Abstract
The high-mobility group A protein 2 (HMGA2) is a non-histone chromatin factor highly expressed in fetal tissue and malignant tumors but rarely detected within normal adult tissues. The clinical implications and biological functions of HMGA2 in endometrial carcinoma are largely unknown. Here we report that HMGA2 expression was barely detected in benign endometrium samples (2 of 28 samples). However, HMGA2 expression increased significantly from precancerous lesion endometrial glandular dysplasia (7 of 17, 41.2%), to serous endometrial intraepithelial carcinoma (5 of 8, 62.5%) and to full blown endometrial serous carcinoma (39 of 59, 66.1%). Functional characterization of HMGA2 revealed that the gene has both tumor growth promotion and metastasis. In addition, HMGA2 induced epithelial-mesenchymal transition (EMT) through modulation vimentin and β-catenin. Furthermore, HMGA2 overexpression started from endometrial serous precancers, non-invasive cancers, as well as in full blown carcinomas in a p53 knockout mouse model we recently established in our laboratory. Our findings suggest that HMGA2 may serve as a useful diagnostic marker in the assessment of endometrial serous cancer and its precursor lesions.
Keywords: HMGA2, endometrial serous carcinoma, tumor growth, metastasis, EMT
Introduction
Endometrial carcinoma is the most common gynecologic malignancy, which has been classified into two different types, based on clinical, pathological and epidemiological observations [1,2]. The rarer yet more clinically aggressive cancer, endometrial serous carcinoma (ESC) accounts for approximately 10-15% of all endometrial cancers but is responsible for a disproportionate 40% of the cancer related death [3]. Despite the significant advances about the carcinogenesis of type I cancer (mainly endometrioid type), progresses about type II carcinogenesis, particularly the development of ESC, is significantly lacking. Therefore, the importance of understanding endometrial serous carcinogenesis is highly needed.
High-mobility group A2 (HMGA2), a member of the high-mobility group family, is a non-histone nuclear-binding oncofetal protein, which can modulate transcription by promoting conformational changes [4]. HMGA2 is highly expressed in embryonic tissue and in many malignant neoplasms including the pancreas [5], thyroid [6], lung [7], and ovary [8], but rare in normal adult tissues [9]. There is strong evidence from previous studies that expression of HMGA2 is associated with tumor aggressive behavior, which is probably due to its biologic function related to epithelial-mesenchymal transition [10] and stem cell self-renewal ability [11].
Previous studies have shown that the overexpression of HMGA2 was a critical molecular event responsible for the tumorigenesis of ovarian serous cancers [12,13]. A recent study demonstrated that HMGA2 was also overexpressed in advanced endometrial cancers by immunohistochemistry [14]. However, whether HMGA2 plays an oncogenic role in the development of ESC remains unclear. The goal of this study was to explore the role of HMGA2 in ESC. For the first time, we have revealed that HMGA2 overexpression started from endometrial serous precancer, to serous endometrial intraepithelial carcinoma, and to full blown ESCs and it was intimately linked to P53 gene disfunction in ESCs.
Materials and methods
Case selection
All evaluated endometrial samples were derived from Departments of Pathology at Yale University and the University of Arizona after obtaining an Institutional Review Board approval. Total of 173 endometrial samples were studied which included benign endometrium (n=28), endometrial glandular dysplasia (EmGD) (n=17), endometrial intraepithelial carcinoma (EIC) (n=8), endometrial serous carcinoma (ESC) (n=59) and endometrial endometrioid carcinoma (EEC) (n=61). All malignant cases were diagnosed using criteria of the International Federation of Gynecology Oncology (FIGO). EmGD was diagnosed as defined by Zheng et al. [15]. All the benign endometriums come from age-matched hysteromyoma patients had hysterectomy or ovarian cancer with normal endometriums.
Cell lines and cell culture
KLE, HEC-1-A and AN3 CA were purchased from the American Type Culture Collection (ATCC). Ishikawa and SPEC-2 cell lines were generously provided by Dr. Wenxin Zheng (The University of Arizona, Tucson, AZ, USA). HEK293T was purchased from China Type Culture Collection (Shanghai, China). HEC-1-A cell line was maintained in McCoy’s 5A modified medium, KLE cell line was maintained in DMEM:F12 medium, AN3 CA and SPEC-2 cell lines were cultured in Eagle’s minimum essential medium, Ishikawa and HEK293T were culture in DMEM. All cell culture media were supplemented with 10% fetal bovine serum (FBS) and culture media for SPEC-2 cells were also supplemented with 100 mM sodium pyruvate. All cell lines were cultured and maintained in 5% CO2 at 37°C.
Plasmids and transfection
HMGA2 overexpression plasmid pBABE-puro-HMGA2 was kindly provided by Dr. Jianjun Wei (Northwest University, Chicago, IL, USA). Retrovirus-pBABE-puro-HMGA2 and the control retrovirus-pBABE-puro were produced in GPZ-293 package cells. All the constructs were validated by sequencing. For stable infection, cells (1×105) were plated in six-well plates without antibiotics for 1 day before incubation. Then, the medium was replaced with 1 mL of retrovirus solution generated from the step mentioned above. The solution was supplement with 10 µg/mL polybrene. After 24 h period, fresh medium containing 2 µg/mL puromycin (Sigma-Aldrich) was added to each well. Multiple colonies were obtained after 2 weeks of puromycin selection.
