Abstract
Breast cancer (BCa) is the most common cancer in Mexican women. Certain risk factors, such as environmental and lifestyle factors have been implicated in BCa initiation and progression. Moreover, genetic factors, such as single nucleotide polymorphisms (SNPs) of the P450 system, have been reported in BCa. In this report, and for the first time in the literature, we analyzed the rs5602 (67730 T > C) polymorphism in the CYP8A1 in patients with BCa and in healthy Mexican women to identify a potential risk between this polymorphism and BCa. Leukocyte cells from 38 control patients and tissue from radical mastectomy surgeries in 64 BCa patients were used for polymorphism analysis using an allelic discrimination assay with TaqMan probes. Links with clinic-pathological characteristics were also analyzed. Statistical analysis was performed using the standard χ2 or Fisher exact test statistic. All CYP8A1 genotypes were detected in patients with BCa and the controls. Significant differences were observed in the distribution of CYP8A1 genotypes between the patients and controls (P=0.0008) and allele C was significantly associated with BCa risk (OR 2.08, 95% CI 1.166-3.72, P=0.0178). All polymorphism frequencies were in Hardy-Weinberg Equilibrium (HWE) in the controls (P > 0.05). We found that variant 67730 T > C was significantly associated with an increased risk of BCa (P < 0.05). We not observed an association of the TT and TC + CC genotypes with the clinical stage, BIRADS, estrogen receptor (ER) status, progesterone receptor (PR) status, HER2 status, p53 status, CD34 status, metastasis or therapy use. These results indicate that the CYP8A1 rs5602 SNP is a possible risk factor for BCa in Mexican women. This study showed an association between the CYP8A1 polymorphism and BCa risk in a Mexican population.
Keywords: Cytochrome P450 8A1, polymorphism, breast cancer, Mexican women
Introduction
Breast cancer (BCa) is the most common malignancy of women and is the leading type of cancer in Mexican females [1,2]. BCa is heterogeneous disease and prognosis varies in individual patients and a number of risk factors have been identified among these, a great number are linked to nutrition, life style and environmental factors [3,4]. Enzyme expression studies have showed that in breast evidence exists for the involvement of a genotoxic mechanism in the carcinogenic process and metabolic activation of suspected carcinogens in the form of DNA adducts [5,6]. The human cytochrome P450 (CYP450) system consists of a number of CYP isoforms that its function has pharmacologic and toxic effects. It has been shown that xenobiotics are mainly metabolized by the CYP 1-3 families suggesting that endogenous substrates are transformed by other families [7]. The CYP450s family has been involved in tumor initiation and promotion, because they can activate or deactivate carcinogens and can influence the response of anti-cancer drugs in tumor cells [8,9].
Searching for prognostic and predictive biomarkers in BCa, allows a molecular characterization of cancer signatures and provides relevant information aimed at personalized treatment. Genetic variability and the occurrence of specific polymorphisms may participate in susceptibility to tumors, and in the type of response to the therapy used [10,11].
The CYP8A1 (Prostacyclin I2 synthase; PGIS) acts as an isomerase and catalyze the formation of prostaglandin H2 (PGH2) into prostaglandin I2 (PGI2) [12]. Prostacyclin play a role in preventing cancer and as CYP8A1 is the responsible enzyme for the production of prostacyclin may be important in cancer prevention [13,14]. CYP8A1 signaling through arachidonic acid metabolism affects tumor cells including suppression of inflammation and cell proliferation, promotion of apoptosis, prevention of metastasis, and reduced growth of established metastases [13,15] PGI2 is a potent anti-metastatic cancer agent [15,16]. The association between CYP8A1 single nucleotide polymorphisms (SNP) and cancer (lung, colorectal, thyroid and breast) has been well documented [17-20]. Homozygotes for the minor alleles of rs5602 (67730 T > C), rs477627 (9650 T > C) and rs6125671 (14110 G > A) were associated with an increased BCa risk and a protective effect was observed for minor allele homozygotes with the rs477627 (9650 T > C) polymorphism in a Caucasian population [20]. For rs6095541 (48110188 C > T) and rs6095543 (48113300 T > C) in women with progesterone positive BCa, an association of BCa with this allele in Caucasian individuals has been shown [21]. Recently, 27 genetic variants in CYP8A1 gene were identified by DNA sequencing analysis in Korean individuals. Of these variations, 19 SNPs were identified in non-codificant regions (four newly identified SNPs). The authors showed no particular intronic SNPs with alternative splicing events [22].
In a past study, we detect the CYP8A1 CC genotype for SNP rs56195291 (60020 C > G) in Mexican women diagnosed with BCa; however, in this study, no association between CYP8A1 polymorphism and BCa risk was detected [23]. The objective of this work was investigate, for the first time, the association between CYP8A1 rs5602 (67730 T > C) polymorphism and the potential risk for BCa susceptibility.
