Abstract
Our previous study demonstrated that high mRNA levels for Seven in Absentia Homolog 2 (SIAH2) correlated with high Estrogen Receptor (ER) mRNA levels and with longer progression-free survival (PFS) after first-line tamoxifen. Others showed high SIAH2 protein levels in ER-negative breast cancer associated with an unfavorable relapse-free survival. In the current study, we investigated SIAH2 protein expression to clarify the discrepancy between protein and mRNA findings and to determine its diagnostic value in breast cancer patients. Tissue microarrays (TMAs) containing core specimens of primary breast tumors were immunohistochemically stained for SIAH2 protein. The TMAs analyzed a cohort of 746 patients with primary breast cancer (PBC) and a cohort of 245 patients with ER-positive metastatic breast cancer (MBC) treated with first-line tamoxifen. SIAH2 staining was scored for intensity and proportion of positive tumor cells and evaluated for its relationship with metastasis-free survival (MFS) and PFS. Multivariate survival analyses included traditional prognostic or predictive factors, respectively. The PBC-cohort had 263 patients with high SIAH2 protein expression and decreased expression of ER protein and mRNA levels (P = 0.005 and P = 0.003, respectively). High SIAH2 levels correlated with significant unfavorable MFS in lymph node negative, ER-positive breast cancer patients. The MBC-cohort had 86 patients with increased SIAH2 protein expression. High SIAH2 expression was associated with an unfavorable PFS after first-line tamoxifen in multivariate analyses (HR = 1.45; 95% CI, 1.07-1.96; P = 0.015). In conclusion, SIAH2 protein expression is especially observed in ER-negative tumors. Its prognostic value in breast cancer does not add to current prognostic markers. The proportion of SIAH2-positive cells can be used as biomarker to predict tamoxifen treatment failure in MBC patients.
Keywords: Seven-in-absentia-homolog 2, breast cancer, endocrine therapy resistance, tissue microarray, immunohistochemistry
Introduction
Each year approximately 1.4 million women worldwide are diagnosed with breast cancer. Despite many new developments in the treatment of breast cancer, the overall survival (OS) of patients with metastatic breast cancer (MBC) remains poor [1]. Currently, Estrogen Receptor (ER)-positive breast cancer patients are treated with anti-hormonal agents such as tamoxifen and aromatase inhibitors. Although tamoxifen treatment results in tumor growth inhibition, in metastatic disease only half of the patients with ER-positive breast tumors responds to this drug. Moreover, those women who initially respond on tamoxifen, will eventually develop progressive disease due to acquired resistance. We revealed 81 genes differentially expressed between ER-positive breast cancer patients with progressive disease and patients who respond to tamoxifen [2]. SIAH2 was one of the genes significantly related with response and progression-free survival (PFS) [3].
SIAH2 is an ubiquitin E3 ligase which ubiquitinates proteins for proteasomal-dependent degradation [3,4] and has target proteins in the RAS and estrogen signaling pathway, DNA damage response, cell growth and differentiation, angiogenesis and hypoxia [4-6]. Studies in breast cancer showed SIAH2 mainly in ER-positive tumors [3,7]. In addition, estrogens upregulated SIAH2 on both mRNA and protein level by a rapid transcriptional response mediated by the ER [8].
Our previous study in primary breast tumors demonstrated a positive relationship between SIAH2 mRNA and ER protein levels [3]. In contrast, Chan et al. found that SIAH2 protein levels were predominantly upregulated in ER-negative breast cancer [9]. They also observed increased SIAH2 protein expression during the transition of carcinoma in situ to invasive cancer and concluded that high SIAH2 protein expression is associated with an unfavorable survival. On the other hand, we observed increased SIAH2 mRNA levels with a favorable disease outcome in patients with ER-positive primary breast cancer [3]. In addition, Confalonieri et al. showed a positive association between SIAH2 mRNA and disease-free survival (DFS) [10]. As yet, it is unknown why SIAH2 protein and mRNA levels have opposed prognostic value in these heterogeneous tumor specimens.
In the present study, we investigated the relationship between SIAH2 and ER protein as well as mRNA expression levels to resolve the observed discrepancy in literature. We also examined the association of SIAH2 protein expression with prognosis and tamoxifen therapy response in ER-positive breast cancer patients.
Patients and methods
Ethics statement
This retrospective study was approved by the medical ethics committee of the Erasmus MC Rotterdam, the Netherlands (MEC 02.953). The study was carried out according the REMARK guidelines [11] and Code of Conduct of the Federation of Medical Scientific Societies in the Netherlands (http://www.fmwv.nl).
Patients and tumor tissues
Formalin-fixed, paraffin-embedded (FFPE) primary breast tumor tissue samples were used from patients with primary operable breast cancer between 1985 and 2000. Two different patient series were included, i.e. patients with primary breast cancer (PBC-cohort) and patients with metastatic breast cancer treated with first-line tamoxifen monotherapy (MBC-cohort).
The PBC-cohort contained 817 patients. Tumors were included for analysis if histologic subtype, tumor differentiation grade according to the Bloom-Richardson score, ER, progesterone receptor (PgR), HER2/neu, epidermal growth factor receptor (EGFR) and cytokeratin 5 (CK5) status were available. After applying these criteria, tumor specimens of 746 patients were analyzed for SIAH2 protein expression.
Three hundred twenty six of these patients (43%) had breast-conserving surgery and 433 patients (57%) underwent modified mastectomy. Two hundred eighty two patients (38%) received adjuvant therapy, of which 108 patients were treated with hormonal therapy, 138 patients with chemotherapy and 36 patients with a combination of hormonal and chemotherapy. Six hundred twenty tumors (82%) were considered as ER-positive. Four hundred sixty four patients (62%) did not receive adjuvant systemic therapy. The median follow-up time of patients alive was 124 months (4 to 326 months) with a median metastasis-free survival (MFS) of 95 months (4 to 328 months). Disease recurrence occurred in 275 patients (36%).
Of the patients who developed metastatic breast cancer, 89 patients (32%) were treated with first-line tamoxifen monotherapy. These 89 patients were also included in the MBC-cohort. The MBC-cohort included 339 patients with metastatic disease and treated with first-line tamoxifen therapy. After applying above inclusion criteria, tumor specimens of 245 patients in this TAM cohort were eligible for further analyses.
Patient and tumor characteristics of this MBC-cohort have been previously reported by us [12]. Briefly, 33 of these 245 patients (13%) underwent breast-conserving surgery and 46 patients (19%) modified mastectomy. Eighty patients (33%) received adjuvant chemotherapy, the remaining 165 patients (67%) were hormone-naive. Median follow-up time of patients alive was 44 months (6 to 282 months). After developing metastatic disease, all 245 patients received first-line tamoxifen monotherapy. From the 162 patients (66%) who benefit from this treatment, 8 patients (5%) had a complete response, 43 patients (27%) had a partial response and 111 patients (69%) had stable disease for more than six months. Therapy failure was observed in 83 patients (34%) of whom 60 patients (72%) showed progressive disease and 23 patients (28%) had stable disease for six months or less.
