ABSTRACT
Dendritic cells (DCs) are major targets of filovirus infection in vivo. Previous studies have shown that the filoviruses Ebola virus (EBOV) and Marburg virus (MARV) suppress DC maturation in vitro. Both viruses also encode innate immune evasion functions. The EBOV VP35 (eVP35) and the MARV VP35 (mVP35) proteins each can block RIG-I-like receptor signaling and alpha/beta interferon (IFN-α/β) production. The EBOV VP24 (eVP24) and MARV VP40 (mVP40) proteins each inhibit the production of IFN-stimulated genes (ISGs) by blocking Jak-STAT signaling; however, this occurs by different mechanisms, with eVP24 blocking nuclear import of tyrosine-phosphorylated STAT1 and mVP40 blocking Jak1 function. MARV VP24 (mVP24) has been demonstrated to modulate host cell antioxidant responses. Previous studies demonstrated that eVP35 is sufficient to strongly impair primary human monocyte-derived DC (MDDC) responses upon stimulation induced through the RIG-I-like receptor pathways. We demonstrate that mVP35, like eVP35, suppresses not only IFN-α/β production but also proinflammatory responses after stimulation of MDDCs with RIG-I activators. In contrast, eVP24 and mVP40, despite suppressing ISG production upon RIG-I activation, failed to block upregulation of maturation markers or T cell activation. mVP24, although able to stimulate expression of antioxidant response genes, had no measurable impact of DC function. These data are consistent with a model where filoviral VP35 proteins are the major suppressors of DC maturation during filovirus infection, whereas the filoviral VP24 proteins and mVP40 are insufficient to prevent DC maturation.
IMPORTANCE The ability to suppress the function of dendritic cells (DCs) likely contributes to the pathogenesis of disease caused by the filoviruses Ebola virus and Marburg virus. To clarify the basis for this DC suppression, we assessed the effect of filovirus proteins known to antagonize innate immune signaling pathways, including Ebola virus VP35 and VP24 and Marburg virus VP35, VP40, and VP24, on DC maturation and function. The data demonstrate that the VP35s from Ebola virus and Marburg virus are the major suppressors of DC maturation and that the effects on DCs of the remaining innate immune inhibitors are minor.
INTRODUCTION
Zaire ebolavirus (EBOV) and Marburg marburgvirus (MARV) belong to the Filovirus family of emerging, zoonotic negative-sense RNA viruses. Both EBOV and MARV cause severe, frequently fatal disease in humans (1). The West African Ebola epidemic, which was first recognized in early 2014, is the largest filovirus outbreak on record with over 28,000 cases worldwide and a reported case fatality rate of approximately 40% (2). The severity of EBOV and MARV infections reflects, at least in part, robust systemic virus replication that is not effectively restrained by host immune responses (3).
Both EBOV and MARV encode multiple proteins that impair innate antiviral immune responses and likely contribute to excessive virus replication (3, 4). Both viruses encode VP35, a double-stranded RNA (dsRNA)-binding protein that carries out multiple functions critical for virus propagation. One such function is the inhibition of signaling by RIG-I and MDA5 through (RLR) signaling, which otherwise triggers alpha/beta interferon (IFN-α/β) gene expression (5–13). For both EBOV VP35 (eVP35) and MARV VP35 (mVP35), point mutations that disrupt VP35 dsRNA-binding activity also greatly impair RLR inhibition activity (5, 9, 14, 15). In vitro studies suggest that dsRNA-binding allows eVP35 to sequester RIG-I stimulatory dsRNAs (8, 9). eVP35 also interacts with the cellular protein PACT, which binds to and facilitates activation of RIG-I (8). Further, mutations that disrupt VP35 antagonism of RIG-I increase cellular antiviral responses and severely attenuate EBOV and MARV replication in cells that effectively mount an IFN-α/β response (9, 14, 16); such mutations also greatly reduce replication and disease in rodent models (9, 17). These observations collectively suggest that inhibition of RIG-I signaling by VP35 is critical for robust systemic filovirus replication and virulence.
Additionally, EBOV and MARV antagonize Jak-STAT signaling triggered by IFNs exogenously added to cells. For EBOV, this inhibition is mediated by its VP24 protein (eVP24), which binds to select karyopherin alpha (KPNA) proteins (18–21). Binding of eVP24 to KPNA1, -5, and -6 blocks interaction between the KPNAs and tyrosine-phosphorylated STAT1 (PY-STAT1), thereby blocking PY-STAT1 nuclear accumulation. In contrast, MARV VP40 (mVP40) and not MARV VP24 (mVP24) blocks Jak-STAT signaling by targeting JAK-1 tyrosine phosphorylation of STAT-1 (22). Both eVP24 and mVP40 impair IFN-induced expression of IFN-stimulated genes (ISGs). Although mVP24 does not block type I IFN signaling, it does modulate cellular signaling pathways. By interacting with cellular protein Keap1, mVP24 expression activates antioxidant response genes and modulates NF-κB, thereby inducing a cytoprotective response whereby cells better survive under stress conditions (23–25).
In vitro studies indicate that EBOV and MARV productively replicate in and impair maturation of dendritic cells (DCs) (26–28). These observations are likely relevant to in vivo infection since DCs have been demonstrated to be targets of infection in animal models and in infected humans. EBOV IFN-antagonist proteins have been implicated as potentially mediating this suppression (28–31). Expression of eVP35 in monocyte-derived DCs (MDDCs) is sufficient to disrupt not only IFN-α/β production but also production of proinflammatory cytokines, upregulation of cell surface expression of DC maturation markers, and activation of T cells following infection of the cells with RIG-I activator Sendai virus (SeV) or MDA5 activator encephalomyocarditis virus (EMCV) (31). Infection of DCs with recombinant EBOVs bearing mutations that disrupt VP35 dsRNA-binding and RLR inhibition results in significant DC maturation, definitively linking eVP35 to DC suppression in the context of EBOV infection (28). Although eVP35 expression is sufficient to suppress DC maturation, studies in DCs infected with mutant EBOVs suggest that eVP24 may also modulate DC responses and that eVP24 cooperates with eVP35 to modulate DC responses to EBOVs (32).
How mVP35, mVP40, mVP24, or eVP24 impact DC maturation has not been explicitly addressed. Structural and biochemical studies suggest that mVP35 interacts with dsRNA differently than does eVP35 (10, 11). Specifically, eVP35 and the related Reston ebolavirus VP35 cap the ends of dsRNAs, whereas mVP35 may not (9–11, 33). Since 5′-triphosphates on the ends of RNAs are a feature recognized by RIG-I, this difference has implications for the mechanism by which eVP35 and mVP35 block RLR signaling. In support of this, EBOV more potently suppressed IFN-α/β responses than does MARV upon infection of THP-1 cells, and eVP35 is a more potent suppressor of SeV-induced RIG-I signaling than is mVP35 (34). By blocking Jak1 function, mVP40 inhibits the tyrosine phosphorylation events downstream of the IFN-α/β receptor (22). This differs substantially from the effects of eVP24, which only blocks PY-STAT1 nuclear import but leaves IFN-induced tyrosine phosphorylation events intact. Furthermore, despite the fact that mVP24 has the ability to antagonize the antioxidant response pathway, it has also been suggested to have a cytoprotective effect that may facilitate viral propagation (24). Whether mVP24 might also impact DC maturation is unclear.
Here, we use a previously described lentivirus delivery system to express individual EBOV and MARV innate immune modulating proteins in human MDDCs and to investigate their effects on MDDC function upon stimulation with RLR activators or with IFN-β. Our results suggest that, while mVP35 suppresses IFN-α/β responses to a modestly lesser extent than does eVP35, each is capable of effectively suppressing DC maturation in response to RLR activating SeV and EMCV. Both eVP24 and mVP40 effectively suppress expression of IFN-inducible genes and mVP24 can activate antioxidant response genes. However, none of these latter three proteins can effectively suppress maturation of DCs by SeV. These data suggest that for MARV, like EBOV, the VP35 protein is the predominant suppressor of DC maturation and function, whereas the filoviral VP24 proteins and the mVP40 protein cannot on their own inhibit DC maturation.
