Abstract
Intraperitoneal chemotherapy together with cytoreductive surgery is the standard of care for a number of peritoneal surface malignancies. However, this approach fails to maintain the complete response and disease recurs due to microscopic residual disease. Although safer than systemic chemotherapy regimens, locoregional treatment with chemotherapeutics can induce toxicity which is a major concern affecting the patient’s treatment protocol and outcome. For an enhanced treatment efficacy, efforts should be made to maximize cytotoxic effects of chemotherapeutic agents on tumor cells while minimizing their toxic effects on host cells. Bromelain and N-acetylcysteine are two natural agents with good safety profiles shown to have anti-cancer effects. However, their interaction with chemotherapeutics is unknown. In this study, we investigated if these agents have the potential to sensitize in vitro gastrointestinal cancer models to cisplatin, paclitaxel, 5-fluorouracil, and vincristine. The drug-drug interaction was also analyzed. Our findings suggest that combination of bromelain and N-acetylcysteine with chemotherapeutic agents could give rise to an improved chemotherapeutic index in therapeutic approaches to peritoneal surface malignancies of gastrointestinal origin so that maximum benefits could result from less toxic and more patient-friendly doses. This represents a potentially efficacious strategy for the enhancement of microscopic cytoreduction and is a promising area for future research.
Keywords: 5-fluorouracil, bromelain, cisplatin, gastrointestinal cancers, N-acetylcysteine, paclitaxel, potentiation, vincristine
Introduction
Chemotherapeutic agents are widely administered intravenously in cancer therapy. Nevertheless, it has been shown in the context of the peritoneal surface malignancies (PSMs) that disease control may be significantly improved when chemotherapy is used through the intraperitoneal route [1]. In agreement, a combination of cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC), with or without early postoperative intraperitoneal chemotherapy (EPIC), has offered long-term benefits in selected patients with PSM [2]. This multimodal strategy is now considered as the standard of care for patients with PMP [3] and advocated as a promising approach to other primary or secondary peritoneal malignancies, including peritoneal carcinomatosis (PC) of gastrointestinal origin [4] and malignant peritoneal mesothelioma (MPM) [5]. However, evidence shows that HIPEC fails to maintain the surgical complete response achieved by CRS [1]. In addition, chemotherapy-induced toxicity even at low plasma levels is always an issue of concern. Thus, HIPEC needs to be supplemented by novel treatments capable of targeting the residual disease. In this regard, locoregional use of safe agents with cytotoxic effects on cancer cells represents a potentially efficacious strategy for the enhancement of microscopic cytoreduction.
Bromelain (BR) and N-acetylcysteine (NAC) are two natural agents with good safety profiles shown to have anti-cancer effects. We previously described the efficacy of BR/NAC in inhibition of gastrointestinal cancer cells’ proliferation and survival [6]. Here, we intended to find out if BR/NAC treatment has the capability to sensitize gastrointestinal cancer cells into chemotherapy. To this end, a number of chemotherapeutic agents of different classes and variable utility in both peritoneal and systemic chemotherapy, including cisplatin, 5-fluorouracil, paclitaxel and vincristine, were used and the influence of the BR/NAC pretreatment on cancer cell response to chemotherapy in sequential therapy was evaluated. Moreover, the interaction between BR/NAC and chemotherapeutic agents in combination therapy was further analyzed. Here, we report that bromelain and NAC in combination with chemotherapeutics potentiate the inhibition of growth and proliferation of gastrointestinal cancer cells in vitro.
Methods
Cell culture
Human gastric carcinoma cell lines MKN45 and KATO-III were obtained from the Cancer Research Campaign Laboratories (University of Nottingham, UK) and the American Type Culture Collection (ATCC, USA), respectively. LS174T colon adenocarcinoma cell line was purchased from Sigma-Aldrich (Sigma-Aldrich, USA). All cell lines were maintained in a humidified atmosphere of 95% air and 5% CO2 at 37°C in their respective media as follows: MKN45 in RPMI-1640 medium, KATO-III in IMDM and LS174T in EMEM (all from Invitrogen, USA). The culture media used were all supplemented with 10% (v/v) fetal bovine serum and 1% (v/v) penicillin-streptomycin (Invitrogen, USA), with the exception of IMDM being supplemented with 20% fetal bovine serum. As per the distributor’s instructions, the culture medium for LS174T was further supplemented with 2 mM Glutamine and 1% Non-Essential Amino Acids.
Drug preparation
Bromelain and NAC were purchased from Sigma-Aldrich (Sigma-Aldrich, USA) and the stock solutions were made with BR and NAC being dissolved in relevant culture media. Cisplatin (Cis) and paclitaxel (PTX) were solubilized in dimethylformamide (DMF) and absolute ethanol, respectively. 5-fluorouracil (5-FU) and vincristine (VCR) were solubilized in methanol. Stock solutions were filtered, pH adjusted (applicable for NAC) and diluted with appropriate medium according to the final treating concentrations required for single agent and combination treatment groups.
Cytotoxicity assay
Single agent treatment
MKN45, KATO-III and LS174T cells were seeded into 96-well plates in triplicate and maintained in their respective medium in a humidified 5% CO2 incubator at 37°C for 72 hours. Cells were then incubated for another 72 hours with the treatment medium containing different concentrations of single agent BR, NAC, Cis, 5-FU, PTX or VCR. Control cells were also included in all plates and maintained in their respective drug-free medium containing the same concentration of the drug solvent as did the treatment medium. Upon completion of the treatment, cells were subjected to Sulforhodamine B (SRB) assay.
Sequential treatment
Sequential treatment was used to evaluate chemosensitizing effects of BR/NAC pretreatment. KATO-III and LS174T cells, seeded into 96-well plates and incubated for 72 hours, were first pretreated with different concentrations of BR/NAC for 2, 4 or 8 hours and then incubated with cytotoxic agents for 72 hours as follows:
KATO-III cells: a. 2 h pretreatment with BR (100, 200, 300 μg/mL) followed by 72 h treatment with Cis (0.5, 1 and 5 μM), 5-FU (10 and 50 μM), PTX (1 and 5 nM) and VCR (1 and 2.5 nM); b. 4 h pretreatment with BR (100, 200, 300 μg/mL) followed by 72 h treatment with Cis (0.5, 1 and 5 μM), 5-FU (10 and 50 μM), PTX (1 and 5 nM) and VCR (1 and 2.5 nM); c. 8 h pretreatment with BR (100, 200, 300 μg/mL) followed by 72 h treatment with Cis (0.5, 1 and 5 μM), 5-FU (10 and 50 μM), PTX (1 and 5 nM) and VCR (1 and 2.5 nM); d. 4 h pretreatment with BR+NAC (50+5 and 100+10) followed by 72 h treatment with Cis (1, 5 and 10 μM), 5-FU (50 and 100 μM), PTX (1 and 5 nM) and VCR (1 and 2.5 nM); e. 8 h pretreatment with BR+NAC (50+5 and 100+10) followed by 72 h treatment with Cis (1, 5 and 10 μM), 5-FU (50 and 100 μM), PTX (1 and 5 nM) and VCR (1 and 2.5 nM).
LSA74T cells: a. 4 h pretreatment with BR (10, 20 and 30 μg/mL) followed by 72 h treatment with Cis (10 and 20 μM), 5-FU (10 and 50 μM), PTX (10 and 50 nM) and VCR (10 and 50 nM); b. 4 h pretreatment with BR+NAC (10+20 and 20+10) followed by 72 h treatment with Cis (10 and 20 μM), 5-FU (10 and 50 μM), PTX (10 and 50 nM) and VCR (10 and 50 nM).
Control cells were also included in all plates and upon completion of the treatment, cells were subjected to SRB assay.
Combination treatment
To examine the capability of BR/NAC in potentiating chemotherapy, MKN45 and LS174T cells were treated with each of the cytotoxic agents in conjunction with nine different combinations of BR and NAC. Untreated control groups were included in all experiments. Upon completion of the treatment, cells were subjected to SRB assay and treating agents were assayed on their own and in combination at a non-constant ratio.
Sulforhodamine B assay
The effect of drugs on growth and proliferation of the cells was investigated by sulforhodamine B assay. Upon completion of the treatment, cells were fixed and proceeded with the SRB assay as described elsewhere [6] and the absorbance was read at 570 nm.
Drug-drug interaction and combination index analyses
The interaction between the drugs in combination treatment was determined by the median effect analysis using CalcuSyn software (Biosoft, UK) and the combination index (CI) was calculated based on the drug concentration and cell viability. CIs less than 0.9 and greater than 1.1 were considered as synergism and antagonism, respectively, and those between 0.9 and 1.1 as additivity.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software Inc., USA). The Student’s t-test was applied for unpaired samples. p values <0.05 were considered significant. All data presented are representative of three independent experiments and depicted as mean ± SE.
Results
Using escalating concentrations of Cis, 5FU, PTX and VCR, a cytotoxicity assay of these chemotherapeutic agents on KATO-III, MKN45 and LS174T cells was first performed using SRB assay. The possible chemosensitizing effects of BR/NAC were next explored in sequential treatment.