Tissue microarray immunohistological staining and evaluation
Preparation of tissue microarrays (TMAs) of FFPE primary breast tumor specimens and immunohistochemical stainings were performed as described previously [12]. For immunohistochemistry the primary antibody against SIAH2 (monoclonal (1:80), Novus Biologicals, Littleton, CO, USA) was incubated for one hour at room temperature. Stainings were scored by at least two observers independently (AT, KvdW, ER). The scoring was performed similar to the method described previously [13] and determined staining intensity and proportion of tumor cells with SIAH2 expression. The proportion of cells with SIAH2 expression was divided into nine groups (0 = negative for SIAH2 staining, 1 = 1-4% SIAH2 positive cells, 2 = 5-10%, 3 = 11-20%, 4 = 21-30%, 5 = 31-40%, 6 = 41-50%, 7 = 51-99%, 8 = 100%). Staining intensity was split into four categories (0 = negative, 1 = weak, 2 = moderate, 3 = strong). The scoring method by Chan et al. was also used to evaluate our SIAH2 staining [9]. This method combines both intensity (same categories as above) and proportion tumor cells stained for SIAH2 (0 = no cells staining positive, 1 ≤ 10%, 2 = 10-50%, 3 ≥ 50%). The scores for intensity and percentage were added up to a maximum of 6. A cut-off of > 2 was considered as SIAH2 positive.
Next to the protein expression levels also mRNA levels of SIAH2 and ER were available for 114 patients from the PBC-cohort [3].
Data analysis and statistics
Statistical analysis were performed with STATA statistical package, release 13.0 (STATA Corp., College Station, TX), similarly as described previously [12]. Log-rank tests for trends and when appropriate the Pearson’s Chi-squared and Mann-Whitney were used to investigate the association between SIAH2 protein expression and clinicopathological factors. Boxplots were generated to illustrate correlations between protein and mRNA levels and scatterplots were used to visualize relationships between mRNA levels. Spearman rank correlation tests were applied to evaluate the relationships between protein and mRNA levels for the different molecular factors. Hazard ratios (HR) with 95% CI were computed by the Cox proportional hazard model in order to analyze the association of SIAH2 protein expression with MFS for the PBC-cohort and with PFS after first-line therapy with tamoxifen for the MBC-cohort. The endpoints MFS and PFS were defined as described previously [14]. Significant findings in univariate analysis for SIAH2 expression were compared in multivariate analysis with our base models of traditional clinicopathological predictors to test for its independent prognostic and predictive value. Survival curves were generated by using the Kaplan-Meier method. The log-rank test was used to test for differences between survival curves. The P-values were two sided and significant. P < 0.05 was considered as statistically significant.
Results
SIAH2 protein expression
Different staining patterns for SIAH2 were observed, as exemplified in Figure 1A-H. Its expression was predominantly detected in the nucleus as described previously [15-18]. The proportion of cells expressing SIAH2 protein and the staining intensity were evaluated separately for all scoring categories in both cohorts of patients (Figure 2). Logrank tests for trend showed that only the categories for proportion but not for intensity were related with MFS in the PBC-cohort (proportion P = 0.013; intensity P = 0.54) and with PFS in the MBC-cohort (proportion P = 0.005; intensity P = 0.67). The method of Chan et al. [9], which combines proportion and intensity resulting in six scores, demonstrated a relationship with PFS (P = 0.049) but not with MFS (P = 0.60). Based on above findings, further analyses of SIAH2 protein expression as dichotomized variable were based on the proportion of SIAH2-positive cells (Table 1). Specimens with ≤ 20% positive cells were considered to express low SIAH2 protein levels, whereas tumors with > 20% positive cells were defined to have high SIAH2 protein expression. This resulted in 263 tumors (35%) and 86 tumors (35%) with high SIAH2 protein expression in the PBC- and MBC-cohort, respectively.
Figure 1.
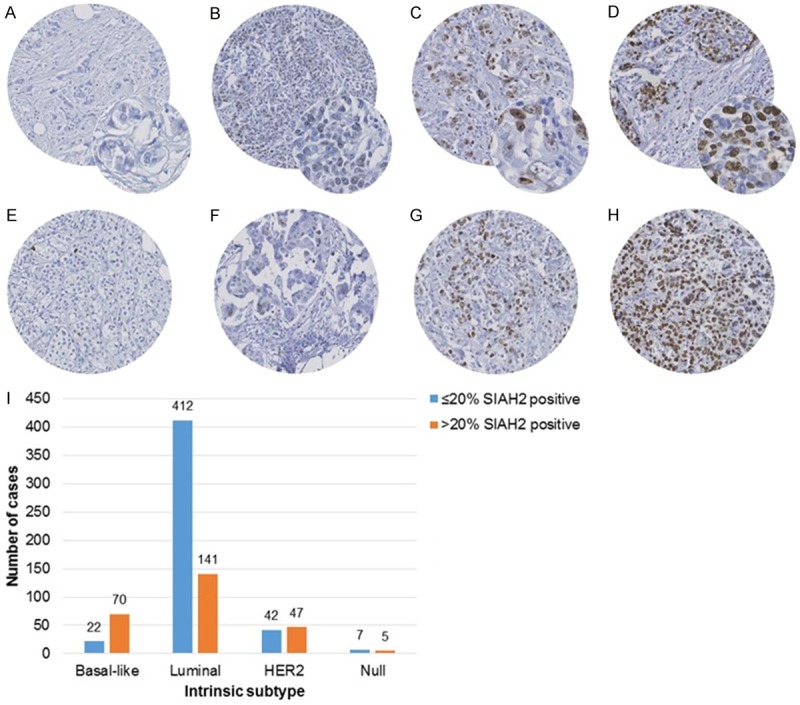
SIAH2 staining patterns and intrinsic subtypes. A to D exemplify the categories for staining intensity, whereas figures E to H those for proportion of SIAH2-positive tumor cells. A. Negative for SIAH2; B. Weak intensity; C. Moderate intensity; D. Strong intensity. E. Less than 5% of the tumor cells positive for SIAH2, strong staining. F. Moderate staining in 11-20% of the tumor cells. G. Moderate staining in 31-40% of the tumor cells. H. 100% of the tumor cells are strongly stained for SIAH2. I. Shows SIAH2 protein expression within the intrinsic subtypes. Analysis was performed on 746 tumors of the PBC-cohort. Protein expression of SIAH2 was dichotomized on the proportion of positive tumor cells, i.e. ≤ 20% SIAH2-positive versus > 20% SIAH-positive. The intrinsic subtypes were defined by ER, HER2/neu, EGFR and Cytokeratin 5 and classified according to Chan et al. [9] as luminal (positive for ER, negative for HER2/neu), HER2 (positive for HER2/neu), basal (positive for EGFR and/or Cytokeratin 5, negative for ER and HER2/neu) and null (negative for all). SIAH2-positive tumors are especially observed in the basal subtype (76%), in contrast to the luminal subtype (25%).
Figure 2.
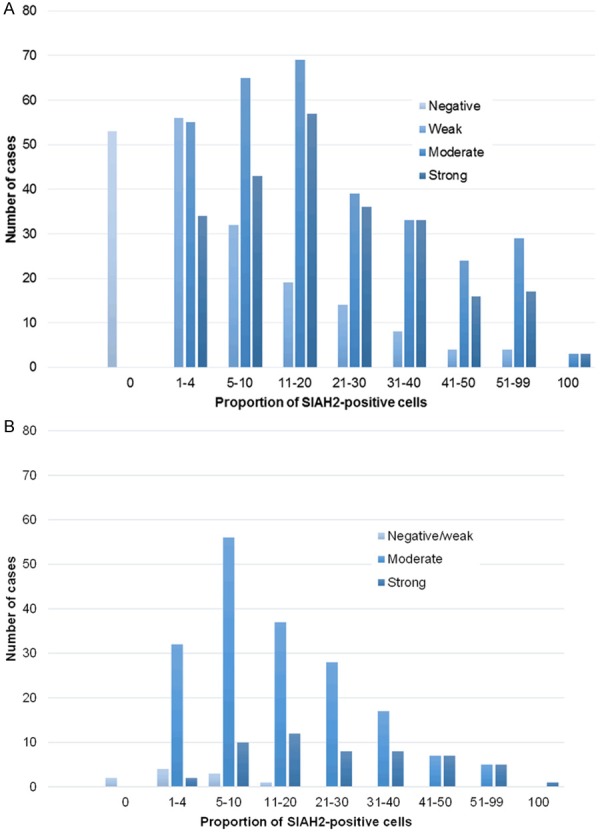
Overview of scoring categories for proportion of SIAH2-positive cells and staining intensity. The upper figure shows the distribution for SIAH2 protein staining intensity and amount of SIAH2-positive tumor cells in 746 tumors of patients from the PBC-cohort. The lower figure shows the staining intensity for SIAH2 and the amount of SIAH2-positive tumor cells in 245 patients from the MBC cohort.