MATERIALS AND METHODS
Isolation of human MDDCs and naive T cells.
Human MDDCs were generated from CD14+ cells purified from concentrated leukocytes of healthy, anonymous human blood-bank blood (New York Blood Center) as described previously (30). Briefly, peripheral blood mononuclear cells were isolated by Ficoll density gradient centrifugation (GE Healthcare, catalog no. 95021-205), and CD14+ cells were purified using CD14 microbeads (Miltenyi Biotech). CD14+ cells, at a concentration of 106 cells/ml, were incubated at 37°C for 5 days in DC medium (RPMI containing 4% human AB serum [Fisher Scientific], 2 mM l-glutamine, 1 mM sodium pyruvate, penicillin [100 U/ml]–streptomycin [100 μg/ml], and 55 μM β-mercaptoethanol) supplemented with 500 U/ml human granulocyte-macrophage colony-stimulating factor (PeproTech) and 500 U/ml human interleukin-4 (PeproTech). Typically, we obtained approximately 107 MDDCs per 20 ml of culture. Naive CD4 T cell isolation was performed using CD4+ isolation kits (Miltenyi Biotech, catalog no. 130-096-533) according to the manufacturer's recommendations.
Generation of filoviral IFN antagonist-encoding lentiviruses and Vpx-bearing viral particles.
Replication-defective lentiviruses were generated as previously described (31, 35). Expression plasmids used were pHCMV-G encoding VSV glycoprotein, packaging plasmid pNL4.3-gag-pol and derivatives of pHR-SIN-CSIGW. Flag-tagged wild-type EBOV-VP35, MARV-VP35, EBOV-VP24, MARV-VP40, or RAVV-VP40 were cloned into pHR-SIN-CSIGW to generate lentiviruses encoding each protein. Vpx-containing viruslike particles (Vpx-VLPs), which greatly enhance transduction by HIV-1-based lentivirus vectors, were generated by cotransfecting expression plasmids for VSV G and pSIV3+, a simian immunodeficiency virus SIV251 gag-pol encoding plasmid containing Vpx (kindly provided by Dan Littman, NYU Medical Center).
Transduction of human MDDCs.
MDDCs were transduced by spinoculation at 1,850 rpm with lentiviruses and Vpx-VLP for 2.5 h and then cultured in fresh medium for 72 h. Transduced MDDCs were harvested to assess for expression by flow cytometry and Western blotting or used in subsequent experiments. “No lentivirus” samples were infected with Vpx-VLPs in the absence of additional lentiviruses.
Infection/stimulation of transduced MDDCs.
SeV strain Cantell was grown by inoculating 10-day-old embryonated chicken eggs and incubating them for 2 days at 37°C. Encephalomyocarditis virus (EMCV) was grown in baby hamster kidney cells. At 72 h postransduction, the MDDCs were mock treated, infected with either SeV or EMCV, or stimulated with 100 U of human-IFN-β (PBL, Piscataway, NJ)/ml.
Allogeneic T cells and transduced DC coculture assays.
Transduced DCs were either mock infected or infected with SeV for 4 h before coculture with purified naive CD4 T cells for 5 days at the indicated ratios. Culture supernatants were then harvested and assayed by enzyme-linked immunosorbent assay (ELISA) for IFN-γ (BD Biosciences) according to the manufacturer's recommendations.
Quantitative reverse transcription-PCR (qRT-PCR).
Total RNA was extracted from MDDCs with TRIzol reagent (Sigma). cDNA was prepared using the SuperScript III first-strand synthesis System (Invitrogen). Relative gene expression was determined by using the PerfeCTa SYBR green FastMix (Quanta Biosciences, Inc.) with a Bio-Rad CFX96 instrument and CFX Manager software (Bio-Rad). This software was also used to analyze the relative mRNA expression levels by the change in threshold cycle (ΔCT) method using the β-actin gene to normalize the results.
Antibodies for Western blotting.
Mouse monoclonal anti-GFP antibody was purchased from Clontech Laboratories (catalog no. 632375). Mouse monoclonal anti-Flag M2 antibody (F3165) and mouse monoclonal anti-β-tubulin antibody (T0198) were purchased from Sigma-Aldrich.
Flow cytometry analysis of DCs.
MDDCs were harvested and stained for cell surface expression of multiple markers of DC maturation by flow cytometry at 20 h after SeV infection. DCs were pelleted and resuspended in fluorescence-activated cell sorting buffer (1× phosphate-buffered saline, 1% bovine serum albumin, and 0.1% sodium azide) and then incubated for 30 min on ice in the dark with the antibodies. Antibodies, including anti-CD11C-PECy7, anti-CD40-PE, anti-CD83-PE, and anti-CD86-PE, were purchased from eBioscience. Flow cytometry data were collected using an LSR II flow cytometer (BD Bioscience) and analyzed using FlowJo software (Tree Star, Ashland, OR).
RESULTS
mVP35 impairs MDDC IFN-α/β and proinflammatory cytokine responses to SeV and EMCV infection.
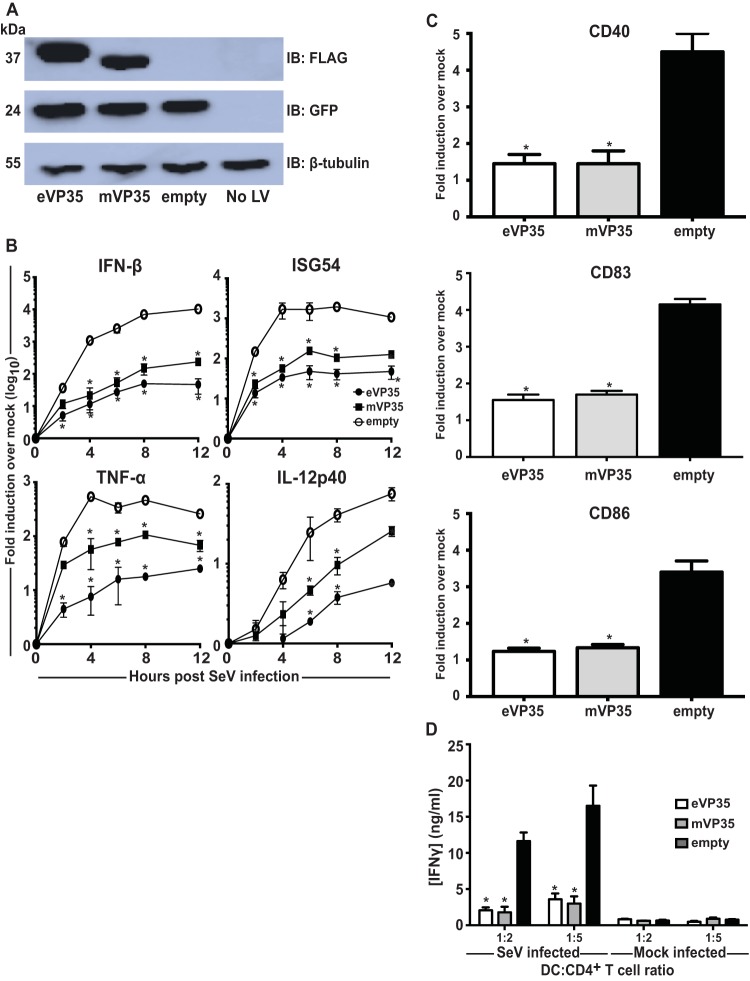
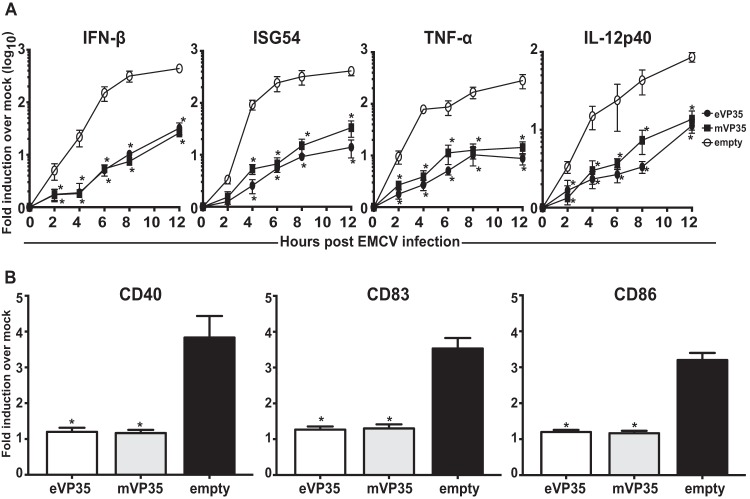