BR/NAC pretreatment sensitizes KATO-III cells to chemotherapy with cisplatin, 5-fluorouracil, paclitaxel or vincristine
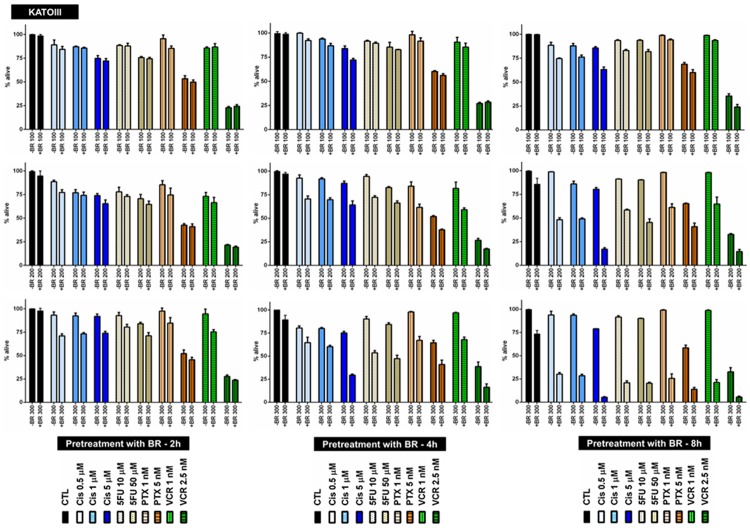
To investigate any potential chemosensitizing effect of BR pretreatment, KATO-III cells in four chemotherapy groups were pretreated with three selected concentrations of BR for 2, 4 or 8 hours (Figure 1), and subsequently treated with three selected concentrations of Cis, or two selected concentrations of 5FU, PTX or VCR for 72 hours. Table 1 summarizes the results of pretreatment with BR. As shown, positive chemosensitization was observed in all treatment groups, except for the two sequentially treated with 100 μg/mL BR and either VCR concentration. However, when BR was used at concentrations of 100 and 200 μg/mL, chemosensitizing effects, with the exception of one instance (BR 200 μg/mL and Cis 0.5 μM), were not statistically significant.
Figure 1.
BR pretreatment of KATO-III cells for 2, 4 or 8 hours followed by chemotherapy. BR pretreatment sensitizes KATO-III cells to chemotherapy with cisplatin, 5-fluorouracil, paclitaxel or vincristine. All data presented are representative of three independent experiments and depicted as mean ± SE.
Table 1.
Chemosensitizing effects of BR pretreatment on KATO-III cells
| SENS2 h | SE4 h | 8 h | ||||
|
|
||||||
| BR 100 | SENS | p values | SENS | p values | SENS | p values |
|
| ||||||
| CTL | NA | n | NA | n | NA | n |
| Cis 0.5 | + | n | + | 0.0128 | + | 0.0111 |
| Cis 1 | + | n | + | n | + | 0.0230 |
| Cis 5 | + | n | + | 0.0188 | + | 0.0016 |
| 5FU 10 | + | n | + | n | + | 0.0022 |
| 5FU 50 | + | n | + | n | + | 0.0065 |
| PTX 1 | + | n | + | n | + | 0.0096 |
| PTX 5 | + | n | + | n | + | n |
| VCR 1 | - | n | + | n | + | 0.0046 |
| VCR 2.5 | - | n | - | n | + | 0.0325 |
|
| ||||||
| BR 200 | ||||||
| CTL | NA | n | NA | n | NA | n |
| Cis 0.5 | + | 0.0252 | + | 0.0090 | + | < 0.0001 |
| Cis 1 | + | n | + | 0.0007 | + | 0.0003 |
| Cis 5 | + | n | + | 0.0093 | + | < 0.0001 |
| 5FU 10 | + | n | + | 0.0012 | + | < 0.0001 |
| 5FU 50 | + | n | + | 0.0026 | + | 0.0003 |
| PTX 1 | + | n | + | 0.0171 | + | 0.0006 |
| PTX 5 | + | n | + | 0.0004 | + | 0.0031 |
| VCR 1 | + | n | + | 0.0333 | + | 0.0106 |
| VCR 2.5 | + | n | + | 0.0105 | + | 0.0018 |
|
| ||||||
| BR 300 | ||||||
| CTL | NA | n | NA | n | NA | 0.0022 |
| Cis 0.5 | + | 0.0053 | + | n | + | 0.0001 |
| Cis 1 | + | 0.0041 | + | 0.0003 | + | < 0.0001 |
| Cis 5 | + | 0.0042 | + | < 0.0001 | + | < 0.0001 |
| 5FU 10 | + | 0.0496 | + | 0.0005 | + | < 0.0001 |
| 5FU 50 | + | 0.0270 | + | 0.0009 | + | < 0.0001 |
| PTX 1 | + | n | + | 0.0019 | + | < 0.0001 |
| PTX 5 | + | n | + | 0.0129 | + | 0.0002 |
| VCR 1 | + | 0.0276 | + | 0.0005 | + | < 0.0001 |
| VCR 2.5 | + | n | + | 0.0211 | + | 0.0040 |
SENS: Sensitization, NA: not applicable; +: chemosensitized (better response compared to “no pretreatment” control); -: not chemosensitized (response similar to or worse than that in “no pretreatment” control); CTL: pretreatment-only control; Cis: cisplatin; 5FU: 5-fluorouracil; PTX: paclitaxel; VCR: vincristine; n: not significant. Significant results (p < 0.05) are shown in bold.
In contrast, the highest concentration of BR (300 μg/mL) induced significant enhancement of response to Cis, 5FU and VCR (1 nM). When pretreatment was applied for a longer period, significant results appeared at lower concentrations of BR, too. As shown in Table 1, significant enhancement of response to all chemotherapeutic agents used was evident after 4 h pretreatment with 200 or 300 μg/mL BR. In this regard, significant sensitization to both concentrations of Cis was also found with 4 hour BR pretreatment at the concentration of 100 μg/mL. Finally, when KATO-III cells were pretreated for 8 hours, BR at all the three concentrations used significantly enhanced response to the four chemotherapeutic agents (Table 1).
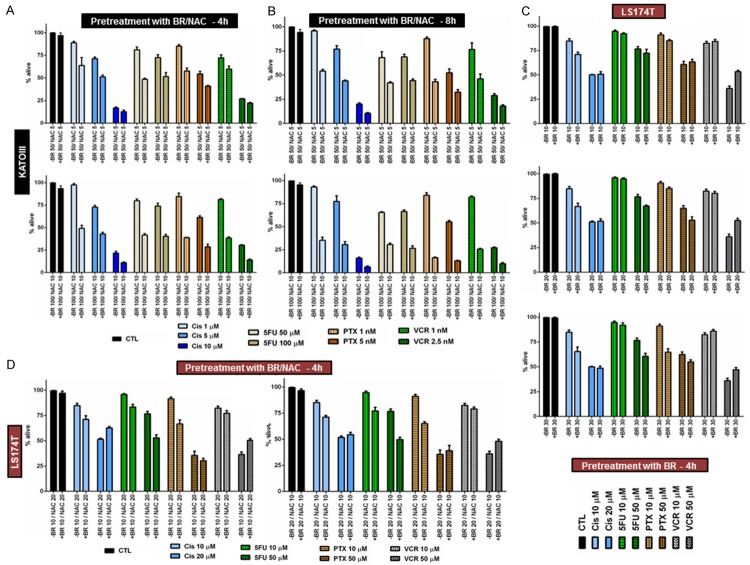
To explore the effect of combined use of BR and NAC, we then pretreated KATO-III cells with two selected combinations of BR and NAC for 4 (Figure 2A) or 8 (Figure 2B) hours and subsequently treated them with single agent Cis, 5FU, PTX or VCR for 72 hours. As shown in Figure 2A and 2B, both 4- and 8-hour pretreatment with BR/NAC positively sensitized KATO-III cells to all the four cytotoxic agents. Statistical analysis of the results indicated that, with the exception of two instances (4 hour pretreatment with 50 μg/mL BR+5 mM NAC followed by 72 hour treatment with 10 μM Cis or 1 nM VCR) the chemosensitizing effects observed were all significant (Table 2).
Figure 2.
BR+NAC pretreatment of KATO-III cells for 4 or 8 hours (A, B), BR pretreatment (C) and BR+NAC pretreatment (D) of LS174T cells for 4 hours followed by chemotherapy. BR/NAC pretreatment sensitizes cells to chemotherapy with cisplatin, 5-fluorouracil, paclitaxel or vincristine. All data presented are representative of three independent experiments and depicted as mean ± SE.
Table 2.
