ABSTRACT
The NF-κB signaling network, which is an ancient signaling pathway, plays a pivotal role in innate immunity and constitutes a first line of defense against invading pathogens, including viruses. However, numerous viruses possess evolved strategies to antagonize the activation of the NF-κB signaling pathway. Our previous study demonstrated that the nonstructural protein 2C of enterovirus 71 (EV71), which is the major pathogen of hand, foot, and mouth disease, inhibits tumor necrosis factor alpha (TNF-α)-mediated activation of NF-κB by suppressing IκB kinase β (IKKβ) phosphorylation. Nevertheless, the mechanism underlying the inhibition of IKKβ phosphorylation by EV71 2C remains largely elusive. We demonstrate that EV71 2C interacts with all isoforms of the protein phosphatase 1 (PP1) catalytic subunit (the PP1α, PP1β, and PP1γ isoforms) through PP1-docking motifs. EV71 2C has no influence on the subcellular localization of PP1. In addition, the PP1-binding-deficient EV71 2C mutant 3E3L nearly completely lost the ability to suppress IKKβ phosphorylation and NF-κB activation was markedly restored in the mutant, thereby indicating that PP1 binding is efficient for EV71 2C-mediated inhibition of IKKβ phosphorylation and NF-κB activation. We further demonstrate that 2C forms a complex with PP1 and IKKβ to dephosphorylate IKKβ. Notably, we reveal that other human enteroviruses, including poliovirus (PV), coxsackie A virus 16 (CVA16), and coxsackie B virus 3 (CVB3), use 2C proteins to recruit PP1, leading to the inhibition of IKKβ phosphorylation. Our findings indicate that enteroviruses exploit a novel mechanism to inhibit IKKβ phosphorylation by recruiting PP1 and IKKβ to form a complex through 2C proteins, which ultimately results in the inhibition of the NF-κB signaling pathway.
IMPORTANCE The innate antiviral immunity system performs an essential function in recognizing and eliminating invading viruses. Enteroviruses include a number of important human pathogens, including poliovirus (PV), EV71, and coxsackieviruses (CVs). As 2C is the most conserved and complex nonstructural protein of enteroviruses, its biological function is largely unclear, whereas the 2A and 3C proteinases of enteroviruses are well characterized. We reveal that EV71 2C forms a complex with PP1 and IKKβ to maintain IKKβ in an unphosphorylated and inactive state, resulting in the inactivation of the TNF-α-mediated NF-κB signaling pathway. We provide evidence that the 2C proteins of the enteroviruses PV, CVA16, and CVB3 suppress IKKβ phosphorylation through the same mechanism involving PP1. We demonstrate that enteroviruses exploit a novel mechanism involving PP1 to regulate innate antiviral immunity, and our findings may be particularly important for understanding the pathogenicity of enteroviruses.
INTRODUCTION
The Enterovirus genus, which belongs to the Picornaviridae family, comprises small, single-stranded, nonenveloped, positive-sense RNA viruses. Enteroviruses include numerous important human pathogens, including poliovirus (PV), coxsackievirus A (CVA), coxsackievirus B (CVB), echoviruses (Echo), and numbered enteroviruses (EVs) (1). Compared with original enteroviruses (like PV, CVA, CVB, and Echo), EV71, known as a common pathogen of hand, foot, and mouth disease (HFMD), is a new type of enterovirus that belongs to the human enterovirus A species (1). EV71 was first isolated in 1969 from the stool of a 9-month-old child with encephalitis in California. Since then, EV71 infection has been reported worldwide, particularly in China and Southeastern Asia (2–4). EV71 mainly infects children aged 6 to 59 months and rarely infects adults. The virus typically causes HFMD with neurological and systemic complications, such as poliomyelitis-like acute flaccid paralysis and brainstem encephalitis; infection with the virus can even be fatal. Thus, EV71 will be considered the most virulent human pathogen after PV has been eradicated (5).
Control of virus infection in the host cell is initiated through the innate immunity system, which is mediated by the cooperation of a variety of pattern recognition receptors (PRRs). The NF-κB signaling network is an ancient signaling pathway that was initially found in unicellular organisms and is a central regulator in innate immunity (6). Its activation is tightly controlled by the IκB kinase (IKK) complex, which consists of two catalytic subunits (IKKα and IKKβ) and the NF-κB essential module NEMO, or IKKγ. The canonical NF-κB signaling pathway is activated by the engagement of various stimuli, such as tumor necrosis factor alpha (TNF-α), interleukin-1, lipopolysaccharide, lipopeptides, and viruses with their receptors. TNF-α-activated signaling serves as a model of the canonical NF-κB pathway (7). The engagement of TNF-α with its receptor, TRAF2, phosphorylates and activates the IKK complex (8), inducing the phosphorylation of IκB and its release from the NF-κB–IκB inhibitory complex and ultimate degradation by the 26S proteasome. The liberated NF-κB molecule, which possesses nuclear localization signal (NLS) regions, subsequently enters the nucleus and activates target gene transcription (9).
To restrict and circumvent the host antiviral immune system, enteroviruses have evolved sophisticated strategies to antagonize type I interferon (IFN) production. IFN antagonizes the activity of enteroviruses, and such antagonism is largely due to viral cleavage to major PRRs or the key molecule of IFN signaling pathways that is mediated by viral 2A and 3C proteinases or virus-induced caspases (10). PV-induced caspases cleave MDA-5 and MAVS (11, 12). The CVB3 3C proteinase cleaves TRIF, MAVS, FAK, and IκB (13–15). Like the PV-induced caspases and CVB3 3C proteinase, the EV71 3C proteinase cleaves IRIF and IRF7 and prevents the association of RIG-I with MAVS (16–18), whereas the EV71 2A proteinase cleaves MAVS (19) and EV71-induced caspases cleave MDA-5 (20). 2C proteins are the most conserved and complex nonstructural proteins of enteroviruses. However, in comparison with the biological functions of viral proteinases, which are well characterized, the biological function of the 2C proteins in innate immunity system remains unclear. We previously demonstrated that the EV71 2C protein interacts with IκB kinase β (IKKβ) and suppresses IKKβ phosphorylation to inhibit the TNF-α-mediated activation of NF-κB (21). However, the molecular mechanism underlying the inhibition of IKKβ phosphorylation is uncertain. As for the other enteroviruses, whether their 2C proteins regulate the NF-κB signaling pathway remains to be fully addressed.
In the current study, we demonstrate that EV71 2C forms a complex with protein phosphatase 1 (PP1) and IKKβ, which results in the inactivation of the TNF-α-mediated NF-κB signaling pathway. We provide evidence that the 2C proteins of PV, CVA16, and CVB3 all interact with PP1 through PP1-binding motifs, leading to the inhibition of IKKβ phosphorylation. Our study identifies a novel mechanism by which enteroviruses regulate the NF-κB signaling pathway through the formation of a PP1-2C-IKKβ complex.
MATERIALS AND METHODS
Virus, cell culture, and transfection.
The human EV71 BrCr strain was obtained from the Institute of Medical Biology, Chinese Academy of Medical Science. Human embryonic kidney 293T (293T) cells (China Center for Type Culture Collection [CCTCC]) and HeLa cells (CCL-2; American Type Culture Collection) were cultured in Dulbecco's modified Eagle's medium (Gibco) containing 10% fetal bovine serum (Gibco) at 37°C with 5% CO2. 293T cells and HeLa cells were transfected with the calcium phosphate ProFection mammalian transfection system (Promega) and the Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions.
Plasmids.
Hemagglutinin-tagged 2C (2C-HA), Flag-tagged 2C (2C-Flag), HA-IKKβ, NF-κB–luciferase (Luc), and pRL-TK were prepared as previously described (21). HA-PP1α was a gift from Thomas Leung (Institute of Molecular and Cell Biology, Singapore) (22), and Flag-PP1α, Flag-PP1β, and Flag-PP1γ were kindly provided by Irene L. Andrulis (Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Canada) (23). The pNTAP-A expression vector was purchased from Stratagene. pNTAP-A-2C was obtained by PCR amplification of the open reading frame (ORF) of EV71 2C and then cloned into the pNTAP-A vector through the use of the BamHI and EcoRI restriction enzymes. EV71 2C mutations (in which the amino acid substitution was in the PP1-binding motifs) Mut1-Flag, Mut2-Flag, Mut3-Flag, and 3E3L-Flag (see Fig. 4A and 5A) were constructed by multistep overlap PCR amplification and subsequently inserted into the pCAGGS expression vector through the use of the XhoI and SmaI restriction enzymes. The ORFs of the PV, CVA16, and CVB3 2C proteins were amplified by PCR from full-length PV, CVA16, and CVB3 cDNA clones, respectively, and then cloned into the pCAGGS expression vector through the use of the XhoI and SmaI restriction enzymes. The resulting constructs were designated PV-2C-Flag, CVA16-2C-Flag, and CVB3-2C-Flag, respectively. Full-length PV, CVA16, and CVB3 cDNA clones were provided courtesy of Panyong Mao (Institute of Infectious Diseases, Beijing 302 Hospital, Beijing, China) (24), Bo Zhang (Wuhan Institute of Virology, Chinese Academy of Sciences, China) (25), Huipeng Chen (Institute of Biotechnology, Academy of Military Medical Science, China) (26), respectively. The sequences of all constructs were verified by DNA sequencing to possess 100% agreement with the original sequence. The primers used in this study are summarized in Table 1.
