Abstract
PURPOSE
We compared signal change on magnetic resonance imaging (MRI) with fat suppression and bone scan activity of lumbar facet joints to determine if these two imaging findings are correlated.
METHODS
We retrospectively identified all patients who underwent imaging of the lumbar spine for pain evaluation using both technetium-99m methylene disphosphonate single-photon emission computed tomography/computed tomography (99mTc-MDP SPECT/CT) and MRI with at least one fat-suppressed T2- or T1-weighted sequence with gadolinium enhancement within a 180-day interval, at our institution between 1 January 2008 and 19 February 2013. Facet joint activity on 99mTc-MDP SPECT/CT and peri-facet signal change on MRI were rated as normal or increased. Agreement between the two examination types were determined with the κ and prevalence-adjusted bias-adjusted κ (PABAK) statistics.
RESULTS
This study included 60 patients (28 male, 47%), with a mean age of 49±19.7 years (range, 12–93 years). The κ value indicated no agreement between 99mTc-MDP SPECT/CT and MRI (κ=−0.026; 95% confidence interval: −0.051, 0.000). The PABAK values were fair to high at each spinal level, which suggests that relatively low disease prevalence lowered the κ values. Together, the κ and PABAK values indicate that there is some degree of intermodality agreement, but that it is not consistent.
CONCLUSION
Overall, facet joint signal change on fat-suppressed MRI did not always correlate with increased 99mTc-MDP SPECT/CT activity. MRI and 99mTc-MDP SPECT/CT for facet joint evaluation should not be considered interchangeable examinations in clinical practice or research.
Low back pain is a common source of chronic pain worldwide and may incur a substantial socioeconomic cost (1, 2). Facet joint pain is believed to be a common cause of low back pain (3). Both clinical examination and findings on anatomic imaging have limited ability to identify specific causes of low back pain, including specific painful facet joints (4). Therefore, identification of physiologic imaging biomarkers to identify treatable causes of low back pain is desirable.
Accurate identification of specific painful facet joints has potential to improve selection of patients for percutaneous treatment and to avert inappropriate interventions, facilitating clinical consideration of alternate causes of pain. In our clinical experience, facet joint interventions are most frequently delivered to the bilateral L4/L5 and bilateral L5/S1 facet joints for low back pain because it is difficult to achieve greater specificity clinically. Imaging biomarkers could select the most appropriate facet joints for intervention, and have potential to decrease the cost and minor risk associated with each incremental injection.
Such a task will ultimately require prospective assessment of the ability of putative imaging biomarkers of facet joint pain to predict response to comparison medial branch blocks. Prior to embarking on such prospective studies, it is critical to perform initial evaluation of potential imaging biomarkers. It is desirable to compare the concordance of candidate biomarkers to determine if they can or cannot act as surrogates in future clinical investigation and ultimately clinical practice.
Two potential imaging markers of facet joint pain are Technetium-99m methylene diphosphonate (99mTc-MDP) activity and peri-facet signal change on fat-suppressed MRI. Prior studies purport that bone scan activity on 99mTc-MDP single photon emission tomography/computed tomography (99mTc-MDP SPECT) can predict both painful facet joints and positive response to percutaneous treatment (5, 6). However, this assertion is unproved; these studies used intra-articular injections, which have a high placebo rate, rather than comparative medial branch blocks, the standard diagnostic method for facet joint pain accepted by pain medicine physicians (7). Limited data also purport that T2 hyperintensity or gadolinium enhancement on MRI may be associated with painful facet joint arthropathy, although this is based on limited indirect evidence, rather than use of comparative medial branch blocks, and is also unproved (8).
Nonetheless, if bone scan activity and hyperintensity on fat-suppressed MRI images were markers of the same condition, painful facet joint arthropathy, these two imaging findings may demonstrate a high degree of concordance within the same patient. In our clinical experience, we have anecdotally observed discrepancies between 99mTc-MDP activity and MRI signal change of specific facet joints. However, to our knowledge there is no prior data to formally assess the degree of concordance of these two potential imaging biomarkers. In this study, we explored the concordance of facet joint activity on 99mTc-MDP SPECT/CT bone scan with facet joint signal change on fat-suppressed MRI.