Transient transfection
HMGA2 siRNAs and control siRNA (Sigma-Aldrich) were used for transfecting endometrial cancer cells with lipofectamine RNAiMAX (Invitrogen) according to the manufacturer’s protocol. The efficiency of HMGA2 inhibition was assessed by western blot.
Migration and invasion assays
For cell migration assay, endometrial cancer cells (5-10×104 cells) were seeded into the upper chambers of a transwell system (24-well, 8 μm pore size, BD falcon) with 200 μl of media containing no FBS. The lower chambers were filled with 500 μl culture media containing 10% FBS as a chemoattractant. After 24-32 hours, cells in the lower surface of the membrane were fixed in methanol for 15 minutes, stained with 0.1% crystal violet for 30 minutes. After removing the noninvading cells with a cotton swab, the successfully invading cells were counted under a light microscope. Invasion assays were performed using Matrigel-coated transwell system according to the manufacture’s protocol. The incubation time was 48 hours.
Western blotting
Cultured cells were harvested and lysed in lysis buffer. The protein concentration was determined by BCA Assay Kit (Thermo Scientific). Cell lysates were separated by SDS-PAGE and transferred to PVDF membrane (Millipore). After blocking with 5% non-fat milk, the membranes were incubated with primary antibody and corresponding horseradish peroxydase-coupled secondary antibody. The protein bands were visualized with chemiluminescence (ECL) (PerkinElmer) and detected by Image Quant LAS 4000 (GE Health care Life Sciences). The rabbit anti-human HMGA2 polyclonal antibody was purchased from Bio Check, Inc. Other antibodies (β-actin, vimentin and β-catenin) were purchased from Cell signaling Technology.
Clonogenic assay
Single-cell suspensions were generated for each cell line and specified numbers of cells (100) were seeded into six-well tissue culture plates. Cells were cultured for 10-14 days, stained with 0.01% crystal violet and colonies with greater than 50 cells were counted.
MTT assay
Cell proliferation was measured using 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide (MTT) assay. Cells were seeded into 96-well plates (1.0-2.0×103 cells/well) and cultured for 1-5 days. At specified time points, 20 μl of MTT (Sigma-Aldrich) solution (0.5 mg/ml) was added to each well, and the cells were incubated for additional 4 hours at 37°C. Then the supernatants were carefully removed and 100 μl of dimethyl sulfoxide (DMSO, Sigma-Aldrich) was added to each well. The absorbance values were evaluated at 490 nm with a Microplate Reader (Thermo Fisher Scientific).
Immunohistochemistry
Formalin-fixed and paraffin-embedded tissues were sectioned at 4 μm. The slides were deparaffinized in xylene and rehydrated in a graded series of ethanol. Antigen retrieval was performed by microwave irradiation in EDTA retrieval solution (PH 8.0). The Endogenous peroxidase activity was blocked by 3% hydrogen peroxidase (SIGMA-ALDRICH; #216763) in methanol. The slides were then incubated overnight at 4°C with HMGA2 (anti-rabbit, BioCheck, Inc) primary antibody at 1:900 dilution. The following steps were performed by Vectastain ABC kit (Rabbit IgG, Vector, PK4001) according to manufacturer’s instruction. The signal was developed by DAB detection system and negative immunohistochemistry controls were routinely employed. The staining results were scored semiquantitatively by extent and intensity.
RNA isolation and qRT-PCR
Total RNA was extracted from endometrial cancer cells using TRIzol Reagent (Ambion) following manufacturer’s instructions. The cDNA was synthesized from miRNA using the One Step PrimeScript miRNA cDNA Synthesis Kit (Takara). Quantitative real-time PCR was performed by StepOne Plus Real-Time PCR System (Applied Biosystems) with SYBR green Premix Ex Taq II (Takara). The expression of GAPDH was used as the endogenous control for miRNA expression level.
Subcutaneous tumor implantation and tail-vein injections in nude mice
SPEC-2 cells with either overexpression or normal control of HMGA2 (normal control: empty vector) were injected subcutaneously into the bilateral flanks of 4-6-week-old female BALB/c nu/nu mice. About 5×106 cells were resuspended in 120 μl of solution mixture containing PBS and Matrigel (5:1, BD falcon). Size of tumors were measured weekly, and mice were euthanized when tumors reached 2.0 cm in diameter. The tumor volume were calculated using the equation a×b2×π/6, where “a” and “b” are the longest and shortest diameter, respectively. Tail-vein injection experiments were performed on 4-6-week-old BALB/c nu/nu female mice by injecting 1×106 cells suspended in 100 μl of PBS into the lateral tail veins. Ten weeks later, the lungs were collected and fixed in 4% formalin. All animal experiments were performed with the approval of Shandong University Animal Care and Use Committee.
