ABSTRACT
The human immunodeficiency virus (HIV-1) envelope glycoproteins (Env) mediate virus entry through a series of complex conformational changes triggered by binding to the receptors CD4 and CCR5/CXCR4. Broadly neutralizing antibodies that recognize conserved Env epitopes are thought to be an important component of a protective immune response. However, to date, HIV-1 Env immunogens that elicit broadly neutralizing antibodies have not been identified, creating hurdles for vaccine development. Small-molecule CD4-mimetic compounds engage the CD4-binding pocket on the gp120 exterior Env and induce Env conformations that are highly sensitive to neutralization by antibodies, including antibodies directed against the conserved Env region that interacts with CCR5/CXCR4. Here, we show that CD4-mimetic compounds sensitize primary HIV-1 to neutralization by antibodies that can be elicited in monkeys and humans within 6 months by several Env vaccine candidates, including gp120 monomers. Monoclonal antibodies directed against the gp120 V2 and V3 variable regions were isolated from the immunized monkeys and humans; these monoclonal antibodies neutralized a primary HIV-1 only when the virus was sensitized by a CD4-mimetic compound. Thus, in addition to their direct antiviral effect, CD4-mimetic compounds dramatically enhance the HIV-1-neutralizing activity of antibodies that can be elicited with currently available immunogens. Used as components of microbicides, the CD4-mimetic compounds might increase the protective efficacy of HIV-1 vaccines.
IMPORTANCE Preventing HIV-1 transmission is a high priority for global health. Eliciting antibodies that can neutralize transmitted strains of HIV-1 is difficult, creating problems for the development of an effective vaccine. We found that small-molecule CD4-mimetic compounds sensitize HIV-1 to antibodies that can be elicited in vaccinated humans and monkeys. These results suggest an approach to prevent HIV-1 sexual transmission in which a virus-sensitizing microbicide is combined with a vaccine.
INTRODUCTION
Preventing sexual transmission of human immunodeficiency virus (HIV-1) is essential for altering the course of the global pandemic of AIDS. Currently, over 36 million people are infected by HIV-1; 2.0 million people are newly infected with the virus annually, and nearly 1.2 million individuals succumb each year to AIDS (1). Hence, there is an urgent need to develop vaccines or other strategies that can prevent HIV-1 transmission.
HIV-1-neutralizing antibodies are an important component of a protective vaccine-induced immune response. Passive administration of HIV-1-neutralizing antibodies protects monkeys from intravenous and mucosal challenge with simian-human immunodeficiency viruses (SHIVs) (2–7). The trimeric envelope glycoprotein (Env) spike on the virion surface is the only HIV-1-specific target accessible to neutralizing antibodies (8–10). The titers of antibodies against a specific region of Env (the gp120 V2 variable region) correlated with the moderate efficacy observed in the RV144 clinical vaccine trial (11–13). A primary focus of HIV-1 vaccine development is the elicitation of antibodies against Env that protect against HIV-1 acquisition.
The HIV-1 Env trimer, which is composed of three gp120 exterior subunits and three gp41 transmembrane subunits, mediates virus entry into host cells (10). The unliganded HIV-1 Env trimer is metastable (14–19). Binding of gp120 to the initial receptor, CD4, triggers Env conformational changes that result in the formation/exposure of two elements: (i) the gp120 binding site for the second receptor, CCR5 or CXCR4, and (ii) the gp41 heptad repeat (HR1) coiled coil (20–35). Binding of gp120 to the CCR5 or CXCR4 coreceptor induces further Env conformational changes that result in the formation of an energetically stable gp41 six-helix bundle that promotes the fusion of the viral and target cell membranes (18–20, 36).
As a successful persistent virus, HIV-1 has evolved Env trimers that exhibit substantial genetic variability and a heavily glycosylated surface, which represent major challenges for the elicitation of broadly neutralizing antibodies (10, 37–40). Most anti-Env antibodies elicited during natural infection do not neutralize HIV-1, and those that do usually are strain restricted, allowing virus escape (31, 41–45). Only after several years of infection in some HIV-1-infected individuals are more broadly neutralizing antibodies generated (43, 46–49). Broadly HIV-1-neutralizing antibodies typically display unusual features, such as long complementarity-determining regions that allow binding to the heavily shielded, conserved epitopes on the surface of the unliganded Env trimer (46, 50, 51). Some neutralizing antibodies with modest breadth bind Env carbohydrate-dependent epitopes (51–58).
Even the best current HIV-1 Env immunogens elicit antibodies that inhibit the infection of only the small subset of primary viruses that are more prone to neutralization (51, 59–62). The sensitivity of HIV-1 strains to antibody neutralization depends upon the integrity of the Env epitope and Env reactivity; the latter property indicates the propensity of unliganded Env to undergo conformational changes (16, 63). A successful HIV-1 vaccine must cover a range of phylogenetically diverse transmitted/founder viruses, most of which have Envs of low reactivity and exhibit low sensitivity to neutralization by antibodies (16, 63–65). Thus, a successful HIV-1/AIDS vaccine should elicit antibodies that recognize conserved elements of the Env trimer in its “closed,” unliganded conformation. Most potently neutralizing antibodies require minimal conformational change in the unliganded Env trimer for their binding (16, 66).
The CD4-bound Env intermediate differs significantly in conformation from the unliganded state (67, 68). The more “open” conformation of the CD4-bound Env results in the exposure of the highly conserved gp120 surface involved in CCR5/CXCR4 binding (23, 24, 69, 70). The conserved coreceptor-binding surface on gp120 overlaps with V3 and CD4-induced (CD4i) epitopes (69–75). Antibodies against these epitopes are efficiently elicited soon after HIV-1 infection in humans (76–78). Moreover, these types of antibodies can be elicited by the immunization of animals and humans with Env proteins (78–84). The ease with which these antibodies are elicited by immunization depends upon the ability of the Env immunogen to bind CD4 in the host; for example, the level of these antibodies was significantly higher in rabbits transgenic for human CD4 than in control rabbits (79, 80). CD4i antibodies were elicited in wild-type rabbits by gp120 cores in proportion to how closely the engineered cores resembled the CD4-bound state (83, 84). CD4i- and V3-directed antibodies also were elicited by HIV-1 Env immunogens in monkeys, whose CD4 is efficiently bound by HIV-1 gp120 (78–82). The CD4i- and V3-directed antibodies exhibit little neutralizing activity against most primary HIV-1, as their epitopes are not exposed in the unliganded state of Env and are sterically inaccessible once the viral Env spike binds CD4 on a target cell (85, 86). If, however, these epitopes can be exposed on a virus that has not yet engaged cellular CD4, CD4i- and V3-directed antibodies effectively neutralize a wide range of HIV-1 and even some heterologous HIV-2 strains (76–79, 82, 86).
Soluble CD4 and CD4 miniproteins have been shown to induce the CD4-bound state on HIV-1 variants and to render them susceptible to neutralization by CD4i and V3 antibodies (76–79, 82, 86). Small-molecule compounds (less than 500 Da) also have been discovered that bind HIV-1 gp120 and block the gp120-CD4 interaction (87). The prototypic CD4-mimetic compounds NBD-556 and NBD-557 only weakly inhibited a few HIV-1 isolates (87–89). NBD-556 binds in a well-conserved pocket on gp120 and can induce conformational changes in gp120 similar to those induced by CD4 (88–91). Structure-based design has led to the improvement of the affinity and antiviral potency and breadth of the CD4-mimetic compounds (92–95). Recently developed analogues have been shown to inhibit infection by a range of HIV-1 primary strains (94, 95). Moreover, at subinhibitory concentrations, these CD4-mimetic compounds induced the exposure of previously cryptic Env epitopes and sensitized primary HIV-1 to neutralization by CD4i- and V3-directed monoclonal antibodies (15, 96). These analogues also sensitized primary HIV-1 to neutralization by serum from rabbits immunized with a gp120 core engineered to be fixed in the CD4-bound state (96). The efficiency with which the gp120 core immunogens elicited rabbit antibodies that neutralized the sensitized HIV-1 correlated with the degree of fixation to resemble the CD4-bound state (83, 84, 96). These results suggested that HIV-1 Env immunogens that could bind CD4 in the immunized host also elicit antibodies that could neutralize primary HIV-1 sensitized by CD4-mimetic compounds. Here, we test this hypothesis in monkeys and humans immunized with multiple HIV-1 vaccine candidates.
MATERIALS AND METHODS
Compounds.
The CD4-mimetic compounds (+)(R,R)BNM-III-170 and (R,R)BNM-IV-147 are referred to as BNM-III-170 and BNM-IV-147, respectively, throughout the manuscript. The compounds were synthesized and the chemical structure characterized as described previously (92–95). The compounds were dissolved in dimethyl sulfoxide (DMSO) at a stock concentration of 10 to 20 mM, aliquoted, and stored at −20°C. Each compound then was diluted to 1 mM in serum-free Dulbecco's modified Eagle's medium (DMEM) and used for different assays.
Cell lines.
293T human embryonic kidney and Cf2Th canine thymocytes (ATCC) were grown at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium (Invitrogen) containing 10% fetal bovine serum (Sigma) and 100 μg/ml penicillin-streptomycin (Mediatech, Inc.). Cf2Th cells stably expressing human CCR5 and CD4 were grown in medium supplemented with 0.4 mg/ml G418 and 0.2 mg/ml hygromycin (Invitrogen).
Recombinant luciferase viruses.
293T human embryonic kidney cells were cotransfected with plasmids expressing the pCMVΔP1Δenv HIV-1 Gag-Pol packaging construct, the HIV-1 envelope glycoproteins, or the envelope glycoprotein of the control amphotropic murine leukemia virus (A-MLV) and the firefly luciferase-expressing vector at a DNA ratio of 1:1:3 μg using the Effectene transfection reagent (Qiagen). Cotransfection produced recombinant, luciferase-expressing viruses capable of a single round of infection. The virus-containing supernatants were harvested 36 to 40 h after transfection, spun, aliquoted, and frozen at −80°C until further use. The reverse transcriptase (RT) levels of all virus stocks were measured as described previously (97).
Infection by single-round luciferase viruses.
Cf2Th-CCR5-CD4 target cells were seeded at a density of 6 × 103 cells/well in 96-well luminometer-compatible tissue culture plates (PerkinElmer) 24 h before infection. On the day of infection, BNM-III-170 or BNM-IV-147 (0 to 100 μM) was incubated with recombinant viruses (10,000 RT units) at 37°C for 30 min. In the case of sensitization assays, a constant concentration of compounds was incubated with virus for 30 min at 37°C, and then 17b or other antibodies (over a range of 0 to 100 μg/ml) or plasma at different dilutions was added to the virus-compound mixture and incubated for an additional 30 min at 37°C. The mixtures then were added to the target cells and incubated for 48 h at 37°C. After this time, the medium was removed from each well and the cells were lysed by the addition of 30 μl passive lysis buffer (Promega) and three freeze-thaw cycles. An EG&G Berthold LB 96V microplate luminometer was used to measure the luciferase activity of each well after the addition of 100 μl of luciferin buffer (15 mM MgSO4, 15 mM KPO4, pH 7.8, 1 mM ATP, and 1 mM dithiothreitol) and 50 μl of 1 mM 99% Firefly d-luciferin free acid (Prolume).
ELISA.
The titer of anti-gp120 antibodies in the plasma of immunized monkeys and humans was measured by enzyme-linked immunosorbent assay (ELISA). Ninety-six-well ELISA plates (REACTI-BIND; Fisher Scientific) were coated with 100 μl of 2 μg/ml purified recombinant HIV-1YU2 gp120 with a C-terminal His-6 tag, which was produced transiently by the transfection of 293F cells. Coated plates were incubated at 4°C overnight. Plates were washed three times with phosphate-buffered saline (PBS) containing 0.1% Tween 20 and blocked with 200 μl/well of 1% bovine serum albumin (BSA) in PBS and incubated at 37°C for 1 h. Blocking buffer was aspirated, and serial dilutions of preimmune or immunized human or monkey plasma were added in duplicate in a final volume of 100 μl/well and incubated at 37°C for 1 h. Plates were washed three times with PBS containing 0.1% Tween 20. Goat anti-human Fc gamma horseradish peroxidase (HRP) (Jackson ImmunoResearch Laboratories) was diluted 1:10,000 with blocking buffer, and 100 μl was added to each well and incubated at 37°C for 1 h. Plates were washed three times with PBS containing 0.1% Tween 20. 3,3′5,5-Tetramethylbenzidine (TMB) single solution substrate (Life Technologies) was added at a final volume of 100 μl/well and incubated at room temperature for 5 min to allow color to develop before reading at an optical density of 450 nm. Mean titer values are reported. The titers of anti-gp120 antibodies that resulted in an ELISA signal 2.5 times the value obtained for the negative control and 5 times the value obtained for the negative control (preimmune) plasma were estimated.