Chemosensitizing effects of BR+NAC pretreatment on KATOIII cells
| SE4 h | 8 h | |||
|
|
||||
| BR 50/NAC 5 | SENS | p values | SENS | p values |
|
| ||||
| CTL | NA | n | NA | n |
| Cis 1 | + | 0.0481 | + | < 0.0001 |
| Cis 5 | + | 0.0014 | + | 0.0009 |
| Cis 10 | + | n | + | 0.0032 |
| 5FU 50 | + | 0.0007 | + | 0.0135 |
| 5FU 100 | + | 0.0175 | + | 0.0016 |
| PTX 1 | + | 0.0016 | + | 0.0001 |
| PTX 5 | + | 0.0102 | + | 0.0133 |
| VCR 1 | + | n | + | 0.0238 |
| VCR 2.5 | + | 0.0034 | + | 0.0090 |
|
| ||||
| BR 100/NAC 10 | ||||
| CTL | NA | n | NA | 0.1130 |
| Cis 1 | + | 0.0002 | + | < 0.0001 |
| Cis 5 | + | 0.0004 | + | 0.0025 |
| Cis 10 | + | 0.0068 | + | 0.0027 |
| 5FU 50 | + | 0.0001 | + | < 0.0001 |
| 5FU 100 | + | 0.0008 | + | 0.0003 |
| PTX 1 | + | 0.0003 | + | < 0.0001 |
| PTX 5 | + | 0.0007 | + | < 0.0001 |
| VCR 1 | + | < 0.0001 | + | < 0.0001 |
| VCR 2.5 | + | < 0.0001 | + | 0.0002 |
SENS: Sensitization, NA: not applicable; +: chemosensitized (better response compared to “no pretreatment” control); CTL: pretreatment-only control; Cis: cisplatin; 5FU: 5-fluorouracil; PTX: paclitaxel; VCR: vincristine; n: not significant. Significant results (p < 0.05) are shown in bold.
BR/NAC pretreatment sensitizes LS174T cells to chemotherapy with cisplatin, 5-fluorouracil, paclitaxel or vincristine
Next, we evaluated the capability of short-term pretreatment with BR or BR+NAC in enhancing response to chemotherapy of LS174T cells. For this purpose, LS174T cells were sequentially exposed to 4 hour pretreatment with BR (Figure 2C) or BR+NAC (Figure 2D) and 72 hour single agent chemotherapy. As tabulated in Table 3, our data indicated that pretreatment differentially affected the cancer cell response to chemotherapy. All the pretreatment protocols significantly enhanced cancer cell sensitivity to 10 μM Cis. Response to the both 5FU concentrations was enhanced by pretreatment which was statistically significant for the higher 5FU concentration (50 μM) after BR pretreatment (20 and 30 μg/mL) as well as for the both concentrations of 5FU (10 and 50 μM) after BR+NAC pretreatment. With the exception of one instance (10 μg/mL BR pretreatment for 50 nM PTX), pretreatment enhanced cancer cell sensitivity to the both concentrations of PTX used, of which response to 10 nM PTX was significantly improved by all protocols. Of the four cytotoxic agents, response to VCR was least affected by BR/NAC pretreatment. In this regard, although pretreatment of cancer cells with 20 μg/mL BR and both combinations of BR+NAC apparently enhanced sensitivity to 10 nM VCR, the results were not statistically significant.
Table 3.
Chemosensitizing effects of BR/NAC pretreatment on LS174T cells
| SE4 h | |||||
|
|
|||||
| BR 10 | SENS | p values | BR 20 | SENS | p values |
|
| |||||
| CTL | NA | n | CTL | NA | n |
| Cis 10 | + | 0.0017 | Cis 10 | + | 0.0012 |
| Cis 20 | - | n | Cis 20 | - | n |
| 5FU 10 | + | n | 5FU 10 | + | n |
| 5FU 50 | + | n | 5FU 50 | + | 0.0037 |
| PTX 10 | + | 0.0211 | PTX 10 | + | 0.0376 |
| PTX 50 | - | n | PTX 50 | + | n |
| VCR 10 | - | n | VCR 10 | + | n |
| VCR 50 | - | 0.0001 | VCR 50 | - | 0.0006 |
|
| |||||
| BR 30 | BR 10/NAC 20 | ||||
| CTL | NA | n | CTL | NA | n |
| Cis 10 | + | 0.0038 | Cis 10 | + | 0.0098 |
| Cis 20 | + | n | Cis 20 | - | < 0.0001 |
| 5FU 10 | + | n | 5FU 10 | + | 0.0027 |
| 5FU 50 | + | 0.0019 | 5FU 50 | + | < 0.0001 |
| PTX 10 | + | < 0.0001 | PTX 10 | + | 0.0002 |
| PTX 50 | + | n | PTX 50 | + | n |
| VCR 10 | - | n | VCR 10 | + | n |
| VCR 50 | - | 0.0085 | VCR 50 | - | 0.0010 |
|
| |||||
| BR 20/NAC 10 | |||||
| CTL | NA | n | |||
| Cis 10 | + | 0.0004 | |||
| Cis 20 | - | n | |||
| 5FU 10 | + | 0.0008 | |||
| 5FU 50 | + | < 0.0001 | |||
| PTX 10 | + | < 0.0001 | |||
| PTX 50 | - | n | |||
| VCR 10 | + | n | |||
| VCR 50 | - | 0.0037 | |||
SENS: Sensitization, NA: not applicable; +: chemosensitized (better response compared to “no pretreatment” control); CTL: pretreatment-only control; Cis: cisplatin; 5FU: 5-fluorouracil; PTX: paclitaxel; VCR: vincristine; n: not significant. Significant results (p < 0.05) are shown in bold.
Concomitant treatment of MKN45 cells with BR+NAC differentially affects response to cisplatin, 5-fluorouracil, paclitaxel or vincristine
Next, we intended to evaluate the effect of concomitant treatment with combined BR and NAC on response to chemotherapy of MKN45 cell lines in combination therapy.
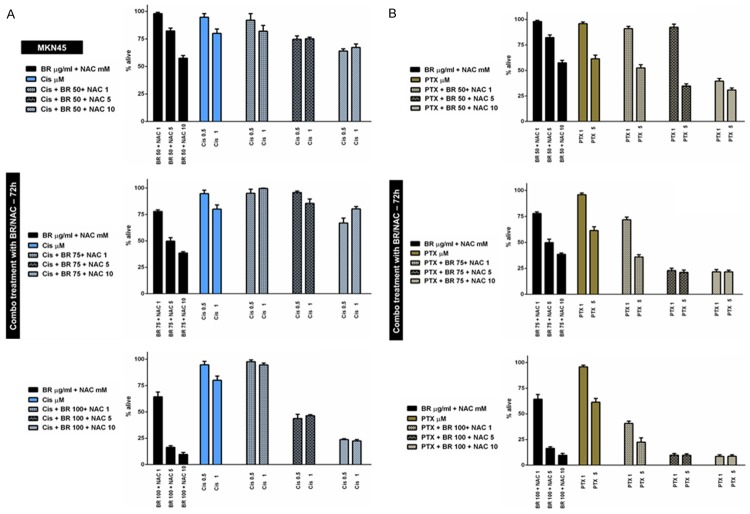
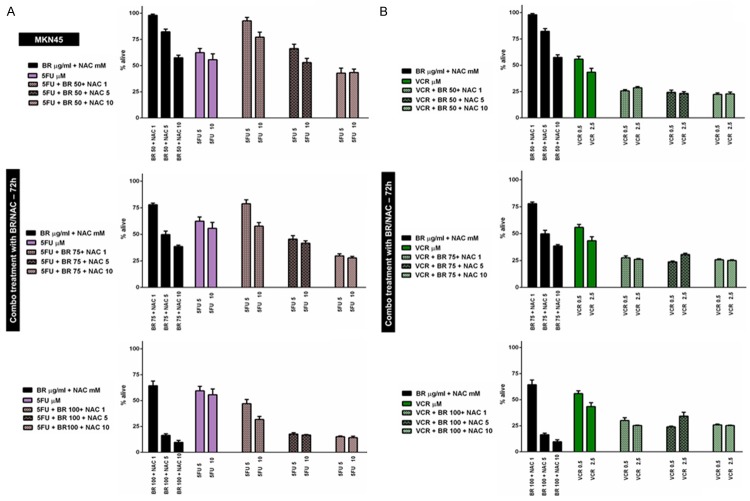
At the first stage, MKN45 cells were treated using 9 possible combinations of three selected concentrations of BR and NAC in conjunction with two different concentrations of Cis (Figure 3A), PTX (Figure 3B), 5FU (Figure 4A) or VCR (Figure 4B) for 72 hours. This created 4 different chemotherapy groups and 72 (4×18) individual treatment subgroups. Our data indicated that BR/NAC treatment differentially affect cancer cell response to concomitant chemotherapy with individual agents. Table 4 summarizes results of the statistical analysis. In this table, colored areas highlight concentrations at which BR/NAC enhanced the effect of chemotherapy. As regards Cis group, BR/NAC enhanced the effect of chemotherapy in 10 out of 18 subgroups, statistically significant in 7 subgroups, including BR 50+NAC 5+Cis 0.5, BR 50+NAC 10+Cis 0.5, BR 75+NAC 10+Cis 0.5, BR 100+NAC 5+Cis 0.5, BR 100+NAC 10+Cis 0.5, BR 100+ NAC 5+Cis 1 and BR 100+NAC 10+Cis 1. BR/NAC also enhanced 5FU-induced cytotoxicity in 13 subgroups, which was significant in 10, including BR 50+NAC 10+5FU 5, BR 75+NAC 5+5FU 5, BR 75+NAC 10+5FU 5, BR 100+NAC 5+5FU 5, BR 100+NAC 10+5FU 5, BR 75+NAC 5+5FU 10, BR 75+NAC 10+5FU 10, BR 100+NAC 1+5FU 10, BR 100+NAC 5+5FU 10 and BR 100+NAC 10+5FU 10. Finally, cytotoxic effects of PTX and VCR were found to be enhanced by BR/NAC in all treatment subgroups. Statistically, results in these two groups were all significant, except for three PTX (BR 50+NAC 1+PTX 1, BR 50+ NAC 5+PTX 1 and BR 50+NAC 1+PTX 5) and one VCR (BR 100+NAC 5+VCR 2.5) subgroups.