Trp53 knockout mice model
Trp53fl/fl mice (B6.129P2-Trp53tm1Brn/J) and Ksp1.3-Cre mice (B6.Cg-Tg (Cdh16-cre) 91Igr/J) were purchased from the Jackson Laboratory to generate +/+; Trp53fl/fl (Wild Type) and Ksp1.3-Cre/+; Trp53fl/fl (hereafter referred to as Trp53∆/∆) mice [16]. Exons 2-10 of Trp53 gene, the mouse homologue of human TP53, were flanked by loxP in this conditional targeted knockout. Genotyping of the Trp53 allele and Ksp1.3-Cre was performed by PCR-based test using the following primer pairs for Trp35 (5’-GGTTAAACCCAGCTTGACCA-3’ and 5’-GGAGGCAGAGACAGTTGGAG-3’) and for Ksp1.3-Cre (5’-GCAGATCTGGCTCTCCAAAG-3’ and 5’-AGGCAAATTTTGGTGTACGG-3’) with an internal positive control (5’-CAAATGTTGCTTGTCTGGTG-3’ and GTCAGTCGAGTGCACAGTTT-3’). The female mice were euthanized gradually at the age of 10-80 weeks and FFPE uteri were submitted to immunohistochemical analysis. All mouse experiments were conducted in accordance with the ethical guidelines of the Canton of Zurich.
Statistical analysis
The statistical analysis was carried out using SPSS V20.0. Student’s t-test was applied to analyze the statistical differences. All the experiments were repeated for at least three times. Significance levels were *p<0.05; **p<0.001.
Results
HMGA2 expression in non-neoplastic endometrial tissues
HMGA2 is rarely detected within normal adult tissues, but is expressed by a variety of benign and malignant tumors [7,11,17]. To evaluate whether HMGA2 is overexpressed in non-neoplastic endometrial tissues, we immunohistochemically examined HMGA2 expression in benign endometrium samples (n=28). We found that most of the benign endometrium samples showed negative expression of HMGA2. Only two cases were positive with one focal staining of a single weakly proliferative endometrial gland and one occasional staining (Figure 1A-C) has any biologic meaning.
Figure 1.
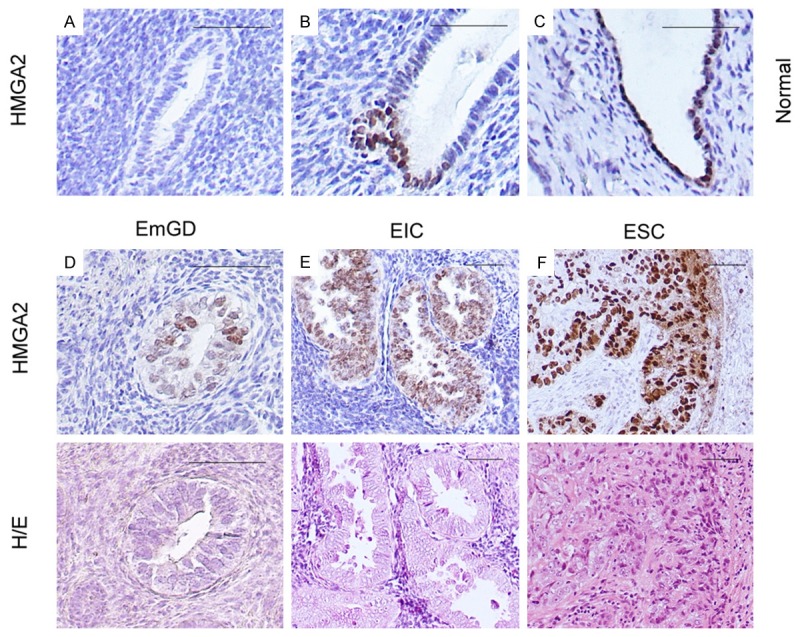
Photomicrographs illustrate some examples of intensity of HMGA2 expression in benign endometrium, endometrial serous carcinomas (ESCs) and their precursor lesions. A-C. HMGA2 expression in cases of benign endometrium. D. Immunohistochemical and hematoxylin and eosin (H/E) staining on serous endometrial glandular dysplasia (EmGD). E. Immunohistochemical and hematoxylin and eosin (H/E) staining on serous endometrial intraepithelial carcinoma (EIC). F. Immunohistochemical and hematoxylin and eosin (H/E) staining on endometrial serous carcinoma (ESC). Scale bars=50 μm.
HMGA2 expression in endometrial cancer and its precursor lesions
We next evaluated HMGA2 expression in endometrial glandular dysplasia (EmGD, n=17), serous endometrial intraepithelial carcinomas (SEIC, n=8), endometrial serous carcinomas (ESC, n=59), endometrial endometrioid carcinomas (EEC, n=61). As shown in Figure 1D-F and Table 1, EmGD and SEIC exhibited a significantly higher rate of HMGA2 expression. Among the 17 cases of EmGD, 1 was diffusely positive (80-100% of the cells), 6 with 50-79% positive cells, 1 with focal staining (25-50% positive cells) and 4 with less than 25% positive cells. In the SEIC category, 7 of 8 were positive with 1 being diffuse, 4 with 50-79%, and 2 with <25% positive cells. Most of these SEICs showed moderate and strong staining intensity. Similar to SEIC, the expression of HMGA2 in ESC was obvious and diffuse. If positivity for HMGA2 is defined as ≥50% staining, irrespective of intensity of staining, it showed an increased level of expression from EmGD (7 of 17, 41.2%) to SEICs (5 of 8, 62.5%) and to ESC (39 of 59, 66.1%). In contrast to type II endometrial carcinomas, EECs exhibited a significantly lower rate of HMGA2 expression. From the 61 EECs, 34 (55.7%) showed complete negative and 9 (14.8%) showed HMGA2 positive in less than 50% endometrial glandular epithelial cells. The positive rate for HMGA2 in EECs was 29.5%. These results suggest that most HMGA2 overexpression was related to the endometrial serous carcinogenesis, rather than the development of EECs.
