Abstract
Since its discovery in 1992, the small, 10.4 kDa calcium-binding protein S100P has gained the attention of researchers from different scientific fields due to its potential roles in both healthy and neoplastic tissues. Although not ubiquitously expressed, in tissues where it is present, S100P is associated with distinct changes in cellular behaviour. In this review we have summarized the evolutionary history of S100P, its expression and involvement in implantation and human embryonic development, as well as important functions in normal tissue and cancer. Finally, we have demonstrated its pivotal role as a potential diagnostic and therapeutic target, which opens promising avenues for further fruitful research on S100P.
Keywords: S100P, embryonic development, cancer
Introduction
S100P is a member of the large family of S100 calcium-binding proteins that mediate Ca2+ dependent signal transduction pathways [1,2]. It was originally isolated from the placenta, (which is reflected in its name “P”) by Becker et al. in 1992 [3,4]. S100P is also relatively novel in evolutionary terms, as it is present only in the genomes of vertebrate species. The expression of this protein has been observed during the rhythmic hormonal fluctuations within the uterine wall, where it may have close association with embryonic implantation, as well as the developing embryo, and plays a functional role in a number of adult human tissues. However, a majority of the published reports describe roles of S100P in diverse human cancers, where it is increasingly recognized as a potential diagnostic and therapeutic target.
Here we present a comprehensive review of the multitude of S100P functions, which are implicated in almost all aspects of cellular behavior.
Ancestral origin
The S100 family (called so due to their solubility in 100% ammonium sulphate at neutral pH) of calcium-binding proteins comprises a large number of proteins with a high degree of structural similarity. Most have shown cell and tissue specific expression, however, some functional redundancy is also possible. Since their discovery in 1965 these proteins have been implicated in a whole host of cellular functions, both intracellularly and as secreted molecules [5,6].
S100s are considered relatively ‘young’ in evolutionary terms, as they are present only in vertebrate species [7]. Over 20 S100 proteins have been identified, but the number might still increase with the rapid accumulation of novel genomic sequences of additional vertebrate species. In the human genome, 16 S100 genes (S100A1-A16) cluster in the human epidermal differentiation complex on chromosome 1q21 [8], while S100B, S100G, S100Z and S100P are present on separate chromosomes [9]. In humans, S100P gene maps on the 4th chromosome (4p16), with its homologs being found in the respective chromosomal locations in chimpanzee, dog, Norwegian rat, and opossum. Interestingly, despite being present in a wide number of mammals, including all primates with available genomic sequences, S100P is not expressed ubiquitously and the gene is missing in a number of species, including majority of rodents [9]. This can be due to either methodological issues such as incomplete genome sequencing e.g. in the cow and the fish, or due to the loss of the corresponding genome sequences during speciation.
S100P in implantation and human embryonic development
A receptive endometrium and viable blastocyst are the two necessary conditions for successful implantation, continuation of progressive cell divisions and further development of a living embryo [10,11]. Interestingly, the rhythmic changes in receptivity of the uterine endometrium correlate with the rise of S100P levels in humans due to hormonal variation, especially with marked increase in progesterone (P4). During the implantation window, which lasts approximately four days, S100P expression surges to levels that are approximately 100 times higher than in other phases of the menstrual cycle [12-14]. This suggests that S100P is potentially a unique biomarker of a receptive endometrium. In addition, it was shown, at least in vitro, that expression of S100P also increases significantly in stromal cells after their co-culture with trophoblast cells [15,16], which implies that S100P may also be involved in interactions at the maternal-fetal interface (Figure 1). Clearly, further exciting work to fully elucidate how S100P may encourage or even permit implantation is still awaited for.
Figure 1.
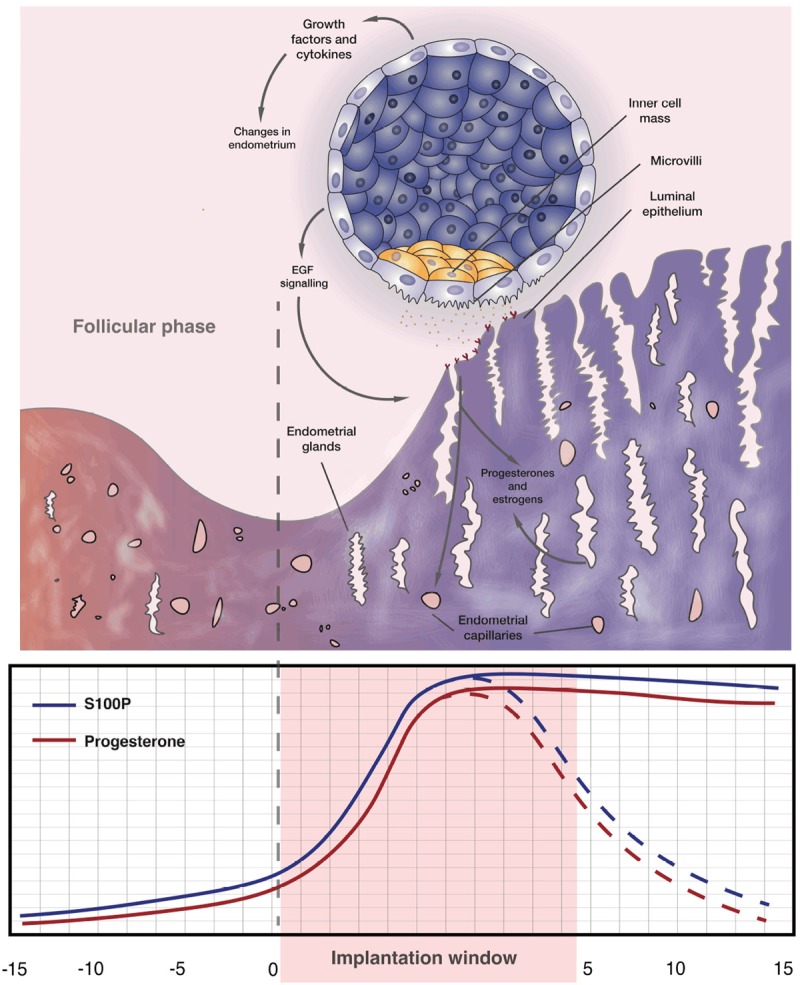
S100P expression during embryonic implantation. During the implantation of the human embryo, S100P expression is closely correlated with the rhythmic hormonal changes in the endometrium, particularly with the levels of progesterone (P4). When a developing blastocyst is implanted, S100P is expressed in both the trophoblastic layer of the embryo, as well as in the endometrium of the uterine wall. When implantation is not achieved, S100P expression in the endometrium returns to a basal level. The graph below the image shows a correlation between progesterone and S100P, and is represented in red and blue, respectively.
In addition to this fundamental role, we have recently discovered that S100P is also expressed in several tissues during embryonic development (Figure 2). This expression was first observed in embryos in Carnegie stage 17 (CS17) onwards, initially in the urogenital sinus (Figure 2A and 2B), and persisted within the structure beyond CS23, as expression was observed in post conception week 10 (PCW10). The expression of S100P then extends continuously across the epithelium both in the developing urethra and bladder (with stronger immunoreactivity observed in the bladder) (Figure 2D and 2F), reaching into the lumen of the renal pelvis and to the developing glomerulus (Figure 2G, 2H and 2L). The embryos in CS19 also showed S100P expression within the allantois (Figure 2C), and in CS21 in the hepatic vein (data not shown). In addition to urogenital system, the S100P is also expressed within the developing gastrointestinal tract from CS17 onwards, where it was observed in the stomach and the pylorus (Figure 2J and 2). This is also seen in the later stages, observed in PCW17 and PCW19 (data not shown), again in the epithelium of the stomach, and in the spleen. Further expression in embryonic development was also observed in the epithelium of the gall bladder from CS21 (Figure 2J and 2K), as well as in adrenal glands in CS21 onwards and spleen in PCW14.
Figure 2.