Previous studies have implicated eVP35 as an inhibitor of DC maturation. To determine whether mVP35 can also inhibit human DC responses upon RIG-I activation, human MDDCs were transduced with empty vector, eVP35, or mVP35 lentiviruses. Transduction of DCs with replication-defective lentiviruses reproducibly achieves efficiencies of >90% and induces little to no IFN-α/β response or other evidence of DC activation (31, 35, 36). After 72 h, the MDDCs were infected with SeV, known to activate DCs in a RIG-I-dependent manner (37). The expression of eVP35 or mVP35 was verified by Western blotting (Fig. 1A). As assessed by qRT-PCR, mVP35 and eVP35 both substantially suppressed IFN-β and ISG54 expression relative to the empty vector control (Fig. 1B). The levels for the proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin-12p40 (IL-12p40) were also suppressed, but for these genes we did observe a trend where mVP35 appeared to be a less potent inhibitor than eVP35 (Fig. 1B). We also sought to determine whether mVP35 expression is sufficient to inhibit upregulation of DC maturation markers in response to SeV infection. Upregulation—relative to mock-infected DCs—of CD40, CD83, and CD86, was suppressed in mVP35-expressing MDDCs compared to SeV-infected empty vector MDDCs, and the extent of suppression was comparable to that seen with eVP35 (Fig. 1C). We further investigated whether mVP35 expression is also sufficient to inhibit the ability of MDDCs to activate naive T cells. Transduced MDDCs were infected with SeV and cocultured with naive allogeneic CD4+ T cells for 5 days at various DC-to-T cell ratios. The supernatants from the cocultures were analyzed for accumulation of IFN-γ, an indication of T cell activation. Compared to T cells cocultured with control MDDCs, T cells cultured with MDDCs expressing mVP35 produced less IFN-γ, and again the level of inhibition was nearly identical to that seen with eVP35 (Fig. 1D).
FIG 1.
mVP35 inhibits human MDDC responses similarly to eVP35 upon SeV infection. (A) Human MDDCs were mock transduced (No LV) or transduced with empty lentiviral vector (empty) or lentiviruses that express either Flag-tagged eVP35 or mVP35. Cells from one representative donor were analyzed by Western blotting with anti-Flag antibody. Probing with β-tubulin antibody served as a loading control. (B) Transduced MDDCs were mock infected or infected with SeV. Total RNA was extracted at the indicated time in hours postinfection, and the IFN-β, ISG54, TNF-α, and IL-12p40 mRNA levels were quantified by qRT-PCR, normalizing their levels to that of β-actin mRNA. The graph indicates the fold change relative to the mock-infected samples. (C) Transduced MDDCs were mock infected or infected with SeV. After 24 h, the cells were assayed by flow cytometry for the cell surface levels of CD40, CD83, and CD86. The change in mean fluorescence intensity (MFI) of infected MDDCs relative to the mock-infected MDDCs from three independent experiments is shown. (D) Transduced MDDCs were mock infected or infected with SeV and then cultured with purified naive CD4 T cells at the indicated ratios for 5 days. The cultured supernatants were harvested, and the amount of IFN-γ was determined by ELISA. Error bars indicate standard deviations for all panels, and an asterisk (*) indicates where P < 0.05 relative to empty vector-transduced MDDCs at the same time point.
To delineate whether there is a differential activity in eVP35 and mVP35 suppression of MDDC response by MDA5, another RLR receptor, we used EMCV, a known MDA5 activator (38), to stimulate the transduced MDDCs. Similar to the case for MDDCs infected with SeV, mVP35 greatly suppressed IFN-β, ISG54, TNF-α, and IL-12p40 expression as measured by qRT-PCR (Fig. 2A). The consistent but small differences between eVP35 and mVP35 that were seen for cytokine expression following SeV infection were largely absent with EMCV infection. DC maturation upon EMCV infection was also suppressed, since mVP35 suppressed the upregulation of DC maturation markers CD40, CD83, and CD86 (Fig. 2B). Cumulatively, these data demonstrate that mVP35, like eVP35, has the ability to inhibit DC responses upon activation of both the RIG-I and MDA5 pathways.
FIG 2.
mVP35 inhibits human MDDC responses similarly to eVP35 upon EMCV infection. (A) Transduced MDDCs were mock infected or infected with EMCV. RNA was extracted at the indicated hours postinfection; IFN-β, ISG54, TNF-α, and IL-12p40 mRNA levels were quantified by qRT-PCR, normalizing their levels to that of β-actin mRNA. The graph indicates the fold change of the EMCV-infected samples relative to the mock-infected samples. (B) Transduced MDDCs were mock infected or infected with EMCV. After 24 h, the cells were assayed by flow cytometry for the cell surface levels of CD40, CD83, and CD86. The change in mean fluorescence intensity (MFI) of infected MDDCs relative to the mock-infected MDDCs from three independent experiments is shown. Error bars indicate standard deviations for all panels, and an asterisk (*) indicates where P < 0.05 relative to empty vector-transduced MDDCs at the same time point.
eVP24 inhibits ISG induction but fails to suppress other MDDC responses.