Table 1.
HMGA2 immunoreactivity in benign endometrium and neoplastic endometrium
| Extent tissue staining | ||||||||||
|
|
||||||||||
| Case/Foci assessed | 80%-100% | 50%-79% | 25%-49% | <25% | 0% | |||||
|
| ||||||||||
| Benign endometrium | 28 | 0 (0%) | 0 (0%) | 0 (0%) | 2 (7.1%) | 26 (92.9%) | ||||
| Precursor and noninvasive lesions | ||||||||||
| Serous EmGD | 17 | 1 (5.9%) | 6 (35.3%) | 1 (5.9%) | 4 (23.5%) | 5 (29.4%) | ||||
| Serous EIC | 8 | 1 (12.5%) | 4 (50%) | 0 (0%) | 2 (25%) | 1 (12.5%) | ||||
| Endometrial cancers | ||||||||||
| Endometrioid | 61 | 6 (9.8%) | 12 (19.7%) | 2 (3.3%) | 7 (11.5%) | 34 (55.7%) | ||||
| Serous | 59 | 16 (27.1%) | 23 (39.0%) | 2 (3.4%) | 11 (18.6%) | 7 (11.9%) | ||||
|
| ||||||||||
| Intensity of the immunoreactivity | ||||||||||
|
|
||||||||||
| Strong | Moderate | Weak | Absent | |||||||
|
| ||||||||||
| Benign endometrium | 28 | 0 (0%) | 1 (3.6%) | 1 (3.6%) | 26 (92.9%) | |||||
| Precursor and noninvasive lesions | ||||||||||
| Serous EmGD | 17 | 2 (11.8%) | 7 (41.2%) | 3 (17.6%) | 5 (29.4%) | |||||
| Serous EIC | 8 | 1 (12.5%) | 5 (62.5%) | 1 (12.5%) | 1 (12.5%) | |||||
| Endometrial cancers | ||||||||||
| Endometrioid | 61 | 0 (0%) | 19 (31.1%) | 8 (13.1%) | 34 (55.7%) | |||||
| Serous | 59 | 15 (25.4%) | 29 (49.2%) | 8 (13.6%) | 7 (11.9%) | |||||
Endometrial cancer cell lines increased proliferation after HMGA2 overexpression in vitro
To investigate whether HMGA2 play an oncogenic role in endometrial carcinoma, we measured HMGA2 baseline expression in six endometrial cancer cell lines by real-time PCR (Figure 2A). Given that HMGA2 was relatively low expression in SPEC-2 cells, which were derived from ESC, we selected the cell line generate HMGA2 overexpressed cells by transfecting HMGA2 overexpression plasmid. Expression efficiency was validated by RT-PCR and Western blot (Figure 2B and 2C). We then measured the effect of HMGA2 on endometrial cancer cells proliferation by clonogenic assay. As shown in Figure 2D, overexpression of HMGA2 significantly promoted colony-forming efficiency of the endometrial cancer cells. The effect of HMGA2 on endometrial cancer cell proliferation was further confirmed by MTT assay. HMGA2 overexpression significantly enhanced endometrial cancer cells growth when compared to the control cells.
Figure 2.
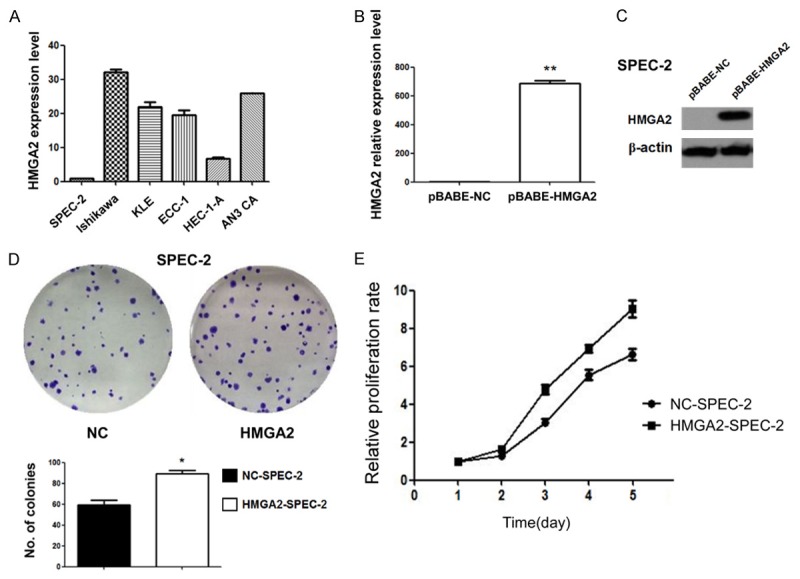
HMGA2 overexpression promote proliferation of endometrial cancer cells in vitro. A. HMGA2 baseline expression in six endometrial cancer cell lines were measured by real-time PCR. B. The overexpression of HMGA2 was validated by qPCR in SPEC-2 cells. C. The overexpression of HMGA2 was validated by Western blot in SPEC-2 cells. D. Clonogenic assay was performed on SPEC-2 cells with HMGA2 overexpression compared to control cells. E. MTT assay was performed on SPEC-2 cells with HMGA2 overexpression compared to control cells.