Table 1.
Overview of SIAH2 protein expression quantity and intensity in patients from the primary breast cancer (PBC) and metastatic breast cancer (MBC) cohort
| SIAH2 protein intensity | SIAH2 protein quantity in primary breast cancer cohort | SIAH2 protein quantity in metastatic breast cancer cohort | ||
|---|---|---|---|---|
|
|
|
|||
| Low SIAH2 protein expression 0-20% | High SIAH2 protein expression > 20% | Low SIAH2 protein expression 0-20% | High SIAH2 protein expression > 20% | |
| Negative | 53 | 0 | ||
| Weak* | 107 | 30 | 10 | 0 |
| Moderate | 189 | 128 | 125 | 57 |
| Strong | 134 | 105 | 24 | 29 |
| Total | 483 | 263 | 159 | 86 |
Weak was defined as negative/weak in the metastatic breast cancer cohort.
Pearson’s Chi-squared test showed a positive correlation between SIAH2 protein intensity and quantity P < 0.001.
Association of SIAH2 protein expression with clinicopathological characteristics
SIAH2 protein expression in the PBC-cohort was significantly associated with all studied clinicopathological factors, except for the number of involved lymph nodes and tumor histology (Table 2). A positive correlation was detected between high SIAH2 protein expression and basal-like, HER2 and null intrinsic subtypes (P < 0.001), as shown in Figure 1I. In the ER-positive MBC-cohort, SIAH2 protein expression correlated with age, menopausal status, tumor differentiation grade, HER2/neu receptor expression and soft tissue metastases (Table 3).
Table 2.
Associations of SIAH2 protein levels with clinicopathological factors of 746 patients from the primary breast cancer (PBC) cohort
| Characteristic | N | % | Low SIAH2 protein expression* | % | High SIAH2 protein expression** | % | P-valueα |
|---|---|---|---|---|---|---|---|
| All patients | 746 | 100 | 483 | 65 | 263 | 35 | |
| Age (years) | |||||||
| ≤ 40 | 73 | 10 | 36 | 7 | 37 | 14 | 0.001β |
| 41-55 | 309 | 41 | 192 | 40 | 117 | 44 | |
| 56-70 | 249 | 33 | 167 | 35 | 82 | 31 | |
| > 70 | 115 | 15 | 88 | 18 | 27 | 10 | |
| Menopausal status | |||||||
| Premenopausal | 347 | 47 | 205 | 42 | 142 | 54 | 0.003 |
| Postmenopausal | 399 | 53 | 278 | 58 | 121 | 46 | |
| Tumor stage | |||||||
| pT1 | 426 | 57 | 299 | 62 | 127 | 48 | 0.001 |
| pT2/9 | 279 | 37 | 158 | 33 | 121 | 46 | |
| pT3/4 | 41 | 5 | 26 | 5 | 15 | 6 | |
| Lymph nodes involved | |||||||
| 0 | 410 | 55 | 259 | 54 | 151 | 57 | 0.132 |
| 1-3 | 217 | 29 | 152 | 31 | 65 | 25 | |
| > 3 | 119 | 16 | 72 | 15 | 47 | 18 | |
| Differentiation grade# | |||||||
| 1 | 142 | 19 | 135 | 28 | 7 | 3 | < 0.001 |
| 2 | 334 | 45 | 248 | 51 | 86 | 33 | |
| 3 | 270 | 36 | 100 | 21 | 170 | 65 | |
| Tumor histology | |||||||
| IDC | 653 | 88 | 426 | 88 | 227 | 86 | 0.055 |
| ILC | 28 | 4 | 22 | 5 | 6 | 2 | |
| other | 65 | 9 | 36 | 7 | 30 | 11 | |
| ER status† | |||||||
| Negative | 136 | 18 | 45 | 9 | 91 | 35 | < 0.001 |
| Positive | 610 | 82 | 438 | 91 | 172 | 65 | |
| PgR status† | |||||||
| Negative | 275 | 37 | 129 | 27 | 146 | 56 | < 0.001 |
| Positive | 471 | 63 | 354 | 73 | 117 | 44 | |
| HER2/neu status† | |||||||
| Negative | 657 | 88 | 441 | 91 | 216 | 82 | < 0.001 |
| Positive | 89 | 12 | 42 | 9 | 47 | 18 | |
| EGFR expression† | |||||||
| Negative | 692 | 93 | 458 | 95 | 234 | 89 | 0.003 |
| Positive | 54 | 7 | 25 | 5 | 29 | 11 | |
| CK5 expression† | |||||||
| Negative | 577 | 77 | 413 | 86 | 164 | 62 | < 0.001 |
| Positive | 169 | 23 | 70 | 14 | 99 | 38 |
Abbreviations: IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; ER, estrogen receptor; PgR, progesterone receptor; EGFR, epidermal growth factor receptor; CK5, cytokeratin 5.
SIAH2 negative defined as ≤ 20% cells positive for SIAH2 staining.
SIAH2 positive defined as > 20% cells positive for SIAH2 staining.
P for Pearson’s Chi-squared test;
Mann-Whitney U test.
According to the Bloom-Richardson score.
As retrieved from TMA.
Table 3.
Associations of SIAH2 protein levels with clincopathological factors of 245 patients from the MBC-cohort
| Characteristic | N | %* | Low SIAH2 protein expression** | % | High SIAH2 protein expression*** | % | P-valueα |
|---|---|---|---|---|---|---|---|
| All patients | 245 | 100 | 159 | 65 | 86 | 35 | |
| Age (years) | |||||||
| ≤ 40 | 17 | 7 | 8 | 5 | 9 | 10 | 0.003β |
| 41-55 | 78 | 32 | 44 | 28 | 34 | 40 | |
| 56-70 | 88 | 36 | 60 | 38 | 28 | 33 | |
| > 70 | 62 | 25 | 47 | 30 | 15 | 17 | |
| Menopausal status | |||||||
| Premenopausal | 66 | 27 | 33 | 21 | 33 | 38 | 0.003 |
| Postmenopausal | 179 | 73 | 126 | 79 | 53 | 62 | |
| Adjuvant chemotherapy | |||||||
| No | 148 | 60 | 97 | 61 | 51 | 59 | 0.790 |
| Yes | 80 | 33 | 50 | 31 | 30 | 35 | |
| Lymph nodes involved | |||||||
| 0 | 86 | 35 | 49 | 31 | 37 | 43 | 0.107 |
| 1-3 | 71 | 29 | 52 | 33 | 19 | 22 | |
| > 3 | 83 | 34 | 53 | 33 | 30 | 35 | |
| Differentiation grade# | |||||||
| 1 | 37 | 15 | 33 | 21 | 4 | 5 | < 0.001 |
| 2 | 131 | 53 | 92 | 58 | 39 | 45 | |
| 3 | 77 | 31 | 34 | 21 | 43 | 50 | |
| Tumor histology | |||||||
| IDC | 210 | 86 | 134 | 84 | 76 | 88 | 0.599 |
| ILC | 20 | 8 | 15 | 9 | 5 | 6 | |
| Other | 15 | 6 | 10 | 6 | 5 | 6 | |
| PgR status† | |||||||
| Negative | 62 | 25 | 40 | 25 | 22 | 26 | 0.942 |
| Positive | 183 | 75 | 119 | 75 | 64 | 74 | |
| HER2/neu status† | |||||||
| Negative | 197 | 80 | 140 | 88 | 57 | 66 | < 0.001 |
| Positive | 48 | 20 | 19 | 12 | 29 | 34 | |
| Dominant site of relapse | |||||||
| Viscera | 92 | 38 | 62 | 39 | 30 | 35 | 0.005 |
| Bone | 124 | 51 | 86 | 54 | 38 | 44 | |
| Soft tissue | 29 | 12 | 11 | 7 | 18 | 21 | |
| Disease-free survival (years) | |||||||
| ≤ 1 | 36 | 15 | 25 | 16 | 11 | 13 | 0.611 |
| 1-3 | 118 | 48 | 73 | 46 | 45 | 52 | |
| > 3 | 91 | 37 | 61 | 38 | 30 | 35 |
Abbreviations: IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; PgR, progesterone receptor.
