ABSTRACT
Maternal vaccination to induce anti-HIV immune factors in breast milk is a potential intervention to prevent postnatal HIV-1 mother-to-child transmission (MTCT). We previously demonstrated that immunization of lactating rhesus monkeys with a modified vaccinia Ankara (MVA) prime/intramuscular (i.m.) protein boost regimen induced functional IgG responses in milk, while MVA prime/intranasal (i.n.) boost induced robust milk Env-specific IgA responses. Yet, recent studies have suggested that prevention of postnatal MTCT may require both Env-specific IgA and functional IgG responses in milk. Thus, to investigate whether both responses could be elicited by a combined systemic/mucosal immunization strategy, animals previously immunized with the MVA prime/i.n. boost regimen received an i.n./i.m. combined C.1086 gp120 boost. Remarkably, high-magnitude Env-specific IgA responses were observed in milk, surpassing those in plasma. Furthermore, 29% of vaccine-elicited Env-specific B cells isolated from breast milk were IgA isotype, in stark contrast to the overwhelming predominance of IgG isotype Env-specific B cells in breast milk of chronically HIV-infected women. A clonal relationship was identified between Env-specific blood and breast milk B cells, suggesting trafficking of that cell population between the two compartments. Furthermore, IgA and IgG monoclonal antibodies isolated from Env-specific breast milk B cells demonstrated diverse Env epitope specificities and multiple effector functions, including tier 1 neutralization, antibody-dependent cellular cytotoxicity (ADCC), infected cell binding, and inhibition of viral attachment to epithelial cells. Thus, maternal i.n./i.m. combined immunization is a novel strategy to enhance protective Env-specific IgA in milk, which is subsequently transferred to the infant via breastfeeding.
IMPORTANCE Efforts to increase the availability of antiretroviral therapy to pregnant and breastfeeding women in resource-limited areas have proven remarkably successful at reducing HIV vertical transmission rates. However, more than 200,000 children are infected annually due to failures in therapy implementation, monitoring, and adherence, nearly half by postnatal HIV exposure via maternal breast milk. Intriguingly, in the absence of antiretroviral therapy, only 10% of breastfed infants born to HIV-infected mothers acquire the virus, suggesting the existence of naturally protective immune factors in milk. Enhancement of these protective immune factors through maternal vaccination will be a critical strategy to reduce the global pediatric AIDS epidemic. We have previously demonstrated that a high magnitude of HIV Env-specific IgA in milk correlates with reduced risk of infant HIV acquisition. In this study, we describe a novel HIV vaccine regimen that induces potent IgA responses in milk and therefore could potentially protect against breast milk HIV MTCT.
INTRODUCTION
More than 200,000 new pediatric human immunodeficiency virus (HIV) infections occur annually via mother-to-child transmission (MTCT), nearly half through breastfeeding (1). Antiretroviral (ARV) drugs can dramatically reduce the rate of MTCT, but in areas of high HIV prevalence, acute HIV infection in pregnant and postpartum women as well as poor access and adherence to ARV treatment throughout the breastfeeding period has limited progress in the prevention of breast milk transmission (2). According to UNAIDS in 2014, only 68% of HIV-infected pregnant women in low- and middle-income countries received ARV therapy during pregnancy, and only 61% of those women continued this therapy postpartum (3). Despite the risk of HIV acquisition, breastfeeding is necessary for infant survival in many regions of the world, as breastfed infants have lower rates of diarrheal and respiratory infections (4). It is well established that antibodies are transferred to infants via the placenta and through breast milk consumption (5); thus, maternal immunization could be an important alternative strategy to allow safe breastfeeding in areas of high HIV prevalence.
The specificity and function of antibodies important to prevent mucosal HIV transmission remain unclear. Despite the high level of total IgA in breast milk, the predominant envelope (Env)-specific antibody response in the breast milk of both HIV-infected women and simian immunodeficiency virus (SIV)-infected rhesus monkeys is IgG (6, 7). The presence of high levels of functional IgG in breast milk, particularly IgG mediating antibody-dependent cellular cytotoxicity (ADCC), has been linked to reduced incidence of MTCT (8). Env-specific IgG in breast milk is likely due to transudate from the systemic compartment, since HIV Env-specific IgG responses in breast milk correlate well with those in plasma, though they are lower by two orders of magnitude (6). Interestingly, the majority of Env-specific B cells in the breast milk of chronically HIV-infected women produce IgG and not IgA (9, 10). Indeed, it has traditionally proven to be exceedingly difficult to induce Env-specific IgA or IgA isotype B cells in the mucosal compartment via vaccination (11), and thus the potential role of IgA in preventing viral transmission at the mucosal surface is poorly understood.
The RV144 ALVAC/AIDSVAX vaccine efficacy trial in Thailand, which demonstrated 31.2% efficacy, suggested a deleterious role of plasma Env-specific IgA responses of certain specificities in protection against sexually transmitted HIV infections (12). Further analysis revealed an inverse correlation between ADCC activity and acquisition risk in vaccinees with low IgA responses (12). It was hypothesized that IgA blocks ADCC by competing for the same binding sites as ADCC-mediating IgG antibodies. Indeed, vaccine-elicited IgA specific for conformational epitopes in the C1 region of Env can block ADCC activity in clinical samples (13). Nevertheless, there is solid evidence that mucosal IgA could contribute to the prevention of HIV transmission. In a recent study, vaccine-elicited mucosal IgA responses were associated with protection against vaginal simian-human immunodeficiency virus (SHIV) challenge (14), and multiple investigations have demonstrated that passive rectal immunization with neutralizing dimeric IgA can protect from subsequent SHIV exposure (15, 16). Similarly, we recently reported an association between a high magnitude of Env-specific IgA and low rates of breast milk HIV transmission (17). Thus, Env-specific IgA responses in the systemic and mucosal compartments seem to play dramatically different physiologic roles.
In previous studies, we demonstrated that HIV vaccination can elicit strong Env-specific antibody responses in milk of rhesus monkeys (18). Hormonally induced lactating animals were immunized with the transmitted/founder Env C.1086 using either a gp140 DNA or modified vaccinia Ankara (MVA) prime followed by two C.1086 gp120 protein boosts delivered either systemically (intramuscularly [i.m.]) or mucosally (intranasally [i.n.]). The systemic and mucosal vaccine regimens elicited comparable levels of plasma Env-binding IgG, yet mucosal immunization induced significantly higher Env-binding IgA responses in breast milk. Furthermore, systemic vaccination induced a much more potent functional IgG response (tier 1 neutralization, ADCC) in breast milk than mucosal vaccination. An optimal vaccine regimen for the prevention of breast milk transmission may need to elicit both strong Env-binding and functional antibody responses in milk (19).
Thus, we sought to design a vaccine strategy that could combine the potent functional antibody response observed in the systemically vaccinated animals with the robust Env-specific IgA response in the mucosally vaccinated animals. Animals previously immunized with the C.1086 MVA prime/i.n. boost regimen received an additional simultaneous i.n./i.m. protein boost. Env-binding and functional responses were then assessed in both plasma and breast milk. To examine the antibody responses in more detail, B cells from both blood and milk were isolated, VH/VL genes sequenced, and IgA and IgG monoclonal antibodies (MAbs) analyzed for specificity and associated function. This study provides a detailed understanding of the antibody-mediated immune responses elicited by combined systemic and mucosal vaccination, enabling critical assessment of this maternal vaccination strategy for the prevention of HIV-1 breast milk transmission.
MATERIALS AND METHODS
Animals and vaccine regimen.
Recombinant MVA expressing the C.1086 Env gp140 gene and recombinant Env gp120 glycoproteins was generated as described previously (18). Lactation was induced in 4 female Indian rhesus monkeys by depot medroxyprogesterone and estradiol injections followed by oral dopamine antagonist administration as described previously (20). The lactating monkeys were initially primed with 109 PFU of recombinant MVA (rMVA) expressing the HIV C.1086 Env gene and boosted twice intranasally (i.n.) at weeks 12 and 16 with 200 μg of HIV C.1086 gp120 protein (100 μg in each nostril) adjuvanted with the Toll-like receptor 7/8 (TLR7/8) agonist R848 (500 μg/animal) (Fig. 1). The i.n. boosted animals were immunized a third time with a combined i.n./i.m. boost technique at week 50 following the original MVA prime. The i.n. component of the combined boost was identical to the previous i.n. boosts (200 μg HIV Env C.1086 gp120 plus 500 μg R848). The intramuscular (i.m.) component of the combined boost consisted of 100 μg HIV Env C.1086 gp120 and 250 μl of the adjuvant STR8S-C (squalene-containing STS base adjuvant plus R848 plus oCpGs) (21). i.n. protein boosts were administered to anesthetized monkeys placed on their backs in 30-μl aliquots at 30-s intervals for a total volume of 150 μl per nostril (22). Between aliquot administrations, the nares were held closed. The i.m. protein boost was administered at a single injection site in the quadriceps of anesthetized monkeys. Blood samples were collected weekly for 4 weeks following immunization and then bimonthly for an additional 1 to 2 months. Milk was collected twice weekly by manual collection (20) and then separated into cellular, supernatant, and lipid fractions by centrifugation (7, 23). Animals were maintained according to the Guide for the Care and Use of Laboratory Animals (grants.nih.gov), and the animal protocol was approved by the Duke University Animal Care and Use Committee.
FIG 1.
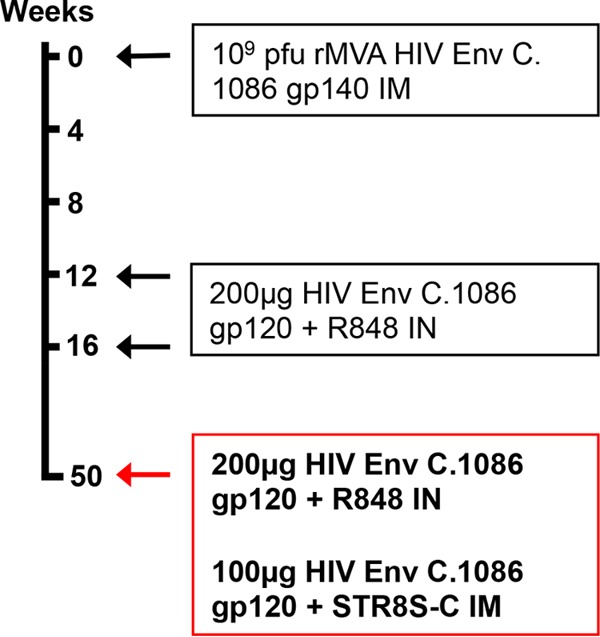
Combined i.n./i.m. HIV-1 Env vaccine regimen. Lactation was hormonally induced in four female rhesus macaques prior to weeks 0, 12, and 50 so that milk samples could be collected at each time point. Animals were primed with recombinant MVA expressing HIV Env C.1086 gp140 and then boosted twice with HIV Env C.1086 gp120 i.n. with R848 adjuvant. The animals were then boosted a third time using a combined i.n./i.m. Env boost with C.1086 gp120 i.n. plus R848 adjuvant as well as C.1086 gp120 i.m. plus STR8S-C adjuvant.
Plasma, milk, and MAb HIV-1 Env recombinant protein ELISA.