Endometrial cancer cell increased ability of migration and invasion after HMGA2 overexpression
To investigate whether HMGA2 overexpression promotes migration and invasion of endometrial cancer cells in vitro, we first performed HMGA2 siRNA knockdown experiments. As shown in Figure 3A, HMGA2 repression by siRNAs in ishikawa cells was measured by Western blot. We then examined the expression of EMT markers by Western blot in SPEC-2 cells with and without HMGA2 overexpression. As shown in Figure 3B, HMGA2 overexpression increased the levels of vimentin and β-catenin. We further performed in vitro invasion and migration by transwell assay. As demonstrated in Figure 3C, we observed a significant increase in both migration and invasion in SPEC-2 cells with HMGA2 overexpression. We also performed transwell assay in HMGA2 knockdown ishikawa cells. Compared with the control cells, HMGA2 knockdown cells exhibited reduced migration and invasion (Figure 3D). These results suggested that HMGA2 promotes migration and invasion of endometrial cancer cells through inducing EMT.
Figure 3.
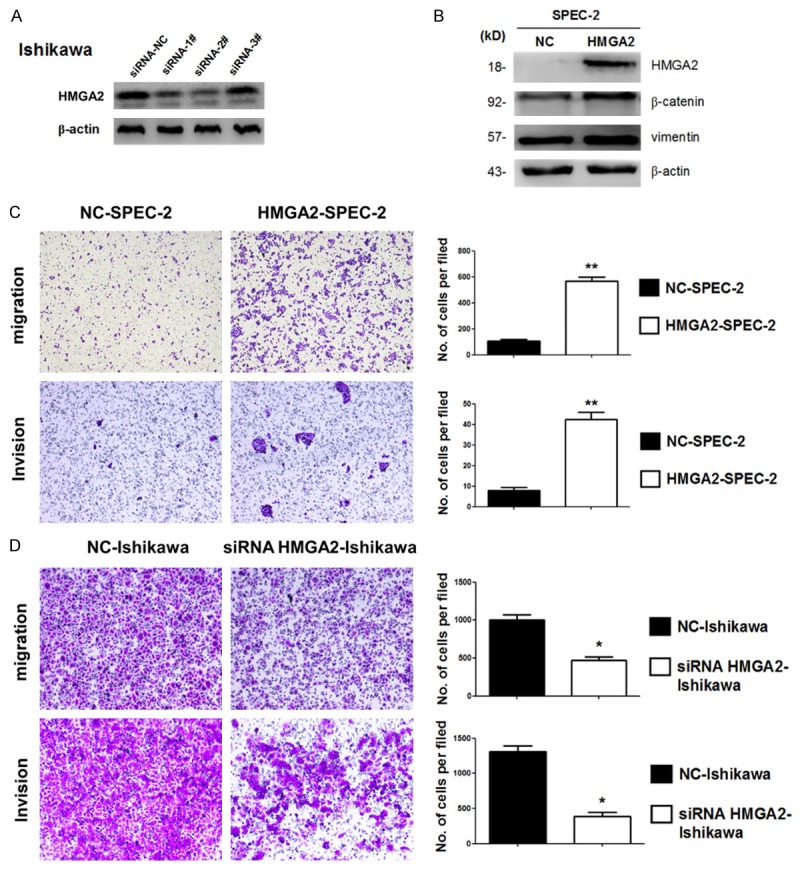
HMGA2 promote migration and invasion of endometrial cancer cells in vitro. A. HMGA2 repression by siRNAs in ishikawa cells was measured by Western blot. B. Western blot analysis confirmed MET related markers showed different expression in SPEC-2 cells with and without HMGA2 overexpression. C. Migration and invasion were measured by transwell assay in SPEC-2 cell lines with and without HMGA2 overexpression. D. Migration and invasion were performed in ishikawa cells with and without HMGA2 depletion by siRNA-2#. *P<0.05, **P<0.01.
HMGA2 promoted tumor growth and metastasis in xenografts
To determine whether HMGA2 could affect tumorigenesis in vivo, SPEC-2 cells with and without HMGA2 overexpression were subcutaneously inoculated into nude mice (n=9 for each group). As demonstrated in Figure 4A, tumor sizes derived from HMGA2 overexpressed cells were bigger than those in the control group. We further performed in vivo metastasis analysis via tail vein injection. SPEC-2 cells with and without HMGA2 overexpression were injected into nude mice via tail vein. After 8 weeks mice were anesthetized and mouse lungs were harvested, fixed and dissected. Five of six mice transplanted with SPEC-2-HMGA2 cells had pulmonary metastasis loci and two of the five had visible metastatic nodules, while two of six mice transplanted with SPEC-2-NC cells had pulmonary metastasis loci and no mouse of the five had visible metastatic nodules (Figure 4B). These results suggest that overexpression of HMGA2 promotes tumor growth and metastasis in vivo.
Figure 4.
