Abstract
MicroRNAs (miRNA) play crucial roles in regulating cell proliferation, differentiation and developmental timing. Aberrantly expressed miRNAs have recently emerged as key regulators of metabolism. However, little is known about its role in tumor metabolism of cervical cancer. In this study, we determined the oncogenic effects of miRNAs on Warburg effect, a metabolic phenotype that allows cancer cells to utilize glucose even under aerobic conditions. A gain-of-function study was performed in 12 down-regulated miRNAs that frequently reported in cervical cancer. We found that miR-34a plays a suppressive role in Warburg effect as evidenced by decreased lactate production and glucose consumption. Knockdown of oncoprotein E6 expression of human papillomavirus in SiHa and HeLa cells by siRNAs lead to an increased protein level of p53, decreased level of miR-34a, as well as reduced Warburg effect. Subsequently, lactate dehydrogenase A (LDHA), which catalyzes the last key step in glycolysis, was identified as a direct target of miR-34a. Silencing of LDHA or introduction of miR-34a significantly attenuated colony formation ability and invasive capacity of SiHa and HeLa cells, and these effects were fully compromised by reintroduction of LDHA. In conclusion, our findings demonstrated that deregulated miR-34a/LDHA axis induced by HPV E6/p53 signaling facilitates tumor growth and invasion through regulating Warburg effect in cervical cancer, and provided new insights into the mechanism by which miR-34a contributes to the development and progression of cervical cancer.
Keywords: Human papillomavirus, miR-34a, Warburg effect, LDHA, cervical cancer
Introduction
Cervical cancer is one of the most common gynecological malignancies in women with an estimated global incidence of 493,243 cases and nearly 273,505 deaths per year (www.who.int/hpvcentre). Cervical cancer ranks as the fourth leading cause of cancer-related death among women worldwide [1]. Persistent human papillomaviruses (HPV) infection with is widely recognized as a cause of both intraepithelial neoplasia (CIN) and cervical cancer, however, the molecular mechanisms underlying HPV-related cervical cancer tumorigenesis and progression are still poorly unexplored [2].
MicroRNAs (miRNAs) are short non-coding RNAs that regulate gene expression at the posttranscriptional level by fully complementary or imperfect base-pairing with nucleotide sequences of target mRNAs [3,4]. Therefore, miRNAs exert profound effects on diverse biological through modulating target gene expression. Indeed, growing evidences suggest that miRNAs play crucial functions in multiple processes of cervical cancer, including cell proliferation [5], invasion [6], differentiation [7], apoptosis [8], and mitophagy [9].
The miR-34 family consists of three members: miR-34a, miR-34b, and miR-34c. miR-34a is encoded on chromosome 1p36.22 and deletions in this region are frequently observed in many human cancers [10]. In mice, miR-34a is ubiquitously expressed but with the highest expression in the brain. In human, apart from highly expressed in the ovary, prostate and testis, miR-34a is also found in brain, lung, thymus and kidney [11]. Notably, miR-34a is frequently down-regulated in tumors like gastric cancer [12], liver cancer [13] and prostate cancer [14]. miR-34a is a key modulator of tumor suppression. It regulates a plethora of target genes involved in cell cycle, differentiation and apoptosis, and antagonizes processes that are essential for cancer cell stemness, metastasis, and chemoresistance [15,16].
Reprogrammed energy metabolism is regarded as an emerging hallmark of cancer [17]. The aerobic glycolysis, known as Warburg effect, refers to a common phenomenon in tumors that cancer cells preferentially utilize glucose through glycolysis over oxidative phosphorylation even under aerobic conditions. Accumulating evidence suggests that various miRNAs are involved in the metabolic phenotypes of cancer cells [18-21]. However, limited knowledge is known about the relationship between deregulated miRNAs and Warburg effect in cervical cancer.
Although the tumor-suppressive role of miR-34a is well known, its implications in cancer metabolism remain unexplored. In this study, by a gain-of-function screen, we identified miR-34a as a regulator in glycolysis. Then we confirmed previous report that reduced levels of miR-34a in cervical cancer as a result from HPV oncoprotein E6 destabilization of p53. Furthermore, we demonstrated that the miR-34a/LDHA axis modulates the glycolytic pathway in cervical cancer, which consequently promotes tumor cell proliferation and invasion.