RESULTS
Direct inhibition of HIV-1 infection by small-molecule CD4-mimetic compounds.
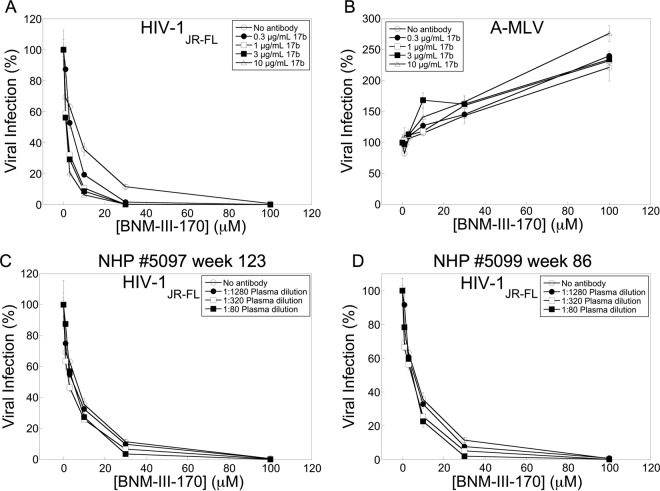
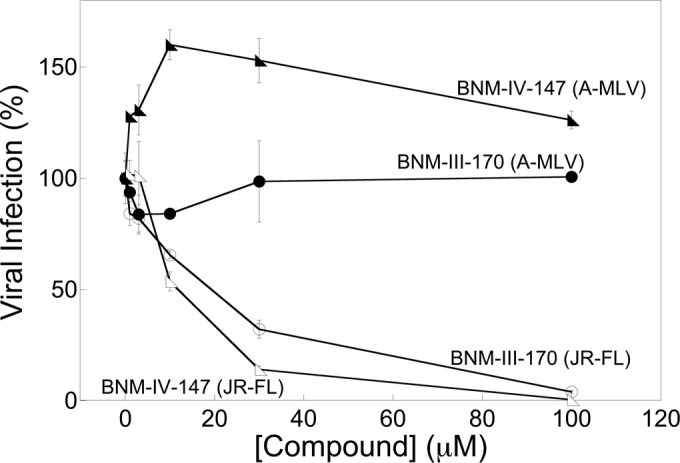
We examined the ability of two recently designed and synthesized CD4-mimetic compounds, BNM-III-170 and BNM-IV-147 (93), to inhibit HIV-1 entry. Recombinant HIV-1 expressing the firefly luciferase gene was pseudotyped with different envelope glycoproteins, either HIV-1JR-FL or HIV-1YU2 Env or, as a control, the amphotropic murine leukemia virus (A-MLV) Env. The recombinant viruses were incubated with cells expressing CD4 and CCR5 in the presence of different concentrations of the compounds. BNM-III-170 and BNM-IV-147 specifically inhibited the wild-type (wt) HIV-1JR-FL virus with 50% inhibitory concentrations (IC50s) of 22 and 7 μM, respectively (Fig. 1 and Table 1). Both compounds inhibited the HIV-1YU2 virus even more potently (Table 1 and and Melillo et al., unpublished). The HIV-1JR-FL S375W and HIV-1YU2 S375W mutants, in which the gp120 Phe 43 cavity is occupied by the indole ring of the substituted tryptophan residue (84, 98), were resistant to BNM-III-170 (Table 1). In contrast, the HIV-1YU2 S375A mutant was even more sensitive to BNM-III-170 than wild-type HIV-1YU2. These results are consistent with the expectation that the antiviral activity of CD4-mimetic compounds depends upon their interaction with the gp120 Phe 43 cavity (88, 91–95).
FIG 1.
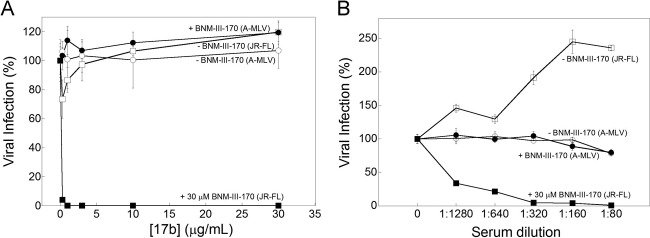
Inhibition of HIV-1JR-FL infection by CD4-mimetic compounds. Recombinant HIV-1 encoding firefly luciferase was pseudotyped with the HIV-1JR-FL Env or the A-MLV Env. Viruses were incubated with Cf2Th-CD4/CCR5 cells in the presence of the indicated concentrations of the CD4-mimetic compounds BNM-III-170 and BNM-IV-147. After 48 h, the luciferase activity in the target cells was measured. The level of infection relative to that seen in the absence of the compound is reported. The means and standard deviations from triplicate samples within a typical experiment are shown.
TABLE 1.
Inhibition of HIV-1 Env variants by CD4-mimetic compounds and the 17b CD4i antibody
| Virus | Clade | IC50 of: |
||||
|---|---|---|---|---|---|---|
| 17ba (μg/ml) | 17b + 50 μM BNM-III-170a (μg/ml) | 17b + 50 μM BNM-IV-147a (μg/ml) | BNM-III-170b (μM) | BNM-IV-147b (μM) | ||
| JR-FL wt | B | >30 | 0.2 ± 0.0 | 0.2 ± 0.0 | 18.9 ± 3.8 | 6.7 ± 0.9 |
| JR-FL S375W | B | >30 | >30 | >30 | >100 | 90.5 |
| YU2 wt | B | >30 | 0.2 ± 0.1 | 0.1 | 1.2 ± 0.1 | 0.4 |
| YU2 S375W | B | >30 | >30 | >30 | >100 | 88.7 |
| YU2 S375A | B | >30 | 0.2 ± 0.0 | 0.4 | 0.6 ± 0.0 | 0.4 |
| A4 | A | >30 | 1.6 ± 0.8 | 11.0 ± 6.7 | 4.5 ± 1.4 | 1.8 ± 0.4 |
| B6 | B | >30 | >30 | >30 | 47.6 ± 19.0 | 15.8 ± 1.4 |
| C5 | C | >30 | 5.7 ± 4.8 | 17.2 ± 8.5 | 9.2 ± 2.7 | 5.9 ± 2.1 |
| C11 | C | >30 | >30 | >30 | 36.6 ± 8.4 | 14.4 ± 0.3 |
| C17 | C | >30 | >30 | 20.1 ± 9.9 | 7.4 ± 0.6 | 4.4 ± 0.9 |
| 821 | D | >30 | 1.5 ± 1.2 | 12.8 ± 8.3 | 0.5 ± 0.0 | 0.5 ± 0.0 |
| 859 | D | >30 | 19.2 ± 9.6 | 10.3 ± 9.9 | 2.5 ± 0.2 | 1.1 ± 0.2 |
| AG266 | AG Subtype | >30 | >30 | 12.9 ± 8.8 | 25.2 ± 1.5 | 7.7 ± 0.2 |
| A-MLV | >30 | 25.5 ± 4.5 | >30 | 88.7 ± 11.2 | 81.2 ± 9.4 | |
Recombinant HIV-1 encoding firefly luciferase was pseudotyped with the indicated HIV-1 Env (or A-MLV Env control). Viruses were incubated with 50 μM CD4-mimetic compound (BNM-III-170 or BNM-IV-147) or with DMSO and then with different concentrations of the 17b antibody. After incubation of the viruses with Cf2Th-CD4/CCR5 cells for 48 h, the cells were lysed and luciferase activity was measured. The inhibitory concentration (IC50) of the 17b antibody is reported. Means and standard deviations from triplicate samples within an experiment are shown. ND, not determined.
The inhibitory concentrations (IC50) for the direct antiviral effect of BNM-III-170 and BNM-IV-147 were determined as in footnote a.
BNM-III-170 sensitizes diverse HIV-1 strains to neutralization by an antibody against a CD4-induced gp120 epitope.
We tested the ability of BNM-III-170 to sensitize different strains of HIV-1 to neutralization by the 17b monoclonal antibody, which recognizes a CD4-induced (CD4i) epitope (73, 74, 86). Recombinant HIV-1 encoding firefly luciferase was pseudotyped with the Envs from different primary HIV-1 strains and then incubated sequentially with a subneutralizing concentration of BNM-III-170 or BNM-IV-147, the 17b antibody, and Cf2Th-CD4/CCR5 cells. After 48 h, the luciferase activity in the target cells was measured as an indication of infection efficiency. As controls, we tested viruses pseudotyped with HIV-1JR-FL and HIV-1YU2 Env mutants containing residues at gp120 position 375 that either fill (S375W) or expand (S375A) the Phe 43 cavity. Recombinant HIV-1 pseudotyped with the A-MLV Env was used as an additional control for specificity. The wild-type primary HIV-1 viruses were resistant to neutralization by the 17b antibody alone, but several viruses became exquisitely sensitive to 17b neutralization in the presence of subneutralizing concentrations of BNM-III-170 or BNM-IV-147 (Fig. 2A and Table 1). After treatment with BNM-III-170, both wild-type HIV-1JR-FL and wild-type HIV-1YU2, as well as the HIV-1YU2 S375A mutant, were neutralized by the 17b antibody (Table 1). In contrast, the HIV-1JR-FL S375W and HIV-1YU2 S375W mutants were not inhibited by the 17b antibody, regardless of the presence of BNM-III-170. As expected, viruses with the A-MLV Env also were resistant to 17b neutralization. These results indicate that gp120 binding by BNM-III-170 is critical for its ability to sensitize HIV-1 to neutralization by the 17b antibody, similar to the results seen for other CD4-mimetic compounds (96).
FIG 2.
BNM-III-170 sensitizes HIV-1JR-FL to neutralization by antibodies. Recombinant HIV-1 encoding firefly luciferase was pseudotyped with the HIV-1JR-FL Env or the A-MLV Env. Viruses were incubated with either DMSO (open symbols) or 30 μM BNM-III-170 (filled symbols) and then with the 17b CD4i antibody at the indicated concentration (A) or with serum from an HIV-1-infected individual at the indicated dilution (B). After incubation of the viruses with Cf2Th-CD4-CCR5 cells for 48 h, the cells were lysed and luciferase activity was measured. The level of infection relative to that seen in the absence of the antibody is shown. The means and standard deviations from triplicate samples within an experiment are shown; the experiment shown in panel A was repeated more than 10 times and the experiment in panel B once, with similar results.
BNM-III-170 sensitizes HIV-1 to neutralization by antibodies generated in an HIV-1-infected individual.
As antibodies directed against CD4-induced Env epitopes are commonly elicited during natural HIV-1-infection (76–78), we tested the neutralization of HIV-1JR-FL by the serum of an HIV-1-infected individual in the absence and presence of BNM-III-170. In the absence of BNM-III-170, higher concentrations of this serum resulted in a specific enhancement of HIV-1JR-FL infection (Fig. 2B). When BNM-III-170 was added at subneutralizing concentrations, HIV-1JR-FL was efficiently neutralized by the serum. Both the enhancement and neutralization of HIV-1JR-FL were specific, as no effect of the serum on infection of viruses with the A-MLV Env was observed. Thus, antibodies that can neutralize HIV-1 after treatment with a CD4-mimetic compound are present in some HIV-1-infected people.
BNM-III-170 sensitizes HIV-1 to neutralization by monkey plasma elicited by Env immunization.
Previous studies demonstrated that CD4-mimetic compounds could sensitize primary HIV-1 strains to neutralization by sera from rabbits immunized with gp120 cores that were modified to stabilize the CD4-bound conformation (84, 96). We tested the plasma of rhesus macaques immunized with multiple HIV-1 Env vaccine candidates for the presence of antibodies that neutralize the primary virus, HIV-1JR-FL, treated with a subinhibitory concentration of BNM-III-170. Five groups of monkeys were studied.
(i) Nonhuman primate (NHP) 62.1 (Center for HIV/AIDS Vaccine Immunology and Immunogen Design [CHAVI-ID]).
