Abstract
PURPOSE
We aimed to assess the effectiveness, benefits, and reliability of percutaneous vertebroplasty (PV) in patients with vertebral involvement of multiple myeloma.
METHODS
PV procedures performed on 166 vertebrae of 41 patients with multiple myeloma were retrospectively evaluated. Most of our patients were using level 3 (moderate to severe pain) analgesics. Magnetic resonance imaging was performed before the procedure to assess vertebral involvement of multiple myeloma. The following variables were evaluated: affected vertebral levels, loss of vertebral body height, polymethylmethacrylate (PMMA) cement amount applied to the vertebral body during PV, PMMA cement leakages, and pain before and after PV as assessed by a visual analogue scale (VAS).
RESULTS
Median VAS scores of patients decreased from 9 one day before PV, to 6 one day after the procedure, to 3 one week after the procedure, and eventually to 1 three months after the procedure (P < 0.001). During the PV procedure, cement leakage was observed at 68 vertebral levels (41%). The median value of PMMA applied to the vertebral body was 6 mL.
CONCLUSION
Being a minimally invasive and easily performed procedure with low complication rates, PV should be preferred for serious back pain of multiple myeloma patients.
Multiple myeloma (MM) is a hematologic malignancy characterized by lytic bone lesions and is usually with vertebral involvement. At the time of diagnosis, vertebral involvement is present in approximately 60% of patients (1). Pathologic vertebral fractures can easily occur in MM. Spinal instability, back pain, neurologic dysfunction and physical symptoms can be observed in patients with MM due to vertebral fractures. As a result, the quality of life of patients is affected significantly. A variety of contemporary therapeutic approaches are available for vertebral involvement in MM. These approaches are chemotherapy, radiotherapy, radioisotope therapy, bisphosphonate therapy, algological treatment and palliative/stabilization surgery. Risks of spinal instability and neural compression can be high with conservative treatment options. While surgery can be suitable for patients with neural compression, its complication rates are high (2).
Percutaneous vertebroplasty (PV) is a minimally invasive treatment method. PV is used to treat back pain caused by vertebral involvement due to osteoporotic vertebral compression fractures, metastasis, multiple myeloma, and aggressive hemangioma (3). PV increases spinal stability by preventing vertebral collapse (2). PV was originally used for treatment of painful vertebral hemangioma by Galibert et al. (4). In several studies, it was reported that PV prevented vertebral height loss and reduced patient pain and need of analgesia use in patients with vertebral involvement due to osteoporosis and metastasis (5–8). PV usage is gradually increasing for vertebral involvement due to MM. However, data on PV usage for MM is limited in the literature (8). PV is preferred because it is more easily performed than surgery, more effective, and has lower rates of serious complications.
The aim of this study was to assess the effectiveness, benefits, and reliability of PV in patients with vertebral involvement of MM.
Methods
Study design and population
In this retrospective study, 41 patients (166 vertebrae) with MM who underwent PV between November 2008 and May 2014 were included. The only indication for PV was severe back pain. Severe pain generally limited body movements of patients and did not respond to different analgesics. Most of our patients were using level 3 (moderate to severe pain) analgesics (opiate analgesics). There was no neurologic deficit in any of the patients. Magnetic resonance imaging (MRI) was performed before the procedure, in order to assess vertebral involvement of MM. Conventional sagittal T1-weighted, T2-weighted and short tau inversion recovery (STIR) images were acquired on a 3.0 T (Achieva TX, Philips Medical systems) or a 1.5 T scanner (Magnetom Vision plus, Siemens Medical systems) using a spine coil. Sagittal postcontrast T1-weighted images were acquired after administration of 0.1 mmol/kg contrast media (Dotarem®; Guerbet or Magnevist®, Bayer Healthcare) when necessary. Vertebral involvement was determined by clinical and radiologic assessments. Presence of back pain and radicular pain raised clinical suspicion for vertebral involvement. The degree of vertebral involvement in MM was assessed using the semiquantitative visual assessment index showing vertebral deformity developed by Genant et al. (9). In this index, loss of height is evaluated as grade 0, normal; grade 1, 20%–25% mild; grade 2, 25%–40% moderate; grade 3, >40% severe. This study was approved by the local clinical research ethics committee.