FIG 4.
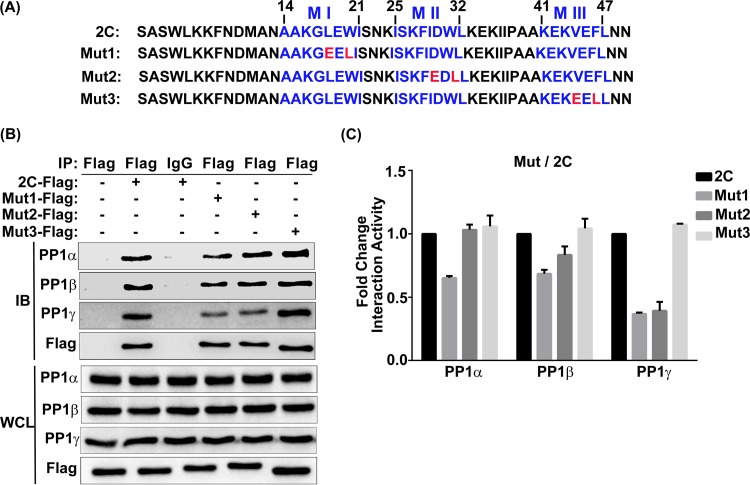
Interaction of the individual mutated EV71 2C PP1-binding motifs with PP1c. (A) Analysis of the protein sequences of wild-type 2C in comparison with those of 2C mutants Mut1, Mut2, and Mut3. The three PP1-binding motifs of 2C are displayed in blue, and the amino acids replaced by E or L are in red. Numbers indicate the amino acid location in the 2C protein. (B) 293T cells were transfected with a vector, 2C-Flag, or a Flag-tagged mutated 2C sequence. At 24 h posttransfection, cells were harvested and lysed. The whole-cell lysate was immunoprecipitated with anti-Flag or control mouse IgG, and the immunoblots were probed using the corresponding antibodies. (C) Quantification of the proteins in the IP panels from panel B using ImageJ software; the results shown (mean ± SD fold change) are representative of those from three independent experiments.
FIG 5.
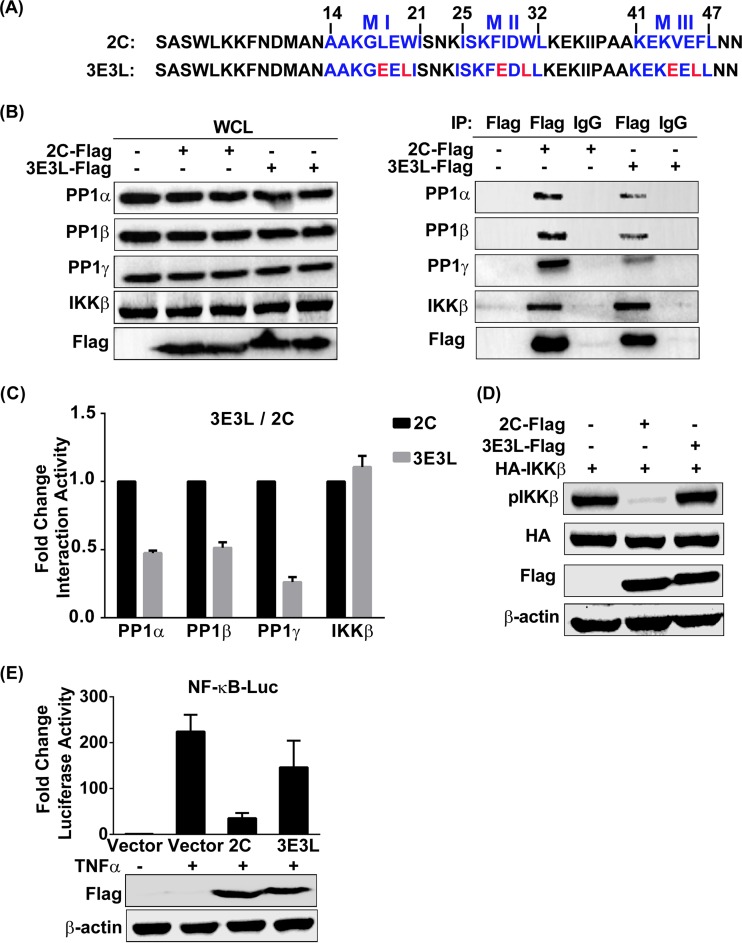
A PP1-binding-deficient mutant of EV71 2C barely inhibits IKKβ phosphorylation and NF-κB activation. (A) Analysis of the protein sequences of wild-type 2C in comparison with those of 2C mutant 3E3L. (B) 293T cells were transfected with the vector, 2C-Flag, or the Flag-tagged 3E3L mutant. At 24 h posttransfection, cells were harvested and lysed. The whole-cell lysate was immunoprecipitated with anti-Flag or control mouse IgG, and the immunoblots were probed with the corresponding antibodies. (Left) Confirmation of expression of the indicated protein by immunoblotting; (right) detection of endogenous PP1 in an immunoprecipitated sample. (C) Quantification of the proteins in the IP panels from panel B by using ImageJ software. The results shown (mean ± SD fold change) are representative of those from three independent experiments. (D) 293T cells were cotransfected with HA-IKKβ and the vector, 2C-Flag, or 3E3L mutant-Flag at 24 h posttransfection, and the whole-cell lysate was immunoblotted by using the indicated antibodies, including a phospho-IKKα/β (Ser-176/180)-specific antibody. (E) 293T cells were transfected with plasmids carrying the indicated protein or the vector alone and then mock treated or treated for 6 h with TNF-α (10 ng/ml) at 24 h posttransfection. Cells were lysed and analyzed by a luciferase reporter assay. The data shown (mean ± SD fold change) are representative of those from three independent experiments.
TABLE 1.
Primers used in the studya
Restriction enzyme sites are shown in bold. Initiation codons and termination codons are in blue. The coding sequences of the 2C proteins of EV71, PV, CVA16, and CVB3 are underlined. Flag tag sequences are in italics. The site of mutagenesis is in red.
Antibodies.
The following primary antibodies were used: anti-EV71 2C rabbit polyclonal antibody, which was produced by us and previously described (21); mouse anti-HA (Abmart); rabbit anti-HA (Sigma); mouse anti-Flag M2 (Sigma); rabbit anti-PP1α (Abcam); rabbit anti-PP1β (Abcam); goat anti-PP1γ (Santa Cruz); rabbit anti-IKKα/β (Santa Cruz); rabbit anti-phospho-IKKα/β (Ser 176/180; Cell Signaling Technology); mouse β-actin (Proteintech); and normal mouse IgG (Santa Cruz).
The following secondary antibodies were used: horseradish peroxidase (HRP)-coupled goat anti-mouse IgG (Pierce), HRP-coupled goat anti-rabbit IgG (Pierce), HRP-coupled donkey anti-goat IgG (Proteintech), fluorescein isothiocyanate (FITC)-coupled goat anti-mouse IgG (Pierce), and tetramethyl rhodamine isocyanate (TRITC)-coupled goat anti-rabbit IgG (Pierce).
TAP, silver staining, and mass spectrometry.
Tandem affinity purification (TAP) assays were performed according to the manufacturer's standard instructions (Stratagene). In brief, 293T cells that had been transfected with pNTAP-A-2C or the pNTAP-A control vector were infected with EV71 (multiplicity of infection [MOI] = 5) at 36 h posttransfection. After 24 h postinfection, the cells were harvested and lysed. EV71 2C-host protein complexes were first purified using washed streptavidin resin and then washed calmodulin resin. The precipitated proteins were eluted by boiling the resin for 10 min and then subjected to SDS-PAGE. The gels were stained with a silver stain kit for mass spectrometry (Thermo) according to the manufacturer's instructions, and the purified host proteins were identified by mass spectrometry, which was performed by Wuhan Institute of Biotechnology, China.
Co-IP and immunoblotting assays.
For one-step coimmunoprecipitation (co-IP), 293T cells, which were transfected with the expression plasmids or vectors indicated below and stimulated by TNF-α (10 ng/ml) for 15 min or infected with EV71 (MOI = 5) for 24 h in certain cases, were harvested and lysed with Western blotting and immunoprecipitation (IP) lysis buffer that contained phenylmethylsulfonyl fluoride and a protease inhibitor cocktail (Roche). Then, the cells were clarified by centrifugation at 16,000 × g for 10 min at 4°C, and the supernatants were used in the subsequent co-IP assays according to the manufacturer's instructions for protein G-agarose (Fast Flow; Millipore). In brief, the antibodies of choice were added to the supernatants, and the reaction mixture was gently shaken overnight at 4°C. Then, 50 μl of a washed protein G agarose bead slurry (25 μl packed beads) was added, and the reaction mixture was gently shaken at 4°C for 2 h. The beads were washed five times with Tris-buffered saline (50 mM Tris-HCl, 500 mM NaCl, pH 7.4). The immunoprecipitates were eluted by boiling the beads for 10 min and subjecting them to SDS-PAGE and immunoblot analysis. The intensities of the IP bands were measured by using ImageJ software.