Env-binding IgG and IgA were assessed for plasma/milk (18) and for monoclonal antibodies (MAbs) (24). For plasma/milk assays, 384-well enzyme-linked immunosorbent assay (ELISA) plates were coated overnight with HIV C.1086 gp120 (30 ng/well) and then blocked with the assay diluent (phosphate-buffered saline [PBS] containing 4% whey, 15% normal goat serum, and 0.5% Tween 20). Dilutions of plasma or milk were then added to the plates. Antibodies were detected with a horseradish peroxidase (HRP)-conjugated polyclonal goat anti-monkey IgG (Rockland, Gilbertsville, PA) or IgA (Rockland, Gilbertsville, PA) and the addition of the ABTS-2 peroxidase substrate system (KPL, Gaithersburg, MD). Macaca mulatta purified IgG and IgA (Nonhuman Primate Reagent Resource) were used to develop standard curves, and the concentration of IgG or IgA antibody was calculated relative to the standard using a 5-parameter fit curve (WorkOut 2.5; PerkinElmer, Waltham, MA). For recombinant MAb ELISA, 384-well ELISA plates (Corning Life Sciences, Lowell, MA) were coated with each antigen at 30 ng/well. Antigens included C.1086 gp120 D7 and A1.con env03 gp140 (12), B.con env03 gp140 (12), C.con env03 gp140 (12), AE.A244 gD gp120 (12), C.1086 V1V2, C.1086 V2, C.1086 V1V2 N156Q, and gp70 B.CaseA2 V1V2 (18), gp70 B.CaseA2 V1V2/169K (18), C.con env03.V3 linear peptide and BioRV144 C1 peptide (13), C.YU2 core, C.YU2 core D368R, RSC3 (25), RSC3 d371/P363N (25), and C.1086 D7 gp120 K160N, ConC gp120, and ConC gp120 N332A. Serial dilutions of monoclonal antibody were distributed to the plates after blocking, and the MAbs were detected using a peroxidase-conjugated goat anti-monkey IgG (Rockland, Gilbertsville, PA) and the SureBlue Reserve tetramethylbenzidine (TMB) peroxidase substrate (KPL, Gaithersburg, MD). For monoclonal antibodies, the 50% effective concentration (EC50) was calculated by the concentration of antibody which resulted in a 50% reduction in optical density (OD) from the maximum value.
Quantitative SIgA ELISA.
ELISA plates (Corning Life Sciences, Lowell, MA) were coated overnight at 4°C with Con6 gp120 B. Dilutions of breast milk were added to the plate following blocking. The antibodies were detected using anti-secretory-chain IgA Ab SC 9H7 CL3 (a mouse-derived monoclonal IgG2A kappa antibody which cross-reacts with rhesus and human secretory components [SCs] though does not react with dimeric IgA alone) (kindly provided by Barton Haynes), followed by an HRP-conjugated polyclonal anti-mouse IgG (Promega, Madison, WI) and then TMB peroxidase substrate. The standard used was b12 secretory IgA (SIgA) prepared by complexing b12 dimeric IgA (dIgA) (NHP reagent program), with rhesus SC followed by an overnight incubation, resulting in a 1:1 molar ratio of dIgA to SC. The concentration of HIV-1 Env-specific SIgA antibody was calculated relative to the standard curve using a 5-parameter fit curve (WorkOut 2.5; PerkinElmer, Waltham, MA). A positive result was defined as the mean OD of preimmune milk samples plus 3 standard deviations (SD). The limit of detection (LOD) in this assay was determined to be 100 ng/ml based upon the lowest concentration of the dIgA-SC standard with a corresponding OD that exceeded 3 times that of the blank wells.
sCD4 and A32 blocking ELISA.
Assessment of antibody specificity for the CD4bs and C1 conformational epitopes was done as described previously (24). Briefly, 384-well plates were coated overnight with C.1086 gp120 (30 ng/well) and then blocked before addition of serially diluted MAbs. For the soluble CD4 (sCD4) blocking ELISA, sCD4 (a gift from Bing Chen) was next added to the plates (6.4 ng/well), followed by addition of a biotinylated anti-CD4 (0.3 ng/well). For the A32 blocking ELISA, biotinylated A32 antibody was added (0.625 ng/well). In both assays, HRP-conjugated streptavidin (at a 1:30,000 dilution) and SureBlue Reserve TMB peroxidase substrate were used to detect the biotinylated antibodies. Background was normalized using negative-control wells that received only assay diluent in place of primary monoclonal antibody. Percent inhibition was calculated as follows: 100 − (serum triplicate mean/no-inhibition control mean) × 100. A reduction of absorbance by >50% by a MAb indicated blocking of sCD4 binding to gp120 or blocking of A32 MAb binding to the C1 region (26).
TNC ELISA.
A 384-well plate was coated with 2 μg/ml of anti-tenascin C (anti-TNC) antibody rabbit polyclonal IgG (H-300; Santa Cruz Biotechnology) and incubated at 4°C overnight. Wells were washed with PBS plus 0.1% Tween 20 and blocked with 7.5% bovine serum albumin (BSA) (Gibco) at room temperature for 1 h. Milk samples delipidized by centrifugation at 21,000 × g were diluted in 7.5% BSA, and a commercially available purified TNC (Millipore) was used as the protein standard, ranging from 5 μg/ml to 5 ng/ml. The standards and samples were added to the plate and incubated for 1 h at room temperature. Plates were washed 2 times, and 1 μg/ml of tenascin C mouse monoclonal antibody (T2H5; Fisher) was added and incubated for 1 h at room temperature. Plates were washed 2 times, and a 1:10,000 dilution of goat anti-mouse HRP-conjugated antibody (Promega) was added to each well and incubated for 1 h at room temperature. Plates were washed 4 times, and SureBlue Reserve TMB microwell substrate (KPL) was added and incubated in the dark at room temperature for 5 min. TMB stop solution (KPL) was added, and plates were read at 450 nm.
Peptide array serum specificity mapping.
Serum epitope mapping of heterologous strains was performed as described previously with minor modifications (27, 28). In short, a peptide library of overlapping peptides (15-mers overlapping by 12), covering 7 full-length HIV-1 gp160 Env consensus sequences (clades A, B, C, and D, group M, CRF1, and CRF2) and 6 vaccine and laboratory strain gp120 sequences (A244_1, TH023_1, MN_B, 1086_C, TV1_C, and ZM651_C), was printed onto epoxy glass slides (provided by JPT Peptide Technologies GmbH [Germany]). Sequences of all peptides in the library have been previously reported (29). Microarray binding was performed using the HS4800 Pro Hybridization Station (Tecan, Männedorf, Switzerland). All arrays were blocked with blocking buffer (PBS, 1% milk, 5% normal goat serum [NGS], 0.05% Tween 20) for 1 h at 30°C, followed by a 2-h incubation at 30°C with serum diluted 1:50 in blocking buffer. Arrays were subsequently incubated for 45 min at 30°C with goat anti-human IgG conjugated with AF647 (Jackson ImmunoResearch, PA) (0.75 μg/ml final concentration) diluted with blocking buffer. Washes between all steps were with PBS containing 0.1% Tween 20. Arrays were scanned at a wavelength of 635 nm using an Axon Genepix 4300 scanner (Molecular Devices, Sunnyvale, CA, USA) at a photomultiplier tube (PMT) setting of 580 and 100% laser power. Images were analyzed using Genepix Pro 7 software (Molecular Devices). The intensity of binding of the postimmunization serum to each peptide was corrected with its own background value, which was defined as the median signal intensity of the prebleed serum for that peptide plus 3 times the standard errors among the 3 subarray replicates present on each slide.
Neutralization.
Neutralization of clade C tier 1 (C.MW965) and autologous tier 2 (C.1086) HIV-1 pseudovirus variants by plasma, milk, and isolated MAbs was measured in TZM-bl cells via a reduction in luciferase reporter gene expression (30). In brief, dilutions of plasma, milk, or MAbs were incubated with an optimized amount of virus for 45 min at 37°C in a 96-well plate, and then freshly trypsinized TZM-bl cells in growth medium were added to each well. Following a subsequent 48-h incubation, the culture medium was removed and replaced with a luciferase reagent (Bright-Glo; Promega), causing cell lysis and luminescence proportional to the amount of infection. The luminescence was measured using a Victor 2 luminometer (PerkinElmer). The 50% inhibitory dilution (ID50) titers were calculated as the dilution that resulted in a 50% reduction in relative luminescence units (RLU) compared to virus control wells. Likewise, the 50% inhibitory concentration (IC50) values were calculated as the concentration of antibody which caused a 50% reduction in RLU (31). MAbs that mediated tier 1 virus neutralization were further screened against a multiclade panel of tier 1 viruses B.BaL.26, B.MN.3, and B.SF162.
ADCC.
The ADCC activity of plasma, delipidized breast milk, purified IgG, and MAbs was assessed using the GranToxiLux (GTL) assay (32). Briefly, CEM.NKRCCR5 cells (NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH; from Alexandra Trkola) (33) were used as targets after coating with recombinant C.1086 gp120 protein (5 μg/ml). Cryopreserved human peripheral blood mononuclear cells (PBMC) from an HIV-seronegative donor with the heterozygous 158F/V genotype for Fc-gamma receptor IIIa were used as the source of the effector cells (34). Serial dilutions of plasma, milk, purified IgG, and MAbs were tested. The maximum percent granzyme B (GzB) activity was defined as the peak proportion of cells positive for proteolytically active GzB out of the total viable target cell population. The final results are expressed after subtraction of the background percent GzB activity observed in wells containing effector and target cells in the absence of antibodies. ADCC endpoint titers and concentrations were determined by interpolating the dilutions of plasma and breast milk, or the concentrations of purified antibodies, that intersect the positive cutoff using GraphPad Prism software version 6.0f (GraphPad Software, Inc., La Jolla, CA).
ADCC inhibition.
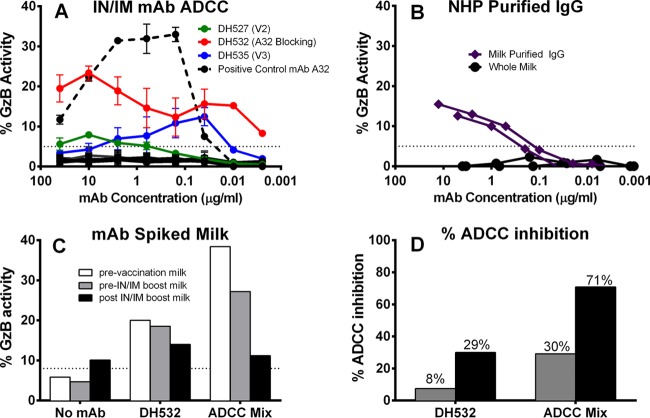
For studies with spiked breast milk (see Fig. 8), a 1:16 dilution of breast milk was spiked with previously determined optimal ADCC concentrations of either DH532 (10 μg/ml) or a mixture of ADCC-mediating antibodies (A32, 2G12, 7B2, and CH44 at 15 ng/ml). The results are reported as ADCC activity (percent granzyme B activity) and percent ADCC inhibition, calculated as the percentage of ADCC activity reduction based on the ADCC mediated by the IgG MAb or MAb mixture in prevaccination milk.
FIG 8.
The ADCC effector function of MAbs is partially inhibited in milk following i.n./i.m. immunization. (A) Three MAbs were determined to mediate ADCC. DH527 (originally IgA, here made with a IgG backbone) has V2 specificity, DH532 is A32 blocking (C1 conformational), and DH535 is V3 specific. (B) Purified IgG from breast milk of animals A6E042 and A6E088 mediates a low level of ADCC activity exceeding that of whole milk from those same animals. (C) ADCC activity of ADCC-mediating monoclonal antibodies spiked into post-i.n./i.m. immunization milk is partially inhibited. (D) ADCC inhibition quantified by the percent change from MAbs spiked into prevaccination milk. The dotted lines indicate the threshold for positivity. Two experimental replicates were completed for panel A; panels B to D represent only a single experiment due to sample limitation.
Isolation of C.1086 Env-specific B cells.