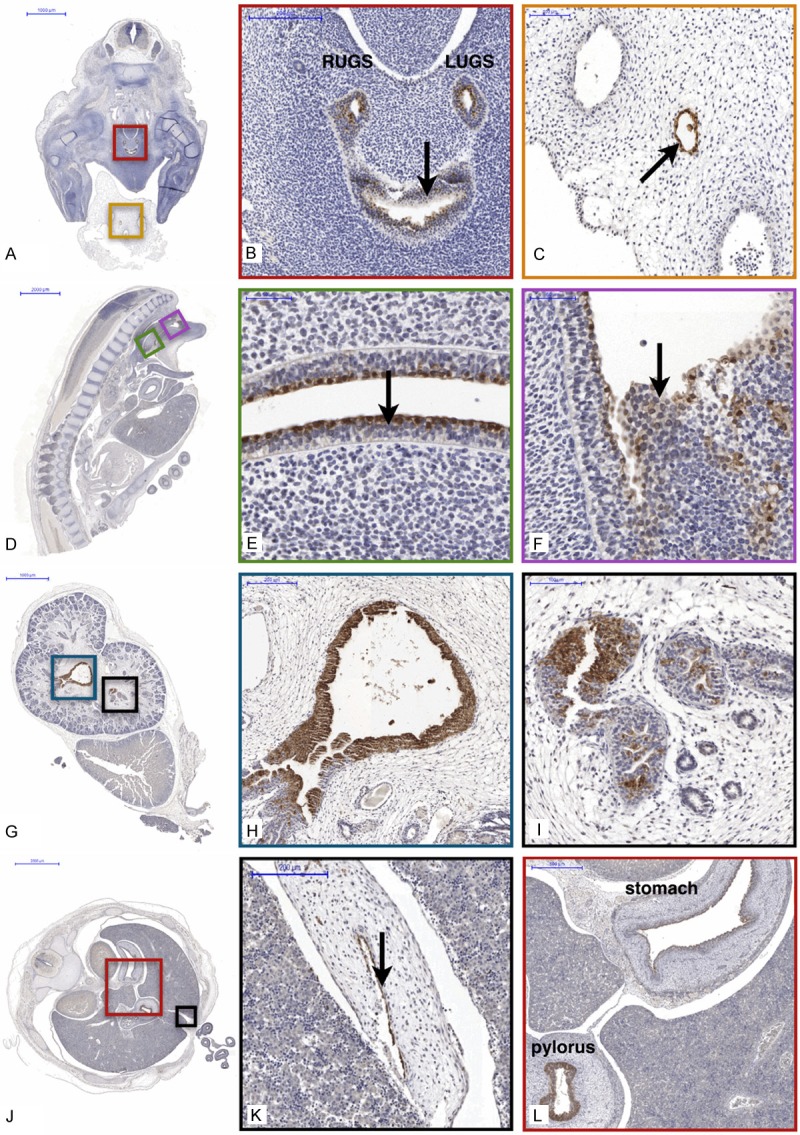
S100P expression in the human embryo. S100P is expressed in the right and left horns of the urogenital sinus (RUGS and LUGS), as well as in the bladder portion of the urogenital sinus (indicated by arrow) in CS17 (A, B), as well as in the allantois (C). In CS21 (D), S100P in the urethral portion of the urogenital sinus (E) shows stronger immunoreactivity than in the bladder portion (F). In the kidney, expression of S100P is seen in the renal pelvis (G, H) as well as in the glomerulus (I) in PCW10. Further expression is seen in the gastrointestinal tract (J-L), including the gallbladder (indicated with arrow) (K) as well as the stomach and pylorus (I) in CS23.
S100P expression in adult tissues
Among the healthy tissues, the highest S100P transcript levels have been observed in the esophagus, particularly in the early stages of differentiation of esophageal epithelium. Moderate mRNA expression has been further seen in the stomach, duodenum and large intestine, as well as in the prostate, trachea, bone marrow and in the leukocytes [17]. At the protein level, the highest S100P levels were seen in the placenta and stomach [18]. Additionally, S100P was shown to increase for a brief period within the prostate during the teenage years, after which its levels decline in adults [19].
S100P in cancer
S100P expression has been found frequently, and at high levels, in a variety of different tumor types [18]. Moreover, a wealth of experimental data from both transcriptomic and proteomic analyses, as well as from the functional assays utilizing S100P-overexpressing or silenced tumor cells both in vitro and in vivo have directly implicated S100P in cancer cell biology. Table 1 provides a comprehensive summary of major reports documenting S100P expression and role in various cancer types.
Table 1.
S100P in cancer
| Basal cell carcinoma of the salivary gland | S100P is used for differentiating basal cell neoplasms from adenoid cystic carcinomas [93]. |
| Breast Cancer | S100P is one of the markers of cancer initiation, is expressed in ductal hyperplasia, in lesions with high-risk of progression, in situ and invasive ductal carcinomas, and is associated with poor prognosis. Its expression correlates with ERBB2/Her2/neu, ER (estradiol) and P4 expression [94-102]. |
| Cholagiocarcinoma | S100P expression is a strong indicator of the early stages of cholangiocarcinoma with increased expression correlating with progression from low to high grade biliary intraepithelial neoplasia (BilIN) [103], and is a sensitive biomarker for detecting cholangicarcinoma [70]. |
| Cervical cancer | S100P is upregulated in all stages of cervical adenocarcinoma [104-107]. |
| Colon cancer | S100P is highly expressed in non-dysplastic tissue from ulcerative colitis patients with high-grade dysplasia [108], and may be used to distinguish flat adenoma from normal mucosa [109,110]. The overexpression of S100P in colorectal cancer cells promotes metastasis [111], and acts as a potential prognostic biomarker [112]. |
| Esophageal cancer | S100P is downregulated in esophageal squamous cell carcinoma [113,114]. |
| Endometrial Cancer | S100P expression is higher in endometrial cancer than in normal endometrium and increases with tumor grade [38]. |
| Gastric cancer | Immunohistochemical analysis of tissue microarray shows S100P expression in >75% of gastric cancers; its downregulation in gastric cancer cell lines leads to apoptosis and inhibition of colony-formation. In contrast, low expression of S100P is linked to poor patients’ outcome [115,116]. |
| Hepatocellular carcinoma | S100P is a novel prognostic factor in HCC that can predict survival in patients with advanced tumor stage or early recurrences [117,118]. |
| Lung cancer | S100P is one of five genes found consistently deregulated in meta-analysis of 12 cDNA array studies. Its expression is observed in early stages of non-small cell lung cancer (NSCLC) and lung adenocarcinoma, and with S100A2 and trypsinogens is predictive of metastatic progression and poor survival in NSCLC [119-122]. |
| Melanoma | S100P, RAGE and ezrin are significantly higher in melanomas than in benign nevus pigmentosus, and metastatic melanoma in comparison to the primary tumor [123]. |
| Oral cancer | S100P is one of the salivary biomarkers in oral squamous carcinoma that can detect cancer recurrence in patients in remission [74,124]. |
| Ovarian cancer | High expression of S100P is correlated with shorter overall survival after chemotherapy [125,126]; conversely, this is also noted in clear cell adenocarcinoma of the ovary which express low levels of S100P [127]. |
| Prostate cancer | S100P is expressed in only 18.5% of prostate cancers, and its expression is significantly lower in cancer than in normal prostate and benign prostate hyperplasia [11]. However, it is one of the highest expressed genes in the androgen – independent CWR22 prostate cancer xenografts [128,129]. Additionally, it correlates with metastatic progression of hormone refractory prostate cancer cells [130]. |
| Pancreatic adenocarcinoma | S100P is expressed in the precursor lesions of pancreatic ductal adenocarcinoma (PDAC), as well as throughout all stages of PDAC development and progression, and is involved in growth and invasion of cancer cells [50,131-134]. |
| Mucinous cystic neoplasms | S100P is expressed in pancreatic mucinous cystic neoplasms (MCN) [59,135]. |
| Intraductal papillary mucinous tumors | Intraductal papillary mucinous tumors (IPMTs) in the pancreas are also expressing S100P [136,137]. |
| Urothelial cancer | S100P is a diagnostic biomarker of urothelial cancer [138,139], and acts as a potential marker for distinguishing urothelial from squamous differentiation [140]. |
The expression of S100P is influenced by several hormones and regulated by a number of transcription factors, and experimental observation has confirmed S100P up-regulation in the presence of androgens [20], SMAD, STAT/CREB and SP/KLF [21,22], as well as progesterone [23,24] and retinoic acid [25]. BMP4 has also been identified as a regulator of S100P expression in in vitro studies where a positive correlation was observed between the two proteins [26]. Additionally, glucocorticoids have been observed to regulate a number of transcripts, including S100P [27].
Interestingly, in metastatic and androgen refractory prostate cancer cells, a correlation between IL-6 and S100P expression has also been observed, and it has been postulated that IL-6 stimulates S100P expression [28]. Several of these S100P regulators are schematically illustrated in the left hand side of Figure 3, shaded in green.
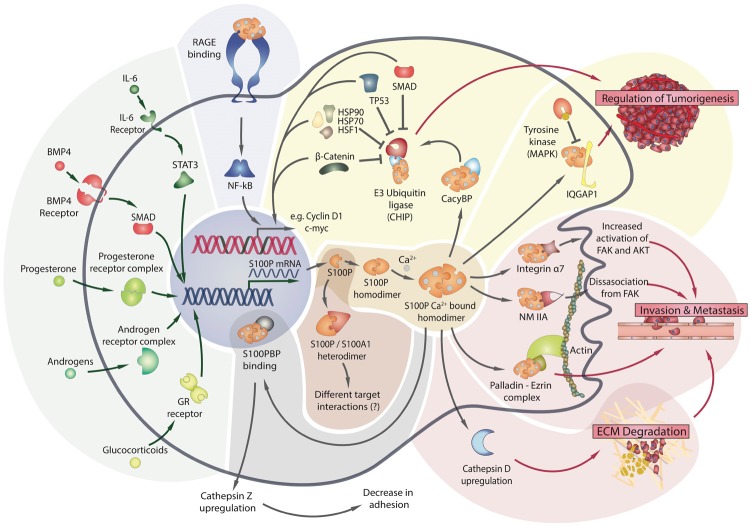
Figure 3.