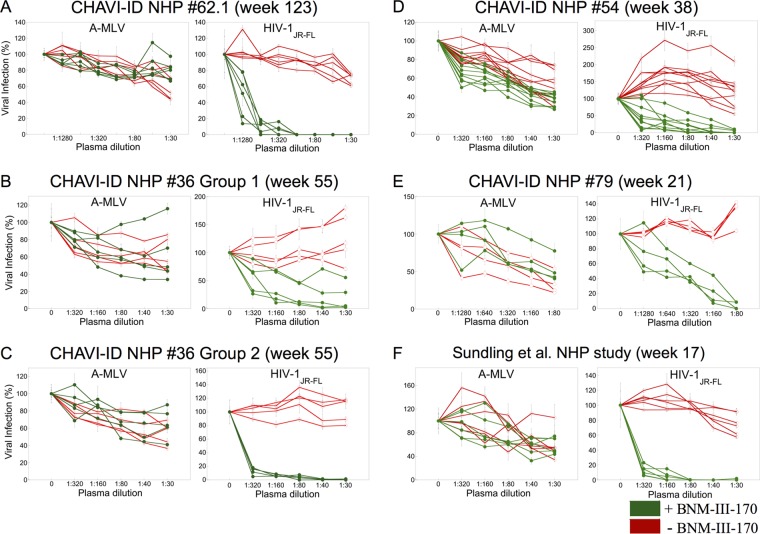
Six rhesus macaques were immunized over the course of 136 weeks with gp120 and gp140C glycoproteins from different transmitted/founder and primary HIV-1 (99). For weeks 0 to 24, the gp120 and gp140C immunogens were selected based on their affinity for the unmutated common ancestor and intermediate ancestors of the CH01-CH04 neutralizing antibody lineage (99, 100). Plasma from week 123 was tested for the ability to neutralize HIV-1JR-FL that had been incubated with either a subinhibitory concentration of BNM-III-170 or a DMSO control. In the absence of BNM-III-170, none of the plasma samples from the six vaccinated monkeys neutralized HIV-1JR-FL (Fig. 3A). In the presence of a subinhibitory concentration of BNM-III-170, all six plasma potently neutralized HIV-1JR-FL. The observed neutralization was specific, as the virus pseudotyped with the A-MLV Env was not inhibited. These results indicate that BNM-III-170 can potently sensitize a primary, neutralization-resistant HIV-1 to antibodies raised in monkeys to gp120/gp140C immunogens.
FIG 3.
Monkeys immunized with different HIV-1 Env immunogens generate antibodies that neutralize HIV-1JR-FL sensitized with BNM-III-170. Recombinant, luciferase-expressing viruses with HIV-1JR-FL Env or A-MLV Env were incubated with 30 μM BNM-III-170 (green) or without compound (red). The viruses subsequently were incubated with the indicated dilution of plasma from immunized monkeys prior to incubation with Cf2Th-CD4/CCR5 cells for 48 h. The level of infection relative to that seen in the absence of plasma is shown. The means and standard deviations from triplicate samples in a typical experiment are shown.
(ii) NHP 36 (CHAVI-ID).
Two groups of 5 rhesus macaques were primed with different ALVAC canarypox vectors followed by three boosts with a combination of the same ALVAC vector and a mixture of two gp120 glycoproteins from a clade B HIV-1 strain and a clade E HIV-1 strain (99, 101). The group 1 monkeys were immunized with an empty ALVAC vector, whereas the group 2 monkeys were immunized with ALVAC VPC, which encodes HIV-1 Gag, Pol, and Env proteins (101). The immunization scheme in the group 2 monkeys recapitulates that used in the RV144 clinical vaccine trial in Thailand, which resulted in ∼32% protection from HIV-1 acquisition (12). Plasma collected at week 55, 2 weeks after the last boost, were tested. In the group 1 monkeys, three of the plasma potently neutralized HIV-1JR-FL pretreated with BNM-III-170, and the other two plasma samples exhibited weak inhibition (Fig. 3B). In the group 2 monkeys, all five plasma potently neutralized HIV-1JR-FL treated with BNM-III-170 (Fig. 3C). None of the plasma neutralized HIV-1JR-FL in the absence of BNM-III-170. The plasma exhibited minimal inhibition of HIV-1 pseudotyped with the A-MLV Env in either the presence or absence of BNM-III-170. These results indicate that an RV144-like immunization regimen, including ALVAC VPC, was more effective at eliciting antibodies that neutralized the sensitized HIV-1JR-FL than a comparable immunization regimen using gp120 glycoproteins with the empty ALVAC vector.
(iii) NHP 54 (CHAVI-ID).
Ten rhesus macaques were immunized with gp140 Envs corresponding to those Env sequences observed at multiple time points in a human infected with clade C HIV-1 who raised broadly neutralizing antibodies (CAP206) (99, 102). Five of the monkeys were immunized with gp140 Envs that sequentially were observed in the CAP206 study, and five of the monkeys were immunized with a mixture of gp140 Envs corresponding in sequence to the swarm of viruses observed in the infected individual (99, 102). Plasma samples collected at week 38, 2 weeks after the last boost, were tested for the ability to neutralize HIV-1JR-FL in the absence or presence of subneutralizing concentrations of BNM-III-170. Plasma from most of the monkeys immunized with sequential or swarm gp140 Envs exhibited the ability to neutralize HIV-1JR-FL after pretreatment with BNM-III-170 (Fig. 3D). No neutralization of HIV-1JR-FL that was not incubated with BNM-III-170 was seen. Minimal effects of the plasma on infection of the virus with the A-MLV Env were observed in the absence and presence of BNM-III-170. Thus, immunization with CAP206 gp140 Env variants elicited plasma antibodies that could neutralize HIV-1 that was sensitized by BNM-III-170. Of note, the titers of neutralizing antibodies against sensitized HIV-1JR-FL were not as high in NHP 54 as those seen in the two monkey studies described above (NHP 62.1 and NHP 36). One variable that could account for this difference is the time at which the immune plasma were collected. An earlier time point was used with NHP 54 than with NHP 62.1 and NHP 36. Another variable is the degree of divergence between the Env immunogen and the HIV-1JR-FL strain used in the neutralization arrays. NHP 54 immunizations were conducted with a clade C HIV-1 Env, whereas the NHP 62.1 and NHP 36 immunizations included a clade B Env immunogen, which belongs to the same clade as HIV-1JR-FL. These possible explanations will be addressed in the studies below.
(iv) NHP 79 (CHAVI-ID).
The CH505 Envs were derived at multiple time points from a clade C HIV-1-infected African subject who generated a broadly neutralizing antibody response (103). In NHP 79 (group 1), four rhesus macaques were immunized with GLA-SE adjuvant and the CH505 gp120 glycoprotein derived on day 7 after infection. Plasma samples from 21 weeks of immunization were tested. The plasma neutralized HIV-1JR-FL that had been incubated with BNM-III-170 but did not inhibit infection of the untreated HIV-1JR-FL (Fig. 3E). Minimal effects of the plasma on A-MLV infection were observed. These results indicate that immunization with a clade C gp120 can elicit antibodies that neutralize the clade B HIV-1JR-FL treated with BNM-III-170. Moreover, these antibodies can be elicited as early as 21 weeks of immunization.
(v) Sundling et al. NHP study (Karolinska Institutet).
Six rhesus macaques were immunized with a soluble HIV-1YU2 gp140-F trimer (with a fibritin foldon) administered in adjuvant (104). All six monkey plasma samples potently neutralized HIV-1JR-FL that was sensitized by BNM-III-170 (Fig. 3F). These plasmas exhibited only weak inhibition of the virus pseudotyped with the A-MLV Env. Control plasma from monkeys immunized with adjuvant alone did not neutralize HIV-1JR-FL in either the presence or absence of BNM-III-170 (data not shown).
Together, the above-described studies demonstrate that various HIV-1 gp120 and/or soluble gp140 Env immunogens can elicit antibodies in monkeys that neutralize a heterologous primary HIV-1 previously treated with subneutralizing concentrations of a CD4-mimetic compound.
Time course of neutralizing antibody elicitation.
The elicitation of neutralizing antibodies against BNM-III-170-sensitized HIV-1JR-FL was documented after an extensive period of immunization in some of the above-described nonhuman primate studies. To investigate whether shorter immunization regimens might elicit these antibodies, we tested the plasma of selected monkeys from earlier time points in the immunization schedule.
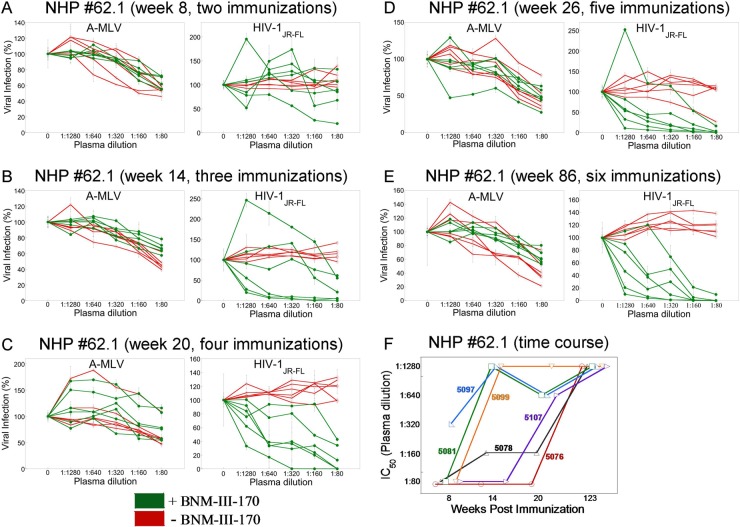
In our initial study of NHP 62.1 monkeys, we evaluated the plasma from animals that received 8 immunizations (week 123). We demonstrated that 6/6 monkeys immunized with gp120 and gp140C glycoproteins had circulating antibodies that could neutralize HIV-1JR-FL that had been sensitized by BNM-III-170 (Fig. 3A). To investigate the time course of elicitation of these antibodies, the plasma from these six monkeys was tested at 8, 14, 20, 26, and 86 weeks following initial immunization. At week 8, after two immunizations, only one of the six monkeys exhibited circulating antibodies that neutralized the BNM-III-170-sensitized HIV-1JR-FL (Fig. 4A). By week 14, after three immunizations, three of the monkeys had these antibodies in their plasma, and a fourth animal exhibited weak activity (Fig. 4B). By week 20, after four immunizations, 4/6 monkeys had antibodies that potently neutralized HIV-1JR-FL in the presence of BNM-III-170, and the remaining two monkeys exhibited weak activity (Fig. 4C). By 26 weeks, after five immunizations, all 6 monkeys had circulating antibodies that neutralized the BNM-III-170-treated HIV-1JR-FL but not the untreated virus (Fig. 4D). These antibodies also were evident in the plasma collected at 86 weeks, after six immunizations, from all 6 monkeys (Fig. 4E). The time course of elicitation of antibodies that neutralized the BNM-III-170-sensitized HIV-1JR-FL is summarized in Fig. 4F.
FIG 4.
Time course of elicitation of antibodies that neutralize sensitized HIV-1JR-FL. (A to E) Plasma samples from NHP 62.1 (CHAVI-ID) collected at the indicated times of immunization were tested for the ability to neutralize recombinant HIV-1 with HIV-1JR-FL or A-MLV Envs, in either the absence (red) or presence (green) of BNM-III-170 (30 μM). The means and standard deviations from triplicate samples are shown. (F) The titers of plasma that neutralized 50% of HIV-1JR-FL were plotted versus the time of immunization for each monkey in NHP 62.1. The numbers identify the individual monkeys in the study.
The group 2 monkeys in NHP 36 received an RV144-like vaccination regimen and generated antibodies that neutralized the BNM-III-170-sensitized HIV-1JR-FL by 55 weeks following the initiation of immunization (Fig. 3C). Plasma taken from 4/5 monkeys at week 27 after initial inoculation, after the monkeys received two ALVAC VPC primes and two gp120 boosts, neutralized the BNM-III-170-sensitized HIV-1JR-FL virus (data not shown). Weaker neutralizing activity was detected in the plasma of the remaining monkey. These studies indicate that by 26 to 27 weeks after inoculation of monkeys with Env immunogens, antibodies capable of neutralizing a BNM-III-170-sensitized primary HIV-1 can be elicited.
Neutralization of different sensitized HIV-1 strains by immunized monkey plasma.
The neutralization of BNM-III-170-sensitized HIV-1AD8, HIV-1YU2, and HIV-1JR-FL by plasma from immunized monkeys was tested. Plasma from monkeys in CHAVI-ID NHP 62.1, NHP 79, and NHP 109 neutralized these viruses in the presence of a subinhibitory concentration of BNM-III-170 but not in the absence of the compound (Table 2). These results indicate that antibodies generated in monkeys by Env immunization are capable of neutralizing diverse HIV-1 strains sensitized by BNM-III-170.
TABLE 2.
