–14904. doi: 10.18632/oncotarget.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paul BT, Blanchard Z, Ridgway M, ElShamy WM. BRCA1-IRIS inactivation sensitizes ovarian tumors to cisplatin. Oncogene. 2015;34:3036–3052. doi: 10.1038/onc.2014.237. [DOI] [PubMed] [Google Scholar]
- 5.Manie E, Popova T, Battistella A, Tarabeux J, Caux-Moncoutier V, Golmard L, Smith NK, Mueller CR, Mariani O, Sigal-Zafrani B, Dubois T, Vincent-Salomon A, Houdayer C, Stoppa-Lyonnet D, Stern MH. Genomic hallmarks of homologous recombination deficiency in invasive breast carcinomas. Int J Cancer. 2016;138:891–900. doi: 10.1002/ijc.29829. [DOI] [PubMed] [Google Scholar]
- 6.Dacheux E, Vincent A, Nazaret N, Combet C, Wierinckx A, Mazoyer S, Diaz JJ, Lachuer J, Venezia ND. BRCA1-Dependent Translational Regulation in Breast Cancer Cells. PLoS One. 2013;8:e67313. doi: 10.1371/journal.pone.0067313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li D, Bi FF, Chen NN, Cao JM, Sun WP, Zhou YM, Cao C, Li CY, Yang Q. Epigenetic repression of phosphatidylethanolamine Nmethyltransferase (PEMT) in BRCA1-mutated breast cancer. Oncotarget. 2014;5:1315–1325. doi: 10.18632/oncotarget.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li D, Bi FF, Cao JM, Cao C, Liu B, Yang Q. Regulation of DNA methyltransferase 1 transcription in BRCA1-mutated breast cancer: a novel crosstalk between E2F1 motif hypermethylation and loss of histone H3 lysine 9 acetylation. Mol Cancer. 2014;13:26. doi: 10.1186/1476-4598-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li D, Chen NN, Cao JM, Sun WP, Zhou YM, Li CY, Wang XX. BRCA1 as a nicotinamide adenine dinucleotide (NAD)-dependent metabolic switch in ovarian cancer. Cell Cycle. 2014;13:2564–2571. doi: 10.4161/15384101.2015.942208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li D, Bi FF, Chen NN, Cao JM, Sun WP, Zhou YM, Li CY, Yang Q. A novel crosstalk between BRCA1 and sirtuin 1 in ovarian cancer. Sci Rep. 2014;4:6666. doi: 10.1038/srep06666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumaraswamy E, Wendt KL, Augustine LA, Stecklein SR, Sibala EC, Li D, Gunewardena S, Jensen RA. BRCA1 regulation of epidermal growth factor receptor (EGFR) expression in human breast cancer cells involves microRNA-146a and is critical for its tumor suppressor function. Oncogene. 2015;34:4333–4346. doi: 10.1038/onc.2014.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li D, Bi FF, Cao JM, Cao C, Li CY, Yang Q. Effect of BRCA1 on epidermal growth factor receptor in ovarian cancer. J Exp Clin Cancer Res. 2013;32:102. doi: 10.1186/1756-9966-32-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin DH, Choi YJ, Park JW. SIRT1 and AMPK mediate hypoxia-induced resistance of non-small cell lung cancers to cisplatin and doxorubicin. Cancer Res. 2014;74:298–308. doi: 10.1158/0008-5472.CAN-13-2620. [DOI] [PubMed] [Google Scholar]
- 14.Aichler M, Motschmann M, Jutting U, Luber B, Becker K, Ott K, Lordick F, Langer R, Feith M, Siewert JR, Walch A. Epidermal growth factor receptor (EGFR) is an independent adverse prognostic factor in esophageal adenocarcinoma patients treated with cisplatin-based neoadjuvant chemotherapy. Oncotarget. 2014;5:6620–6632. doi: 10.18632/oncotarget.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li D, Bi FF, Chen NN, Cao JM, Sun WP, Zhou YM, Li CY, Yang Q. A novel crosstalk between BRCA1 and poly (ADP-ribose) polymerase 1 in breast cancer. Cell Cycle. 2014;13:3442–3449. doi: 10.4161/15384101.2014.956507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seoane S, Arias E, Sigueiro R, Sendon-Lago J, Martinez-Ordonez A, Castelao E, Eiro N, Garcia-Caballero T, Macia M, Lopez-Lopez R, Maestro M, Vizoso F, Mourino A, Perez-Fernandez R. Pit-1 inhibits BRCA1 and sensitizes human breast tumors to cisplatin and vitamin D treatment. Oncotarget. 2015;6:14456–14471. doi: 10.18632/oncotarget.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun C, Li N, Yang Z, Zhou B, He Y, Weng D, Fang Y, Wu P, Chen P, Yang X, Ma D, Zhou J, Chen G. miR-9 regulation of BRCA1 and ovarian cancer sensitivity to cisplatin and PARP inhibition. J Natl Cancer Inst. 2013;105:1750–1758. doi: 10.1093/jnci/djt302. [DOI] [PubMed] [Google Scholar]
- 18.Tan X, Peng J, Fu Y, An S, Rezaei K, Tabbara S, Teal CB, Man YG, Brem RF, Fu SW. miR-638 mediated regulation of BRCA1 affects DNA repair and sensitivity to UV and cisplatin in triple-negative breast cancer. Breast Cancer Res. 2014;16:435. doi: 10.1186/s13058-014-0435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joshi PM, Sutor SL, Huntoon CJ, Karnitz LM. Ovarian cancer-associated mutations disable catalytic activity of CDK12, a kinase that promotes homologous recombination repair and resistance to cisplatin and poly(ADP-ribose) polymerase inhibitors. J Biol Chem. 2014;289:9247–9253. doi: 10.1074/jbc.M114.551143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ponnusamy M, Zhou X, Yan Y, Tang J, Tolbert E, Zhao TC, Gong R, Zhuang S. Blocking sirtuin 1 and 2 inhibits renal interstitial fibroblast activation and attenuates renal interstitial fibrosis in obstructive nephropathy. J Pharmacol Exp Ther. 2014;350:243–256. doi: 10.1124/jpet.113.212076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ponnusamy M, Zhuang MA, Zhou X, Tolbert E, Bayliss G, Zhao TC, Zhuang S. Activation of Sirtuin-1 Promotes Renal Fibroblast Activation and Aggravates Renal Fibrogenesis. J Pharmacol Exp Ther. 2015;354:142–151. doi: 10.1124/jpet.115.224386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asaka R, Miyamoto T, Yamada Y, Ando H, Mvunta DH, Kobara H, Shiozawa T. Sirtuin 1 promotes the growth and cisplatin resistance of endometrial carcinoma cells: a novel therapeutic target. Lab Invest. 2015;95:1363–1373. doi: 10.1038/labinvest.2015.119. [DOI] [PubMed] [Google Scholar]
- 23.Cao B, Shi Q, Wang W. Higher expression of SIRT1 induced resistance of esophageal squamous cell carcinoma cells to cisplatin. J Thorac Dis. 2015;7:711–719. doi: 10.3978/j.issn.2072-1439.2015.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim KK, Han A, Yano N, Ribeiro JR, Lokich E, Singh RK, Moore RG. Tetrathiomolybdate mediates cisplatin-induced p38 signaling and EGFR degradation and enhances response to cisplatin therapy in gynecologic cancers. Sci Rep. 2015;5:15911. doi: 10.1038/srep15911. [DOI] [PMC free article] [PubMed] [Google Scholar]