For two-step co-IP assays, 293T cells were cotransfected with HA-IKKβ and 2C-Flag or the vector and lysed at 36 h posttransfection. The supernatants were subjected to the first step of co-IP using an EZview Red anti-Flag M2 affinity gel (Sigma) according to the manufacturer's instructions. Different from the one-step co-IP, the immunoprecipitates of the first step of co-IP were eluted with the Flag peptide (Sigma) by gently shaking the reaction mixture at 4°C for 2 to 4 h. The eluate from the first step of co-IP was used to perform the second step of co-IP using the same procedure described above, as well as the one-step co-IP assay described above.
Immunofluorescence and confocal microscopy.
HeLa cells were transfected with Flag-PP1α, Flag-PP1β, or Flag-PP1γ using the Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. In certain cases, the catalytic subunit of protein phosphatase 1 (PP1c) was cotransfected with 2C-HA or cells were infected with EV71 (MOI = 5). At 24 h posttransfection, the cells were washed with 3% normal goat serum (NGS) in phosphate-buffered saline (PBS) (all subsequent washing steps used 3% NGS in PBS), fixed with 4% paraformaldehyde for 15 min, washed, permeabilized with 0.2% Triton X-100 in PBS for 15 min, washed, blocked with 5% NGS plus 2% bovine serum albumin in PBS for 2 h, and washed thrice. For immunostaining of 2C-HA, EV71-expressed 2C, and three Flag-tagged PP1c isoforms, the cells were incubated with rabbit anti-HA antibody (recognizing 2C-HA), rabbit anti-EV71 2C antibody (recognizing EV71-expressed 2C), and mouse anti-Flag antibody (recognizing Flag-tagged PP1c) overnight at 4°C. After five washes (10 min each), the cells were incubated with TRITC-coupled goat anti-rabbit IgG (recognizing rabbit anti-HA antibody and rabbit anti-EV71 2C antibody) and FITC-coupled goat anti-mouse IgG (recognizing mouse anti-Flag antibody) for 1 h at room temperature, respectively. After five washes (10 min each), the cell nuclei were stained with Hoechst 33258 (Beyotime Institute of Biotechnology, China) and washed thrice (5 min each). Fluorescent images were obtained with a PerkinElmer UltraView VOX confocal microscope equipped with a 405-nm laser line (for violet fluorescence of the stained cell nuclei), a 488-nm laser line (for green fluorescence), and a 561-nm laser line (for red fluorescence). The cells were observed with a 60× oil immersion objective.
Luciferase reporter gene assay.
Luciferase reporter gene assays were performed by using a dual-luciferase assay system kit (Promega). In brief, 293T cells grown on six-well plates were cotransfected with the NF-κB luciferase reporter plasmid and pRL-TK, 2C-Flag, 3E3L-Flag, or the control vector plasmid and were mock treated or treated with TNF-α (10 ng/ml) for 6 h at 24 h posttransfection. The cells were lysed with passive lysis buffer, the supernatants were collected by centrifugation at 12,000 × g for 30 s, and luciferase activities were measured according to the manufacturer's instructions (Promega). For all assays, experiments were performed in triplicate.
RESULTS
EV71 2C interacts with three isoforms of PP1c.
To gain insight into the molecular mechanism underlying the inhibition of IKKβ phosphorylation mediated by EV71 2C, we performed tandem affinity purification (TAP) screening and a mass spectrometry assay to identify 2C-interacting host factors using TAP-tagged 2C and control vector pNTAP-A in transfected 293T cells in the context of EV71 infection. From the results of mass spectrometry, we found 11 fragments of the catalytic subunit of protein phosphatase 1 (PP1c) appearing in the purifications with TAP-tagged 2C (Table 2) but not in the purifications with control vector pNTAP-A.
TABLE 2.
Mass spectrometry-based identification results for TAP-tagged 2C-purified PP1c
| Fragment no. | UniProtKB accession no. | Name |
|---|---|---|
| 1 | P62136 | Serine/threonine-protein phosphatase PP1-alpha catalytic subunit |
| 2 | E9PMD7 | Serine/threonine-protein phosphatase (fragment) |
| 3 | P62140 | Serine/threonine-protein phosphatase PP1-beta catalytic subunit |
| 4 | E7ETD8 | Serine/threonine-protein phosphatase (fragment) |
| 5 | C9JP48 | Serine/threonine-protein phosphatase (fragment) |
| 6 | C9J9S3 | Serine/threonine-protein phosphatase (fragment) |
| 7 | F8W0W8 | Serine/threonine-protein phosphatase |
| 8 | F8VYE8 | Serine/threonine-protein phosphatase |
| 9 | F8VR82 | Serine/threonine-protein phosphatase |
| 10 | P36873 | Serine/threonine-protein phosphatase PP1-gamma catalytic subunit |
| 11 | F8W0V8 | Serine/threonine-protein phosphatase PP1-gamma catalytic subunit |
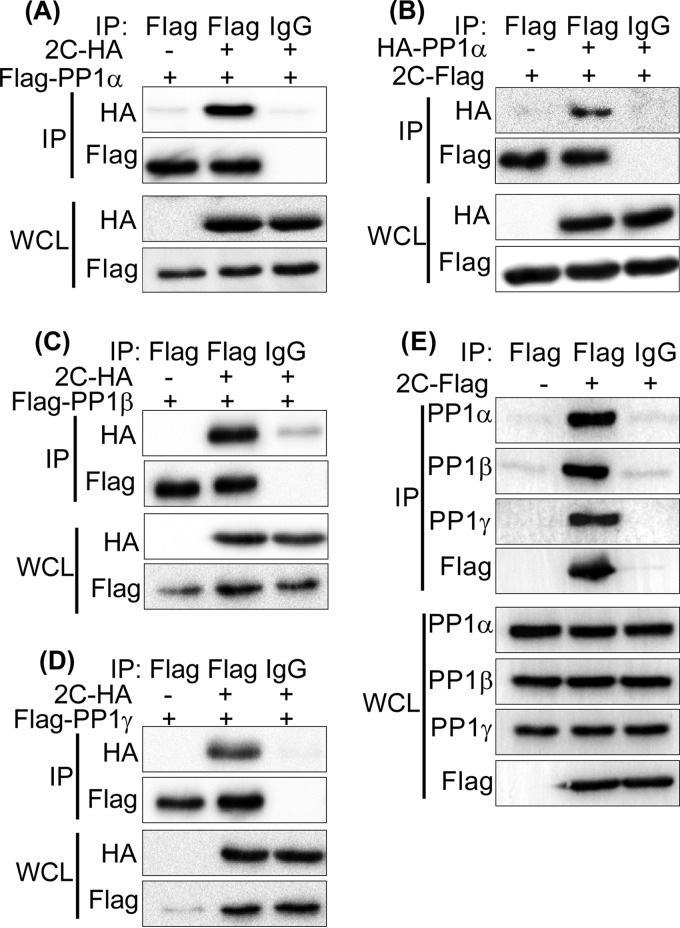
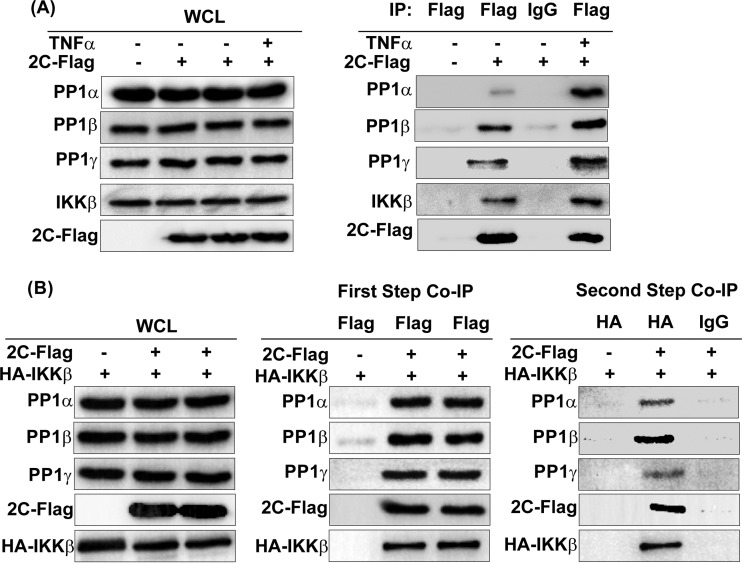
To confirm the EV71 2C-PP1 interaction under physiological conditions, we performed co-IP assays with transfected 293T cells by using epitope-tagged 2C and PP1. As shown in Fig. 1A, Flag-PP1α and the control mouse IgG but not the control vector and the control mouse IgG coprecipitated with 2C-HA. 2C-Flag, in turn, consistently coprecipitated with HA-PP1α (Fig. 1B). The amino acid sequences of the three PP1c isoforms are about 90% identical. Except for the main differences, located at the N and C termini (27), we sought to test whether EV71 2C also binds PP1β and PP1γ. As shown in Fig. 1C and D, both Flag-PP1β and PP1γ interacted with 2C-HA. We also found endogenous PP1α-, PP1β-, and PP1γ-bound 2C-Flag (Fig. 1E).