Inhibition of primary HIV-1 Env isolates by CD4-mimetic compounds in the presence of 17b CD4i antibody or plasma from immunized monkeys
| Antibody/compound | IC50 fora: |
|||
|---|---|---|---|---|
| HIV-1JR-FL | HIV-1YU2 | HIV-1AD8 | A-MLV | |
| BNM-III-170b | 17.4 μM | 1.9 μM | 3.6 μM | >100 μM |
| 17b | >30 μg/ml | >30 μg/ml | >30 μg/ml | >30 μg/ml |
| NHP 79-5356, wk 21 | >1:80 | >1:80 | >1:80 | 1:160 |
| NHP 62.1-5097, wk 123 | >1:80 | >1:80 | >1:80 | 1:160 |
| NHP 109-6117, wk 71 | >1:80 | >1:80 | >1:80 | >1:80 |
| NHP 109-6204, wk 71 | >1:80 | >1:80 | >1:80 | 1:160 |
| 17b + BNM-III-170 | 0.2 μg/ml | <0.2 μg/ml | 0.2 μg/ml | >30 μg/ml |
| NHP 79-5356, wk 21, + BNM-III-170 | 1:320 | 1:640 | 1:640 | 1:80 |
| NHP 62.1-5097, wk 123, + BNM-III-170 | 1:640 | 1:1,280 | 1:1,280 | >1:80 |
| NHP 109-6117, wk 71, + BNM-III-170 | 1:1,280 | 1:1,280 | 1:1,280 | >1:80 |
| NHP 109-6204, wk 71, + BNM-III-170 | 1:640 | 1:1,280 | 1:1,280 | 1:160 |
Recombinant HIV-1 encoding firefly luciferase was pseudotyped with the indicated HIV-1 Env (or A-MLV Env control), as described in the footnotes to Table 1. Viruses were incubated with 10 μM BNM-III-170 (YU2 and AD8), 50 μM BNM-III-170 (JR-FL and A-MLV), or DMSO and then with different concentrations of the 17b antibody or dilution of plasma. The nonhuman primates (NHP) 6117 and 6204 were subjects in CHAVI-ID NHP 109, and both were members of group 1, which received basiliximab (1 mg) after immunization with CH505 T/F gp120 in GLA-SE adjuvant. The concentration of the 17b antibody (μg/ml) or the titer of the plasma that neutralized 50% of the virus infection is indicated. Means and standard deviations from triplicate samples within an experiment are shown.
The inhibitory concentration (IC50) for the direct antiviral effect of BNM-III-170 was determined as described in footnote a.
Elicitation of antibodies that neutralize sensitized HIV-1 in vaccinated humans.
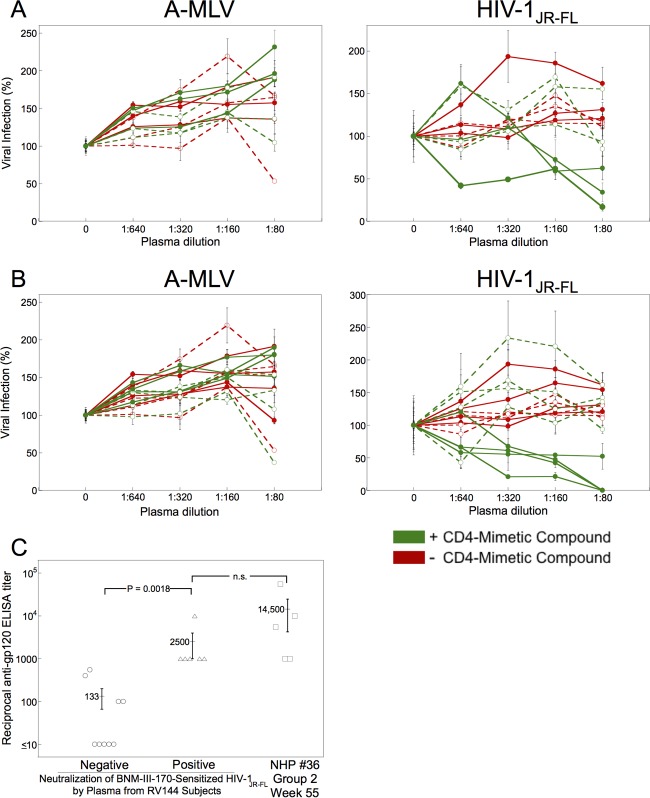
The above-described study of monkeys (NHP 36) suggested that an immunization regimen similar to that used in the RV144 clinical vaccine trial (12, 99) could elicit antibodies that efficiently neutralize BNM-III-170-sensitized HIV-1JR-FL (Fig. 3C). We investigated whether Env-vaccinated humans in the RV144 trial raised such antibodies. We tested preimmune and immune plasma from 15 randomly selected RV144 subjects for the ability to neutralize HIV-1JR-FL in the absence and presence of BNM-III-170 and BNM-IV-147 (Fig. 5). None of the plasma significantly inhibited HIV-1JR-FL in the absence of the compounds (Table 3 and Fig. 5). Plasma from five of the Env-vaccinated individuals specifically neutralized HIV-1JR-FL but not the A-MLV Env pseudotype in the presence of subinhibitory concentrations of BNM-III-170 and BNM-IV-147. Preimmune plasma from these individuals did not neutralize HIV-1JR-FL in the presence of BNM-III-170 and BNM-IV-147. These results suggest that some humans primed with ALVAC VPC and boosted twice with ALVAC VPC plus clade B/E gp120 glycoproteins generate antibodies that neutralize HIV-1JR-FL sensitized by BNM-III-170 and BNM-IV-147.
FIG 5.
Some RV144 vaccinees generate antibodies that neutralize sensitized HIV-1. (A) Recombinant, luciferase-expressing viruses with HIV-1JR-FL Env or A-MLV Env were incubated with 30 μM BNM-III-170 (green) or without compound (red). The viruses subsequently were incubated with the indicated dilution of plasma from human subjects in the RV144 HIV-1 vaccine trial. Preimmune plasma samples are designated by open symbols and dashed lines and immune plasma samples by closed symbols and unbroken lines. The virus-plasma mixtures were incubated with Cf2Th-CD4/CCR5 cells for 48 h, after which the cells were lysed and luciferase activity measured. The level of infection relative to that seen in the absence of plasma is shown. The means and standard deviations from triplicate samples within an experiment are shown; the experiment was performed twice with similar results. (B) Neutralization assays were performed as described for panel A, except that 30 μM BNM-IV-147 was used instead of BNM-III-170. (C) The reciprocal titers of anti-gp120 antibodies measured by ELISA are shown for immune plasma from RV144 vaccinees that are either negative or positive for the neutralization of BNM-III-170-sensitized HIV-1JR-FL. The anti-gp120 antibody reciprocal titers of plasma from monkeys in group 2 of the NHP 36 study are shown for comparison. Differences between the groups were analyzed by the Mann-Whitney U (t) test; the P value is shown for the comparison where statistical significance was achieved. n.s., not significant.
TABLE 3.
Inhibition of HIV-1JR-FL by plasma from RV144 subjects
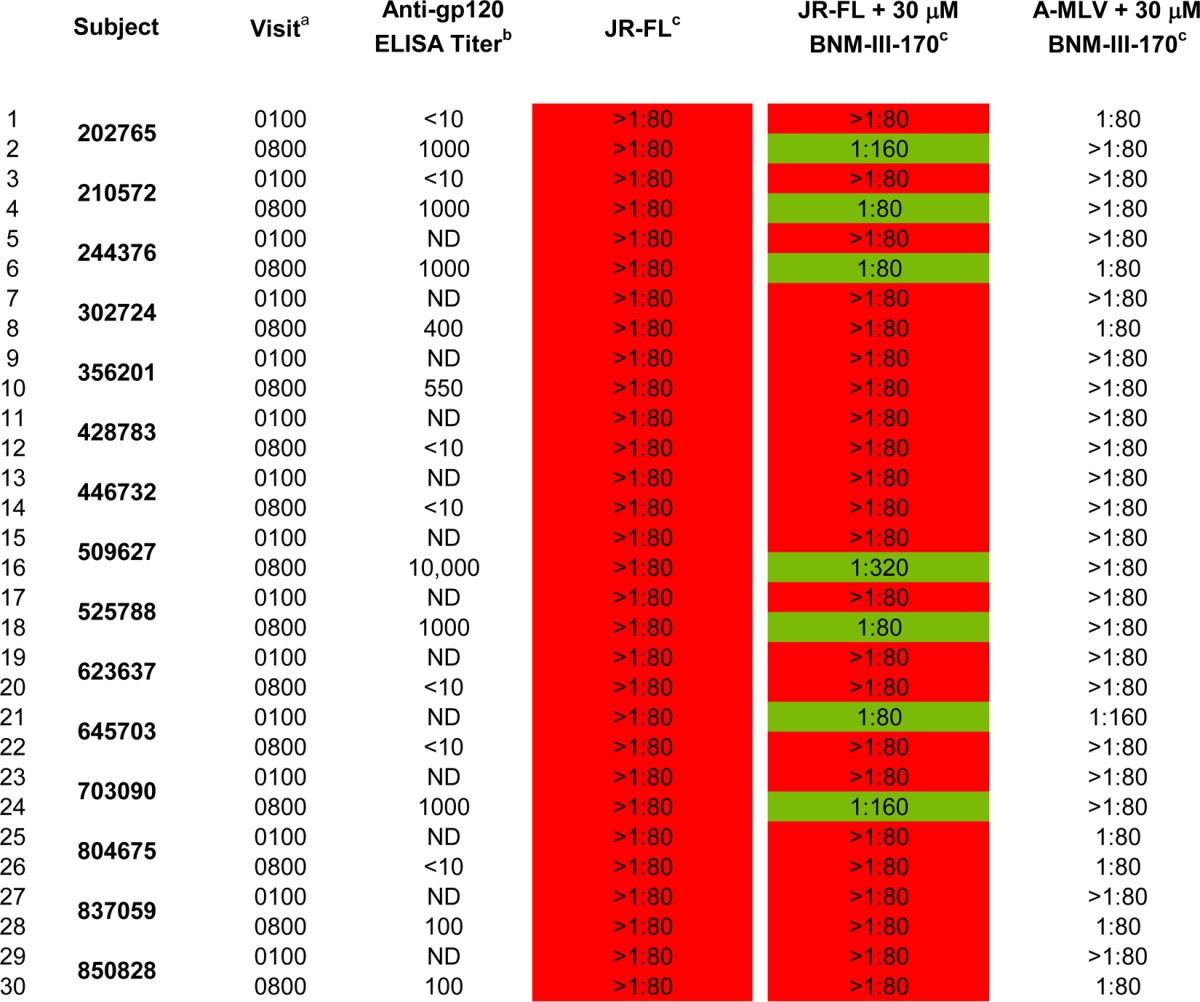
a The 0100 visits occurred prior to vaccination and allowed the collection of preimmune plasma samples. The 0800 visit occurred at 26 weeks after the initiation of vaccination, 2 weeks after the fourth vaccination (ALVAC-HIV twice, ALVAC-HIV + AIDSVAX-B/E twice).
b The reciprocal anti-gp120 ELISA titers of the plasma samples were determined as described in Materials and Methods.
c The titer of plasma that neutralized 50% of HIV-1JR-FL or A-MLV infection was determined, as described in the Materials and Methods.
Compared with the monkeys in NHP 36 (Fig. 3C), the human subjects in this RV144 cohort exhibited lower levels and frequencies of antibodies that neutralized the BNM-III-170-sensitized HIV-1JR-FL (Fig. 5 and Table 3). We used an ELISA to measure the levels of gp120-reactive antibodies in the plasma of the RV144 vaccinees and the immunized monkeys in group 2 of NHP 36. A correlation was observed between the presence of antibodies that neutralized the BNM-III-170-sensitized HIV-1JR-FL and the titer of anti-gp120 antibodies in the plasma of the RV144 vaccinees (Fig. 5C). Only the RV144 subjects with reciprocal anti-gp120 titers of at least 1,000 had plasma antibodies that neutralized the BNM-III-170-sensitized HIV-1JR-FL. These anti-gp120 titers were in the lower range of those observed in the monkeys in group 2 of the NHP 36 study (Fig. 5C). These results suggest that individual differences in the antibody response to the RV144 immunization regimen among human vaccinees likely contribute to the observed differences in the levels of antibodies that neutralize the BNM-III-170-sensitized HIV-1JR-FL.
Env epitopes targeted by antibodies that neutralize sensitized HIV-1.
Monoclonal antibodies directed against conserved CD4i and V3 epitopes have been shown to neutralize primary HIV-1 strains that have been exposed to a CD4-mimetic compound (15, 96). CD4i and anti-V3 antibodies can be elicited by vaccination of monkeys with some Env immunogens (76, 78). We hypothesized that these types of antibodies in the plasma of the Env-vaccinated monkeys contributed to the observed neutralization of the BNM-III-170-sensitized HIV-1JR-FL. To test this hypothesis, we examined the ability of monoclonal antibodies isolated from the immunized monkeys to neutralize HIV-1JR-FL in the absence and presence of BNM-III-170.
Monoclonal antibodies were derived from monkeys immunized in CHAVI-ID NHP 62.1: 900973 (directed against the gp120 C terminus); 900974, 900990, and 902067 (directed against the gp120 V3 region); and 902090 (directed against the gp120 V2 region). Another V3 region-directed antibody, GE2.JG8, was generated from monkey F124 immunized in the Sundling et al. study (104) after sorting single Env-specific memory B cells (105). Two human monoclonal antibodies (CH22 and CH23) directed against the gp120 V3 region were derived from subjects in the RV135 clinical HIV-1 vaccine trial (106). The 830A antibody, derived from an HIV-1-infected individual, recognizes a discontinuous epitope comprising residues from the gp120 V2 region (107, 108). None of the monoclonal antibodies neutralized untreated HIV-1JR-FL (Table 4). However, in the presence of subneutralizing concentrations of BNM-III-170, all of the antibodies except 900973 (directed against the gp120 C terminus) potently neutralized HIV-1JR-FL. These results indicate that some of the antibodies that mediate neutralization of HIV-1JR-FL sensitized by BNM-III-170 and that are generated by vaccination of monkeys and humans are directed against the gp120 V2 and V3 regions.
