Abstract
S100A4 represents an important member of the S100 family of small calcium-binding proteins. Increased expression of S100A4 has been observed in chronic inflammatory and autoimmune diseases, such as idiopathic inflammatory myopathies. The majority of studies of S100A4 are focused on cancer research; however, the oncogenic roles of S100A4 in epithelial ovarian cancer (EOC) remain largely unexplored. In this study, S100A4 expression is significantly up-regulated in ovarian cancer and associated with the clinical stage of EOC patients. Attenuation of S100A4 expression results in decreased cell mobility and metastatic capacity, whereas overexpression of S100A4 enhanced the invasive ability of EOC cells. Then by an integrated informatics analysis and luciferase reporter assay, we identify that miR-296 is a critical upstream regulator of S100A4. In addition, deregulated miR-296/S100A4 axis facilitates epithelial-mesenchymal transition (EMT) process as demonstrated by altered expression of EMT-related markers. In conclusion, our study reveals that deregulated miR-296/S100A4 promotes tumor progression in EOC, and provides evidence that miR-296/S100A4 axis-related signaling may represent a potential target for EOC therapy.
Keywords: S100A4, miR-296, epithelial ovarian cancer, epithelial mesenchymal transition
Introduction
Epithelial ovarian cancer (EOC) is the leading cause of gynecologic cancer-related death worldwide [1,2]. The 5-year survival ratio for all stages of EOC has been estimated to be 45.6% [3]. Specifically, the 5-year survival rate in patients diagnosed at early stage is more than 70%, and this ratio declines to 35% in patients at advanced stage. Unfortunately, only 19% of EOC are diagnosed at the early stage [4]. The highly aggressive phenotypes of EOC cells are critically involved in this poor prognosis. Therefore, elucidation of the molecular mechanisms underlying EOC progression, and discovery of valuable predictive biomarkers are essential for developing efficient therapies [5-7].
S100A4, also known as metastasis-associated protein MTS1, is a small calcium-binding protein belong to the S100 protein family, which characterized by the two EF-hand Ca2+ binding motifs [8,9]. Recently, it has been demonstrated that S100A4 is critically implicated in the development and progression of fibrosis in many organs [10-12], as well as chronic inflammatory and autoimmune diseases, including muscle tissue from patients with idiopathic inflammatory myopathies [13-15]. S100A4 is frequently up-regulated in multiple human cancers, including gastrointestinal cancers [16-20], breast cancer [21], prostate cancer [22] and lung cancer [23], and contributes to the oncogenic transformation, angiogenesis, mobility and metastasis of tumor cells. In EOC, nuclear S100A4 expression is higher in solid tumors than that in effusions, and is associated with more aggressive clinical disease in primary carcinoma [24]. Treatment with recombinant S100A4 resulted in enhancement of invasiveness, which was associated with the up-regulation of small GTPase RhoA [25]. However, the reasons for upregulated S100A4 are poorly known and the effects of endogeneous S100A4 remain largely unexplored in EOC.
Epithelial mesenchymal transition (EMT) is biological process that epithelial cell acquires the properties of mesenchymal cell, thereby promoting the invasive capacity of tumor cells. S100A4 is also an EMT-related maker and plays an important role in EMT. For example, S100A4 is involved in EMT mediated by the sonic hedgehog-Gli1 signaling pathway in pancreatic cancer [26]. And S100A4 participates in EMT in breast cancer via targeting MMP2 [27]. However, whether S100A4-mediated EMT is involved in the progression EOC remains unknown.
In current study, we firstly determine the expression pattern of S100A4 in EOC and find that S100A4 is significantly upregulated in EOC tissues and positively associated with clinical TNM stage. By loss and gain function study, we show that S100A4 promotes cell mobility and metastatic capacity of EOC cells. Furthermore, an integrated analysis identifies miR-296 as a critical upstream regulator of S100A4. Mechanistically, miR-296/S100A4 axis facilitates EMT by altering the expression of EMT-related molecules, including E-cadherin, Vimentin, N-cadherin, Snail1 and MMP9.
Materials and methods
Cell culture and EOC tissues
Human EOC cells (SK-OV-3, HO8910, HO8910-PM, OVCAR-3) were all obtained from Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The Caov-3 was purchased from American Type Culture Collection (ATCC). All cells were cultured in DMEM (Invitrogen, USA) supplemented with 10% fetal bovine serum (Gibco, USA) and 1% antibiotics (penicillin and streptomycin) at 37°C and 5% CO2 in a humidified atmosphere. A tissue microarray (OV809) containing seventy cases of EOC tissues and ten cases of normal ovarian tissues were purchased from Xi’an Alenabio Inc (Xi’an, China).