Due to missing information numbers do not always add up to 100%.
SIAH2 negative defined as ≤ 20% cells positive for SIAH2 staining.
SIAH2 positive defined as > 20% cells positive for SIAH2 staining.
P for Pearson’s Chi-squared test;
Mann-Whitney U test.
According to the Bloom-Richardson score.
As retrieved from TMA.
Correlations between SIAH2 and ER on protein and mRNA levels in the PBC-cohort
We previously reported increased SIAH2 mRNA expression in ER-positive compared to ER-negative tumors [3], whereas in this study higher SIAH2 protein levels in ER negative tumors were detected (Table 2; P < 0.001). To evaluate this discrepancy, we investigated mRNA levels with protein levels for both genes, which were available for 114 patients. For SIAH2 alone, mRNA levels had an inverse relation with protein expression (Figure 3; P = 0.089). Combined, SIAH2 protein levels were inversely associated with ER protein and mRNA levels (Figure 3B; P = 0.005 and P = 0.003, respectively), whereas SIAH2 mRNA levels showed a positive correlation with mRNA and protein levels of ER (Figure 3B; P < 0.001).
Figure 3.
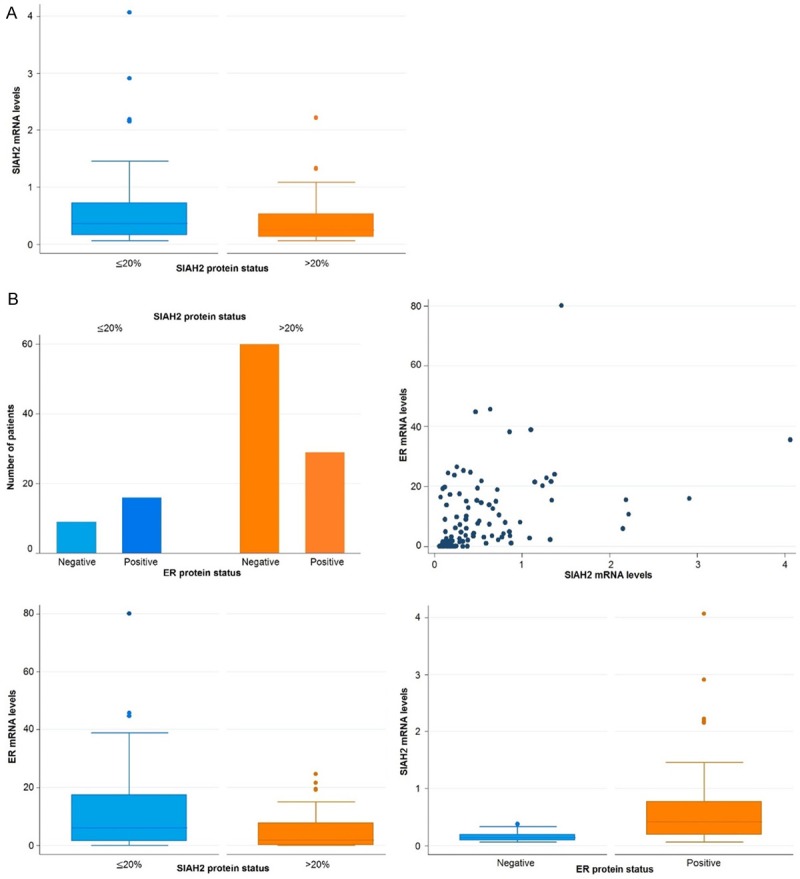
Overview of the correlations between protein and mRNA expression for SIAH2 and estrogen receptor (ER). Analysis was performed in 114 tumors of patients from the PBC-cohort. A Shows the correlation between SIAH2 mRNA and protein expression levels. SIAH2 protein expression is inversely correlated with SIAH2 mRNA levels, although not significant in a two-sample t-test correlation between SIAH2 and ER protein and mRNA expression levels. Upper left figure: SIAH2 protein expression is significantly inversely correlated with ER protein status significantly positive relationship was found between SIAH2 and ER mRNA levels. A significantly inverse association between SIAH2 protein expression and ER mRNA levels was found (P = 0.003). Lower right figure: SIAH2 mRNA levels were positively associated with ER protein expression (P < 0.001).
Association of SIAH2 protein expression with MFS
High SIAH2 protein expression in the PBC-cohort was associated with an unfavorable MFS (HR = 1.41, CI 1.11-1.80, P = 0.005; Table 4). This association between SIAH2 protein expression and MFS continued to be significant in ER-positive tumors of 610 patients (HR = 1.42, CI 1.07-1.89, P = 0.014; Table 5) and within the prognostic subset of 336 lymph node-negative adjuvant systemic therapy naïve patients (HR = 1.76, CI, 1.15-2.67; P = 0.009; Table 6). The prognostic value of SIAH2 protein expression was visualized with a Kaplan-Meier curve in Figure 4A. SIAH2 protein expression and MFS were not significantly related in multivariate analysis, when corrected for traditional prognostic factors such as age, menopausal status, tumor stage, differentiation grade, PgR and HER2/neu status. Tumors with high SIAH2 protein expression were only related with OS for the whole set (HR = 1.29, CI 1.01-1.65; P = 0.039) but not for the subsets (HR = 1.26, P = 0.19 and HR = 1.36, P = 0.160 for the ER-positive and prognostic subset, respectively).
Table 4.