S100P interactions. A summary of known S100P pathways within and outside of the cell is illustrated, starting with transcriptional regulators of S100P expression (green shading). The protein is capable of forming heterodimers (in brown) as well as homodimers (in orange), with most known interactions occurring with the latter. The intercellular interactions are divided into those associated with tumorigenesis (in yellow), regulation of migration, invasion and metastasis (red shading) and translocation into the nucleus (in grey). The extracellular interaction with RAGE is shown in blue.
Structure and function of S100P
Structurally, S100P belongs to a family of small dimeric members of the large EF-hand super-family of calcium-binding proteins, although it has been shown to bind other divalent metal ions, like Mg2+, Cu2+ and Zn2+ [1,2]. It is a 95 amino acid residue protein, comprising two EF-hands, first one with the low affinity for calcium binding and the second, canonical one, which binds calcium with high affinity. S100P monomers readily interact with one another with high affinity, and homodimer formation is deemed obligatory for S100P functions [29]. Binding of calcium to the EF-binding sites opens the C-terminus of the S100P protein and enables the interactions with other proteins [1,3]. However, heterodimers of S100P with other S100s, e.g. S100A1 have also been observed [30]. The differing interfaces between S100P homodimers and S100P/S100A1 heterodimers could, through changing the conformation of the adjacent C-terminal region, potentially modulate the interaction of S100P with its target proteins, with important functional repercussions. However, this needs to be further established [30].
S100P interacts, directly or indirectly, with a number of different proteins. Through these interactions, S100P integrates and regulates various signaling pathways with a number of important functional outputs that are schematically summarized in Figure 3.
S100P (and several other S100s) has previously been demonstrated to bind to tetratricopeptide repeat region (TPR) domains of proteins in a Ca2+-dependent manner. Recently, S100A2 and S100P were shown to interact with a TPR containing U-box E3 ubiquitin ligase CHIP (‘C terminus of Hsc70- interacting protein’), resulting in inhibition of CHIP-mediated ubiquitination and proteasome degradation of Hsp70, Hsp90, HSF1, Smad1 and mutated p53 [31]. The importance of heat shock proteins not only in stress response but also in oncogenesis [32-34], Smad1 in TGF-β signaling, and multifaceted roles of p53, all testify to S100P as a powerful modifier of carcinogenesis involved in tumor cell proliferation, differentiation, apoptosis, invasion and metastasis.
The E3 ubiquitin ligase also regulates β-catenin degradation [35,36]. β-catenin is a constituent part of a protein complex with E-cadherin and α-catenin that form the adherens junctions [37], which are necessary for the creation and maintenance of epithelial cell barriers and cell-cell adhesion; adherent junctions form a dynamic link to the actin cytoskeleton [37].
Increased S100P affects β-catenin in an additional way, through stimulation of its cytoplasmic to nuclear translocation (at least in endometrial cancer cells) where β-catenin interacts with T-cell factor/lymphoid enhancing factor (TCF/LEF), resulting in increase in the expression of Cyclin D1 and c-myc. Both of these genes are critically involved in cellular proliferation and differentiation [38,39]. Similarly, S100P downregulation leads to a concomitant downregulation of Cyclin D1 and CDK2, resulting in suppressed cellular growth and increased apoptosis in hepatocellular carcinoma [40].
Finally, a ubiquitinylation complex comprising Siah-1, CacyBP/SIP and Skp1 proteins (CacyBP is a calcyclin i.e. S100A6-binding protein which binds several S100 proteins, including S100P), is involved in β-catenin degradation [41], suggesting that several S100s, including S100P connects calcium homeostasis with protein ubiquitinylation and degradation [42].
S100P is also an important interacting partner of IQGAP1 [43], an ubiquitously expressed multidomain scaffolding protein involved in transducing signaling pathways downstream of various cell surface receptors, such as receptor tyrosine kinases, G protein-coupled receptors and several integrins, with wide repercussions on regulation of actin cytoskeleton, microtubule dynamics, and cell-cell contacts. This is particularly important in cancer, where IQGAP1 is thought to contribute to the transformed cell phenotype by regulating signaling pathways involved in cell proliferation and transformation, weakening of cell : cell adhesion contacts, stimulation of cell motility and invasion [44]. Heil et al. have shown that after EGF stimulation of HeLa cells, which increases intracellular Ca2+, S100P binds to IQGAP1 and interferes with its tyrosine phosphorylation. This impairs the B-Raf binding to IQGAP1, and through reducing the MEK1/2 intermediate, modulates MAPK signaling cascade. The interaction between S100P and IQGAP1 was shown to occur through the IQ domain of IQGAP1 and the first EF hand loop of S100P, rather than C-terminus, thus representing a new structural principle of interaction of S100P with the target protein [43,45].
Ca2+-bound S100P is known to bind to and activate dormant ezrin, a multidomain regulatory protein that links cytoskeleton to plasma membrane allowing its interaction with F-actin, which facilitated transendothelial migration of lung squamous carcinoma cells [46,47]. Similarly, through interaction with cytoskeletal protein nonmuscle myosin II (NMIIA), increased levels of S100P reduced the number of FAS (focal adhesion sites), reducing thus adhesion and increasing cell migration [48]. In addition, in lung cancer cells, S100P affects migration and invasion through interaction with integrin Alpha 7 (α7), which is mediated by FAK/AKT-ZEB signaling [49]. In pancreatic cancer cells, S100P was also recently shown to interact with another binding partner, S100PBP, a protein with no homology to any characterized protein, and which, through regulation of cathepsin Z and integrin αvb5 modulates cell adhesion [50,51]. Furthermore, S100P overexpression was also shown to correlate with increased expression of another S100 family member, S100A6, as well as the aspartic protease cathepsin D, both of which are involved in migration and invasion of pancreatic adenocarcinoma cells [52].
Finally, S100P can act in an autocrine manner via Receptor for Activated Glycation End Products (RAGE) to stimulate cell proliferation and survival via the NF-kB pathway [53,54]. S100P (along with several other S100 proteins [55]) acts as initial activator of the pathway via NF-κB/Rel complexes that translocate to the nucleus and induce the expression of a large number of diverse target genes [56]. The S100P-RAGE interaction has recently been employed as a novel therapeutic strategy and will be further discussed in the Therapeutic section of this review.
S100P and diagnostics
Due to its expression in neoplastic lesions and absence in most healthy tissues, S100P has been evaluated as a potential biomarker for detection of several cancers, most commonly using immunohistochemistry approaches. Several studies have highlighted S100P as a marker of pancreatic adenocarcinoma (PDAC) as its expression increases as precursor lesions PanINs (pancreatic intraepithelial neoplasias) progress [50,57]; moreover, S100P has been identified as a possible marker of intraductal papillary mucinous neoplasms (IPMNs) [58] as well as mucinous cystic neoplasms [59], additional potential precursor lesions for PDAC. As a member of a panel, e.g. with mesothelin and/or KOC, S100P showed potential in correct differentiation of true PDACs from borderline cases in cytological assessment of EUS obtained biopsies or surgical resections [60-62]. S100P, mesothelin and IMP3 have also been found as useful biomarkers in gallbladder adenocarcinoma [63], as well as in extrahepatic bile duct carcinoma [64,65].
In cholangiocarcinomas, S100P was proposed to be an effective diagnostic marker in combination with maspin, pVHL and insulin-like growth factor II mRNA-binding protein 3 in bile duct biopsies [66], in distinguishing adenocarcinoma from benign biliary epithelium on endoscopic bile duct biopsy specimens, as well as for distinguishing between cholangiolar-type intrahepatic from bile duct intrahepatic cholangiocarcinomas. Furthermore, bile levels of S100P were significantly higher in cholangiocarcinoma patients compared to those with cholelithiasis [26,67-70].
Interestingly, S100P has also been identified as potential non-invasive biomarker of oral squamous carcinoma in saliva [71-74]. Finally, S100P has been observed in patients with early stage breast cancer, and its expression has been associated with poor prognosis and survival [75]; particularly important is its potential value as a diagnostic marker of triple negative breast cancer [76].
S100P as a therapeutic target
Because of its established functional roles in cancer, S100P has been considered a valuable therapeutic target. Several attempts have been made to inhibit either S100P, its targets, or its interactions, of which one has gained much attention recently - the interaction between S100P and RAGE [77,78]. In vitro attempts to inhibit this interaction was first achieved with cromolyn, and more successfully with its 5-methyl analogue [79,80], both of which bind to the C-domain of S100P, interfering thus with its binding to RAGE [81].
However, a recurring issue with cromolyn is lack of specificity for S100P as it binds to other S100 proteins, as well as its low biodistribution and bioavailability.
Alternative methods of inhibiting S100P, now intracellularly, have been conducted using antisense mRNA retroviral transfection in colon [82], gastric [36], breast [83], and glioblastoma [84] cancer cell lines, and have resulted in a decrease of cellular motility and metastatic potential. Finally, anti-S100P antibodies have been tested both in vitro and in vivo and have shown promising results as both single agents and in combination with chemotherapeutic drugs, such as gemcitabine in pancreatic cancer [85].