Immunohistochemical staining
The section of EOC tissue microarray was deparaffinized in xylene and boiled in 0.01 M citrate buffer (pH 6.0) for 15 min in a microwave oven. Then the slide was treated with 0.3% hydrogen peroxide to neutralize endogenous peroxidase and incubated with 10% BSA (Sangon, Shanghai, China) at room temperature for 1 h. After washing with phosphate-buffered saline (PBS), the section was incubated with an anti-S100A4 primary antibody (Novus Biologicals, USA, 1:200) at 4°C overnight, followed by incubation with a second antibody labeled by HRP (rabbit) at room temperature for 1 h. Finally, the immunoreactivity were visualized with 3,3’-diaminobenzidine tetrahydrochloride and counterstained was done with hematoxylin. The staining score of tumor cells were estimated based on the percent of positive cells and staining intensity. The percent of positive cells was classified as: 0-5% positive cells scored 0; 5-30% positive cells scored 1; 30-50% positive cells scored 2 and more than 50% scored to 3. The staining intensity was revealed as follows: no staining scored 0, weakly staining scored 1, moderately staining scored 2 and strongly staining scored 3. The final score was calculated as the percent of positive cell score × staining intensity score. The evaluation of immunostaining was carried out independently by two independent observers, who are unaware of the information of the patient or the tissue site.
Total RNA extraction and real-time PCR
Total RNA was extracted using TRIzol reagent (TaKaRa, Biotech Co., Ltd, Dalian, China) and RNeasy protect mini kit (Qiagen, Hilden, Germany). Complementary DNA (cDNA) was synthesized using a first strand cDNA synthesis kit (TaKaRa, Dalian, China). Real-time PCR was performed with SYBR Premix Ex TaqTM (TaKaRa, Biotech Co., Ltd, Dalian, China). PCR primers used in this study were listed in Table 1. Each sample was run in triplicate. Relative expression was standardized using the quantity of β-actin and data were analyzed according to the 2-ΔΔCt formula.
Table 1.
Primers used in this study
| Gene | Sequence | |
|---|---|---|
| S100A4 | Forward | GATGAGCAACTTGGACAGCAA |
| Reverse | CTGGGCTGCTTATCTGGGAAG | |
| E-cadherin | Forward | ATTTTTCCCTCGACACCCGAT |
| Reverse | TCCCAGGCGTAGACCAAGA | |
| Vimentin | Forward | AGTCCACTGAGTACCGGAGAC |
| Reverse | CATTTCACGCATCTGGCGTTC | |
| E-cadherin | Forward | AGCCAACCTTAACTGAGGAGT |
| Reverse | GGCAAGTTGATTGGAGGGATG | |
| Snail1 | Forward | TCGGAAGCCTAACTACAGCGA |
| Reverse | AGATGAGCATTGGCAGCGAG | |
| MMP9 | Forward | GGGACGCAGACATCGTCATC |
| Reverse | TCGTCATCGTCGAAATGGGC | |
| β-actin | Forward | CATGTACGTTGCTATCCAGGC |
| Reverse | CTCCTTAATGTCACGCACGAT | |
Small interfering RNA, plasmid construction and transfection with miRNA
The small interfering RNA (siRNA) sequences targeting S100A4 mRNA (GenePharma, Shanghai, China) were transfected into EOC cells for silencing. The nontargeting siRNA was used as a negative control. The transfection was conducted with Lipofectamine 2000 (Invitrogen, USA) according to manufacturer’s instructions. The medium was replaced with complete medium after 8 h of transfection. After incubating for 48 h, cells were plated for invasion and migration assays or harvested for Western blotting. The coding sequences of S100A4 were cloned into pcDNA3.1 (+) to generate S100A4 expression vector and verified by sequencing. The knockdown or overexpression efficacy was demonstrated by Western blotting. MiR-149, miR-296, miR-505 and scrambled control oligos were purchased from Invitrogen (Invitrogen, USA). Transfection was performed following the manufacturer’s instructions with the mimic concentration at 50 nM. Cells were incubated in the medium containing the transfection mixture for 48 h before each experiment.
Luciferase reporter assays
The predicted 3’-UTR segment of S100A4 was inserted into the pmirGLO Dual-Luciferase miRNA target expression vector (Promega, Cat. E1330, USA). And the humanized Renilla luciferase-neomycin resistance cassette (hRluc-neo) was used as a control reporter for normalization. Cells were seeded to reach 90% confluence and transiently transfected with the reporter plasmid. After 48 h, the cells were harvested and lysed, and luciferase activity was analyzed by the Dual-Glo® Luciferase Assay System (Promega, Cat. E2920, USA) using a multifunctional microplate reader. The experiments were performed independently in five replicates.
Cell migration and invasion assay
The effects of siRNA-mediated S100A4-knockdown and miR-296 on the migration and invasion of EOC cells were detected by transwell model (Corning, NY, USA). In brief, for cell migration assay, 8000 cells in 100 μl serum-free medium were seeded into the upper compartment of the transwell inserts. The lower compartment of the chamber was supplemented with 10% fetal bovine serum as a chemo-attractant. After incubation for 24 h, cells on the upper compartment were scrubbed and washed with PBS. The invaded cell on the membrane were fixed with methanol and stained with 0.1% crystal violet. For invasion assay, the procedures were the same to migration assay apart from the chamber was replaced with matrigel-coated filters (BD Bioscience, USA). Cell invasive ability was quantified by counting the stained cell number in six individual fields by microscopy. All experiments were performed in triplicate.