Methods
Patients
Institutional Review Board approval was obtained for this retrospective study. We searched our imaging database for the records of all patients who underwent both 99mTc-MDP SPECT/CT and MRI of the lumbar spine with at least one fat-suppressed sequence, which were performed within 180 days of each other, at our institution, between 1 January 2008 and 19 February 2013. The database search selected patients with an indication of pain rather than tumor, infection, or trauma. The initial database search identified 73 patients with both a 99mTc-MDP SPECT/CT and MRI of the lumbar spine with at least one fat-suppressed sequence performed within 180 days of each other with the indication of pain.
Exclusion criteria included metallic hardware; surgery of any type in the lumbar spine within six months of one of the imaging examinations; surgery of any type in the lumbar spine with scar tissue in contact with facet joint(s); incomplete coverage of the facet joints on one side on sagittal MRI images with fat suppression; and poor image quality. Incomplete coverage of only the upper-level facet joints, which may occasionally occur with focused 99mTc-MDP SPECT/CT examinations, was not an exclusion criterion, although this finding was recorded and is reported.
Additionally, since the effects of percutaneous intervention and treatment on facet joint imaging features are unknown, any percutaneous facet joint treatment performed in the interval between the two imaging examinations was documented.
Imaging examinations
SPECT/CT examinations of the lumbar spine were performed three to four hours after injection of 740 MBq (±10%) 99mTc-MDP. All examinations were performed on either a 6-slice or 16-slice Precedence scanner (SKYLight SPECT system with a Brilliance CT scanner; Philips Healthcare). CT parameters were as follows: 120 kVp, 60 mAs per slice, 3 mm slice thickness, and 3 mm increments. SPECT parameters were as follows: 128×128 word mode matrix, 64 views at 20 seconds per view, 1.46 zoom factor, step and shoot angular step of 3, body contouring, and low-energy all-purpose collimator.
The MRI examination protocol varied depending on the MRI unit used (various scanners either GE or Siemens) and radiologist preference. The following parameters were recorded for each MRI examination: magnet strength; presence or absence of gadolinium administration; fat-suppression technique used, such as short tau inversion recovery (STIR), chemical fat saturation, or Dixon technique; and the imaging plane(s) of fat-suppressed sequences.
Image analysis
For consistency, the lowest presacral vertebral body was designated L5 for each case, including cases of a transitional lumbosacral vertebral body or nonstandard lumbar vertebral body numbers. All readers were masked to clinical information, radiology reports, and results of the imaging test that they did not rate.
Two radiologists—one, who had successfully completed the Nuclear Medicine Board Examination and one, with a Certificate of Added Qualification in nuclear radiology by the American Board of Radiology—who had two and five years of postfellowship experience, respectively, independently rated each 99mTc-MDP SPECT/CT examination using aycan OsiriX Pro (Aycan Medical Systems) viewed on a MacBook Pro (Apple) with tandem Apple Thunderbolt displays. Each reader was free to view the fused images in axial, sagittal, and coronal planes. Planar whole-body images were also available.
No widely used and validated grading scale exists for facet joint 99mTc-MDP activity. Therefore, each reader first rated each facet joint of the lumbar spine, T12/L1 through L5/S1, dichotomously as having either increased activity or normal activity. This approach was used because it reflects actual clinical practice in which facet joint evaluation is a subjective comparison of target activity to background activity within areas of normal bone. The dichotomous approach was used to facilitate comparison with MRI and because a dichotomous approach has been used in prior studies of facet joint bone activity for guidance of percutaneous treatment (5, 6).