S100P expression was found to be associated with cancer resistance to several chemotherapeutic agents, and its silencing sensitized the cancer cells in vitro to doxorubicin [86], cisplatin [87] and oxaliplatin [88]. Furthermore, S100P has also been associated with drug resistance in gastric [89] and pancreatic cancers [90]. Therefore, blocking S100P function might also be expected to improve responses to other therapeutic treatments. However, this needs to be carefully assessed, as some conflicting reports exist, for example, in ovarian and gastric cancer cells, where, at least in vitro, overexpression of S100P led to sensitization of cancer cells to carboplatin and paclitaxel [91], and oxaliplatin, respectively [92].
Despite this, S100P appears to represent a potentially very effective anti-cancer target, at least for in some cancer types, and further development of anti-S100P specific therapies will likely prove to be a fruitful and productive field of investigation.
Conclusion
In this review, we have summarized the current knowledge on S100P with the addition of our own recent observations of S100P expression in human embryonic development.
Despite its relatively short evolutionary history, functions of S100P in vertebrates are vital, from involvement in the earliest steps of embryonic implantation and subsequent embryonic development to exerting the important roles in both healthy adult and cancer tissues. It is, however, for the latter, that S100P has gained most of its existing attention, as it can be potentially utilized as both a diagnostic/prognostic marker and a promising therapeutic target. Since its roles have far-reaching cross-disciplinary implications, spanning from reproductive physiology and embryonic development to inflammation and oncology, studying S100P will thus continue to be an important and fruitful research topic.
Acknowledgements
We would like to thank Dr Steven Lisgo from the Institute of Genetic Medicine, Newcastle University, and Dr Dianne Gerelli from the Institute of Child Health, University College London for their assistance with the human embryonic and foetal material which was provided by the joint MRC/Wellcome Trust grant#099175/Z/12/Z human Developmental Biology Resource (http://hdbr.org).
Disclosure of conflict of interest
None.
References
- 1.Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001;33:637–668. doi: 10.1016/s1357-2725(01)00046-2. [DOI] [PubMed] [Google Scholar]
- 2.Gribenko A, Lopez MM, Richardson JM 3rd, Makhatadze GI. Cloning, overexpression, purification, and spectroscopic characterization of human S100P. Protein Sci. 1998;7:211–215. doi: 10.1002/pro.5560070123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker T, Gerke V, Kube E, Weber K. S100P, a novel Ca(2+)-binding protein from human placenta. cDNA cloning, recombinant protein expression and Ca2+ binding properties. Eur J Biochem. 1992;207:541–547. doi: 10.1111/j.1432-1033.1992.tb17080.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhu HY, Tong XM, Lin XN, Jiang LY, Wang JX, Zhang SY. Expression and Distribution of Calcium-Binding Protein S100P in Human Placenta during Pregnancy. Int J Fertil Steril. 2015;8:445–452. doi: 10.22074/ijfs.2015.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bresnick AR, Weber DJ, Zimmer DB. S100 proteins in cancer. Nat Rev Cancer. 2015;15:96–109. doi: 10.1038/nrc3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore BW. A soluble protein characteristic of the nervous system. Biochem Biophys Res Commun. 1965;19:739–744. doi: 10.1016/0006-291x(65)90320-7. [DOI] [PubMed] [Google Scholar]
- 7.Zimmer DB, Eubanks JO, Ramakrishnan D, Criscitiello MF. Evolution of the S100 family of calcium sensor proteins. Cell Calcium. 2013;53:170–179. doi: 10.1016/j.ceca.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Kypriotou M, Huber M, Hohl D. The human epidermal differentiation complex: cornified envelope precursors, S100 proteins and the ‘fused genes’ family. Exp Dermatol. 2012;21:643–649. doi: 10.1111/j.1600-0625.2012.01472.x. [DOI] [PubMed] [Google Scholar]
- 9.Shang X, Cheng H, Zhou R. Chromosomal mapping, differential origin and evolution of the S100 gene family. Genet Sel Evol. 2008;40:449–464. doi: 10.1186/1297-9686-40-4-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update. 2006;12:731–746. doi: 10.1093/humupd/dml004. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, Zhang JG, Wang W. Expression and significance of S100P, CD147, and OCT4 in different prostate cancer tissue TNM stages. Genet Mol Res. 2015;14:6844–6851. doi: 10.4238/2015.June.18.27. [DOI] [PubMed] [Google Scholar]
- 12.Kodaman PH, Taylor HS. Hormonal regulation of implantation. Obstet Gynecol Clin North Am. 2004;31:745–766. ix. doi: 10.1016/j.ogc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 1999;340:1796–1799. doi: 10.1056/NEJM199906103402304. [DOI] [PubMed] [Google Scholar]
- 14.Zhang D, Ma C, Sun X, Xia H, Zhang W. S100P expression in response to sex steroids during the implantation window in human endometrium. Reprod Biol Endocrinol. 2012;10:106. doi: 10.1186/1477-7827-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popovici RM, Betzler NK, Krause MS, Luo M, Jauckus J, Germeyer A, Bloethner S, Schlotterer A, Kumar R, Strowitzki T, von Wolff M. Gene expression profiling of human endometrial-trophoblast interaction in a coculture model. Endocrinology. 2006;147:5662–5675. doi: 10.1210/en.2006-0916. [DOI] [PubMed] [Google Scholar]
- 16.Burrows TD, King A, Loke YW. Trophoblast migration during human placental implantation. Hum Reprod Update. 1996;2:307–321. doi: 10.1093/humupd/2.4.307. [DOI] [PubMed] [Google Scholar]
- 17.Sato N, Hitomi J. S100P expression in human esophageal epithelial cells: Human esophageal epithelial cells sequentially produce different S100 proteins in the process of differentiation. Anat Rec. 2002;267:60–69. doi: 10.1002/ar.10085. [DOI] [PubMed] [Google Scholar]
- 18.Parkkila S, Pan PW, Ward A, Gibadulinova A, Oveckova I, Pastorekova S, Pastorek J, Martinez AR, Helin HO, Isola J. The calciumbinding protein S100P in normal and malignant human tissues. BMC Clin Pathol. 2008;8:2. doi: 10.1186/1472-6890-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhanasekaran SM, Dash A, Yu J, Maine IP, Laxman B, Tomlins SA, Creighton CJ, Menon A, Rubin MA, Chinnaiyan AM. Molecular profiling of human prostate tissues: insights into gene expression patterns of prostate development during puberty. FASEB J. 2005;19:243–245. doi: 10.1096/fj.04-2415fje. [DOI] [PubMed] [Google Scholar]
- 20.Averboukh L, Liang P, Kantoff PW, Pardee AB. Regulation of S100P expression by androgen. Prostate. 1996;29:350–355. doi: 10.1002/(SICI)1097-0045(199612)29:6<350::AID-PROS2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 21.Gibadulinova A, Oveckova I, Parkkila S, Pastorekova S, Pastorek J. Key promoter elements involved in transcriptional activation of the cancer-related gene coding for S100P calcium-binding protein. Oncol Rep. 2008;20:391–396. [PubMed] [Google Scholar]
- 22.Gibadulinova A, Tothova V, Pastorek J, Pastorekova S. Transcriptional regulation and functional implication of S100P in cancer. Amino Acids. 2011;41:885–892. doi: 10.1007/s00726-010-0495-5. [DOI] [PubMed] [Google Scholar]
- 23.Tong XM, Lin XN, Song T, Liu L, Zhang SY. Calcium-binding protein S100P is highly expressed during the implantation window in human endometrium. Fertil Steril. 2010;94:1510–1518. doi: 10.1016/j.fertnstert.2009.07.1667. [DOI] [PubMed] [Google Scholar]
- 24.Chandramouli A, Mercado-Pimentel ME, Hutchinson A, Gibadulinova A, Olson ER, Dickinson S, Shanas R, Davenport J, Owens J, Bhattacharyya AK, Regan JW, Pastorekova S, Arumugam T, Logsdon CD, Nelson MA. The induction of S100p expression by the Prostaglandin E(2) (PGE(2))/EP4 receptor signaling pathway in colon cancer cells. Cancer Biol Ther. 2010;10:1056–1066. doi: 10.4161/cbt.10.10.13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shyu RY, Huang SL, Jiang SY. Retinoic acid increases expression of the calcium-binding protein S100P in human gastric cancer cells. J Biomed Sci. 2003;10:313–319. doi: 10.1007/BF02256450. [DOI] [PubMed] [Google Scholar]