Procedural technique
PV was performed in sterile conditions under analgosedation (midazolam 0.03 mg/kg intravenous [IV] and/or fentanyl 1 μ/kg IV and/or ketamine 1 mg/kg IV and/or propofol 3–5 mg/kg IV and/or pethidine 1 mg/kg intramuscular), in a biplane, flat-paneled angiography unit (AXIOM Artis FD Biplane Angiosuite, Siemens). Ampicillin 1000 mg/sulbactam 500 mg IV combination was administered for preprocedural antibioprophylaxis. Patients were laid on the angiography table in a prone position. During the procedure, a cement vertebroplasty system (OptiMed Medical Devices, Ettlingen) or kyphon vertebroplasty kit (Kyphon Inc.) involving 10- or 13-gauge single-use-only bone biopsy needles was used. Biopsy needles were placed with the help of anteroposterior and/or lateral fluoroscopic imaging and left transpedicular, right transpedicular, and bipedicular approaches were used. Polymethylmethacrylate (PMMA) (Cemento Fixx, Optimed) bone cement was prepared and applied to the vertebral body using biopsy needles in a slow and controlled way manually or using an injection gun. The cement was administered principally to the lytic zone of the vertebrae. A maximum number of four sessions was performed on a single patient. After the procedure, patients were held in the observation room for three hours and subsequently discharged within the same day.
Pain assessment
In order to assess the pain scores of MM patients with vertebral involvement, a visual analogue scale (VAS) was used. VAS scores of the patients were recorded one day before, one day after, one week after, and three months after PV. VAS involved the standard pain scale between 0 and 10 (0, no pain; 10, intolerable, the most severe pain ever felt in patient’s life) in order to determine the level of pain objectively. Before and after VAS scores were assessed by talking to the patients face to face or contacting them by phone.
Statistical analysis
Statistical analysis of data was done using the SPSS 22.0 statistical package program. Descriptive statistics were determined in terms of average±standard deviation or median (minimum-maximum). Categorical data was determined as frequency and percentage. Wilcoxon signed rank test was used to compare dependent groups. Repeated VAS measurements of groups were compared by taking difference values between them into consideration. The level of significance was determined as α=0.05.
Results
Between November 2008 and May 2014, 24 men (58.5%) and 17 women (41.5%) with MM underwent PV. The average age of the patients was 60.63±11.24 (range, 39–84 years).
PV was performed on 166 vertebrae, of which, 86 were thoracic (51.8%) and 80 were lumbar (48.2%). The PV procedure was performed at T4-L5 vertebral levels. PV was most frequently performed at the L1 level (n=22/166; 13.3%) for lumbar and at the T12 level (n=19/166; 11.5%) for thoracic vertebrae. According to the semiquantitative visual assessment index developed by Genant et al. (9), loss of height was grade 0 in 12 vertebrae (7.2%), grade 1 in 47 vertebrae (28.3%), grade 2 in 50 vertebrae (30.1%), and grade 3 in 57 vertebrae (34.4%).
The PV procedure was performed in a single session on 27 patients. Two or more sessions were performed on 14 patients (nine patients had two sessions, four patients had three sessions, and one patient had four sessions).
The median number of PV-performed vertebrae per session was one (range, 1–4), and three vertebrae received PV (range, 1–11) per patient. The median duration per session was 43.5 minutes (range, 18–78). The median volume of PMMA injected into the vertebral body during PV was 6 mL (range, 3–10).
The effectiveness of PV was assessed using VAS pain scores before and after PV, the amount of PMMA applied to the vertebral body and PMMA leakages during the procedure.
