Abstract
Quercetin, a natural existing polyphenol compound, has shown anticancer capacity for liver, breast, nasopharyngeal and prostate carcinoma but has not been clinically approved yet. This might be due to lack of clear mechanistic picture. Bladder cancer is one of the most common cancers of the urinary tract in the world. In China, bladder cancer has the highest rate of incidence out of all malignancies of the urinary system. The anticancer application of quercetin on bladder cancer has not been investigated either. This study was aimed to examine the mechanisms of quercetin on inhibition of bladder cancer. First, two human and one murine bladder cancer cell lines were tested in vitro for inhibitory sensitivity by MTT and cologenic assays. Second, AMPK pathway including 4E-BP1 and S6K were examined by western blot. Quercetin induces apoptosis and inhibits migration. We are the first to show that quercetin displays potent inhibition on bladder cancer cells via activation of AMPK pathway.
Keywords: Quercetin, bladder cancer, AMPK
Introduction
Bladder cancer is one of the most frequent cancers of the urinary tract, it accounted for about 74,000 new cases and 16,000 deaths in the United States in 2015 [1]. Even though transurethral resection has served as the standard treatment, recurrence and metastasis are often seen in clinic [2]. The commonest way to prevent recurrence and progression is supplemented with intravesical chemotherapy or immunosuppressive agents [3,4]. Despite such approaches, outcomes have changed very little for the past three decades [5], yet recurrence and metastasis still tend to occur. Therefore, in addition to current treatment, novel therapies, such as targeted therapy which has made great progress in cancer treatment [6-8] may be an important strategy for treatment of bladder cancer. To characterize efficient targets, a number of large-scale molecular studies have been conducted in bladder cancer [9-12], including AMPK [13]. AMPK plays an important role in regulating cellular metabolism, preserving cellular energy homeostasis, and is involved in many other cellular processes, including cell apoptosis [14,15]. Recently, compelling evidence shows that AMPK actually plays a critical role in tumor survival and growth [16,17]. Furthermore, AMPK has been considered as a potential therapeutic target for the treatment of cancer [18].
Quercetin, a plant-derived aglycone form of flavonoid glycosides, has been used as a nutritional supplement and may prevent occurrence of various diseases, including cancer. However, its therapeutic effect is unclear and molecular mechanisms of quercetin on cancer treatment have largely not been explored yet. Couple of reports demonstrates that NF-kappaB is a key inflammatory modulator for quercetin to kill cancer cells including melanoma and lung cancer. [19-23] and AMPK pathway may get involved in the anticancer event on various tumors [24,25]. However, whether this is true in bladder cancer is unknown. Thus, we use the two human and one murine bladder cancer cell lines to examine the killing effect of quercetin and the underlying mechanisms.
Materials and methods
Quercetin purchased from Aladdin chemistry Co. Ltd and Adamas Reagent Co. Ltd was dissolved in DMSO to prepare the stock solution of 50 mM. Antibodies for the protein characteristics were against total p70S6 kinase, phosphor-p70 S6 kinase (Thr389), total AMPK, phosphor-AMPK (Thr172), total 4E-BP1, phosphor-4EBP1, NF-κB p65, NF-κB and β-actin were purchased from Cell Signaling Technology. Compound C was from Selleckchem (Houston, Texas, USA).
Cell lines and culture conditions
Bladder cancer cell lines provided by Dr. P. Guo (Institute of Urology, Xi’an Jiaotong University, Xi’an, Shaanxi, P. R. China) was cultured in DMEM supplemented (Hyclone, Logan, UT, USA) with 10% of FBS (Hyclone, Logan, UT, USA) and 1% of penicillin-streptomycin at 37°C, in humidified air containing 5% of CO2.
Cell viability assay
Cell viability was assessed using a tetrazolium-based assay. Cells were seeded at 6×103 per well in 96-well culture plates and incubated in medium containing 10% FBS. Different seeding densities were optimized at the beginning of the experiments. After 24 hours, cells were treated with different concentrations of quercetin (0 μM, 5 µM, 10 µM, 20 µM, 40 µM, 80 µM, 160 µM) and incubated for 48 hours. 50 μl of MTT tetrazolium salt (Sigma) dissolved in Hank’s balanced solution at a concentration of 2 mg/ml was added to each well with indicated treatment and incubated in CO2 incubator for 5 hours. Finally, the medium was aspirated from each well and 150 μl of DMSO (Sigma) was added to dissolve formazan crystals and the absorbance of each well was obtained using a Dynatech MR5000 plate reader at a test wavelength of 490 nm with a reference wavelength of 630 nm.
Clonogenic assay
Clonogenic survival was defined as the ability of the cells to maintain their clonogenic capacity and to form colonies. 8×103 cells were seeded into 24-well dishes in 0.5 ml of medium for 24 hours. Cells were further treated with different concentrations of quercetin (0 μM, 5 µM, 10 µM, 20 µM, 40 µM, 80 µM), and then maintained for 6-8 days in a CO2 incubator. Finally, the cells were fixed with 10% formaldehyde solution and then stained with 0.1% crystal violet, each experiment was performed in triplicate and colony numbers calculated through spectral scanning to determine the maximum absorption wavelength as 550 nm and measured its absorbance through area scanning. Statistical analysis was prepared with the SPSS software program. The ANOVA (analysis of variance) was used to compare colony numbers and determine the significance of these differences.