The C.1086 Env-specific B cell population was identified in blood, breast milk, and gastrointestinal (GI) tissue of all 4 monkeys via flow cytometry. Data were collected using the FACSAria2 instrument (BD Biosciences) with the FACSDiVa software and were analyzed by manual gating with FlowJo software. The complete gating strategy for the total B cell population was defined as CD3− CD14− CD16− CD20+. The total numbers of identified B cells from blood and breast milk of each monkey were as follows: A6E030, 81,191 and 1,383; A6E042, 73,258 and 97; A6E088, 107,300 and 248; and AU71S, 34,859 and 14. Env-specific B cells were obtained from B cell population by conjugation of C.1086 Env to dyes AF647 and Pac-Blue (Invitrogen) and selection for double-positive B cells.
MAb genetic characterization and small-scale testing.
Following B cell sorting, the expressed Ig VH and VL genes were amplified by reverse transcription and nested PCR as described previously (35–37). The PCR products were purified and sequenced. Ig isotype was determined by sequence homology. Somatic hypermutation frequencies, inferred V(D)J rearrangement, and CDR3 length were determined from Clonalyst (40). Overlapping PCR was performed to coexpress the variable-region PCR products with full-length IgG1 (heavy chain) and kappa or lambda (light chain) cassettes, and the PCR products were transiently transfected in 293T cells without the need for a cloning step. The supernatants of transfected cells were screened for reactivity against the following HIV Env proteins by small-scale ELISA: C.1086 gp140, C.10868 gp120, M.ConS gp120, B.MN gp120, A.9004SS gp120, M.ConS gp140, A.92RW020 gp140, B.BxB/Bal gp140, and C.CH505TFqODwV3. Six IgA and six IgG antibodies were selected for large-scale production based upon (i) the strength of binding to the immunogen gp120 region (those with higher OD against gp140 construct were excluded) and (ii) broad clade binding specificity. Heavy and light chains for the 12 MAbs selected were cloned into the pcDNA3.1+ mammalian expression vector. The heavy chain for all antibodies, including IgA, included an IgG backbone. Plasmids were transiently transfected into 293F cells, and the antibody was purified using protein A beads as described previously (35).
Epithelial cell binding.
The ability of breast milk Env-specific MAbs to impede infectious virus binding to colonic epithelial cells was assessed (24). In short, colonic HT-29 cells (ATCC) were grown to confluence on a 96-well flat-bottom plate in modified McCoy's 5A medium supplemented with 10% FBS and antibiotics. The HT-29 cells were washed once with serum-free medium and treated with 100 μl of 50 μg/ml mitomycin C for 1 h to prevent further division, followed by two washes. The isolated MAbs then were diluted in serum-free medium, incubated with 10× concentrated HIV pseudovirus MW965 for 1 h at 37°C, added in quadruplicate to the colonic epithelial cell monolayer, and then finally incubated at 37°C for 4 h. Monolayers were washed twice with PBS to remove free virus, and 104 TZM-bl reporter cells were added to each well of the virus-bound monolayer. After 48 h, luciferase reagent (Bright-Glo; Promega) was added to the well, and the RLU were measured. Percent inhibition was calculated by dividing the RLU of each well by the median RLU of epithelium-bound virus that was not preincubated with isolated rhesus Abs. The IgG isoform of the influenza virus MAb CH65 was used as a negative control, whereas the IgG isoform of broadly neutralizing CD4 binding site MAb VRC01 was used as a positive control. The cutoff value was determined by the mean plus 2 SD of CH65 IgG relative to no antibody.
Binding of MAbs to infected cells.
The ability of MAbs to recognize CEM.NKRCCR5 infected with a C.1086 infectious molecular clone (IMC) was assessed (32). CEM.NKRCCR5 cells were infected with a C.1086 IMC that encodes the Renilla luciferase reporter gene and preserves all HIV-1 open reading frames (38) using dextran-DEAE as described previously (32). At 48 h postinfection, the ability of MAbs to recognize the HIV-infected cells was measured by indirect surface immunofluorescence analysis. The infected CEM.NKRCCR5 cells were incubated with 10 μg/ml of the rhesus-derived MAbs in R10 medium for 2 h at 37°C and then stained with a vital dye (Live/Dead Fixable Aqua Dead Cell Stain; Invitrogen) to exclude nonviable cells from subsequent analyses. Primary Ab binding was detected by secondary labeling with fluorescein isothiocyanate (FITC)-conjugated goat anti-rhesus IgG (SouthernBiotech Inc., Birmingham, AL), and HIV-1-infected cells were identified by staining for intracellular expression of p24 (KC57-RD1; Beckman Coulter) using standard methods. Infected cell binding was evaluated by gating on live, p24+ events using FlowJo version 9.8 software (TreeStar, Inc., Ashland, OR).
IgG purification and concentration.
IgG was isolated from plasma and breast milk using protein G columns (6) (protein G resin prepacked into 96-well depletion plates [GE Healthcare]). Plasma was diluted 2-fold with Tris-buffered saline (TBS) (pH 7.5), and 200 μl of the diluted sample was added per well. For breast milk, 300 μl of undiluted sample was added to each well. The plates were incubated at room temperature, with shaking, for 1 h. The unbound fractions were removed by centrifugation at 700 × g for 3 min. Wells were then washed 3 times with 400 μl of TBS to remove loosely bound material. The IgG bound to the resin was eluted with 200 μl of 2.5% glacial acetic acid (pH 2.51) and immediately neutralized with 120 μl of 1 M Tris-HCl (pH 9.0). The eluted IgG fractions were concentrated using Amicon Ultra centrifugal filters (Millipore) with a 30,000-molecular-weight cutoff. The sample volume was reduced to 50 μl by centrifugation at 14,000 × g in a microcentrifuge precooled to 4°C. A buffer exchange was then performed using 2.0 volumes of PBS, pH 7.5. The concentrated IgG was assayed for protein concentration using a NanoDrop 8000 spectrophotometer (Thermo Fisher Scientific) using the IgG reference setting and then diluted to 1 mg/ml with PBS.
Statistical methods.
Exact Wilcoxon signed rank tests were performed for all outcome and time point comparisons described in the text. R software version 2.141.1 was used for calculations (R Foundation for Statistical Computing, Vienna, Austria). Note that 0.13 is the lowest P value attainable with 4 experimental animals.
RESULTS
Combined i.n./i.m. Env immunization increases Env-specific IgA responses in breast milk.
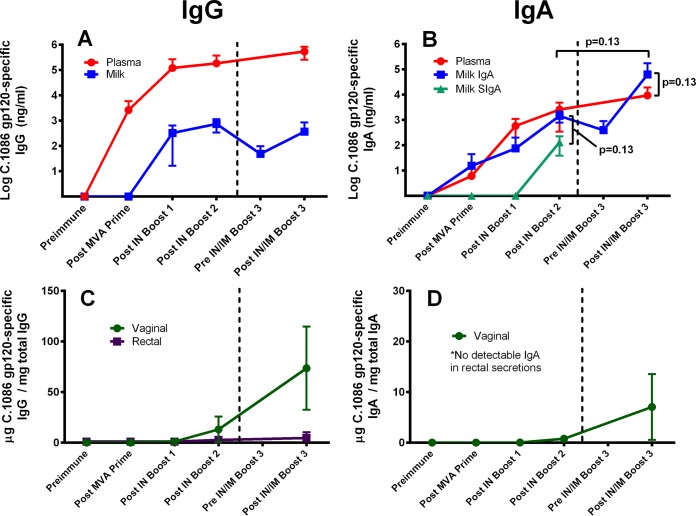
In an attempt to elicit both functional IgG and Env-specific IgA responses in breast milk, four female Indian rhesus monkeys previously immunized with an MVA prime/i.n. boost regimen were boosted a third time with a combined i.n./i.m. approach 50 weeks after the original MVA prime (Fig. 1). Lactation was hormonally induced in the rhesus monkeys prior to immunization (20). In both plasma and milk we observed an increase in Env-specific IgA over the response following i.n. Env boost alone. This increase was more pronounced in milk yet was not statistically significant (median milk IgA after boosts 2 and 3, 1,712 ng/ml and 7,641 ng/ml, respectively; P = 0.13) (Fig. 2). The magnitude of the Env-specific IgA response elicited in breast milk following i.n./i.m. boost is remarkable and distinct from that found in chronically HIV-infected individuals, as median breast milk Env-specific IgA levels exceeded those in plasma (median milk IgA = 7,641 ng/ml and plasma IgA = 6,200 ng/ml; P = 0.13). Interestingly, the vaccine-elicited antibody responses were quite durable over the 34 weeks prior to i.n./i.m. boost 3 (week 50), with high-magnitude Env-specific IgG and IgA measured in the breast milk of vaccinated monkeys prior to the final boost. We next investigated what proportion of the vaccine-elicited Env-specific IgA response was secretory (SIgA) by assessing the amount of secretory component in milk (Fig. 2B). Due to sample limitation after the third protein boost, SIgA in the breast milk was measured only preimmunization and following boosts 1 and 2. Env-specific SIgA was detected in 3 of 4 animals following i.n. boost 2, comprising only 9% of the total Env-specific IgA in breast milk (median IgA = 1,712 ng/ml and SIgA = 152 ng/ml; P = 0.13). This finding suggests that the majority of vaccine-elicited Env-specific IgA did not traffic to the breast milk compartment via poly-Ig receptor interaction. Thus, the predominance of vaccine-elicited Env-specific IgA antibodies in milk was likely either passively transcytosed through mammary epithelia or produced locally. The vaccine-elicited IgA responses in other mucosal compartments were also assessed. Following i.n./i.m. immunization, Env-binding IgA increased in the vaginal compartment, surpassing levels previously attained following i.n. Env boosts (18). There was no detectable IgA in rectal secretions (Fig. 2C and D).
FIG 2.
The combined i.n./i.m. HIV-1 Env boost enhances IgA responses in mammary and vaginal compartments over those elicited by i.n. boost alone. The gp120-specific IgG responses of animals given the MVA prime/i.n. boost regimen plus the combined i.m./i.n. boost are shown for plasma/milk (A) and vaginal/rectal secretions (C), while gp120-specific IgA responses are shown for plasma/milk (B) and vaginal/rectal secretions (D). Data points represent mean value for 4 animals, with standard deviations indicated by error bars. Plasma is in red, milk in blue, vaginal secretions in green, and rectal secretions in purple. The secretory IgA (SIgA) fraction of the gp120-specific IgA responses is in turquoise (B). IgA was not detectable in rectal secretions for any time point.
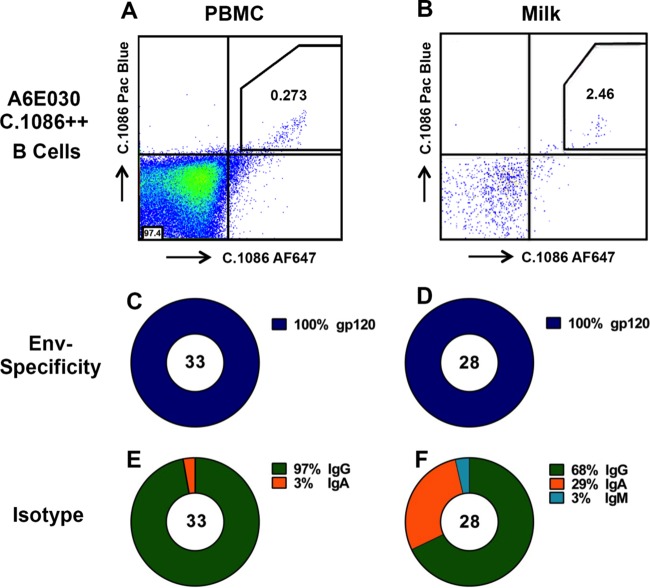
High proportion of Env-specific IgA isotype memory B cells isolated from breast milk of i.n./i.m. Env-immunized lactating monkey A6E030.