Cox univariate and multivariate analysis for metastasis-free survival of SIAH2 protein expression levels. Analysis was performed in all 746 patients from the PBC-cohort
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Factor of base model | N | % | HR | 95% CI | P-value | HR | 95% CI | P-value |
| All patients | 746 | 100 | ||||||
| Age (years) | ||||||||
| ≤ 40 | 73 | 10 | 1.00 | 1.00 | ||||
| 41-55 | 309 | 41 | 0.66 | 0.46 to 0.95 | 0.027 | 0.80 | 0.55 to 1.16 | 0.243 |
| 56-70 | 249 | 33 | 0.58 | 0.40 to 0.86 | 0.006 | 0.91 | 0.48 to 1.71 | 0.767 |
| > 70 | 115 | 15 | 0.57 | 0.36 to 0.92 | 0.023 | 0.86 | 0.42 to 1.75 | 0.673 |
| Menopausal status | ||||||||
| Premenopausal | 347 | 47 | 1.00 | 1.00 | ||||
| Postmenopausal | 399 | 53 | 0.79 | 0.62 to 1.01 | 0.057 | 0.82 | 0.49 to 1.37 | 0.452 |
| Tumor stage | ||||||||
| pT1 | 426 | 57 | 1.00 | 1.00 | ||||
| pT2/9 | 279 | 37 | 1.85 | 1.44 to 2.37 | < 0.001 | 1.50 | 1.15 to 1.94 | 0.002 |
| pT3/4 | 41 | 5 | 2.75 | 1.76 to 4.30 | < 0.001 | 1.89 | 1.19 to 3.00 | 0.007 |
| Lymph nodes involved | ||||||||
| 0 | 410 | 55 | 1.00 | 1.00 | ||||
| 1-3 | 217 | 29 | 1.61 | 1.22 to 2.13 | 0.001 | 1.49 | 1.12 to 1.97 | 0.006 |
| > 3 | 119 | 16 | 2.92 | 2.16 to 3.93 | < 0.001 | 1.76 | 1.76 to 3.31 | < 0.001 |
| Differentiation grade | ||||||||
| 1 | 85 | 25 | 1.00 | 1.00 | ||||
| 2 | 164 | 49 | 2.44 | 1.30 to 4.59 | 0.006 | 2.30 | 1.21 to 4.34 | 0.011 |
| 3 | 87 | 26 | 3.24 | 1.67 to 6.30 | 0.001 | 3.14 | 1.58 to 6.27 | 0.001 |
| ER status† | ||||||||
| Negative | 136 | 18 | 1.00 | 1.00 | ||||
| Positive | 610 | 82 | 0.79 | 0.59 to 1.07 | 0.124 | 0.95 | 0.64 to 1.41 | 0.800 |
| PgR status† | ||||||||
| Negative | 275 | 37 | 1.00 | 1.00 | ||||
| Positive | 471 | 63 | 0.91 | 0.71 to 1.16 | 0.429 | 1.18 | 0.85 to 1.64 | 0.316 |
| HER2 status† | ||||||||
| Negative | 657 | 88 | 1.00 | 1.00 | ||||
| Positive | 89 | 12 | 1.39 | 0.99 to 1.95 | 0.056 | 1.25 | 0.88 to 1.78 | 0.208 |
| SIAH2 protein expression | Added to the base model | |||||||
| Low (≤ 20% positive cells) | 483 | 65 | 1.00 | 1.00 | ||||
| High (> 20% positive cells) | 263 | 35 | 1.41 | 1.11 to 1.80 | 0.005 | 1.09 | 0.82 to 1.43 | 0.565 |
Abbreviations: HR, hazard ratio; ER, estrogen receptor; PgR, progesterone receptor.
As retrieved from TMA.
Table 5.
Cox univariate and multivariate analysis for progression-free survival of SIAH2 protein expression levels. Analysis was performed in 610 patients with ER-positive breast cancer from the PBC-cohort
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Factor of base model | N | % | HR | 95% CI | P-value | HR | 95% CI | P-value |
| All patients | 610 | 100 | ||||||
| Age (years) | ||||||||
| ≤ 40 | 53 | 9 | 1.00 | 1.00 | ||||
| 41-55 | 250 | 41 | 0.64 | 0.42 to 0.97 | 0.034 | 0.74 | 0.48 to 1.13 | 0.165 |
| 56-70 | 208 | 34 | 0.54 | 0.35 to 0.83 | 0.006 | 0.64 | 0.31 to 1.31 | 0.221 |
| > 70 | 99 | 16 | 0.57 | 0.33 to 0.97 | 0.037 | 0.67 | 0.30 to 1.49 | 0.330 |
| Menopausal status | ||||||||
| Premenopausal | 280 | 46 | 1.00 | 1.00 | ||||
| Postmenopausal | 330 | 54 | 0.83 | 0.63 to 1.08 | 0.159 | 1.04 | 0.58 to 1.86 | 0.898 |
| Tumor stage | ||||||||
| pT1 | 361 | 59 | 1.00 | 1.00 | ||||
| pT2/9 | 221 | 36 | 1.88 | 1.43 to 2.48 | < 0.001 | 1.51 | 1.13 to 2.01 | 0.005 |
| pT3/4 | 28 | 5 | 2.50 | 1.45 to 4.29 | 0.001 | 1.81 | 1.04 to 3.18 | 0.037 |
| Lymph nodes involved | ||||||||
| 0 | 336 | 55 | 1.00 | 1.00 | ||||
| 1-3 | 180 | 30 | 1.52 | 1.12 to 2.08 | 0.008 | 1.37 | 1.00 to 1.88 | 0.048 |
| > 3 | 94 | 15 | 2.77 | 1.98 to 3.87 | < 0.001 | 2.30 | 1.61 to 3.29 | < 0.001 |
| Differentiation grade | ||||||||
| 1 | 138 | 23 | 1.00 | 1.00 | ||||
| 2 | 307 | 50 | 2.04 | 1.36 to 3.07 | 0.001 | 1.70 | 1.12 to 2.57 | 0.013 |
| 3 | 165 | 27 | 2.86 | 1.76 to 4.39 | < 0.001 | 2.47 | 1.58 to 3.86 | < 0.001 |
| PgR status† | ||||||||
| Negative | 146 | 24 | 1.00 | 1.00 | ||||
| Positive | 464 | 76 | 0.94 | 0.69 to 1.28 | 0.690 | 1.11 | 0.79 to 1.54 | 0.552 |
| HER2 status† | ||||||||
| Negative | 553 | 91 | 1.00 | 1.00 | ||||
| Positive | 57 | 9 | 1.53 | 1.02 to 2.31 | 0.041 | 1.25 | 0.81 to 1.92 | 0.318 |
| SIAH2 protein expression | Added to the base model | |||||||
| Low (≤ 20% positive cells) | 438 | 72 | 1.00 | 1.00 | ||||
| High (> 20% positive cells) | 172 | 28 | 1.42 | 1.07 to 1.89 | 0.014 | 1.00 | 0.72 to 1.37 | 0.977 |
Abbreviations: HR, hazard ratio; PgR, progesterone receptor.
As retrieved from TMA.
Table 6.
Cox univariate and multivariate analysis for metastasis-free survival of SIAH2 protein expression levels. Analysis was performed in 336 patients with lymph node-negative ER-positive breast cancer from the PBC-cohort
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Factor of base model | N | % | HR | 95% CI | P-value | HR | 95% CI | P-value |
| All patients | 336 | 100 | ||||||
| Age (years) | ||||||||
| ≤ 40 | 25 | 7 | 1.00 | 1.00 | ||||
| 41-55 | 133 | 40 | 0.53 | 0.29 to 0.99 | 0.048 | 0.63 | 0.33 to 1.20 | 0.162 |
| 56-70 | 120 | 36 | 0.34 | 0.18 to 0.69 | 0.002 | 0.25 | 0.09 to 0.74 | 0.012 |
| > 70 | 58 | 17 | 0.54 | 0.26 to 1.17 | 0.119 | 0.38 | 0.12 to 1.20 | 0.101 |
| Menopausal status | ||||||||
| Premenopausal | 147 | 44 | 1.00 | 1.00 | ||||
| Postmenopausal | 189 | 56 | 0.80 | 0.53 to 1.20 | 0.282 | 1.75 | 0.76 to 4.02 | 0.184 |
| Tumor stage | ||||||||
| pT1 | 235 | 70 | 1.00 | 1.00 | ||||
| pT2/9 | 93 | 28 | 1.58 | 1.02 to 2.46 | 0.041 | 1.34 | 0.85 to 2.11 | 0.211 |
| pT3/4 | 8 | 2 | 4.39 | 1.88 to 10.2 | 0.001 | 3.18 | 1.29 to 7.82 | 0.012 |
| Differentiation grade | ||||||||
| 1 | 85 | 25 | 1.00 | 1.00 | ||||
| 2 | 164 | 49 | 2.44 | 1.30 to 4.59 | 0.006 | 2.30 | 1.21 to 4.34 | 0.011 |
| 3 | 87 | 26 | 3.24 | 1.67 to 6.30 | 0.001 | 3.14 | 1.58 to 6.27 | 0.001 |
| PgR status† | ||||||||
| Negative | 78 | 23 | 1.00 | 1.00 | ||||
| Positive | 258 | 77 | 1.03 | 0.63 to 1.68 | 0.916 | 1.11 | 0.66 to 1.90 | 0.687 |
| HER2 status† | ||||||||
| Negative | 301 | 90 | 1.00 | 1.00 | ||||
| Positive | 35 | 10 | 1.69 | 0.96 to 2.99 | 0.070 | 1.21 | 0.65 to 2.27 | 0.553 |
| SIAH2 protein expression | Added to the base model | |||||||
| Low (≤ 20% positive cells) | 241 | 72 | 1.00 | 1.00 | ||||
| High (> 20% positive cells) | 95 | 28 | 1.76 | 1.15 to 2.67 | 0.009 | 1.10 | 0.66 to 1.83 | 0.707 |
Abbreviations: HR, hazard ratio; PgR, progesterone receptor.