Assessment of apoptosis
Apoptosis was detected by flow cytometry via the examination of altered plasma membrane phospholipid packing by lipophilic dye Annexin V as described elsewhere. Treated cells were harvested by trypsin, washed twice with PBS, and then suspended in binding buffer at a concentration of 1×106 cells/mL according to the manufacturer’s instruction. Thereafter, 5 µl of Annexin V-FITC and 5 µlM of propidium iodide were added into 100 µl of cell suspension and incubated for 30 minutes at room temperature in the dark. After adding 400 µl of binding buffer, labeled cells were counted by flow cytometry within 1 h. All early apoptotic cells (Annexin V-positive, propidium iodide-negative), necrotic/late apoptotic cells (double positive), as well as living cells (double negative) were detected by FACSC alibur flow cytometer and subsequently analyzed by Cell Quest software (Becton Dickinson). Argon laser excitation wavelength was 488 nm, whereas emission data were acquired at wavelength 530 nm (FL-1 channel) for fluorescein isothiocyanate (FITC) and 670 nm (FL-3 c3 channel) for propidium iodide. At the same time, we analyzed it by fluorescence microscopy by seeding a total of 1.2×104 cells onto 96-well culture plates and after 24 hours treated cells with different concentrations of quercetin (40 μM, 80 μM, 160 μM). 24 hours later, 100 μl binding buffer, 1 μL of Annexin V-FITC and 1 μL of propidium iodide were added to cells at room temperature in the dark for 15 minutes, kept in a low temperature and then observed by fluorescence microscopy.
Measurement of cell migration
A total of 5×103 cells were seeded onto a six-well plate and allowed to reach full confluence. The monolayer was wounded using a cocktail stick. Cells were incubated with serum-free DMEM medium, for the time period as stated. Digital images were taken at times 0, 6 h, 12 h and 24 h. The mean area was calculated using Image J software. The experiments were repeated three times.
Protein characterization
Western blot assessment was performed using regular procedure. Primary antibody was added in BSA and allowed to incubate overnight at 4°C, washed with TBS/0.05% Tween-20 for 5 times (10 min per time) before the secondary antibody was added and then incubated for an additional hour at room temperature. The membrane was again washed 3 times before adding Pierce Super Signal chemiluminescent substrate (Rockford, IL, USA) and then immediately imaged on Chemi Doc (Bio-Rad, Hercules, CA, USA). The films were scanned using EPSON PERFECTION V500 PHOTO and quantified by Image J (NIH, Bethesda, MD, USA).
Statistical analysis
All data are presented as mean±SEM. Statistical analyses were carried out using ANOVA analysis and statistical significance was assumed at a value of p<0.05.
Results
Quercetin inhibits bladder cancer cell lines proliferation
In order to determine whether quercetin affected the proliferation of the bladder cancer cell lines in vitro, we analyzed the effects of the quercetin on the bladder cancer cell lines using MTT approach. The cell line was grown in 10% FBS and was exposed to quercetin (0 μM, 5 µM, 10 µM, 20 µM, 40 µM, 80 µM, 160 µM; Figure 1) and cell viability determined by tetrazolium-based assay after 48 h. In the experiment, we found that quercetin led to a dose-dependent inhibition on cell proliferation, and IC50 was shown in Table 1, the most sensitive cell line is UMUC3 with IC50 42.29 μM.
Figure 1.
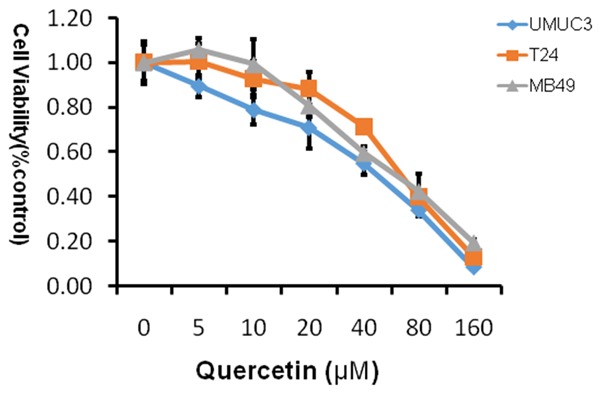
MTT proliferation assays. Treatment with quercetin on cell proliferation of human bladder cancer cell lines T24, UMUC3 and murine bladder cancer cell line MB49. Cell viability was assessed with 48 hour quercetin treatment at concentrations ranging from 0 to 160 µM using a tetrazolium-based assay. Results are presented as the median of 3 independent experiments.
Table 1.
Inhibitory concentration 50% (IC50) of quercetin
| Cells | MB49 | T24 | UMUC3 |
|---|---|---|---|
| IC50 (µM) | 58.63 | 64.02 | 42.29 |
Quercetin suppresses colony formation
The ability of cell lines to form colonies in the presence of quercetin, was studied and we discovered quercetin led to dose-dependent inhibition of colony formation as shown in Figure 2. At a concentration of 80 µM, colony numbers were significantly decreased at three bladder cancer cells, and the most sensitive cell line was UMUC3 which is consistent with the results of MTT experimental.
Figure 2.