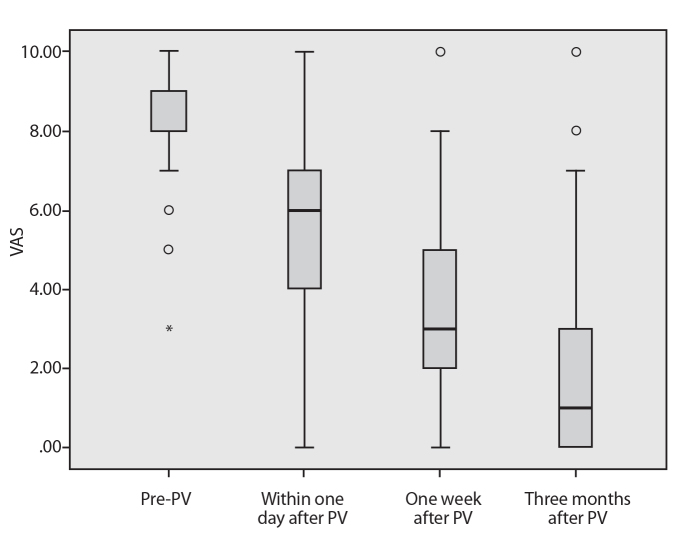
Median VAS scores of patients decreased from 9 (range, 3–10) one day before the procedure, to 6 (range, 0–10) one day after the procedure, to 3 (range, 0–10) one week after the procedure, and eventually to 1 (range, 0–10) three months after the procedure (P < 0.001) (Fig. 1). There was a significant difference between the average VAS scores at one day before and one day after the procedure, one day before and at one week after the procedure and one day before and three months after the procedure (P < 0.001, for all).
Figure 1.
VAS scores of pain obtained one day before, one day after, one week after, and three months after the PV procedure. Tukey box plot represents median (horizontal line), interquartile range (IQR, box), 1.5×IQR, and outliers (open circles). A significant decrease can be observed in VAS values after PV (P < 0.001, for comparison of all post-PV time points with before PV).
No complications were observed in 98 vertebral levels (59%). There were PMMA leakages in a total of 68 vertebrae (41%); 25 vertebrae (15.1%) had leakages into the disc, 36 vertebrae (21.7%) into the epidural or paravertebral vein, and seven vertebrae (4.2%) into both the disc and the epidural or paravertebral vein. No neurologic deficit or clinical symptom was observed because of these leakages.
VAS scores measured at one day, one week, and three months after the procedure were significantly reduced compared with the preprocedure score in patients with and without complications (P < 0.001, P < 0.001, and P < 0.001 in the group with complications and P = 0.007, P = 0.007, and P = 0.007 in the group with no complications, respectively). No statistically significant difference was observed with regard to changes of VAS scores obtained before and after the procedure between the two groups (P = 0.086, P = 0.777, P = 0.127, and P = 0.051, for one day before, one day after, one week after, and three months after, respectively). There was no statistically significant relationship between the applied cement volume and the VAS score decrease after the procedure (P = 0.797, P = 0.257, P = 0.732, and P = 0.864, for one day before, one day after, one week after, and three months after, respectively). There was no statistically significant relationship between vertebral height loss and the decrease of VAS scores after the procedure (P = 0.394, P = 0.247, P = 0.052, and P = 0.113 for one day before, one day after, one week after, and three months after, respectively).
Table shows a detailed analysis of the PV procedure in 41 patients with MM. Figs. 2 and 3 give case examples.
Table.
Detailed analysis of 41 patients with multiple myeloma who had percutaneous vertebroplasty
| Variables | Value |
|---|---|
| Sex (men/women), n (%) | 24 (58.5)/17 (41.5) |
| Age (years), mean±SD (range) | 60.63±11.24 (39–84) |
| VAS score one day before PV, median (range) | 9 (3–10) |
| VAS score one day after PV, median (range) | 6 (0–10) |
| VAS score one week after PV, median (range) | 3 (0–10) |
| VAS score three months after PV, median (range) | 1 (0–10) |
| Number of vertebrae treated, n (%) | 166 (100) |
| Thoracic level, number of vertebrae (%) | 86 (51.8) |
| T4 | 1 (0.6) |
| T5 | 2 (1.2) |
| T6 | 4 (2.4) |
| T7 | 8 (4.8) |
| T8 | 12 (7.2) |
| T9 | 10 (6) |
| T10 | 12 (7.2) |
| T11 | 18 (10.9) |
| T12 | 19 (11.5) |
| Lumbar level, number of vertebrae (%) | 80 (48.2) |
| L1 | 22 (13.3) |
| L2 | 17 (10.2) |
| L3 | 21 (12.7) |
| L4 | 12 (7.2) |
| L5 | 8 (4.8) |
| Loss of vertebral height, number of vertebrae (%) | 166 (100) |
| Grade 0 | 12 (7.2) |
| Grade 1 | 47 (28.3) |
| Grade 2 | 50 (30.1) |
| Grade 3 | 57 (34.4) |
| Complications, number of vertebrae (%) | 68 (41) |
| Leaks into the disc | 25 (15.1) |
| Leaks into the epidural or paravertebral vein | 36 (21.7) |
| Leaks into the disc and epidural or paravertebral vein | 7 (4.2) |
| PMMA amount applied during the procedure (mL), median (range) | 6 (3–10) |
| Patients who had one session of the procedure, n | 27 |
| Patients who had 2, 3 or 4 sessions of the procedure, n | 14 |
| Number of vertebrae receiving PV per session, median (range) | 1 (1–4) |
| Number of vertebrae receiving PV per patient, median (range) | 3 (1–11) |
| Duration of session (min), median (range) | 43.5 (18–78) |
SD, standard deviation; VAS, visual analogue scale (0, no pain; 10, the most acute pain); PV, percutaneous vertebroplasty; PMMA, polymethylmethacrylate.