Materials and methods
Cell culture
The human cervical cancer cell line, HeLa and SiHa, were cultured in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% fetal bovine serum and 100 IU/mL penicillin, and 100 μg/mL streptomycin (Invitrogen, USA) at 37°C and 5% CO2 in a humidified incubator.
Transfection
miRNA mimics and inhibitors were synthesized by Ambion (Austin, USA). A total of 100 nM of miR-34a mimics or inhibitors and corresponding negative controls were diluted separately in 200 μL of Opti-MEM medium. After incubation for 15 min at room temperature, the oligonucleotides were added and the cells were incubated at 37°C in 5% CO2 for 48 hr. Small interfering RNAs (siRNAs) targeting LDHA were synthesized by GenePharma (Shanghai, China). The sequences of siRNAs targeting LDHA were as follows: 5’-TTGTTGATGCATCGAGG-3’ (sense) and 5’-GGGTCCTTGGGGAACATG-3’ (sense). A synthetic double-stranded siRNA targets the HPV16 E6 coding region was generated as described by Tang et al. [22]. Cells were transfected with Lipofectamine® RNAiMAX reagent (Invitrogen, USA) following the manufacturer’s instructions.
Measurement of lactate production and glucose consumption
Cancer cells were incubated in DMEM without phenol red for 24 hr, and the culture medium was then harvested for measurement of lactate or glucose concentrations. Lactate levels were determined using the Lactate Assay kit (BioVision, USA) and glucose levels were measured using a glucose assay kit (Sigma-Aldrich, USA). The lactate or glucose concentration was calculated using a standard calibration curve prepared under the same condition and reported in a microplate reader. All values were normalized to total protein levels (BCA Protein Assay Kit, Thermo Scientific, USA).
Western blotting
Cells lysate was was prepared using RIPA lysis buffer in the presence of protease inhibitors. The protein concentrations were determined by BCA Protein Assay Kit (Thermo Scientific, USA). Antibody for p53 and Tubulin was purchased from Abcam. Proteins were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and transferred into a nitrocellulose membrane (Bio-Rad, USA). Then, the membrane was blocked with 5% non-fat milk and incubated with primary antibodies. The proteins were detected using enhanced chemiluminescence reagents (Thermo Scientific). After rinsing with TBS, the membrane was incubated overnight at 4°C with a primary antibody. The immunoreactivity was detected with an enhanced chemiluminescence substrate (Thermo Scientific, USA).
Northern blot analysis
The Northern blot analysis was performed as described by Wang et al. [22]. The antisense oligodeoxynucleotide probes were designed based on individual miRNA sequences derived from miRBase (http://www.mirbase.org/). An antisense oligodeoxynucleotide (oST197, 59-AAAATATGGA ACGCTTCACGA-39) targeting U6 snRNA was used as a control.
Total RNA extraction and real-time PCR
Total RNA was extracted from SiHa or HeLa cells using TRIzol® reagent (TaKaRa, Biotech Co., Ltd, Dalian, China). Complementary DNA (cDNA) was synthesized using Complementary DNA was synthesized using the PrimeScript™ RT reagent Kit (TaKaRa, Tokyo, Japan). Quantitative real-time PCR was performed with were performed using SYBR Premix Ex Taq II (TaKaRa, Biotech Co., Ltd, Dalian, China) according to the manufacturer’s instruction. The relative mRNA expression was calculated and normalized to β-actin using the comparative CT method. Primers used in this study were deposited in primerBank (http://pga.mgh.harvard.edu/primerbank/). The miRNAs were detected with specific primers and probes using TaqMan® MicroRNA Assays (Applied Biosystems, USA). The relative miRNA expression was calculated and normalized to U6B snRNA.
Luciferase reporter assays
SiHa or HeLa cells (1 × 104 cells per well) were planted in 96-well plates and transfected with indicated miR-34a mimics or inhibitors by using Lipofectamine 2000 (Thermo Fisher Scientific, USA) according to the manufacturer’s instructions. After incubation for 24 hr, the cells were cotransfected with the testing firefly luciferase reporter plasmid together with a Renilla luciferase plasmid for another 24 hr. Then the cells were harvested and supernatant of the cell lysate was analyzed for dualluciferase activities using Dual-Glo® Luciferase Assay System (Promega, Cat. E2920). Relative luciferase activity was calculated by dividing the light unit readings obtained from a firefly luciferase reporter construct by the light unit readings obtained from the Renilla luciferase reporter.