Figure 3.
Concomitant treatment of MKN45 cells with BR+NAC plus cisplatin (A) or paclitaxel (B) for 72 hours. BR+NAC treatment differentially affect cancer cell response to concomitant chemotherapy with individual agents. (A) BR/NAC enhanced the effect of cisplatin in 10 out of 18 subgroups. (B) Cytotoxic effects of paclitaxel were found to be enhanced by BR+NAC in all treatment subgroups. All data presented are representative of three independent experiments and depicted as mean ± SE.
Figure 4.
Concomitant treatment of MKN45 cells with BR+NAC plus 5-fluorouracil (A) or vincristine (B) for 72 hours. (A) BR+NAC increased 5FU-induced cytotoxicity in 13 out of 18 subgroups. (B) BR+NAC potentiate cytotoxic effects of vincristine in all treatment subgroups. All data presented are representative of three independent experiments and depicted as mean ± SE.
Table 4.
Concomitant treatment of MKN45 cells with BR+NAC
| MKN45 Combo | Cis | PTX | ||
|
| ||||
| 0.5 | 1 | 1 | 5 | |
|
| ||||
| BR 50 + NAC 1 | n | n | n | n |
| BR 50 + NAC 5 | 0.0101 | n | n | < 0.0001 |
| BR 50 + NAC 10 | 0.0012 | n | < 0.0001 | < 0.0001 |
| BR 75 + NAC 1 | n | n | < 0.0001 | 0.0001 |
| BR 75 + NAC 5 | n | n | < 0.0001 | < 0.0001 |
| BR 75 + NAC 10 | 0.0080 | n | < 0.0001 | < 0.0001 |
| BR 100 + NAC 1 | n | n | < 0.0001 | < 0.0001 |
| BR 100 + NAC 5 | 0.0006 | 0.0011 | < 0.0001 | < 0.0001 |
| BR 100 + NAC 10 | < 0.0001 | 0.0002 | < 0.0001 | < 0.0001 |
|
| ||||
| 5FU | VCR | |||
|
|
||||
| 5 | 10 | 0.5 | 2.5 | |
|
| ||||
| BR 50 + NAC 1 | n | n | < 0.0001 | 0.0034 |
| BR 50 + NAC 5 | n | 0.6957 | < 0.0001 | 0.0005 |
| BR 50 + NAC 10 | 0.0122 | 0.0865 | < 0.0001 | 0.0005 |
| BR 75 + NAC 1 | n | n | < 0.0001 | 0.0010 |
| BR 75 + NAC 5 | 0.0085 | 0.0417 | < 0.0001 | 0.0082 |
| BR 75 + NAC 10 | < 0.0001 | 0.0007 | < 0.0001 | 0.0006 |
| BR 100 + NAC 1 | n | 0.0035 | < 0.0001 | 0.0006 |
| BR 100 + NAC 5 | < 0.0001 | < 0.0001 | < 0.0001 | n |
| BR 100 + NAC 10 | < 0.0001 | < 0.0001 | < 0.0001 | 0.0006 |
Cis: cisplatin; 5FU: 5-fluorouracil; PTX: paclitaxel; VCR: vincristine; n: not significant. Italic text or digits highlight concentrations at which BR/NAC enhanced the effect of chemotherapy. Significant results (p < 0.05) are shown in bold.
Concomitant treatment of LS174T cells with BR+NAC enhances response to cisplatin, 5-fluorouracil, paclitaxel or vincristine
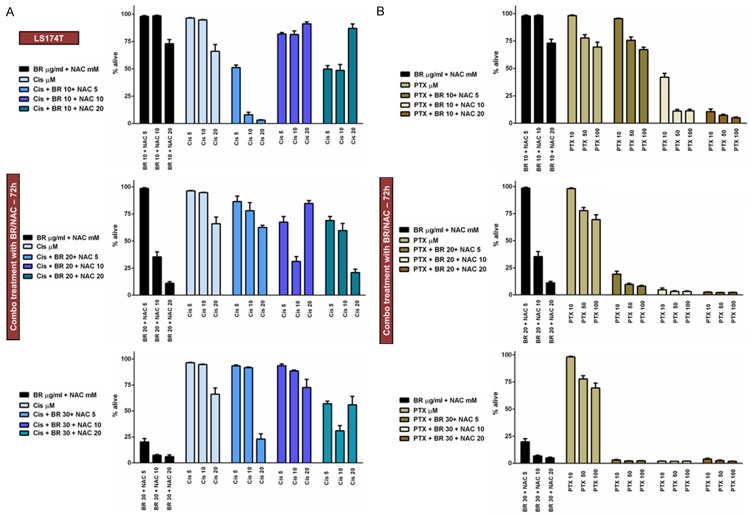
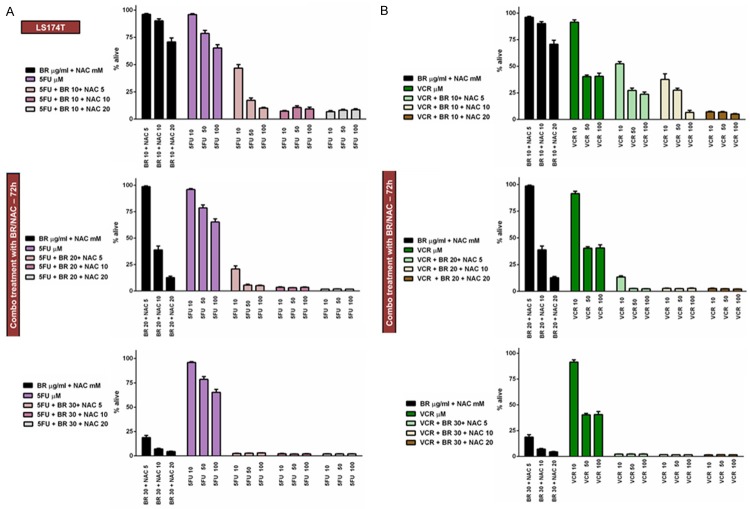
Using similar experimental design, we then examined how BR+NAC influence cytotoxic effects of the four chemotherapeutic agents in combination treatment of LS174T cells. We used 9 possible combinations of three selected concentrations of BR (10, 20 and 30 μg/mL) and NAC (5, 10 and 20 mM) in conjunction with three different concentrations of Cis (Figure 5A), PTX (Figure 5B), 5FU (Figure 6A), or VCR (Figure 6B). Hence, LS174T cells were treated in 4 different chemotherapy groups and 108 (4×27) individual treatment subgroups. As summarized in Table 5, the data indicated that BR/NAC treatment enhanced cancer cell response to concomitant chemotherapy in 104 out of 108 treatment subgroups. Except for four subgroups of Cis group (BR 20+NAC 5+Cis 5, BR 30+NAC 10+Cis 5, BR 20+NAC 5+Cis 20 and BR 30+NAC 20+Cis 20) and two subgroups of PTX (BR 10+NAC 5+PTX 50 and BR 10+NAC 5+PTX 100), BR+NAC-induced enhancement of chemotherapy was statistically significant.
Figure 5.
Concomitant treatment of LS174T cells with BR+NAC plus cisplatin (A) or paclitaxel (B) for 72 hours. (A) BR+NAC treatment enhanced cancer cell response to concomitant chemotherapy with cisplatin in 23 out of 27 subgroups. (B) All 27 treatment subgroups showed enhanced response to paclitaxel. All data presented are representative of three independent experiments and depicted as mean ± SE.
Figure 6.
Concomitant treatment of LS174T cells with BR+NAC plus 5-fluorouracil (A) or vincristine (B) for 72 hours. BR+NAC potentiate cytotoxic effects of 5-fluorouracil (A) and vincristine (B) in all treatment subgroups. All data presented are representative of three independent experiments and depicted as mean ± SE.
Table 5.
Concomitant treatment of LS174T cells with BR+NAC
| LS174T Combo | Cis | PTX | ||||
|
| ||||||
| 5 | 10 | 20 | 10 | 50 | 100 | |
|
| ||||||
| BR 10 + NAC 5 | < 0.0001 | < 0.0001 | < 0.0001 | 0.0113 | n | n |
| BR 10 + NAC 10 | < 0.0001 | 0.0028 | n | < 0.0001 | < 0.0001 | < 0.0001 |
| BR 10 + NAC 20 | < 0.0001 | < 0.0001 | n | < 0.0001 | < 0.0001 | < 0.0001 |
| BR 20 + NAC 5 | n | 0.0495 | 0.6338 | < 0.0001 | < 0.0001 | < 0.0001 |
| BR 20 + NAC 10 | 0.0002 | < 0.0001 | n | < 0.0001 | < 0.0001 | < 0.0001 |
| BR 20 + NAC 20 | < 0.0001 | 0.0003 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| BR 30 + NAC 5 | 0.0143 | 0.0032 | 0.0003 | < 0.0001 | < 0.0001 | < 0.0001 |
| BR 30 + NAC 10 | n | < 0.0001 | n | < 0.0001 | < 0.0001 | < 0.0001 |
| BR 30 + NAC 20 | < 0.0001 | < 0.0001 | 0.3427 | < 0.0001 | < 0.0001 | < 0.0001 |
|
| ||||||
| 5FU | VCR | |||||
|
|
||||||
| 10 | 50 | 100 | 10 | 50 | 100 | |
|
| ||||||
| BR 10 + NAC 5 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | 0.0004 | 0.0008 |
| BR 10 + NAC 10 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | 0.0002 | < 0.0001 |
| BR 10 + NAC 20 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| BR 20 + NAC 5 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| BR 20 + NAC 10 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| BR 20 + NAC 20 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| BR 30 + NAC 5 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| BR 30 + NAC 10 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| BR 30 + NAC 20 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
Cis: cisplatin; 5FU: 5-fluorouracil; PTX: paclitaxel; VCR: vincristine; n: not significant. Italic text or digits highlight concentrations at which BR/NAC enhanced theeffect of chemotherapy. Significant results (p < 0.05) are shown in bold.