FIG 1.
2C protein of EV71 binds PP1α, PP1β, and PP1γ. (A to D) 293T cells were cotransfected with 2C-HA and Flag-PP1α (A), Flag-PP1β (C), Flag-PP1γ (D), or 2C-Flag with HA-PP1α (B). At 24 h posttransfection, the cells were harvested and lysed, the whole-cell lysate (WCL) was immunoprecipitated with anti-Flag or control mouse IgG, and immunoblotting was conducted with the corresponding antibodies. (E) 293T cells were transfected with a vector or 2C-Flag. At 24 h posttransfection, the cells were harvested and lysed. The whole-cell lysate was immunoprecipitated with anti-Flag or control mouse IgG, and the immunoblots were probed with the corresponding antibodies. The results shown are representative of those from three independent experiments.
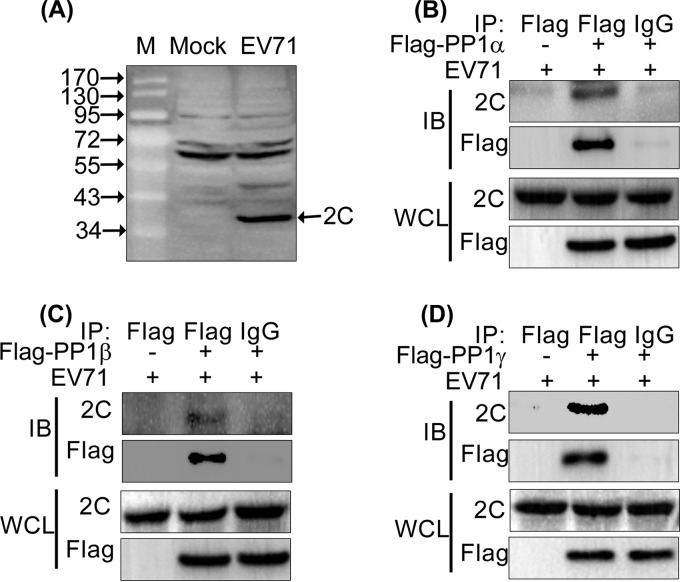
To further determine the interaction between EV71-expressed 2C and PP1 under the conditions of EV71 infection, we first characterized the specificity of an EV71 2C antibody, which was produced by us and was previously described (21). Figure 2A shows that the EV71 2C antibody specifically recognized EV71-expressed 2C (about 37 kDa). Then, we conducted co-IP assays, in which 293T cells were transfected with the Flag-tagged PP1c isoforms and subsequently infected with EV71 for 24 h. As shown in Fig. 2B to D, the EV71-expressed 2C protein interacted with all three PP1c isoforms. The results indicate that 2C interacts with PP1c.
FIG 2.
EV71 2C binds PP1α, PP1β, and PP1γ. (A) Identification of the specificity of the EV71 2C antibody. 293T cells were either not infected (mock) or infected with EV71 (MOI = 5) for 24 h, and the whole-cell lysate was probed with an anti-EV71 2C rabbit polyclonal antibody. Lane M, protein size marker (the numbers to the left of gel are molecular masses, in kilodaltons). (B to D) 293T cells were transfected with Flag-PP1α (B), Flag-PP1β (C), Flag-PP1γ (D), or a vector. At 24 h posttransfection, the cells were infected with EV71 (MOI = 5) for 24 h. Whole-cell lysates were immunoprecipitated with anti-Flag or control mouse IgG, and the immunoblots (IBs) were probed with the corresponding antibodies.
EV71 2C does not change the localization of PP1c.
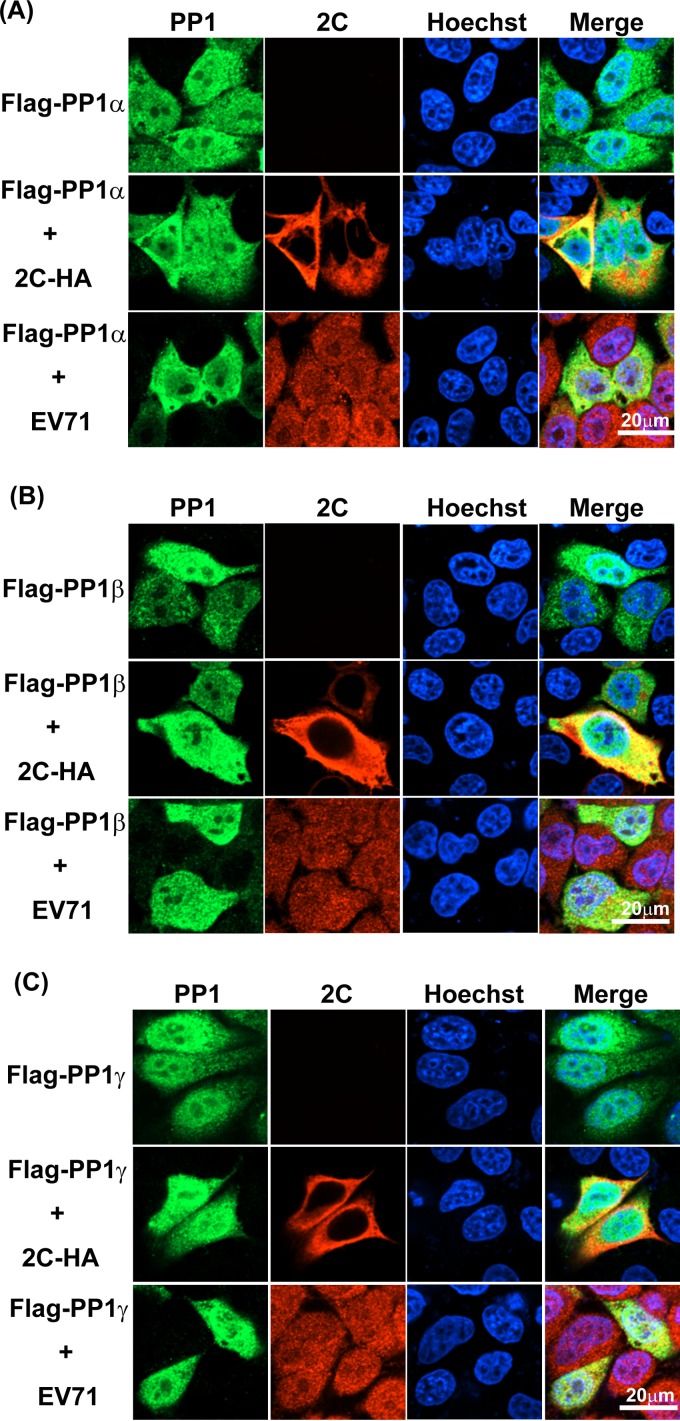
Some PP1-interacting proteins reportedly regulate the subcellular localization of PP1 (28–31). We intended to investigate whether EV71 2C affects PP1 localization in living cells by an immunofluorescence assay. As shown in Fig. 3 and consistent with our previous findings (21), overexpressed 2C-Flag (red) was localized exclusively in the cytoplasm. EV71-expressed 2C was located in the entire cell. The three PP1c isoforms (Fig. 3, green) were distributed in both the cytoplasm and the nucleus. The distribution of PP1c remained unchanged under the conditions of 2C-Flag overexpression or EV71 infection, suggesting that EV71 2C does not influence the cellular localization of PP1c.
FIG 3.
EV71 2C does not influence the location of PP1c. HeLa cells were transfected with Flag-PP1α (A), Flag-PP1β (B), or Flag-PP1γ (C); cotransfected with 2C-HA and Flag-PP1α (A), Flag-PP1β (B), or Flag-PP1γ (C); or transfected with Flag-PP1α (A), Flag-PP1β (B), or Flag-PP1γ (C) and infected with EV71 (MOI = 5). After 24 h, immunofluorescence staining with the corresponding antibodies was performed, and the nuclei were subsequently stained with Hoechst 33258. After three washes (5 to 10 min each), the cells were observed using a 60× oil immersion objective. Fluorescent images were taken under a PerkinElmer UltraView VOX confocal microscope. Each image is representative of the findings for the majority of cells observed in several fields. The results shown are representative of those from two independent experiments.