Colony formation assays
Single SiHa or HeLa cells (500 cells per well) were planted in 35 mm well plates overnight for cells attachment. Cells with indicated treatment were allowed to growth for another two weeks. Then SiHa or HeLa cells were fixed with 4% paraformaldehyde for 1 hr, and stained with 0.5% crystal violet. After gently washing with PBS for three times, the number of colonies was evaluated under microscope. And cell cluster with more than 30 cells was considered as a colony. The experiments were performed independently in three replicates.
Transwell invasion assay
Transwell (Corning Costar, USA) were coated with 100 μL Matrigel (BD Biosciences, USA) diluted 1:6 in serum-free DMEM. The lower chamber of Transwell was supplemented with 5% FBS as chemoattractant. After Matrigel solidification, 1 × 104 SiHa or HeLa cells in 200 μL serum-free DMEM were added into Transwell inserts and incubated for 48 hr. The cells on the top chamber of the Transwell filter were removed and cells on the bottom side of the filter were stained by 0.5% crystal violet. The number of invaded cells was counted in at least six randomized fields.
Statistical analysis
Each experiment was repeated for at least three times independently. All the values were reported as means ± SDs. Differences between experimental and control groups were analyzed by Student’s t-test or one-way ANOVA. P < 0.05 was considered as statistically significant.
Results
miR-34a inhibits Warburg effect in cervical cancer
To extensively assess the effects of miRNAs on the aberrant glycolytic activity of cervical cancer cells, a screening of 12 well known deregulated miRNAs in cervical cancer was initially performed. As an indicator of glycolysis, lactate level was used to measure the effect induced by miRNAs in HeLa (Figure 1A) and SiHa (Figure 1B) cells. Several miRNAs (miR-23b, miR-34a, miR-101, miR-143, miR-572) involved in glycolysis were deregulated in cervical cancer. Two miRNAs (miR-34a and miR-143) significantly reduced lactate production in both HeLa and SiHa cells. In this study, we focused on the roles of miR-34a. To further confirm the correlation between miR-34a and Warburg effect, we detected the glucose consumption in the presence of miR-34a mimics (Figure 1C and 1D). Compared to negative control cells, cervical cancer cells with exogenous miR-34a consumed less glucose. And expectedly, suppression of miR-34a significantly promoted glucose consumption in HeLa and SiHa cells. Taken together, these data above suggest that deregulated miR-34a is critically associated with Warburg effect in cervical cancer.
Figure 1.
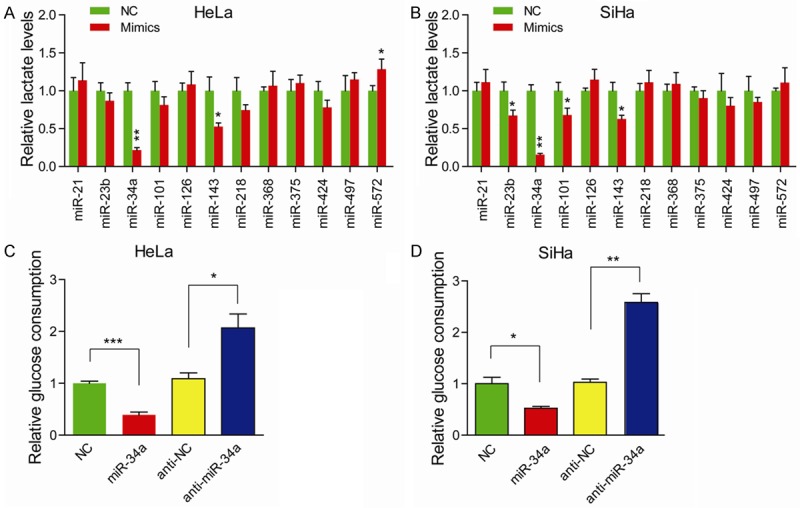
miR-34a inhibits Warburg effect in cervical cancer. Lactate production was measured in HeLa (A) and SiHa (B) cells after transfection of indicated miRNAs mimics; NC versus Mimics, *P < 0.05, **P < 0.01. Glucose consumption was measured in HeLa (C) and SiHa (D) cells with miR-34a introduction or inhibition; *P < 0.05, **P < 0.01, ***P < 0.001.