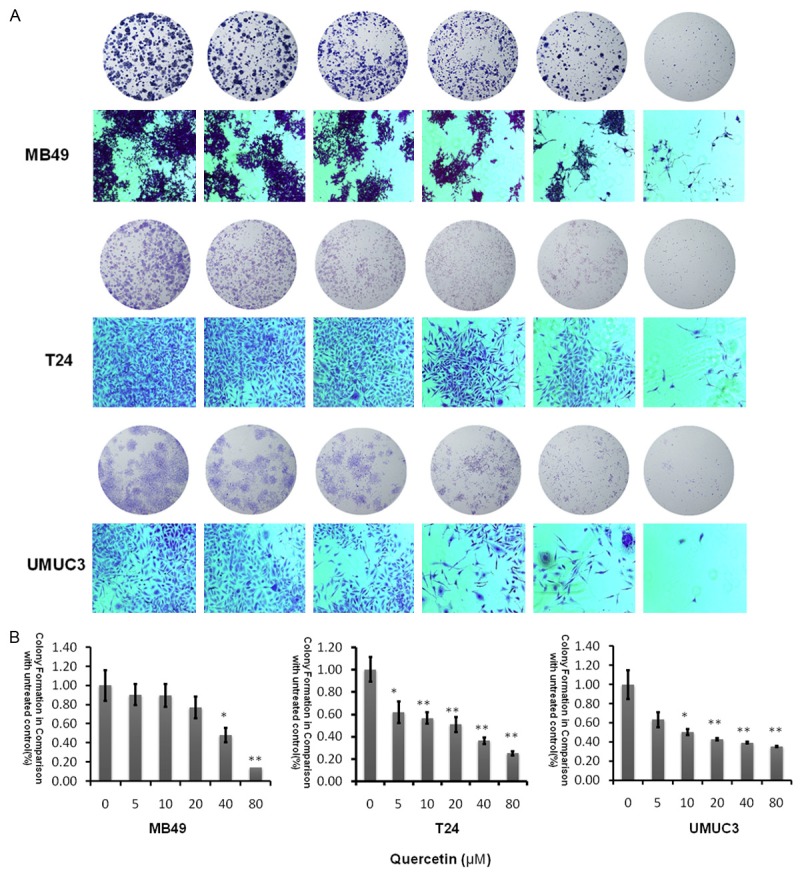
Evaluation of colony suppression of quercetin on bladder cancer cell lines. A. Clonogenic assay was assessed after 7 day quercetin treatment at various concentrations (0-80 µM) and pictures of migration on an inverted microscope with ×10 magnification. B. Bar chart shows quantitative data of average of 3 independent experiments (*P<0.05, **P<0.01 compared with control). Quantitative data were determined by solubilization of crystal violet and spectrophotometric reading at O D 550 nm. Results are presented as the median of 3 independent experiments (Comparison was drawn by the t-test (two-tailed). Data represent means ± SD; *P<0.05).
Assessment of apoptosis
As shown in Figure 3, cellular apoptosis was considerably increased in the quercetin-treated groups compared to the control group using FITC as an apoptosis marker in a dose dependent fashion. At 160 µM, quercetin induced significance apoptotic cells positively marked with both FITC and PI. There is little difference in the three cell lines. This demonstrated that quercetin is able to induce apoptosis in bladder cancer cells.
Figure 3.
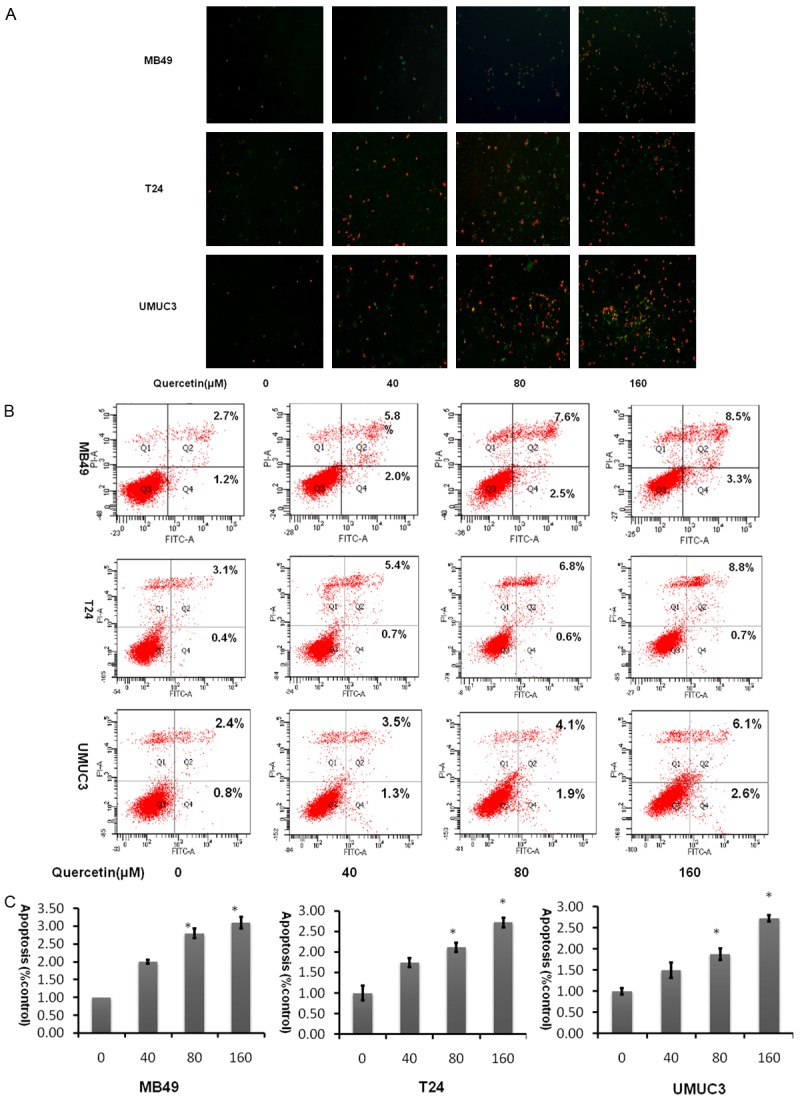
Quercetin induces apoptosis in bladder cancer cell lines in a dose dependent manner. A. Analysis of FITC and PI staining by fluorescent microscopic. B. Representative flow cytometry scatter plots of propidium iodide (PI) (Y axis) vs Annexin V-fluorescein isothiocyanate (FITC) (X axis). C. Bar chart shows quantitative data of average of 3 independent flow cytometry experiments (*P<0.05, **P<0.01 compared with control).