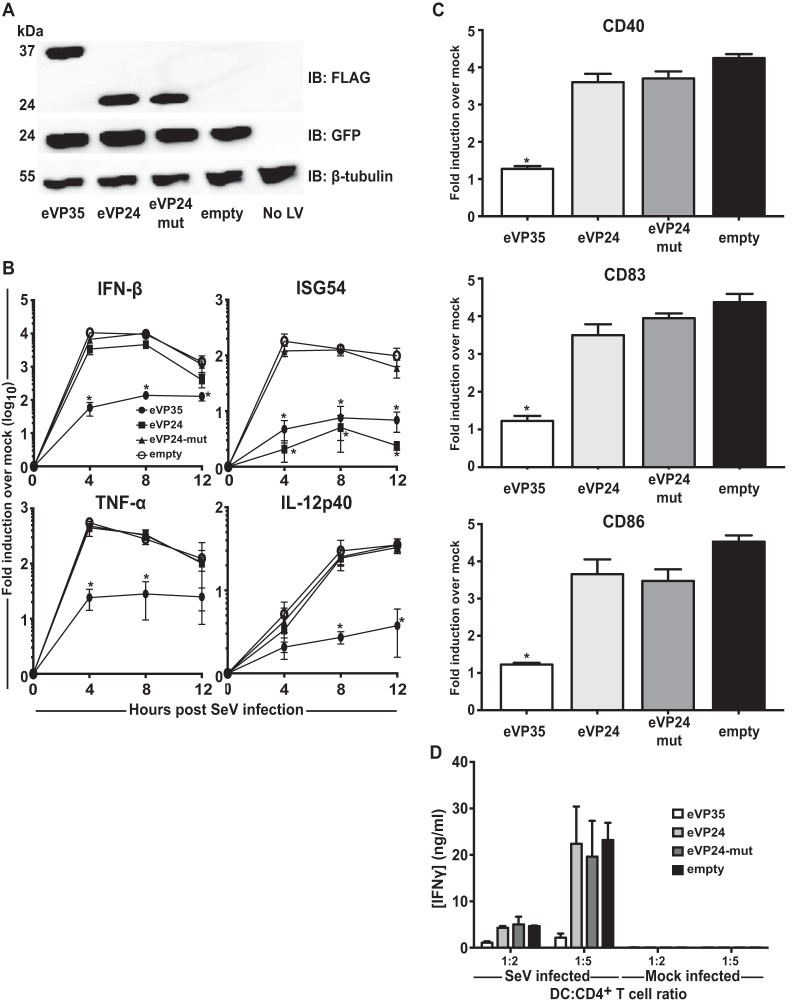
Next, we expanded our study to investigate the role of filoviral IFN antagonists other than eVP35 and mVP35. eVP24 antagonizes IFN-α/β and IFN-γ signaling pathways by inhibiting the nuclear translocation of PY-STAT1, thereby inhibiting ISG transcription (21). In addition, EBOVs with mutated eVP24s trigger altered DC responses (28, 32). However, whether eVP24 is sufficient to inhibit DC responses has not been investigated. MDDCs were transduced with empty vector lentivirus or lentiviruses expressing eVP35, wild-type eVP24 (eVP24), or an eVP24 mutant (Q184A/N185A/H186A) with impaired binding to KPNA5 and impaired suppression of IFN signaling (21). Expression of each protein was confirmed, and wild-type and mutant eVP24s expressed to similar levels, as assessed by Western blot (Fig. 3A). At 72 h postransduction, the cells were infected with SeV and analyzed by qRT-PCR. Whereas eVP35 nearly completely suppressed IFN-β mRNA expression, eVP24 and an eVP24 mutant did not significantly suppress accumulation of IFN-β, TNF-α, and IL-12p40 mRNAs (Fig. 3B). eVP24 potently suppressed the expression of ISG54 whereas the eVP24 mutant did not, a finding consistent with the described capacity of eVP24 to inhibit IFN-induced gene expression by a mechanism that involves KPNA interaction (Fig. 3B). Despite the suppression of IFN-β and ISG54 mRNAs, eVP24 did not suppress upregulation of DC maturation markers CD40, CD83, or CD86 upon SeV infection (Fig. 3C) and did not significantly affect allogeneic T cell activation (Fig. 3D). These data suggest that eVP24 is not sufficient to antagonize DC maturation.
FIG 3.
eVP24 does not prevent SeV-induced human MDDC maturation. (A) Human MDDCs were mock transduced (No LV) or transduced with empty lentiviral vector (empty) or lentivirus that expresses either Flag-tagged eVP35, eVP24, or VP24-mut. Cells from one representative donor were analyzed by Western blotting with anti-Flag antibody. Probing with β-tubulin antibody served as a loading control. (B) Transduced MDDCs were mock infected or infected with SeV. RNA was extracted at the indicated times in hours postinfection; the IFN-β, ISG54, TNF-α, and IL-12p40 mRNA levels were quantified qRT-PCR, normalizing their levels to that of β-actin mRNA. The graph indicates the fold change relative to the mock-infected samples. (C) Transduced MDDCs were mock infected or infected with SeV. After 24 h, the cells were assayed by flow cytometry for the cell surface levels of CD40, CD83, and CD86. The change in mean fluorescence intensity (MFI) of infected MDDCs relative to the mock-infected MDDCs from three independent experiments is shown. (D) Transduced MDDCs were mock infected or infected with SeV and then cultured with purified naive CD4 T cells at the indicated ratios for 5 days. Error bars indicate standard deviations for all panels, and an asterisk (*) indicates where P < 0.05 relative to empty vector-transduced MDDCs at the same time point.
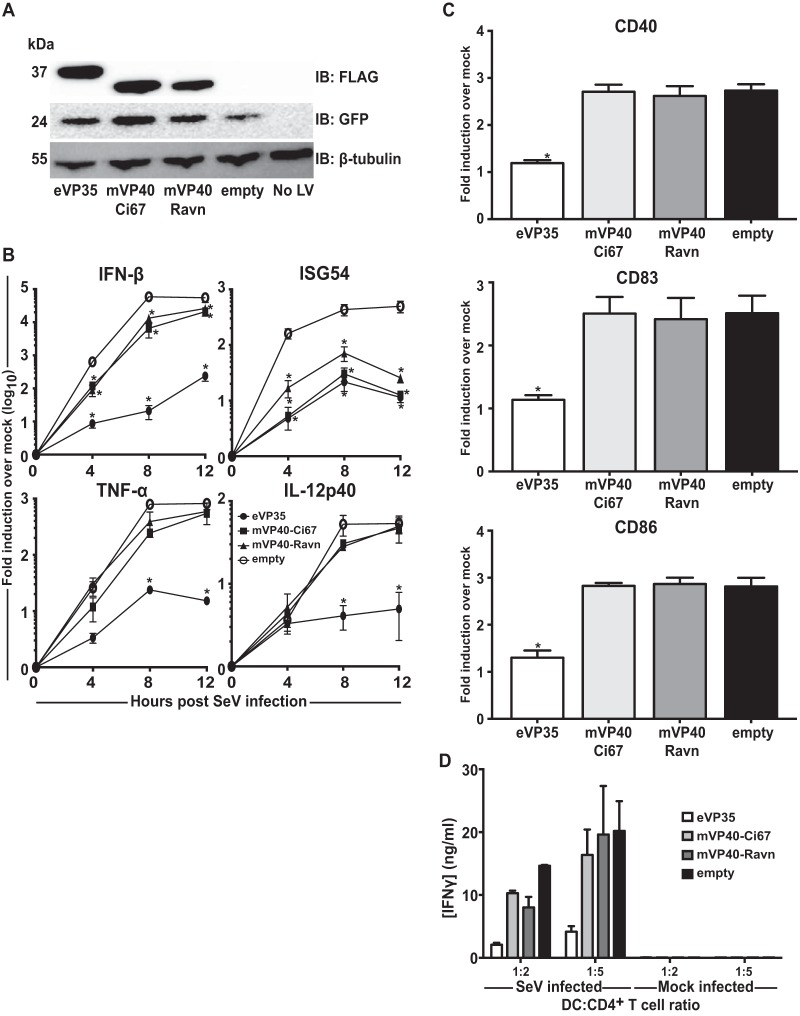
mVP40 can inhibit ISG induction but also fails to suppress MDDC responses.
mVP40 has also been demonstrated to inhibit IFN-α/β signaling by blocking the IFN-induced signaling of tyrosine kinase Jak1 (22). We asked whether mVP40 from two separate MARV clades, the Musoke strain (mVP40-Ci67) or the Ravn strain (mVP40-Ravn) can inhibit human DC responses. MDDCs were transduced with empty, eVP35, mVP40-Ci67 or mVP40-Ravn lentiviruses and subsequently infected with SeV (Fig. 4A). Analysis of qRT-PCR results demonstrated that mVP40-Ci67 and mVP40-Ravn significantly decreased IFN-β mRNA expression, although the decrease in induction was quite modest at all time points (Fig. 4B). This inhibition may reflect suppression of the IFN-α/β induced amplification loop whereby IFN-α/β induces RIG-I and IRF-7. Both mVP40s decreased the amount of ISG54 induction compared to control MDDCs, similar to the case with eVP24. Neither mVP40 blocked induction of TNF-α or IL-12p40 (Fig. 4B). Furthermore, mVP40-Ci67 and mVP40-Ravn did not suppress DC maturation upon SeV infection (Fig. 4C), nor did they inhibit allogeneic T cell activation (Fig. 4D). These data further demonstrate that mVP40 proteins, despite their ability to antagonize IFN signaling, are not sufficient to block DC maturation.