In addition, the activity of each facet joint was evaluated quantitatively, with one reader placing a circular region of interest (ROI) of 1 cm diameter centered over the middle of the facet joint in the axial plane. To account for background bone marrow activity, 1 cm ROI measures of activity were placed over each of the iliac crests. Comparison of facet joint activity to bone tracer uptake in the adjacent vertebral body was not used because the activity of vertebral bodies is often heterogeneous due to marked uptake at sites of degenerative disc disease and disc osteophyte complexes.
Facet joints for which both of the independent primary readers agreed were assigned a consensus diagnosis of either increased or normal activity. A third radiologist, who completed an independent nuclear medicine residency and successfully completed the Nuclear Medicine Board Examination, as well as a Certificate of Added Qualification in nuclear medicine, with 15 years of postfellowship experience, evaluated the specific facet joints that had discrepant assignments between the two primary readers. This third rating served as a tie-breaking analysis to assign a consensus diagnosis of either increased or normal activity.
Two neuroradiologists with Certificates of Added Qualification and 15 and six years of postfellowship experience, respectively, independently rated each MRI examination using a GE PACS workstation (GE Healthcare). To facilitate comparison with 99mTc-MDP SPECT/CT ratings, each facet joint was first rated as either positive for signal change on fat-suppressed MRI or normal. Each positive facet joint was assigned a grade of 1 to 4 using a grading scale described previously by Czervionke and Fenton (8) and used in other prior investigation (9). This grading scale can be applied to either T2-weighted images with fat suppression or T1-weighted images with fat suppression and intravenous gadolinium administration. Isolated fluid within the joint space itself without evidence of gadolinium enhancement or peri-facet signal change is not graded because it may not represent active inflammation. This is consistent with other diagnostic criteria for joints in the axial skeleton, since fluid and synovitis within the joint cannot be differentiated by fluid signal/T2 hyperintensity on T2-weighted images alone (10). Although the facet joint evaluation and grading were based on findings on fat-suppressed sequences, the readers were free to view and cross-reference all sequences available.
Facet joints in which both of the independent primary readers agreed were assigned a consensus diagnosis of either increased or normal activity. A third neuroradiologist with a Certificate of Added Qualification and 17 years of postfellowship experience evaluated the specific facet joints that had discrepant assignments between the two primary readers. Again, this third rating served as a tie-breaking analysis to assign a consensus diagnosis of either increased or normal activity.
Facet joint signal change on fat-suppressed images has been shown to increase prevalence at sites of biomechanical stress such as scoliosis, anterolisthesis, or vertebral body compression fracture (9, 11), and prevalence could vary by level (11). Since the agreement between imaging studies compared a given facet joint only to itself rather than to other facet joints under different degrees of stress, these potential causes of biomechanical stress were not assessed. However, a level-by-level analysis was performed.
Statistical analysis
Concordance between the consensus MRI diagnosis and the consensus 99mTc-MDP SPECT/CT diagnosis for each studied level of the spine was assessed using measures of agreement. In many levels of the spine, the vast majority of facet joint assignments were normal, skewing the marginal distribution of abnormal to normal facet joints toward normal. This is known to cause significant attenuation in the magnitude of the κ statistic, because even a single disagreement could substantially lower the calculated κ score (12). To account for this in the analysis, the prevalence-adjusted bias-adjusted κ (PABAK) was used to quantify the agreement between the consensus MRI diagnosis and consensus 99mTc-MDP SPECT/CT diagnosis in addition to the standard Cohen κ statistic (13). The PABAK score factors in the prevalence index, which accounts for the prevalence of facet joint abnormality, and the bias index, which accounts for differences in reader threshold for assigning abnormality.
κ values were interpreted according to standard thresholds: <0.20, no agreement to slight agreement; 0.21 to 0.40, fair agreement; 0.41 to 0.60, moderate agreement; 0.61 to 0.80, substantial agreement; and 0.81 to 1.0, almost perfect agreement (14). Generalized estimating equations were used to account for correlation between multiple facet joints from each patient. κ values obtained with the different fat-suppression technique were compared by using each fat suppression technique as a stratum (15). Statistical analyses were conducted using SAS software version 9.3 (SAS Institute, Inc.). P <0.05 was considered statistically significant.