PP1c binding is crucial for EV71 2C-mediated inhibition of IKKβ phosphorylation and NF-κB activation.
To investigate whether PP1c binding to EV71 2C causes the suppression of TNF-α-mediated NF-κB activation, we sought to determine the key sites of the 2C protein that are required for the initial binding of PP1. Most PP1c-interacting proteins (more than 95%) contain the canonical PP1-binding motif (R/K)(0,1)(V/I/L)(^P)(F/W) (commonly termed the RVXF motif) [(0,1) indicates no or any one residue and (^P) means any residue but proline (P)], which directly interacts with a hydrophobic pocket on the surface of PP1. Within the consensus PP1-binding motif, the most conserved valine (V)/isoleucine (I)/leucine (L) and phenylalanine (F)/tryptophan (W) residues appear to anchor the binding of PP1 interactors to PP1, whereas the other residues mediate the specificity of recognition of the various PP1 interactors (32, 33). Prediction of the functional sites in the EV71 2C protein was performed by using the eukaryotic linear motif (ELM) resource (http://elm.eu.org). Indeed, we found that the 2C protein possesses three highly conserved PP1-binding motifs (designated motifs I, II, and III) (Fig. 4A and 5A, top), which are located at amino acids (aa) 14 to 21 (AAKGLEWI), amino acids 25 to 32 (ISKFIDWL), and amino acids 41 to 47 (KEKVEFL), respectively.
First, to analyze the PP1-binding motifs individually, we constructed three mutants with EV71 2C mutations, namely, Mut1 (in which L18 and W20 in motif I were mutated to E [glutamic acid] or L), Mut2 (in which I29 and W31 in motif II were mutated to E or L), and Mut3 (in which V44 and F46 in motif III were mutated to E or L) (Fig. 4A, bottom). Through flowing co-IP assays (Fig. 4B) and quantification of the proteins in the IP panels (Fig. 4C), we found that, in comparison with the level of 2C binding to PP1c isoforms PP1α, PP1β, and PP1γ, Mut1 showed binding activity of 65.21%, 68.47%, and 36.81%, respectively. Mut2 showed an evident decrease only in the interaction (39.21%) with PP1γ, and Mut3 did not influence PP1 binding. Taken together, these data suggest that three PP1-binding motifs perform complementary functions to a certain degree. The PP1γ-binding activity of EV71 2C appears to be more sensitive to the motif I or II mutation.
Then, six key residues within PP1-binding motifs were mutated to E or L, and the mutant was named 3E3L (Fig. 5A, bottom). Thereafter, the ability of the 3E3L mutant to interact with endogenous PP1 and IKKβ was assessed (Fig. 5B and C). Wild-type 2C efficiently bound the three isoforms of PP1c (Fig. 5B, second lane), and the 3E3L mutant showed significantly decreased PP1c-binding activity (47.47% binding to PP1α, 51.36% binding to PP1β, and 26.20% binding to PP1γ) (Fig. 5B, fourth lane, and C). In contrast, the 3E3L mutant demonstrated IKKβ-binding activity similar to that of wild-type 2C. These data together indicate that the canonical PP1-binding motifs are efficient for the binding of 2C to PP1c but do not affect IKKβ binding.
We further introduced PP1c-binding-deficient mutant 3E3L into IKKβ phosphorylation and luciferase reporter assays. Consistent with the findings of our previous investigation (21), wild-type 2C markedly inhibited IKKβ phosphorylation (Fig. 5D, second lane), whereas the 3E3L mutant did not inhibit IKKβ phosphorylation (Fig. 5D, third lane). Furthermore, as shown in Fig. 5E, the 3E3L mutant significantly restored the level of NF-κB activation stimulated by TNF-α. Taken together, these results support the notion that PP1c binding is crucial for EV71 2C-mediated inhibition of IKKβ phosphorylation and NF-κB activation.
EV71 2C recruits PP1c and IKKβ to form a complex.
We subsequently investigated the functions of PP1c in 2C-mediated inhibition of IKKβ phosphorylation. We first used 2C-Flag or control vector-transfected 293T cells to test the effect of TNF-α-stimulated NF-κB activation on the interaction of 2C and PP1c or IKKβ. As shown in Fig. 6A, 2C interacted with both endogenous IKKβ and PP1c in the absence of TNF-α stimulation. After 15 min of TNF-α stimulation, the quantity of IKKβ and the three isoforms of PP1c precipitated by 2C significantly increased, suggesting that the TNF-α-stimulated activation of the NF-κB signaling pathway promotes the binding of 2C to PP1c and IKKβ. We and other researchers previously revealed that the 125 N-terminal amino acids of 2C mediate the interaction of 2C with IKKβ (21) and that PP1c interacts with IKKβ (34). Taken together, our data indicate that 2C, PP1c, and IKKβ interact with each other in pairs, implying that 2C likely recruits PP1c and IKKβ to form a complex.
FIG 6.
EV71 2C, IKKβ, and the three isoforms of PP1c form a tripolymer. (A) TNF-α promotes the interaction of 2C and endogenous IKKβ or PP1c. 293T cells were transfected with the vector or 2C-Flag. At 24 h posttransfection, cells were unstimulated or stimulated for 15 min with TNF-α (10 ng/ml) and subsequently harvested and lysed. The whole-cell lysate was immunoprecipitated with anti-Flag or control mouse IgG and immunoblotted with the corresponding antibodies. (B) Two-step coimmunoprecipitation assay. 293T cells were cotransfected with HA-IKKβ and the vector or 2C-Flag. At 24 h posttransfection, the cells were harvested and lysed, the whole-cell lysate was immunoprecipitated with an anti-Flag-labeled affinity gel, and the Flag fusion protein was eluted by competition with Flag peptide under native conditions. Samples that eluted from the first immunoprecipitation were used to perform the second immunoprecipitation with mouse HA antibody or control mouse IgG antibody. Finally, the HA fusion protein was eluted by the use of SDS-PAGE sample buffer. The immunoblots were probed with the corresponding antibodies. The data shown are representative of those from three independent experiments.
To this end, we performed a two-step co-IP assay by using HA-IKKβ- and 2C-Flag- or control vector-cotransfected 293T cells. Whole-cell lysates were first immunoprecipitated with an anti-Flag-labeled affinity gel, and the Flag fusion protein was eluted by competition with the Flag peptide under native conditions. The samples that eluted from the first immunoprecipitation were used for the second immunoprecipitation with mouse HA antibody or control mouse IgG antibody. Finally, the HA fusion protein was eluted by SDS-PAGE sample buffer under denaturing conditions. As shown in Fig. 6B, we clearly detected all three endogenous PP1c isoforms in the samples of the second co-IP, suggesting that 2C, PP1c, and IKKβ can form a complex.
These results collectively suggest that the EV71 2C protein recruits PP1c to IKKβ and subsequently form a 2C-PP1-IKKβ complex to suppress IKKβ phosphorylation and the NF-κB signaling pathway.
The 2C proteins of PV, CVA16, and CVB3 suppress IKKβ phosphorylation by recruiting PP1.
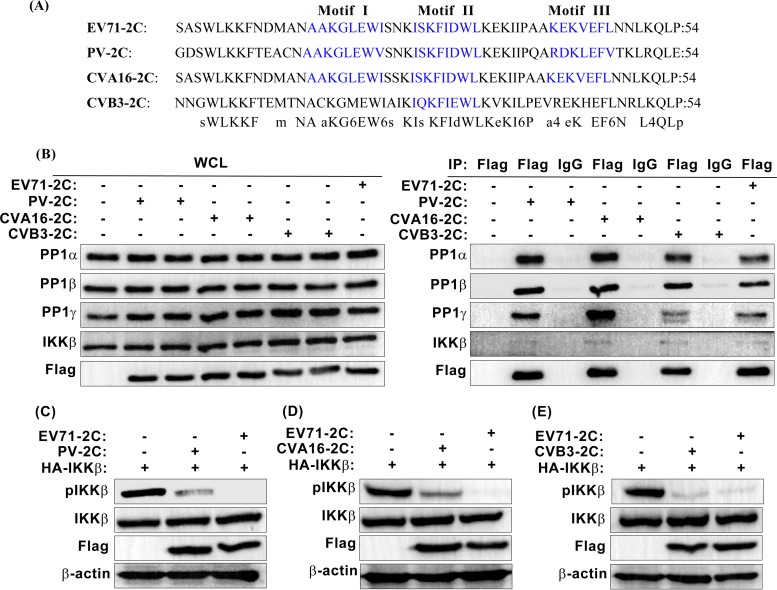
Given that 2C is a highly conserved viral protein in human enteroviruses, we inquired whether the 2C proteins of other enteroviruses also regulate the NF-κB signaling pathway via inhibition of IKKβ phosphorylation. We investigated three additional enteroviruses, namely, PV, CVA16, and CVB3, which possess clinical significance. Through protein sequence alignment using the Clustal W method of MegAlign software, we found that, like EV71 2C, the PV and CVA16 2C proteins contain three PP1-binding motifs, whereas CVB3 2C possesses only one PP1-binding motif (Fig. 7A). As expected, owing to the existence of PP1-binding motifs, the 2C proteins of PV, CVA16, and CVB3 all interacted with the three endogenous isoforms of PP1c (Fig. 7B). However, the interaction activity between CVB3 2C and PP1γ was significantly decreased. This reduction may be due to the single PP1-binding motif of the CVB3 2C protein, which corresponds to EV71 2C PP1-binding motif II. EV71 2C PP1-binding motif I, like motif II, performed an important function in PP1γ binding since the mutant with a mutation of PP1-binding motif I (Mut1) showed only 36.81% PP1γ-binding activity (Fig. 4B and C). The absence of motif I likely accounts for why the interaction between CVB3 2C and PP1γ is significantly reduced. In addition to PP1 binding, endogenous IKKβ was identified within the co-IP eluate (Fig. 7B). In an immunoblotting assay, we observed that the PV, CVA16, and CVB3 2C proteins decreased the level of IKKβ phosphorylation (Fig. 7C to E). Taken together, these results demonstrate that PV, CVA16, and CVB3 2C suppress IKKβ phosphorylation by using the same mechanism that EV71 2C uses.