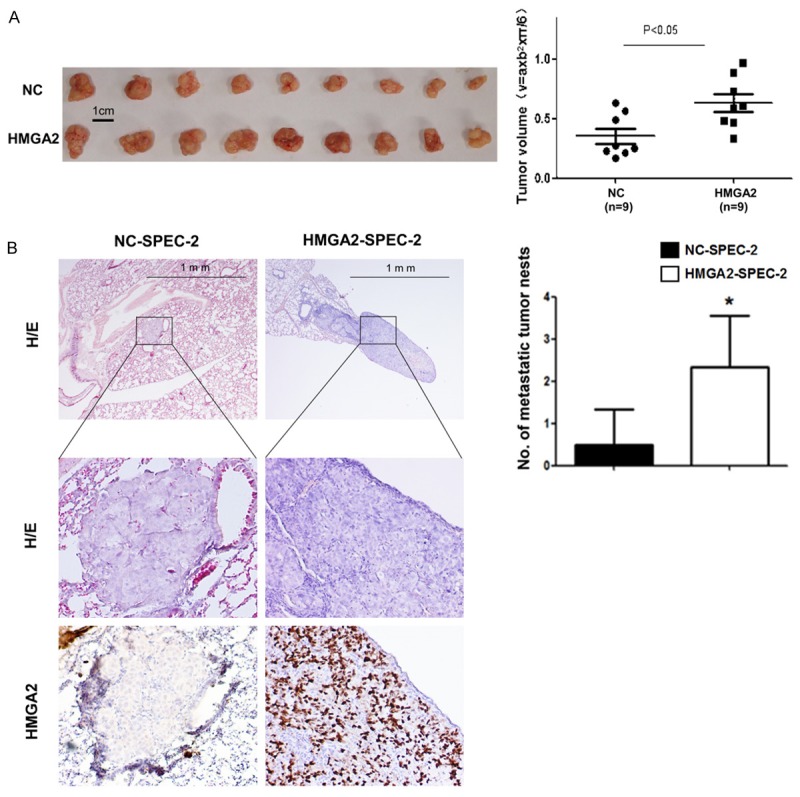
HMGA2 promote proliferation and invasion of endometrial cancer cells in vivo. A. Photographs illustrate representative tumors in xenografts of SPEC-2 cell lines with and without HMGA2 overexpression. B. Hematoxylin and eosin (HE)-staining and IHC of lungs isolated from mice that received tail vein injection of SPEC-2 cell line with and without HMGA2 overexpression (left). Each group contains 6 mice. Plot graph illustrates the numbers of pulmonary metastatic nodules under microscope were counted and analyzed with Student’s t-test (right). All data are shown as mean ± SD. *P<0.05.
HMGA2 expression in conditional Trp53 knockout mouse model of type II endometrial carcinoma
It was reported that mice with endometrium-specific deletion of the Trp53 gene initially exhibited precursor lesions of type II endometrial carcinomas in humans and later developed carcinomas representing all type II subtypes [16]. We established conditional Trp53 knockout mouse model according to this report in our laboratory. We also found tumor formation in mice at around 65-week old and EmGD were found at around 30-week old consistent with Wild’s report [16]. We then examined HMGA2 expression by IHC staining in mice and found HMGA2 first appeared expression in 34 week-old mice (Figure 5C). HMGA2 expression was also found in EmGD (Figure 5D), EIC (Figure 5E) and endometrial carcinomas (Figure 5F) in mice. These results suggest that a gain of HMGA2 expression is a very early event in endometrial serous carcinomas.
Figure 5.
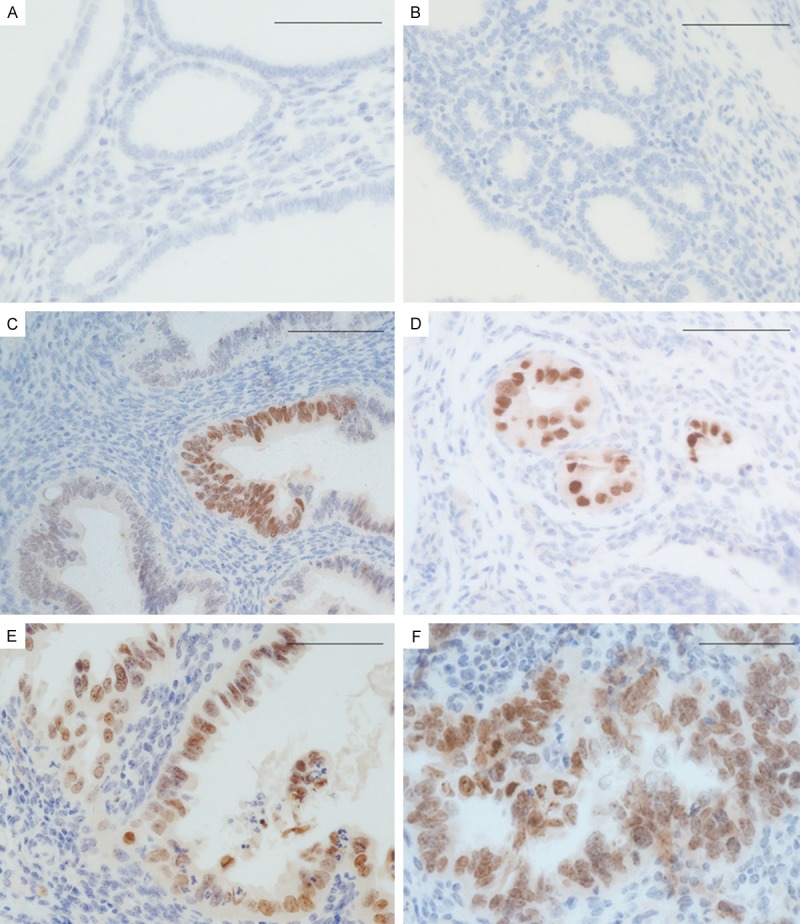
Photomicrographs illustrate some examples of HMGA2 expression in conditional Trp53 knockout mouse model of type II endometrial carcinoma. A. HMGA2 expression in benign endometrium of wild type mice. B and C. HMGA2 expression in benign endometrium of conditional Trp53 knockout mice. D. HMGA2 expression in serous endometrial glandular dysplasia (EmGD). E. serous endometrial intraepithelial carcinoma (EIC) and F, endometrial serous carcinoma (ESC) of conditional Trp53 knockout mice. Scale bars=50 μm.