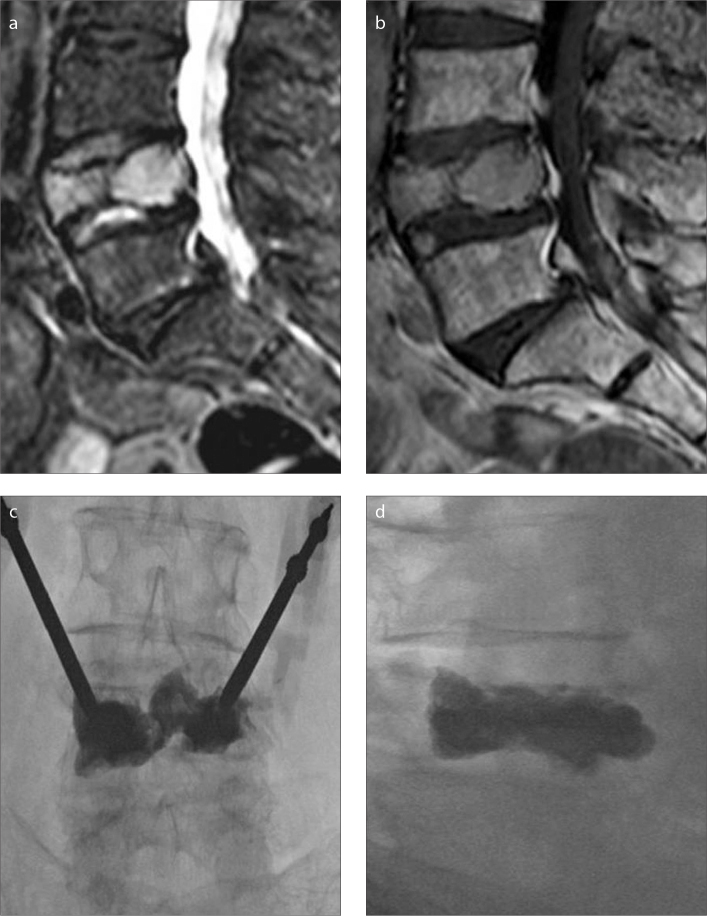
Figure 2. a–d.
A 76-year-old-female patient with multiple myeloma. Grade 3 vertebral height loss on L4 vertebral body is shown as hyperintense signal on STIR sagittal image (a) and isointense signal on sagittal T1-weighted image (b). PV procedure performed on L4 vertebral body with bipedicular approach (c, d).