- 26.Hamada S, Satoh K, Hirota M, Fujibuchi W, Kanno A, Umino J, Ito H, Satoh A, Kikuta K, Kume K, Masamune A, Shimosegawa T. Expression of the calcium-binding protein S100P is regulated by bone morphogenetic protein in pancreatic duct epithelial cell lines. Cancer Sci. 2009;100:103–110. doi: 10.1111/j.1349-7006.2008.00993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kino T, Manoli I, Kelkar S, Wang Y, Su YA, Chrousos GP. Glucocorticoid receptor (GR) beta has intrinsic, GRalpha-independent transcriptional activity. Biochem Biophys Res Commun. 2009;381:671–675. doi: 10.1016/j.bbrc.2009.02.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammacher A, Thompson EW, Williams ED. Interleukin-6 is a potent inducer of S100P, which is up-regulated in androgen-refractory and metastatic prostate cancer. Int J Biochem Cell Biol. 2005;37:442–450. doi: 10.1016/j.biocel.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Koltzscher M, Gerke V. Identification of hydrophobic amino acid residues involved in the formation of S100P homodimers in vivo. Biochemistry. 2000;39:9533–9539. doi: 10.1021/bi000257+. [DOI] [PubMed] [Google Scholar]
- 30.Wang G, Zhang S, Fernig DG, Spiller D, Martin-Fernandez M, Zhang H, Ding Y, Rao Z, Rudland PS, Barraclough R. Heterodimeric interaction and interfaces of S100A1 and S100P. Biochem J. 2004;382:375–383. doi: 10.1042/BJ20040142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimamoto S, Kubota Y, Yamaguchi F, Tokumitsu H, Kobayashi R. Ca2+/S100 proteins act as upstream regulators of the chaperone-associated ubiquitin ligase CHIP (C terminus of Hsc70-interacting protein) J Biol Chem. 2013;288:7158–7168. doi: 10.1074/jbc.M112.436758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130:1005–1018. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendillo ML, Santagata S, Koeva M, Bell GW, Hu R, Tamimi RM, Fraenkel E, Ince TA, Whitesell L, Lindquist S. HSF1 drives a transcriptional program distinct from heat shock to support highly malignant human cancers. Cell. 2012;150:549–562. doi: 10.1016/j.cell.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hay-Koren A, Caspi M, Zilberberg A, Rosin-Arbesfeld R. The EDD E3 ubiquitin ligase ubiquitinates and up-regulates beta-catenin. Mol Biol Cell. 2011;22:399–411. doi: 10.1091/mbc.E10-05-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ning X, Sun S, Hong L, Liang J, Liu L, Han S, Liu Z, Shi Y, Li Y, Gong W, Zhang S, Chen Y, Guo X, Cheng Y, Wu K, Fan D. Calcyclin-binding protein inhibits proliferation, tumorigenicity, and invasion of gastric cancer. Mol Cancer Res. 2007;5:1254–1262. doi: 10.1158/1541-7786.MCR-06-0426. [DOI] [PubMed] [Google Scholar]
- 37.Brembeck FH, Rosario M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr Opin Genet Dev. 2006;16:51–59. doi: 10.1016/j.gde.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Guo L, Chen S, Jiang H, Huang J, Jin W, Yao S. The expression of S100P increases and promotes cellular proliferation by increasing nuclear translocation of beta-catenin in endometrial cancer. Int J Clin Exp Pathol. 2014;7:2102–2112. [PMC free article] [PubMed] [Google Scholar]
- 39.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 40.Kim JK, Jung KH, Noh JH, Eun JW, Bae HJ, Xie HJ, Ahn YM, Ryu JC, Park WS, Lee JY, Nam SW. Targeted disruption of S100P suppresses tumor cell growth by down-regulation of cyclin D1 and CDK2 in human hepatocellular carcinoma. Int J Oncol. 2009;35:1257–1264. [PubMed] [Google Scholar]
- 41.Matsuzawa SI, Reed JC. Siah-1, SIP, and Ebi collaborate in a novel pathway for betacatenin degradation linked to p53 responses. Mol Cell. 2001;7:915–926. doi: 10.1016/s1097-2765(01)00242-8. [DOI] [PubMed] [Google Scholar]
- 42.Filipek A, Jastrzebska B, Nowotny M, Kuznicki J. CacyBP/SIP, a calcyclin and Siah-1-interacting protein, binds EF-hand proteins of the S100 family. J Biol Chem. 2002;277:28848–28852. doi: 10.1074/jbc.M203602200. [DOI] [PubMed] [Google Scholar]
- 43.Heil A, Nazmi AR, Koltzscher M, Poeter M, Austermann J, Assard N, Baudier J, Kaibuchi K, Gerke V. S100P is a novel interaction partner and regulator of IQGAP1. J Biol Chem. 2011;286:7227–7238. doi: 10.1074/jbc.M110.135095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson M, Sharma M, Henderson BR. IQGAP1 regulation and roles in cancer. Cell Signal. 2009;21:1471–1478. doi: 10.1016/j.cellsig.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 45.Nammalwar RC, Heil A, Gerke V. Ezrin interacts with the scaffold protein IQGAP1 and affects its cortical localization. Biochim Biophys Acta. 2015;1853:2086–2094. doi: 10.1016/j.bbamcr.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 46.Koltzscher M, Neumann C, Konig S, Gerke V. Ca2+-dependent binding and activation of dormant ezrin by dimeric S100P. Mol Biol Cell. 2003;14:2372–2384. doi: 10.1091/mbc.E02-09-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Austermann J, Nazmi AR, Muller-Tidow C, Gerke V. Characterization of the Ca2+ -regulated ezrin-S100P interaction and its role in tumor cell migration. J Biol Chem. 2008;283:29331–29340. doi: 10.1074/jbc.M806145200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Du M, Wang G, Ismail TM, Gross S, Fernig DG, Barraclough R, Rudland PS. S100P dissociates myosin IIA filaments and focal adhesion sites to reduce cell adhesion and enhance cell migration. J Biol Chem. 2012;287:15330–15344. doi: 10.1074/jbc.M112.349787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsu YL, Hung JY, Liang YY, Lin YS, Tsai MJ, Chou SH, Lu CY, Kuo PL. S100P interacts with integrin alpha7 and increases cancer cell migration and invasion in lung cancer. Oncotarget. 2015;6:29585–29598. doi: 10.18632/oncotarget.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dowen SE, Crnogorac-Jurcevic T, Gangeswaran R, Hansen M, Eloranta JJ, Bhakta V, Brentnall TA, Luttges J, Kloppel G, Lemoine NR. Expression of S100P and its novel binding partner S100PBPR in early pancreatic cancer. Am J Pathol. 2005;166:81–92. doi: 10.1016/S0002-9440(10)62234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lines KE, Chelala C, Dmitrovic B, Wijesuriya N, Kocher HM, Marshall JF, Crnogorac-Jurcevic T. S100P-binding protein, S100PBP, mediates adhesion through regulation of cathepsin Z in pancreatic cancer cells. Am J Pathol. 2012;180:1485–1494. doi: 10.1016/j.ajpath.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 52.Whiteman HJ, Weeks ME, Dowen SE, Barry S, Timms JF, Lemoine NR, Crnogorac-Jurcevic T. The role of S100P in the invasion of pancreatic cancer cells is mediated through cytoskeletal changes and regulation of cathepsin D. Cancer Res. 2007;67:8633–8642. doi: 10.1158/0008-5472.CAN-07-0545. [DOI] [PubMed] [Google Scholar]
- 53.Arumugam T, Simeone DM, Schmidt AM, Logsdon CD. S100P stimulates cell proliferation and survival via receptor for activated glycation end products (RAGE) J Biol Chem. 2004;279:5059–5065. doi: 10.1074/jbc.M310124200. [DOI] [PubMed] [Google Scholar]
- 54.Fuentes MK, Nigavekar SS, Arumugam T, Logsdon CD, Schmidt AM, Park JC, Huang EH. RAGE activation by S100P in colon cancer stimulates growth, migration, and cell signaling pathways. Dis Colon Rectum. 2007;50:1230–1240. doi: 10.1007/s10350-006-0850-5. [DOI] [PubMed] [Google Scholar]
- 55.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 56.Pahl HL. Activators and target genes of Rel/NFkappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 57.Hu H, Zhang Q, Huang C, Shen Y, Chen X, Shi X, Tang W. Diagnostic value of S100P for pancreatic cancer: a meta-analysis. Tumour Biol. 2014;35:9479–9485. doi: 10.1007/s13277-014-2461-4. [DOI] [PubMed] [Google Scholar]