Statistical analysis
All experiments were performed in triplicate and data were presented as the means ± SD. All statistical analyses were performed using the SPSS 16.0 software and graphical representations were performed with GraphPad Prism 5 (San Diego, CA) software. The Student’s t-test was used to determine differences between two groups, and one-way ANOVA was used to analyze the differences among multiple groups. P < 0.05 was considered to be statistically significant.
Results
S100A4 expression is significantly upregulated in EOC and associated with clinical stage of EOC patients
Expression of S100A4 in EOC was firstly examined in Oncomine datasets. Data from eight individual datasets showed that S100A4 was frequently expressed in ovarian cancer, which was significantly different from that in normal ovarian epithelial (OE) tissues (Figure 1A). Next, we examined the expression of S100A4 in a tissue microarray by immunohistochemical staining. As shown in Figure 1B, elevated protein expression of S100A4 was also observed in EOC tissues. Indeed, S100A4 expression was positively associated with advanced TNM stages of EOC patients. And consistent with this, the S100A4 mRNA expression in EOC cell lines was also higher than that in OE tissues (Figure 1C), indicating an underlying oncogenic role of S100A4 in the progression of EOC.
Figure 1.
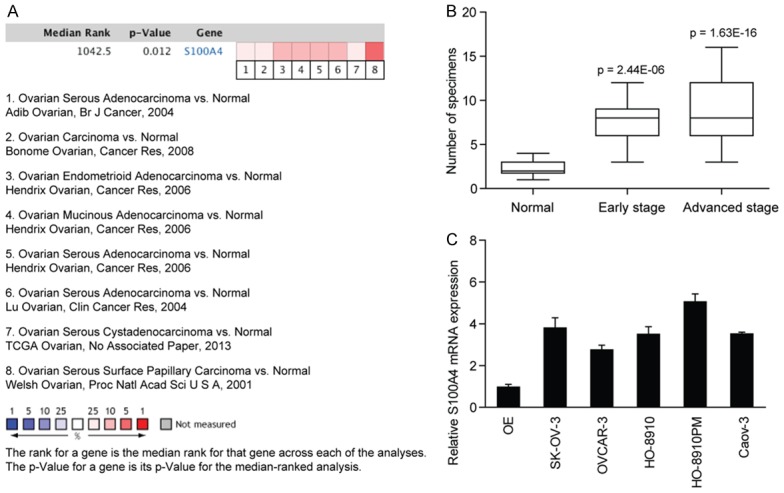
S100A4 expression is significantly up-regulated in ovarian cancer and associated with clinical stage of EOC patients. A. Analysis of Oncomine datasets demonstrated a statistically significant increase in S100A4 expression in EOC tissues compared with the normal ovarian tissues. B. The protein expression of S100A4 in an EOC tissue microarray containing 10 normal ovarian tissues, 15 EOC specimens at early TNM stage and 55 EOC specimens at advanced TNM stage. The p value was calculated to determine the difference in S100A4 expression between normal ovarian tissues and EOC specimens at early or advanced stages. C. The mRNA expression of S100A4 in EOC cell lines was detected by real-time PCR.
S100A4 promotes cell migration and invasion in ovarian cancer cells in vitro
To determine the underlying cellular functions of S100A4 in EOC, siRNA-mediated S100A4 knockdown was performed. Western blotting demonstrated that S100A4 expression was markedly decreased in SK-OV-3 and HO-8910PM cells after targeted siRNA treatment (Figure 2A). By transwell model, we found that suppression of S100A4 expression significantly inhibited cell migration (Figure 2B) and invasion (Figure 2C) ability of EOC cells.
Figure 2.
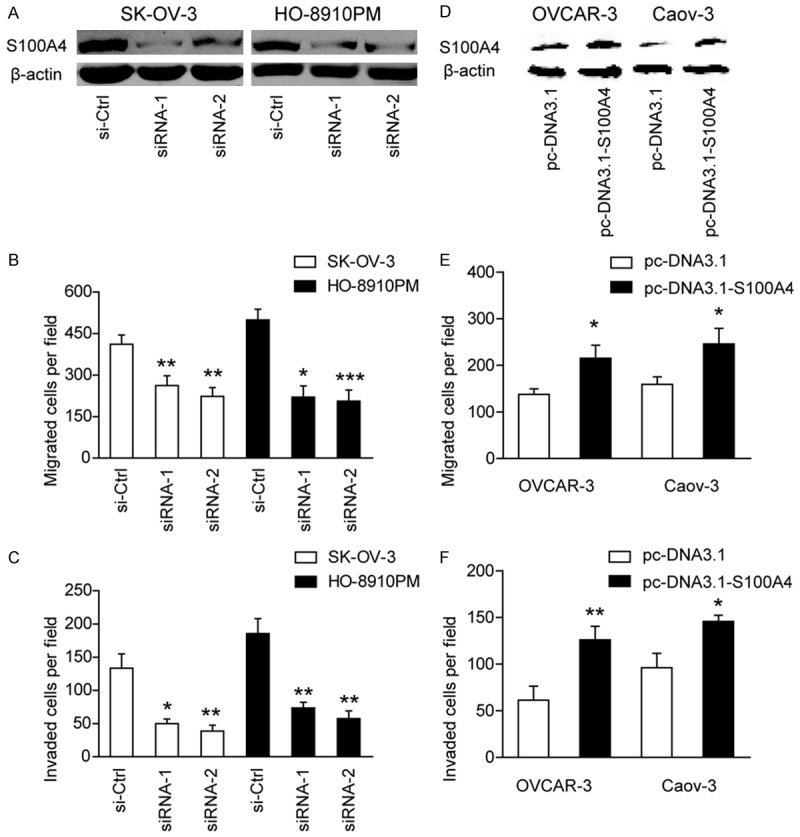
S100A4 promotes cell migration and invasion in ovarian cancer cells in vitro. (A) The interfere efficacy of S100A4 in SK-OV-3 and HO-8910PM cells was demonstrated by Western blotting. (B) The influence of S100A4 knockdown on cell migration (B) and cell invasion (C) of SK-OV-3 and HO-8910PM cells. si-Ctrl versus siRNA-1 or siRNA-2, *P < 0.05, **P < 0.01, ***P < 0.001. (D) The overexpression efficacy of S100A4 in OVCAR-3 and CaoV-3 cells was demonstrated by Western blotting. The influence of S100A4 overexpression on cell migration (E) and cell invasion (F) was measured in OVCAR-3 and CaoV-3 cells. pc-DNA3.1 versus pc-DNA3.1-S100A4, *P < 0.05, **P < 0.01.