TABLE 4.
Neutralization of BNM-III-170-treated HIV-1JR-FL by monoclonal antibodies from vaccinated monkeys and humansa
| Monoclonal antibody | gp120 epitope | IC50 (μg/ml) for: |
|||
|---|---|---|---|---|---|
| HIV-1JR-FL |
A-MLV |
||||
| Without BNM-III-170 | With BNM-III-170 | Without BNM-III-170 | With BNM-III-170 | ||
| 17b | CD4i | >30 | 0.6 ± 0.4 | >30 | >30 |
| 902090 | V2 (171–177) | >30 | 5.0 ± 2.4 | >30 | >30 |
| 830A Fab | Discontinuous V2i epitope | >10 | 0.6 | >10 | >10 |
| 900973 | C terminus (491–501) | >30 | >30 | >30 | >30 |
| 900974 | V3 (304–317) | >30 | 0.2 ± 0.0 | >30 | >30 |
| 900990 | V3 (318–327) | >30 | 5.1 | >30 | >30 |
| 902067 | V3 (304–314) | >30 | 0.2 ± 0.0 | >30 | >30 |
| GE2 JG8 | V3 (301–317) | >100 | 0.6 | >100 | >100 |
| CH22 | V3 (304–320) | >30 | 0.2 | >30 | >30 |
| CH23 | V3 (302–318) | >30 | 3.3 | >30 | 22.1 |
The antibody concentration (IC50) that inhibited the infection of the recombinant viruses by 50% in the absence or presence of 30 μM BNM-III-170 is reported. In this assay, the IC50s of BNM-III-170 alone were 20.7 ± 7.6 μM for HIV-1JR-FL and >100 μM for A-MLV.
Cooperativity between a CD4-mimetic compound and antibodies.
To test whether CD4-mimetic compounds and antibodies synergize to inhibit primary HIV-1, we examined the neutralization of HIV-1JR-FL and A-MLV over a range of concentrations of BNM-III-170 and 17b antibody (Fig. 6A and B). The results in Fig. 6A show that, at every concentration of BNM-III-170 tested, the presence of the 17b antibody resulted in a lower level of virus infection than that seen in the absence of antibody. Because little or no neutralization of HIV-1JR-FL by the 17b antibody alone was observed, our results imply that BNM-III-170 positively cooperates with the 17b antibody to inhibit HIV-1 infection. Moreover, the sensitization of HIV-1JR-FL to the inhibitory effects of the 17b antibody were observed over the entire range of BNM-III-170 concentrations tested. These observations are consistent with a model in which the small molecule's direct antiviral effect requires interaction with the Env trimer at a higher stoichiometry than that which promotes sensitization of the virus to neutralization by the 17b antibody.
FIG 6.
Cooperativity in HIV-1 inhibition between a CD4-mimetic compound and antibodies. Recombinant, luciferase-expressing viruses with HIV-1JR-FL Env (A, C, and D) or A-MLV Env (B) were incubated with the indicated concentrations of BNM-III-170, followed by incubation with the indicated concentration of either the 17b antibody (A and B) or the indicated dilution of plasma from two monkeys in CHAVI-ID NHP 62.1 (C and D). The viruses were used to infect Cf2Th-CD4/CCR5 cells, and the level of infection was measured as described in Materials and Methods. The means and standard deviations from triplicate samples are shown.
We also tested the potential cooperativity in HIV-1 inhibition between BNM-III-170 and two of the plasma samples from monkeys immunized in the NHP 62.1 study (described above). The results were qualitatively similar to those obtained with the 17b antibody (Fig. 6C and D). At every concentration of added BNM-III-170, the monkey plasma inhibited HIV-1JR-FL infection, although the inhibitory effects of the plasma were less than those of the 17b antibody. At the same dilutions, in the absence of BNM-III-170, the monkey plasma did not significantly inhibit HIV-1JR-FL infection. Apparently, BNM-III-170 can positively cooperate with antibodies elicited by immunization of monkeys to inhibit HIV-1 infection.
Stability of HIV-1 sensitization by CD4-mimetic compounds.
A previous study suggested that direct HIV-1 inactivation by CD4-mimetic compounds is irreversible (14). To evaluate whether the sensitization of HIV-1 to antibody neutralization can be reversed, we incubated HIV-1 with a subneutralizing concentration of BNM-III-170 for 2 h at 37°C. A control virus was incubated with DMSO for the same time period. Virus preparations were divided into two. One set of viruses was incubated with increasing concentrations of the 17b antibody, maintaining the original concentration of DMSO or BNM-III-170. The second set of viruses was pelleted, resuspended in buffer without DMSO or BNM-III-170, and then incubated with different concentrations of the 17b antibody. Infection of target cells then was measured for all of the viruses.
The sensitization of HIV-1JR-FL to neutralization by the 17b antibody was comparable for the virus continuously exposed to BNM-III-170 and for the BNM-III-170-treated virus that subsequently was washed and resuspended in compound-free medium (Fig. 7). This result suggests that the sensitized state of HIV-1 is sufficiently long-lived to withstand washing.
FIG 7.
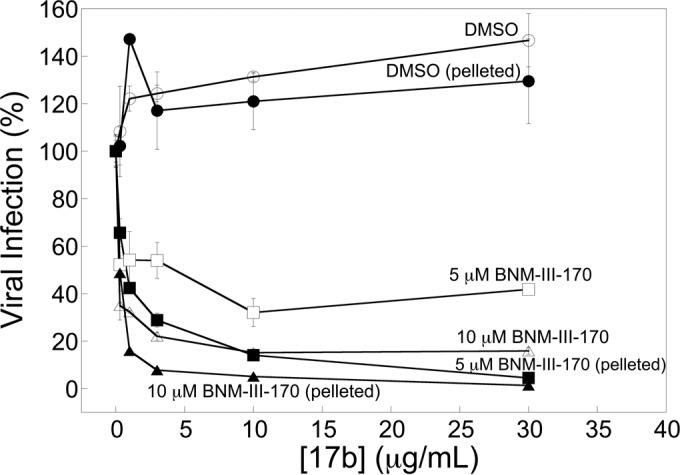
Stability of the BNM-III-170-sensitized state of HIV-1JR-FL. Recombinant, luciferase-expressing HIV-1JR-FL was incubated with either BNM-III-170 or the appropriate concentration of DMSO for 2 h at 37°C. Half of the virus preparation was incubated with the indicated concentration of the 17b antibody, maintaining the original concentration of DMSO and BNM-III-170. The other half of the virus preparation was pelleted by centrifugation, resuspended in buffer without DMSO or BNM-III-170, and then incubated with different concentrations of the 17b antibody. The infection of Cf2Th-CD4/CCR5 cells by the viruses was determined as described in Materials and Methods. The level of infection relative to that observed in the absence of the 17b antibody is reported, with means and standard deviations derived from triplicate samples.
DISCUSSION
Controlling the global HIV-1/AIDS pandemic likely will require the implementation of modalities to prevent sexual transmission of the virus. In this regard, HIV-1 Env trimers represent attractive targets due to their accessibility and functional lability, but they also present challenges arising from interstrain variability and glycan shielding. Vaccine strategies that generate antibodies in uninfected individuals capable of potently neutralizing a wide range of transmitted/founder HIV-1 variants have yet to be developed (60–62). The HIV-1 Env immunogens tested to date raise antibodies that fail to inhibit most primary HIV-1 strains, which exhibit low Env reactivity (63, 64). Our results confirm that various Env immunization regimens in primates, some quite prolonged and involving multiple immunogens, elicited only very low or undetectable levels of antibodies capable of neutralizing the primary HIV-1JR-FL isolate. HIV-1JR-FL in particular exhibits a low Env reactivity and generally is difficult to neutralize by anti-HIV-1 antibodies (16, 96, 109, 110). However, HIV-1JR-FL is not unique in this respect; the unliganded conformations of many primary HIV-1 Envs, including those from transmitted/founder viruses (64), present only a limited number of conserved epitopes for antibody binding and virus neutralization (51, 60–62). In contrast, the induction of the CD4-bound state on a virus that has not yet engaged the target cell results in a shortened infectious half-life and a dramatic increase in susceptibility to neutralization by antibodies against the conserved coreceptor-binding site (14, 15, 76, 86, 96). Recent structure-based design and synthesis has increased the potency and breadth of small-molecule CD4-mimetic compounds, which can induce the CD4-bound conformation of Env (88–95). Here, we demonstrate that antibodies elicited in monkeys and humans by several different Env immunogens potently neutralize primary HIV-1 treated with subneutralizing concentrations of a CD4-mimetic compound, BNM-III-170.
Importantly, antibodies that neutralize primary HIV-1 that has been sensitized by CD4-mimetic compounds recognize Env conformations closer to the CD4-bound state rather than the unliganded state of Env (95). In this and a previous study (96), monoclonal antibodies raised against gp120 CD4i and V3 epitopes neutralized primary HIV-1 only in the presence of CD4-mimetic compounds. These conserved gp120 elements are not formed/exposed in the unliganded state of Env but become so after binding to CD4 or the CD4-mimetic compounds (70, 73–76, 86, 89, 95). Our results with the 830A monoclonal antibody from HIV-1-infected humans (107, 108) and the 902090 monoclonal antibody from an immunized monkey also suggest that some V2 epitopes on gp120 become readily available for antibody binding after Env exposure to BNM-III-170 binding. The V2i antibody 830A recognizes a discontinuous epitope on the surface of a β-barrel composed of V2 strands (108). The C strand (residues 171 to 177) of this V2 β-barrel is recognized by the 902090 antibody. Therefore, the V2 β-barrel is formed and at least partly exposed after BNM-III-170 treatment; this is not the case in the unliganded state of the HIV-1JR-FL Env.
Given that several antibodies neutralizing sensitized HIV-1 recognize Env conformations close to or identical to the CD4-bound conformation, Env immunogens that can achieve and present this conformation to the host immune system would be expected to raise these antibodies more effectively. Indeed, in a prior study of rabbits, HIV-1 gp120 cores engineered to remain in the CD4-bound state elicited antibodies that neutralized sensitized HIV-1 more efficiently (84, 96). The results presented here demonstrate that these antibodies can be elicited in monkeys and humans by a variety of Env immunogens. In these hosts, which naturally express a CD4 molecule capable of binding HIV-1 Env (78–81), Env immunogens able to bind CD4 apparently can present the CD4-induced conformation of Env to the immune system. In primates, unlike the situation in rabbits, any Env immunogen that retains the ability to bind CD4 could elicit antibodies capable of neutralizing HIV-1 sensitized by a CD4-mimetic compound. For these immunogens, no additional measures are required to elicit the desired antibodies. Sensitization by CD4-mimetic compounds may extend the prophylactic efficacy of any Env vaccine formulation that is not 100% effective at preventing HIV-1 acquisition. Future studies also should test the possibility that, even in primates, immunization with Envs engineered to prefer the CD4-bound conformation will elicit antibodies that neutralize sensitized HIV-1 more efficiently.
The binding site for the CD4-mimetic compounds on the gp120 glycoprotein is well conserved among group M HIV-1, except for clade AE recombinant viruses (111). The prototypic CD4-mimetic compounds have been extensively modified to achieve more contacts with gp120, resulting in improvements in antiviral potency and breadth (88–95). For example, nearly all clade B HIV-1 and most clade C HIV-1 variants tested can be directly inhibited by the recently developed CD4-mimetic compounds, such as BNM-III-170 and BNM-IV-147 (95). Of particular note, all of the HIV-1 strains that were sensitized to neutralization by antibodies at subinhibitory concentrations of BNM-III-170 were efficiently inhibited by higher concentrations of the compound. Because the vaccine immunogens elicit antibodies that themselves have little or no neutralizing activity against most primary HIV-1 strains, these antibodies primarily contribute to the potency and not to the breadth of the CD4-mimetic compound.
The positive effects of BNM-III-170 on HIV-1 sensitization to neutralizing antibodies were evident even at low concentrations of the CD4-mimetic compound, hinting that sensitization can occur at a low stoichiometry of the compound bound to the Env trimer. Thus, our results are consistent with a model in which the sensitization of HIV-1 to antibody neutralization occurs at a lower stoichiometry of compound binding to the Env trimer compared with that of direct antiviral inhibition. We observed multiple instances where plasma or antibody inhibited HIV-1 infection only in the presence of BNM-III-170, indicating that the CD4-mimetic compound can increase the potency and breadth of vaccine-induced antibodies, presumably by enhancing antibody binding to the Env spike. Neutralization by antibodies that recognize on-pathway Env conformations beyond the unliganded state, up to and including the CD4-bound state, could hypothetically benefit from exposure of the virus to the CD4-mimetic compound.