- 58.Nakata K, Nagai E, Ohuchida K, Hayashi A, Miyasaka Y, Aishima S, Oda Y, Mizumoto K, Tanaka M, Tsuneyoshi M. S100P is a novel marker to identify intraductal papillary mucinous neoplasms. Hum Pathol. 2010;41:824–831. doi: 10.1016/j.humpath.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 59.Fukushima N, Fukayama M. Mucinous cystic neoplasms of the pancreas: pathology and molecular genetics. J Hepatobiliary Pancreat Surg. 2007;14:238–242. doi: 10.1007/s00534-006-1168-3. [DOI] [PubMed] [Google Scholar]
- 60.Dim DC, Jiang F, Qiu Q, Li T, Darwin P, Rodgers WH, Peng HQ. The usefulness of S100P, mesothelin, fascin, prostate stem cell antigen, and 14-3-3 sigma in diagnosing pancreatic adenocarcinoma in cytological specimens obtained by endoscopic ultrasound guided fineneedle aspiration. Diagn Cytopathol. 2014;42:193–199. doi: 10.1002/dc.21684. [DOI] [PubMed] [Google Scholar]
- 61.Kato K, Kamada H, Fujimori T, Aritomo Y, Ono M, Masaki T. Molecular Biologic Approach to the Diagnosis of Pancreatic Carcinoma Using Specimens Obtained by EUSGuided Fine Needle Aspiration. Gastroenterol Res Pract. 2012;2012:243524. doi: 10.1155/2012/243524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ali A, Brown V, Denley S, Jamieson NB, Morton JP, Nixon C, Graham JS, Sansom OJ, Carter CR, McKay CJ, Duthie FR, Oien KA. Expression of KOC, S100P, mesothelin and MUC1 in pancreatico-biliary adenocarcinomas: development and utility of a potential diagnostic immunohistochemistry panel. BMC Clin Pathol. 2014;14:35. doi: 10.1186/1472-6890-14-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi J, Liu H, Wang HL, Prichard JW, Lin F. Diagnostic utility of von Hippel-Lindau gene product, maspin, IMP3, and S100P in adenocarcinoma of the gallbladder. Hum Pathol. 2013;44:503–511. doi: 10.1016/j.humpath.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 64.Kawashima H, Itoh A, Ohno E, Miyahara R, Ohmiya N, Tanaka T, Shimoyama Y, Nakamura S, Ebata T, Nagino M, Goto H, Hirooka Y. Diagnostic and prognostic value of immunohistochemical expression of S100P and IMP3 in transpapillary biliary forceps biopsy samples of extrahepatic bile duct carcinoma. J Hepatobiliary Pancreat Sci. 2013;20:441–447. doi: 10.1007/s00534-012-0581-z. [DOI] [PubMed] [Google Scholar]
- 65.Schmidt MT, Himmelfarb EA, Shafi H, Lin F, Xu H, Wang HL. Use of IMP3, S100P, and pVHL immunopanel to aid in the interpretation of bile duct biopsies with atypical histology or suspicious for malignancy. Appl Immunohistochem Mol Morphol. 2012;20:478–487. doi: 10.1097/PAI.0b013e318245e05b. [DOI] [PubMed] [Google Scholar]
- 66.Chen L, Huang K, Himmelfarb EA, Zhai J, Lai JP, Lin F, Wang HL. Diagnostic value of maspin in distinguishing adenocarcinoma from benign biliary epithelium on endoscopic bile duct biopsy. Hum Pathol. 2015;46:1647–54. doi: 10.1016/j.humpath.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 67.Maeda S, Morikawa T, Takadate T, Suzuki T, Minowa T, Hanagata N, Onogawa T, Motoi F, Nishimura T, Unno M. Mass spectrometrybased proteomic analysis of formalin-fixed paraffin-embedded extrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2015;22:683–691. doi: 10.1002/jhbp.262. [DOI] [PubMed] [Google Scholar]
- 68.Liau JY, Tsai JH, Yuan RH, Chang CN, Lee HJ, Jeng YM. Morphological subclassification of intrahepatic cholangiocarcinoma: etiological, clinicopathological, and molecular features. Mod Pathol. 2014;27:1163–1173. doi: 10.1038/modpathol.2013.241. [DOI] [PubMed] [Google Scholar]
- 69.Sato Y, Harada K, Sasaki M, Nakanuma Y. Clinicopathological significance of S100 protein expression in cholangiocarcinoma. J Gastroenterol Hepatol. 2013;28:1422–1429. doi: 10.1111/jgh.12247. [DOI] [PubMed] [Google Scholar]
- 70.Hamada S, Satoh K, Hirota M, Kanno A, Ishida K, Umino J, Ito H, Kikuta K, Kume K, Masamune A, Katayose Y, Unno M, Shimosegawa T. Calcium-binding protein S100P is a novel diagnostic marker of cholangiocarcinoma. Cancer Sci. 2011;102:150–156. doi: 10.1111/j.1349-7006.2010.01757.x. [DOI] [PubMed] [Google Scholar]
- 71.Nagler RM. Saliva as a tool for oral cancer diagnosis and prognosis. Oral Oncol. 2009;45:1006–1010. doi: 10.1016/j.oraloncology.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 72.Li Y, St John MA, Zhou X, Kim Y, Sinha U, Jordan RC, Eisele D, Abemayor E, Elashoff D, Park NH, Wong DT. Salivary transcriptome diagnostics for oral cancer detection. Clin Cancer Res. 2004;10:8442–8450. doi: 10.1158/1078-0432.CCR-04-1167. [DOI] [PubMed] [Google Scholar]
- 73.Martin JL, Gottehrer N, Zalesin H, Hoff PT, Shaw M, Clarkson JH, Haan P, Vartanian M, McLeod T, Swanick SM. Evaluation of Salivary Transcriptome Markers for the Early Detection of Oral Squamous Cell Cancer in a Prospective Blinded Trial. Compend Contin Educ Dent. 2015;36:365–373. [PubMed] [Google Scholar]
- 74.Brinkmann O, Kastratovic DA, Dimitrijevic MV, Konstantinovic VS, Jelovac DB, Antic J, Nesic VS, Markovic SZ, Martinovic ZR, Akin D, Spielmann N, Zhou H, Wong DT. Oral squamous cell carcinoma detection by salivary biomarkers in a Serbian population. Oral Oncol. 2011;47:51–55. doi: 10.1016/j.oraloncology.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maciejczyk A, Lacko A, Ekiert M, Jagoda E, Wysocka T, Matkowski R, Halon A, Gyorffy B, Lage H, Surowiak P. Elevated nuclear S100P expression is associated with poor survival in early breast cancer patients. Histol Histopathol. 2013;28:513–524. doi: 10.14670/HH-28.513. [DOI] [PubMed] [Google Scholar]
- 76.Maierthaler M, Kriegsmann M, Peng C, Jauch S, Szabo A, Wallwiener M, Rom J, Sohn C, Schneeweiss A, Sinn HP, Yang R, Burwinkel B. S100P and HYAL2 as prognostic markers for patients with triple-negative breast cancer. Exp Mol Pathol. 2015;99:180–187. doi: 10.1016/j.yexmp.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 77.Penumutchu SR, Chou RH, Yu C. Structural insights into calcium-bound S100P and the V domain of the RAGE complex. PLoS One. 2014;9:e103947. doi: 10.1371/journal.pone.0103947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Donato R. RAGE: a single receptor for several ligands and different cellular responses: the case of certain S100 proteins. Curr Mol Med. 2007;7:711–724. doi: 10.2174/156652407783220688. [DOI] [PubMed] [Google Scholar]
- 79.Arumugam T, Ramachandran V, Logsdon CD. Effect of cromolyn on S100P interactions with RAGE and pancreatic cancer growth and invasion in mouse models. J Natl Cancer Inst. 2006;98:1806–1818. doi: 10.1093/jnci/djj498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arumugam T, Ramachandran V, Sun D, Peng Z, Pal A, Maxwell DS, Bornmann WG, Logsdon CD. Designing and developing S100P inhibitor 5-methyl cromolyn for pancreatic cancer therapy. Mol Cancer Ther. 2013;12:654–662. doi: 10.1158/1535-7163.MCT-12-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Penumutchu SR, Chou RH, Yu C. Interaction between S100P and the anti-allergy drug cromolyn. Biochem Biophys Res Commun. 2014;454:404–409. doi: 10.1016/j.bbrc.2014.10.048. [DOI] [PubMed] [Google Scholar]
- 82.Jiang L, Lai YK, Zhang J, Wang H, Lin MC, He ML, Kung HF. Targeting S100P inhibits colon cancer growth and metastasis by Lentivirusmediated RNA interference and proteomic analysis. Mol Med. 2011;17:709–716. doi: 10.2119/molmed.2011.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beissel B, Silva ID, Pesquero JB, Russo J, Schor N, Bellini MH. S-phase reduction in T47D human breast cancer epithelial cells induced by an S100P antisense-retroviral construct. Oncol Rep. 2007;17:611–615. [PubMed] [Google Scholar]
- 84.Sims JN, Graham B, Pacurari M, Leggett SS, Tchounwou PB, Ndebele K. Di-ethylhexylphthalate (DEHP) modulates cell invasion, migration and anchorage independent growth through targeting S100P in LN-229 glioblastoma cells. Int J Environ Res Public Health. 2014;11:5006–5019. doi: 10.3390/ijerph110505006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dakhel S, Padilla L, Adan J, Masa M, Martinez JM, Roque L, Coll T, Hervas R, Calvis C, Messeguer R, Mitjans F, Hernandez JL. S100P antibody-mediated therapy as a new promising strategy for the treatment of pancreatic cancer. Oncogenesis. 2014;3:e92. doi: 10.1038/oncsis.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bertram J, Palfner K, Hiddemann W, Kneba M. Elevated expression of S100P, CAPL and MAGE 3 in doxorubicin-resistant cell lines: comparison of mRNA differential display reverse transcription-polymerase chain reaction and subtractive suppressive hybridization for the analysis of differential gene expression. Anticancer Drugs. 1998;9:311–317. doi: 10.1097/00001813-199804000-00004. [DOI] [PubMed] [Google Scholar]