Drug-drug interaction analysis of the combination treatments
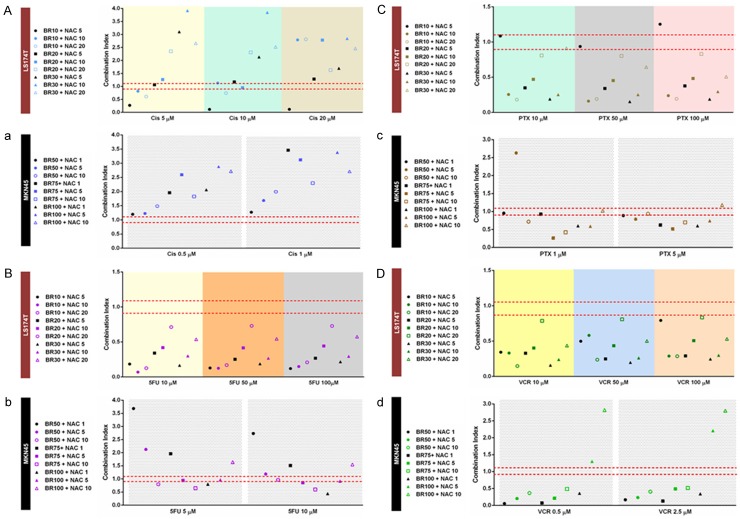
We analyzed how BR+NAC interact with each cytotoxic agent at concentrations used for combination treatment and compared the results in individual chemotherapy groups. Figure 7Aa illustrate the results of drug-drug interaction in Cis group. As seen, the outcome of this interaction in combination treatment of MKN45 cells was antagonistic, with the lowest concentrations of BR+NAC (BR 50+NAC 1 and 5) indicating the weakest antagonism with Cis. As regards LS174T cells, synergy and additivity appeared at given concentrations. Synergistic interactions were found with BR 10+NAC 5, 10 and 20 in combination with Cis 5, BR 10+NAC 5 and 20 in combination with Cis 10, and BR 10+NAC 5 in combination with Cis 20. The strongest synergism was observed when any of the three Cis concentrations was combined with the lowest concentrations of BR and NAC. When BR 20+NAC 5 and BR 20+NAC 10 were used in combination with Cis 5 and 10, respectively, additive interaction resulted. In addition, Cis 10 in combination with BR 10+NAC 10 and BR 20+NAC 5 showed a borderline interaction.
Figure 7.
Drug-drug interaction analysis between cisplatin (A, a), 5-fluorouracil (B, b), paclitaxel (C, c) or vincristine (D, d) and BR+NAC drugs in LS174T (A-D) and MKN45 (a-d) cells. Drug-drug interaction analysis revealed synergism and additivity as the predominant patterns of interaction between bromelain and NAC in combination therapy with chemotherapeutics. As regards cisplatin, the outcome of this interaction in combination treatment of MKN45 cells was antagonistic. The combination index (CI) was calculated based on the drug concentration and cell viability. CIs less than 0.9 and greater than 1.1 were considered as synergism and antagonism, respectively, and those between 0.9 and 1.1 as additivity.
Our data analysis for 5FU group is depicted in Figure 7Bb. As shown, drug-drug interaction in the majority of the combination formulations used for the treatment of MKN45 cells was synergistic or additive. Formulations with synergistic interaction included BR 75+NAC 10 with 5FU 5 and 10, BR 100+NAC 1 with 5FU 5 and 10, BR 50+NAC 10 with 5FU 5, and BR 75+NAC 5 with 5FU 10. Additive interactions were present between BR 50+NAC 10 and 5FU 10, BR 75+NAC 5 and 5FU 5, as well as between BR 100+NAC 5 and 5FU 5 and 10. When BR and NAC were used at the lowest concentrations (BR 50+NAC 1), the strongest antagonism with 5FU appeared. With respect to LS174T cells, drug-drug interaction in all formulations used was synergistic. In this regard, an increase in the concentration of NAC in combination with a given concentration of BR weakened the resultant interaction with 5FU, following a similar pattern in combination with different concentrations of 5FU.
With regard to PTX group, as demonstrated in Figure 7Cc, our results indicated that synergy and, less frequently, additivity are the predominant models of drug-drug interaction in both cell lines. BR 50+NAC 5 and BR 100+NAC 10 in combination with PTX 1 and 5, respectively, were the only formulations with antagonistic interaction in MKN45 cells. Formulations with additivity included BR 50+NAC 1, BR 75+NAC 1 and BR 100+NAC 10 in combination with PTX 1, as well as BR 50+NAC 10 in combination with PTX 5. The remaining formulations all showed synergistic interaction, among which BR 75+NAC 5 had the strongest synergy with PTX. In LS174T cells, when the lowest concentrations of BR+NAC (BR 10+NAC 5) were used, the weakest interaction with PTX resulted. This was present as two additive patterns (in combination with PTX 10 and 50) and the only antagonistic interaction (in combination with PTX 100). The interaction between BR+NAC and PTX in all the remaining formulations was synergistic which followed a similar pattern for different concentrations of PTX.
As shown in Figure 7Dd, VCR group indicated the most favorable drug-drug interaction, with synergistic interaction found in 7 and 9 out of 9 combination formulations used for the treatment of MKN45 and LS174T cells, respectively. The only antagonistic interactions with VCR (0.5 and 2.5 μM) were present in combination with the highest concentrations of BR+NAC (BR 100+NAC 5 and 10). In MKN45 cells, the remaining patterns were all synergistic and similar for both concentrations of VCR. As with 5FU, the interaction between BR+NAC and VCR in all formulations used for LS174T cells was synergistic. BR 20+NAC 20 represented the weakest interaction with both VCR concentrations.
Discussion
The peritoneal component of malignancies is often a major source of morbidity and mortality. In the context of PSM, surgery per se has shown limited curative effectiveness and thus needs to be combined with chemotherapy. On the other hand, the existence of the peritoneal-blood barrier, a diffusion barrier consisting of the mesothelium, interstitium and submesothelial capillary wall [7], and the paucity of subperitoneal blood vessels prevent systemic chemotherapy from delivering therapeutic concentrations to the superficial tumor deposits on the peritoneal lining. Hence, systemic chemotherapy has proven to be minimally effective in this context. In contrast, one can take advantage of the blood-peritoneal barrier to achieve a much higher drug concentration in the peritoneal cavity by intraperitoneal administration of chemotherapeutic agents [reviewed in [8]]. By this approach, not only tumor deposits and peritoneal free cancer cells are targeted, but also tumor cells growing in the submesothelial lymphatic sinus are exposed to high concentrations of drugs absorbed from the lymphatic orifices [8,9]. Therefore, use of intraperitoneal chemotherapy in conjunction with surgery is rational in PSM. For an enhanced treatment efficacy, efforts should be made to maximize cytotoxic effects of chemotherapeutic agents on tumor cells while minimizing their toxic effects on host cells. Since the penetration of intraperitoneally administered agents into peritoneal nodules, even with hyperthermia, is limited to 2-5 mm, CRS is essential to reduce the tumor volume to minimum [2]. In addition, locoregional chemotherapy after complete dissection of an adhesive process and before the onset of wound healing and organization of fibrinous deposits minimizes nonuniform distribution of chemotherapeutic agents and facilitates their access to residual disease and peritoneal free cancer cells [10]. It has been demonstrated that the capillary wall and the surrounding interstitial matrix, but not the mesothelial lining, are the principal barriers for clearance of molecules from the abdominopelvic space [7,11]. Thus, the extent of the peritoneal resection aimed in CRS only minimally affects the pharmacokinetics of the intraperitoneally administered agents [12]. Finally, hyperthermia is believed to enhance cytotoxic effects of selected agents [13] and to improve drug penetration [14]. On this basis, HIPEC is advocated as the standard, or preferable, chemotherapy in selected patients with peritoneal dissemination of malignancies. For clinically stable patients without any evidence of early postoperative complications, HIPEC might be followed by EPIC. As follows, evidence also suggests that intravenous chemotherapy administered simultaneously with intraperitoneal perfusion gains pharmacokinetic advantages. In this regard, it was shown that perfused peritoneal solution rapidly became saturated by intravenously administered cytotoxic agent through large peritoneal and subperitoneal surface blood circulation. This “sink” phenomenon in the absence of enzymatic metabolism provides persistently high levels of intraperitoneal drug [15]. Hence, adjuvant and neoadjuvant bidirectional chemotherapy, too, has been proposed as a treatment option following a major cytoreductive procedure [1,16].