Results
Of the 73 patients identified,13 were excluded due to metallic hardware (n=6), postoperative scar tissue in contact with lumbar facet joints (n=3), and poor image quality (n=4). Of the 60 patients included in the study cohort, 28 (47%) were male, and the mean age was 49±19.7 years (range, 12–93 years).
Most MRI examinations (49 of 60, 82%) were performed at 1.5 T and 11 (18%) were at 3.0 T. Of these 60 examinations, 33 (55%) were performed with T2 with chemical fat suppression, 18 (30%) were performed with STIR, two (3%) were performed with the Dixon technique, and 11 (18%) were performed with T1 with gadolinium and fat suppression. All MRI studies but one had sagittal images with fat suppression, one (2%) had only axial images with fat suppression, and seven (11.7%) had both axial and sagittal fat-suppressed images.
Overall, 720 facet joints were characterized by MRI and 716 facet joints were rated on 99mTc-MDP SPECT/CT. The four facet joints not rated on 99mTc-MDP SPECT/CT were because the readers did not rate the upper level of facet joints between two rib-bearing vertebral bodies in one patient with four lumbar-type vertebral bodies, and the T12/L1 facet joints were out of the coverage area in a second patient. Since the presence or absence of ribs is more difficult to determine on MRI, the MRI rated these four facet joints as the T12/L1 level. Fifty-three of 60 patients (88%) had five lumbar-type vertebral bodies, four (7%) had four lumbar-type vertebral bodies (including three patients with riblets at T12), and three (5%) had six lumbar-type vertebral bodies; the lowest mobile presacral vertebral body was designated L5 in these cases. Five patients (8%) had transitional lumbosacral vertebral bodies, all designated L5. The mean time between the MRI and 99mTc-MDP SPECT/CT examinations was 46±49 days.
Of 720 facet joints, 149 (20.7%) were rated positive on MRI by consensus—2.5 facet joints per patient on average. Fifty-two of 716 facet joints (7.3%) were rated positive on 99mTc-MDP SPECT/CT by consensus—0.9 facet joints per patient. Forty-one of 60 patients (68%) had at least one facet joint with increased signal on MRI, whereas 27 of 60 patients (45%) had at least one facet joint with increased activity on 99mTc-MDP SPECT/CT.
Most positive facet joints were at L4/5 and L5/S1, 60 (50% of all examined joints at this level) and 36 (30%), respectively, on MRI, and 27 (22.5%) and 11 (9.2%), respectively, on 99mTc-MDP SPECT/CT. A total of 122 facet joints were rated positive on MRI only, 25 facet joints were rated positive on 99mTc-MDP SPECT/CT only, and 27 facet joints were rated positive on both MRI and 99mTc-MDP SPECT/CT.
The agreement between MRI and 99mTc-MDP SPECT/CT by level is shown in Table 1. The frequency of concordance in ratings between the imaging modalities ranged from 64% to 95% over the six studied spinal levels; however, the preponderance of normal facet joints resulted in substantial attenuation of the κ statistic. The largest observed value for κ was 0.28, despite high concordance in ratings. The distribution of the ratings is shown by the prevalence index. For example, the T12/L1 regions had a 95% increased probability of reporting a normal finding, with a prevalence index of −0.95. After accounting for the skewed distribution of normal to increased activity, the adjusted PABAK values indicated reasonably high agreement across the spinal regions except for the L4/L5 and L5/S1 regions. In both of these regions, agreement was fair, with κ values of 0.28 (95% CI, 0.11–0.45) and 0.35 (95% CI, 0.18–0.52), respectively.
Table 1.