As retrieved from TMA.
Figure 4.
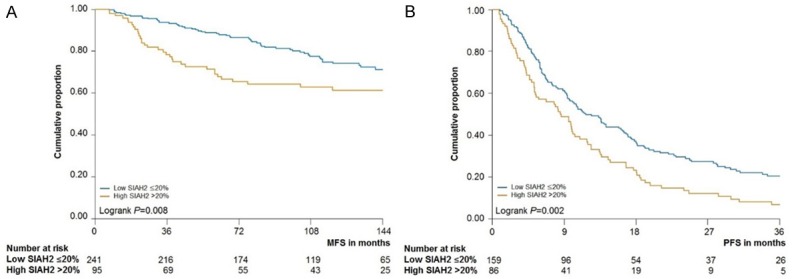
Kaplan-meier curves of MFS and PFS as a function of SIAH2 protein. A. Relationship between SIAH2 protein expression and metastasis-free survival in PBC cohort. Analysis was performed in 336 patients with lymph node negative, ER-positive breast cancer. SIAH2 protein expression was divided in tumors with low (≤ 20%, blue line) and high expression (> 20%, orange line). B. Relationship between SIAH2 protein expression and PFS analyzed in 245 MBC patients treated with first-line tamoxifen. SIAH2 protein expression was divided in tumors with low (≤ 20%) and high expression (> 20%).
Association of SIAH2 protein expression with PFS after first-line tamoxifen monotherapy
The MBC-cohort showed a significant relationship between SIAH2 protein expression and PFS (HR = 1.55, CI 1.18-2.04; P = 0.002; Table 7 and Figure 4B) but not with OS after start of first-line therapy (HR = 1.21, CI 0.90-1.63, P = 0.202). When added to the multivariate base model including the traditional predictive factors age, menopausal status, adjuvant therapy, dominant site of relapse, DFS, PgR, and HER2/neu status, tumors with high SIAH2 protein expression associated with a poor PFS (HR = 1.45, CI 1.07-1.96; P = 0.015).
Table 7.
Cox univariate and multivariate analysis for progression-free survival of SIAH2 protein expression levels. Analysis was performed in 245 ER-positive tumors from patients with MBC treated with first-line tamoxifen monotherapy
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Factor of base model | N | %* | HR | 95% CI | P-value | HR | 95% CI | P-value |
| All patients | 245 | 100 | ||||||
| Age (years) | ||||||||
| ≤ 40 | 17 | 7 | 1.00 | 1.00 | ||||
| 41-55 | 78 | 32 | 1.21 | 0.71 to 2.08 | 0.487 | 1.19 | 0.68 to 2.07 | 0.545 |
| 56-70 | 88 | 36 | 0.69 | 0.40 to 1.18 | 0.173 | 0.53 | 0.26 to 1.07 | 0.076 |
| > 70 | 63 | 26 | 0.56 | 0.32 to 0.98 | 0.044 | 0.44 | 0.21 to 0.95 | 0.038 |
| Menopausal status | ||||||||
| Premenopausal | 66 | 27 | 1.00 | 1.00 | ||||
| Postmenopausal | 180 | 73 | 0.64 | 0.48 to 0.86 | 0.003 | 1.15 | 0.73 to 1.80 | 0.544 |
| Adjuvant chemotherapy | ||||||||
| No | 149 | 61 | 1.00 | 1.00 | ||||
| Yes | 80 | 33 | 1.49 | 1.12 to 1.99 | 0.006 | 0.99 | 0.70 to 1.40 | 0.950 |
| Dominant site of relapse | ||||||||
| Viscera | 93 | 38 | 1.00 | 1.00 | ||||
| Bone | 124 | 51 | 0.81 | 0.61 to 1.07 | 0.144 | 0.70 | 0.52 to 0.95 | 0.021 |
| Soft tissue | 29 | 12 | 0.49 | 0.30 to 0.78 | 0.003 | 0.45 | 0.28 to 0.73 | 0.001 |
| Disease-free survival (years) | ||||||||
| ≤ 1 | 36 | 15 | 1.00 | 1.00 | ||||
| 1-3 | 118 | 48 | 0.78 | 0.53 to 1.15 | 0.218 | 0.69 | 0.41 to 1.16 | 0.157 |
| > 3 | 92 | 38 | 0.70 | 0.47 to 1.05 | 0.083 | 0.63 | 0.37 to 1.10 | 0.103 |
| PgR status† | ||||||||
| Negative | 62 | 25 | 1.00 | 1.00 | ||||
| Positive | 184 | 75 | 0.87 | 0.64 to 1.17 | 0.357 | 0.79 | 0.58 to 1.09 | 0.152 |
| HER2/neu status† | ||||||||
| Negative | 198 | 81 | 1.00 | 1.00 | ||||
| Positive | 48 | 20 | 1.15 | 0.82 to 1.60 | 0.414 | 0.98 | 0.69 to 1.39 | 0.901 |
| SIAH2 protein expression | Added to the base model | |||||||
| Low (≤ 20% positive cells) | 86 | 35 | 1.00 | 1.00 | ||||
| High (> 20% positive cells) | 159 | 65 | 1.55 | 1.18 to 2.04 | 0.002 | 1.45 | 1.07 to 1.96 | 0.015 |
Abbreviations: HR, hazard ratio; PgR, progesterone receptor.
Due to missing information numbers do not always add up to 100%.
As retrieved from TMA.
Discussion
In the current study, we investigated the relationship between SIAH2 and ER status and the prognostic and predictive value of SIAH2 in breast cancer patients. We show that SIAH2 protein expression is inversely related with ER protein expression. In contrast, we found a positive correlation between SIAH2 mRNA levels and ER protein expression. Furthermore, we demonstrated that high SIAH2 protein expression is associated with an unfavorable MFS in PBC patients and PFS in MBC patients treated with first-line tamoxifen, respectively.
The observed inverse correlation between SIAH2 and ER protein expression has been described earlier in basal subtype of breast cancer by Chan et al. [9]. We now confirmed their findings in a larger subset of breast cancer patients and demonstrate high SIAH2 protein expression in especially ER-negative tumors. However, our previous study reported high SIAH2 mRNA levels in tumors with high ER mRNA levels [3]. Exploratory analyses in a subset of patients for which mRNA as well as protein expression data were available confirmed the positive correlation at mRNA but the inverse relation at protein level between SIAH2 and ER. Discordance between protein and mRNA expression levels have also been observed for other genes [19]. Moreover, the proteo-genomic characterization of colon cancer revealed that mRNA transcript abundance is not automatically translated into protein abundance; only 32% of the genes had a significant positive mRNA-protein correlation [20].