Scratch wound healing assay
Cell scratch assay was used to analyze whether quercetin affects the migration of bladder cancer cells. As shown in Figure 4, cell free area of the quercetin-treated group was significantly wider than that of control in three cell lines at 24 hours (P<0.05), demonstrating that quercetin inhibits bladder cancer cell migration.
Figure 4.
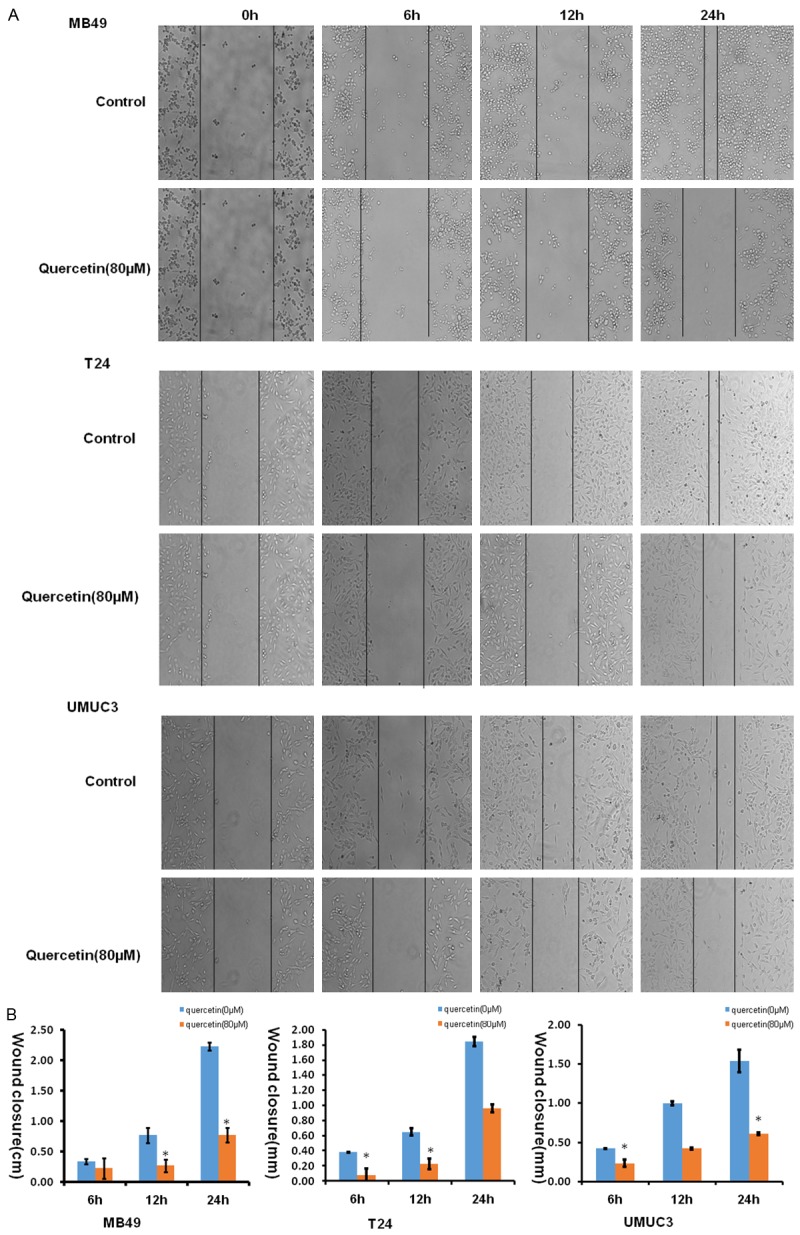
Quercetin showed impaired migration in wound healing assays. A. Images showed the gap of the scratched region of the different group cells; B. Bar chart shows wound closure distances data of average of 3 independent wound healing assays experiments (*P<0.05, **P<0.01 compared with control).