- 87.Zhang YW, Zheng Y, Wang JZ, Lu XX, Wang Z, Chen LB, Guan XX, Tong JD. Integrated analysis of DNA methylation and mRNA expression profiling reveals candidate genes associated with cisplatin resistance in non-small cell lung cancer. Epigenetics. 2014;9:896–909. doi: 10.4161/epi.28601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tang H, Liu YJ, Liu M, Li X. Establishment and gene analysis of an oxaliplatin-resistant colon cancer cell line THC8307/L-OHP. Anticancer Drugs. 2007;18:633–639. doi: 10.1097/CAD.0b013e3280200428. [DOI] [PubMed] [Google Scholar]
- 89.Ge F, Wang C, Wang W, Wu B. S100P predicts prognosis and drug resistance in gastric cancer. Int J Biol Markers. 2013;28:e387–392. doi: 10.5301/jbm.5000034. [DOI] [PubMed] [Google Scholar]
- 90.Hamada S, Masamune A, Miura S, Satoh K, Shimosegawa T. MiR-365 induces gemcitabine resistance in pancreatic cancer cells by targeting the adaptor protein SHC1 and proapoptotic regulator BAX. Cell Signal. 2014;26:179–185. doi: 10.1016/j.cellsig.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 91.Wang Q, He Z, Gao J, Hu S, Huang M, Liu M, Zheng J, Tang H. S100P sensitizes ovarian cancer cells to carboplatin and paclitaxel in vitro. Cancer Lett. 2008;272:277–284. doi: 10.1016/j.canlet.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 92.Zhao X, Bai Z, Wu P, Zhang Z. S100P enhances the chemosensitivity of human gastric cancer cell lines. Cancer Biomark. 2013;13:1–10. doi: 10.3233/CBM-130330. [DOI] [PubMed] [Google Scholar]
- 93.Jung MJ, Roh JL, Choi SH, Nam SY, Kim SY, Lee SW, Cho KJ. Basal cell adenocarcinoma of the salivary gland: a morphological and immunohistochemical comparison with basal cell adenoma with and without capsular invasion. Diagn Pathol. 2013;8:171. doi: 10.1186/1746-1596-8-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Adam Maciejczyk A. New prognostic factors in breast cancer. Adv Clin Exp Med. 2013;22:5–15. [PubMed] [Google Scholar]
- 95.Barraclough DL, Platt-Higgins A, de Silva Rudland S, Barraclough R, Winstanley J, West CR, Rudland PS. The metastasis-associated anterior gradient 2 protein is correlated with poor survival of breast cancer patients. Am J Pathol. 2009;175:1848–1857. doi: 10.2353/ajpath.2009.090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bray JD, Jelinsky S, Ghatge R, Bray JA, Tunkey C, Saraf K, Jacobsen BM, Richer JK, Brown EL, Winneker RC, Horwitz KB, Lyttle CR. Quantitative analysis of gene regulation by seven clinically relevant progestins suggests a highly similar mechanism of action through progesterone receptors in T47D breast cancer cells. J Steroid Biochem Mol Biol. 2005;97:328–341. doi: 10.1016/j.jsbmb.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 97.Carlsson H, Petersson S, Enerback C. Cluster analysis of S100 gene expression and genes correlating to psoriasin (S100A7) expression at different stages of breast cancer development. Int J Oncol. 2005;27:1473–1481. [PubMed] [Google Scholar]
- 98.Chung L, Shibli S, Moore K, Elder EE, Boyle FM, Marsh DJ, Baxter RC. Tissue biomarkers of breast cancer and their association with conventional pathologic features. Br J Cancer. 2013;108:351–360. doi: 10.1038/bjc.2012.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guerreiro Da Silva ID, Hu YF, Russo IH, Ao X, Salicioni AM, Yang X, Russo J. S100P calcium-binding protein overexpression is associated with immortalization of human breast epithelial cells in vitro and early stages of breast cancer development in vivo. Int J Oncol. 2000;16:231–240. [PubMed] [Google Scholar]
- 100.Mackay A, Jones C, Dexter T, Silva RL, Bulmer K, Jones A, Simpson P, Harris RA, Jat PS, Neville AM, Reis LF, Lakhani SR, O’Hare MJ. cDNA microarray analysis of genes associated with ERBB2 (HER2/neu) overexpression in human mammary luminal epithelial cells. Oncogene. 2003;22:2680–2688. doi: 10.1038/sj.onc.1206349. [DOI] [PubMed] [Google Scholar]
- 101.Russo J, Hu YF, Silva ID, Russo IH. Cancer risk related to mammary gland structure and development. Microsc Res Tech. 2001;52:204–223. doi: 10.1002/1097-0029(20010115)52:2<204::AID-JEMT1006>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 102.Schor AP, Carvalho FM, Kemp C, Silva ID, Russo J. S100P calcium-binding protein expression is associated with high-risk proliferative lesions of the breast. Oncol Rep. 2006;15:3–6. [PubMed] [Google Scholar]
- 103.Aishima S, Fujita N, Mano Y, Kubo Y, Tanaka Y, Taketomi A, Shirabe K, Maehara Y, Oda Y. Different roles of S100P overexpression in intrahepatic cholangiocarcinoma: carcinogenesis of perihilar type and aggressive behavior of peripheral type. Am J Surg Pathol. 2011;35:590–598. doi: 10.1097/PAS.0b013e31820ffdf1. [DOI] [PubMed] [Google Scholar]
- 104.Chao A, Wang TH, Lai CH. Overview of microarray analysis of gene expression and its applications to cervical cancer investigation. Taiwan J Obstet Gynecol. 2007;46:363–373. doi: 10.1016/S1028-4559(08)60005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chao A, Wang TH, Lee YS, Hsueh S, Chao AS, Chang TC, Kung WH, Huang SL, Chao FY, Wei ML, Lai CH. Molecular characterization of adenocarcinoma and squamous carcinoma of the uterine cervix using microarray analysis of gene expression. Int J Cancer. 2006;119:91–98. doi: 10.1002/ijc.21813. [DOI] [PubMed] [Google Scholar]
- 106.Jakubickova L, Barathova M, Pastorekova S, Pastorek J, Gibadulinova A. Expression of S100P gene in cervical carcinoma cells is independent of E7 human papillomavirus oncogene. Acta Virol. 2005;49:133–137. [PubMed] [Google Scholar]
- 107.Mills AM, Karamchandani JR, Vogel H, Longacre TA. Endocervical fibroblastic malignant peripheral nerve sheath tumor (neurofibrosarcoma): report of a novel entity possibly related to endocervical CD34 fibrocytes. Am J Surg Pathol. 2011;35:404–412. doi: 10.1097/PAS.0b013e318208f72e. [DOI] [PubMed] [Google Scholar]
- 108.Brentnall TA, Pan S, Bronner MP, Crispin DA, Mirzaei H, Cooke K, Tamura Y, Nikolskaya T, Jebailey L, Goodlett DR, McIntosh M, Aebersold R, Rabinovitch PS, Chen R. Proteins That Underlie Neoplastic Progression of Ulcerative Colitis. Proteomics Clin Appl. 2009;3:1326. doi: 10.1002/prca.200900061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kita H, Hikichi Y, Hikami K, Tsuneyama K, Cui ZG, Osawa H, Ohnishi H, Mutoh H, Hoshino H, Bowlus CL, Yamamoto H, Sugano K. Differential gene expression between flat adenoma and normal mucosa in the colon in a microarray analysis. J Gastroenterol. 2006;41:1053–1063. doi: 10.1007/s00535-006-1894-y. [DOI] [PubMed] [Google Scholar]
- 110.Liu F, Guo JB, Shen ZY, Mu TY, Zhi PK, Li GX. [Application of genome-wide microarray for screening genes related to peritoneal metastasis of colorectal cancer] . Nan Fang Yi Ke Da Xue Xue Bao. 2012;32:400–403. [PubMed] [Google Scholar]
- 111.Dong L, Wang F, Yin X, Chen L, Li G, Lin F, Ni W, Wu J, Jin R, Jiang L. Overexpression of S100P promotes colorectal cancer metastasis and decreases chemosensitivity to 5-FU in vitro. Mol Cell Biochem. 2014;389:257–264. doi: 10.1007/s11010-013-1947-5. [DOI] [PubMed] [Google Scholar]
- 112.Wang Q, Zhang YN, Lin GL, Qiu HZ, Wu B, Wu HY, Zhao Y, Chen YJ, Lu CM. S100P, a potential novel prognostic marker in colorectal cancer. Oncol Rep. 2012;28:303–310. doi: 10.3892/or.2012.1794. [DOI] [PubMed] [Google Scholar]
- 113.Ji J, Zhao L, Wang X, Zhou C, Ding F, Su L, Zhang C, Mao X, Wu M, Liu Z. Differential expression of S100 gene family in human esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2004;130:480–486. doi: 10.1007/s00432-004-0555-x. [DOI] [PubMed] [Google Scholar]