Agreement summary for consensus MRI and 99mTc-MDP SPECT/CT
| Joint | Concordance | Cohen κ (95% CI) | Prevalence index | Bias index | PABAK (95% CI) |
|---|---|---|---|---|---|
| T12/L1a | 0.95 | 0.000b | −0.95 | −0.05 | 0.90 (0.81, 0.98) |
| L1/L2 | 0.92 | −0.027 (−0.058, 0.004) | −0.92 | −0.05 | 0.83 (0.73, 0.93) |
| L2/L3 | 0.80 | −0.009 (−0.158, 0.139) | −0.78 | −0.10 | 0.60 (0.46, 0.74) |
| L3/L4 | 0.79 | −0.071 (−0.124, −0.019) | −0.79 | −0.13 | 0.58 (0.44, 0.73) |
| L4/L5 | 0.64 | 0.283 (0.140, 0.427) | −0.28 | −0.28 | 0.28 (0.11, 0.45) |
| L5/S1 | 0.68 | 0.035 (−0.051, 0.182) | −0.61 | −0.21 | 0.35 (0.18, 0.52) |
| Overall | 0.026 (−0.051, 0.000) |
MRI, magnetic resonance imaging; 99mTc-MDP SPECT/CT, technetium Tc 99m methylene disphosphonate single-photon emission computed tomography/computed tomography; CI, confidence interval; PABAK, prevalence-adjusted bias-adjusted κ.
Two patients (4 facets) did not have readings for T12/L1, and there were no positive consensus assessments for 99mTc-MDP SPECT/CT diagnosis for T12/L1.
CI was not estimable from the data.
For the subset of 26 patients who had MRI and 99mTc-MDP SPECT/CT examinations within a 20-day period, there was slight agreement when grouping all joints, with a κ of 0.18 (95% CI, 0.07–0.30), PABAK of 0.56 (95% CI, 0.47–0.65), and prevalence index of 0.70. The agreement between MRI and 99mTc-MDP SPECT/CT by level for this subset of 26 patients is shown in Table 2. Figs. 1 and 2 illustrate instances of disagreement between MRI and 99mTc-MDP SPECT/CT.
Table 2.
Agreement summary for consensus MRI and 99mTc-MDP SPECT/CT for patients where scans are no more than 20 days apart
| Joint | Concordance | Cohen κ (95% CI) | Prevalence index | Bias index | PABAK (95% CI) |
|---|---|---|---|---|---|
| T12/L1a | 0.98 | 0.00b | −0.98 | −0.02 | 0.98 (0.89, 1.00) |
| L1/L2 | 0.91 | −0.031 (−0.080, 0.018) | −0.91 | −0.06 | 0.81 (0.66, 0.97) |
| L2/L3 | 0.72 | −0.12 (−0.21,−0.028) | −0.72 | −0.13 | 0.44 (0.21, 0.68) |
| L3/L4a | 0.78 | 0.00b | −0.77 | −0.22 | 0.55 (0.33, 0.77) |
| L4/L5 | 0.72 | 0.43 (0.22, 0.65) | −0.28 | −0.24 | 0.44 (0.21, 0.68) |
| L5/S1 | 0.61 | −0.04(−0.21, 0.13) | −0.57 | −0.28 | 0.22 (−0.04, 0.48) |
| Overall | −0.03 (−0.24, 0.18) |
MRI, magnetic resonance imaging; 99mTc-MDP SPECT/CT, technetium Tc 99m methylene disphosphonate single-photon emission computed tomography/computed tomography; CI, confidence interval; PABAK, prevalence-adjusted bias-adjusted κ.
Two patients (4 facets) did not have readings for T12/L1, and there were no positive consensus assessments for 99mTc-MDP SPECT/CT diagnosis for T12/L1 and L3/L4.
CI was not estimable from the data.