Discrepancies between protein and mRNA levels have been explained by different mechanisms [21]. In our subset of tumors with high SIAH2 mRNA levels, we observed a trend towards decreased SIAH2 protein levels. This finding might be explained by auto-ubiquitination, since most RING-finger domain E3 ubiquitin ligases, like SIAH2, have the capacity to ubiquitinate themselves, resulting in their own degradation and its limited availability to ubiquitinate target substrates [22]. Another possible explanation for the difference in SIAH2 protein and mRNA levels is that SIAH2 mRNA sequences are complementary bound by microRNAs (miRNAs). These miRNAs are involved in the post-transcriptional (down) regulation of gene expression and affects both translation and stability of target mRNAs. Several miRNAs have been identified to be differentially expressed between the molecular subtypes of breast cancer [30]. Until now, only miR-146b-5p has been functionally shown to target and down-regulate SIAH2 mRNA in vitro and in vivo [23,24] and was detected in breast cancer [25,26].
In the present study, we demonstrate that high SIAH2 protein expression in ER-positive primary tumors is associated with unfavorable MFS in PBC patients and with PFS after first-line tamoxifen monotherapy in MBC patients. The diagnostic value of SIAH2 has previously been investigated in several other studies. Chan et al. also showed in 246 tumors, including basal and luminal subtypes, that high levels of SIAH2 protein expression associated with an unfavorable survival [9]. At the mRNA level, our previous study showed that high SIAH2 levels were related with prolonged PFS [3] and others associated high SIAH2 levels with a longer DFS, MFS and OS [3,10]. These differences in prognostic and predictive value of SIAH2 protein versus transcript in the various studies can be explained by our observed inverse relation between SIAH2 protein and mRNA expression levels.
Our results suggest that tamoxifen resistance in tumor specimens can be defined by low mRNA levels and high protein expression for SIAH2. A role of SIAH2 in endocrine therapy resistance might be anticipated because SIAH2 expression is upregulated by estrogens via ER [8]. Since high SIAH2 protein levels are correlated with low ER expression in this study, the question arises whether SIAH2 controls in a feedback loop the ER protein levels. The resistance to tamoxifen might then be explained by a lack of target due to SIAH2 mediated degradation of ER. Further studies are needed to investigate this hypothesis and to determine the role of SIAH2 in endocrine therapy resistance.
Compounds that target SIAH2 protein might help to overcome endocrine therapy resistance. Next to general proteasome inhibitors that might be applicable, only one SIAH2 specific protein inhibitor has been identified and tested in vivo. The compound menadione (vitamin K3) attenuates SIAH2 auto-ubiquitination which resulted in inhibition of tumor growth in mice with xenografted human melanomas [27]. Reducing SIAH2 activity by menadione in breast cancer might repress ER-signaling due to accumulation of SIAH2 targets N-CoR and HDAC3 [28,29], two ER transcriptional repressor proteins. On the other hand, our in vitro results showed that SIAH2 silencing was associated with decreased sensitivity to the pure anti-estrogen ICI164,384 [3]. The effect of SIAH2 inhibition could also be realized by reducing hypoxia-inducible factor 1α, which can enable tumor growth and metastasis by inducing angiogenesis [30].
To summarize, we have demonstrated an inverse relationship between SIAH2 mRNA and protein expression levels. High SIAH2 protein expression is especially observed in ER-negative breast cancer. In ER-positive breast cancer, high levels of SIAH2 protein associate with unfavorable outcome in PBC and treatment outcome in MBC. Together with the mRNA findings in our previous study, we are the first to report a relationship between SIAH2 protein expression and outcome to tamoxifen in MBC. Assessment of SIAH2 mRNA and protein levels could allow a better selection of patients for tamoxifen when our findings are confirmed.
Acknowledgements
This work was supported by the European Framework Program (FP7-CAREMORE to AT, FP7-DNA damage response to AT-J), the European Research Council (ERC-Advanced to ML and RF), Cancer Genomics Netherlands to JM, Top Institute Pharma (T3-108, T3-502 to EB and MJ) and KNAW Van Walree (KvdW).
Disclosure of conflict of interest
None.
References
- 1.Allemani C, Minicozzi P, Berrino F, Bastiaannet E, Gavin A, Galceran J, Ameijide A, Siesling S, Mangone L, Ardanaz E, Hedelin G, Mateos A, Micheli A, Sant M EUROCARE Working Group. Predictions of survival up to 10 years after diagnosis for European women with breast cancer in 2000-2002. Int J Cancer. 2013;132:2404–2412. doi: 10.1002/ijc.27895. [DOI] [PubMed] [Google Scholar]
- 2.Jansen MP, Foekens JA, van Staveren IL, Dirkzwager-Kiel MM, Ritstier K, Look MP, Meijer-van Gelder ME, Sieuwerts AM, Portengen H, Dorssers LC, Klijn JG, Berns EM. Molecular classification of tamoxifen-resistant breast carcinomas by gene expression profiling. J. Clin. Oncol. 2005;23:732–740. doi: 10.1200/JCO.2005.05.145. [DOI] [PubMed] [Google Scholar]
- 3.Jansen MP, Ruigrok-Ritstier K, Dorssers LC, van Staveren IL, Look MP, Meijer-van Gelder ME, Sieuwerts AM, Helleman J, Sleijfer S, Klijn JG, Foekens JA, Berns EM. Downregulation of SIAH2, an ubiquitin E3 ligase, is associated with resistance to endocrine therapy in breast cancer. Breast Cancer Res Treat. 2009;116:263–271. doi: 10.1007/s10549-008-0125-z. [DOI] [PubMed] [Google Scholar]
- 4.House CM, Moller A, Bowtell DD. Siah proteins: novel drug targets in the Ras and hypoxia pathways. Cancer Res. 2009;69:8835–8838. doi: 10.1158/0008-5472.CAN-09-1676. [DOI] [PubMed] [Google Scholar]
- 5.Nakayama K, Frew IJ, Hagensen M, Skals M, Habelhah H, Bhoumik A, Kadoya T, Erdjument-Bromage H, Tempst P, Frappell PB, Bowtell DD, Ronai Z. Siah2 regulates stability of prolylhydroxylases, controls HIF1alpha abundance, and modulates physiological responses to hypoxia. Cell. 2004;117:941–952. doi: 10.1016/j.cell.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Wong CS, Moller A. Siah: a promising anticancer target. Cancer Res. 2013;73:2400–2406. doi: 10.1158/0008-5472.CAN-12-4348. [DOI] [PubMed] [Google Scholar]
- 7.Ciechanover A. Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol. 2005;6:79–87. doi: 10.1038/nrm1552. [DOI] [PubMed] [Google Scholar]