Although CRS combined with HIPEC has brought about long-term benefits in selected patients with PSM, this multimodal curative approach remains associated with treatment failures attributed to the inadequacy of HIPEC to maintain the surgical complete response. This indicates the need for the development of supplementary strategies [1]. In this regard, our preliminary findings on cytotoxic effects of BR/NAC on gastrointestinal cancer cells provided evidence in support of potential utility of this compound in microscopic cytoreduction for PSM of gastrointestinal origin [6]. Here, we investigated whether BR/NAC also has the capability to enhance chemotherapy-induced cytotoxicity if used on their own as pretreatment or in combination with individual chemotherapeutic agents of different families, including cisplatin, 5FU, PTX and VCR. Cisplatin, 5FU and PTX are commonly used agents in intraperitoneal chemotherapy of PSM [2]. HIPEC with cisplatin is particularly employed for PCs from gastric [17] and ovarian cancer [18]. When administered via hyperthermic peritoneal perfusion, cisplatin gains pharmacological advantages that result from not only higher peritoneal concentration and lower systemic absorption and toxicity [19], but enhanced penetration to peritoneal tumors [20,21], rapid absorption [22], and heat synergy [13,23]. 5FU and PTX display relatively high area under the curve of intraperitoneal to intravenous exposure (AUC IP/IV) ratios [24]. 5FU is considered as the cornerstone of the perioperative chemotherapy for peritoneal carcinomatosis of gastrointestinal origin [15]. Due to its large particle size and prolonged retention in the peritoneal cavity, PTX is considered to be suitable for intraperitoneal chemotherapy [25]. Moreover, the bidirectional administration was shown to maintain effective concentrations of PTX in the peritoneal cavity for over 72 hours [26]. Intraperitoneal and bidirectional administration of PTX has been reported to be clinically safe and effective in patients with PC from gastric cancer [27]. 5FU and PTX are also frequently used in EPIC for PSM [2]. VCR is also a widely used intravenous chemotherapeutic agent in human oncology, including combination therapy of CRC [28] and primary colonic lymphoma [29]. As with the aforementioned agents, intraperitoneal administration of VCR has been shown in vivo to provide good clinical results and high bioavailability of the drug with no specific side effects and suggested as a safe and effective alternative for VCR chemotherapy [30-32].
Our data indicated that BR/NAC pretreatment has the potential to sensitize KATO-III and LS174T cells to chemotherapy. At the concentrations used, BR/NAC and individual chemotherapeutic agents were found to differentially interact with one another in combination treatment of either cell line, with resultant interaction ranging from synergy to additivity to antagonism. The most favorable interactions were observed in 5FU group of LS174, as well as in PTX and VCR groups of both cell lines. Synergistic and additive interactions were also evident in other groups, except for Cis group of MKN45. Even in this group, treatment subgroups with minimal antagonism were present. The capability of BR in potentiating the cytotoxic effects of anticancer agents has been shown in a limited number of studies. According to the anecdotal clinical studies in early 1970s, oral administration of BR in doses of over 1000 mg daily in combination with chemotherapeutic agents, such as 5FU and VCR, resulted in tumor regression [33,34]. Oishi et al., however, were the first to observe in vitro that cytotoxicity on KATO-III cells of 5FU, mitomycin-C, doxorubicin and cisplatin was enhanced by the addition of BR [[35] in [36] and [37]]. Similarly, BR has been found to enhance cisplatin cytotoxicity on MPM cells [38].
Evidence also shows that NAC improves the utility of chemotherapy through enhancing the cytotoxic effects of chemotherapeutic agents and/or protecting the host tissues against their toxic effects. Initially, Kline et al. reported that NAC enhanced therapeutic effects of ifosfamide in prolonging the survival of mice with early L1210 leukemia while protecting against chemotherapy-induced toxicity [39]. Using murine models of lung metastasis by malignant melanoma cells, De Flora et al. reported that NAC not only on its own, but also in synergy with doxorubicin prevented tumorigenicity and metastases [40]. Consistently, they later showed that NAC interacted with doxorubicin to inhibit B16-BL6 melanoma cell tumorigenicity and metastasis in mice and prevented doxorubicin-induced toxicity [41]. In agreement, Adeyemo et al. reported that NAC and vitamin E enhanced the susceptibility of Colo201 and Colo205 colon carcinoma cells to 5FU, in vitro [42]. These results were supported by a separate study in vivo wherein NAC increased activity of 5FU against HCT-15 colorectal cancer xenografts in nude mice [43]. Exploring the role of DNA damage response (DDR) defects and ataxia telangiectasia mutated (ATM)/p53 inactivation in lymphomagenesis and chemoresistance in Eµ-myc transgenic mice model of B-cell lymphomas, Reimann et al. found that tumors developed under NAC therapy not only retained a functional ATM-governed DDR, but also maintained sensitivity to chemotherapy (cyclophosphamide and doxorubicin) and indicated a profoundly improved long-term outcome [44]. In line with this, Brum et al. recently reported that NAC pretreatment of CaOV3 ovarian cancer cells potentiates doxorubicin-induced activation of p53 and ATM, leading to reorganization of cytoskeletal networks, inhibition of mTOR activity, and inhibition of cell proliferation and migration [45]. In a study of the underlying role of CXCL12/CXCR4 signaling in chemoresistance to gemcitabine in first-line therapy of pancreatic cancer, Arora et al. found that gemcitabine promotes chemoresistance, migration and invasion of MiaPaCa and Colo357 pancreatic cancer cells through NFκB- and HIF1α-mediated upregulation of CXCR4, a mechanism which was abrogated by NAC pretreatment [46]. In this connection, a recent study by Qanungo et al. consistently revealed that gemcitabine failed to inhibit the growth of MIA PaCa-2 xenografts in nude mice, individually. However, combination treatment with NAC resulted in a reduction of approximately 50% in tumor growth, where NAC markedly enhanced tumor apoptosis [47]. As a chemoprotectant, NAC has been shown to provide protection against toxic effects of a variety of chemotherapeutic agents, including cisplatin [48,49], 5FU [50,51], cyclophosphamide [52,53], ifosfamide [39,54], oxaliplatin [55], methotrexate [56], doxorubicin [41,57], and combined carboplatin, melphalan and etoposide phosphate [58].
In vitro models used in this study represent mucin-expressing carcinoma cell lines with gastric (MKN45 and KATO-III) or intestinal (LS174T) mucin phenotype. While MKN45 and KATO-III cells express the prototypical membrane-associated mucin MUC1 along with the secreted mucin MUC5AC, LS174T expresses the secreted mucins specific to the intestinal goblet cells, primarily MUC2. Evidence shows that both membrane-associated and secreted mucins are involved in diverse biological mechanisms that underpin resistance to chemotherapy. To this end, mucins are thought to form a physical barrier to cellular drug uptake, to alter drug metabolism, to promote resistance to apoptosis, and to contribute to cell stemness and epithelial–mesenchymal transition (EMT) [59]. The mucin-depleting effects of BR/NAC has been observed in our lab [60]. Collectively, chemosensitizing effects of BR/NAC treatment on mucin-expressing gastrointestinal carcinoma cells may be justified in part by their role in depriving tumor cells of their mucins. We thus postulate that utility of this treatment in a locoregional approach after cytoreductive surgery can enhance microscopic cytoreduction through direct cytotoxic effects, chemosensitization and mucin depletion.
In conclusion, our findings supported by results from the aforementioned studies suggest that BR/NAC may have a role as monotherapy in its own right, therapy to facilitate complete cytoreduction through its physico-chemical effects, or as an additive agent to intraperitoneal chemotherapy. It might also be used as pre-conditioning prior to peritonectomy/HIPEC. This represents a promising area for future research. Taking into consideration the aberrant expression of mucins in carcinomas with contributory roles in the development of resistance to chemotherapy, chemosensitizing effects of this novel treatment might be resulted, at least in part, from its mucin-depleting potential.
Disclosure of conflict of interest
Noce.