The demonstration that antibodies elicited in primates by several different Env immunogens potently neutralize primary HIV-1 sensitized by a CD4-mimetic compound has potential practical implications. Antibodies that neutralize the sensitized HIV-1 are consistently elicited in vaccinated primates that generate a robust anti-gp120 response; such antibodies arise relatively early in the course of vaccination. These observations suggest that combining a vaccine with a CD4-mimetic compound, for example, used as a microbicide, might achieve the requisite level of prophylactic efficacy. The stimulation of antibody-dependent cell cytotoxicity (ADCC) against HIV-1-infected cells by CD4-mimetic compounds (112) could provide an additional level of protection in this setting. Challenge models employing SHIVs in monkeys can be used to investigate the relevance of the observed HIV-1 sensitization to protection against mucosal exposure to virus. Future efforts should be directed toward improving the coverage of a greater range of HIV-1 variants by this combined prophylactic approach.
ACKNOWLEDGMENTS
We thank Yvette McLaughlin, Elizabeth Carpelan, and Julia Barnes for manuscript preparation. We thank Irwin Chaiken and Wayne Hendrickson for valuable discussions and input. We thank Charla Andrews and Robert O'Connell at the U.S. Military HIV Research Program and Sandhya Vasan at the Armed Forces Research Institute of Medical Sciences for their assistance in providing the RV144 specimens.
This study was supported by the National Institutes of Health (GM56550 and AI24755) and the Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery (CHAVI-ID) (AI100645), the International AIDS Vaccine Initiative, and the late William F. McCarty-Cooper. N.M. was supported by amfAR grant 107431-45-RFNT, NIH grant AI090682, and a Ragon Institute Innovation Award.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.United Nations Programme on HIV/AIDS. June 2014. UNAIDS report on the global HIV/AIDS epidemic. United Nations Programme on HIV/AIDS, Geneva, Switzerland: http://www.unaids.org. [Google Scholar]
- 2.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med 6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 3.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho MW, Martin MA. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med 5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 4.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, Marx PA, Burton DR. 2007. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 5.Hessell AJ, Rakasz EG, Tehrani DM, Huber M, Weisgrau KL, Landucci G, Forthal DN, Koff WC, Poignard P, Watkins DI, Burton DR. 2009. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog 5:e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruprecht RM. 2009. Passive immunization with human neutralizing monoclonal antibodies against HIV-1 in macaque models: experimental approaches. Methods Mol Biol 525:559–566. doi: 10.1007/978-1-59745-554-1_31. [DOI] [PubMed] [Google Scholar]
- 7.Burton DR, Hessell AJ, Keele BF, Klasse PJ, Ketas TA, Moldt B, Dunlop DC, Poignard P, Doyle LA, Cavacini L, Veazey RS, Moore JP. 2011. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc Natl Acad Sci U S A 108:11181–11186. doi: 10.1073/pnas.1103012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allan JS, Coligan JE, Barin F, McLane MF, Sodroski JG, Rosen CA, Haseltine WA, Lee TH, Essex M. 1985. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science 228:1091–1094. doi: 10.1126/science.2986290. [DOI] [PubMed] [Google Scholar]
- 9.Robey WG, Safai B, Oroszlan S, Arthur LO, Gonda MA, Gallo RC, Fischinger PJ. 1985. Characterization of envelope and core structural gene products of HTLV-III with sera from AIDS patients. Science 228:593–595. doi: 10.1126/science.2984774. [DOI] [PubMed] [Google Scholar]
- 10.Wyatt R, Sodroski J. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 11.Karasavvas N, Billings E, Rao M, Williams C, Zolla-Pazner S, Bailer RT, Koup RA, Madnote S, Anworn D, Shen X, Tomaras GD, Currier JR, Jiang M, Magaret C, Andrews C, Gottardo R, Gilbert P, Cardozo TJ, Rerks-Ngarm S, Nitayaphan S, Pitisuttithum P, Kaewkungwal J, Paris R, Greene K, Gao H, Gurunathan S, Tartaglia J, Sinangil F, Korber BT, Montefiori DC, Mascola JR, Robb ML, Haynes BF, Ngauy V, Michael NL, Kim JH, de Souza MS. 2012. The Thai Phase III HIV Type 1 Vaccine trial (RV144) regimen induces antibodies that target conserved regions within the V2 loop of gp120. MOPH TAVEG Collaboration. AIDS Res Hum Retrovir 28:1444–1457. doi: 10.1089/aid.2012.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rerks-Ngarm S, Pittisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birs DL, Chunsutiwat S, Khamboonruang C, Thongsharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 13.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yaes NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haim H, Si Z, Madani N, Wang L, Courter JR, Princiotto A, Kassa A, DeGrace M, McGee-Estrada K, Mefford M, Gabuzda D, Smith AB III, Sodroski J. 2009. Soluble CD4 and CD4-mimetic compounds inhibit HIV-1 infection by induction of a short-lived activated state. PLoS Pathog 5:e1000360. doi: 10.1371/journal.ppat.1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshimura K, Harada S, Shibata J, Hatada M, Yamada Y, Ochiai C, Tamamura H, Matsushita S. 2010. Enhanced exposure of human immunodeficiency virus type 1 primary isolate neutralization epitopes through binding of CD4 mimetic compounds. J Virol 84:7558–7568. doi: 10.1128/JVI.00227-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haim H, Salas I, McGee K, Eichelberger N, Winter E, Pacheco B, Sodroski J. 2013. Modeling virus- and antibody-specific factors to predict human immunodeficiency virus neutralization efficiency. Cell Host Microbe 14:547–558. doi: 10.1016/j.chom.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon YD, Finzi A, Wu X, Dogo-Isonagie C, Lee L, Moore LR, Schmidt SD, Stuckey J, Yang Y, Zhou T, Zhu J, Vicic DA, Debnath AIK, Shapiro L, Bewley CA, Mascola JR, Sodroski J, Kwong PD. 2012. Unliganded HIV-1 gp120 core structures assume the CD4-bound conformation with regulation by quaternary interactions and variable loops. Proc Natl Acad Sci U S A 109:5663–5668. doi: 10.1073/pnas.1112391109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan DC, Fass D, Berger JM, Kim PS. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263–273. doi: 10.1016/S0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 19.Weissenhorn W, Dessen A, Harrison SC, Skehel JJ, Wiley DC. 1997. Atomic structure of the ectodomain from HIV-1 gp41. Nature 387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 20.Tan K, Liu J, Wang J, Shen S, Lu M. 1997. Atomic structure of a thermostable subdomain of HIV-1 gp41. Proc Natl Acad Sci U S A 94:12303–12308. doi: 10.1073/pnas.94.23.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman JC, Montagnier L. 1984. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 22.Dalgleish AG, Beverley PC, Clapham PR, Crawford DH, Greaves MF, Weiss RA. 1984. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 23.Wu L, Gerard N, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A, Desjardins E, Newman W, Gerard C, Sodroski J. 1996. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR5. Nature 384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 24.Trkola A, Dragic T, Arthos J, Binley JM, Olson WC, Allaway GP, Cheng-Mayer C, Robinson J, Maddon PJ, Moore JP. 1996. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature 384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 25.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath PD, Wu L, Mackay CR, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. 1996. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 85:1135–1148. doi: 10.1016/S0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 26.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. 1996. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 27.Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 28.Doranz BJ, Rucker J, Yi Y, Smyth RJ, Samson M, Peiper SC, Parmentier M, Collman RG, Doms RW. 1996. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell 85:1149–1158. doi: 10.1016/S0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 29.Feng Y, Broder CC, Kennedy PE, Berger EA. 1996. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 30.Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. 1996. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 31.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 32.Furuta RA, Wild CT, Weng Y, Weiss CD. 1998. Capture of an early fusion-active conformation of HIV-1 gp41. Nat Struct Biol 5:276–279. doi: 10.1038/nsb0498-276. [DOI] [PubMed] [Google Scholar]
- 33.Koshiba T, Chan DC. 2003. The prefusogenic intermediate of HIV-1 gp41 contains exposed C-peptide regions. J Biol Chem 278:7573–7579. doi: 10.1074/jbc.M211154200. [DOI] [PubMed] [Google Scholar]
- 34.He Y, Vassell R, Zaitseva M, Nguyen N, Yang Z, Weng Y, Weiss CD. 2003. Peptides trap the human immunodeficiency virus type 1 envelope glycoprotein fusion intermediate at two sites. J Virol 77:1666–1671. doi: 10.1128/JVI.77.3.1666-1671.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Si Z, Madani M, Cox JM, Chruma JJ, Klein JC, Schön A, Phan N, Wang L, Biorn AC, Cocklin S, Chaiken I, Freire E, Smith AB III, Sodroski J. 2004. Small-molecule inhibitors of HIV-1 entry block receptor-induced conformational changes in the viral envelope glycoproteins. Proc Natl Acad Sci U S A 101:5036–5041. doi: 10.1073/pnas.0307953101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melikyan GB, Markosyna RM, Hemmati H, Delmedico MK, Lambert DM, Cohen FS. 2000. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle conformation, induces membrane fusion. J Cell Biol 151:413–423. doi: 10.1083/jcb.151.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA, Majeed S, Steenbeke TD, Venturi M, Chaiken I, Fung M, Katinger H, Parren P, Robinson J, Wang L, Burton DR, Freire E, Wyatt R, Sodroski J, Hendrickson WA, Arthos J. 2002. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 420:678–682. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- 38.McCaffrey RA, Saunders C, Hensel M, Stamatatos L. 2004. N-linked glycosylation of the V3 loop and the immunologically silent face of gp120 protects human immunodeficiency virus type 1 SF162 from neutralization by anti-gp120 and anti-gp41 antibodies. J Virol 78:3279–3295. doi: 10.1128/JVI.78.7.3279-3295.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wyatt R, Kwong PD, Desjardins E, Sweet RW, Robinson J, Hendrickson WA, Sodroski JG. 1998. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 40.Reitter JN, Means RE, Desrosiers RC. 1998. A role for carbohydrates in immune evasion in AIDS. Nat Med 4:679–684. doi: 10.1038/nm0698-679. [DOI] [PubMed] [Google Scholar]
- 41.Frost SD, Wrin T, Smith DM, Kosakovsky Pond S, Liu Y, Paxinos E, Chappey C, Galovich J, Beauchaine J, Petropouos CJ, Little SJ, Richman DD. 2005. Neutralizing antibody responses drive the evolution of human immunodeficiency virus type 1 envelope during recent HIV infection. Proc Natl Acad Sci U S A 102:18514–18519. doi: 10.1073/pnas.0504658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fischer W, Ganusov W, Giorgi EE, Hraber PT, Keele BF, Leitner T, Han CS, Gleasner CD, Green L, Lo CC, Nag A, Wallstrom TC, Wang S, McMichael AJ, Haynes BF, Hahn BH, Perelson AS, Borrow P, Shaw M, Bhattacharya T, Korber BT. 2010. Transmission of single HIV-1 genomes and dynamics of early immune escape revealed by ultra-deep sequencing. PLoS One 5:e12303. doi: 10.1371/journal.pone.0012303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao F, Bonsignori M, Liao HX, Kumar A, Xia SM, Lu X, Cai F, Hwang KK, Song H, Zhou T, Lynch RM, Alam SM, Moody MA, Ferrari G, Berrong M, Kelsoe G, Shaw GM, Hahn BH, Montefiori DC, Kamanga G, Cohen MS, Hraber P, Kwong PD, Korber BT, Mascola JR, Kepler TB, Haynes BF. 2014. Cooperation of B cell lineages in induction of HIV-1-broadly neutralizing antibodies. Cell 158:481–491. doi: 10.1016/j.cell.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bar KJ, Tsao CY, Iyer SS, Decker JM, Yang Y, Bonsignori M, Chen X, Hwang KK, Montefiori DC, Liao HX, Hraber P, Fischer W, Li H, Wang S, Sterrett S, Keele BF, Ganusov VV, Perelson AS, Korber BT, Georgiev I, McLellan JS, Pavlicek JW, Gao F, Haynes BF, Hahn BH, Kwong PD, Shaw GM. 2012. Early low-titer neutralizing antibodies impede HIV-1 replication and select for virus escape. PLoS Pathog 8:e1002721. doi: 10.1371/journal.ppat.1002721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore PL, Ranchobe N, Lambson BE, Gray ES, Cave E, Abrahams MR, Bandawe G, Mlisana K, Abdool Karim SS, Williamson C, Morris L, CAPRISA 002 Study, NIAID Center for HIV/AIDS Vaccine Immunology (CHAVI). 2009. Limited neutralizing antibody specificities drive neutralization escape in early HIV-1 subtype C infection. PLoS Pathog 5:e1000598. doi: 10.1371/journal.ppat.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stamatatos L, Morris L, Burton DR, Mascola JR. 2009. Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nat Med 15:866–870. [DOI] [PubMed] [Google Scholar]