FIG 4.
mVP40 does not inhibit SeV-induced human MDDC responses. (A) Human MDDCs were mock transduced (No LV) or transduced with empty lentiviral vector (empty) or lentiviruses that express either Flag-tagged eVP35, mVP40-Ci67, or mVP40-Ravn. Cells from a representative donor were analyzed by Western blotting with anti-Flag antibody. Probing with β-tubulin antibody served as a loading control. (B) RNA was isolated from transduced MDDCs that were either mock infected or infected with SeV. Total RNA was extracted at the indicated times in hours postinfection; IFN-β, ISG54, TNF-α, and IL-12p40 mRNA levels were quantified by qRT-PCR, normalizing their levels to that of β-actin mRNA. The graph indicates the fold change relative to the mock-infected samples. (C) Transduced MDDCs were mock infected or infected with SeV. After 24 h, the cells were assayed by flow cytometry for the cell surface levels of CD40, CD83, and CD86. The change in mean fluorescence intensity (MFI) of infected MDDCs relative to the mock-infected MDDCs from three independent experiments is shown. (D) Transduced MDDCs were mock infected or infected with SeV and then cultured with purified naive CD4 T cells at the indicated ratios for 5 days. Error bars indicate standard deviations for all panels, and an asterisk (*) indicates where P < 0.05 relative to empty vector-transduced MDDCs at the same time point.
eVP24 and mVP40 inhibit IFN signaling in human MDDCs.
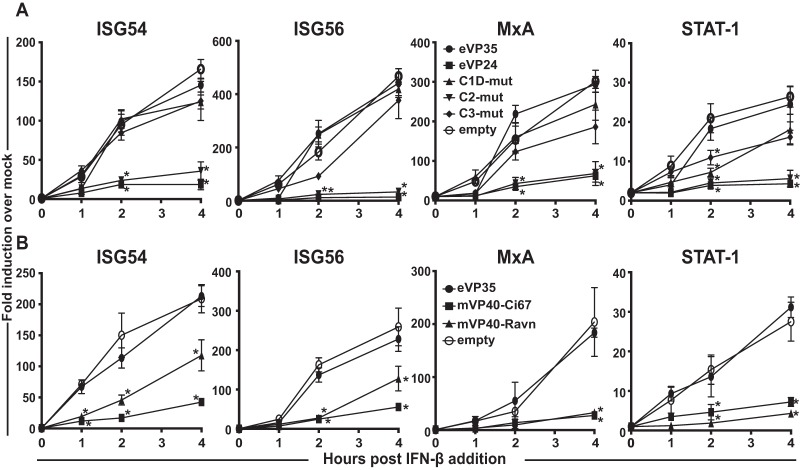
The suppression of ISG54 gene expression in response to SeV infection seen above is consistent with inhibition of IFN-induced Jak-STAT signaling in response to IFN-α/β. To more directly address whether eVP24 and mVP40 effectively block responses to IFN-α/β in primary DCs, experiments similar to those described above were performed, except human IFN-β was used to trigger MDDC responses. Consistent with published experiments in cell lines, eVP24 inhibited the induction of the ISGs ISG54, ISG56, MxA, and STAT1, whereas eVP35 did not affect induction of these ISGs (Fig. 5). Prior work identified three clusters of residues within eVP24 that directly contact KPNA5 (21). Mutant eVP24s termed cluster 1D, cluster 2, and cluster 3 differentially impacted eVP24-KPNA5 interaction, as well as eVP24 suppression of IFN signaling, with cluster 1D and cluster 3 mutants, resulting in a substantial loss of IFN antagonist activity in IFN-induced reporter gene assays (21). When tested in MDDCs that were stimulated with human IFN-β, the cluster 1D and cluster 3 mutants did not block expression of ISG54, ISG56, MxA, or STAT-1 (Fig. 5A). Consistent with its more modest effects on eVP24-KPNA5 binding, the cluster 2 mutant retained suppressing activity toward ISG induction (Fig. 5A). These data correlate inhibition of ISG expression by eVP24 in MDDCs with the eVP24-KPNA5 interaction, demonstrate that potent suppression of IFN signaling is retained in MDDCs, and indicate that suppression of ISG induction is not adequate to block MDDC maturation when SeV is used as the stimulus.
FIG 5.
Filoviral IFN antagonists inhibit IFN-induced gene expression in human MDDCs. (A) Human MDDCs were transduced with empty lentiviral vector (empty) or lentiviruses that express either Flag-tagged eVP35, eVP24, or indicated VP24 mutants. At 3 days after lentiviral transduction, the MDDCs were stimulated with 100 U of human IFN-β/ml. At the indicated time in hours posttreatment, total RNA was isolated and analyzed by qRT-PCR to quantify ISG54, ISG56, MxA, and STAT1 mRNA levels, which were normalized to the β-actin mRNA levels. Similar assays were performed for panel B, but the MDDCs were transduced with mVP40-Ci67 and mVP40-Ravn. Error bars indicate standard deviations for all panels, and an asterisk (*) indicates where P < 0.05 relative to empty vector-transduced MDDCs at the same time point.
We extended these analyses to mVP40. Expression of mVP40-Ci67 or mVP40-Ravn blocked human IFN-β-induced ISG expression, an observation consistent with the role of mVP40s as IFN signaling inhibitors (Fig. 5B). As with eVP24, this function appears insufficient to suppress DC maturation induced by SeV infection.
mVP24 does not inhibit human DC responses upon SeV infection.
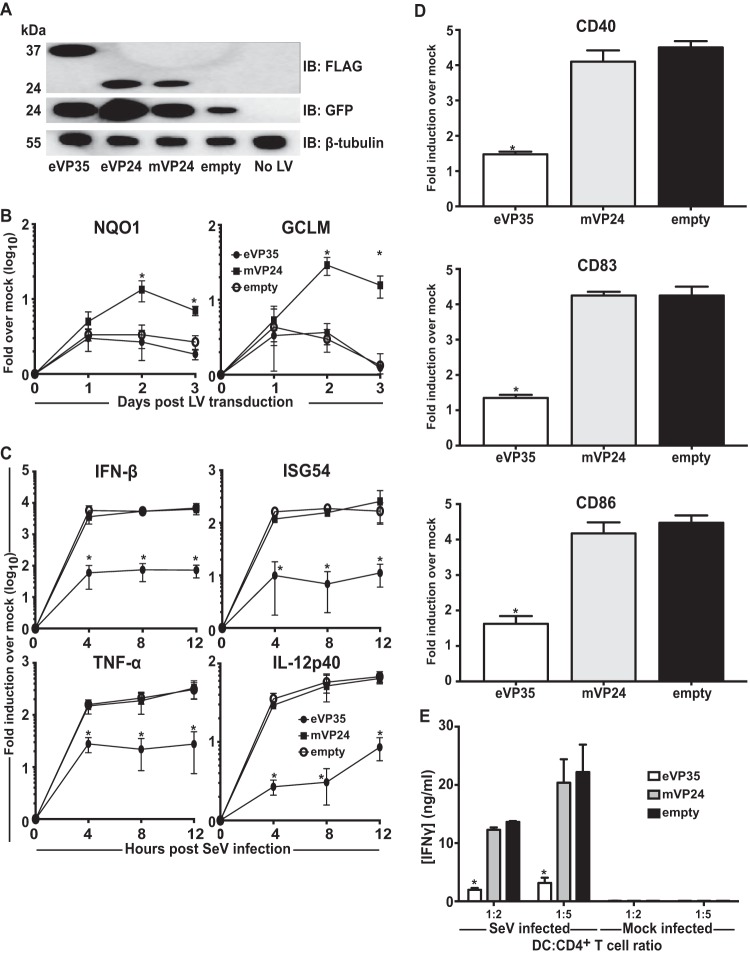
mVP24, unlike eVP24, does not block IFN signaling pathways. However, mVP24 has been shown to interact with cellular protein Keap1. This dissociates transcription factor Nrf2 from Keap1, allowing Nrf2 nuclear accumulation and induction of antioxidant response gene expression (23, 24). mVP24-Keap1 interaction may also promote NF-κB activation (25). Transduction of MDDCs with eVP35, eVP24, and mVP24 lentiviruses resulted in the expression of each protein (Fig. 6A). DCs expressing mVP24 did upregulate representative antioxidant response genes NQO1 and HO-1, whereas DCs expressing eVP35 or control DCs did not (Fig. 6B). However, mVP24 had no detectable effect on SeV-induced expression of IFN-β, ISG54, TNF-α, and IL-12p40 mRNA (Fig. 6C), nor did it suppress DC maturation (Fig. 6D) or allogeneic T cell activation (Fig. 6E). These data demonstrate that, despite affecting ARE genes, MARV-VP24 does not significantly alter DC responses to SeV infection.