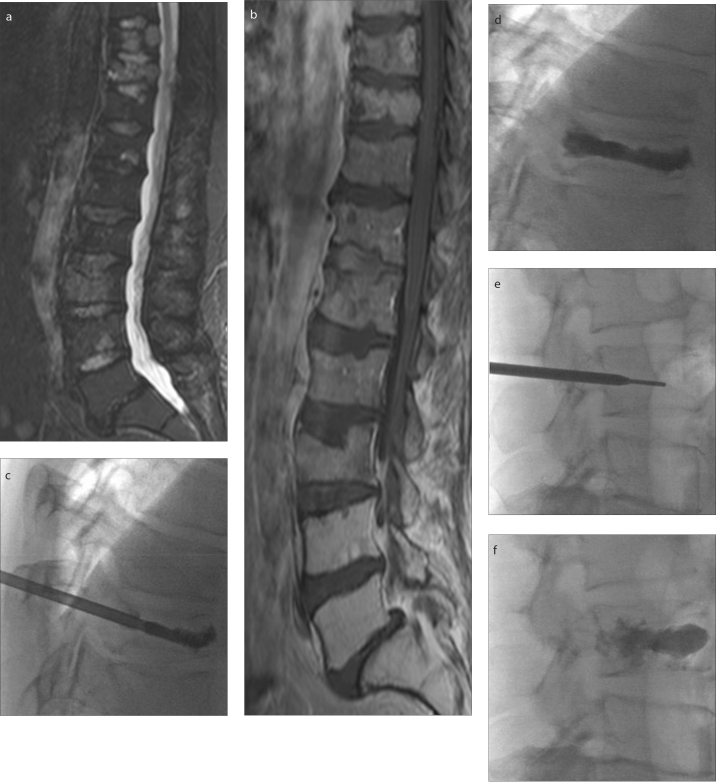
Figure 3. a–f.
Focal and diffuse involvement of multiple myeloma in thoracic (T10) and lumbar (L3) vertebrae is shown as hyperintense signal on STIR sagittal image (a) and iso-hypointense diffuse involvement on sagittal T1-weighted image (b). PV was performed on T10 (c, d) and L3 vertebrae (e, f).
Discussion
This study demonstrates that PV decreases back pain due to vertebral involvement and that it is an effective method for patients with MM. One of the main reasons for morbidity in MM is skeletal system involvement. MM usually affects the spinal column and causes vertebral collapse and acute pain (10). Patients need to be bedridden for weeks, use high doses of opioid analgesics and rarely receive palliative radiotherapy. Vertebral fractures can increase morbidity and mortality by causing spinal deformity, resistant pain, and spinal cord compression. The main aim of treating vertebral fractures due to MM should be to reduce pain and to provide functional restoration (11). Most of our patients had been using level 3 (moderate to severe pain) analgesics (opiate analgesics) and all patients had pain unresponsive to these analgesics in the preprocedural period. The need for analgesics decreased after the procedure.
PV is a minimally invasive procedure where PMMA bone cement is injected into the vertebral body. Strengthening vertebra with PV helps support the vertebral structure in vertebral fractures which may relieve, pain due to fractures (12). Chen et al. (11) performed PV on 36 vertebrae of 24 patients who had vertebral fractures secondary to MM. They showed that the mean VAS score before the procedure was 9 and it decreased to 3.8 one day after, to 3.5 three months after, and to 4.7 one year after the procedure. Anselmetti et al. (13) reported that the median score of 106 patients with vertebral fractures due to MM decreased from 9 (4–10) to 1 (0–9) after PV. In a study of 64 myeloma-associated vertebral levels, Simony et al. (14) observed the VAS score decrease from 7.6 in the preoperative period to 3.2 three months after PV. In our study, a significant decrease was present in median VAS scores of 41 patients (166 vertebrae) before PV compared with the scores after PV. This significant decrease in VAS scores after the procedure is in accordance with the limited amount of literature data on the subject (11, 13, 14). PV provides effective and fast relief for patients with vertebral pain due to MM.
The effectiveness of PV can be related to many factors. The most important one of which being the biomechanical mechanism (11). PMMA used during PV helps with stabilizing microfractures and strengthening the treated vertebra (15, 16). PMMA can cause damage in nerve endings and pain receptors because of the heat released during polymerization. Coagulation of tumoral tissue can also be directly induced. In addition, direct cytotoxic effects can cause tumor necrosis. For these reasons, a small amount of cement can induce a significant reduction in pain (17). Yang et al. (2) used 3–9.5 mL of cement in PV procedures for MM. In our study, the PMMA amount used to reach adequate vertebral stiffness ranged 3–10 mL.