HPV oncoprotein E6 reduces miR-34a expression by regulating p53
High-risk HPV infection contributes to aberrant expression of cellular oncogenic miRNAs. It has been widely demonstrated that E6 and E7 oncoproteins can respectively inactivate p53 and pRB, two major cellular tumor suppressors, thereby contributing to progression of cervical cancer [23,24]. As miR-34a was a direct target of p53, we determined whether the E6/p53 axis contributed to aberrant expression of miR-34a. Indeed, protein level of p53 was remarkably reduced by knockdown of E6 oncoprotein (Figure 2A). Meanwhile, silencing of p53 by specific siRNAs significantly upregulated miR-34a expression (Figure 2B and 2C). Furthermore, knockdown of E6 also decreased the lactate production (Figure 2D) and glucose consumption (Figure 2E) in SiHa and HeLa cells, indicating an oncogenic role of E6 oncoprotein in Warburg effect through targeting miR-34a.
Figure 2.
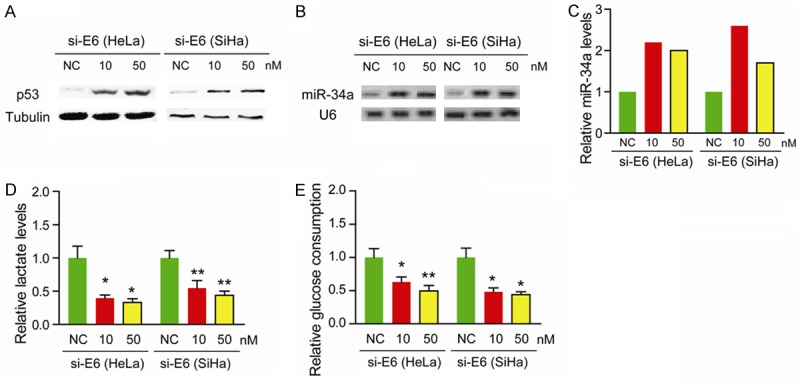
HPV oncoprotein E6 reduces miR-34a expression by regulating p53. (A) Protein level of p53 in E6 siRNA-treated HeLa and SiHa cells was measured by Western blotting. Tubulin was loaded as a control. (B) The expression of miR-34a in E6 siRNA-treated HeLa and SiHa cells was measured by miRNA ligation analysis. (C) Bar graph showing relative miR-34a levels after being normalized to U6 snRNA. Lactate production (D) and glucose consumption (E) were measured in E6 siRNA-treated HeLa and SiHa cells; *P < 0.05, **P < 0.01.
LDHA is a direct target of miR-34a
The lactate dehydrogenase A (LDHA), which is responsible for the inter-conversion of pyruvate and L-lactate, plays a critical branch point in metabolism of tumor cells. By a miRNA target prediction programs, miRBase, we found LDHA was a potential target of miR-34a (Figure 3A). Treated HeLa and SiHa cells with miR-34a significantly downregulated LDHA expression at both mRNA level (Figure 3B) and protein level (Figure 3C). Then the wild type and mutant type of 3’-untranslated regions (UTRs) of LDHA were introduced into luciferase reporter plasmids. The result showed that miR-34a significantly decreased the luciferase activity of the plasmid with wild type LDHA 3’-UTR in HeLa (Figure 3D) and SiHa (Figure 3E) cells, whereas the activity of the mutant plasmid remained unaltered, indicating that LDHA is a direct target for miR-34a in cervical cancer.
Figure 3.