Western blot
Quercetin activates rather than suppress NF-kappaB
Transcription factor NF-κB is a very well-known pro-inflammatory biomarker. Previous reports have demonstrated that quercetin is able to suppress the activation of NF-κB [26] and then inhibit cancer cell growth [27]. To validate whether this pathway was involved in inhibitory effect of quercetin on bladder cancer cells, we performed a similar examination. However, surprisingly as shown in Figure 5, NF-κB was significantly activated in a dose dependent manner whether in human bladder cancer T24 or murine bladder cancer MB49 can activate NF-κB. This implies that quercetin does not suppress NF-κB associated inflammatory pathway.
Figure 5.
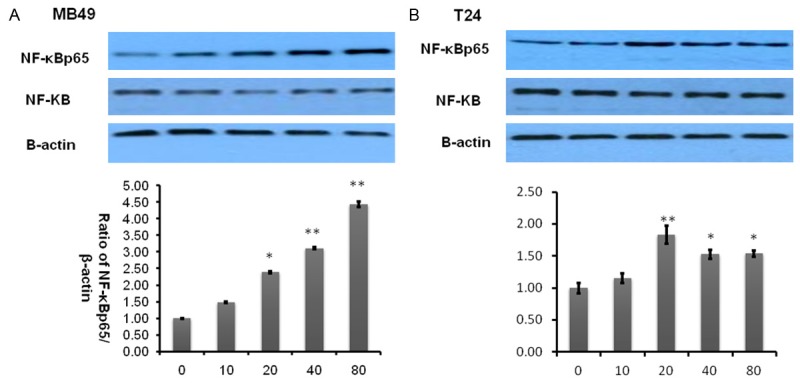
Quercetin activates NF-κBp65. A, B. Represent western blottings of NF-κBp65 and NF-κB after MB49 and T24 bladder cancer cell lines treated by quercetin for 2 h. β-actin was included as a loading control. The ratio of different proteins to β-actin was calculated by the band density of Western blots of each cell line using Image J software (*P<0.05, **P<0.01 compared with control).
Quercetin activates AMPK signaling pathway
AMPK, a central energy sensor, is known as a crucial factor in the interaction between metabolism and cancer [28]. Quercetin is known to activate AMPK in some cancer cell lines, we therefore sought to examine whether pathways known to be influenced by AMPK activity might be affected in bladder cancer cell lines by quercetin treatment. As activation of AMPK correlates tightly with phosphorylation at Thr-172 (p-AMPK), we assessed activation of AMPK by determining p-AMPK and its primary downstream targeting enzyme including p-p70s6k and p-4EBP1. Treatment of bladder cancer cells with quercetin for 2 hours induced a significant increase in the level of phosphorylation of p-AMPK while p-p70s6k, p-4EBP1 decreased as shown in Figure 6A-C.
Figure 6.
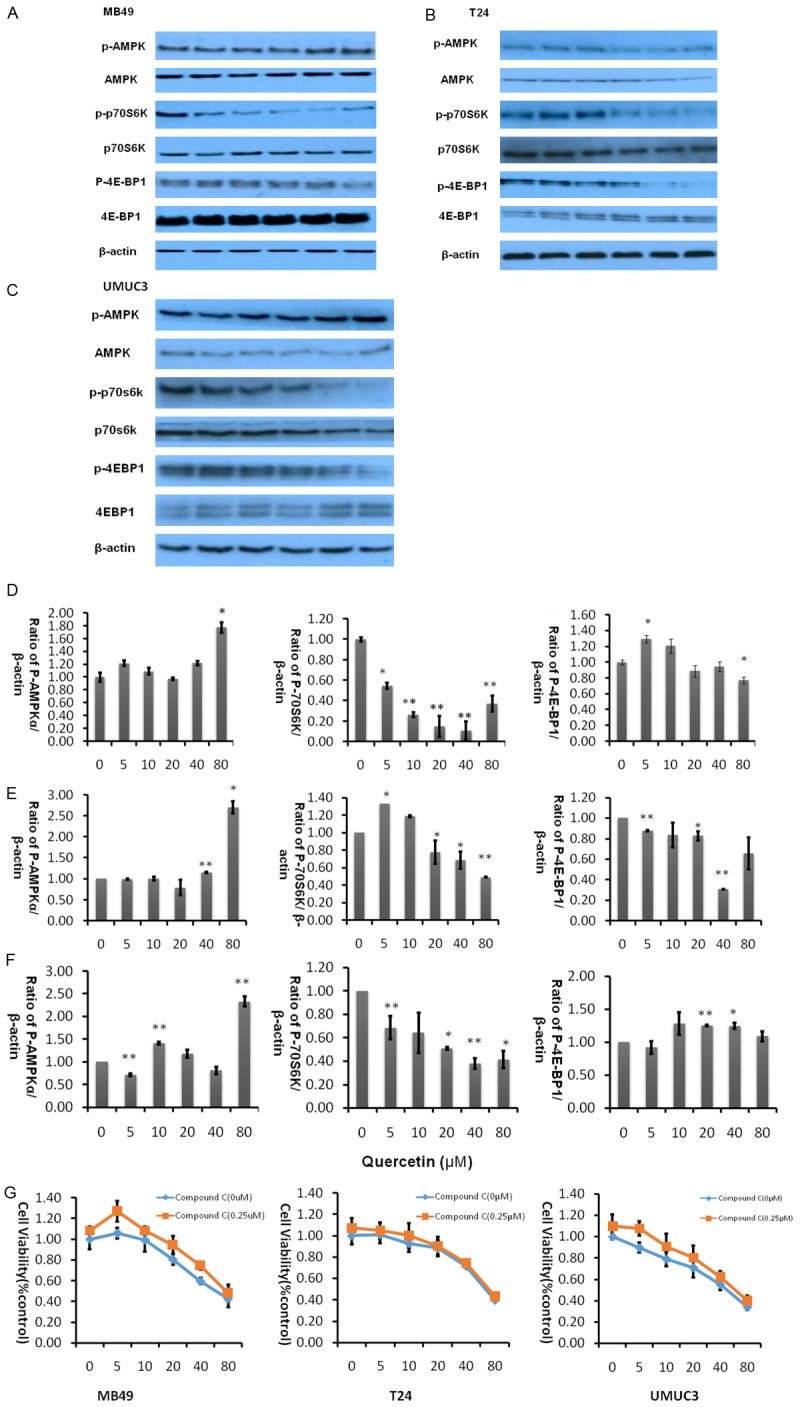
Quercetin activates AMPK intracellular signaling pathways at various concentrations. A-C. Represent western blot of p-AMPK, p-p70S6K, p-4EBP1, t-AMPK, t-p70S6K and t-4EBP1 of three bladder cancer cell lines MB49, T24, and UMUC3 respectively treated with quercetin (0-80 µM) for 2 hours. β-actin was included as a loading control. D. Represents the ratio of proteins of interests to β-actin was calculated by the band density of western blots of MB49 cell line using Image J software (*P<0.05, **P<0.01 compared with control). E. Represent the ratio of proteins of interests to β-actin was calculated by the band density of western blots of T24 cell line using Image J software. *P<0.05, **P<0.01 compared with control. F. Represent the ratio of proteins of interests to β-actin was calculated by the band density of western blots of MB49 cell line using Image J software. (*P<0.05, **P<0.01 compared with control). G. Cell viability was assessed after treatment of quercetin alone or quercetin combined with AMPK antagonist compound C for 48 hours. Data were presented as cell proliferation curves.