- 8.Frasor J, Danes JM, Funk CC, Katzenellenbogen BS. Estrogen down-regulation of the corepressor N-CoR: mechanism and implications for estrogen derepression of N-CoR-regulated genes. Proc Natl Acad Sci U S A. 2005;102:13153–13157. doi: 10.1073/pnas.0502782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan P, Moller A, Liu MC, Sceneay JE, Wong CS, Waddell N, Huang KT, Dobrovic A, Millar EK, O’Toole SA, McNeil CM, Sutherland RL, Bowtell DD, Fox SB. The expression of the ubiquitin ligase SIAH2 (seven in absentia homolog 2) is mediated through gene copy number in breast cancer and is associated with a basal-like phenotype and p53 expression. Breast Cancer Res. 2011;13:R19. doi: 10.1186/bcr2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Confalonieri S, Quarto M, Goisis G, Nuciforo P, Donzelli M, Jodice G, Pelosi G, Viale G, Pece S, Di Fiore PP. Alterations of ubiquitin ligases in human cancer and their association with the natural history of the tumor. Oncogene. 2009;28:2959–2968. doi: 10.1038/onc.2009.156. [DOI] [PubMed] [Google Scholar]
- 11.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM Statistics Subcommittee of NCIEWGoCD. Reporting recommendations for tumor MARKer prognostic studies (REMARK) Breast Cancer Res Treat. 2006;100:229–235. doi: 10.1007/s10549-006-9242-8. [DOI] [PubMed] [Google Scholar]
- 12.Reijm EA, Timmermans AM, Look MP, Meijervan Gelder ME, Stobbe CK, van Deurzen CH, Martens JW, Sleijfer S, Foekens JA, Berns PM, Jansen MP. High protein expression of EZH2 is related to unfavorable outcome to tamoxifen in metastatic breast cancer. Ann Oncol. 2014;25:2185–2190. doi: 10.1093/annonc/mdu391. [DOI] [PubMed] [Google Scholar]
- 13.Phillips T, Murray G, Wakamiya K, Askaa J, Huang D, Welcher R, Pii K, Allred DC. Development of standard estrogen and progesterone receptor immunohistochemical assays for selection of patients for antihormonal therapy. Appl Immunohistochem Mol Morphol. 2007;15:325–331. doi: 10.1097/01.pai.0000213135.16783.bc. [DOI] [PubMed] [Google Scholar]
- 14.Ramirez-Ardila DE, Helmijr JC, Look MP, Lurkin I, Ruigrok-Ritstier K, van Laere S, Dirix L, Sweep FC, Span PN, Linn SC, Foekens JA, Sleijfer S, Berns EM, Jansen MP. Hotspot mutations in PIK3CA associate with first-line treatment outcome for aromatase inhibitors but not for tamoxifen. Breast Cancer Res Treat. 2013;139:39–49. doi: 10.1007/s10549-013-2529-7. [DOI] [PubMed] [Google Scholar]
- 15.Qi J, Tripathi M, Mishra R, Sahgal N, Fazli L, Ettinger S, Placzek WJ, Claps G, Chung LW, Bowtell D, Gleave M, Bhowmick N, Ronai ZA. The E3 ubiquitin ligase Siah2 contributes to castration-resistant prostate cancer by regulation of androgen receptor transcriptional activity. Cancer Cell. 2013;23:332–346. doi: 10.1016/j.ccr.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rizzardi AE, Rosener NK, Koopmeiners JS, Isaksson Vogel R, Metzger GJ, Forster CL, Marston LO, Tiffany JR, McCarthy JB, Turley EA, Warlick CA, Henriksen JC, Schmechel SC. Evaluation of protein biomarkers of prostate cancer aggressiveness. BMC Cancer. 2014;14:244. doi: 10.1186/1471-2407-14-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frew IJ, Hammond VE, Dickins RA, Quinn JM, Walkley CR, Sims NA, Schnall R, Della NG, Holloway AJ, Digby MR, Janes PW, Tarlinton DM, Purton LE, Gillespie MT, Bowtell DD. Generation and analysis of Siah2 mutant mice. Mol Cell Biol. 2003;23:9150–9161. doi: 10.1128/MCB.23.24.9150-9161.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt RL, Park CH, Ahmed AU, Gundelach JH, Reed NR, Cheng S, Knudsen BE, Tang AH. Inhibition of RAS-mediated transformation and tumorigenesis by targeting the downstream E3 ubiquitin ligase seven in absentia homologue. Cancer Res. 2007;67:11798–11810. doi: 10.1158/0008-5472.CAN-06-4471. [DOI] [PubMed] [Google Scholar]
- 19.Chen G, Gharib TG, Huang CC, Taylor JM, Misek DE, Kardia SL, Giordano TJ, Iannettoni MD, Orringer MB, Hanash SM, Beer DG. Discordant protein and mRNA expression in lung adenocarcinomas. Mol Cell Proteomics. 2002;1:304–313. doi: 10.1074/mcp.m200008-mcp200. [DOI] [PubMed] [Google Scholar]
- 20.Zhang B, Wang J, Wang X, Zhu J, Liu Q, Shi Z, Chambers MC, Zimmerman LJ, Shaddox KF, Kim S, Davies SR, Wang S, Wang P, Kinsinger CR, Rivers RC, Rodriguez H, Townsend RR, Ellis MJ, Carr SA, Tabb DL, Coffey RJ, Slebos RJ, Liebler DC, Nci C. Proteogenomic characterization of human colon and rectal cancer. Nature. 2014;513:382–387. doi: 10.1038/nature13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Celis JE, Kruhoffer M, Gromova I, Frederiksen C, Ostergaard M, Thykjaer T, Gromov P, Yu J, Palsdottir H, Magnusson N, Orntoft TF. Gene expression profiling: monitoring transcription and translation products using DNA microarrays and proteomics. FEBS Lett. 2000;480:2–16. doi: 10.1016/s0014-5793(00)01771-3. [DOI] [PubMed] [Google Scholar]
- 22.Scortegagna M, Subtil T, Qi J, Kim H, Zhao W, Gu W, Kluger H, Ronai ZA. USP13 enzyme regulates Siah2 ligase stability and activity via noncatalytic ubiquitin-binding domains. J Biol Chem. 2011;286:27333–27341. doi: 10.1074/jbc.M111.218214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nata T, Fujiya M, Ueno N, Moriichi K, Konishi H, Tanabe H, Ohtake T, Ikuta K, Kohgo Y. MicroRNA-146b improves intestinal injury in mouse colitis by activating nuclear factor-kappaB and improving epithelial barrier function. J Gene Med. 2013;15:249–260. doi: 10.1002/jgm.2717. [DOI] [PubMed] [Google Scholar]
- 24.Liao Y, Zhang M, Lonnerdal B. Growth factor TGF-beta induces intestinal epithelial cell (IEC-6) differentiation: miR-146b as a regulatory component in the negative feedback loop. Genes Nutr. 2013;8:69–78. doi: 10.1007/s12263-012-0297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia AI, Buisson M, Bertrand P, Rimokh R, Rouleau E, Lopez BS, Lidereau R, Mikaelian I, Mazoyer S. Down-regulation of BRCA1 expression by miR-146a and miR-146b-5p in triple negative sporadic breast cancers. EMBO Mol Med. 2011;3:279–290. doi: 10.1002/emmm.201100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhaumik D, Scott GK, Schokrpur S, Patil CK, Campisi J, Benz CC. Expression of microRNA-146 suppresses NF-kappaB activity with reduction of metastatic potential in breast cancer cells. Oncogene. 2008;27:5643–5647. doi: 10.1038/onc.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah M, Stebbins JL, Dewing A, Qi J, Pellecchia M, Ronai ZA. Inhibition of Siah2 ubiquitin ligase by vitamin K3 (menadione) attenuates hypoxia and MAPK signaling and blocks melanoma tumorigenesis. Pigment Cell Melanoma Res. 2009;22:799–808. doi: 10.1111/j.1755-148X.2009.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Guenther MG, Carthew RW, Lazar MA. Proteasomal regulation of nuclear receptor corepressor-mediated repression. Genes Dev. 1998;12:1775–1780. doi: 10.1101/gad.12.12.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma D, Saxena NK, Davidson NE, Vertino PM. Restoration of tamoxifen sensitivity in estrogen receptor-negative breast cancer cells: tamoxifen-bound reactivated ER recruits distinctive corepressor complexes. Cancer Res. 2006;66:6370–6378. doi: 10.1158/0008-5472.CAN-06-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moller A, House CM, Wong CS, Scanlon DB, Liu MC, Ronai Z, Bowtell DD. Inhibition of Siah ubiquitin ligase function. Oncogene. 2009;28:289–296. doi: 10.1038/onc.2008.382. [DOI] [PMC free article] [PubMed] [Google Scholar]