Furthermore, we overexpressed S100A4 expression in two cell lines (OVCAR-3 and Caov-3) with lower S100A4 expression (Figure 2D). Expectedly, overexpression of S100A4 significantly promoted the invasive ability of OVCAR-3 and Caov-3 cells as demonstrated by cell migration (Figure 2E) and invasion (Figure 2F) assay, respectively. Taken together, these results indicated that upregulated S100A4 promoted the invasive potential of EOC cells and this prerequisite further facilitated tumor progression.
miR-296 is a critical upstream regulator of S100A4
To identify the potential upstream miRNA regulator of S100A4, two miRNA target prediction programs, TargetScan and Miranda, were used. As a result, three candidate miRNAs (miR-149, miR-296 and miR-505) were selected for next investigation. By treatment with mimics of those three miRNAs, we found miR-296 but not miR-149 or miR-505 can markedly down-regulate S100A4 mRNA expression (Figure 3A). Consistently, the protein level of S100A4 in SK-OV-3 and HO-8910PM cells was also reduced by miR-296, indicating miR-296 might be an upstream regulator of S100A4 (Figure 3B). To further confirm this finding, we then cloned a sequence containing the predicted 3’-UTR target site of S100A4 mRNA and its mutated sequence into the pGL3 luciferase reporter gene to generate wild type and mutation vector, respectively. The result showed that luciferase activity in wild type SK-OV-3 and HO-8910PM cells co-transfected with miR-296 was significantly decreased compared with the control (Figure 3C), whereas no significant difference was found in mutant type. Next, cell migration and invasion assays were performed to determine whether miR-296 is the functional mediator of S100A4. Consistent with the phenomenon observed in siRNA-mediated S100A4 knockdown, cell migration and invasion ability of EOC cells was also reduced by miR-296 (Figure 3D). Collectively, these findings suggested that miR-296 was a critical upstream regulator of S100A4.
Figure 3.
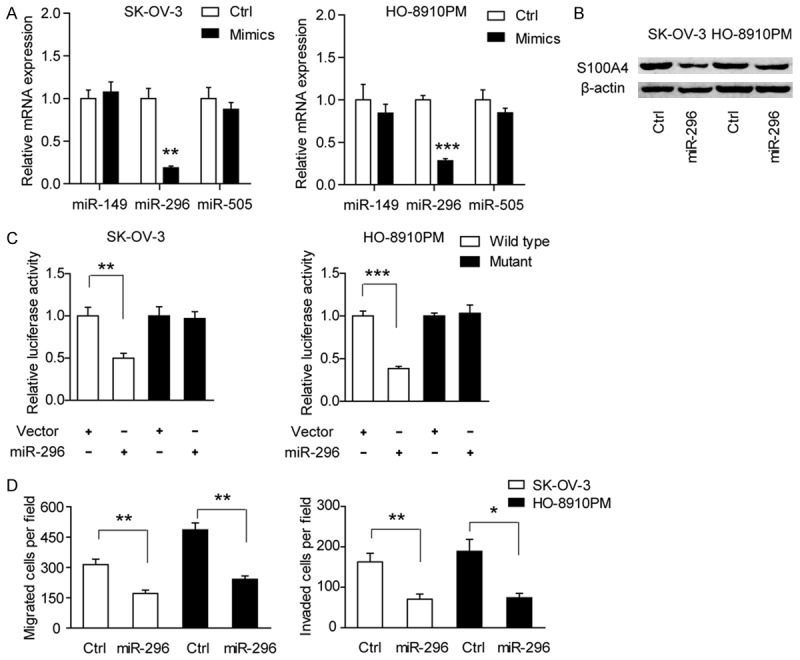
miR-296 is a critical upstream regulator of S100A4. A. The mRNA expression of S100A4 was detected in the presence of miR-149, miR-296 and miR-505 mimics. B. The protein expression of S100A4 was detected in the presence of miR-296 mimics in SK-OV-3 and HO-8910PM cells. C. Luciferase reporter assay demonstrated the direct interaction between miR-296 and S100A4 3’UTR in SK-OV-3 and HO-8910PM cells. The data are shown as the means ± S.D. of three replicates (vector versus miR-296, **P < 0.01, ***P < 0.001). D. The influence of miR-296 mimics on cell migration and cell invasion of SK-OV-3 and HO-8910PM cells. Ctrl versus miR-296, *P < 0.05, **P < 0.01.