Abbreviations
- 5FU
5-fluorouracil
- AUC IP/IV
area under the curve of intraperitoneal to intravenous exposure ratio
- BR
bromelain, Cis, cisplatin
- CRS
cytoreductive surgery
- DDR
DNA damage response
- DMF
dimethylformamide
- EPIC
early postoperative intraperitoneal chemotherapy
- HIPEC
hyperthermic intraperitoneal chemotherapy
- MPM
malignant peritoneal mesothelioma
- NAC
N-acetylcysteine
- PC
peritoneal carcinomatosis
- PSMs
peritoneal surface malignancies
- PTX
paclitaxel
- VCR
vincristine
References
- 1.Sugarbaker PH, Bijelic L. Adjuvant bidirectional chemotherapy using an intraperitoneal port. Gastroenterol Res Pract. 2012;2012:752643. doi: 10.1155/2012/752643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohamed F, Cecil T, Moran B, Sugarbaker P. A new standard of care for the management of peritoneal surface malignancy. Curr Oncol. 2011;18:e84–96. doi: 10.3747/co.v18i2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006;7:69–76. doi: 10.1016/S1470-2045(05)70539-8. [DOI] [PubMed] [Google Scholar]
- 4.Sugarbaker PH. Cytoreductive surgery plus hyperthermic perioperative chemotherapy for selected patients with peritoneal metastases from colorectal cancer: a new standard of care or an experimental approach? Gastroenterol Res Pract. 2012;2012:309417. doi: 10.1155/2012/309417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan TD, Deraco M, Baratti D, Kusamura S, Elias D, Glehen O, Gilly FN, Levine EA, Shen P, Mohamed F, Moran BJ, Morris DL, Chua TC, Piso P, Sugarbaker PH. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J. Clin. Oncol. 2009;27:6237–6242. doi: 10.1200/JCO.2009.23.9640. [DOI] [PubMed] [Google Scholar]
- 6.Amini A, Masoumi-Moghaddam S, Ehteda A, Morris DL. Bromelain and N-acetylcysteine inhibit proliferation and survival of gastrointestinal cancer cells in vitro: significance of combination therapy. J Exp Clin Cancer Res. 2014;33:92. doi: 10.1186/s13046-014-0092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacquet P, Sugarbaker PH. Peritoneal-plasma barrier. Cancer Treat Res. 1996;82:53–63. doi: 10.1007/978-1-4613-1247-5_4. [DOI] [PubMed] [Google Scholar]
- 8.Rubino MS, Abdel-Misih RZ, Bennett JJ, Petrelli NJ. Peritoneal surface malignancies and regional treatment: a review of the literature. Surg Oncol. 2012;21:87–94. doi: 10.1016/j.suronc.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Yonemura Y, Endo Y, Obata T, Sasaki T. Recent advances in the treatment of peritoneal dissemination of gastrointestinal cancers by nucleoside antimetabolites. Cancer Sci. 2007;98:11–18. doi: 10.1111/j.1349-7006.2006.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugarbaker PH. Cytoreductive surgery and perioperative intraperitoneal chemotherapy: a new standard of care for appendiceal mucinous tumors with peritoneal dissemination. Clin Colon Rectal Surg. 2005;18:204–214. doi: 10.1055/s-2005-916281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugarbaker PH, Van der Speeten K, Anthony Stuart O, Chang D. Impact of surgical and clinical factors on the pharmacology of intraperitoneal doxorubicin in 145 patients with peritoneal carcinomatosis. Eur J Surg Oncol. 2011;37:719–726. doi: 10.1016/j.ejso.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 12.de Lima Vazquez V, Stuart OA, Mohamed F, Sugarbaker PH. Extent of parietal peritonectomy does not change intraperitoneal chemotherapy pharmacokinetics. Cancer Chemother Pharmacol. 2003;52:108–112. doi: 10.1007/s00280-003-0626-8. [DOI] [PubMed] [Google Scholar]
- 13.Urano M, Kuroda M, Nishimura Y. For the clinical application of thermochemotherapy given at mild temperatures. Int J Hyperthermia. 1999;15:79–107. doi: 10.1080/026567399285765. [DOI] [PubMed] [Google Scholar]
- 14.Jacquet P, Averbach A, Stuart OA, Chang D, Sugarbaker PH. Hyperthermic intraperitoneal doxorubicin: pharmacokinetics, metabolism, and tissue distribution in a rat model. Cancer Chemother Pharmacol. 1998;41:147–154. doi: 10.1007/s002800050721. [DOI] [PubMed] [Google Scholar]
- 15.Van der Speeten K, Stuart OA, Mahteme H, Sugarbaker PH. Pharmacology of perioperative 5-fluorouracil. J Surg Oncol. 2010;102:730–735. doi: 10.1002/jso.21702. [DOI] [PubMed] [Google Scholar]
- 16.Yonemura Y, Endou Y, Shinbo M, Sasaki T, Hirano M, Mizumoto A, Matsuda T, Takao N, Ichinose M, Mizuno M, Miura M, Ikeda M, Ikeda S, Nakajima G, Yonemura J, Yuuba T, Masuda S, Kimura H, Matsuki N. Safety and efficacy of bidirectional chemotherapy for treatment of patients with peritoneal dissemination from gastric cancer: Selection for cytoreductive surgery. J Surg Oncol. 2009;100:311–316. doi: 10.1002/jso.21324. [DOI] [PubMed] [Google Scholar]
- 17.Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, Zhou YF, Xiong B, Yonemura Y, Li Y. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18:1575–1581. doi: 10.1245/s10434-011-1631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deraco M, Kusamura S, Virzi S, Puccio F, Macri A, Famulari C, Solazzo M, Bonomi S, Iusco DR, Baratti D. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy as upfront therapy for advanced epithelial ovarian cancer: multi-institutional phase-II trial. Gynecol Oncol. 2011;122:215–220. doi: 10.1016/j.ygyno.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Cho HK, Lush RM, Bartlett DL, Alexander HR, Wu PC, Libutti SK, Lee KB, Venzon DJ, Bauer KS, Reed E, Figg WD. Pharmacokinetics of cisplatin administered by continuous hyperthermic peritoneal perfusion (CHPP) to patients with peritoneal carcinomatosis. J Clin Pharmacol. 1999;39:394–401. doi: 10.1177/00912709922007967. [DOI] [PubMed] [Google Scholar]
- 20.Los G, Mutsaers PH, van der Vijgh WJ, Baldew GS, de Graaf PW, McVie JG. Direct diffusion of cis-diamminedichloroplatinum(II) in intraperitoneal rat tumors after intraperitoneal chemotherapy: a comparison with systemic chemotherapy. Cancer Res. 1989;49:3380–3384. [PubMed] [Google Scholar]
- 21.Los G, Verdegaal EM, Mutsaers PH, McVie JG. Penetration of carboplatin and cisplatin into rat peritoneal tumor nodules after intraperitoneal chemotherapy. Cancer Chemother Pharmacol. 1991;28:159–165. doi: 10.1007/BF00685503. [DOI] [PubMed] [Google Scholar]
- 22.Cashin PH, Ehrsson H, Wallin I, Nygren P, Mahteme H. Pharmacokinetics of cisplatin during hyperthermic intraperitoneal treatment of peritoneal carcinomatosis. Eur J Clin Pharmacol. 2013;69:533–540. doi: 10.1007/s00228-012-1405-4. [DOI] [PubMed] [Google Scholar]
- 23.Barlogie B, Corry PM, Drewinko B. In vitro thermochemotherapy of human colon cancer cells with cis-dichlorodiammineplatinum(II) and mitomycin C. Cancer Res. 1980;40:1165–1168. [PubMed] [Google Scholar]
- 24.Sugarbaker PH, Mora JT, Carmignani P, Stuart OA, Yoo D. Update on chemotherapeutic agents utilized for perioperative intraperitoneal chemotherapy. Oncologist. 2005;10:112–122. doi: 10.1634/theoncologist.10-2-112. [DOI] [PubMed] [Google Scholar]
- 25.Soma D, Kitayama J, Ishigami H, Kaisaki S, Nagawa H. Different tissue distribution of paclitaxel with intravenous and intraperitoneal administration. J Surg Res. 2009;155:142–146. doi: 10.1016/j.jss.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 26.Ishigami H, Kitayama J, Otani K, Kamei T, Soma D, Miyato H, Yamashita H, Hidemura A, Kaisaki S, Nagawa H. Phase I pharmacokinetic study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer. Oncology. 2009;76:311–314. doi: 10.1159/000209277. [DOI] [PubMed] [Google Scholar]
- 27.Emoto S, Sunami E, Yamaguchi H, Ishihara S, Kitayama J, Watanabe T. Drug development for intraperitoneal chemotherapy against peritoneal carcinomatosis from gastrointestinal cancer. Surg Today. 2014;44:2209–20. doi: 10.1007/s00595-014-0848-x. [DOI] [PubMed] [Google Scholar]
- 28.Fleischer I, Wainstein R, de Gibson AS. [Treatment of advanced cancer of the colon and rectum with the combination of 5-fluorouracil, imidazolecarboxamide and vincristine] . Medicina (B Aires) 1983;43:143–146. [PubMed] [Google Scholar]
- 29.Tang TC, Kuo MC, Chang H, Dunn P, Wang PN, Wu JH, Lin TL, Hung YS, Kuo TT, Shih LY. Primary colonic lymphoma: an analysis of 74 cases with localized large-cell lymphoma. Eur J Haematol. 2011;87:28–36. doi: 10.1111/j.1600-0609.2011.01632.x. [DOI] [PubMed] [Google Scholar]
- 30.Bairy KL, Sanath S, Jagetia GC, Somayaji SN, Vidyasagar MS, Baliga MS. Evaluation of intraperitoneal vincristine in malignant peritoneal effusion. Indian J Physiol Pharmacol. 2003;47:270–278. [PubMed] [Google Scholar]