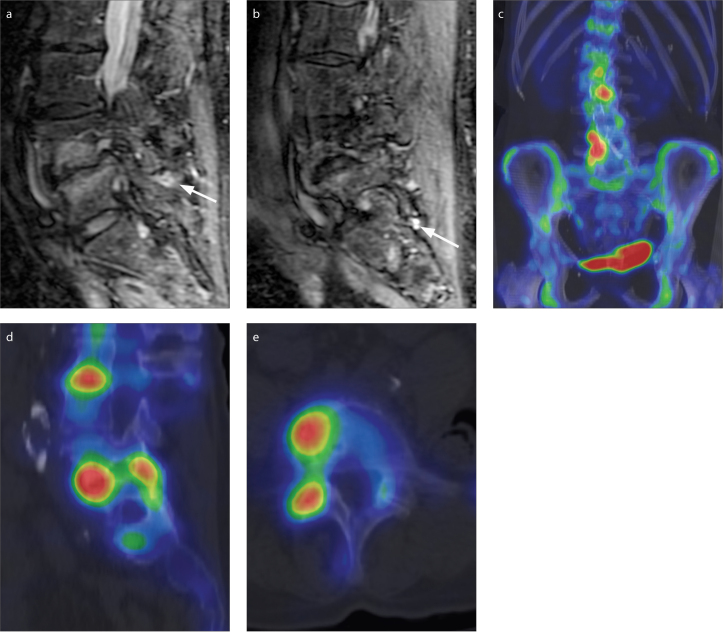
Figure 1. a–e.
MRI and 99mTc-MDP SPECT/CT images of a 67-year-old woman taken 10 days apart. The right L4/L5 and L5/S1 facet joints were rated as normal by consensus on MRI but as having increased activity on 99mTc-MDP SPECT/CT. Sagittal short tau inversion recovery images (a, b) focused on the right L4/L5 (a) and L5/S1 (b) facet joints demonstrate no substantial peri-facet signal change. There is minimal fluid signal within the inferior recesses of the facet joints (arrows), which is not considered sufficient for designation of peri-facet signal change and is not factored in the grading scale. Coronal (c) and right sagittal (d) 99mTc-MDP SPECT/CT images of the lumbar spine demonstrate increased activity around the right L4/L5 and L5/S1 facet joints relative to adjacent facet joints in the lumbar spine. Axial 99mTc-MDP SPECT/CT at the L4/L5 level (e) demonstrates increased activity of the right facet joint.
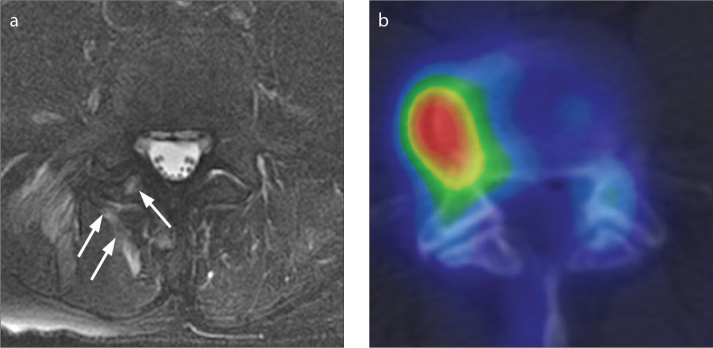
Figure 2. a, b.
MRI and 99mTc MDP SPECT/CT images of a 64-year-old man taken six days apart. The facet joint was rated as abnormal on MRI and normal on 99mTc-MDP SPECT/CT. Axial T2-weighted MRI with fat saturation (a) demonstrates T2 hyperintensity within the inferior articular process of the right L4/L5 facet joint and the surrounding soft tissues (arrows). An axial 99mTc-MDP SPECT/CT (b) image of the lumbar spine demonstrates increased activity within a right lateral osteophyte at L4/L5, but normal activity within the right L4/L5 facet joint itself.
The agreement between patients receiving different types of MRI fat suppression was similar. For the six patients (72 facet joints, all levels pooled) with only gadolinium-enhanced fat-suppressed images, κ was 0.46 (95% CI, 0.24–0.67). For the 33 patients (396 facets) with only T2 fat-suppressed MRI, κ was 0.42 (95% CI, 0.32–0.53). For the 16 patients (192 facet joints) having only STIR fat-suppressed images, κ was 0.29 (95% CI, 0.24–0.46). Using each fat suppression technique as a stratum the overall κ was 0.40 (95% CI, 0.32–0.49) and the data does not support the κ values being different (P = 0.42).