FIG 7.
The 2C proteins of PV, CVA16, and CVB3 bind PP1 and inhibit IKKβ phosphorylation. (A) Alignment of the EV71, PV, CVA16, and CVB3 2C protein sequences. Alignment was performed using the Clustal W method of MegAlign software. PP1-binding motifs are displayed in blue. (B) Interaction between the 2C proteins of PV, CVA16, CVB3, and EV71 and the three isoforms of PP1c or IKKβ in transfected 293T cells. EV71 2C was used as a positive control. At 24 h posttransfection, cells were harvested and lysed. The whole-cell lysate was immunoprecipitated with anti-Flag or control mouse IgG and immunoblotted with the corresponding antibodies. (C to E) Phosphorylation of IKKβ in 293T cells expressing HA-IKKβ, the Flag-tagged 2C protein of PV (C), CVA16 (D), or CVB3 (E), and Flag-tagged EV71 2C. At 24 h posttransfection, the whole-cell lysate was analyzed by immunoblotting using the indicated antibodies, including a phospho-IKKα/β (Ser-176/180)-specific antibody. The data shown are representative of those from three independent experiments.
DISCUSSION
The IKK complex not only is the master regulator of the NF-κB signaling pathway but also is engaged in cross talk with other signaling mechanisms, such as proantiapoptotic, proinflammatory, and proliferative pathway-independent NF-κB activation (35). Multiple biological functions render the kinase activity of the IKK complex to be tightly controlled by phosphorylation, nondegradative ubiquitination, adaptor protein interactions, and higher-order oligomerization events and to be an excellent target for various viruses (36). The reversible phosphorylation of the IKK complex, especially IKKβ, is mediated by autophosphorylation or upstream IKKs and protein phosphatases, including PP1, PP2, PP4, and PP6 (37, 38). To suppress NF-κB activation, many viruses can use the components of the IKKβ upstream signaling network or directly bind IKKβ to inhibit IKKβ phosphorylation (39). However, the mechanism by which these viruses directly target and dephosphorylate IKKβ has yet to be fully dissected. In this study, on the basis of our previous findings, which state that EV71 2C suppresses the TNF-α-mediated NF-κB signaling pathway by directly binding IKKβ and inhibiting IKKβ phosphorylation (21), we reveal that the 2C proteins of enteroviruses, including EV71, PV, CVA16, and CVB3, exploit a novel mechanism to inhibit IKKβ phosphorylation by recruiting PP1 through its classical PP1-binding motifs and IKKβ to form a complex. This mechanism ultimately results in the inhibition of the NF-κB signaling pathway.
Our data obtained in this study provide clear evidence that, instead of a physical block of IKKβ phosphorylation sites, EV71 2C-mediated PP1 recruitment is efficient for the inhibition of IKKβ phosphorylation and NF-κB activation. According to our previous studies, we speculated that the binding between the EV71 2C protein and the kinase domain (KD) of IKKβ may interrupt the upstream kinase to interact with and phosphorylate Ser177 and Ser181 within the KD and ultimately lead to the inhibition of IKKβ phosphorylation (21). Unexpectedly, we found in this study that the mutant with the PP1-binding-deficient 2C mutation 3E3L, which does not influence IKKβ-binding activity, markedly restored IKKβ phosphorylation and NF-κB activation, suggesting that, in comparison with IKKβ binding, PP1 binding performs a predominant function in EV71 2C-mediated NF-κB inactivation.
We previously revealed that the N-terminal 125 aa of EV71 2C are responsible for the inhibition of IKKβ and NF-κB activation (21). Du et al. further determined that aa 105 to 121 are the binding area of IKKβ (40). Our data obtained in the current study indicate that the domain from aa 1 to 47, which contains three PP1-binding motifs, is responsible for PP1c binding. Together with the findings in the report of Du et al. (40), our findings indicate that both the three PP1-binding motifs from aa 1 to 47 and the IKKβ-binding area from aa 105 to 121 within the N-terminal 125 aa are critical for the inhibition of IKKβ phosphorylation. Compared with the function of herpes simplex virus (HSV) γ134.5, which serves as the bridging protein via the different N- and C-terminal ends (41), EV71 2C appears to serve as an adaptor between PP1 and IKKβ via the two N-terminal adjacent areas (PP1 and the IKKβ-binding area). This function of 2C may facilitate PP1 and IKKβ interaction and result in PP1-mediated IKKβ dephosphorylation. Considering that PP1-binding proteins generally regulate the subcellular localization, activity, or substrate specificity of PP1 (42), we performed immunofluorescence assays and observed that, unlike the HIV-1 Tat protein, which enhances the nucleus targeting of PP1γ (30), EV71 2C does not regulate the localization of PP1. Future study is needed to define whether 2C directly regulates PP1 enzyme activity.
Using a one-step co-IP assay, Jin et al. found that the HSV γ134.5 protein recruits PP1 and IKKβ, which form a complex to dephosphorylate IKKβ, resulting in the inactivation of NF-κB (41). Likewise, Qu et al. reported that the host protein hCINAP negatively regulates NF-κB signaling by forming an hCINAP-PP1-IKKβ complex (38). However, neither study fully proved the model in which PP1, IKKβ, and γ134.5 (or hCINAP) can form a tripolymer complex. In the current study, we used a two-step co-IP assay, which is a more rigorous method for confirming the tripolymer formation of the three proteins, which were found to interact with each other in a pairwise manner. Our data indicate that EV71 2C recruits all isoforms of PP1 and forms a complex with IKKβ.
Although several protein phosphatases, including PP1, PP2, PP4, and PP6, have been reported to be regulators for type I signaling pathways, only a few viruses have recently been reported to use PP1 as a target to modulate host innate immune responses (41, 43–46). Gack and colleagues (43–45) showed that measles virus (MV) inhibits type I IFN production by targeting PP1α and PP1γ but not PP1β, thus inhibiting the PP1-MDA5 interaction and PP1 phosphatase activity to prevent RLR dephosphorylation and activation. They revealed a model in which viral proteins bind PP1 and interfere with the dephosphorylation activity (43–45). In contrast, our results suggested a distinct model in which protein 2C promotes the dephosphorylation of the phosphorylated IKKβ by recruiting all three isoforms of PP1c and IKKβ. Although the HSV γ134.5 protein has been reported to recruit PP1α and IKKβ to promote the dephosphorylation of the phosphorylated IKKβ, the functions of PP1β and PP1γ in the γ134.5-mediated inhibition of IKKβ phosphorylation have not been determined (41, 47). Our data presented here provide evidence that all three isoforms of PP1c (PP1α, PP1β, and PP1γ) perform a critical function in the inhibition of IKKβ phosphorylation mediated by EV71 2C. We further confirmed the EV71 2C-PP1-IKKβ complex model by studying other classical enteroviruses, including PV, CVA16, and CVB3. Although enteroviruses and picornaviruses, such as foot-and-mouth disease virus, CVB3, and PV, reportedly use L proteases, 3C proteases, and 3A, respectively, to antagonize the NF-κB signaling pathway, PP1 is not involved in the above-mentioned processes (15, 48, 49). Taken together, our study reveals a novel mechanism by which enterovirus 2C proteins that lack protease activity regulate the NF-κB signaling pathway through the formation of a PP1-2C-IKKβ complex.