Material and methods
Biological samples
All samples were collected from the Service of Pathology, Military Hospital for Women and Neonatology, Secretaría de la Defensa Nacional (SEDENA) in Mexico City. This study included 64 samples from radical mastectomy surgeries of patients with BCa (mean age of 56.2±11.8 years from 31 to 81 years old). In addition, 38 samples of peripheral blood from healthy women (mean age of 67.5±4.9 years from 55 to 84 years old) were recruited as control. All samples were obtained for polymorphism analysis and informed written consent was obtained to participate in this study. Collection information of demographic status, tumor characteristics, as well as anthropometric measures, reproductive and medical history and lifestyle behavior in patients was used. None of the healthy women had cancer or a family history of cancer. The Ethical approval was provided by the Bioethics and Research committees with registration number SI-378. The human experimentation guidelines of these committees were followed.
Allelic discrimination assay
Isolation of genomic DNA was performed using the DNeasy Blood & Tissue Kit (Qiagen; Germantown, MD, USA) according to the manufacturer’s protocol. The DNA concentration and purity were measured using Nanodrop Spectrophotometer (Delaware, USA). All extracted DNAs were stored at -80°C.
Genotyping of the CYP8A1 (67730 T > C) rs5602 polymorphism was performed using PCR and sequencing. The primer and probe sequences used were examined by BLAST to confirm their specificity. The synthesized primers and probes (Applied Biosystems; CA, USA) were optimized at 61°C. Primers and probes were designed according to the sequence of CYP8A1 as follows: forward primer 5’-GCACTTCAGTATCTCAGGTAAC-3’ and reverse primer 5’-CCTTGGAACCACAGTCATTAG-3’. T-allele probe: 5’-FAM-CCTGGTGGGAGCACATCTTTTCCTTGA-TAMRA-3’ and C-allele probe VIC: 5’-VIC-CCTGGTGGGAGCACGTCTTTTCCTTGA-TAMRA-3. PCR amplicon was 96 bp. PCR was performed in a final volume of 20 µL (95°C for 5 min and 30 cycles of 95°C for 15 s and 61°C for 1 min). The reaction mixture contained 12.5 µL of TaqMan 2X Genotyping Master Mix (Applied Biosystems; CA, USA), 200 nM FAM-labeled probe, 200 nM VIC-labeled probe, 0.4 µM forward primer, 0.4 µM reverse primer and 100 ng of genomic DNA.
DNA sequencing
To confirm the results of DNA genotyping, 20 randomly selected RT-PCR products were cleaned with ExoSAP-IT (Affymetrix, USB-Products, CA, USA). Presences of the polymorphism were determined by PCR sequencing using the BigDye® Terminator v. 3.1 Sequencing Kit (Applied Biosystems; CA, USA). The sequencing reaction contained 2 μL of PCR product, 2 μM of primer and 2 μL of sequencing buffer. Cycling conditions were 96°C for 1 min and 25 cycles at 96°C for 30 s, 50°C for 15 s and 60°C for 3 min and extension products were diluted with 5 μL of nuclease-free water and purified with CENTRI-SEP™ Spin Columns (Applied Biosystems; CA, USA). Finally, was added 10 μL of Hi-Di™ Formamide (Applied Biosystems; CA, USA). Analysis was performed on a capillary automated sequencer ABI PRISM® 3100 Avant Genetic Analyzer (Applied Biosystems; CA, USA).
Statistical analysis
The Hardy-Weinberg (HWE) equilibrium test (in the controls) was used as a quality control measure for genotyping using the standard χ2 statistic. χ2 or exact Fisher test were used for calculate odds ratio (OR) and confidence intervals (CI) and evaluate the relation to the CYP8A1 polymorphism between BCa risk and clinic pathological factors. All statistical analysis was performed using GraphPad Prism version 6.0 software. A P-value less than 0.05 was considered statistically significant.
Results
Clinic-pathological data from 64 patients were included and are presented in the Table 1. The mean age was 56.2 years (SD=11.8) and the age range was 31-81 years. A total of 50 women (78.1%) were diagnosed with invasive ductal BCa, 9 (14.1%) with invasive lobular and 5 (7.8%) with invasive mixed BCa.
Table 1.