miR-296/S100A4 axis is a novel regulator of EMT in ovarian cancer
The process of epithelial-mesenchymal transition (EMT) is a key step in tumor metastasis by which epithelial cells lose their cell polarity and cell-cell adhesion, and further gain invasive properties to become mesenchymal cells. However, whether the oncogenic functions of the miR-296/S100A4 axis were dependent on EMT in EOC remain unclear. Here, we analyzed the effects of S100A4 silencing on the gene expression profile of EMT markers, including E-cadherin, Vimentin, N-cadherin, Snail1 and MMP9 in SK-OV-3 and HO-8910PM cells. The result revealed that E-cadherin, the epithelial marker, was significantly up-regulated by silencing of S100A4. Meanwhile, the mesenchymal markers, including Vimentin, N-cadherin and Snail1, were down-regulated by silencing of S100A4 (Figure 4A, 4B). Furthermore, the alternations of these markers at protein level were detected by miR-296 treatment. Expectedly, miR-296 reversed the EMT phenotype of SK-OV-3 and HO-8910PM cells by up-regulating the epithelial marker and down-regulating the mesenchymal markers (Figure 4C, 4D). And as shown in Figure 5A, restoration of S100A4 expression completely recovered the altered expression of E-cadherin and N-cadherin induced by miR-296. And expectedly, ectopic expression of S100A4 rescued the suppressive roles of miR-296 on cell migration (Figure 5B) and invasion (Figure 5C) in both SK-OV-3 and HO-8910PM cells. Taken together, these data above suggested that miR-296/S100A4 axis was a novel regulator of EMT in EOC.
Figure 4.
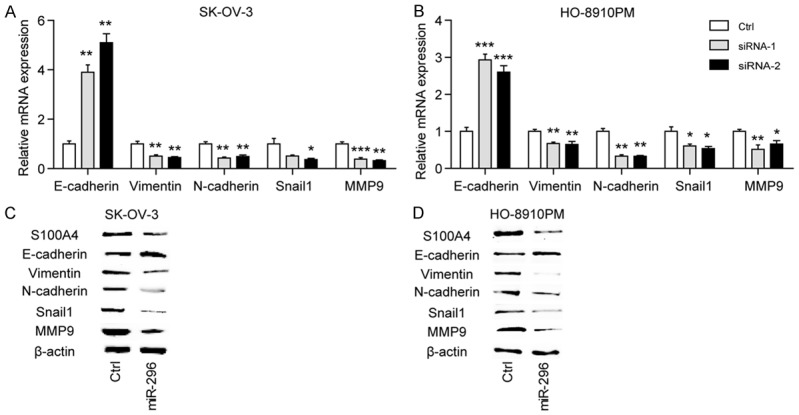
miR-296/S100A4 axis is a novel regulator of EMT in ovarian cancer. The influence of S100A4 knockdown on the mRNA expression of E-cadherin, Vimentin, N-cadherin, Snail1 and MMP9 in SK-OV-3 (A) and HO-8910PM cells (B). si-Ctrl versus siRNA-1 or siRNA-2, *P < 0.05, **P < 0.01, ***P < 0.001. Western blotting analysis of the effects of miR-296 mimics on the expression of S100A4, E-cadherin, Vimentin, N-cadherin, Snail1 and MMP9 in SK-OV-3 (C) and HO-8910PM cells (D).
Figure 5.
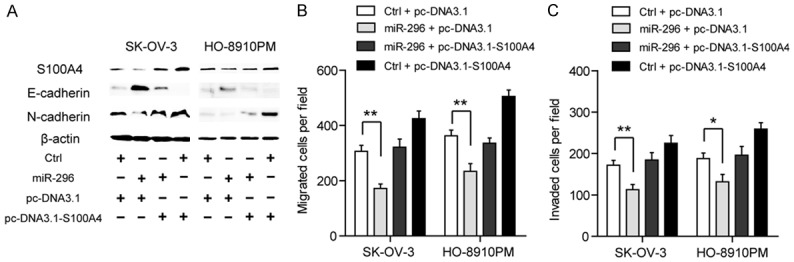
Restoration of S100A4 blocks the role of miR-296 in EMT, cell migration and invasion. (A) The protein expression of E-cadherin and N-cadherin was detected in the presence of miR-149 and restoration of S100A4. Restoration of S100A4 abolished the inhibitory effects of miR-296 on cell migration (B) and cell invasion (C) in SK-OV-3 and HO-8910PM cells; *P < 0.05, **P < 0.01.