- 114.Zhi H, Zhang J, Hu G, Lu J, Wang X, Zhou C, Wu M, Liu Z. The deregulation of arachidonic acid metabolism-related genes in human esophageal squamous cell carcinoma. Int J Cancer. 2003;106:327–333. doi: 10.1002/ijc.11225. [DOI] [PubMed] [Google Scholar]
- 115.Jia SQ, Niu ZJ, Zhang LH, Zhong XY, Shi T, Du H, Zhang GG, Hu Y, Su XL, Ji JF. Identification of prognosis-related proteins in advanced gastric cancer by mass spectrometry-based comparative proteomics. J Cancer Res Clin Oncol. 2009;135:403–411. doi: 10.1007/s00432-008-0474-3. [DOI] [PubMed] [Google Scholar]
- 116.Zhang Q, Hu H, Shi X, Tang W. Knockdown of S100P by lentiviral-mediated RNAi promotes apoptosis and suppresses the colony-formation ability of gastric cancer cells. Oncol Rep. 2014;31:2344–2350. doi: 10.3892/or.2014.3104. [DOI] [PubMed] [Google Scholar]
- 117.Ko CH, Cheng CF, Lai CP, Tzu TH, Chiu CW, Lin MW, Wu SY, Sun CY, Tseng HW, Wang CC, Kuo ZK, Wang LM, Chen SF. Differential proteomic analysis of cancer stem cell properties in hepatocellular carcinomas by isobaric tag labeling and mass spectrometry. J Proteome Res. 2013;12:3573–3585. doi: 10.1021/pr4004294. [DOI] [PubMed] [Google Scholar]
- 118.Yuan RH, Chang KT, Chen YL, Hsu HC, Lee PH, Lai PL, Jeng YM. S100P expression is a novel prognostic factor in hepatocellular carcinoma and predicts survival in patients with high tumor stage or early recurrent tumors. PLoS One. 2013;8:e65501. doi: 10.1371/journal.pone.0065501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Amelung JT, Buhrens R, Beshay M, Reymond MA. Key genes in lung cancer translational research: a meta-analysis. Pathobiology. 2010;77:53–63. doi: 10.1159/000278292. [DOI] [PubMed] [Google Scholar]
- 120.Bartling B, Rehbein G, Schmitt WD, Hofmann HS, Silber RE, Simm A. S100A2-S100P expression profile and diagnosis of non-small cell lung carcinoma: impairment by advanced tumour stages and neoadjuvant chemotherapy. Eur J Cancer. 2007;43:1935–1943. doi: 10.1016/j.ejca.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 121.Diederichs S, Bulk E, Steffen B, Ji P, Tickenbrock L, Lang K, Zanker KS, Metzger R, Schneider PM, Gerke V, Thomas M, Berdel WE, Serve H, Muller-Tidow C. S100 family members and trypsinogens are predictors of distant metastasis and survival in early-stage non-small cell lung cancer. Cancer Res. 2004;64:5564–5569. doi: 10.1158/0008-5472.CAN-04-2004. [DOI] [PubMed] [Google Scholar]
- 122.Rehbein G, Simm A, Hofmann HS, Silber RE, Bartling B. Molecular regulation of S100P in human lung adenocarcinomas. Int J Mol Med. 2008;22:69–77. [PubMed] [Google Scholar]
- 123.Zhu L, Ito T, Nakahara T, Nagae K, Fuyuno Y, Nakao M, Akahoshi M, Nakagawa R, Tu Y, Uchi H, Furue M. Upregulation of S100P, receptor for advanced glycation end products and ezrin in malignant melanoma. J Dermatol. 2013;40:973–979. doi: 10.1111/1346-8138.12323. [DOI] [PubMed] [Google Scholar]
- 124.Cheng YS, Jordan L, Rees T, Chen HS, Oxford L, Brinkmann O, Wong D. Levels of potential oral cancer salivary mRNA biomarkers in oral cancer patients in remission and oral lichen planus patients. Clin Oral Investig. 2014;18:985–993. doi: 10.1007/s00784-013-1041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Surowiak P, Maciejczyk A, Materna V, Drag-Zalesinska M, Wojnar A, Pudelko M, Kedzia W, Spaczynski M, Dietel M, Zabel M, Lage H. Unfavourable prognostic significance of S100P expression in ovarian cancers. Histopathology. 2007;51:125–128. doi: 10.1111/j.1365-2559.2007.02714.x. [DOI] [PubMed] [Google Scholar]
- 126.Wang X, Tian T, Li X, Zhao M, Lou Y, Qian J, Liu Z, Chen H, Cui Z. High expression of S100P is associated with unfavorable prognosis and tumor progression in patients with epithelial ovarian cancer. Am J Cancer Res. 2015;5:2409–2421. [PMC free article] [PubMed] [Google Scholar]
- 127.Umezaki Y, Ito M, Nakashima M, Mihara Y, Naruke Y, Kurohama H, Yatsunami N, Yasuhi I. S100P is a useful marker for differentiation of ovarian mucinous tumors. Eur J Gynaecol Oncol. 2015;36:138–141. [PubMed] [Google Scholar]
- 128.Mousses S, Bubendorf L, Wagner U, Hostetter G, Kononen J, Cornelison R, Goldberger N, Elkahloun AG, Willi N, Koivisto P, Ferhle W, Raffeld M, Sauter G, Kallioniemi OP. Clinical validation of candidate genes associated with prostate cancer progression in the CWR22 model system using tissue microarrays. Cancer Res. 2002;62:1256–1260. [PubMed] [Google Scholar]
- 129.Amler LC, Agus DB, LeDuc C, Sapinoso ML, Fox WD, Kern S, Lee D, Wang V, Leysens M, Higgins B, Martin J, Gerald W, Dracopoli N, Cordon-Cardo C, Scher HI, Hampton GM. Dysregulated expression of androgen-responsive and nonresponsive genes in the androgen-independent prostate cancer xenograft model CWR22-R1. Cancer Res. 2000;60:6134–6141. [PubMed] [Google Scholar]
- 130.Basu GD, Azorsa DO, Kiefer JA, Rojas AM, Tuzmen S, Barrett MT, Trent JM, Kallioniemi O, Mousses S. Functional evidence implicating S100P in prostate cancer progression. Int J Cancer. 2008;123:330–339. doi: 10.1002/ijc.23447. [DOI] [PubMed] [Google Scholar]
- 131.Arumugam T, Simeone DM, Van Golen K, Logsdon CD. S100P promotes pancreatic cancer growth, survival, and invasion. Clin Cancer Res. 2005;11:5356–5364. doi: 10.1158/1078-0432.CCR-05-0092. [DOI] [PubMed] [Google Scholar]
- 132.Crnogorac-Jurcevic T, Missiaglia E, Blaveri E, Gangeswaran R, Jones M, Terris B, Costello E, Neoptolemos JP, Lemoine NR. Molecular alterations in pancreatic carcinoma: expression profiling shows that dysregulated expression of S100 genes is highly prevalent. J Pathol. 2003;201:63–74. doi: 10.1002/path.1418. [DOI] [PubMed] [Google Scholar]
- 133.Missiaglia E, Blaveri E, Terris B, Wang YH, Costello E, Neoptolemos JP, Crnogorac-Jurcevic T, Lemoine NR. Analysis of gene expression in cancer cell lines identifies candidate markers for pancreatic tumorigenesis and metastasis. Int J Cancer. 2004;112:100–112. doi: 10.1002/ijc.20376. [DOI] [PubMed] [Google Scholar]
- 134.Naidoo K, Jones R, Dmitrovic B, Wijesuriya N, Kocher H, Hart IR, Crnogorac-Jurcevic T. Proteome of formalin-fixed paraffin-embedded pancreatic ductal adenocarcinoma and lymph node metastases. J Pathol. 2012;226:756–763. doi: 10.1002/path.3959. [DOI] [PubMed] [Google Scholar]
- 135.Fukushima N, Sato N, Prasad N, Leach SD, Hruban RH, Goggins M. Characterization of gene expression in mucinous cystic neoplasms of the pancreas using oligonucleotide microarrays. Oncogene. 2004;23:9042–9051. doi: 10.1038/sj.onc.1208117. [DOI] [PubMed] [Google Scholar]
- 136.Terris B, Ponsot P, Paye F, Hammel P, Sauvanet A, Molas G, Bernades P, Belghiti J, Ruszniewski P, Flejou JF. Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol. 2000;24:1372–1377. doi: 10.1097/00000478-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 137.Ohuchida K, Mizumoto K, Egami T, Yamaguchi H, Fujii K, Konomi H, Nagai E, Yamaguchi K, Tsuneyoshi M, Tanaka M. S100P is an early developmental marker of pancreatic carcinogenesis. Clin Cancer Res. 2006;12:5411–5416. doi: 10.1158/1078-0432.CCR-06-0298. [DOI] [PubMed] [Google Scholar]
- 138.Yao R, Lopez-Beltran A, Maclennan GT, Montironi R, Eble JN, Cheng L. Expression of S100 protein family members in the pathogenesis of bladder tumors. Anticancer Res. 2007;27:3051–3058. [PubMed] [Google Scholar]
- 139.Mohanty SK, Smith SC, Chang E, Luthringer DJ, Gown AM, Aron M, Amin MB. Evaluation of contemporary prostate and urothelial lineage biomarkers in a consecutive cohort of poorly differentiated bladder neck carcinomas. Am J Clin Pathol. 2014;142:173–183. doi: 10.1309/AJCPK1OV6IMNPFGL. [DOI] [PubMed] [Google Scholar]
- 140.Gulmann C, Paner GP, Parakh RS, Hansel DE, Shen SS, Ro JY, Annaiah C, Lopez-Beltran A, Rao P, Arora K, Cho Y, Herrera-Hernandez L, Alsabeh R, Amin MB. Immunohistochemical profile to distinguish urothelial from squamous differentiation in carcinomas of urothelial tract. Hum Pathol. 2013;44:164–172. doi: 10.1016/j.humpath.2012.05.018. [DOI] [PubMed] [Google Scholar]