Clinic-pathological characterization of the patients
| Characteristics | Total (N=64) |
|---|---|
| Average age (years) | 56.2±11.8 |
| Alcohol | |
| Yes | 7 (11%) |
| No | 57 (89%) |
| Tobacco | |
| Yes | 7 (11%) |
| No | 57 (89%) |
| Drugs | |
| Yes | 0 (0%) |
| No | 64 (100%) |
| Exposure to biomass | |
| Yes | 10 (15.6%) |
| No | 54 (84.4%) |
| Pregnancies | |
| 0-2 | 12 (18.8%) |
| > 2 | 52 (81.2%) |
| Menarche | |
| < 12 years | 10 (15.6%) |
| ≥ 12 years | 54 (84.4%) |
| Oral contraceptives or HRT use | |
| Yes | 15 (23.4%) |
| No | 49 (76.6%) |
| Chronic diseases | |
| Yes | 28 (43.8%) |
| No | 36 (56.2%) |
| Family history of cancer | |
| Yes | 19 (29.7%) |
| No | 45 (70.3%) |
| Place of residence | |
| North | 7 (10.9%) |
| Center | 53 (82.8%) |
| South | 4 (6.3%) |
| Birthplace | |
| North | 3 (4.7%) |
| Center | 56 (87.5%) |
| South | 5 (7.8%) |
| ER status | |
| Positive | 40 (62.5%) |
| Negative | 24 (37.5%) |
| PR status | |
| Positive | 39 (60.9%) |
| Negative | 25 (39.1%) |
| HER2 status | |
| Positive | 34 (53.1%) |
| Negative | 30 (46.9%) |
| Ki67 status | |
| Positive | 64 (100%) |
| Negative | 0 (0%) |
| p53 status | |
| Positive | 36 (56.3%) |
| Negative | 28 (43.7%) |
| CD34 status | |
| < 10-15 vessels | 17 (26.6%) |
| ≥ 10-15 vessels | 47 (73.4%) |
| Histological grade | |
| Grade I | 4 (6.3%) |
| Grade II | 10 (15.6%) |
| Grade III | 50 (78.1%) |
| BIRADS | |
| < 3 | 2 (3.1%) |
| ≥ 3 | 62 (96.9%) |
| BMI (kg/m2) | |
| 12-18.4 | 0 (0%) |
| 18.5-24.9 | 15 (23.4%) |
| 25-29.9 | 25 (39.1%) |
| ≥ 30 | 24 (37.5%) |
| Menopausal status | |
| < 52 years | 40 (62.5%) |
| ≥ 52 years | 16 (25%) |
| Not menopausal | 8 (12.5%) |
| Metastasis | |
| Yes | 17 (26.6%) |
| No | 47 (73.4%) |
| Therapy | |
| Yes | 31 (48.4%) |
| No | 33 (51.6%) |
| Average age of menarche (years) | 13.1±1.6 |
| Average age of menopausal (years) | 47.1±5.2 |
| Average number of pregnancies | 5±3 |
| Average BMI (kg/m2) | 28.3±4.6 |
HRT: Hormone replacement therapy; ER: Estrogen receptor, PR: Progesterone receptor, BMI: Body Mass Index. The values of average age, average age of menarche, average age of menopause, average number of pregnancies and average BMI represent the mean ± SD.
The allele and genotype frequencies for the CYP8A1 polymorphisms (CC, TC, and TT) detected in the patients and controls are summarized in Table 2. All polymorphism frequencies were in HWE in the controls (P > 0.05). Genotype distribution exhibited a statistically significant difference between the cases and control groups (P=0.0008). The allele distribution also exhibited significant difference and allele C was associated with BCa risk (OR 2.08, 95% CI 1.17-3.72, P=0.0178). The DNA genotyping results were consistent with the sequencing results (Figure 1). Overall, we found 9.4% wild type sequences and 48.4% polymorphic sequences in patients with BCa, while in control group the frequency was 39.5% wild type sequences and 23.7% polymorphic sequences for CYP8A1. We found that variant 67730 T > C was significantly associated with an increased risk of BCa. Significant differences were observed in the distributions of CYP8A1: OR 0.12, 95% CI 0.035-0.39, P=0.0003 and OR 0.21, 95% CI 0.07-0.65, P=0.0074 for TC and CC genotypes, respectively between the patients and controls (Table 3).
Table 2.
Genotype and allele frequencies of CYP8A1 rs5602 (67730 T > C) polymorphism in BCa patients and control patients
| Genotypes/Alleles | BCa patients (N=64) | Controls (N=38) | P value | ||
|---|---|---|---|---|---|
|
|
|||||
| N (%) | Frequency | N (%) | Frequency | ||
| Genotype | |||||
| CC | 27 (42.2) | 0.422 | 14 (36.8) | 0.368 | 0.0008 |
| TC | 31 (48.4) | 0.484 | 9 (23.7) | 0.237 | |
| TT | 6 (9.4) | 0.094 | 15 (39.5) | 0.395 | |
| Alleles | |||||
| C | 85 (66) | 0.66 | 37 (49) | 0.49 | 0.0178* |
| T | 43 (34) | 0.34 | 39 (51) | 0.51 | |
OR=2.08, 95% CI=1.17-3.72.