Env-specific B cells were isolated from blood, milk, and intestinal tissue using a fluorescently labeled C.1086 Env probe (data not shown). Populations of Env-specific B cells were identified in the blood and intestines of all four animals, comprising an average of 0.12% and 0.11% of all B cells isolated in each respective compartment. Two animals had low B cell counts in breast milk (<100 total CD20+ cells). However, intriguingly, in the remaining two animals with a significant population of breast milk B cells, 2.64% on average were Env specific (Table 1). Based upon the large number of breast milk B cells in monkey A6E030, this animal was selected for investigation of the vaccine-elicited Env-specific breast milk B cell repertoire. In total, 33 Env-specific B cells were isolated from blood and 28 from breast milk. The isotype distribution of the blood Env-specific MAbs was 97% IgG and 3% IgA (Fig. 3E). In contrast, 29% of Env-specific MAbs in the breast milk compartment were IgA, whereas 68% were IgG and 3% IgM (Fig. 3F). This relatively high proportion of IgA-producing B cells in milk suggests that i.n./i.m. vaccination may induce localization of Env-specific IgA isotype B cells to the mammary compartment. A similar analysis was conducted for breast milk B cells isolated from animal A6E088; however, only 5 Env-specific B cells were isolated from blood and 5 from breast milk. Though it is difficult to draw conclusions from the small number of MAbs isolated from A6E088, 20% (1 of 5) of Env-specific B cells were IgA isotype in both blood and breast milk
TABLE 1.
Percentages of Env-specific memory B cells isolated from blood, milk, and intestines of four i.n./i.m. Env-vaccinated lactating monkeys
| Animal | C.1086+ B cells (%) in: |
||
|---|---|---|---|
| Blood | Milk | Intestine | |
| A6E030 | 0.27 | 2.46 | 0.07 |
| A6E042 | 0.06 | NDa | 0.07 |
| A6E088 | 0.10 | 2.82 | 0.22 |
| AU71S | 0.06 | ND | 0.07 |
| Avg | 0.12 | 2.64b | 0.11 |
ND, none detected (CD20+ total cell count of <100).
Average excluding values not detected due to low total CD20+ cell count.
FIG 3.
Isolation of C.1086 Env-specific IgA-producing B cells from breast milk of animal A6E030. (A and B) Env-specific B cells were isolated by selection of cells from blood (A) and milk (B) that are double positive for C.1086 Env labeled with two different fluorophores. (C and D) All MAbs isolated from Env-specific B cells in blood (C) and breast milk (D) were confirmed to be gp120 specific by ELISA. (E and F) While the blood gp120-specific MAbs were predominantly IgG isotype (E), those in milk were 29% IgA isotype (F). An identical analysis was performed for animal A6E088, though only 5 MAbs were isolated from plasma B cells and 5 MAbs from breast milk B cells.
The i.n./i.m. Env vaccine regimen increases both the magnitude and breadth of the systemic Env epitope-specific IgG response.
To determine the impact of our immunization regimen on the fine specificity of the plasma IgG response, we used a peptide array assay to assess binding to overlapping linear peptides covering 7 consensus strains and 6 vaccine strains of Env gp160. The magnitude and breadth of the IgG response against Env peptides increased following each vaccination (Table 2). In all animals, anti-V3 binding was the dominant response observed; however, all vaccinated animals also developed binding responses to other linear regions, including C1, C2, V3, C4, and C5. The animal with the highest-magnitude IgG response (A6E030) developed notable epitope breadth with binding responses against all these regions. The greatest increase in gp120 peptide binding magnitude was seen following the first i.n. Env boost, as evidenced in the two animals for which the preimmune and i.n. boost 1 time points were measured. However, the i.n./i.m. Env boost introduced robust epitope expansion in all animals, with the exception of monkey AU71S.
TABLE 2.
i.n./i.m. vaccine regimen increases both linear epitope magnitude and breadth of systemic gp120-specific IgG responses
| HIV Env epitope (peptide range, aa) | Signal intensitya for the indicated monkey and time point |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A6E030 |
A6E042 |
AU71S |
A6E088 |
|||||||||
| Prime | i.n.1 | i.n.2 | i.n./i.m.3 | Prime | i.n.1 | i.n.2 | i.n./i.m.3 | i.n.2 | i.n./i.m. 3 | i.n.2 | i.n./i.m.3 | |
| C1.1 (28–29) | 1 | 94 | 2,369 | 13,162 | 1 | 1 | 1 | 1 | 50 | 46 | 264 | 1,472 |
| C1.2 (32–36) | 1 | 10 | 2,128 | 7,091 | 1 | 1 | 3,646 | 22,843 | 37 | 21 | 4,558 | 20,443 |
| C2.1 (83–84) | 1 | 42 | 1,115 | 4,050 | 1 | 1 | 62 | 347 | 17 | 4 | 43 | 39 |
| C2.2 (91) | 1 | 6,599 | 7 | 31,884 | 1 | 501 | 18,187 | 18,831 | 15 | 205 | 43 | 7,344 |
| V3 (93–106) | 6,115 | 41,303 | 50,112 | 65,186 | 4,321 | 60,998 | 57,211 | 60,520 | 18,498 | 22,535 | 54,913 | 57,958 |
| V4 (126–127) | 168 | 207 | 11,719 | 9,690 | 1 | 1 | 1 | 1 | 162 | 102 | 471 | 649 |
| C4 (136–139) | 1,671 | 2,361 | 31,085 | 47,253 | 1 | 232 | 1 | 113 | 7,846 | 7,153 | 562 | 313 |
| V5 = C5 (147–151) | 1 | 472 | 3,155 | 8,040 | 1 | 1 | 721 | 293 | 106 | 29 | 9,176 | 13,172 |
| C5.1 (156–159) | 1 | 6,594 | 24,739 | 23,126 | 1 | 115 | 504 | 1,610 | 68 | 164 | 6,153 | 10,711 |
| gp41 ID (187–196) | 96 | 781 | 524 | 1,495 | 1 | 56 | 1 | 512 | 597 | 518 | 117 | 114 |
| MPER (212–216) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 111 | 56 | 8 | 4 |
Maximum binding (signal intensity) to a single peptide within each identified epitope. Italic, signal intensity of 1,000 to 10,000; bold italic, signal intensity of 10,000 to 30,000; underlined bold italic, signal intensity of >30,000. Plasma samples for the postprime and post-i.n. boost 1 time points were not available for animals AU71S and A6E088 due to sample limitation.
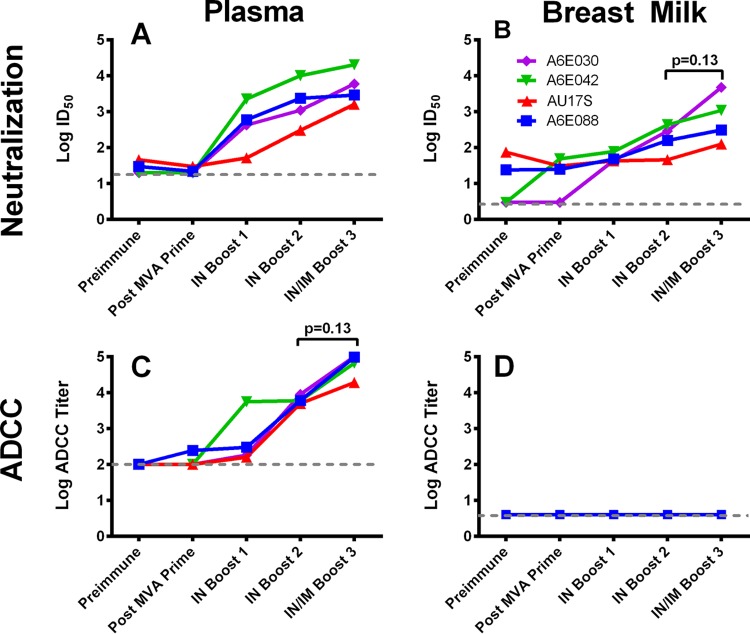
Combined i.n./i.m. Env boost increases neutralizing activity in breast milk but does not elicit ADCC responses.
We previously observed that systemic (i.m.) Env immunization, but not mucosal (i.n.) immunization, was able to elicit neutralizing and ADCC-mediating responses in the breast milk of lactating rhesus macaques (18). Thus, we measured these functional antibody responses following combined i.n./i.m. Env immunization in both plasma and breast milk (Fig. 4). In each vaccinated, lactating monkey, the neutralization response against a tier 1 clade C variant, MW965, was augmented in milk following the combined i.n./i.m. Env boost (median ID50s after boosts 2 and 3, 226 and 702, respectively; P = 0.13) (Fig. 4B). Surprisingly one animal (A6E030) had comparable neutralization activity in plasma and breast milk (ID50s of 5,974 and 4,796, respectively). The plasma of all animals demonstrated ADCC antibody responses, which is consistent with results seen in the MVA prime/i.n. boost group prior to administration of the i.n./i.m. combined boost (18). ADCC responses were enhanced in plasma following the i.n./i.m. boost (median titers after boots 2 and 3, 5,994 and 81,498, respectively; P = 0.13) (Fig. 4C). However, no ADCC activity was detectable in breast milk following the i.n./i.m. boost.
FIG 4.
Functional HIV-1 Env-specific antibody responses elicited in milk and plasma following combined i.n./i.m. Env immunization. (A and B) Neutralization responses against the tier 1 clade C virus MW965 in TZM-bl cells in plasma (A) and milk (B). Each colored line represents a different vaccinated animal. (C and D) ADCC activity against HIV-1 C.1086 gp120-coated CEM.NKR cells in plasma (C) and milk (D) following the combined i.n./i.m. Env boost. The dashed line indicates the starting dilution. Four of four animals had detectable ADCC activity in plasma, compared to zero of four animals with detectable activity in breast milk.
The concentration of TNC does not increase in response to vaccination.
Tenascin C (TNC) is an innate protein found in breast milk, which we have previously described to have HIV-1 neutralizing function (39). Thus, we investigated whether an increase in innate factors like tenascin C might play a role in the enhanced neutralization titers following each immunization observed in this study. However, measurement of breast milk TNC concentration across time points of the immunization schedule revealed that while TNC was detectable in rhesus monkey milk, it was comparable between all 4 animals and 4 time points (Table 3). We have previously found that the concentration of TNC in human breast milk ranges from 2.2 to 671 μg/ml and that TNC has a neutralization IC50 of 82 to 158 μg/ml (39). Therefore, the low level of tenascin C detected in the vaccinated monkey breast milk across all time points (0.53 to 3.94 μg/ml) suggests that the sequential increase in neutralization titers throughout the vaccine schedule is not due to a change in the composition of innate breast milk factors.
TABLE 3.
The concentration of innate HIV-neutralizing factor tenascin C in breast milk does not significantly change during the vaccination schedule
| Animal | Tenascin C concn (μg/ml) at time point: |
|||
|---|---|---|---|---|
| Postprime | Post-boost 1 | Post-boost 2 | Pre-boost 3 | |
| A6E030 | 0.53 | 1.47 | 1.37 | 2.47 |
| A6E042 | 0.91 | 1.92 | 0.90 | 3.94 |
| A6E088 | 1.30 | 0.66 | 2.69 | 2.22 |
| AU71S | 3.62 | 2.81 | 3.19 | NAa |
NA, sample not available.
Genetic characteristics of IgA and IgG MAbs produced from breast milk and blood Env-specific memory B cells.