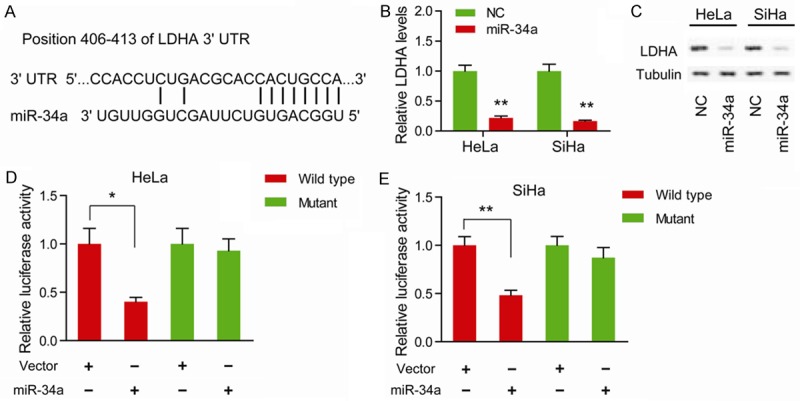
LDHA is a direct target of miR-34a. (A) In silico algorithms predicted LDHA contains one 7-mer putative miR-34a-binding site on its 3’-untranslated regions. (B) The mRNA level of LDHA in miR-34a mimics-treated HeLa and SiHa cells was measured by quantitative real-time PCR; NC versus Mimics, *P < 0.05, **P < 0.01. (C) The protein level of LDHA in miR-34a mimics-treated HeLa and SiHa cells was measured by Western blotting. Tubulin was loaded as a control. The direct physical interaction between miR-34a and LDHA 3’-untranslated regions in HeLa (D) and SiHa (E) cells was demonstrated by luciferase reporter assay; mutation of putative miR-34a binding site on LDHA 3’-untranslated region blocked the inhibitory effect of miR-34a on luciferase activity; *P < 0.05, **P < 0.01.
miR-34a/LDHA axis promotes tumor progression of cervical cancer
Upregulated expression of LDHA has been reported in many human malignancies [25-28]. To uncover the oncogenic roles of LDHA in cervical cancer, loss-of-function study was performed. Two specific siRNAs targeting LDHA resulted in evidently decrease in the protein level of LDHA (Figure 4A). Given the important role of LDHA in cancer cell metabolism, we further investigated the cellular function of LDHA. As expected, silencing of LDHA significantly decreased the colony formation ability (Figure 4B) and invasive capacity (Figure 4C) in HeLa and SiHa cells, whereas overexpression of LDHA promoted tumor progression by facilitating cell proliferation and invasion (Figure 4E and 4F). Introduction of miR-34a fully recapitulated the suppressive effects induced by inhibition of LDHA. Moreover, restoration of LDHA rescued the miR-34a-induced decrease in colony formation ability (Figure 4E) and invasive capacity (Figure 4F). Representative immunoblots of LDHA protein upon introduction of miR-34a or overexpression of LDHA were shown in Figure 4D. Taken together, these results suggest that miR-34a might regulate glycolysis by modulating LDHA expression in cervical cancer cells.
Figure 4.
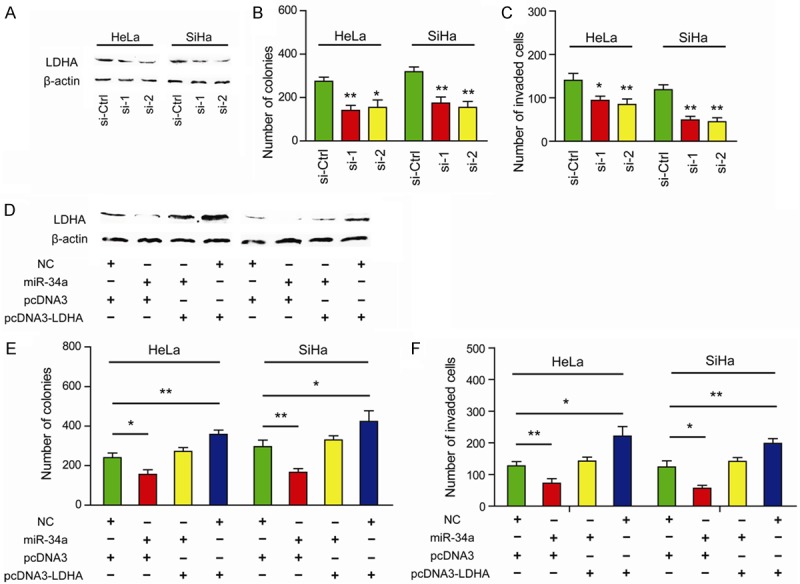
miR-34a/LDHA axis promotes tumor progression of cervical cancer. (A) The protein level of LDHA in LDHA siRNA-treated HeLa and SiHa cells was detected by quantitative real-time PCR. (B) Colony formation ability of HeLa and SiHa cells was also significantly reduced upon LDHA knockdown; si-Ctrl versus si-1 or si-2, *P < 0.05, **P < 0.01. (C) Knockdown of LDHA inhibited cell invasive capacity as revealed by Matrigel invasion chamber; si-Ctrl versus si-1 or si-2, *P < 0.05, **P < 0.01. (D) Representative immunoblots of LDHA protein upon introduction of miR-34a or overexpression of LDHA. Ectopic expression of miR-34a inhibited colony formation ability (E) and cell invasive capacity (F) in HeLa and SiHa cells, and restoration of LDHA completely abolished miR-34a-mediated effects; *P < 0.05, **P < 0.01.