Figure 1.
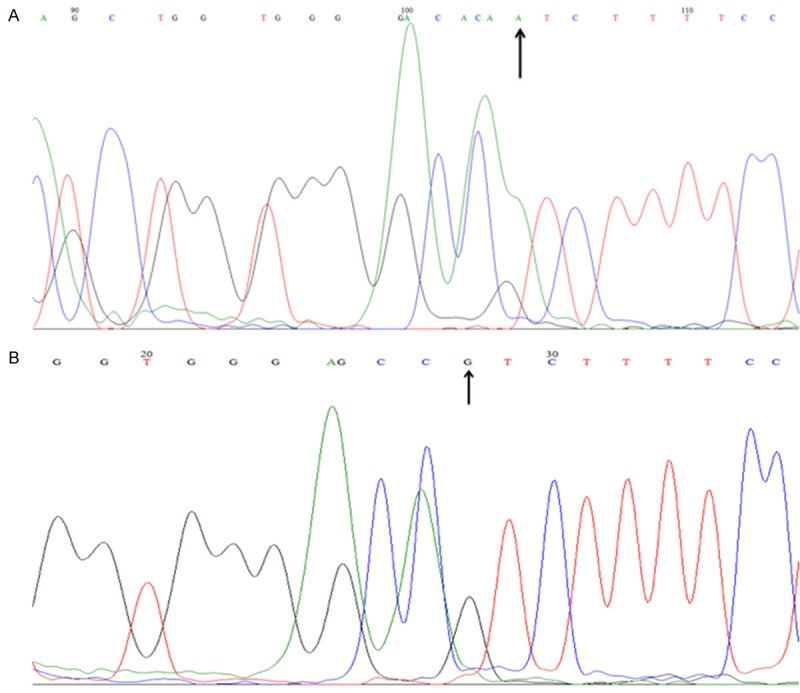
Sequencing chromatograms of CYP8A1 rs5602 (67730 T > C) polymorphism. The figure showed the reverse complement DNA sequencing results of the TC genotype (A) and CC genotype (B).
Table 3.
Association of CYP8A1 rs5602 (67730 T > C) polymorphism with the risk of BCa
| Genotypes | BCa (%) N=64 | Controls (%) N=38 | OR | 95% CI |
|---|---|---|---|---|
| TT | 6 (9.4%) | 15 (39.5%) | 1.0 (reference) | |
| TC | 31 (48.4%) | 9 (23.7%) | 0.12a | 0.035-0.39 |
| CC | 27 (42.2%) | 14 (36.8%) | 0.21b | 0.07-0.65 |
P=0.0003 compared with CYP8A1 TT genotype;
P=0.0074 compared with CYP8A1 TT genotype.
In Table 4 is showed an association of the TT and TC + CC genotypes with the clinic-pathological characteristics, including clinical stage, BIRADS, ER status, PR status, HER2 status, p53 status, CD34 status, metastasis and therapy use. We not observed a statistically significance effect between the C allele and an increased risk in some of this factors.
Table 4.
Association analysis between rs5602 (67730 T > C) polymorphism and the clinic-pathological characteristics
| Genotype | |||||
|---|---|---|---|---|---|
|
|
|||||
| Clinical data information | All (%) | TT (%) | TC + CC (%) | P value | OR (95% CI) |
| Clinical stage | |||||
| I | 4 (6.3) | 0 (0) | 4 (6.3) | 0.5065 | 0.93 (0.045-19.35) |
| > I | 60 (93.7) | 6 (9.4) | 54 (84.3) | ||
| BIRADS | |||||
| < 3 | 2 (3.1) | 0 (0) | 2 (3.1) | 0.6440 | 1.74 (0.075-40.32) |
| ≥ 3 | 62 (96.9) | 6 (9.4) | 56 (87.5) | ||
| ER status | |||||
| Positive | 40 (62.5) | 5 (7.8) | 35 (54.7) | 0.2682 | 3.29 (0.36-29.98) |
| Negative | 24 (37.5) | 1 (1.5) | 23 (36) | ||
| PR Status | |||||
| Positive | 39 (60.9) | 5 (7.8) | 34 (53.1) | 0.2376 | 3.53 (0.39-32.18) |
| Negative | 25 (39.1) | 1 (1.6) | 24 (37.5) | ||
| HER2 Status | |||||
| Positive | 34 (53.1) | 3 (4.7) | 31 (48.4) | 0.8720 | 0.87 (0.16-4.68) |
| Negative | 30 (46.9) | 3 (4.7) | 27 (42.2) | ||
| p53 Status | |||||
| Positive | 36 (56.3) | 3 (4.7) | 33 (51.6) | 0.7458 | 0.76 (0.14-4.08) |
| Negative | 28 (43.7) | 3 (4.7) | 25 (39) | ||
| CD34 status | |||||
| < 10-15 vessels | 17 (26.6) | 2 (3.2) | 15 (23.4) | 0.6932 | 1.43 (0.24-8.64) |
| ≥ 10-15 vessels | 47 (73.4) | 4 (6.2) | 43 (67.2) | ||
| Metastasis | |||||
| Yes | 17 (26.6) | 2 (3.2) | 15 (23.4) | 0.6932 | 1.43 (0.24-8.64) |
| No | 47 (73.4) | 4 (6.2) | 43 (67.2) | ||
| Therapy | |||||
| Yes | 31 (48.4) | 2 (3.2) | 29 (45.3) | 0.4368 | 0.5 (0.08-2.95) |
| No | 33 (51.6) | 4 (6.2) | 29 (45.3) | ||
Discussion
The PGI2 synthetic enzyme, which is known as PGIS, was proposed to be a cytochrome P450 (CYP8A1) by Ullrich et al. and was confirmed by DeWitt and Smith [24,25]. CYP8A1 is predominantly expressed in vascular endothelial and smooth muscle cells, and gene is localized on chromosome 20q13. [26]. The CYP8A1 gene plays a central role in inflammation, vascular and pulmonary diseases and has been shown to be an anti-tumor regulator in cancer [27,28].