Discussion
HMGA2 is abundantly expressed during embryogenesis, but it is either undetectable or its expression remains at low levels in normal adult tissues [18]. Interestingly, HMGA2 is highly expressed in most malignant epithelial tumors [19]. Overexpression of HMGA2 is found to be an early-stage biomarker in High-grade serous ovarian carcinoma [13]. In this study, we have demonstrated that HMGA2 expression was detected occasionally in benign endometrium samples (2 of 28 samples). HMGA2-positive normal appearing endometrial epithelia may be an early event in the pathogenesis of endometrial serous carcinoma. Importantly, overexpression of HMGA2 showed an increased pattern from serous EmGDs (7 of 17, 41.2%) to serous EICs (5 of 8, 62.5%) and to ESCs (39 of 59, 66.1%). HMGA2 positivity in EmGDs and EICs suggest that it is implicated in the early development of endometrial serous carcinoma.
Our results are concordant a previous study [14], which indicated that HMGA2 was overexpressed in more than 50% of endometrial serous carcinomas. HMGA2 may play an oncogenic role in tumorigenesis and progression of endometrial serous carcinoma. The oncogenic properties of HMGA2 are reported to be involved in tumor transformation [20], tumor growth [21] and tumor metastasis [22]. We investigated the oncogenic properties of HMGA2 in endometrial cancer cells through in vitro and in vivo study. We found overexpression of HMGA2 in endometrial cancer cells significantly promoted proliferation, migration and invasion in vitro and enhanced tumor growth and metastasis in vivo. Importantly, HMGA2 induced epithelial-mesenchymal transition (EMT) through modulation vimentin and β-catenin.
HMGA2 overexpression was reported to associate with p53-dominant mutations in high-grade ovarian serous carcinoma [12]. Our previous study showed alteration of p53 is an early event in the development of endometrial serous carcinoma [23]. In Trp53 knockout mouse model, HMGA2 become overexpression even from morphologically benign endometrium. HMGA2 may be another useful early-stage diagnostic marker of ESCs. Future studies are needed to elucidate the relationship and mechanism between P53 and HMGA2.
In summary, our results in this study suggest that HMGA2 overexpression is closely associated with endometrial serous cancer. HMGA2 may serve as a useful diagnostic marker in the assessment of endometrial cancers and their precursor lesions. We further demonstrated that HMGA2 overexpression is closely associated with tumor growth and metastasis. Importantly HMGA2 induced epithelial-mesenchymal transition (EMT) of endometrial cancer cells. Future studies are needed to assess the correlations between HMGA2 and clinicopathologic features and prognosis.
Acknowledgements
This study was partially supported by awards from National 863 Program (2014AA020605), and The National Natural Science Foundation of China (81472432, 81272857, 81171897).
Disclosure of conflict of interest
None.
References
- 1.Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. Lancet Oncol. 2014;15:e268–278. doi: 10.1016/S1470-2045(13)70591-6. [DOI] [PubMed] [Google Scholar]
- 2.Zheng W, Xiang L, Fadare O, Kong B. A proposed model for endometrial serous carcinogenesis. Am J Surg Pathol. 2011;35:e1–e14. doi: 10.1097/PAS.0b013e318202772e. [DOI] [PubMed] [Google Scholar]
- 3.Fader AN, Santin AD, Gehrig PA. Early stage uterine serous carcinoma: management updates and genomic advances. Gynecol Oncol. 2013;129:244–250. doi: 10.1016/j.ygyno.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Wu J, Wei JJ. HMGA2 and high-grade serous ovarian carcinoma. J Mol Med (Berl) 2013;91:1155–1165. doi: 10.1007/s00109-013-1055-8. [DOI] [PubMed] [Google Scholar]
- 5.Hristov AC, Cope L, Reyes MD, Singh M, Iacobuzio-Donahue C, Maitra A, Resar LM. HMGA2 protein expression correlates with lymph node metastasis and increased tumor grade in pancreatic ductal adenocarcinoma. Mod Pathol. 2009;22:43–49. doi: 10.1038/modpathol.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belge G, Meyer A, Klemke M, Burchardt K, Stern C, Wosniok W, Loeschke S, Bullerdiek J. Upregulation of HMGA2 in thyroid carcinomas: a novel molecular marker to distinguish between benign and malignant follicular neoplasias. Genes Chromosomes Cancer. 2008;47:56–63. doi: 10.1002/gcc.20505. [DOI] [PubMed] [Google Scholar]
- 7.Sarhadi VK, Wikman H, Salmenkivi K, Kuosma E, Sioris T, Salo J, Karjalainen A, Knuutila S, Anttila S. Increased expression of high mobility group A proteins in lung cancer. J Pathol. 2006;209:206–212. doi: 10.1002/path.1960. [DOI] [PubMed] [Google Scholar]