Next, we added AMPK specific inhibitor compound C to determine whether activating AMPK signaling pathway is essential for quercetin to exert anti-tumor effect. As shown in Figure 6G, in three bladder cancer cell lines, quercetin and Compound C have shown varying degrees of antagonism, the ability of quercetin to inhibit cancer cell proliferation was reduced after adding compound C, implying that inhibition of AMPK accelerates proliferation of cancer cells.
Discussion
Bladder cancer remains one of the main malignancies that affect the genitourinary tract [29-31]. In fact, the main problem of this disease is that even if it is superficial at diagnostic moment, the current treatment, which is transurethral resection followed by BCG intravesical administration, is not fully effective in preventing tumor recurrence and progression. Therefore, fairly amount of research groups has studied several natural products, produced by secondary metabolism of plants, in an attempt to find new and better treatments to bladder tumors. Quercetin, which exists in nearly all plants, has a long history as a normal part of the human diet [32]. An increasing number of studies have revealed the biological and pharmacological activities of quercetin, which are beneficial to human health including its selective antiproliferative effects and cell death, predominantly through an apoptotic mechanism in cancer cell lines but not in normal cells [33]. Recent data from literature indicate the potential role of quercetin for pharmacologic treatment, including its activity against bladder cancer [28,34]. However, the underlying molecular mechanisms remain poorly understood. In the present study, we found that quercetin inhibited cell proliferation and induced cell death via activating AMPK signaling pathway.
In this work, proliferation data obtained from MTT assays revealed that quercetin treatment significantly suppressed three bladder cancer cells proliferation. Furthermore, colony information and migration data obtained from clonogenic assay and scratch wound assay confirmed the suppression effect of quercetin on bladder cancer cell proliferation or motility. The mechanism of quercetin in bladder cancer cells was analysized by Western blots and the results showed that the expression of p-AMPK was increased while p-p70s6k was strongly decreased after treated with quercetin. These data collectively demonstrate that quercetin exerts anticancer effect on bladder cancer via AMPK signaling pathway. We also found out that quercetin led to a dose-dependent cell apoptosis through FITC apoptosis assay. Wound healing assay also reveals that quercetin suppresses migration of bladder cancer cells.
In conclusion, the results of this study show the anti-proliferative potential of quercetin on tumor cells by activating AMPK signaling pathway and provides solid foundation on the clinical application of quercetin in bladder cancer treatment.