Discussion
The Warburg effect, an emerging hallmark of cancer cells, draws great attentions in recent years due to its crucial role in the maintenance and development of tumor cells. Although a spectrum of aberrant miRNAs has been revealed in cervical cancer, little is known about their role in Warburg effect. In this study, we demonstrated that HPV E6-mediated down-regulation of miR-34a inhibited Warburg effect through targeting LDHA.
Given lactate is a representative product in glycolysis and has been widely detected by commercial kit, we therefore used the extracellular lactate level as the indicator of Warburg effect. Based on gain-of-function study, we found that miR-34a inhibited the lactate production in both HeLa and SiHa cells. Inhibition of miR-34a increased glucose consumption, indicating that miR-34a was critically involved in Warburg effect. Notably, miR-143 was also found statistically significant in suppression of lactate production. Previously, it has been reported that miR-143 inhibits prostate cancer progression through targeting hexokinase 2, which is a key enzyme in glucose metabolism [29]. However, whether this observation is exist in cervical cancer remains further investigation.
HPV16 and HPV18 are two most common genotypes that contribute to more than 70% of all cervical cancer. Two early viral oncoproteins, E6 and E7, of HPV16 and HPV18 are responsible for HPV-associated cervical cancer [30]. It has been widely demonstrated that E6 and E7 oncoproteins respectively inactivate the tumor suppressor gene p53 and pRb [23,24]. And many cellular transcriptional factors, especially p53, have been demonstrated to regulate miRNA transcription [31]. Given E6 oncoprotein could induce degradation of p53, it is reasonable that viral E6 causes deregulated expression of cellular miRNAs. As a direct target of p53, miR-34a has emerged as a tumor suppressor in various malignancies through inhibition of multiple targets [15]. Consistent with these notions, knockdown of E6 oncoprotein resulted in reduced level of p53 and miR-34a. And in line with miR-34a-mediated suppression of Warburg effect, knockdown of E6 oncoprotein also caused a drastic decrease in lactate production and glucose consumption.
Furthermore, we demonstrated that miR-34a reprogrammed the glycolysis of cervical cancer by directly targeting LDHA, which is frequently up-regulated in human cancers [32-34]. Ectopic expression of miR-34a or knockdown of LDHA attenuated tumor growth and invasion. As Warburg effect not only provides cancer cells with building blocks through pentose phosphate pathways (PPP), but also alters extracellular acidification through increased production of lactate, it is convincible that miR-34a/LDHA axis exhibits a tumor-suppressive role in cervical cancer [35,36].
In conclusion, our results showed for the first time that miR-34a inhibits tumor progression of cervical cancer by influencing Warburg effect. Surprisingly, the miR-34a mimic has become the first miRNA to reach Phase1 clinical trial in 2013 (http://clinicaltrials.gov/ct2/show/NCT01829971). Thus our study provide a proof of principle that miR-34a is a potential target in cervical cancer.