FIG 6.
MARV-VP24 does not detectably modulate human MDDC responses to SeV infection. (A) Human MDDCs were mock transduced (No LV) or transduced with empty lentiviral vector (empty) or lentivirus vectors that express either Flag-tagged eVP35, eVP24, or mVP24. Cells from a representative donor were analyzed for Flag-tagged protein expression by Western blotting with anti-Flag antibody. (B) RNA was isolated from transduced MDDCs daily. The GCLM and NQO1 mRNA levels were quantified by qRT-PCR, normalizing their levels to that of β-actin mRNA. (C) RNA was isolated from transduced MDDCs that were either mock infected or infected with SeV. RNA was extracted at the indicated time in hours postinfection; the IFN-β, ISG54, TNF-α, and IL-12p40 mRNA levels were quantified as in panel B. (D) Transduced MDDCs were mock infected or infected with SeV. After 24 h, the cells were assayed by flow cytometry for the cell surface levels of CD40, CD83, and CD86. The change in the mean fluorescence intensity (MFI) of infected MDDCs relative to the mock-infected MDDCs from three independent experiments is shown. (E) Transduced MDDCs were mock infected or infected with SeV and then cultured with either purified naive CD4 T cells at the indicated ratios for 5 days. The cultured supernatants were harvested, and the amount of IFN-γ was determined by ELISA. Error bars indicate standard deviations for all panels, and an asterisk (*) indicates where P < 0.05 relative to the empty vector-transduced MDDCs at the same time point.
DISCUSSION
DCs are significant targets of filovirus infection in vivo, and in vitro studies have demonstrated the productive replication of EBOV and MARV in MDDCs (26–28, 32, 39). In spite of active replication in these cells, EBOV-infected MDDCs fail to undergo maturation and do not activate naive T cells (26–28). It is proposed that effective suppression of DC maturation may dampen adaptive immunity and contribute to a failure of the host to control infection (40). Previous studies demonstrated that expression of eVP35 alone is sufficient to strongly impair DC function upon infection with SeV or EMCV, as evidenced by lack of IFN-α/β production, reduced cytokine production, the absence of cell surface marker upregulation, and the failure to stimulate T cell responses (31). Introduction into recombinant EBOVs of mutations known to impair eVP35 inhibition of RIG-I results in viruses that do stimulate DC maturation, confirming a role for VP35 in suppression of DC responses in the context of EBOV infection (28).
Whether and to what extent other filoviral proteins contribute to suppression of DC maturation and function has been less clear. eVP35 and mVP35 each inhibit RIG-I signaling and, for each protein, mutations that disrupt dsRNA binding activity largely ablate this inhibitory activity (5, 9–11). However, a combination of structural and biochemical approaches suggest differences in how eVP35 and mVP35 interact with dsRNAs. More specifically, eVP35 “caps” the ends of dsRNA molecules, whereas mVP35 likely does not (9, 10, 33, 34). The capping activity of eVP35 can mask a 5′-triphosphate, which would otherwise be recognized by and contribute to activation of RIG-I. The lack of end-capping by mVP35 correlates with a less potent suppression of RIG-I signaling (34). This correlates with a greater activation of IFN-α/β responses in MARV-infected THP-1 cells than in EBOV-infected THP-1 cells (34). It might also be expected to impair suppression of DC maturation by mVP35. Further, because MDA5 is activated by long dsRNA molecules and does not recognize 5′-triphosphates, the absence of end-capping by mVP35 might have less impact on suppression induced by the MDA5 activator EMCV (38).
In our experimental system, eVP35 and mVP35 each strongly suppressed IFN-α/β and cytokine responses relative to the empty vector controls. Statistical analysis demonstrated significant suppression of SeV-induced IFN-β, IFN-inducible ISG54, and proinflammatory cytokine (TNF-α and IL-12p40) expression levels by both eVP35 and mVP35. Similar results were obtained in EMCV-infected cells. In the present experimental system, where lentivirus vectors are used to express the VP35s, it is difficult to vary expression levels. It is therefore likely that modest differences in eVP35 versus mVP35 activities are masked by expression levels. Our data therefore indicate that, when expressed to a sufficient level, either eVP35 or mVP35 is sufficient to potently suppress RLR-induced DC maturation.
In prior studies, mutations that disrupt eVP35 RIG-I inhibitory function allowed EBOV to trigger DC maturation; however, a K142A eVP24 point mutation that impairs interaction with KPNAs and decreases eVP24 inhibition of IFN signaling also affected the inhibition of DC maturation, leading to the conclusion that eVP35 and eVP24 work in cooperation to impair DC function (28). Global analysis of DC gene expression after infection with wild-type and mutant eVP35 and eVP24 viruses demonstrated little host response to wild-type virus infection and identified an impact of eVP24 mutations that was kinetically different from changes induced by the eVP35 virus (32). These results support the possibility that eVP24 IFN signaling inhibition contributes to suppression of DC maturation.
Interestingly, in our system wild-type eVP24 had little to no effect on IFN-β, TNF-α, or IL-12p40 mRNA levels compared to the control lentivirus. However, ISG54 mRNA induction by SeV was suppressed by eVP24 but not by a mutant with impaired capacity to interact with KPNA5, a finding consistent with the fact that KPNA interaction is responsible for eVP24 inhibition of IFN-induced Jak-STAT signaling and results in suppression of IFN-stimulated gene (ISG) expression (18–21).
To more fully address the function of eVP24 as an IFN-antagonist in a biologically relevant primary human cell, we tested eVP24 and additional mutants previously demonstrated to impair interaction with KPNA and to decrease eVP24 suppression of ISG expression in response to exogenously added IFN-β (21). Our results demonstrate that eVP24 does effectively block IFN-α/β-induced signaling in MDDCs and that the inhibition occurs by the established mechanism, whereby inhibition requires interaction with KPNAs that otherwise promote nuclear accumulation of tyrosine phosphorylated STAT1. Despite the potent inhibition of ISG expression, eVP24 did not impair proinflammatory cytokine responses, the upregulation of maturation markers, or T cell stimulation. These data suggest that eVP24 expression is not sufficient on its own to block DC maturation.