Acknowledgements
This work is in part supported by Advanced Fund of Hunan Provincial Education Department (No. 15A117) and Xiaoxiang Endowed University Professor Fund of Hunan Normal University (No. 840140-008) to X.Y.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Sternberg CN, Bellmunt J, Sonpavde G, Siefker-Radtke AO, Stadler WM, Bajorin DF, Dreicer R, George DJ, Milowsky MI, Theodorescu D, Vaughn DJ, Galsky MD, Soloway MS, Quinn DI International Consultation on Urologic Disease-European Association of Urology Consultation on Bladder Cancer 2012. ICUD-EAU International Consultation on Bladder Cancer 2012: Chemotherapy for urothelial carcinoma-neoadjuvant and adjuvant settings. Eur Urol. 2013;63:58–66. doi: 10.1016/j.eururo.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Rouprêt M, Babjuk M, Compérat E, Zigeuner R, Sylvester RJ, Burger M, Cowan NC, Böhle A, Van Rhijn BW, Kaasinen E, Palou J, Shariat SF. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Cell Carcinoma. 2015 Update. Eur Urol. 2015;68:868–79. doi: 10.1016/j.eururo.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 4.Serretta V, Morgia G, Altieri V, Di Lallo A, Ruggiero G, Salzano L, Battaglia M, Falsaperla M, Zito A, Sblendorio D, Melloni D, Allegro R members of Gruppo Studi Tumori Urologici (GSTU) Foundation. A 1-year maintenance after early adjuvant intravesical chemotherapy has a limited efficacy in preventing recurrence of intermediate risk non-muscle-invasive bladder cancer. BJU Int. 2010;106:212–217. doi: 10.1111/j.1464-410X.2009.09153.x. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan AL, Litwin MS, Chamie K. The future of bladder cancer care in the USA. Nat Rev Urol. 2014;11:59–62. doi: 10.1038/nrurol.2013.180. [DOI] [PubMed] [Google Scholar]
- 6.Lu Y, Liu L, Wang Y, Li F, Zhang J, Ye M, Zhao H, Zhang X, Zhang M, Zhao J, Yan B, Yang A, Feng H, Zhang R, Ren X. siRNA delivered by EGFRspecific scFv sensitizes EGFR-TKI-resistant human lung cancer cells. Biomaterials. 2016;76:196–207. doi: 10.1016/j.biomaterials.2015.10.036. [DOI] [PubMed] [Google Scholar]
- 7.Ahir M, Bhattacharya S, Karmakar S, Mukhopadhyay A, Mukherjee S, Ghosh S, Chattopadhyay S, Patra P, Adhikary A. Bhattacharya S Tailored-CuO-nanowire decorated with folic acid mediated coupling of the mitochondrial-ROS generation and miR425-PTEN axis in furnishing potent anti-cancer activity in human triple negative breast carcinoma cells. Biomaterials. 2016;76:115–32. doi: 10.1016/j.biomaterials.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 8.Wong DJ, Ribas A. Targeted Therapy for Melanoma. Cancer Treat Res. 2016;167:251–62. doi: 10.1007/978-3-319-22539-5_10. [DOI] [PubMed] [Google Scholar]
- 9.Aine M, Eriksson P, Liedberg F, Höglund M, Sjödahl G. On Molecular Classification of Bladder Cancer: Out of One, Many. Eur Urol. 2015;68:921–3. doi: 10.1016/j.eururo.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira-Teixeira M, Parada B, Rodrigues-Santos P, Alves V, Ramalho JS, Caramelo F, Sousa V, Reis F, Gomes CM. Functional and molecular characterization of cancer stem-like cells in bladder cancer: a potential signature for muscle-invasive tumors. Oncotarget. 2015;6:36185–201. doi: 10.18632/oncotarget.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dyrskjøt L, Zieger K, Real FX, Malats N, Carrato A, Hurst C, Kotwal S, Knowles M, Malmström PU, Torre M, Wester K, Allory Y, Vordos D, Caillault A, Radvanyi F, Hein AM, Jensen JL, Jensen KM, Marcussen N, Orntoft TF. Gene expression signatures predict outcome in non-muscle-invasive bladder carcinoma: A multicenter validation study. Clin Cancer Res. 2007;13:3545–51. doi: 10.1158/1078-0432.CCR-06-2940. [DOI] [PubMed] [Google Scholar]
- 12.Moon HS, Batirel S, Mantzoros CS. Alpha linolenic acid and oleic acid additively down-regulate malignant potential and positively crossregulate AMPK/S6 axis in OE19 and OE33 esophageal cancer cells. Metabolism. 2014;63:1447–54. doi: 10.1016/j.metabol.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Faubert B, Vincent EE, Poffenberger MC, Jones RG. The AMP-activated protein kinase (AMPK) and cancer: many faces of a metabolic regulator. Cancer Lett. 2015;356:165–70. doi: 10.1016/j.canlet.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 14.Hardie DG, Alessi DR. LKB1 and AMPK and the cancer-metabolism link - ten years after. BMC Biol. 2013;11:36. doi: 10.1186/1741-7007-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang J, Mills GB. AMPK: a contextual oncogene or tumor suppressor? Cancer Res. 2013;73:2929–35. doi: 10.1158/0008-5472.CAN-12-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinberg GR, Kemp BE. Kemp: AMPK in Health and Disease. Physiol Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 17.Viollet B, Horman S, Leclerc J, Lantier L, Foretz M, Billaud M, Giri S, Andreelli F. AMPK inhibition in health and disease. Crit Rev Biochem Mol Biol. 2010;45:276–295. doi: 10.3109/10409238.2010.488215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang JT, Kwon DY, Yoon SH. AMP-activated protein kinase: a potential target for the diseases prevention by natural occurring polyphenols. N Biotechnol. 