Acknowledgements
Project Supported by the Natural Science Foundation of China (No. 81472431) and grant from Medical Science and Technology Development Foundation, Nanjing Department of Health (No. ykk14154).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Castle PE. Gynaecological cancer: New standard of care-HPV testing for cervical cancer screening. Nat Rev Clin Oncol. 2015;12:194–196. doi: 10.1038/nrclinonc.2015.37. [DOI] [PubMed] [Google Scholar]
- 3.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 4.Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan Z, Cui H, Xu X, Lin Z, Zhang X, Kang L, Han B, Meng J, Yan Z, Yan X, Jiao S. MiR-125a suppresses tumor growth, invasion and metastasis in cervical cancer by targeting STAT3. Oncotarget. 2015;6:25266–25280. doi: 10.18632/oncotarget.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang HW, Wang F, Wei Q, Zhao YF, Liu M, Li X, Tang H. miR-20a promotes migration and invasion by regulating TNKS2 in human cervical cancer cells. FEBS Lett. 2012;586:897–904. doi: 10.1016/j.febslet.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 7.Leung CO, Deng W, Ye TM, Ngan HY, Tsao SW, Cheung AN, Pang RT, Yeung WS. miR-135a leads to cervical cancer cell transformation through regulation of beta-catenin via a SIAH1-dependent ubiquitin proteosomal pathway. Carcinogenesis. 2014;35:1931–1940. doi: 10.1093/carcin/bgu032. [DOI] [PubMed] [Google Scholar]
- 8.Wang YD, Cai N, Wu XL, Cao HZ, Xie LL, Zheng PS. OCT4 promotes tumorigenesis and inhibits apoptosis of cervical cancer cells by miR-125b/BAK1 pathway. Cell Death Dis. 2013;4:e760. doi: 10.1038/cddis.2013.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li QQ, Zhang L, Wan HY, Liu M, Li X, Tang H. CREB1-driven expression of miR-320a promotes mitophagy by down-regulating VDAC1 expression during serum starvation in cervical cancer cells. Oncotarget. 2015;6:34924–34940. doi: 10.18632/oncotarget.5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henrich KO, Schwab M, Westermann F. 1p36 tumor suppression--a matter of dosage? Cancer Res. 2012;72:6079–6088. doi: 10.1158/0008-5472.CAN-12-2230. [DOI] [PubMed] [Google Scholar]
- 11.Agostini M, Knight RA. miR-34: from bench to bedside. Oncotarget. 2014;5:872–881. doi: 10.18632/oncotarget.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S, Chen P, Huang Z, Hu X, Chen M, Hu S, Hu Y, Cai T. Sirt7 promotes gastric cancer growth and inhibits apoptosis by epigenetically inhibiting miR-34a. Sci Rep. 2015;5:9787. doi: 10.1038/srep09787. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Gougelet A, Sartor C, Bachelot L, Godard C, Marchiol C, Renault G, Tores F, Nitschke P, Cavard C, Terris B, Perret C, Colnot S. Antitumour activity of an inhibitor of miR-34a in liver cancer with beta-catenin-mutations. Gut. 2015 doi: 10.1136/gutjnl-2014-308969. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, Wiggins JF, Bader AG, Fagin R, Brown D, Tang DG. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Misso G, Di Martino MT, De Rosa G, Farooqi AA, Lombardi A, Campani V, Zarone MR, Gulla A, Tagliaferri P, Tassone P, Caraglia M. Mir-34: a new weapon against cancer? Mol Ther Nucleic Acids. 2014;3:e194. doi: 10.1038/mtna.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010;17:193–199. doi: 10.1038/cdd.2009.56. [DOI] [PubMed] [Google Scholar]
- 17.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Jin LH, Wei C. Role of microRNAs in the Warburg effect and mitochondrial metabolism in cancer. Asian Pac J Cancer Prev. 2014;15:7015–7019. doi: 10.7314/apjcp.2014.15.17.7015. [DOI] [PubMed] [Google Scholar]
- 19.Gao P, Sun L, He X, Cao Y, Zhang H. MicroRNAs and the Warburg Effect: new players in an old arena. Curr Gene Ther. 2012;12:285–291. doi: 10.2174/156652312802083620. [DOI] [PubMed] [Google Scholar]