- 47.Gray ES, Madiga MC, Hermanus T, Moore PL, Wibmer CK, Tumba NL, Werner L, Mlisana K, Sibeko S, Williamson C, Abdool Karim SS, Morris L, CAPRISA 002 Study Team. 2011. The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J Virol 85:4828–4840. doi: 10.1128/JVI.00198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wibmer CK, Bhiman JN, Gray ES, Tumba N, Abdool Karim SS, Williamson C, Morris L, Moore PL. 2013. Viral escape from HIV-1 neutralizing antibodies drives increased plasma neutralization breadth through sequential recognition of multiple epitopes and immunotypes. PLoS Pathog 9:e1003738. doi: 10.1371/journal.ppat.1003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sather DN, Armann J, Ching LK, Mayrantoni A, Sellhorn G, Caldwell Z, Yu X, Wood B, Self S, Kalams S, Stamatatos L. 2009. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J Virol 83:757–769. doi: 10.1128/JVI.02036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klein F, Diskin R, Scheid JF, Gaebler C, Mouquet H, Georgiev IS, Pancera M, Zhou T, Incesu RB, Fu BZ, Gnanapragasam PN, Oliveira TY, Seaman MS, Kwong PD, Bjorkman PJ, Nussenzweig MC. 2013. Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell 153:126–138. doi: 10.1016/j.cell.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S, Godzik A, Kaufmann DE, McElrath MJ, Nussenzweig MC, Pulendran B, Scanlan CN, Schief WR, Silvestri G, Streeck H, Walker BD, Walker LM, Ward AB, Wilson IA, Wyatt R. 2012. A blueprint for HIV vaccine discovery. Cell Host Microbe 12:396–407. doi: 10.1016/j.chom.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pejchal R, Doores KJ, Walker LM, Khayat R, Huang PS, Wang SK, Stanfield RL, Julien JP, Ramos A, Crispin M, Depetris R, Katpally U, Marozsan A, Cupo A, Maloveste S, Liu Y, McBride R, Ito Y, Sanders RW, Ogohara C, Paulson JC, Feizi T, Scanlan CN, Wong CH, Moore JP, Olson WC, Ward AB, Poignard P, Schief WR, Burton DR, Wilson IA. 2011. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science 334:1097–1103. doi: 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walker LM, Phogat SK, Chan-Hui PY, Wanger D, Phung P, Goss JL, Wring T, Simek MD, Fling S, Mitchem JL, Lehman JK, Priddy RH, Olsen OA, Frey SM, Hammond PW, Protocol G Principal Investigators, Mitro G, Serwanga J, Pozniak A, McPhee D, Manigart O, Mwananyanda L, Karita E, Inwoley A, Jacko W, Dehovitz J, Bekker LG, Pitisuttithum P, Paris R, Allen S, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julian JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong CH, Phogat S, Wrin T, Simek MD, Protocol G Principal Investigators, Koff WC, Wilson IA, Burton DR, Poignard P. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kong L, Lee JH, Doores KJ, Murin CD, Julien JP, McBride R, Liu Y, Marozsan A, Cupo A, Klasse PJ, Hoffenberg S, Caulfield M, King CR, Hua Y, Le KM, Khayat R, Deller MC, Clayton T, Tien H, Feizi T, Sanders RW, Paulson JC, Moore JP, Stanfield RL, Burton DR, Ward AB, Wilson IA. 2013. Supersite of immune vulnerability on the glycosylated face of HIV-1 envelope glycoprotein gp120. Nat Struct Mol Biol 20:796–803. doi: 10.1038/nsmb.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doores KJ, Fulton Z, Huber M, Wilson IA, Burton DR. 2010. Antibody 2G12 recognizes di-mannose equivalently in domain- and nondomain-exchanged forms but only binds the HIV-1 glycan shield if domain exchanged. J Virol 84:10690–10699. doi: 10.1128/JVI.01110-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calarese DA, Scanlan CN, Zwick MB, Deechongkit S, Mimura Y, Kunert R, Zhu P, Wormald MR, Stanfield RL, Rous KH, Kelly JW, Rudd PM, Dwek RA, Katinger H, Burton DR, Wilson IA. 2003. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science 300:2065–2071. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 58.McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, O'Dell S, Patel N, Shahzad-ul Hussan S, Yang Y, Zhang B, Zhou T, Zhu J, Boyington JC, Chuang GY, Diwanji D, Georgiev I, Kwon YD, Lee D, Louder MK, Moquin S, Schmidt SD, Yang ZY, Bonsignori M, Crump JA, Kapiga J, Bewley CA, Mascola JR, Nabel GJ, Schief WR, Ward AB, Wilson IA, Kwong PD. 2011. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature 480:336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liao HX, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, Kozink DM, Hwang KK, Chen X, Tsao CY, Liu P, Lu X, Parks RJ, Montefiori DC, Ferrari G, Pollara J, Rao M, Peachman KK, Santra S, Letvin NL, Karasavvas N, Yang ZY, Dai K, Pancera M, Gorman J, Wiehe K, Nicely NI, Rerks-Ngarm S, Nitayaphan S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Sinangil F, Kim JH, Michael NL, Kepler TB, Kwong PD, Mascola JR, Nabel GJ, Pinter A, Zolla-Pazner S, Haynes BF. 2013. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity 38(1):176–186. doi: 10.1016/j.immuni.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walker BD, Ahmed R, Plotkin S. 2011. Moving ahead an HIV vaccine: use both arms to beat HIV. Nat Med 17:1194–1195. doi: 10.1038/nm.2529. [DOI] [PubMed] [Google Scholar]
- 61.Hoxie JA. 2010. Toward an antibody-based HIV-1 vaccine. Annu Rev Med 61:135–152. doi: 10.1146/annurev.med.60.042507.164323. [DOI] [PubMed] [Google Scholar]
- 62.Haynes BT, McElrath MJ. 2013. Progress in HIV-1 vaccine development. Curr Opin HIV AIDS 8:326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haim H, Strack B, Kassa A, Madani N, Wang L, Courter JR, Princiotto A, McGee K, Pacheco B, Seaman MS, Smith AB III, Sodroski J. 2011. Contribution of intrinsic reactivity of the HIV-1 envelope glycoproteins to CD4-independent infection and global inhibitor sensitivity. PLoS Pathog 7:e1002101. doi: 10.1371/journal.ppat.1002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A 105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ping LH, Joseph SB, Anderson JA, Abrahams MR, Salazar-Gonzalez JF, Kincer LP, Treurnicht FK, Arney L, Ojeda S, Zhang M, Keys J, Potter EL, Chu H, Moore P, Salazar M, Iyer S, Jabara C, Kirchherr J, Mapanje C, Ngandu N, Seoighe C, Hoffman I, Gao F, Tang Y, Labranche C, Lee B, Savile A, Vermeulen M, Fiscus S, Morris L, Karim SA, Haynes BF, Shaw GM, Korber BT, Hahn BH, Cohen MS, Montefiori D, Williamson C, Swanstrom R, CAPRISA Acute Infection Study and the Center for HIV-AIDS Vaccine Immunology Consortium. 2013. Comparison of viral Env proteins from acute and chronic infections with subtype C human immunodeficiency virus type 1 identifies differences in glycosylation and CCR5 utilization and suggests a new strategy for immunogen design. J Virol 87:7218–7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guttman M, Cupo A, Julien JP, Sanders RW, Wilson IA, Moore JP, Lee KK. 2015. Antibody potency relates to the ability to recognize the closed pre-fusion form of HIV Env. Nat Commun 6:6144. doi: 10.1038/ncomms7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu J, Bartesaghi A, Borgnia MJ, Sapiro G, Subramaniam S. 2008. Molecular architecture of native HIV-1 gp120 trimers. Nature 455:109–113. doi: 10.1038/nature07159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.White TA, Bartesaghi A, Borgnia MJ, Myerson JR, de la Cruz MJ, Bess JW, Nandwani R, Hoxie JA, Lifson JD, Milne JL, Subramaniam S. 2010. Molecular architectures of trimeric SIV and HIV-1 envelope glycoprotein on intact viruses: Strain-dependent variation in quaternary structure. PLoS Pathog 5:e1001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rizzuto C, Wyatt R, Hernandez-Ramos N, Sun Y, Kwong PD, Hendrickson WA, Sodroski J. 1998. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science 280:1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- 71.Huang CC, Tang M, Zhang M-Y, Majeed S, Montabana E, Stanfield RL, Dimitrov D, Korber B, Sodroski J, Wilson IA, Wyatt R, Kwong PD. 2005. Structure of a V3-containing HIV-1 gp120 core. Science 310:1025–1028. doi: 10.1126/science.1118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang CC, Lam SN, Acharya P, Tang M, Xiang S-H, Shahzad-ul Hassan S, Stanfield RL, Robinson J, Sodroski J, Wilson IA, Wyatt R, Bewley CA, Kwong PD. 2007. Structures of the CCR5 N terminus and of a tyrosine-sulfated antibody with HIV-1 gp120 and CD4. Science 317:1930–1934. doi: 10.1126/science.1145373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thali M, Moore JP, Furman C, Charles M, Ho DD, Robinson J, Sodroski J. 1993. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol 67:3978–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xiang SH, Doka N, Choudhary RK, Sodroski J, Robinson JE. 2002. Characterization of CD4-induced epitopes on the HIV type 1 gp120 envelope glycoprotein recognized by neutralizing human monoclonal antibodies. AIDS Res Hum Retrovir 18:1207–1217. doi: 10.1089/08892220260387959. [DOI] [PubMed] [Google Scholar]
- 75.Xiang SH, Wang L, Abreu M, Huang C-C, Kwong PD, Rosenberg E, Robinson JE, Sodroski J. 2003. Epitope mapping and characterization of a novel CD4-induced human monoclonal antibody capable of neutralizing primary HIV-1 strains. Virology 315:124–134. doi: 10.1016/S0042-6822(03)00521-X. [DOI] [PubMed] [Google Scholar]
- 76.Decker JM, Bibollet-Ruche F, Wei X, Wang S, Levy DN, Wang W, Delaporte E, Peeters M, Derdeyn CA, Allen S, Hunter E, Saag MS, Hoxie JA, Hahn BH, Kwong PD, Robinson JE, Shaw GM. 2005. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J Exp Med 201:1407–1419. doi: 10.1084/jem.20042510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davis KL, Bibollet-Ruche F, Li H, Decker JM, Kutsch O, Morris L, Salomon A, Pinter A, Hoxie JA, Hahn BH, Kwong PD, Shaw GM. 2009. Human immunodeficiency virus type 2 (HIV-2)/HIV-1 envelope chimeras detect high titers of broadly reactive HIV-1 V3-specific antibodies in human plasma. J Virol 83:1240–1259. doi: 10.1128/JVI.01743-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davis KL, Gray ES, Moore PL, Decker JM, Salomon A, Montefiori DC, Graham BS, Keefer MC, Pinter A, Morris L, Hahn BH, Shaw GM. 2009. High titer HIV-1 V3-specific antibodies with broad reactivity but low neutralizing potency in acute infection and following vaccination. Virology 387:414–426. doi: 10.1016/j.virol.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Forsell MN, Dey B, Mörner A, Svehla K, O'Dell S, Högerkorp CM, Voss G, Thorstensson R, Shaw GM, Mascola JR, Karlsson Hedestam GB, Wyatt RT. 2008. B cell recognition of the conserved HIV-1 co-receptor binding site is altered by endogenous primate CD4. PLoS Pathog 4:e1000171. doi: 10.1371/journal.ppat.1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Forsell MN, McKee K, Feng Y, Mascola JR, Wyatt RT. 2014. HIV-1 envelope glycoprotein trimer immunogenicity elicited in the presence of human CD4 alters the neutralization profile. AIDS Res Hum Retrovir 30:1089–1098. doi: 10.1089/aid.2014.