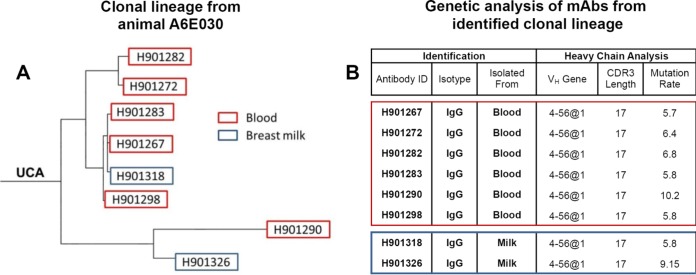
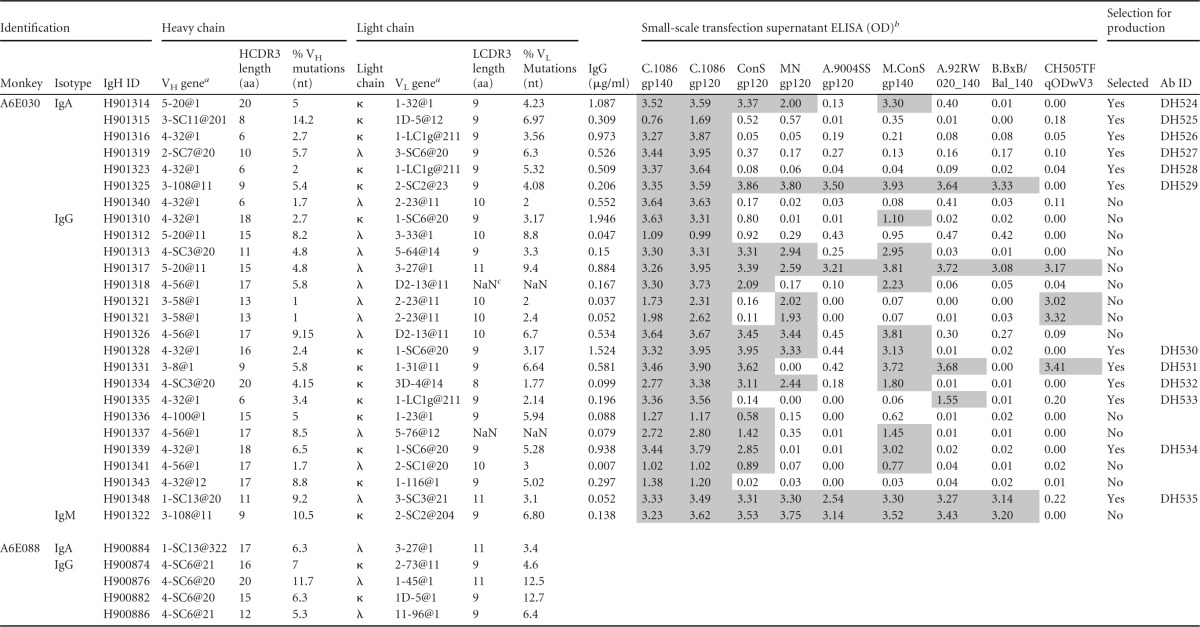
Genetic analysis of paired heavy and light chains from Env-specific blood and breast milk B cells was conducted using Cloanalyst to determine the utilized VH and VL genes, complementarity-determining region 3 (CDR3) length, and mutation frequency (40). Interestingly, we identified a B cell clonal lineage spanning the systemic and mammary compartments (Fig. 5). This clonal lineage was comprised of 8 IgG MAbs, including 6 from blood and 2 from breast milk, which strongly suggests trafficking of vaccine-elicited Env-specific IgG isotype B cells between the two compartments. We found that the VH4 gene family was predominant in blood and breast milk compartments (66% of all MAbs). The median heavy-chain CDR3 (HCDR3) length and mean percent VH mutation frequency were 15 amino acids (aa) and 5% in blood and 13 aa and 5% in breast milk. Among the 28 MAbs isolated from A6E030 breast milk B cells, the median HCDR3 length and mean percent VH mutation frequency were 9 aa and 5% for IgA but 15 aa and 5% for IgG (Table 4). From these 28 milk MAbs, 6 IgA and 6 IgG MAbs were selected for subsequent study based on the magnitude and breadth of the Env-binding profile following transient transfection. The HCDR3 length and percent VH mutation frequency of the 6 IgA and 6 IgG MAbs selected were representative of the total breast milk MAb population (Table 5).
FIG 5.
Clonal relationship of vaccine-elicited Env-specific blood and breast milk IgG isotype B cells. (A) A clonal lineage comprising monoclonal antibodies isolated from C.1086 Env-specific blood and breast milk B cells was identified in animal A6E030 following combined i.n./i.m. immunization. UCA, unmutated common ancestor. (B) Heavy-chain genes, HCDR3 length, and mutation frequency were determined for pairings using Cloanalyst.
TABLE 4.
Genetic data and Env specificity screening of MAbs produced in small scale from Env-specific B cells isolated from breast milk of monkeys A6E030 and A6E088
a VH and VL gene usage obtained from Clonalyst.
b An OD of >2 times that for mock-transfected wells was considered positive and is highlighted by shading. Small-scale screening not conducted for A6E088 MAbs.
c NaN, “not a number,” indicating that Clonalyst could not computationally infer the percentage of mutations.
TABLE 5.
Genetic characterization, specificity, and function of Env-specific monoclonal antibodies isolated from C.1086 Env-specific blood and milk B cells following combined i.n./i.m. Env immunization
| Identification |
Genetic data |
Fine specificity |
Epitope blocking |
Neutralization (IC50, μg/ml) |
ADCC (titer, μg/ml)b | Infected cell binding (avg FITC MFI)c | Mean % inhibition of epithelial cell bindingd | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isotype | Antibody | VH gene | HCDR3 length (aa) | % VH mutations (nt) | Light chain | VL gene | LCDR3 length (aa) | % VL mutations (nt) | Targeta | EC50 (μg/ml) | Target | % blocking | C.MW965 | C.1086 | |||
| IgA | DH524 | 5-20@1 | 20 | 5 | κ | 1-32@1 | 9 | 4.23 | CD4 | 76 | >50 | >50 | >40 | 652 | 45 | ||
| DH525 | 3-SC11@201 | 8 | 14.2 | κ | 1D-5@12 | 9 | 6.97 | Lin C4 | 0.028 | >50 | >50 | >40 | 73 | 32 | |||
| DH526 | 4-32@1 | 6 | 2.7 | κ | 1-LC1g@211 | 9 | 3.56 | >50 | >50 | >40 | 29 | 22 | |||||
| DH527 | 2-SC7@20 | 10 | 5.7 | λ | 3-SC6@20 | 9 | 6.3 | V2 | 0.002 | >50 | >50 | 0.588 | 327 | 23 | |||
| DH528 | 4-32@1 | 6 | 2 | κ | 1-LC1g@211 | 9 | 5.32 | >50 | >50 | >40 | 12 | 17 | |||||
| DH529 | 3-108@11 | 9 | 5.4 | κ | 2-SC2@23 | 9 | 4.08 | Lin C1 | 0.014 | >50 | >50 | >40 | 31 | 23 | |||
| IgG | DH530 | 4-32@1 | 16 | 2.4 | κ | 1-SC6@20 | 9 | 3.17 | CD4 | 76 | >50 | >50 | >40 | 472 | 15 | ||
| DH531 | 3-8@1 | 9 | 5.8 | κ | 1-31@11 | 9 | 6.64 | >50 | >50 | >40 | 15 | 15 | |||||
| DH532 | 4-SC3@20 | 20 | 4.15 | κ | 3D-4@14 | 8 | 1.77 | A32 (C1) | 70 | >50 | >50 | 0.002 | 1,154 | 15 | |||
| DH533 | 4-32@1 | 6 | 3.4 | κ | 1-LC1g@211 | 9 | 2.14 | >50 | >50 | >40 | 8 | 26 | |||||
| DH534 | 4-32@1 | 18 | 6.5 | κ | 1-SC6@20 | 9 | 5.28 | CD4 | 80 | >50 | >50 | >40 | 723 | 35 | |||
| DH535 | 1-SC13@20 | 11 | 9.2 | λ | 3-SC3@21 | 11 | 3.1 | V3 | 0.07 | 0.024e | >50 | 0.013 | 1,460 | 91 | |||
V, variable loop; C, conserved region; Lin, linear.
Bold indicates ADCC activity exceeding the 8% granzyme B specific activity threshold.
Bold indicates value above the cutoff of a MFI of 100.
Bold indicates value above the cutoff of 31% inhibition (2 standard deviations above value for the negative control, CH65).
MAb DH535 was also screened for neutralization against other viruses: B.Bal.26 (IC50 = 2.01μg/ml), B.MN.3 (IC50 = 0.06μg/ml), and B.SF162 (IC50 = 0.13μg/ml).
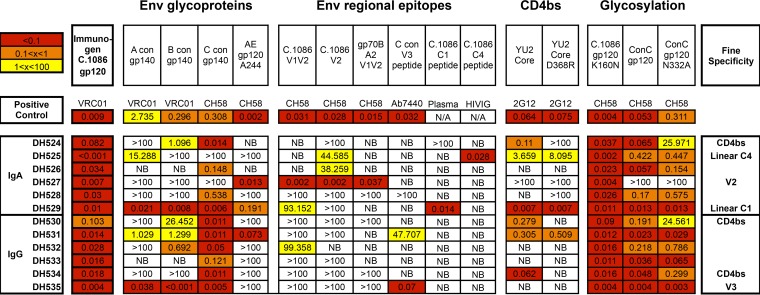
i.n./i.m. Env vaccine-elicited IgA and IgG MAbs isolated from breast milk B cells have variable Env epitope specificity.
First, the Env-binding breadth of the 6 IgA and 6 IgG antibodies chosen for large-scale production was examined by ELISA using consensus clade A, B, C, and AE gp140 proteins. As anticipated, the vaccine-elicited breast milk MAbs showed highest binding strength to clade C Env proteins; however, 8 of the 12 MAbs bound Env proteins of multiple clades (Fig. 6). Next, epitope specificity of the MAbs was evaluated against a panel of Env peptides. Individual IgA MAbs showed strong binding to the V2 (DH527, EC50 = 0.002 μg/ml), C1 (DH529, EC50 = 0.014 μg/ml), and C4 (DH525, EC50 = 0.028 μg/ml) regions. Additionally, one IgG MAb was specific for V3 linear peptide (DH535, EC50 = 0.07 μg/ml) (Fig. 6). To determine whether any of the breast milk MAbs were specific for the CD4-binding site, binding to YU2 core gp120 alongside the CD4-binding site-specific mutant (YU2 D368R) was assessed. One IgA and two IgG vaccine-elicited MAbs demonstrated strong binding to the wild-type YU2 core but not to the YU2 D368R mutant (DH524, DH530, and DH534) (Fig. 6). In addition, these three MAbs were able to block binding of soluble CD4 (sCD4) to Env protein in a competitive ELISA (Table 5). Glycan dependence was assessed by measuring binding to gp120 proteins with and without mutations at well-described glycosylation sites (C.1086 gp120/C.1086 gp120 K160N and ConC gp120/ConC gp120 N332A). None of the vaccine-elicited MAbs appeared to depend on these glycosylation sites for Env binding (Fig. 6). Finally, MAbs were tested for the ability to block Env binding of A32, an ADCC-mediating MAb directed against a C1 conformational epitope. One IgG MAb (DH532) demonstrated 70% blocking, suggesting conformational C1 specificity (Table 5). Two IgA and two IgG vaccine-elicited breast milk MAbs were unable to be epitope mapped and presumably bind to conformational epitopes (Fig. 6; Table 5).
FIG 6.
Binding profile and determined specificities for 6 IgA and 6 IgG vaccine-elicited Env-specific MAbs isolated from breast milk B cells and selected for functional characterization. EC50s (in μg/ml) obtained by ELISA are shown. Tested antigens are grouped into categories (Env glycoproteins, Env regional epitopes, CD4bs epitopes, and glycosylation mutants). NB, no binding detected.
i.n./i.m. Env vaccine-elicited MAbs isolated from breast milk B cells have diverse antiviral function.