- 20.Wei Z, Cui L, Mei Z, Liu M, Zhang D. miR-181a mediates metabolic shift in colon cancer cells via the PTEN/AKT pathway. FEBS Lett. 2014;588:1773–1779. doi: 10.1016/j.febslet.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 21.Masui K, Tanaka K, Akhavan D, Babic I, Gini B, Matsutani T, Iwanami A, Liu F, Villa GR, Gu Y, Campos C, Zhu S, Yang H, Yong WH, Cloughesy TF, Mellinghoff IK, Cavenee WK, Shaw RJ, Mischel PS. mTOR complex 2 controls glycolytic metabolism in glioblastoma through FoxO acetylation and upregulation of c-Myc. Cell Metab. 2013;18:726–739. doi: 10.1016/j.cmet.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Wang HK, McCoy JP, Banerjee NS, Rader JS, Broker TR, Meyers C, Chow LT, Zheng ZM. Oncogenic HPV infection interrupts the expression of tumor-suppressive miR-34a through viral oncoprotein E6. RNA. 2009;15:637–647. doi: 10.1261/rna.1442309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez SL, Stremlau M, He X, Basile JR, Munger K. Degradation of the retinoblastoma tumor suppressor by the human papillomavirus type 16 E7 oncoprotein is important for functional inactivation and is separable from proteasomal degradation of E7. J Virol. 2001;75:7583–7591. doi: 10.1128/JVI.75.16.7583-7591.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xian ZY, Liu JM, Chen QK, Chen HZ, Ye CJ, Xue J, Yang HQ, Li JL, Liu XF, Kuang SJ. Inhibition of LDHA suppresses tumor progression in prostate cancer. Tumour Biol. 2015;36:8093–8100. doi: 10.1007/s13277-015-3540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu H, Jackson AL, Kilgore JE, Zhong Y, Chan LL, Gehrig PA, Zhou C, Bae-Jump VL. JQ1 suppresses tumor growth through downregulating LDHA in ovarian cancer. Oncotarget. 2015;6:6915–6930. doi: 10.18632/oncotarget.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arseneault R, Chien A, Newington JT, Rappon T, Harris R, Cumming RC. Attenuation of LDHA expression in cancer cells leads to redoxdependent alterations in cytoskeletal structure and cell migration. Cancer Lett. 2013;338:255–266. doi: 10.1016/j.canlet.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 28.Yao F, Zhao T, Zhong C, Zhu J, Zhao H. LDHA is necessary for the tumorigenicity of esophageal squamous cell carcinoma. Tumour Biol. 2013;34:25–31. doi: 10.1007/s13277-012-0506-0. [DOI] [PubMed] [Google Scholar]
- 29.Zhou P, Chen WG, Li XW. MicroRNA-143 acts as a tumor suppressor by targeting hexokinase 2 in human prostate cancer. Am J Cancer Res. 2015;5:2056–2063. [PMC free article] [PubMed] [Google Scholar]
- 30.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 31.Zheng ZM, Wang X. Regulation of cellular miRNA expression by human papillomaviruses. Biochim Biophys Acta. 2011;1809:668–677. doi: 10.1016/j.bbagrm.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao D, Zou SW, Liu Y, Zhou X, Mo Y, Wang P, Xu YH, Dong B, Xiong Y, Lei QY, Guan KL. Lysine-5 acetylation negatively regulates lactate dehydrogenase A and is decreased in pancreatic cancer. Cancer Cell. 2013;23:464–476. doi: 10.1016/j.ccr.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Wang H, Liu A, Fang C, Hao J, Wang Z. Lactate dehydrogenase A negatively regulated by miRNAs promotes aerobic glycolysis and is increased in colorectal cancer. Oncotarget. 2015;6:19456–19468. doi: 10.18632/oncotarget.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheng SL, Liu JJ, Dai YH, Sun XG, Xiong XP, Huang G. Knockdown of lactate dehydrogenase A suppresses tumor growth and metastasis of human hepatocellular carcinoma. FEBS J. 2012;279:3898–3910. doi: 10.1111/j.1742-4658.2012.08748.x. [DOI] [PubMed] [Google Scholar]
- 35.Xu XD, Shao SX, Jiang HP, Cao YW, Wang YH, Yang XC, Wang YL, Wang XS, Niu HT. Warburg effect or reverse Warburg effect? A review of cancer metabolism. Oncol Res Treat. 2015;38:117–122. doi: 10.1159/000375435. [DOI] [PubMed] [Google Scholar]
- 36.Asgari Y, Zabihinpour Z, Salehzadeh-Yazdi A, Schreiber F, Masoudi-Nejad A. Alterations in cancer cell metabolism: the Warburg effect and metabolic adaptation. Genomics. 2015;105:275–281. doi: 10.1016/j.ygeno.2015.03.001. [DOI] [PubMed] [Google Scholar]