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Douagi I, Forsell MN, Sundling C, O'Dell S, Feng Y, Dosenovic P, Li Y, Seder R, Loré K, Mascola JR, Wyatt RT, Karlsson Hedestam GB. 2010. Influence of novel CD4 binding-defective HIV-1 envelope glycoprotein immunogens on neutralizing antibody and T-cell responses in nonhuman primates. J Virol 84:1683–1695. doi: 10.1128/JVI.01896-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mörner A, Douagi I, Forsell MN, Sundling C, Dosenovic P, O'Dell Dey B, Kwong PD, Voss G, Thorstensson R, Mascola JR, Wyatt RT, Karlsson Hedestam GB. 2009. Human immunodeficiency virus type 1 env trimer immunization of macaques and impact of priming with viral vector or stabilized core protein. J Virol 83:540–541. doi: 10.1128/JVI.01102-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dey B, Pancera M, Svehla K, Shu Y, Xiang SH, Vainshtein J, Li Y, Sodroski J, Kwong PD, Mascola JR, Wyatt R. 2007. Characterization of human immunodeficiency virus type 1 monomeric and trimeric gp120 glycoproteins stabilized in the CD4-bound state: antigenicity, biophysics, and immunogenicity. J Virol 81:5579–5593. doi: 10.1128/JVI.02500-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dey B, Svehla K, Xu L, Wycuff D, Zhou T, Voss G, Phogat A, Chakrabarti BK, Li Y, Shaw G, Kwong PD, Nabel GJ, Mascola JR, Wyatt RT. 2009. Structure-based stabilization of HIV-1 gp120 enhances humoral immune responses to the induced co-receptor binding site. PLoS Pathog 5:e1000445. doi: 10.1371/journal.ppat.1000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Labrijn A, Poignard P, Raja A, Zwick MB, Delgado K, Franti M, Binley J, Vivona V, Grundner C, Huang C-C, Venturi M, Petropoulos C, Wrin T, Dimitrov D, Robinson J, Kwong PD, Wyatt RT, Sodroski J, Burton D. 2003. Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J Virol 77:10557–10565. doi: 10.1128/JVI.77.19.10557-10565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sullivan N, Sun Y, Sattentau S, Thali M, Wu D, Denisova G, Gershoni J, Robinson J, Moore J, Sodroski J. 1998. CD4-induced conformational changes in the human immunodeficiency virus type 1 gp120 glycoprotein: consequences for virus entry and neutralization. J Virol 72:4694–4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao Q, Ma L, Jiang S, Lu H, Liu S, He Y, Strick N, Neamati N, Debnath AK. 2005. Identification of N-phenyl-N′-(2,2,6,6-tetramethyl-piperidin-4-yl)-oxalamides as a new class of HIV-1 entry inhibitors that prevent gp120 binding to CD4. Virology 339:213–225. doi: 10.1016/j.virol.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 88.Madani N, Schön A, Princiotto AM, LaLonde JM, Courter JR, Soeta T, Ng D, Wang L, Brower ET, Xiang SH, Kwon YD, Huang CC, Wyatt R, Kwong PD, Freire E, Smith AB III, Sodroski J. 2008. Small-molecule CD4 mimics interact with a highly conserved pocket on HIV-1 gp120. Structure 16:1689–1701. doi: 10.1016/j.str.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schön A, Madani N, Klein JC, Hubicki A, Ng D, Yang X, Smith AB III, Sodroski J, Freire E. 2006. Thermodynamics of binding of a low-molecular-weight CD4 mimetic to HIV-1 gp120. Biochemistry 45:10973–10980. doi: 10.1021/bi061193r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Curreli F, Kwon YD, Zhang H, Yang Y, Scacalossi D, Kwong PD, Debnath AK. 2014. Binding mode characterization of NBD series CD4-mimetic HIV-1 entry inhibitors by X-ray structure and resistance study. Antimicrob Agents Chemother 58:5478–5491. doi: 10.1128/AAC.03339-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kwon YD, LaLonde JM, Yang Y, Elban MA, Sugawara A, Courter JR, Jones DM, Smith AB III, Debnath AK, Kwong PD. 2014. Crystal structures of HIV-1 gp120 envelope glycoprotein in complex with NBD analogues that target the CD4-binding site. PLoS One 9:e85940. doi: 10.1371/journal.pone.0085940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.LaLonde JM, Elban MA, Courter JR, Sugawara A, Soeta T, Madani N, Princiotto AM, Kwon YD, Kwong PD, Schön A, Freire E, Sodroski J, Smith AB III. 2011. Design, synthesis, and biological evaluation of small molecule inhibitors of CD4-gp120 binding based on virtual screening. Bioorg Med Chem 19:91–101. doi: 10.1016/j.bmc.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.LaLonde JM, Kwon YD, Jones DM, Sun AW, Courter JR, Soeta T, Kobayashi T, Princiotto AM, Wu X, Schön A, Freire E, Kwong PD, Mascola JR, Sodroski J, Madani N, Smith AB III. 2012. Structure-based design, synthesis, and characterization of dual hotspot small-molecule HIV-1 entry inhibitors. J Med Chem 55:4382–4396. doi: 10.1021/jm300265j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lalonde JM, Le-Khac M, Jones DM, Courter JR, Park J, Schön A, Princiotto AM, Wu X, Mascola JR, Freire E, Sodroski J, Madani N, Hendrickson WA, Smith AB III. 2013. Structure-based design and synthesis of an HIV-1 entry inhibitor exploiting X-ray and thermodynamic characterization. ACS Med Chem Lett 4:338–343. doi: 10.1021/ml300407y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Melillo B, Liang S, Park J, Schön A, Courter JR, LaLonde JM, Wendler DJ, Princiotto AM, Seaman MS, Freire E, Sodroski J, Madani N, Hendrickson WA, Smith AB III. 2016. Small-molecule CD4-mimics: Structure-based optimization of HIV-1 entry inhibition. ACS Med Chem Lett 7:330–334. doi: 10.1021/acsmedchemlett.5b00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Madani N, Princiotto AM, Schön A, LaLonde J, Feng Y, Freire E, Park J, Courter JR, Jones DM, Robinson J, Liao H-X, Moody MA, Permar S, Haynes B, Smith AB III, Wyatt R, Sodroski J. 2014. CD4-mimetic small molecules sensitize human immunodeficiency virus (HIV-1) to vaccine-elicited antibodies. J Virol 88:6542–6555. doi: 10.1128/JVI.00540-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rho HM, Poiesz B, Ruscetti FW, Gallo RC. 1981. Characterization of the reverse transcriptase from a new retrovirus (HTLV) produced by a human cutaneous T-cell lymphoma cell line. Virology 112:355–360. doi: 10.1016/0042-6822(81)90642-5. [DOI] [PubMed] [Google Scholar]
- 98.Xiang SH, Kwong PD, Gupta R, Rizzuto CD, Casper DJ, Wyatt R, Wang L, Hendrickson WA, Doyle ML, Sodroski J. 2002. Mutagenic stabilization/disruption of a CD4-bound state reveals distinct conformations of the human immunodeficiency virus (HIV-1) gp120 envelope glycoprotein. J Virol 76:9888–9899. doi: 10.1128/JVI.76.19.9888-9899.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wiehe K, Easterhoff D, Luo K, Nicely NI, Bradley T, Jaeger FH, Dennison SM, Zhang R, Lloyd KE, Stolarchuk C, Parks R, Sutherland LL, Scearce RM, Morris L, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Sinangil F, Phogat S, Michael NL, Kim JH, Kelsoe G, Montefiori DC, Tomaras GD, Bonsignori M, Santra S, Kepler TB, Alam SM, Moody MA, Liao HX, Haynes BF. 2014. Antibody light-chain-restricted recognition of the site of immune pressure in the RV144 HIV-1 vaccine trial is phylogenetically conserved. Immunity 41:909–918. doi: 10.1016/j.immuni.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bonsignori M, Hwang KK, Chen X, Tsao CY, Morris L, Gray E, Marshall DJ, Crump JA, Kapiga SH, Sam NE, Sinangil F, Pancera M, Yongping Y, Zhang B, Zhu J, Kwong PD, O'Dell S, Mascola JR, Wu L, Nabel GJ, Phogat S, Seaman MS, Whitesides JF, Moody MA, Kelsoe G, Yang X, Sodroski J, Shaw GM, Montefiori DC, Kepler TB, Tomaras GD, Alam SM, Liao HX, Haynes BF. 2011. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J Virol 85:9998–10009. doi: 10.1128/JVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pitisuttithum P, Rerks-Ngarm S, Bussaratid V, Dhitavat J, Maekanantawat W, Pungpak S, Suntharasamai P, Vanijanonta S, Nitayapan S, Kaewkungwal J, Benenson M, Morgan P, O'Connell RJ, Berenberg J, Gurunathan S, Francis DP, Paris R, Chiu J, Stablein D, Michael NL, Excler JL, Robb ML, Kim JH. 2011. Safety and reactogenicity of canarypox ALVAC-HIV (vCP1521) and HIV-1 gp120 AIDSVAX B/E vaccination in an efficacy trial in Thailand. PLoS One 6:e27837. doi: 10.1371/journal.pone.0027837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gray ES, Madiga MC, Moore PL, Mlisana K, Abdool Karim SS, Binley JM, Shaw GM, Mascola JR, Morris L. 2009. Broad neutralization of human immunodeficiency virus type 1 mediated by plasma antibodies against the gp41 membrane proximal external region. J Virol 83:11265–11274. doi: 10.1128/JVI.01359-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liao HX, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, Zhu J, Shapiro L, Program NCS, Mullikin JC, Gnanakaran S, Hraber P, Wiehe K, Kelsoe G, Yang G, Xia SM, Montefiori DC, Parks R, Lloyd KE, Scearce RM, Soderberg KA, Cohen M, Kamanga G, Louder MK, Tran LM, Chen Y, Cai F, Chen S, Moquin S, Du X, Joyce MG, Srivatsan S, Zhang B, Zheng A, Shaw GM, Hahn BH, Kepler TB, Korber BT, Kwong PD, Mascola JR, Haynes BF. 2013. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature 496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sundling C, Forsell M, O'Dell S, Feng Y, Chakralarti B, Rao S, Lore K, Mascola JR, Wyatt R, Douagi I, Karlsson Hedestam GB. 2010. Soluble HIV-1 Env trimers in adjuvant elicit potent and diverse functional B cell responses in primates. J Exp Med 207:2003–2017. doi: 10.1084/jem.20100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Phad GE, Vázquez Bernat N, Feng Y, Ingale J, Martinez Murillo PA, O'Dell S, Li Y, Mascola JR, Sundling C, Wyatt RT, Karlsson Hedestam GB. 2015. Diverse antibody genetic and recognition properties revealed following HIV-1 envelope glycoprotein immunization. J Immunol 194:5903–5914. doi: 10.4049/jimmunol.1500122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Montefiori DC, Karnasuta C, Huang Y, Ahmed H, Gilbert P, de Souza MS, McLinden R, Tovanabutra S, Laurence-Chenine A, Sanders-Buell E, Moody MA, Bonsignori M, Ochsenbauer C, Kappes J, Tang H, Greene K, Gao H, LaBranche CC, Andrews C, Polonis VR, Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Self SG, Berman PW, Francis D, Sinangil F, Lee C, Tartaglia J, Robb ML, Haynes BF, Michael NL, Kim JH. 2012. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J Infect Dis 206:431–441. doi: 10.1093/infdis/jis367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gorny MK, Pan R, Williams C, Wang XH, Volsky B, O'Neal T, Spurrier B, Sampson JM, Li L, Seaman MS, Kong XP, Zolla-Pazner S. 2012. Functional and immunochemical cross-reactivity of V2-specific monoclonal antibodies from HIV-1-infected individuals. Virology 427:198–207. doi: 10.1016/j.virol.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pan R, Gorny MK, Zolla-Pazner S, Kong XP. 2015. The V1V2 region of HIV-1 gp120 forms a five-stranded beta barrel. J Virol 89:8003–8010. doi: 10.1128/JVI.00754-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Seaman MS, Janes H, Hawkins N, Grandpre LE, Devoy C, Giri A, Coffey RT, Harris L, Wood B, Daniels MG, Bhattacharya T, Lapedes A, Polonis VR, McCutchan FE, Gilbert PB, Self SG, Korber BT, Montefiori DC, Mascola JR. 2010. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J Virol 84:1439–1452. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mascola JR, D'Souza P, Gilbert P, Hahn BH, Haigwood NL, Morris L, Petropoulos CJ, Polonis VR, Sarzotti M, Montefiori DC. 2005. Recommendations for the design and use of standard virus panels to assess neutralizing antibody responses elicited by candidate human immunodeficiency virus type 1 vaccines. J Virol 79:10103–10107. doi: 10.1128/JVI.79.16.10103-10107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Foley B, Leitner T, Apetrei C, Hahn B, Mizrachi I, Mullins J, Rambaut A, Wolinsky S, Korber B (ed). 2013. HIV sequence compendium. LA-UR-13–26007. Los Alamos National Laboratory, Theoretical Biology and Biophysics, Los Alamos, NM. [Google Scholar]
- 112.Richard J, Veillette M, Brassard N, Iyer SS, Roger M, Martin L, Pazgier M, Schön A, Freire E, Routy J-P, Smith AB III, Park J, Jones DM, Courter JR, Melillo BN, Kaufmann DE, Hahn BH, Permar SR, Haynes BF, Madani N, Sodroski JG, Finzi A. 2015. CD4 mimetics sensitize HIV-1-infected cells to ADCC. Proc Natl Acad Sci U S A 112:E2687–E2694. doi: 10.1073/pnas.1506755112. [DOI] [PMC free article] [PubMed] [Google Scholar]