The neutralization potency of the isolated milk Env-specific MAbs was assessed in TZM-bl cells against four tier 1 viruses (C.MW965, B.SF162, B.BaL.26, and B.MN.3), as well as the autologous tier 2 virus C.1086. One V3-specific IgG MAb, DH535, neutralized all the tier 1 viruses tested but not the tier 2 autologous virus (Table 5). The ability of MAbs to bind CEM.NKRCCR5 cells infected with C.1086 autologous virus was measured by immunofluorescence. Six MAbs (2 IgA and 4 IgG) were observed to bind the surface of C.1086-infected cells: DH524, DH530, DH534 (sCD4 blocking), DH527 (V2), DH532 (A32 blocking), and DH535 (V3) (Table 5; Fig. 7). Three of these MAbs also demonstrated ADCC activity against CEM.NKRCCR5 cells coated with C.1086 gp120: DH527 (V2), DH532 (A32 blocking), and DH535 (V3) (Table 5; Fig. 8A). Finally, the ability of the MAbs to inhibit binding of HIV C.MW965 virions to epithelial cells was assessed (41). Four MAbs (2 IgA and 2 IgG) blocked epithelial cell binding: DH524 and DH534 (sCD4 blocking), DH525 (linear C4), and DH535 (V3) (Table 5; Fig. 7). Thus, vaccine-elicited breast milk MAbs had a wide array of antiviral functionalities which could contribute to blocking viral infection at mucosal surfaces.
FIG 7.
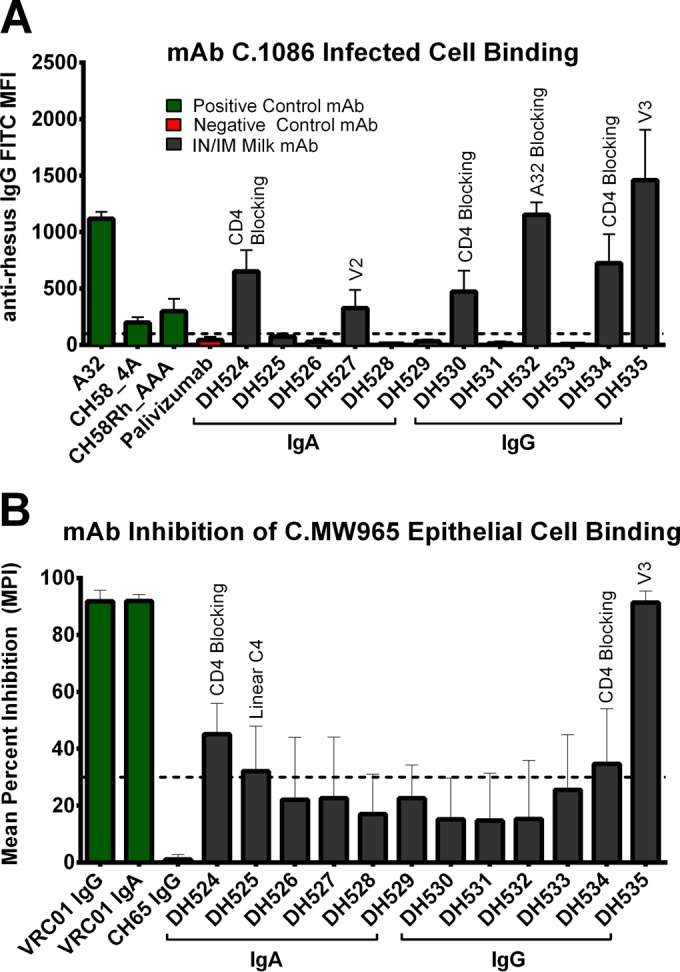
Vaccine-elicited Env-specific MAbs from breast milk B cells bind infected cells and prevent viral binding to epithelial cells. (A) 6 MAbs (2 IgA and 4 IgG) demonstrated binding to the surface of C.1086 HIV-infected CEM.NKRCCR5 cells. (B) 4 MAbs (2 IgA, 2 IgG) prevented C.MW965 from binding to GI epithelial cells. Dashed lines indicate the cutoff for positivity, set at a mean fluorescence intensity (MFI) of 100 for infected cell binding and a mean percent inhibition (MPI) of 31 (mean plus 2 SD for negative control) for inhibition of epithelial cell binding. The specificity of i.n./i.m. milk MAbs with a positive result is denoted above the error bars.
Postimmunization breast milk has a moderate inhibitory effect on MAb ADCC function.
Since vaccine-elicited IgA in plasma has previously been observed to interfere with the IgG effector function of antibodies with the same specificity (13), we next sought to determine whether the robust i.n./i.m.-elicited IgA responses in breast milk could inhibit ADCC activity by milk IgG. Though ADCC was not observed in the unfractionated breast milk of i.n./i.m. immunized animals, IgG purified from the same breast milk was found to mediate a low level of ADCC activity (approximate endpoint concentrations of 0.2 μg/ml for A6E042 and 0.1 μg/ml for A6E088) (Fig. 8B). This result suggests a potential ADCC-blocking effect of the high-magnitude Env-specific IgA response, but another possibility is the presence of an unidentified blocking factor in milk. To further investigate this phenomenon of ADCC inhibition, pre- and post-i.n./i.m. vaccination breast milk was spiked with 10 μg/ml of DH532 (the A32-blocking, vaccine-elicited milk MAb that mediates robust ADCC) or 15 ng/ml of an ADCC MAb mix consisting of A32, 2G12, 7B2, and CH44 (Fig. 8C). Partial inhibition of ADCC effector function was observed in the post-i.n./i.m. vaccination milk spiked with both DH532 (29% inhibition) and the ADCC MAb mix (71% inhibition) but not in the pre-i.n./i.m. milk sample (Fig. 8D). Thus, the high-magnitude IgA response or unidentified factors in post-i.n./i.m. vaccination milk may account for the inability to detect ADCC activity in unfractionated milk of i.n./i.m. Env-immunized monkeys.
DISCUSSION
An inherent challenge in the prevention of breast milk HIV MTCT is elicitation of robust immune responses more potent than those following natural infection. Breast milk from HIV-infected women contains protective Env-specific antibodies (6, 8, 17, 42), the presence of which makes development of a maternal HIV vaccine to enhance these preexisting responses an attractive strategy to prevent breastfeeding transmission of HIV. In this study, we gained further understanding of the isotypes, specificities, and functions of HIV-specific breast milk antibody responses induced by i.n./i.m. immunization and then compared them to previously described responses that may be important to impede postnatal transmission.
Although breast milk from both HIV-infected humans and SIV-infected rhesus monkeys contains higher-magnitude Env-specific IgG than IgA, in our recent analysis of breast milk antibody responses of women from the Breastfeeding Antiretrovirals and Nutrition (BAN) study, only breast milk HIV envelope gp140-specific IgA was associated with reduced risk of postnatal HIV-1 acquisition (6, 7, 17). These results suggest that a maternal vaccine with the ability to elicit robust IgA responses in milk could potentially be protective. It has traditionally proven very difficult to induce Env-specific IgA in mucosal compartments by HIV vaccination (11). Yet, the combined systemic and mucosal vaccination regimen (i.n./i.m.) used in this study elicited remarkably high-magnitude Env-specific IgA responses in breast milk, with a concentration exceeding that in plasma. This provides proof of principle that a vaccine can selectively elicit strong IgA responses in the mammary compartments. It is intriguing that although secretory IgA typically constitutes the majority of IgA in mucosal secretions (43), following i.n. boost 2, SIgA represented only approximately 9% of the total Env-specific IgA in breast milk. This finding indicates that the majority of Env-specific breast milk IgA was either produced locally or passively transudated across epithelial cells rather than secreted across mammary epithelia. However, despite the observed differences in antibody response magnitude, it is important to note that these differences were not statistically significant given the small number of experimental animals.
In addition to the increased IgA-binding responses, i.n./i.m. immunization also enhanced neutralization titers in breast milk. Many innate factors in breast milk, such as tenascin C (TNC) and lactoferrin, have been described to have an HIV-1-neutralizing function (39, 44). To investigate whether an increased production of these innate factors following immunization could contribute to the observed increase in neutralization titers, we determined the concentrations of tenascin C at multiple time points during the immunization schedule. We observed that the concentrations were quite low compared to human levels, far below TNC neutralization IC50 levels previously observed (39), and were similar between all animals and across all samples. These findings suggest that the increase in neutralizing titer following immunization is the result of an enhancement in Env-specific IgG and IgA responses rather than other innate factors. Interestingly, this investigation is the first to report the presence of TNC in breast milk of rhesus monkeys.
Concordant with the high magnitude of Env-specific IgA in milk, a relatively high proportion of Env-specific breast milk B cells were found to be IgA isotype. Previous studies have indicated that IgA isotype B cells comprise 75 to 80% of all mammary gland-associated B cells (45), and there is a body of evidence which supports the existence of a gut-mammary axis in primates by which gut IgA-producing plasmablasts home to lactating mammary tissue (46, 47). Quite counterintuitively, we and others have observed that in the setting of chronic HIV infection, HIV Env-specific memory B cells in breast milk are overwhelmingly IgG isotype (9, 10). Therefore, the relatively high proportion (29%) of Env-specific IgA-producing B cells induced by i.n./i.m. vaccination in breast milk is a novel finding and suggests that this vaccine regimen led to trafficking of clonally proliferating Env-specific B cells to the mammary compartment. The immune function of these B cells in breast milk once ingested by the infant is poorly understood. Intriguingly, activated mononuclear cells have been observed to persist in the mammalian neonatal gut, bind to the brush border, and infiltrate neonatal tissues (48, 49). This observation leaves open the possibility that ingested Env-specific memory B cells could serve as immune sentinels or further differentiate into antibody-producing plasma cells in the infant GI tract.
Among our limited panel of 12 breast milk MAbs (6 IgA and 6 IgG), there was no clear association of antibody isotype with epitope specificity, breadth, or function. As these MAbs were isolated from a single immunized animal, it is unknown whether our results are representative of all immunized animals. Nevertheless, our results clearly demonstrate that i.n./i.m. HIV vaccination can induce IgA isotype memory B cells of diverse specificities in the mucosal compartment and that vaccine-elicited IgA MAbs are capable of mediating functions such as infected cell binding and inhibition of viral binding to epithelial cells. Importantly, this study is the first to isolate and characterize vaccine-elicited IgA from mucosal Env-specific memory B cells. The significant epitope and functional variability observed among our MAb panel suggests that breast milk B cells are not restricted in their epitope specificity. Given our previous discovery that a higher proportion of breast milk B cells are gp120 directed in breast milk than in plasma during chronic HIV-1 infection (9), we anticipate that gp120 i.n./i.m. immunization of a chronically infected woman could further enrich the proportion of gp120-specific B cells.
Interestingly, one of the breast milk IgG MAbs (DH535) was able to mediate multiple effector functions. This V3-specific MAb was determined to have broad tier 1 neutralization function, bind to the surface of C.1086 infected cells, mediate ADCC, and inhibit viral binding to epithelial cells. In a cohort of 248 HIV-infected mothers, the magnitude of the V3-specific IgG in maternal plasma was recently associated with a decreased risk of perinatal MTCT (50). The role of a V3-binding antibody in the prevention of postnatal transmission has not clearly been established, yet it is possible that the polyfunctionality demonstrated by DH535 is required to block mucosal HIV transmission. Indeed, a comparison between two HIV clinical trials (RV144 and VAX003) demonstrated that the ability of a vaccine to induce multiple, coordinated antiviral responses is associated with lower rates of HIV transmission (51, 52).