With respect to the studied clinic-pathological factors in the patients, the average age, average age of menarche and menopausal, place of residence and birthplace in Mexico are consistent with the data. In Mexico, the age range of the risk for BCa is between 40-69 years, the median age of menarche is 12 years and the median age of menopause has been reported to be between 47 and 48.2 years. Moreover, BCa is more frequent in the northern and central regions of Mexico [29-31]. In the literature, lifestyle factors such as smoking, alcohol, oral contraceptives or HRT use, family history of cancer, chronic conditions (hypertension or diabetes), obesity or overweight have been associated with BCa risk [30,32-35]. In our results we observed that the majority of patients not presented alcohol, tobacco, drugs and oral contraceptives or HRT use, exposure to biomass, chronic diseases and family history of cancer but they presented overweight and obesity in a 76.6% of the cases. Our results also suggested that pregnancy or menstruation cessation does not protected against BCa development (98.4 and 87.5% of patients showed pregnancies and menopausal status, respectively) probably by the hormonal changes associated with pregnancy or menopause appear to have little influence on BCa prognosis [36]. Furthermore, we observed that over 50% of the patients showed a positive status in estrogen receptor (ER), progesterone receptor (PR), HER2 and p53. It have been demonstrated that high tumor grade in BCa not wholly dependent on steroid receptor expression and which may involve other oncogenic events as p53 protein stabilization and HER2 overexpression [37]. Possibly, this is the reason of the presence of high histological tumor grade in 78.1% of analyzed women.
In our study we determined an association between CYP8A1 rs5603 polymorphism with the risk of BCa but no an association between the C allele and an increased risk in some clinical pathological factor. This polymorphism is in 3-unstraslated region (3’-UTR). The 3’-UTR of CYP8A1 gene contains multiple polyadenilation signals. RNA blot analysis with the 3’-UTR regions as probes showed that two mRNA of 6 and 3.3 kb for human CYP8A1 contained approximately 4 and 1.5 kb of untraslated regions, respectively. However it is not known what mechanisms are involved in the formation of these transcripts [38]. Currently, the biological meaning of polyadenylation signals in human CYP8A1 is unclear. The 1.5-kb sequence of the 5’-upstream of the translational initiation site contained both GC-rich and pyrimidine-rich regions and consensus sequences of the transcription factor recognition sites such as Sp1, AP-2, the interferon-γ response element, GATA, NF-κB, the CACCC box, and the glucocorticoid response element [39].
In a recent work, Cho et al. showed that 11 SNPs of CYP8A1 gene in 5’-UTR in Korean individuals not interfere with RNA splicing or affect transcription factor-binding sites [22]. However, in other studies have been demonstrated the role of polymorphisms in 3’-UTR of cytochromes in BCa risk or its treatment. Liu et al. showed that rs4646 polymorphism in CYP19A1 was associated with efficacy of anastrozole in BCa in Asiatic population [39]; Tuerxun et al. showed that 6235 T > C polymorphism in CYP1A1 was associated with BCa development in Chinese population [40] and García-Casado et al. showed that rs4646 polymorphism in CYP19A1 was associated with a poor response to letrozole in BCa Caucasian patients [41]. The regulation of gene expression in eukaryotes occurs at multiple levels. Post-transcriptional gene regulation is an effective means to alter the expression [42]. Various RNA-binding proteins stabilize mRNA by association with the AU-rich elements (AREs) in the 3’-UTR [43]. We hypothesize that the presence of polymorphisms in 3’-UTR could possibly regulate the expression of CYP8A1 mRNA through of ARE-binding proteins in BCa.