During the PV procedure, low viscosity cement needs to be applied to collapsed vertebra in a quick and effective way and with high pressure. In this case, there is a risk of cement leakage outside the vertebra (18). Neural compression, radiculopathy, and pulmonary embolism can be observed due to cement leakage outside the vertebra. Cement leakage into the disc or paravertebral area can also be observed. These complications are usually asymptomatic. Complications during PV can be prevented by bringing the needle tip into the proper position under fluoroscopy (11). Other possible complications include vertebral transverse process or pedicle fractures, paravertebral hematoma, epidural abscess, pneumothorax, cerebrospinal fluid leakage, seizure because of oversedation or respiratory arrest and death (19). La Maida et al. (10) reported a cement leakage rate of 27.7% during PV on 18 vertebral fracture levels due to MM. Anselmetti et al. (13) reported a cement leakage rate of 22.9% on vertebral fractures due to MM after PV procedure on 106 patients. In our study, cement leakage was observed in 68 of 166 vertebral levels (41%) during the PV procedure. This high rate of cement leakage can be due to high pressure application of the low viscosity cement into the vertebra. VAS scores within one day, one week, and three months after the procedure were significantly reduced compared with the score one day before the procedure in both groups with and without complications. No statistically significant difference was observed with regard to changes of VAS scores obtained before and after (within one day, one week, and three months) the procedure between the two groups. Khan et al. (8) determined no relationship between VAS score decrease and the presence of cement leakage in their systemic literature review, and this was compatible with our results.
Our study has some limitations. It is a retrospective study without a control group, follow-up with most patients is inadequate because of their primary malignancies, and some patients were reached only by phone, although face to face communication would have been more effective. Another limitation is the assessment of VAS scores at a maximum of three months after the procedure. The reasons for this short interval were difficulties in reaching some patients by phone and in assessing them on control examination.
In conclusion, PV is a simple, effective, reliable, easy to perform and minimally invasive procedure. For this reason, we believe that PV should be preferred to treat acute back pain due to vertebral fractures and to stabilize the vertebra in patients with MM. Treatment of vertebral fractures can be performed effectively and safely with PV.
Main points.
Our study shows that median pain scores of multiple myeloma patients decreased significantly following percutaneous vertebroplasty (PV).
PV decreases back pain due to vertebral involvement in multiple myeloma patients.
PV is an effective and safe method for patients with multiple myeloma.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Trumm C, Jakobs T, Pahl A, et al. CT fluoroscopy-guided percutaneous vertebroplasty in patients with multiple myeloma: analysis of technical results from 44 sessions with 67 vertebrae treated. Diagn Interv Radiol. 2012;18:111–120. doi: 10.4261/1305-3825.DIR.4226-11.1. [DOI] [PubMed] [Google Scholar]
- 2.Yang Z, Tan J, Xu Y, et al. Treatment of MM-associated spinal fracture with percutaneous vertebroplasty (PVP) and chemotherapy. Eur Spine J. 2012;21:912–919. doi: 10.1007/s00586-011-2105-y. http://dx.doi.org/10.1007/s00586-011-2105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez-Catarino M, Blimark C, Willén J, et al. Percutaneous vertebroplasty at C2: case report of a patient with multiple myeloma and a literature review. Eur Spine J. 2007;16:242–249. doi: 10.1007/s00586-006-0256-z. http://dx.doi.org/10.1007/s00586-006-0256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galibert P, Deramond H, Rosat P, et al. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie. 1987;33:166–168. [PubMed] [Google Scholar]