The role of ADCC in protection against breast milk HIV transmission remains unclear. While Mabuka et al. reported an association between ADCC-mediating antibodies in milk and a reduced rate of transmission (8), in our analysis of the BAN cohort, ADCC responses in milk were not associated with protection (17). Important differences between the cohorts of HIV-infected women could explain these discordant results, including sample size, the virus clade, and the maternal immune status. Surprisingly, combined systemic/mucosal immunization did not elicit ADCC activity in milk, though multiple ADCC-mediating MAbs were isolated from breast milk B cells. As IgA blocking of ADCC effector function was demonstrated in the RV144 vaccine trial analysis, we sought to determine if the lack of detectable ADCC activity in milk was due to IgA interference. Purified IgG from post-i.n./i.m. vaccination breast milk was able to mediate low levels of ADCC activity, suggesting a possible IgA-blocking effect. Furthermore, when post-i.n./i.m. vaccination breast milk was spiked with ADCC-mediating antibodies, there was a reduction in the potency of ADCC effector activity. Therefore, the high concentration of Env-specific IgA in breast milk could be an important factor affecting ADCC-mediated antibody function after i.n./i.m. vaccination. Nevertheless, the biological relevance of this ADCC blocking for virus transmission remains to be determined.
Since Env-specific IgA and ADCC activities have been associated with a reduced risk of postnatal HIV transmission, these responses are the benchmarks we have to judge the success or failure of maternal HIV vaccination strategies to induce potentially protective immune responses in breast milk. It is remarkable that the i.n./i.m. vaccination regimen both elicited a high level of Env-specific breast milk IgA and induced local IgA isotype breast milk B cells, which is a quite distinct immune response from that following natural infection. The ability of these robust vaccine-elicited Env-specific IgA responses to prevent breast milk HIV transmission should be further investigated in a nonhuman primate model through infant oral SHIV challenge in the setting of exposure to breast milk of a vaccinated dam. These studies would address the potential in vivo protection of high-magnitude Env-specific IgA responses in breast milk and also interrogate any impact of these IgA responses on ADCC functionality. Furthermore, a nonhuman primate model has the potential to address the feasibility of administering this proposed immunization regimen during pregnancy, as well as the safety of an MVA prime for a developing fetus. If the procedure is proven efficacious and safe, such studies would enable identification of novel immune correlates of protection for postnatal HIV MTCT and would suggest that a maternal vaccination strategy such as i.n./i.m. combined immunization given during pregnancy has potential to curb the ongoing pediatric HIV epidemic.
ACKNOWLEDGMENTS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors have no commercial affiliations or financial conflicts of interest to disclose.
We acknowledge the following individuals for their technical contributions and support: Josh Amos, Brooke Liebl, Carrie Ho, R. Glenn Overman, Robert Parks, Krissey Lloyd, Ashley Trama, Dawn Marshall, Ashley Allen, Lawrence Armand, David Martinez, and Norman Letvin.
Funding Statement
The funders had no role in study design, data collection and interpretation, decision to publish, or the preparation of the manuscript.
REFERENCES
- 1.Kourtis AP, Butera S, Ibegbu C, Belec L, Duerr A. 2003. Breast milk and HIV-1: vector of transmission or vehicle of protection? Lancet Infect Dis 3:786–793. doi: 10.1016/S1473-3099(03)00832-6. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro RL, Hughes MD, Ogwu A, Kitch D, Lockman S, Moffat C, Makhema J, Moyo S, Thior I, McIntosh K, van Widenfelt E, Leidner J, Powis K, Asmelash A, Tumbare E, Zwerski S, Sharma U, Handelsman E, Mburu K, Jayeoba O, Moko E, Souda S, Lubega E, Akhtar M, Wester C, Tuomola R, Snowden W, Martinez-Tristani M, Mazhani L, Essex M. 2010. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med 362:2282–2294. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO, UNICEF, UNAIDS. 2014. Global HIV/AIDS response. Epidemic update and health sector progress towards universal access. Progress report 2014. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 4.American Academy of Pediatrics Work Group on Breastfeeding. 1997. Breastfeeding and the use of human milk. Pediatrics 100:1035–1039. doi: 10.1542/peds.100.6.1035. [DOI] [PubMed] [Google Scholar]
- 5.Munoz FM, Englund JA. 2000. A step ahead. Infant protection through maternal immunzation. Pediatr Clin North Am 47:449–463. [DOI] [PubMed] [Google Scholar]
- 6.Fouda GG, Yates NL, Pollara J, Shen X, Overman GR, Mahlokozera T, Wilks AB, Kang HH, Salazar-Gonzalez JF, Salazar MG, Kalilani L, Meshnick SR, Hahn BH, Shaw GM, Lovingood RV, Denny TN, Haynes B, Letvin NL, Ferrari G, Montefiori DC, Tomaras GD, Permar SR, Center for HIV/AIDS Vaccine Immunology. 2011. HIV-specific functional antibody responses in breast milk mirror those in plasma and are primarily mediated by IgG antibodies. J Virol 85:9555–9567. doi: 10.1128/JVI.05174-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Permar SR, Wilks AB, Ehlinger EP, Kang HH, Mahlokozera T, Coffey RT, Carville A, Letvin NL, Seaman MS. 2010. Limited contribution of mucosal IgA to simian immunodeficiency virus (SIV)-specific neutralizing antibody response and virus envelope evolution in breast milk of SIV-infected, lactating rhesus monkeys. J Virol 84:8209–8218. doi: 10.1128/JVI.00656-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. 2012. HIV-specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads. PLoS Pathog 8:e1002739. doi: 10.1371/journal.ppat.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sacha CR, Vandergrift N, Jeffries TL Jr, McGuire E, Fouda GG, Liebl B, Marshall DJ, Gurley TC, Stiegel L, Whitesides JF, Friedman J, Badiabo A, Foulger A, Yates NL, Tomaras GD, Kepler TB, Liao HX, Haynes BF, Moody MA, Permar SR. 2015. Restricted isotype, distinct variable gene usage, and high rate of gp120 specificity of HIV-1 envelope-specific B cells in colostrum compared with those in blood of HIV-1-infected, lactating African women. Mucosal Immunol 8:316–326. doi: 10.1038/mi.2014.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuaillon E, Valea D, Becquart P, Al Tabaa Y, Meda N, Bollore K, Van de Perre P, Vendrell JP. 2009. Human milk-derived B cells: a highly activated switched memory cell population primed to secrete antibodies. J Immunol 182:7155–7162. doi: 10.4049/jimmunol.0803107. [DOI] [PubMed] [Google Scholar]
- 11.Mestecky J, Jackson S, Moldoveanu Z, Nesbit LR, Kulhavy R, Prince SJ, Sabbaj S, Mulligan MJ, Goepfert PA. 2004. Paucity of antigen-specific IgA responses in sera and external secretions of HIV-type 1-infected individuals. AIDS Res Hum Retroviruses 20:972–988. doi: 10.1089/aid.2004.20.972. [DOI] [PubMed] [Google Scholar]
- 12.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomaras GD, Ferrari G, Shen X, Alam SM, Liao HX, Pollara J, Bonsignori M, Moody MA, Fong Y, Chen X, Poling B, Nicholson CO, Zhang R, Lu X, Parks R, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Gilbert PB, Kim JH, Michael NL, Montefiori DC, Haynes BF. 2013. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci U S A 110:9019–9024. doi: 10.1073/pnas.1301456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bomsel M, Tudor D, Drillet AS, Alfsen A, Ganor Y, Roger MG, Mouz N, Amacker M, Chalifour A, Diomede L, Devillier G, Cong Z, Wei Q, Gao H, Qin C, Yang GB, Zurbriggen R, Lopalco L, Fleury S. 2011. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity 34:269–280. doi: 10.1016/j.immuni.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Watkins JD, Sholukh AM, Mukhtar MM, Siddappa NB, Lakhashe SK, Kim M, Reinherz EL, Gupta S, Forthal DN, Sattentau QJ, Villinger F, Corti D, Ruprecht RM, CAVD Project Group. 2013. Anti-HIV IgA isotypes: differential virion capture and inhibition of transcytosis are linked to prevention of mucosal R5 SHIV transmission. AIDS 27:F13–20. doi: 10.1097/QAD.0b013e328360eac6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sholukh AM, Watkins JD, Vyas HK, Gupta S, Lakhashe SK, Thorat S, Zhou M, Hemashettar G, Bachler BC, Forthal DN, Villinger F, Sattentau QJ, Weiss RA, Agatic G, Corti D, Lanzavecchia A, Heeney JL, Ruprecht RM. 2015. Defense-in-depth by mucosally administered anti-HIV dimeric IgA2 and systemic IgG1 mAbs: complete protection of rhesus monkeys from mucosal SHIV challenge. Vaccine 33:2086–2095. doi: 10.1016/j.vaccine.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollara J, McGuire E, Fouda GG, Rountree W, Eudailey J, Overman RG, Seaton KE, Deal A, Edwards RW, Tegha G, Kamwendo D, Kumwenda J, Nelson JA, Liao HX, Brinkley C, Denny TN, Ochsenbauer C, Ellington S, King CC, Jamieson DJ, van der Horst C, Kourtis AP, Tomaras GD, Ferrari G, Permar SR. 2015. Association of HIV-1 envelope-specific breast milk IgA responses with reduced risk of postnatal mother-to-child transmission of HIV-1. J Virol 89:9952–9961. doi: 10.1128/JVI.01560-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fouda GG, Amos JD, Wilks AB, Pollara J, Ray CA, Chand A, Kunz EL, Liebl BE, Whitaker K, Carville A, Smith S, Colvin L, Pickup DJ, Staats HF, Overman G, Eutsey-Lloyd K, Parks R, Chen H, Labranche C, Barnett S, Tomaras GD, Ferrari G, Montefiori DC, Liao HX, Letvin NL, Haynes BF, Permar SR. 2013. Mucosal immunization of lactating female rhesus monkeys with a transmitted/founder HIV-1 envelope induces strong Env-specific IgA antibody responses in breast milk. J Virol 87:6986–6999. doi: 10.1128/JVI.00528-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fouda GG, Moody MA, Permar SR. 2015. Antibodies for prevention of mother-to-child transmission of HIV-1. Curr Opin HIV AIDS 10:177–182. doi: 10.1097/COH.0000000000000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Permar SR, Kang HH, Carville A, Mansfield KG, Gelman RS, Rao SS, Whitney JB, Letvin NL. 2008. Potent simian immunodeficiency virus-specific cellular immune responses in the breast milk of simian immunodeficiency virus-infected, lactating rhesus monkeys. J Immunol 181:3643–3650. doi: 10.4049/jimmunol.181.5.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moody MA, Santra S, Vandergrift NA, Sutherland LL, Gurley TC, Drinker MS, Allen AA, Xia SM, Meyerhoff RR, Parks R, Lloyd KE, Easterhoff D, Alam SM, Liao HX, Ward BM, Ferrari G, Montefiori DC, Tomaras GD, Seder RA, Letvin NL, Haynes BF. 2014. Toll-like receptor 7/8 (TLR7/8) and TLR9 agonists cooperate to enhance HIV-1 envelope antibody responses in rhesus macaques. J Virol 88:3329–3339. doi: 10.1128/JVI.03309-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozlowski PA, Williams SB, Lynch RM, Flanigan TP, Patterson RR, Cu-Uvin S, Neutra MR. 2002. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J Immunol 169:566–574. doi: 10.4049/jimmunol.169.1.566. [DOI] [PubMed] [Google Scholar]