The nonstructural protein 2C of the Picornaviridae family (329 aa and 37.5 kDa) is one of the most highly conserved viral proteins. The protein is multifunctional and performs important functions in host cell membrane alteration, virus RNA binding and replication, morphogenesis, and encapsidation (50–53). More recently, Zhou and colleagues reported that the EV71 and CVA16 2CATPase functions as both an RNA helicase and an ATP-independent RNA chaperone, which is critical for the RNA replication and viability of enteroviruses (54). The N-terminal region (aa 1 to 72 or 1 to 88) of PV 2C contains membrane, nucleoside triphosphate, and RNA binding motifs and is sufficient for membrane binding. This finding suggests that this region is indispensable for poliovirus (55, 56). EV71 2C is closely related to PV 2C in homology, and the N-terminal region may also perform important functions in the life cycle of EV71. The N-terminal region (aa 5 to 43) is known to interact with reticulon 3, and this interaction is required for viral replication (53). In the current study, we identified three PP1-binding motifs in the N-terminal region (aa 1 to 47) of 2C. When all PP1-binding motifs were mutated to generate the 3E3L mutation, the ability of 2C to bind to all three PP1c isoforms was affected (Fig. 5B), thereby indicating that all motifs can mediate PP1c binding. Moreover, introduction of the 3E3L mutation into the EV71 cDNA infectious clone resulted in the dysfunction of EV71 2C rescue (data not shown), suggesting that the N-terminal PP1-binding motifs (aa 1 to 47) of EV71 2C are critical for virus viability. In this regard, we cannot rule out the possibility that 2C-PP1c interaction may perform other functions aside from inhibiting IKKβ phosphorylation.
As a major Ser/Thr phosphatase that is ubiquitously expressed in all eukaryotic cells, PP1 is involved in cell cycle progression, protein synthesis, skeletal muscle contraction, cellular glycogen metabolism, transcription, and membrane receptor and ion channel formation, in addition to regulation of the innate immune system (57–59). We reveal that all isoforms of PP1c serve as targets for enteroviruses. Enteroviruses probably regulate a broad range of host cellular processes through the targeting of different isoforms of PP1. Taken together, our findings demonstrate that the model of the 2C-PP1-IKKβ antergic complex inhibition of NF-κB signaling pathway described here represents a novel strategy by which enteroviruses escape innate immunity and provides the basis for further understanding of viral pathogenicity.
ACKNOWLEDGMENTS
We thank Thomas Leung for kindly providing the plasmid HA-PP1α and Irene L. Andrulis for providing plasmids Flag-PP1α, Flag-PP1β, and Flag-PP1γ. We thank Panyong Mao, Bo Zhang, and Huipeng Chen for providing the full-length PV, CVA16, and CVB3 cDNA clones, respectively. Lastly, we thank Xi Chen (Wuhan Institute of Biotechnology) for excellent technical assistance with mass spectrometry-based identification.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Schmidt NJ, Lennette EH, Ho HH. 1974. An apparently new enterovirus isolated from patients with disease of the central nervous system. J Infect Dis 129:304–309. doi: 10.1093/infdis/129.3.304. [DOI] [PubMed] [Google Scholar]
- 2.Yip CC, Lau SK, Woo PC, Yuen KY. 2013. Human enterovirus 71 epidemics: what's next? Emerg Health Threats J 6:19780. doi: 10.3402/ehtj.v6i0.19780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang PN, Shih SR. 2014. Update on enterovirus 71 infection. Curr Opin Virol 5:98–104. doi: 10.1016/j.coviro.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH. 2010. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis 10:778–790. doi: 10.1016/S1473-3099(10)70194-8. [DOI] [PubMed] [Google Scholar]
- 5.McMinn PC. 2002. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev 26:91–107. doi: 10.1111/j.1574-6976.2002.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 6.Janeway CA., Jr 1989. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harbor Symp Quant Biol 54(Pt 1):1–13. [DOI] [PubMed] [Google Scholar]
- 7.Hayden MS, Ghosh S. 2014. Regulation of NF-kappaB by TNF family cytokines. Semin Immunol 26:253–266. doi: 10.1016/j.smim.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karin M. 1999. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene 18:6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 9.Karin M, Ben-Neriah Y. 2000. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol 18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 10.Harris KG, Coyne CB. 2013. Enter at your own risk: how enteroviruses navigate the dangerous world of pattern recognition receptor signaling. Cytokine 63:230–236. doi: 10.1016/j.cyto.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barral PM, Morrison JM, Drahos J, Gupta P, Sarkar D, Fisher PB, Racaniello VR. 2007. MDA-5 is cleaved in poliovirus-infected cells. J Virol 81:3677–3684. doi: 10.1128/JVI.01360-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rebsamen M, Meylan E, Curran J, Tschopp J. 2008. The antiviral adaptor proteins Cardif and Trif are processed and inactivated by caspases. Cell Death Differ 15:1804–1811. doi: 10.1038/cdd.2008.119. [DOI] [PubMed] [Google Scholar]
- 13.Mukherjee A, Morosky SA, Delorme-Axford E, Dybdahl-Sissoko N, Oberste MS, Wang T, Coyne CB. 2011. The coxsackievirus B 3C protease cleaves MAVS and TRIF to attenuate host type I interferon and apoptotic signaling. PLoS Pathog 7:e1001311. doi: 10.1371/journal.ppat.1001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bozym RA, Delorme-Axford E, Harris K, Morosky S, Ikizler M, Dermody TS, Sarkar SN, Coyne CB. 2012. Focal adhesion kinase is a component of antiviral RIG-I-like receptor signaling. Cell Host Microbe 11:153–166. doi: 10.1016/j.chom.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaragoza C, Saura M, Padalko EY, Lopez-Rivera E, Lizarbe TR, Lamas S, Lowenstein CJ. 2006. Viral protease cleavage of inhibitor of kappaBalpha triggers host cell apoptosis. Proc Natl Acad Sci U S A 103:19051–19056. doi: 10.1073/pnas.0606019103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lei X, Sun Z, Liu X, Jin Q, He B, Wang J. 2011. Cleavage of the adaptor protein TRIF by enterovirus 71 3C inhibits antiviral responses mediated by Toll-like receptor 3. J Virol 85:8811–8818. doi: 10.1128/JVI.00447-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lei X, Xiao X, Xue Q, Jin Q, He B, Wang J. 2013. Cleavage of interferon regulatory factor 7 by enterovirus 71 3C suppresses cellular responses. J Virol 87:1690–1698. doi: 10.1128/JVI.01855-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lei X, Liu X, Ma Y, Sun Z, Yang Y, Jin Q, He B, Wang J. 2010. The 3C protein of enterovirus 71 inhibits retinoid acid-inducible gene I-mediated interferon regulatory factor 3 activation and type I interferon responses. J Virol 84:8051–8061. doi: 10.1128/JVI.02491-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang B, Xi X, Lei X, Zhang X, Cui S, Wang J, Jin Q, Zhao Z. 2013. Enterovirus 71 protease 2Apro targets MAVS to inhibit anti-viral type I interferon responses. PLoS Pathog 9:e1003231. doi: 10.1371/journal.ppat.1003231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo RL, Kao LT, Lin SJ, Wang RY, Shih SR. 2013. MDA5 plays a crucial role in enterovirus 71 RNA-mediated IRF3 activation. PLoS One 8:e63431. doi: 10.1371/journal.pone.0063431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng Z, Li H, Zhang Z, Meng J, Mao D, Bai B, Lu B, Mao P, Hu Q, Wang H. 2011. Enterovirus 71 2C protein inhibits TNF-alpha-mediated activation of NF-kappaB by suppressing IkappaB kinase beta phosphorylation. J Immunol 187:2202–2212. doi: 10.4049/jimmunol.1100285. [DOI] [PubMed] [Google Scholar]
- 22.Tan I, Ng CH, Lim L, Leung T. 2001. Phosphorylation of a novel myosin binding subunit of protein phosphatase 1 reveals a conserved mechanism in the regulation of actin cytoskeleton. J Biol Chem 276:21209–21216. doi: 10.1074/jbc.M102615200. [DOI] [PubMed] [Google Scholar]