Conclusions
In conclusion we found, for the first time, that CYP8A1 rs5602 (67730 T > C) polymorphism is related with BCa susceptibility in Mexican woman. This study showed an association between CYP8A1 gene polymorphism and BCa risk in a Mexican population. We suggest that variant of CYP8A1 could be a marker for the identification of BCa-susceptible patients or serve as potential target for future therapies.
Acknowledgements
This work was supported by grants from Consejo Nacional de Ciencia y Tecnología SALUD-2010-01-140535 and partially by Instituto Politécnico Nacional SIP 20113894.
Disclosure of conflict of interest
None.
References
- 1.Voutsadakis IA. The network of pluripotency, epithelial-mesenchymal transition, and prognosis of breast cancer. Breast Cancer (Dove Med Press) 2015;7:303–319. doi: 10.2147/BCTT.S71163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GLOBOCAN 2012: www.globocan.iarc.fr. Accesed in October 2, 2015.
- 3.Chlebowski RT. Nutrition and physical activity influence on breast cancer incidence and outcome. Breast. 2013;22:S30–S37. doi: 10.1016/j.breast.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Ferrini K, Ghelfi F, Mannucci R, Titta L. Lifestyle, nutrition and breast cancer: facts and presumptions for consideration. E Cancer Med Sci. 2015;9:557. doi: 10.3332/ecancer.2015.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams JA, Phillips DH. Mammary expression of xenobiotic-metabolising enzymes and their potential role in breast cancer: a review. Cancer Res. 2000;60:4667–4677. [PubMed] [Google Scholar]
- 6.Williams JA. Single nucleotide polymorphisms, metabolic activation and environmental carcinogenesis: why molecular epidemiologists should think about enzyme expression. Carcinogenesis. 2001;22:209–214. doi: 10.1093/carcin/22.2.209. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura M, Yaguti H, Yoshitsugu H, Naito S, Satoh T. Tissue distribution of mRNA expression of human cytochrome P450 isoforms assessed by high-sensitivity real-time reverse transcription PCR. Yakugaku Zasshi. 2003;123:369–375. doi: 10.1248/yakushi.123.369. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez FJ, Gelboin HV. Role of human cytochromes P450 in the metabolic activation of chemical carcinogens and toxins. Drug Metab Rev. 1994;26:165–183. doi: 10.3109/03602539409029789. [DOI] [PubMed] [Google Scholar]
- 9.Kivistoé KT, Kroemer HK, Eichelbaum M. The role of human cytochrome P450 enzymes in the metabolism of anticancer agents: implications for drug interactions. Br J Clin Pharmacol. 1995;40:523–530. doi: 10.1111/j.1365-2125.1995.tb05796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziegler A, Koch A, Krockenberger K, Grosshennig A. Personalized medicine using DNA biomarkers: a review. Hum Genet. 2012;131:1627–1638. doi: 10.1007/s00439-012-1188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nalejska E, Mączyńska E, Lewandowska MA. Prognostic and predictive biomarkers: tools in personalized oncology. Mol Diagn Ther. 2014;18:273–284. doi: 10.1007/s40291-013-0077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ullrich V, Castle L, Weber P. Spectral evidence for the cytochrome P450 nature of prostacyclin synthetase. Biochem Pharmacol. 1981;30:2033–2036. doi: 10.1016/0006-2952(81)90218-5. [DOI] [PubMed] [Google Scholar]
- 13.Lim H, Dey SK. A novel pathway of prostacyclin signaling-hanging out with nuclear receptors. Endocrinology. 2002;143:3207–3210. doi: 10.1210/en.2002-220159. [DOI] [PubMed] [Google Scholar]
- 14.Pradono P, Tazawa R, Maemondo M, Tanaka M, Usui K, Saijo Y, Hagiwara K, Nukiwa T. Gene transfer of thromboxane A(2) synthase and prostaglandin I(2) synthase antithetically altered tumor angiogenesis and tumor growth. Cancer Res. 2002;62:63–66. [PubMed] [Google Scholar]
- 15.Honn KV, Cicone B, Skoff A. Prostacyclin: a potent antimetastatic agent. Science. 1981;212:1270–1272. doi: 10.1126/science.7015512. [DOI] [PubMed] [Google Scholar]
- 16.Keith RL, Miller YE, Hoshikawa Y, Moore MD, Gesell TL, Gao B, Malkinson AM, Golpon HA, Nemenoff RA, Geraci MW. Manipulation of pulmonary prostacyclin synthase expression prevents murine lung cancer. Cancer Res. 2002;62:734–740. [PubMed] [Google Scholar]