- 23.Wilks AB, Christian EC, Seaman MS, Sircar P, Carville A, Gomez CE, Esteban M, Pantaleo G, Barouch DH, Letvin NL, Permar SR. 2010. Robust vaccine-elicited cellular immune responses in breast milk following systemic simian immunodeficiency virus DNA prime and live virus vector boost vaccination of lactating rhesus monkeys. J Immunol 185:7097–7106. doi: 10.4049/jimmunol.1002751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeffries TL Jr, Sacha CR, Pollara J, Himes J, Jaeger FH, Dennison SM, McGuire E, Kunz E, Eudailey JA, Trama AM, LaBranche C, Fouda GG, Wiehe K, Montefiori DC, Haynes BF, Liao HX, Ferrari G, Alam SM, Moody MA, Permar SR. 5 August 2015. The function and affinity maturation of HIV-1 gp120-specific monoclonal antibodies derived from colostral B cells. Mucosal Immunol doi: 10.1038/mi.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O'Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alam SM, Liao HX, Tomaras GD, Bonsignori M, Tsao CY, Hwang KK, Chen H, Lloyd KE, Bowman C, Sutherland L, Jeffries TL Jr, Kozink DM, Stewart S, Anasti K, Jaeger FH, Parks R, Yates NL, Overman RG, Sinangil F, Berman PW, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Karasavva N, Rerks-Ngarm S, Kim JH, Michael NL, Zolla-Pazner S, Santra S, Letvin NL, Harrison SC, Haynes BF. 2013. Antigenicity and immunogenicity of RV144 vaccine AIDSVAX clade E envelope immunogen is enhanced by a gp120 N-terminal deletion. J Virol 87:1554–1568. doi: 10.1128/JVI.00718-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomaras GD, Binley JM, Gray ES, Crooks ET, Osawa K, Moore PL, Tumba N, Tong T, Shen X, Yates NL, Decker J, Wibmer CK, Gao F, Alam SM, Easterbrook P, Abdool Karim S, Kamanga G, Crump JA, Cohen M, Shaw GM, Mascola JR, Haynes BF, Montefiori DC, Morris L. 2011. Polyclonal B cell responses to conserved neutralization epitopes in a subset of HIV-1-infected individuals. J Virol 85:11502–11519. doi: 10.1128/JVI.05363-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen X, Duffy R, Howington R, Cope A, Sadagopal S, Park H, Pal R, Kwa S, Ding S, Yang OO, Fouda GG, Le Grand R, Bolton D, Esteban M, Phogat S, Roederer M, Amara RR, Picker LJ, Seder RA, McElrath MJ, Barnett S, Permar SR, Shattock R, DeVico AL, Felber BK, Pavlakis GN, Pantaleo G, Korber BT, Montefiori DC, Tomaras GD. 2015. Vaccine-induced linear epitope-specific antibodies to simian immunodeficiency virus SIVmac239 envelope are distinct from those induced to the human immunodeficiency virus type 1 envelope in nonhuman primates. J Virol 89:8643–8650. doi: 10.1128/JVI.03635-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gottardo R, Bailer RT, Korber BT, Gnanakaran S, Phillips J, Shen X, Tomaras GD, Turk E, Imholte G, Eckler L, Wenschuh H, Zerweck J, Greene K, Gao H, Berman PW, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Robb ML, Michael NL, Kim JH, Zolla-Pazner S, Haynes BF, Mascola JR, Self S, Gilbert P, Montefiori DC. 2013. Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PLoS One 8:e75665. doi: 10.1371/journal.pone.0075665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarzotti-Kelsoe M, Bailer RT, Turk E, Lin CL, Bilska M, Greene KM, Gao H, Todd CA, Ozaki DA, Seaman MS, Mascola JR, Montefiori DC. 2014. Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J Immunol Methods 409:131–146. doi: 10.1016/j.jim.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montefiori DC. 2009. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol Biol 485:395–405. doi: 10.1007/978-1-59745-170-3_26. [DOI] [PubMed] [Google Scholar]
- 32.Pollara J, Hart L, Brewer F, Pickeral J, Packard BZ, Hoxie JA, Komoriya A, Ochsenbauer C, Kappes JC, Roederer M, Huang Y, Weinhold KJ, Tomaras GD, Haynes BF, Montefiori DC, Ferrari G. 2011. High-throughput quantitative analysis of HIV-1 and SIV-specific ADCC-mediating antibody responses. Cytometry A 79:603–612. doi: 10.1002/cyto.a.21084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trkola A, Matthews J, Gordon C, Ketas T, Moore JP. 1999. A cell line-based neutralization assay for primary human immunodeficiency virus type 1 isolates that use either the CCR5 or the CXCR4 coreceptor. J Virol 73:8966–8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koene HR, Kleijer M, Algra J, Roos D, von dem Borne AE, de Haas M. 1997. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood 90:1109–1114. [PubMed] [Google Scholar]
- 35.Liao HX, Chen X, Munshaw S, Zhang R, Marshall DJ, Vandergrift N, Whitesides JF, Lu X, Yu JS, Hwang KK, Gao F, Markowitz M, Heath SL, Bar KJ, Goepfert PA, Montefiori DC, Shaw GC, Alam SM, Margolis DM, Denny TN, Boyd SD, Marshal E, Egholm M, Simen BB, Hanczaruk B, Fire AZ, Voss G, Kelsoe G, Tomaras GD, Moody MA, Kepler TB, Haynes BF. 2011. Initial antibodies binding to HIV-1 gp41 in acutely infected subjects are polyreactive and highly mutated. J Exp Med 208:2237–2249. doi: 10.1084/jem.20110363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonsignori M, Hwang KK, Chen X, Tsao CY, Morris L, Gray E, Marshall DJ, Crump JA, Kapiga SH, Sam NE, Sinangil F, Pancera M, Yongping Y, Zhang B, Zhu J, Kwong PD, O'Dell S, Mascola JR, Wu L, Nabel GJ, Phogat S, Seaman MS, Whitesides JF, Moody MA, Kelsoe G, Yang X, Sodroski J, Shaw GM, Montefiori DC, Kepler TB, Tomaras GD, Alam SM, Liao HX, Haynes BF. 2011. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J Virol 85:9998–10009. doi: 10.1128/JVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phad GE, Vazquez Bernat N, Feng Y, Ingale J, Martinez Murillo PA, O'Dell S, Li Y, Mascola JR, Sundling C, Wyatt RT, Karlsson Hedestam GB. 2015. Diverse antibody genetic and recognition properties revealed following HIV-1 envelope glycoprotein immunization. J Immunol 194:5903–5914. doi: 10.4049/jimmunol.1500122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edmonds TG, Ding H, Yuan X, Wei Q, Smith KS, Conway JA, Wieczorek L, Brown B, Polonis V, West JT, Montefiori DC, Kappes JC, Ochsenbauer C. 2010. Replication competent molecular clones of HIV-1 expressing Renilla luciferase facilitate the analysis of antibody inhibition in PBMC. Virology 408:1–13. doi: 10.1016/j.virol.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fouda GG, Jaeger FH, Amos JD, Ho C, Kunz EL, Anasti K, Stamper LW, Liebl BE, Barbas KH, Ohashi T, Moseley MA, Liao HX, Erickson HP, Alam SM, Permar SR. 2013. Tenascin-C is an innate broad-spectrum, HIV-1-neutralizing protein in breast milk. Proc Natl Acad Sci U S A 110:18220–18225. doi: 10.1073/pnas.1307336110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kepler TB, Munshaw S, Wiehe K, Zhang R, Yu JS, Woods CW, Denny TN, Tomaras GD, Alam SM, Moody MA, Kelsoe G, Liao HX, Haynes BF. 2014. Reconstructing a B-cell clonal lineage. II. Mutation, selection, and affinity maturation. Front Immunol 5:170. doi: 10.3389/fimmu.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fouda GG, Mahlokozera T, Salazar-Gonzalez JF, Salazar MG, Learn G, Kumar SB, Dennison SM, Russell E, Rizzolo K, Jaeger F, Cai F, Vandergrift NA, Gao F, Hahn B, Shaw GM, Ochsenbauer C, Swanstrom R, Meshnick S, Mwapasa V, Kalilani L, Fiscus S, Montefiori D, Haynes B, Kwiek J, Alam SM, Permar SR. 2013. Postnatally-transmitted HIV-1 envelope variants have similar neutralization-sensitivity and function to that of nontransmitted breast milk variants. Retrovirology 10:3. doi: 10.1186/1742-4690-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braibant M, Barin F. 2013. The role of neutralizing antibodies in prevention of HIV-1 infection: what can we learn from the mother-to-child transmission context? Retrovirology 10:103. doi: 10.1186/1742-4690-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Telemo E, Hanson LA. 1996. Antibodies in milk. J Mammary Gland Biol Neoplasia 1:243–249. doi: 10.1007/BF02018077. [DOI] [PubMed] [Google Scholar]
- 44.Moriuchi M, Moriuchi H. 2001. A milk protein lactoferrin enhances human T cell leukemia virus type I and suppresses HIV-1 infection. J Immunol 166:4231–4236. doi: 10.4049/jimmunol.166.6.4231. [DOI] [PubMed] [Google Scholar]
- 45.Brandtzaeg P. 1983. The secretory immune system of lactating human mammary glands compared with other exocrine organs. Ann N Y Acad Sci 409:353–382. doi: 10.1111/j.1749-6632.1983.tb26883.x. [DOI] [PubMed] [Google Scholar]
- 46.Ahlstedt S, Carlsson B, Hanson LA, Goldblum RM. 1975. Antibody production by human colostral cells. I. Immunoglobulin class, specificity, and quantity. Scand J Immunol 4:535–539. [DOI] [PubMed] [Google Scholar]
- 47.Allardyce RA, Shearman DJ, McClelland DB, Marwick K, Simpson AJ, Laidlaw RB. 1974. Appearance of specific colostrum antibodies after clinical infection with Salmonella typhimurium. Br Med J 3:307–309. doi: 10.1136/bmj.3.5926.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiler IJ, Hickler W, Sprenger R. 1983. Demonstration that milk cells invade the suckling neonatal mouse. Am J Reprod Immunol 4:95–98. doi: 10.1111/j.1600-0897.1983.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 49.Head JR, Beer AE, Billingham RE. 1977. Significance of the cellular component of the maternal immunologic endowment in milk. Transplant Proc 9:1465–1471. [PubMed] [Google Scholar]
- 50.Permar SR, Fong Y, Vandergrift N, Fouda GG, Gilbert P, Parks R, Jaeger FH, Pollara J, Martelli A, Liebl BE, Lloyd K, Yates NL, Overman RG, Shen X, Whitaker K, Chen H, Pritchett J, Solomon E, Friberg E, Marshall DJ, Whitesides JF, Gurley TC, Von Holle T, Martinez DR, Cai F, Kumar A, Xia SM, Lu X, Louzao R, Wilkes S, Datta S, Sarzotti-Kelsoe M, Liao HX, Ferrari G, Alam SM, Montefiori DC, Denny TN, Moody MA, Tomaras GD, Gao F, Haynes BF. 2015. Maternal HIV-1 envelope-specific antibody responses and reduced risk of perinatal transmission. J Clin Invest 125:2702–2706. doi: 10.1172/JCI81593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, Dugast AS, Schoen MK, Rolland M, Suscovich TJ, Mahan AE, Liao L, Streeck H, Andrews C, Rerks-Ngarm S, Nitayaphan S, de Souza MS, Kaewkungwal J, Pitisuttithum P, Francis D, Michael NL, Kim JH, Bailey-Kellogg C, Ackerman ME, Alter G. 2014. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci Transl Med 6:228ra238. [DOI] [PubMed] [Google Scholar]
- 52.Yates NL, Liao HX, Fong Y, deCamp A, Vandergrift NA, Williams WT, Alam SM, Ferrari G, Yang ZY, Seaton KE, Berman PW, Alpert MD, Evans DT, O'Connell RJ, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Pinter A, Zolla-Pazner S, Gilbert PB, Nabel GJ, Michael NL, Kim JH, Montefiori DC, Haynes BF, Tomaras GD. 2014. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med 6:228ra239. [DOI] [PMC free article] [PubMed] [Google Scholar]