Discussion
It has been well established that S100A4 is associated with a variety of human malignancies. In the present study, we show that S100A4 is also involved in the metastatic progression of EOC. Previously, Horiuchi A et al. reported that hypoxia-induced hypomethylation is associated with up-regulation of S100A4 expression in ovarian carcinoma cell lines and tissues [28]. And up-regulated nuclear S100A4 expression in primary carcinomas is correlated with advanced clinical stage and poor response to chemotherapy [25]. Consistent with these findings, we also demonstrate that increased expression of S100A4 in EOC tissues and cell lines compared with the normal counterparts at both mRNA and protein level. Due to limited follow-ups of the commercial tissue microarray, however, we fail to analyze prognostic value of S100A4 based on its nuclear and cytoplasmic expression in the EOC tissues. MicroRNAs (miRNAs) are short non-coding RNAs that usually 21-25 nucleotides in length, which function by binding to the 3’-UTRs of targeted mRNA and lead to the repression of translation or degradation of the coding transcript [29]. Recently, growing evidences suggest that miRNAs play a functional role in regulating development and progression in ovarian cancer [30,31]. Here we show for the first time that miR-296 is a critical modulator of S100A4 expression.
The role of S100A4 in EOC has not yet been well studied. To gain insight into S100A4-mediated oncogenic functions, we silence the endogeneous expression of S100A4 by siRNAs. Consistent with the results in laryngeal cancer [32] and gastric cancer [33], attenuation of S100A4 compromises the metastatic potential of EOC cells. And overexpression of S100A4 in S100A4 low-expressing cells enhances their metastatic capacity.
In line with this, S100A4 overexpression is associated with invasion and metastasis of papillary thyroidcarcinoma [34], and progression of colorectal cancer [35]. As miR-296 contributes to up-regulated S100A4 expression, we further determine whether miR-296 is the functional mediator of S100A4 in EOC. Indeed, induction of miR-296 markedly decreases S100A4 expression and invasive ability of EOC cells. MiR-296 functions as a tumor suppressor in prostate cancer by directly targeting Pin1 [36], and regulates cell growth and multi-drug resistance of human glioblastoma by targeting EAG1 [37]. Therefore, we cannot exclude the potential pro-metastatic roles of other targets of miR-296 in this study.
It was documented that EMT plays an important role in the migratory and invasive ability of tumor cells. And accumulating evidences have indicated that S100A4 is critically involved in the process of EMT. In esophageal cancer, S100A4 could regulate motility and invasiveness of cancer cells through modulating the AKT/Slug signal pathway [38]. In colon cancer, S100A4 is a direct target of β-catenin/T-cell factor signaling and acts as a master mediator of the EMT [39]. Similarly, we demonstrate that deregulated miR-296/S100A4 axis promotes the process of EMT through altering the expression of EMT-related markers. Since the E-cadherin/β-catenin complex is associated with EMT, further investigations are required to confirm that whether β-catenin/T-cell factor signaling is involved in the regulation of the oncogenic functions of miR-296/S100A4 axis.
In conclusion, the current work supports the role of S100A4 in EOC progression, and provides novel insights into the EMT process by which S100A4 promotes metastatic capacity of EOC cells. These results suggest that targeting the miR-296/S100A4 axis may represent a novel therapeutic approach for EOC treatment.
Acknowledgements
This work was supported by the grant from projects of medical and health technology development program in Zhejiang Province, China (No. 2014kya198).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Matulonis U, Abrahm JL. Cancer of the ovary. N Engl J Med. 2005;352:1268–1269. doi: 10.1056/NEJM200503243521222. author reply 1268-1269. [DOI] [PubMed] [Google Scholar]
- 3.Cress RD, Chen YS, Morris CR, Petersen M, Leiserowitz GS. Characteristics of Long-Term Survivors of Epithelial Ovarian Cancer. Obstet Gynecol. 2015;126:491–497. doi: 10.1097/AOG.0000000000000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 5.Wright AA, Cronin A, Milne DE, Bookman MA, Burger RA, Cohn DE, Cristea MC, Griggs JJ, Keating NL, Levenback CF, Mantia-Smaldone G, Matulonis UA, Meyer LA, Niland JC, Weeks JC, O’Malley DM. Use and Effectiveness of Intraperitoneal Chemotherapy for Treatment of Ovarian Cancer. J. Clin. Oncol. 2015;33:2841–2847. doi: 10.1200/JCO.2015.61.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lheureux S, Karakasis K, Kohn EC, Oza AM. Ovarian cancer treatment: The end of empiricism? Cancer. 2015;121:3203–3211. doi: 10.1002/cncr.29481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tremollieres F, Lopes P, Gompel A Groupe d’Etude sur la Menopause et le Vieillissement hormonal. Hormone therapy and ovarian cancer. Lancet. 2015;386:1038. doi: 10.1016/S0140-6736(15)00139-7. [DOI] [PubMed] [Google Scholar]