We also sought to determine whether the MARV IFN signaling inhibitor protein, mVP40, or the MARV homologue to eVP24, mVP24, might also affect DC maturation. We tested mVP40 from the Ci67 and Ravn viruses, which belong to different clades but which each inhibit IFN-α/β signaling by inhibiting tyrosine kinase Jak1 (22, 41). Like eVP24, mVP40s could potently block IFN-β- or SeV-induced ISG expression. SeV-induced IFN-β mRNA expression was modestly but significantly impaired in the presence of mVP40s relative to the empty vector control. Because IFN-β triggers a positive-feedback loop that enhances the IFN-α/β response, for example by upregulating RIG-I and transcription factor IRF-7, and because SeV infection causes the rapid expression of IFN-β, it seems likely that mVP40 suppression of IFN-β gene expression following SeV infection reflects a suppression of this positive feedback loop. Such a mechanism would also account for the incomplete suppression of IFN-β expression and the nearly complete suppression of the IFN-inducible ISG54 gene. For the mVP40 set of experiments, the inhibition by eVP35 was less absolute than in some other experiments. Although the reason for this difference is not known, this experimental variation may diminish the impact of mVP40 on IFN-β expression. Nonetheless, these data, as well as the maturation marker and T cell stimulation data, indicate that, like eVP24, mVP40 is not able to suppress DC maturation on its own. The combined data with eVP24 and mVP40 indicate that blocking IFN-α/β-induced Jak-STAT signaling is in sufficient to prevent DC maturation and function, at least when a potent RIG-I activator is the stimulus driving the maturation. Some studies have identified a role for IFN-α/β signaling in DC responses to stimuli such as TLR agonists (42). However, other studies that used SeV found DC responses to occur independently of IFN-α/β signaling (43).
mVP24 was able to stimulate expression of antioxidant response genes in MDDCs, a finding consistent with its previously reported function, whereby it activates transcription factor Nrf2 through interaction with Keap1, an Nrf2 inhibitor (23, 24). However, it had neither a positive nor a negative effect on any measure of MDDC maturation or function. This is despite the fact that mVP24 can, in transfection studies, modestly upregulate NF-κB responses, presumably by blocking the degradation of IKKβ by Keap1 (25). It is likely that any effects on IKKβ are masked by the strong NF-κB response triggered by SeV infection.
Cumulatively, these data suggest that the VP35 proteins of EBOV and MARV are the primary suppressors of DC maturation and that this suppression occurs through inhibition of RLR signaling. The IFN signaling inhibitors, although functional in DCs, play an auxiliary role in DCs by blocking the IFN-induced positive-feedback loop that promotes upregulation of RLRs. Previous work had shown that it is possible to circumvent eVP35 inhibition by activating other non-RLR immune stimulatory pathways (31). Thus, it would of interest to identify ligands that can stimulate non-RLR pathways and result in both IFN-α/β production and activation of human DCs to enhance immunity during filoviral infection.
ACKNOWLEDGMENTS
This study was supported by NIH-NIAID grants R01AI059536 and U19AI109945 to C.F.B.
We thank Christine Schwall-Pecci for critical reading of the manuscript and the Department of Microbiology at Icahn School of Medicine at Mount Sinai for insightful discussions and reagents. We thank Dan Littman (New York University and Howard Hughes Medical Institute) for the gift of the pSIV3+ plasmid.
REFERENCES
- 1.Feldmann H, Geisbert TW. 2011. Ebola haemorrhagic fever. Lancet 377:849–862. doi: 10.1016/S0140-6736(10)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. 2016. Ebola situation report, 17 February 2016. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 3.Messaoudi I, Amarasinghe GK, Basler CF. 2015. Filovirus pathogenesis and immune evasion: insights from Ebola virus and Marburg virus. Nat Rev Microbiol 13:663–676. doi: 10.1038/nrmicro3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basler CF, Amarasinghe GK. 2009. Evasion of interferon responses by Ebola and Marburg viruses. J Interferon Cytokine Res 29:511–520. doi: 10.1089/jir.2009.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardenas WB, Loo YM, Gale M Jr, Hartman AL, Kimberlin CR, Martinez-Sobrido L, Saphire EO, Basler CF. 2006. Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling. J Virol 80:5168–5178. doi: 10.1128/JVI.02199-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prins KC, Cardenas WB, Basler CF. 2009. Ebola virus protein VP35 impairs the function of interferon regulatory factor-activating kinases IKKεand TBK-1. J Virol 83:3069–3077. doi: 10.1128/JVI.01875-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang TH, Kubota T, Matsuoka M, Jones S, Bradfute SB, Bray M, Ozato K. 2009. Ebola Zaire virus blocks type I interferon production by exploiting the host SUMO modification machinery. PLoS Pathog 5:e1000493. doi: 10.1371/journal.ppat.1000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luthra P, Ramanan P, Mire CE, Weisend C, Tsuda Y, Yen B, Liu G, Leung DW, Geisbert TW, Ebihara H, Amarasinghe GK, Basler CF. 2013. Mutual antagonism between the Ebola virus VP35 protein and the RIG-I activator PACT determines infection outcome. Cell Host Microbe 14:74–84. doi: 10.1016/j.chom.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung DW, Prins KC, Borek DM, Farahbakhsh M, Tufariello JM, Ramanan P, Nix JC, Helgeson LA, Otwinowski Z, Honzatko RB, Basler CF, Amarasinghe GK. 2010. Structural basis for dsRNA recognition and interferon antagonism by Ebola VP35. Nat Struct Mol Biol 17:165–172. doi: 10.1038/nsmb.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramanan P, Edwards MR, Shabman RS, Leung DW, Endlich-Frazier AC, Borek DM, Otwinowski Z, Liu G, Huh J, Basler CF, Amarasinghe GK. 2012. Structural basis for Marburg virus VP35-mediated immune evasion mechanisms. Proc Natl Acad Sci U S A 109:20661–20666. doi: 10.1073/pnas.1213559109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bale S, Julien JP, Bornholdt ZA, Kimberlin CR, Halfmann P, Zandonatti MA, Kunert J, Kroon GJ, Kawaoka Y, MacRae IJ, Wilson IA, Saphire EO. 2012. Marburg virus VP35 can both fully coat the backbone and cap the ends of dsRNA for interferon antagonism. PLoS Pathog 8:e1002916. doi: 10.1371/journal.ppat.1002916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bale S, Julien JP, Bornholdt ZA, Krois AS, Wilson IA, Saphire EO. 2013. Ebolavirus VP35 coats the backbone of double-stranded RNA for interferon antagonism. J Virol 87:10385–10388. doi: 10.1128/JVI.01452-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basler CF, Wang X, Muhlberger E, Volchkov V, Paragas J, Klenk HD, Garcia-Sastre A, Palese P. 2000. The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc Natl Acad Sci U S A 97:12289–12294. doi: 10.1073/pnas.220398297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartman AL, Dover JE, Towner JS, Nichol ST. 2006. Reverse genetic generation of recombinant Zaire Ebola viruses containing disrupted IRF-3 inhibitory domains results in attenuated virus growth in vitro and higher levels of IRF-3 activation without inhibiting viral transcription or replication. J Virol 80:6430–6440. doi: 10.1128/JVI.00044-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartman AL, Towner JS, Nichol ST. 2004. A C-terminal basic amino acid motif of Zaire ebolavirus VP35 is essential for type I interferon antagonism and displays high identity with the RNA-binding domain of another interferon antagonist, the NS1 protein of influenza A virus. Virology 328:177–184. doi: 10.1016/j.virol.