- 31.Voorhorst MJ, van Maarseveen EM, van Lankveld AJ, Teske E. Bioavailability of cyclophosphamide and vincristine after intraperitoneal administration in cats. Anticancer Drugs. 2014;25:1211–1214. doi: 10.1097/CAD.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 32.Teske E, van Lankveld AJ, Rutteman GR. Intraperitoneal antineoplastic drug delivery: experience with a cyclophosphamide, vincristine and prednisolone protocol in cats with malignant lymphoma. Vet Comp Oncol. 2014;12:37–46. doi: 10.1111/j.1476-5829.2012.00329.x. [DOI] [PubMed] [Google Scholar]
- 33.Gerard G. [Anticancer treatment and bromelains] . Agressologie. 1972;13:261–274. [PubMed] [Google Scholar]
- 34.Nieper HA. A program for the treatment of cancer. Krebs. 1974;6:124–127. [Google Scholar]
- 35.Oishi N, Batkin S, Taussig S, Vaught L, Szekerczes J. Paper presented at: The Coulter Electronic Flow Cytometry Meeting. 1985. Enhancement of cell cycle perturbation with bromelain. [Google Scholar]
- 36.Batkin S, Taussig S, Szekerczes J. Modulation of pulmonary metastasis (Lewis lung carcinoma) by bromelain, an extract of the pineapple stem (Ananas comosus) Cancer Invest. 1988;6:241–242. doi: 10.3109/07357908809077053. [DOI] [PubMed] [Google Scholar]
- 37.Taussig SJ, Batkin S. Bromelain, the enzyme complex of pineapple (Ananas comosus) and its clinical application. An update. J Ethnopharmacol. 1988;22:191–203. doi: 10.1016/0378-8741(88)90127-4. [DOI] [PubMed] [Google Scholar]
- 38.Pillai K, Ehteda A, Akhter J, Chua TC, Morris DL. Anticancer effect of bromelain with and without cisplatin or 5-FU on malignant peritoneal mesothelioma cells. Anticancer Drugs. 2014;25:150–160. doi: 10.1097/CAD.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 39.Kline I, Gang M, Woodman RJ, Cysyk RL, Venditti JM. Protection with N-acetyl-L-cysteine (NSC-111180) against isophosphamide (NSC-109724) toxicity and enhancement of therapeutic effect in early murine L1210 leukemia. Cancer Chemother Rep. 1973;57:299–304. [PubMed] [Google Scholar]
- 40.De Flora S, D’Agostini F, Masiello L, Giunciuglio D, Albini A. Synergism between N-acetylcysteine and doxorubicin in the prevention of tumorigenicity and metastasis in murine models. Int J Cancer. 1996;67:842–848. doi: 10.1002/(SICI)1097-0215(19960917)67:6<842::AID-IJC14>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 41.D’Agostini F, Bagnasco M, Giunciuglio D, Albini A, De Flora S. Inhibition by oral N-acetylcysteine of doxorubicin-induced clastogenicity and alopecia, and prevention of primary tumors and lung micrometastases in mice. Int J Oncol. 1998;13:217–224. doi: 10.3892/ijo.13.2.217. [DOI] [PubMed] [Google Scholar]
- 42.Adeyemo D, Imtiaz F, Toffa S, Lowdell M, Wickremasinghe RG, Winslet M. Antioxidants enhance the susceptibility of colon carcinoma cells to 5-fluorouracil by augmenting the induction of the bax protein. Cancer Lett. 2001;164:77–84. doi: 10.1016/s0304-3835(00)00720-5. [DOI] [PubMed] [Google Scholar]
- 43.Bach SP, Williamson SE, Marshman E, Kumar S, O’Dwyer ST, Potten CS, Watson AJ. The antioxidant n-acetylcysteine increases 5-fluorouracil activity against colorectal cancer xenografts in nude mice. J Gastrointest Surg. 2001;5:91–97. doi: 10.1016/s1091-255x(01)80018-4. [DOI] [PubMed] [Google Scholar]
- 44.Reimann M, Loddenkemper C, Rudolph C, Schildhauer I, Teichmann B, Stein H, Schlegelberger B, Dorken B, Schmitt CA. The Mycevoked DNA damage response accounts for treatment resistance in primary lymphomas in vivo. Blood. 2007;110:2996–3004. doi: 10.1182/blood-2007-02-075614. [DOI] [PubMed] [Google Scholar]
- 45.Brum G, Carbone T, Still E, Correia V, Szulak K, Calianese D, Best C, Cammarata G, Higgins K, Ji F, Di W, Wan Y. N-acetylcysteine potentiates doxorubicin-induced ATM and p53 activation in ovarian cancer cells. Int J Oncol. 2013;42:211–218. doi: 10.3892/ijo.2012.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arora S, Bhardwaj A, Singh S, Srivastava SK, McClellan S, Nirodi CS, Piazza GA, Grizzle WE, Owen LB, Singh AP. An undesired effect of chemotherapy: gemcitabine promotes pancreatic cancer cell invasiveness through reactive oxygen species-dependent, nuclear factor kappaB- and hypoxia-inducible factor 1alpha-mediated up-regulation of CXCR4. J Biol Chem. 2013;288:21197–21207. doi: 10.1074/jbc.M113.484576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qanungo S, Uys JD, Manevich Y, Distler AM, Shaner B, Hill EG, Mieyal JJ, Lemasters JJ, Townsend DM, Nieminen AL. N-acetyl-Lcysteine sensitizes pancreatic cancers to gemcitabine by targeting the NFkappaB pathway. Biomed Pharmacother. 2014;68:855–864. doi: 10.1016/j.biopha.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dickey DT, Wu YJ, Muldoon LL, Neuwelt EA. Protection against cisplatin-induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular, and in vivo levels. J Pharmacol Exp Ther. 2005;314:1052–1058. doi: 10.1124/jpet.105.087601. [DOI] [PubMed] [Google Scholar]
- 49.Yoo J, Hamilton SJ, Angel D, Fung K, Franklin J, Parnes LS, Lewis D, Venkatesan V, Winquist E. Cisplatin otoprotection using transtympanic L-N-acetylcysteine: a pilot randomized study in head and neck cancer patients. Laryngoscope. 2014;124:E87–94. doi: 10.1002/lary.24360. [DOI] [PubMed] [Google Scholar]
- 50.Numazawa S, Sugihara K, Miyake S, Tomiyama H, Hida A, Hatsuno M, Yamamoto M, Yoshida T. Possible involvement of oxidative stress in 5-fluorouracil-mediated myelosuppression in mice. Basic Clin Pharmacol Toxicol. 2011;108:40–45. doi: 10.1111/j.1742-7843.2010.00621.x. [DOI] [PubMed] [Google Scholar]
- 51.Al-Hamdany MZ, Al-Hubaity AY. Protective effects of N-acetylcysteine against 5-fluorouracil-induced pulmonary toxicity in albino rats. Iraqi Journal of Medical Sciences. 2014;12:139–149. [Google Scholar]
- 52.Berrigan MJ, Gurtoo HL, Sharma SD, Struck RF, Marinello AJ. Protection by N-acetylcysteine of cyclophosphamide metabolism - related in vivo depression of mixed function oxygenase activity and in vitro denaturation of cytochrome P-450. Biochem Biophys Res Commun. 1980;93:797–803. doi: 10.1016/0006-291x(80)91147-x. [DOI] [PubMed] [Google Scholar]
- 53.Palma PC, Villaca Junior CJ, Netto Junior NR. N-acetylcysteine in the prevention of cyclophosphamide induced haemorrhagic cystitis. Int Surg. 1986;71:36–37. [PubMed] [Google Scholar]
- 54.Hanly L, Rieder MJ, Huang SH, Vasylyeva TL, Shah RK, Regueira O, Koren G. N-acetylcysteine rescue protocol for nephrotoxicity in children caused by ifosfamide. J Popul Ther Clin Pharmacol. 2013;20:e132–145. [PubMed] [Google Scholar]
- 55.Lin PC, Lee MY, Wang WS, Yen CC, Chao TC, Hsiao LT, Yang MH, Chen PM, Lin KP, Chiou TJ. N-acetylcysteine has neuroprotective effects against oxaliplatin-based adjuvant chemotherapy in colon cancer patients: preliminary data. Support Care Cancer. 2006;14:484–487. doi: 10.1007/s00520-006-0018-9. [DOI] [PubMed] [Google Scholar]
- 56.Caglar Y, Ozgur H, Matur I, Yenilmez ED, Tuli A, Gonlusen G, Polat S. Ultrastructural evaluation of the effect of N-acetylcysteine on methotrexate nephrotoxicity in rats. Histol Histopathol. 2013;28:865–874. doi: 10.14670/HH-28.865. [DOI] [PubMed] [Google Scholar]
- 57.Kockar MC, Naziroglu M, Celik O, Tola HT, Bayram D, Koyu A. N-acetylcysteine modulates doxorubicin-induced oxidative stress and antioxidant vitamin concentrations in liver of rats. Cell Biochem Funct. 2010;28:673–677. doi: 10.1002/cbf.1707. [DOI] [PubMed] [Google Scholar]
- 58.Neuwelt EA, Pagel MA, Kraemer DF, Peterson DR, Muldoon LL. Bone marrow chemoprotection without compromise of chemotherapy efficacy in a rat brain tumor model. J Pharmacol Exp Ther. 2004;309:594–599. doi: 10.1124/jpet.103.063347. [DOI] [PubMed] [Google Scholar]
- 59.Jonckheere N, Skrypek N, Van Seuningen I. Mucins and tumor resistance to chemotherapeutic drugs. Biochim Biophys Acta. 2014;1846:142–151. doi: 10.1016/j.bbcan.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 60.Amini A, Masoumi-Moghaddam S, Ehteda A, Liauw W, Morris DL. Depletion of mucin in mucin-producing human gastrointestinal carcinoma: Results from in vitro and in vivo studies with bromelain and N-acetylcysteine. Oncotarget. 2015;6:33329–33344. doi: 10.18632/oncotarget.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]