- 8.Kim EJ, Helfman DM. Characterization of the metastasis-associated protein, S100A4. Roles of calcium binding and dimerization in cellular localization and interaction with myosin. J Biol Chem. 2003;278:30063–30073. doi: 10.1074/jbc.M304909200. [DOI] [PubMed] [Google Scholar]
- 9.Wang G, Rudland PS, White MR, Barraclough R. Interaction in vivo and in vitro of the metastasis-inducing S100 protein, S100A4 (p9Ka) with S100A1. J Biol Chem. 2000;275:11141–11146. doi: 10.1074/jbc.275.15.11141. [DOI] [PubMed] [Google Scholar]
- 10.Tamaki Y, Iwanaga Y, Niizuma S, Kawashima T, Kato T, Inuzuka Y, Horie T, Morooka H, Takase T, Akahashi Y, Kobuke K, Ono K, Shioi T, Sheikh SP, Ambartsumian N, Lukanidin E, Koshimizu TA, Miyazaki S, Kimura T. Metastasis-associated protein, S100A4 mediates cardiac fibrosis potentially through the modulation of p53 in cardiac fibroblasts. J Mol Cell Cardiol. 2013;57:72–81. doi: 10.1016/j.yjmcc.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Osterreicher CH, Penz-Osterreicher M, Grivennikov SI, Guma M, Koltsova EK, Datz C, Sasik R, Hardiman G, Karin M, Brenner DA. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc Natl Acad Sci U S A. 2011;108:308–313. doi: 10.1073/pnas.1017547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plestilova L, Mann H, Andres Cerezo L, Pecha O, Vencovsky J, Senolt L. The metastasis promoting protein S100A4 levels associate with disease activity rather than cancer development in patients with idiopathic inflammatory myopathies. Arthritis Res Ther. 2014;16:468. doi: 10.1186/s13075-014-0468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nasr R, Reed AM, Peterson EJ. Update: biomarkers for idiopathic inflammatory myopathies. Curr Opin Rheumatol. 2012;24:609–615. doi: 10.1097/BOR.0b013e3283585731. [DOI] [PubMed] [Google Scholar]
- 15.Cerezo LA, Kuncova K, Mann H, Tomcik M, Zamecnik J, Lukanidin E, Neidhart M, Gay S, Grigorian M, Vencovsky J, Senolt L. The metastasis promoting protein S100A4 is increased in idiopathic inflammatory myopathies. Rheumatology (Oxford) 2011;50:1766–1772. doi: 10.1093/rheumatology/ker218. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Shi J, Luo Y, Liao Q, Niu Y, Zhang F, Shao Z, Ding Y, Zhao L. LIM and SH3 protein 1 induces TGFbeta-mediated epithelialmesenchymal transition in human colorectal cancer by regulating S100A4 expression. Clin Cancer Res. 2014;20:5835–5847. doi: 10.1158/1078-0432.CCR-14-0485. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Tang W, Wang J, Xie L, Li T, He Y, Qin X, Li S. Clinicopathological and prognostic significance of S100A4 overexpression in colorectal cancer: a meta-analysis. Diagn Pathol. 2013;8:181. doi: 10.1186/1746-1596-8-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chai J, Jamal MM. S100A4 in esophageal cancer: is this the one to blame? World J Gastroenterol. 2012;18:3931–3935. doi: 10.3748/wjg.v18.i30.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Zhou LB, Li XH. S100A4 expression and prognosis of gastric cancer: a metaanalysis. Genet Mol Res. 2014;13:10398–10403. doi: 10.4238/2014.December.12.1. [DOI] [PubMed] [Google Scholar]
- 20.Che P, Yang Y, Han X, Hu M, Sellers JC, Londono-Joshi AI, Cai GQ, Buchsbaum DJ, Christein JD, Tang Q, Chen D, Li Q, Grizzle WE, Lu YY, Ding Q. S100A4 promotes pancreatic cancer progression through a dual signaling pathway mediated by Src and focal adhesion kinase. Sci Rep. 2015;5:8453. doi: 10.1038/srep08453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Wang X, Liang Y, Diao X, Chen Q. S100A4 promotes invasion and angiogenesis in breast cancer MDA-MB-231 cells by upregulating matrix metalloproteinase-13. Acta Biochim Pol. 2012;59:593–598. [PubMed] [Google Scholar]
- 22.Saleem M, Kweon MH, Johnson JJ, Adhami VM, Elcheva I, Khan N, Bin Hafeez B, Bhat KM, Sarfaraz S, Reagan-Shaw S, Spiegelman VS, Setaluri V, Mukhtar H. S100A4 accelerates tumorigenesis and invasion of human prostate cancer through the transcriptional regulation of matrix metalloproteinase 9. Proc Natl Acad Sci U S A. 2006;103:14825–14830. doi: 10.1073/pnas.0606747103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bai H, Qian JL, Han BH. S100A4 is an independent prognostic factor for patients with lung cancer: a meta-analysis. Genet Test Mol Biomarkers. 2014;18:371–374. doi: 10.1089/gtmb.2013.0471. [DOI] [PubMed] [Google Scholar]