- 17.Keith RL, Miller YE, Hudish TM, Girod CE, Sotto-Santiago S, Franklin WA, Nemenoff RA, March TH, Nana-Sinkam SP, Geraci MW. Pulmonary prostacyclin synthase overexpression chemoprevents tobacco smoke lung carcinogenesis in mice. Cancer Res. 2004;64:5897–5904. doi: 10.1158/0008-5472.CAN-04-1070. [DOI] [PubMed] [Google Scholar]
- 18.Kajita S, Ruebel KH, Casey MB, Nakamura N, Lloyd RV. Role of COX-2, thromboxane A2 synthase, and prostaglandin I2 synthase in papillary thyroid carcinoma growth. Mod Pathol. 2005;18:221–227. doi: 10.1038/modpathol.3800285. [DOI] [PubMed] [Google Scholar]
- 19.Gustafsson A, Hansson E, Kressner U, Nordgren S, Andersson M, Lonnroth C, Lundholm K. Prostanoid receptor expression in colorectal cancer related to tumor stage, differentiation and progression. Acta Oncol. 2007;46:1107–1112. doi: 10.1080/02841860701403061. [DOI] [PubMed] [Google Scholar]
- 20.Abraham JE, Harrington P, Driver KE, Tyrer J, Easton DF, Dunning AM, Pharoah PD. Common polymorphisms in the prostaglandin pathway genes and their association with breast cancer susceptibility and survival. Clin Cancer Res. 2009;15:2181–2191. doi: 10.1158/1078-0432.CCR-08-0716. [DOI] [PubMed] [Google Scholar]
- 21.Mavaddat N, Dunning AM, Ponder BA, Easton DF, Pharoah PD. Common genetic variation in candidate genes and susceptibility to subtypes of breast cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:255–259. doi: 10.1158/1055-9965.EPI-08-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho SA, Rohn-Glowacki KJ, Jarrar YB, Yi M, Kim WY, Shin JG, Lee SJ. Analysis of genetic polymorphism and biochemical characterization of a functionally decreased variant in prostacyclin synthase gene (CYP8A1) in humans. Arch Biochem Biophys. 2015;569:10–18. doi: 10.1016/j.abb.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Cárdenas-Rodríguez N, Lara-Padilla E, Bandala C, López-Cruz J, Uscanga-Carmona C, Lucio-Monter PF, Floriano-Sánchez E. CYP2W1, CYP4F11 and CYP8A1 polymorphisms and interaction of CYP2W1 genotypes with risk factors in Mexican women with breast cancer. Asian Pac J Cancer Prev. 2012;13:837–846. doi: 10.7314/apjcp.2012.13.3.837. [DOI] [PubMed] [Google Scholar]
- 24.Ullrich V, Castle L, Weber P. Spectral evidence for the cytochrome P450 nature of prostacyclin synthetase. Biochem Pharmacol. 1981;30:2033–2036. doi: 10.1016/0006-2952(81)90218-5. [DOI] [PubMed] [Google Scholar]
- 25.DeWitt DL, Smith WL. Purification of prostacyclin synthase from bovine aorta by immunoaffinity chromatography. Evidence that the enzyme is a hemoprotein. J Biol Chem. 1983;258:3285–3293. [PubMed] [Google Scholar]
- 26.McLemore TL, Hubbard WC, Litterst CL, Liu MC, Miller S, McMahon NA, Eggleston JC, Boyd MR. Profiles of prostaglandin biosynthesis in normal lung and tumor tissue from lung cancer patients. Cancer Res. 1988;48:3140–3147. [PubMed] [Google Scholar]
- 27.Stearman RS, Dwyer-Nield L, Zerbe L, Blaine SA, Chan Z, Bunn PA Jr, Johnson GL, Hirsch FR, Merrick DT, Franklin WA, Baron AE, Keith RL, Nemenoff RA, Malkinson AM, Geraci MW. Analysis of orthologous gene expression between human pulmonary adenocarcinoma and a carcinogen-induced murine model. Am J Pathol. 2005;1676:1763–1775. doi: 10.1016/S0002-9440(10)61257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cathcart MC, Reynolds JV, O’Byrne KJ, Pidgeon GP. The role of prostacyclin synthase and thromboxane synthase signaling in the development and progression of cancer. Biochim Biophys Acta. 2010;1805:153–166. doi: 10.1016/j.bbcan.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Garrido LF, Lazcano-Ponce EC, Lopez CL, Hernández-Avila M. Age of natural menopause among women in Mexico City. Int J Gynaecol Obstetr. 1996;53:159–166. doi: 10.1016/0020-7292(96)02655-0. [DOI] [PubMed] [Google Scholar]
- 30.Rice MS, Bertrand KA, Lajous M, Tamimi RM, Torres G, López-Ridaura R, Romieu I. Reproductive and lifestyle risk factors and mammographic density in Mexican women. Ann Epidemiol. 2015;25:868–873. doi: 10.1016/j.annepidem.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dirección General de Información en Salud, SSa. (2000-2008): www. dgis.salud.gob.mx.