The normalized ROI ratio of joints rated normal by consensus was 0.71 (95% CI, 0.63–0.79), whereas the normalized ROI ratio of the facet joints rated as having increased activity was 2.25 (95% CI, 1.94–2.56), which was significantly different (P < 0.001) as assessed using generalized estimating equations.
No facet joints were designated positive by 99mTc-MDP SPECT/CT in patients younger than 46 years, whereas 13 patients younger than 30 years had facet joints with signal change on fat-suppressed MRI. Percutaneous facet joint intervention during the interval between MRI and 99mTc-MDP SPECT/CT examinations was only performed in eight facet joints in three patients. There was disagreement between MRI and 99mTc-MDP SPECT/CT in only one of these facet joints.
Discussion
The primary finding of this study is that facet joint activity on 99mTc-MDP SPECT/CT bone scan do not always correlate with the signal changes on fat-suppressed MRI. There was only fair intermodality agreement (κ) at one spinal level and slight to no agreement at other levels. The κ value indicated no agreement between 99mTc-MDP SPECT/CT and MRI (κ= −0.026). The PABAK values were fair to high at each spinal level, which suggests that relatively low disease prevalence lowered the κ values. Together, the κ and PABAK values indicate that there is some degree of intermodality agreement, but that it is not highly consistent. Overall, the results indicate that MRI and 99mTc-MDP SPECT/CT for facet joint evaluation should not be considered interchangeable examinations in clinical practice or research.
To our knowledge, there is no prior literature that directly compares peri-facet signal change on fat-suppressed MRI with 99mTc-MDP bone scan activity. Kim et al. (16) reported that the appearance of facet joint synovium and presence of osteophytes on anatomic MRI without fat suppression correlated with increased activity on 99mTc-MDP bone scan, but did assess signal change on fat suppressed MRI. Interestingly, while that study found that anatomic findings on MRI are correlated to 99mTc-MDP activity, we found that physiologic findings on fat-suppressed MRI that presumably indicate active inflammation are not always correlated to 99mTc-MDP activity. While we can only speculate the reasons for these differing results, it is possible that the mechanism of 99mTc-MDP bone scan activity in some patients may be more reflective of anatomic pathology rather than active inflammation. It is also possible that fat-suppressed T2 images lack sensitivity for isolated synovitis without significant bone or peri-articular edema, although further work would be needed to confirm these possibilities. For pars interarticularis stress reactions, Campbell et al. (17) reported that 99mTc-MDP bone scan activity correlated with signal changes on fat-suppressed MRI in many, but not all cases. This study found that there were instances with signal changes on fat-suppressed MRI without bone scan activity and vice versa. Similar to our study, this finding supports the concept that 99mTc-MDP bone scan activity and signal changes on fat-suppressed MRI should not be considered completely interchangeable examinations for identification of imaging biomarkers of back pain.
Despite the conclusion of prior studies that bone scan activity and signal change on fat-suppressed MRI may be markers of facetogenic pain on the basis of indirect evidence, the findings of this study support the notion that the significance of these imaging findings is incompletely understood. If both findings predict facet joint pain with high sensitivity and specificity, these should demonstrate high concordance. The discordance identified in this study may reflect differences in mechanism since 99mTc-MDP activity reflects blood flow and chemisorption (18) and, in some cases, growth of an osteophyte (19). In contrast, little is known about the pathologic mechanism(s) underlying facet joint signal changes on fat-suppressed MRI. Peri-facet T2 hyperintensity and enhancement have been presumed to represent inflammation (8), similar to subchondral T2 hyperintensity and enhancement in Modic 1 change (20).