- 5.Papanastassiou ID, Phillips FM, Van Meirhaeghe J, et al. Comparing effects of kyphoplasty, vertebroplasty, and non-surgical management in a systematic review of randomized and non-randomized controlled studies. Eur Spine J. 2012;21:1826–1843. doi: 10.1007/s00586-012-2314-z. http://dx.doi.org/10.1007/s00586-012-2314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGirt MJ, Parker SL, Wolinsky JP, et al. Vertebroplasty and kyphoplasty for the treatment of vertebral compression fractures: an evidenced-based review of the literature. Spine J. 2009;9:501–508. doi: 10.1016/j.spinee.2009.01.003. http://dx.doi.org/10.1016/j.spinee.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Tseng YY, Lo YL, Chen LH, et al. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of pain induced by metastatic spine tumor. Surg Neurol. 2008;7:78–83. doi: 10.1016/j.surneu.2008.08.078. http://dx.doi.org/10.1016/j.surneu.2008.08.078. [DOI] [PubMed] [Google Scholar]
- 8.Khan OA, Brinjikji W, Kallmes DF. Vertebral augmentation in patients with multiple myeloma: a pooled analysis of published case series. AJNR Am J Neuroradiol. 2014;35:207–210. doi: 10.3174/ajnr.A3622. http://dx.doi.org/10.3174/ajnr.A3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genant HK, Wu CY, van Kuijk C, et al. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–1148. doi: 10.1002/jbmr.5650080915. http://dx.doi.org/10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 10.La Maida GA, Giarratana LS, Acerbi A, et al. Cement leakage: safety of minimally invasive surgical techniques in the treatment of multiple myeloma vertebral lesions. Eur Spine J. 2012;21:61–68. doi: 10.1007/s00586-012-2221-3. http://dx.doi.org/10.1007/s00586-012-2221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen LH, Hsieh MK, Niu CC, et al. Percutaneous vertebroplasty for pathological vertebral compression fractures secondary to multiple myeloma. Arch Orthop Trauma Surg. 2012;132:759–764. doi: 10.1007/s00402-012-1474-y. http://dx.doi.org/10.1007/s00402-012-1474-y. [DOI] [PubMed] [Google Scholar]
- 12.Mailli L, Filippiadis DK, Brountzos EN, et al. Clinical outcome and safety of multilevel vertebroplasty: clinical experience and results. Cardiovasc Intervent Radiol. 2013;36:183–191. doi: 10.1007/s00270-012-0379-z. http://dx.doi.org/10.1007/s00270-012-0379-z. [DOI] [PubMed] [Google Scholar]
- 13.Anselmetti GC, Manca A, Montemurro F, et al. Percutaneous vertebroplasty in multiple myeloma: prospective long-term follow-up in 106 consecutive patients. Cardiovasc Intervent Radiol. 2012;35:139–145. doi: 10.1007/s00270-011-0111-4. http://dx.doi.org/10.1007/s00270-011-0111-4. [DOI] [PubMed] [Google Scholar]
- 14.Simony A, Hansen EJ, Gaurilcikas M, et al. Pain reduction after percutaneous vertebroplasty for myeloma-associated vertebral fractures. Dan Med J. 2014;61:A4945. [PubMed] [Google Scholar]
- 15.Jensen ME, Kallmes DE. Percutaneous vertebroplasty in the treatment of malignant spine disease. Cancer J. 2002;8:194–206. doi: 10.1097/00130404-200203000-00013. http://dx.doi.org/10.1097/00130404-200203000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Belkoff SM, Mathis JM, Jasper LE, et al. The biomechanics of vertebroplasty. The effect of cement volume on mechanical behavior. Spine. 2001;26:1537–1541. doi: 10.1097/00007632-200107150-00007. http://dx.doi.org/10.1097/00007632-200107150-00007. [DOI] [PubMed] [Google Scholar]
- 17.Gangi A, Guth S, Imbert JP, et al. Percutaneous vertebroplasty: indications, technique, and results. Radiographics. 2003;23:e10. doi: 10.1148/rg.e10. http://dx.doi.org/10.1148/rg.e10. [DOI] [PubMed] [Google Scholar]
- 18.Phillips FM, Todd Wetzel F, Lieberman I, et al. An in vivo comparison of the potential for extravertebral cement leakage after vertebroplasty and kyphoplasty. Spine. 2002;27:2173–2178. doi: 10.1097/00007632-200210010-00018. http://dx.doi.org/10.1097/00007632-200210010-00018. [DOI] [PubMed] [Google Scholar]
- 19.Jensen ME, McGraw JK, Cardella JF, et al. Position statement on percutaneous vertebral augmentation: a consensus statement developed by the American Society of Interventional and Therapeutic Neuroradiology, Society of Interventional Radiology, American Association of Neurological Surgeons/Congress of Neurological Surgeons, and American Society of Spine Radiology. AJNR Am J Neuroradiol. 2007;28:1439–1443. http://dx.doi.org/10.1016/j.jvir.2007.01.014. [PMC free article] [PubMed] [Google Scholar]