- 32.Bostick RM, Sprafka JM, Virnig BA, Potter JD. Predictors of cancer prevention attitudes and participation in cáncer screening examinations. Prev Med. 1994;23:816–826. doi: 10.1006/pmed.1994.1139. [DOI] [PubMed] [Google Scholar]
- 33.Boone SD, Baumgartner KB, Baumgartner RN, Connor AE, John EM, Giuliano AR, Hines LM, Rai SN, Riley EC, Pinkston CM, Wolff RK, Slattery ML. Active and passive cigarette smoking and mortality among Hispanic and non-Hispanic white women diagnosed with invasive breast cancer. Ann Epidemiol. 2015;25:824–831. doi: 10.1016/j.annepidem.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodwin PJ, Ambrosone CB, Hong CC. Modifiable lifestyle factors and breast cancer outcomes: current controversies and research recommendations. Adv Exp Med Biol. 2015;862:177–192. doi: 10.1007/978-3-319-16366-6_12. [DOI] [PubMed] [Google Scholar]
- 35.Vijayvergia N, Denlinger CS. Lifestyle factors in cancer survivorship: where we are and where we are headed. J Pers Med. 2015;5:243–263. doi: 10.3390/jpm5030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Von Schoultz E, Johansson H, Wilking N, Rutqvist LE. Influence of prior and subsequent pregnancy on breast cancer prognosis. J. Clin. Oncol. 1995;13:430–434. doi: 10.1200/JCO.1995.13.2.430. [DOI] [PubMed] [Google Scholar]
- 37.Bouras T, Southey MC, Venter DJ. Overexpression of the steroid receptor coactivator AIB1 in breast cancer correlates with the absence of estrogen and progesterone receptors and positivity for p53 and HER2/neu. Cancer Res. 2001;61:903–907. [PubMed] [Google Scholar]
- 38.Yokoyama C, Yabuki T, Inoue H, Tone Y, Hara S, Hatae T, Nagata M, Takahashi EI, Tanabe T. Human gene encoding prostacyclin synthase (PTGIS): genomic organization, chromosomal localization, and promoter activity. Genomics. 1996;36:296–304. doi: 10.1006/geno.1996.0465. [DOI] [PubMed] [Google Scholar]
- 39.Liu L, Bai YX, Zhou JH, Sun XW, Sui H, Zhang WJ, Yuan HH, Xie R, Wei XL, Zhang TT, Huang P, Li YJ, Wang JX, Zhao S, Zhang QY. A polymorphism at the 3’-UTR region of the aromatase gene is associated with the efficacy of the aromatase inhibitor, anastrozole, in metastatic breast carcinoma. Int J Mol Sci. 2013;14:18973–18988. doi: 10.3390/ijms140918973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tuerxun M, Hamulati W, Bai L, Peng XM, Dolikun M. Study of cytochrome P450 1A1 gene 3’-UTR 6235T-C polymorphism and susceptibility to breast cancer with Uighur medicine. Zhonghua Yi Xue Za Zhi. 2011;91:86–91. [PubMed] [Google Scholar]
- 41.Garcia-Casado Z, Guerrero-Zotano A, Llombart-Cussac A, Calatrava A, Fernandez-Serra A, Ruiz-Simon A, Gavila J, Climent MA, Almenar S, Cervera-Deval J, Campos J, Albaladejo CV, Llombart-Bosch A, Guillem V, Lopez-Guerrero JA. A polymorphism at the 3’-UTR region of the aromatase gene defines a subgroup of postmenopausal breast cancer patients with poor response to neoadjuvant letrozole. BMC Cancer. 2010;10:36. doi: 10.1186/1471-2407-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 43.Aparicio LA, Abella V, Valladares M, Figueroa A. Posttranscriptional regulation by RNA-binding proteins during epithelial-to-mesenchymal transition. Cell Mol Life Sci. 2013;70:4463–4477. doi: 10.1007/s00018-013-1379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