- 8.Malek A, Bakhidze E, Noske A, Sers C, Aigner A, Schafer R, Tchernitsa O. HMGA2 gene is a promising target for ovarian cancer silencing therapy. Int J Cancer. 2008;123:348–356. doi: 10.1002/ijc.23491. [DOI] [PubMed] [Google Scholar]
- 9.Galdiero F, Romano A, Pasquinelli R, Pignata S, Greggi S, Vuttariello E, Bello AM, Calise C, Scaffa C, Pisano C, Losito NS, Fusco A, Califano D, Chiappetta G. Detection of high mobility group A2 specific mRNA in the plasma of patients affected by epithelial ovarian cancer. Oncotarget. 2015;6:19328–19335. doi: 10.18632/oncotarget.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu J, Liu Z, Shao C, Gong Y, Hernando E, Lee P, Narita M, Muller W, Liu J, Wei JJ. HMGA2 overexpression-induced ovarian surface epithelial transformation is mediated through regulation of EMT genes. Cancer Res. 2011;71:349–359. doi: 10.1158/0008-5472.CAN-10-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishino J, Kim I, Chada K, Morrison SJ. Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16Ink4a and p19Arf Expression. Cell. 2008;135:227–239. doi: 10.1016/j.cell.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahajan A, Liu Z, Gellert L, Zou X, Yang G, Lee P, Yang X, Wei JJ. HMGA2: a biomarker significantly overexpressed in high-grade ovarian serous carcinoma. Mod Pathol. 2010;23:673–681. doi: 10.1038/modpathol.2010.49. [DOI] [PubMed] [Google Scholar]
- 13.Wei JJ, Wu J, Luan C, Yeldandi A, Lee P, Keh P, Liu J. HMGA2: a potential biomarker complement to P53 for detection of early-stage high-grade papillary serous carcinoma in fallopian tubes. Am J Surg Pathol. 2010;34:18–26. doi: 10.1097/PAS.0b013e3181be5d72. [DOI] [PubMed] [Google Scholar]
- 14.Romero-Perez L, Castilla MA, Lopez-Garcia MA, Diaz-Martin J, Biscuola M, Ramiro-Fuentes S, Oliva E, Matias-Guiu X, Prat J, Cano A, Moreno-Bueno G, Palacios J. Molecular events in endometrial carcinosarcomas and the role of high mobility group AT-hook 2 in endometrial carcinogenesis. Hum Pathol. 2013;44:244–254. doi: 10.1016/j.humpath.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Fadare O, Zheng W. Endometrial Glandular Dysplasia (EmGD): morphologically and biologically distinctive putative precursor lesions of Type II endometrial cancers. Diagn Pathol. 2008;3:6. doi: 10.1186/1746-1596-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wild PJ, Ikenberg K, Fuchs TJ, Rechsteiner M, Georgiev S, Fankhauser N, Noske A, Roessle M, Caduff R, Dellas A, Fink D, Moch H, Krek W, Frew IJ. p53 suppresses type II endometrial carcinomas in mice and governs endometrial tumour aggressiveness in humans. EMBO Mol Med. 2012;4:808–824. doi: 10.1002/emmm.201101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter DS, Klotzbucher M, Kugoh H, Cai SL, Mullen JP, Manfioletti G, Fuhrman U, Walker CL. Aberrant expression of HMGA2 in uterine leiomyoma associated with loss of TSC2 tumor suppressor gene function. Cancer Res. 2002;62:3766–3772. [PubMed] [Google Scholar]
- 18.Rogalla P, Drechsler K, Frey G, Hennig Y, Helmke B, Bonk U, Bullerdiek J. HMGI-C expression patterns in human tissues. Implications for the genesis of frequent mesenchymal tumors. Am J Pathol. 1996;149:775–779. [PMC free article] [PubMed] [Google Scholar]
- 19.Sun M, Song CX, Huang H, Frankenberger CA, Sankarasharma D, Gomes S, Chen P, Chen J, Chada KK, He C, Rosner MR. HMGA2/TET1/HOXA9 signaling pathway regulates breast cancer growth and metastasis. Proc Natl Acad Sci U S A. 2013;110:9920–9925. doi: 10.1073/pnas.1305172110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayr C, Hemann MT, Bartel DP. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science. 2007;315:1576–1579. doi: 10.1126/science.1137999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Liu X, Li AY, Chen L, Lai L, Lin HH, Hu S, Yao L, Peng J, Loera S, Xue L, Zhou B, Zhou L, Zheng S, Chu P, Zhang S, Ann DK, Yen Y. Overexpression of HMGA2 promotes metastasis and impacts survival of colorectal cancers. Clin Cancer Res. 2011;17:2570–2580. doi: 10.1158/1078-0432.CCR-10-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, Huang Y, Hu X, Su F, Lieberman J, Song E. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Liang SX, Jia L, Chen N, Fadare O, Schwartz PE, Kong B, Zheng W. Molecular identification of “latent precancers” for endometrial serous carcinoma in benign-appearing endometrium. Am J Pathol. 2009;174:2000–2006. doi: 10.2353/ajpath.2009.081085. [DOI] [PMC free article] [PubMed] [Google Scholar]