- 24.Maelandsmo GM, Florenes VA, Nguyen MT, Flatmark K, Davidson B. Different expression and clinical role of S100A4 in serous ovarian carcinoma at different anatomic sites. Tumour Biol. 2009;30:15–25. doi: 10.1159/000199447. [DOI] [PubMed] [Google Scholar]
- 25.Kikuchi N, Horiuchi A, Osada R, Imai T, Wang C, Chen X, Konishi I. Nuclear expression of S100A4 is associated with aggressive behavior of epithelial ovarian carcinoma: an important autocrine/paracrine factor in tumor progression. Cancer Sci. 2006;97:1061–1069. doi: 10.1111/j.1349-7006.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X, Su B, Xie C, Wei S, Zhou Y, Liu H, Dai W, Cheng P, Wang F, Xu X, Guo C. Sonic hedgehog-Gli1 signaling pathway regulates the epithelial mesenchymal transition (EMT) by mediating a new target gene, S100A4, in pancreatic cancer cells. PLoS One. 2014;9:e96441. doi: 10.1371/journal.pone.0096441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu H, Li M, Zhou Y, Wang F, Li X, Wang L, Fan Q. S100A4 participates in epithelial-mesenchymal transition in breast cancer via targeting MMP2. Tumour Biol. 2015 doi: 10.1007/s13277-015-3709-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Horiuchi A, Hayashi T, Kikuchi N, Hayashi A, Fuseya C, Shiozawa T, Konishi I. Hypoxia upregulates ovarian cancer invasiveness via the binding of HIF-1alpha to a hypoxia-induced, methylation-free hypoxia response element of S100A4 gene. Int J Cancer. 2012;131:1755–1767. doi: 10.1002/ijc.27448. [DOI] [PubMed] [Google Scholar]
- 29.Cheng CJ, Bahal R, Babar IA, Pincus Z, Barrera F, Liu C, Svoronos A, Braddock DT, Glazer PM, Engelman DM, Saltzman WM, Slack FJ. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature. 2015;518:107–110. doi: 10.1038/nature13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu N, Zhong L, Zeng J, Zhang X, Yang Q, Liao D, Wang Y, Chen G, Wang Y. Upregulation of microRNA-200a associates with tumor proliferation, CSCs phenotype and chemosensitivity in ovarian cancer. Neoplasma. 2015;62:550–559. doi: 10.4149/neo_2015_066. [DOI] [PubMed] [Google Scholar]
- 31.Lin KT, Yeh YM, Chuang CM, Yang SY, Chang JW, Sun SP, Wang YS, Chao KC, Wang LH. Glucocorticoids mediate induction of microRNA-708 to suppress ovarian cancer metastasis through targeting Rap1B. Nat Commun. 2015;6:5917. doi: 10.1038/ncomms6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang DP, Huang TQ, Li SJ, Chen ZJ. Knockdown of S100A4 chemosensitizes human laryngeal carcinoma cells in vitro through inhibition of Slug. Eur Rev Med Pharmacol Sci. 2014;18:3484–3490. [PubMed] [Google Scholar]
- 33.Hua J, Chen D, Fu H, Zhang R, Shen W, Liu S, Sun K, Sun X. Short hairpin RNA-mediated inhibition of S100A4 promotes apoptosis and suppresses proliferation of BGC823 gastric cancer cells in vitro and in vivo. Cancer Lett. 2010;292:41–47. doi: 10.1016/j.canlet.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Zou M, Al-Baradie RS, Al-Hindi H, Farid NR, Shi Y. S100A4 (Mts1) gene overexpression is associated with invasion and metastasis of papillary thyroid carcinoma. Br J Cancer. 2005;93:1277–1284. doi: 10.1038/sj.bjc.6602856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho YG, Kim CJ, Nam SW, Yoon SH, Lee SH, Yoo NJ, Lee JY, Park WS. Overexpression of S100A4 is closely associated with progression of colorectal cancer. World J Gastroenterol. 2005;11:4852–4856. doi: 10.3748/wjg.v11.i31.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee KH, Lin FC, Hsu TI, Lin JT, Guo JH, Tsai CH, Lee YC, Lee YC, Chen CL, Hsiao M, Lu PJ. MicroRNA-296-5p (miR-296-5p) functions as a tumor suppressor in prostate cancer by directly targeting Pin1. Biochim Biophys Acta. 2014;1843:2055–2066. doi: 10.1016/j.bbamcr.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Bai Y, Liao H, Liu T, Zeng X, Xiao F, Luo L, Guo H, Guo L. MiR-296-3p regulates cell growth and multi-drug resistance of human glioblastoma by targeting ether-a-go-go (EAG1) Eur J Cancer. 2013;49:710–724. doi: 10.1016/j.ejca.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 38.Zhang K, Zhang M, Zhao H, Yan B, Zhang D, Liang J. S100A4 regulates motility and invasiveness of human esophageal squamous cell carcinoma through modulating the AKT/Slug signal pathway. Dis Esophagus. 2012;25:731–739. doi: 10.1111/j.1442-2050.2012.01323.x. [DOI] [PubMed] [Google Scholar]
- 39.Stein U, Arlt F, Walther W, Smith J, Waldman T, Harris ED, Mertins SD, Heizmann CW, Allard D, Birchmeier W, Schlag PM, Shoemaker RH. The metastasis-associated gene S100A4 is a novel target of beta-catenin/T-cell factor signaling in colon cancer. Gastroenterology. 2006;131:1486–1500. doi: 10.1053/j.gastro.2006.08.041. [DOI] [PubMed] [Google Scholar]