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Albarino CG, Wiggleton Guerrero L, Spengler JR, Uebelhoer LS, Chakrabarti AK, Nichol ST, Towner JS. 2015. Recombinant Marburg viruses containing mutations in the IID region of VP35 prevent inhibition of Host immune responses. Virology 476:85–91. doi: 10.1016/j.virol.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartman AL, Bird BH, Towner JS, Antoniadou ZA, Zaki SR, Nichol ST. 2008. Inhibition of IRF-3 activation by VP35 is critical for the high level of virulence of Ebola virus. J Virol 82:2699–2704. doi: 10.1128/JVI.02344-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mateo M, Reid SP, Leung LW, Basler CF, Volchkov VE. 2010. Ebolavirus VP24 binding to karyopherins is required for inhibition of interferon signaling. J Virol 84:1169–1175. doi: 10.1128/JVI.01372-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reid SP, Leung LW, Hartman AL, Martinez O, Shaw ML, Carbonnelle C, Volchkov VE, Nichol ST, Basler CF. 2006. Ebola virus VP24 binds karyopherin α1 and blocks STAT1 nuclear accumulation. J Virol 80:5156–5167. doi: 10.1128/JVI.02349-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reid SP, Valmas C, Martinez O, Sanchez FM, Basler CF. 2007. Ebola virus VP24 proteins inhibit the interaction of NPI-1 subfamily karyopherin alpha proteins with activated STAT1. J Virol 81:13469–13477. doi: 10.1128/JVI.01097-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu W, Edwards MR, Borek DM, Feagins AR, Mittal A, Alinger JB, Berry KN, Yen B, Hamilton J, Brett TJ, Pappu RV, Leung DW, Basler CF, Amarasinghe GK. 2014. Ebola virus VP24 targets a unique NLS binding site on karyopherin alpha 5 to selectively compete with nuclear import of phosphorylated STAT1. Cell Host Microbe 16:187–200. doi: 10.1016/j.chom.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valmas C, Grosch MN, Schumann M, Olejnik J, Martinez O, Best SM, Krahling V, Basler CF, Muhlberger E. 2010. Marburg virus evades interferon responses by a mechanism distinct from Ebola virus. PLoS Pathog 6:e1000721. doi: 10.1371/journal.ppat.1000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page A, Volchkova VA, Reid SP, Mateo M, Bagnaud-Baule A, Nemirov K, Shurtleff AC, Lawrence P, Reynard O, Ottmann M, Lotteau V, Biswal SS, Thimmulappa RK, Bavari S, Volchkov VE. 2014. Marburgvirus hijacks nrf2-dependent pathway by targeting nrf2-negative regulator keap1. Cell Rep 6:1026–1036. doi: 10.1016/j.celrep.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 24.Edwards MR, Johnson B, Mire CE, Xu W, Shabman RS, Speller LN, Leung DW, Geisbert TW, Amarasinghe GK, Basler CF. 2014. The Marburg virus VP24 protein interacts with Keap1 to activate the cytoprotective antioxidant response pathway. Cell Rep 6:1017–1025. doi: 10.1016/j.celrep.2014.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwards MR, Basler CF. 2015. Marburg virus VP24 protein relieves suppression of the NF-κB pathway through interaction with Kelch-like ECH-associated protein 1. J Infect Dis 212(Suppl 2):S154–S159. doi: 10.1093/infdis/jiv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bosio CM, Aman MJ, Grogan C, Hogan R, Ruthel G, Negley D, Mohamadzadeh M, Bavari S, Schmaljohn A. 2003. Ebola and Marburg viruses replicate in monocyte-derived dendritic cells without inducing the production of cytokines and full maturation. J Infect Dis 188:1630–1638. doi: 10.1086/379199. [DOI] [PubMed] [Google Scholar]
- 27.Mahanty S, Hutchinson K, Agarwal S, McRae M, Rollin PE, Pulendran B. 2003. Cutting edge: impairment of dendritic cells and adaptive immunity by Ebola and Lassa viruses. J Immunol 170:2797–2801. doi: 10.4049/jimmunol.170.6.2797. [DOI] [PubMed] [Google Scholar]
- 28.Lubaki NM, Ilinykh P, Pietzsch C, Tigabu B, Freiberg AN, Koup RA, Bukreyev A. 2013. The lack of maturation of Ebola virus-infected dendritic cells results from the cooperative effect of at least two viral domains. J Virol 87:7471–7485. doi: 10.1128/JVI.03316-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin H, Yan Z, Prabhakar BS, Feng Z, Ma Y, Verpooten D, Ganesh B, He B. 2010. The VP35 protein of Ebola virus impairs dendritic cell maturation induced by virus and lipopolysaccharide. J Gen Virol 91:352–361. doi: 10.1099/vir.0.017343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leung LW, Park MS, Martinez O, Valmas C, Lopez CB, Basler CF. 2011. Ebolavirus VP35 suppresses IFN production from conventional but not plasmacytoid dendritic cells. Immunol Cell Biol 89:792–802. doi: 10.1038/icb.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yen B, Mulder LC, Martinez O, Basler CF. 2014. Molecular basis for ebolavirus VP35 suppression of human dendritic cell maturation. J Virol 88:12500–12510. doi: 10.1128/JVI.02163-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ilinykh PA, Lubaki NM, Widen SG, Renn LA, Theisen TC, Rabin RL, Wood TG, Bukreyev A. 2015. Different temporal effects of Ebola virus VP35 and VP24 proteins on global gene expression in human dendritic cells. J Virol 89:7567–7583. doi: 10.1128/JVI.00924-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimberlin CR, Bornholdt ZA, Li S, Woods VL Jr, MacRae IJ, Saphire EO. 2010. Ebolavirus VP35 uses a bimodal strategy to bind dsRNA for innate immune suppression. Proc Natl Acad Sci U S A 107:314–319. doi: 10.1073/pnas.0910547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edwards MR, Liu G, Mire CE, Sureshchandra S, Luthra P, Yen B, Shabman RS, Leung DW, Messaoudi I, Geisbert TW, Amarasinghe GK, Basler CF. 2016. Differential regulation of interferon responses by Ebola and Marburg virus VP35 proteins. Cell Rep 14:1632–1640. doi: 10.1016/j.celrep.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berger G, Durand S, Goujon C, Nguyen XN, Cordeil S, Darlix JL, Cimarelli A. 2011. A simple, versatile and efficient method to genetically modify human monocyte-derived dendritic cells with HIV-1-derived lentiviral vectors. Nat Protoc 6:806–816. doi: 10.1038/nprot.2011.327. [DOI] [PubMed] [Google Scholar]
- 36.Manel N, Hogstad B, Wang Y, Levy DE, Unutmaz D, Littman DR. 2010. A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature 467:214–217. doi: 10.1038/nature09337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okano S, Yonemitsu Y, Shirabe K, Kakeji Y, Maehara Y, Harada M, Yoshikai Y, Inoue M, Hasegawa M, Sueishi K. 2011. Provision of continuous maturation signaling to dendritic cells by RIG-I-stimulating cytosolic RNA synthesis of Sendai virus. J Immunol 186:1828–1839. doi: 10.4049/jimmunol.0901641. [DOI] [PubMed] [Google Scholar]
- 38.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 39.Geisbert TW, Hensley LE, Larsen T, Young HA, Reed DS, Geisbert JB, Scott DP, Kagan E, Jahrling PB, Davis KJ. 2003. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. Am J Pathol 163:2347–2370. doi: 10.1016/S0002-9440(10)63591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bray M, Geisbert TW. 2005. Ebola virus: the role of macrophages and dendritic cells in the pathogenesis of Ebola hemorrhagic fever. Int J Biochem Cell Biol 37:1560–1566. doi: 10.1016/j.biocel.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 41.Feagins AR, Basler CF. 2015. Amino acid residue 79 of Marburg virus VP40 confers interferon-antagonism in mouse cells. J Infect Dis 212(Suppl 2):S219–S225. doi: 10.1093/infdis/jiv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gautier G, Humbert M, Deauvieau F, Scuiller M, Hiscott J, Bates EE, Trinchieri G, Caux C, Garrone P. 2005. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med 201:1435–1446. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopez CB, Garcia-Sastre A, Williams BR, Moran TM. 2003. Type I interferon induction pathway, but not released interferon, participates in the maturation of dendritic cells induced by negative-strand RNA viruses. J Infect Dis 187:1126–1136. doi: 10.1086/368381. [DOI] [PubMed] [Google Scholar]