2009;26:17–22. doi: 10.1016/j.nbt.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Feitelson MA, Arzumanyan A, Kulathinal RJ, Blain SW, Holcombe RF, Mahajna J, Marino M, Martinez-Chantar ML, Nawroth R, Sanchez-Garcia I, Sharma D, Saxena NK, Singh N, Vlachostergios PJ, Guo S, Honoki K, Fujii H, Georgakilas AG, Bilsland A, Amedei A, Niccolai E, Amin A, Ashraf SS, Boosani CS, Guha G, Ciriolo MR, Aquilano K, Chen S, Mohammed SI, Azmi AS, BhAkta D, Halicka D, Keith WN, Nowsheen S. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin Cancer Biol. 2015;35S:S25–S54. doi: 10.1016/j.semcancer.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen W, Padilla MT, Xu X, Desai D, Krzeminski J, Amin S, Lin Y. Quercetin inhibits multiple pathways involved in interleukin 6 secretion from human lung fibroblasts and activity in bronchial epithelial cell transformation induced by benzo[a] pyrene diol epoxide. Mol Carcinog. 2015 doi: 10.1002/mc.22434. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.AlQathama A, Prieto JM. Natural products with therapeutic potential in melanoma metastasis. Nat Prod Rep. 2015;32:1170–82. doi: 10.1039/c4np00130c. [DOI] [PubMed] [Google Scholar]
- 22.Manu KA, Shanmugam MK, Ramachandran L, Li F, Siveen KS, Chinnathambi A, Zayed ME, Alharbi SA, Arfuso F, Kumar AP, Ahn KS, Sethi G. Isorhamnetin augments the anti-tumor effect of capeciatbine through the negative regulation of NF-κB signaling cascade in gastric cancer. Cancer Lett. 2015;363:28–36. doi: 10.1016/j.canlet.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 23.Vidya Priyadarsini R, Senthil Murugan R, Maitreyi S, Ramalingam K, Karunagaran D, Nagini S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-kappa B inhibition. Eur J Pharmacol. 2010;649:84–91. doi: 10.1016/j.ejphar.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Li N, Huang D, Lu N, Luo L. Role of the LKB1/AMPK pathway in tumor invasion and metastasis of cancer cells (Review) Oncol Rep. 2015;34:2821–6. doi: 10.3892/or.2015.4288. [DOI] [PubMed] [Google Scholar]
- 25.Gasparrini M, Giampieri F, Alvarez Suarez JM, Mazzoni L, Forbes Hernandez TY, Quiles JL, Bullon P, Battino M. AMPK as a New Attractive Therapeutic Target for Disease Prevention: the Role of Dietary Compounds. Curr Drug Targets. 2015 doi: 10.2174/1573399811666150615150235. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Wilczynski J, Duechler M, Czyz M. Targeting NF-κB and HIF-1 pathways for the treatment of cancer: part I. Arch Immunol Ther Exp (Warsz) 2011;59:289–99. doi: 10.1007/s00005-011-0131-4. [DOI] [PubMed] [Google Scholar]
- 27.Prasad S, Phromnoi K, Yadav VR, Chaturvedi MM, Aggarwal BB. Targeting inflammatory pathways by flavonoids for prevention and treatment of cancer. Planta Med. 2010;76:1044–63. doi: 10.1055/s-0030-1250111. [DOI] [PubMed] [Google Scholar]
- 28.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma L, Feugang JM, Konarski P, Wang J, Lu J, Fu S, Ma B, Tian B, Zou C, Wang Z. Growth inhibitory effects of quercetin on bladder cancer cell. Front Biosci. 2006;11:2275–85. doi: 10.2741/1970. [DOI] [PubMed] [Google Scholar]
- 30.Swellam T, Miyanaga N, Onozawa M, Hattori K, Kawai K, Shimazui T, Akaza H. Antineoplastic activity of honey in an experimental bladder cancer implantation model: In vivo and in vitro studies. Int J Urol. 2003;10:213–9. doi: 10.1046/j.0919-8172.2003.00602.x. [DOI] [PubMed] [Google Scholar]
- 31.Mori K, Enokida H, Kagara I, Kawakami K, Chiyomaru T, Tatarano S, Kawahara K, Nishiyama K, Seki N, Nakagawa M. CpG hypermethylation of colla gen type I2 contributes to proliferation and migration activity of human bladder cancer. Int J Oncol. 2009;34:1593–602. doi: 10.3892/ijo_00000289. [DOI] [PubMed] [Google Scholar]
- 32.Latruffe N, Menzel M, Delmas D, Buchet R, Lançon A. Compared binding properties between resveratrol and other polyphenols to plasmatic albumin: consequences for the health protecting effect of dietary plant microcomponents. Molecules. 2014;19:17066–17077. doi: 10.3390/molecules191117066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Senthilkumar K, Arunkumar R, Elumalai P, Sharmila G, Gunadharini DN, Banudevi S, Krishnamoorthy G, Benson CS, Arunakaran J. Quercetin inhibits invasion, migration and signaling molecules involved in cell survival and proliferation of prostate cancer cell line (PC-3) Cell Biochem Funct. 2011;29:87–95. doi: 10.1002/cbf.1725. [DOI] [PubMed] [Google Scholar]
- 34.Kim Y, Kim WJ, Cha EJ. Quercetin-induced Growth Inhibition in Human Bladder Cancer Cells Is Associated with an Increase in Ca-activated K Channels. Korean J Physiol Pharmacol. 2011;15:279–83. doi: 10.4196/kjpp.2011.15.5.279. [DOI] [PMC free article] [PubMed] [Google Scholar]