- 23.Winter SL, Bosnoyan-Collins L, Pinnaduwage D, Andrulis IL. 2007. The interaction of PP1 with BRCA1 and analysis of their expression in breast tumors. BMC Cancer 7:85. doi: 10.1186/1471-2407-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen H, Bai B, Hou J, Sun Y, Hu Y, Duan Q, Gao R, Zhu H, Kong W, Xu D, Zhao J, Wang H, Mao P. 2012. OPV-like poliovirus type 1 detection in patients with severe acute respiratory syndrome. Infection 40:455–458. doi: 10.1007/s15010-012-0244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng C, Li X, Liu S, Xu L, Ye H, Qin CF, Zhang B. 2015. Development and characterization of a clinical strain of coxsackievirus A16 and an eGFP infectious clone. Virol Sin 30:269–276. doi: 10.1007/s12250-015-3610-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Zheng Z, Shu B, Liu X, Zhang Z, Liu Y, Bai B, Hu Q, Mao P, Wang H. 2013. Human astrocytic cells support persistent coxsackievirus B3 infection. J Virol 87:12407–12421. doi: 10.1128/JVI.02090-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rebelo S, Santos M, Martins F, da Cruz ESEF, da Cruz ESOA. 2015. Protein phosphatase 1 is a key player in nuclear events. Cell Signal 27:2589–2598. doi: 10.1016/j.cellsig.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Bollen M, Peti W, Ragusa MJ, Beullens M. 2010. The extended PP1 toolkit: designed to create specificity. Trends Biochem Sci 35:450–458. doi: 10.1016/j.tibs.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heroes E, Lesage B, Gornemann J, Beullens M, Van Meervelt L, Bollen M. 2013. The PP1 binding code: a molecular-Lego strategy that governs specificity. FEBS J 280:584–595. doi: 10.1111/j.1742-4658.2012.08547.x. [DOI] [PubMed] [Google Scholar]
- 30.Ammosova T, Jerebtsova M, Beullens M, Lesage B, Jackson A, Kashanchi F, Southerland W, Gordeuk VR, Bollen M, Nekhai S. 2005. Nuclear targeting of protein phosphatase-1 by HIV-1 Tat protein. J Biol Chem 280:36364–36371. doi: 10.1074/jbc.M503673200. [DOI] [PubMed] [Google Scholar]
- 31.Takagi M, Nishiyama Y, Taguchi A, Imamoto N. 2014. Ki67 antigen contributes to the timely accumulation of protein phosphatase 1gamma on anaphase chromosomes. J Biol Chem 289:22877–22887. doi: 10.1074/jbc.M114.556647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Egloff MP, Johnson DF, Moorhead G, Cohen PT, Cohen P, Barford D. 1997. Structural basis for the recognition of regulatory subunits by the catalytic subunit of protein phosphatase 1. EMBO J 16:1876–1887. doi: 10.1093/emboj/16.8.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi Y. 2009. Serine/threonine phosphatases: mechanism through structure. Cell 139:468–484. doi: 10.1016/j.cell.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Li HY, Liu H, Wang CH, Zhang JY, Man JH, Gao YF, Zhang PJ, Li WH, Zhao J, Pan X, Zhou T, Gong WL, Li AL, Zhang XM. 2008. Deactivation of the kinase IKK by CUEDC2 through recruitment of the phosphatase PP1. Nat Immunol 9:533–541. doi: 10.1038/ni.1600. [DOI] [PubMed] [Google Scholar]
- 35.Hinz M, Scheidereit C. 2014. The IkappaB kinase complex in NF-kappaB regulation and beyond. EMBO Rep 15:46–61. doi: 10.1002/embr.201337983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheidereit C. 2006. IkappaB kinase complexes: gateways to NF-kappaB activation and transcription. Oncogene 25:6685–6705. doi: 10.1038/sj.onc.1209934. [DOI] [PubMed] [Google Scholar]
- 37.Liu F, Xia Y, Parker AS, Verma IM. 2012. IKK biology. Immunol Rev 246:239–253. doi: 10.1111/j.1600-065X.2012.01107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qu L, Ji Y, Zhu X, Zheng X. 2015. hCINAP negatively regulates NF-kappaB signaling by recruiting the phosphatase PP1 to deactivate IKK complex. J Mol Cell Biol 7:529–542. doi: 10.1093/jmcb/mjv041. [DOI] [PubMed] [Google Scholar]
- 39.Amaya M, Keck F, Bailey C, Narayanan A. 2014. The role of the IKK complex in viral infections. Pathog Dis 72:32–44. doi: 10.1111/2049-632X.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du H, Yin P, Yang X, Zhang L, Jin Q, Zhu G. 2015. Enterovirus 71 2C protein inhibits NF-kappaB activation by binding to RelA(p65). Sci Rep 5:14302. doi: 10.1038/srep14302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin H, Yan Z, Ma Y, Cao Y, He B. 2011. A herpesvirus virulence factor inhibits dendritic cell maturation through protein phosphatase 1 and Ikappa B kinase. J Virol 85:3397–3407. doi: 10.1128/JVI.02373-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chatterjee J, Kohn M. 2013. Targeting the untargetable: recent advances in the selective chemical modulation of protein phosphatase-1 activity. Curr Opin Chem Biol 17:361–368. doi: 10.1016/j.cbpa.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 43.Wies E, Wang MK, Maharaj NP, Chen K, Zhou S, Finberg RW, Gack MU. 2013. Dephosphorylation of the RNA sensors RIG-I and MDA5 by the phosphatase PP1 is essential for innate immune signaling. Immunity 38:437–449. doi: 10.1016/j.immuni.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis ME, Wang MK, Rennick LJ, Full F, Gableske S, Mesman AW, Gringhuis SI, Geijtenbeek TB, Duprex WP, Gack MU. 2014. Antagonism of the phosphatase PP1 by the measles virus V protein is required for innate immune escape of MDA5. Cell Host Microbe 16:19–30. doi: 10.1016/j.chom.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mesman AW, Zijlstra-Willems EM, Kaptein TM, de Swart RL, Davis ME, Ludlow M, Duprex WP, Gack MU, Gringhuis SI, Geijtenbeek TB. 2014. Measles virus suppresses RIG-I-like receptor activation in dendritic cells via DC-SIGN-mediated inhibition of PP1 phosphatases. Cell Host Microbe 16:31–42. doi: 10.1016/j.chom.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seya T. 2014. Measles virus takes a two-pronged attack on PP1. Cell Host Microbe 16:1–2. doi: 10.1016/j.chom.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 47.Zhang C, Tang J, Xie J, Zhang H, Li Y, Zhang J, Verpooten D, He B, Cao Y. 2008. A conserved domain of herpes simplex virus ICP34.5 regulates protein phosphatase complex in mammalian cells. FEBS Lett 582:171–176. doi: 10.1016/j.febslet.2007.11.082. [DOI] [PubMed] [Google Scholar]
- 48.de Los Santos T, Diaz-San Segundo F, Grubman MJ. 2007. Degradation of nuclear factor kappa B during foot-and-mouth disease virus infection. J Virol 81:12803–12815. doi: 10.1128/JVI.01467-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neznanov N, Kondratova A, Chumakov KM, Angres B, Zhumabayeva B, Agol VI, Gudkov AV. 2001. Poliovirus protein 3A inhibits tumor necrosis factor (TNF)-induced apoptosis by eliminating the TNF receptor from the cell surface. J Virol 75:10409–10420. doi: 10.1128/JVI.75.21.10409-10420.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Argos P, Kamer G, Nicklin MJ, Wimmer E. 1984. Similarity in gene organization and homology between proteins of animal picornaviruses and a plant comovirus suggest common ancestry of these virus families. Nucleic Acids Res 12:7251–7267. doi: 10.1093/nar/12.18.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang C, Ma HC, Wimmer E, Jiang P, Paul AV. 2014. A C-terminal, cysteine-rich site in poliovirus 2C(ATPase) is required for morphogenesis. J Gen Virol 95:1255–1265. doi: 10.1099/vir.0.062497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J, Wu Z, Jin Q. 2012. COPI is required for enterovirus 71 replication. PLoS One 7:e38035. doi: 10.1371/journal.pone.0038035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang WF, Yang SY, Wu BW, Jheng JR, Chen YL, Shih CH, Lin KH, Lai HC, Tang P, Horng JT. 2007. Reticulon 3 binds the 2C protein of enterovirus 71 and is required for viral replication. J Biol Chem 282:5888–5898. doi: 10.1074/jbc.M611145200. [DOI] [PubMed] [Google Scholar]
- 54.Xia H, Wang P, Wang GC, Yang J, Sun X, Wu W, Qiu Y, Shu T, Zhao X, Yin L, Qin CF, Hu Y, Zhou X. 2015. Human enterovirus nonstructural protein 2CATPase functions as both an RNA helicase and ATP-independent RNA chaperone. PLoS Pathog 11:e1005067. doi: 10.1371/journal.ppat.1005067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Echeverri A, Banerjee R, Dasgupta A. 1998. Amino-terminal region of poliovirus 2C protein is sufficient for membrane binding. Virus Res 54:217–223. doi: 10.1016/S0168-1702(98)00016-1. [DOI] [PubMed] [Google Scholar]
- 56.Echeverri AC, Dasgupta A. 1995. Amino terminal regions of poliovirus 2C protein mediate membrane binding. Virology 208:540–553. doi: 10.1006/viro.1995.1185. [DOI] [PubMed] [Google Scholar]
- 57.Ceulemans H, Bollen M. 2004. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol Rev 84:1–39. doi: 10.1152/physrev.00013.2003. [DOI] [PubMed] [Google Scholar]
- 58.Cohen PT. 2002. Protein phosphatase 1—targeted in many directions. J Cell Sci 115(Pt 2):241–256. [DOI] [PubMed] [Google Scholar]
- 59.Zhang F, Moon A, Childs K, Goodbourn S, Dixon LK. 2010. The African swine fever virus DP71L protein recruits the protein phosphatase 1 catalytic subunit to dephosphorylate eIF2alpha and inhibits CHOP induction but is dispensable for these activities during virus infection. J Virol 84:10681–10689. doi: 10.1128/JVI.01027-10. [DOI] [PMC free article] [PubMed] [Google Scholar]