Despite probable mechanistic differences, facet joint activity and signal change could still be concordant if they both reflected different manifestations of the same underlying process, sometimes referred to as facet joint synovitis. This study, however, suggests that a common cause of these two separate imaging findings is not universal. Such variability indicates that these two imaging tests must be independently studied if they are to be used for facet joint evaluation and the results from one modality cannot simply be applied to the other. To our knowledge, the prevalence of these physiologic imaging findings in asymptomatic controls has not been established to determine specificity.
This primary finding also indicates that further evaluation of the significance of these two imaging findings is needed. Although prior studies purport that 99mTc-MDP activity predicts facet joint pain, the methods used have additional shortcomings beyond the lack of use of comparative medial branch blocks, which have been detailed previously (5, 6, 21). It will be useful to evaluate 99mTc-MDP activity and peri-facet signal change independently in future investigations.
Interestingly, the percentage of patients in our study with abnormal facet joints on both MRI and 99mTc-MDP SPECT/CT, particularly low-grade peri-facet joint signal change on MRI, was higher than the reported 15% to 40% prevalence of facet joint pain in patients with low back pain (22). This indicates that this finding can give false-positive results if used to identify painful facet joints. Similarly, there is evidence that vertebral body T2 hyperintensity can persist after successful treatment of vertebral body compression fractures, but it is unknown whether signal change on MRI can persist after active facet inflammation subsides (23). Since facet joint activity is the most frequent finding on 99mTc-MDP SPECT/CT of the spine (24) and clinical evaluation of facet joint pain is limited (4), accurate imaging markers of facet joint pain are desirable and further evaluation of 99mTc-MDP SPECT/CT may be useful.
We have observed that use of fat-suppressed MRI on routine lumbar spine studies has increased recently. Prior studies purporting that fat-suppressed MRI predicts facet joint pain have correlated the side of pain to the side of signal change (8) and have found an increased prevalence of signal change in patients with low back pain (11). However, signal change in specific facet joints predicting pain and response to percutaneous treatment has not been demonstrated. Such investigation would be useful if findings on fat-suppressed MRI are used to identify pain generators and to direct treatment. Additionally, it is important to note that in the former study, the side of pain only correlated to single-level, unilateral, high-grade peri-facet signal change, although lower-grade signal change was more prevalent (8).
This study has some limitations. Because of the retrospective analysis and variability in practice patterns, the MRI technique, including type of fat suppression and administration of gadolinium, was variable. However, the kappa values were similar across different fat suppression techniques and do not support a difference in interobserver variability on the basis of technique. This study lacks histologic correlation and the imaging findings on MRI and 99mTc-MDP SPECT/CT could represent inflammatory changes or could potentially be related to other anatomic findings such as a growing osteophyte. This study strictly compared two imaging findings and did not assess relationship of anatomic findings on MRI or CT. The requirement for patients to undergo both MRI and 99mTc-MDP SPECT/CT may have been selected for patients with complex clinical pictures. It is possible that the duration of peri-facet signal changes in the setting of inflammation differs from that of increased 99mTc-MDP activity, which may lead to falsely discordant cases within the 180-day window. However, the results were similar for the subset of patients undergoing the two imaging exams within a 20-day interval, which supports our conclusions. Discrimination between normal and abnormal facet joint signal or activity, even with use of standardized methods, is subjective in both research and clinical studies.
In conclusion, our study suggests that MRI and 99mTc-MDP SPECT/CT for facet joint evaluation should not be considered interchangeable examinations in clinical practice or research. Future studies may include prospective evaluation with patients undergoing both imaging exams at a simultaneous session. Additionally, future studies testing the ability of both of these independent imaging techniques to predict response to medial branch blocks may be useful.
Main points.
Facet joint activity on 99mTc MDP SPECT/CT do not always correlate with signal change on MRI with fat suppression.
MRI and 99mTc MDP SPECT/CT readers agreed on the status of most facet joints, which were rated normal. However, many of the facet joints rated positive on one exam were rated normal on the other.
Despite the conclusion of prior studies that bone scan activity and signal change on fat-suppressed MRI may be markers of facetogenic pain on the basis of indirect evidence, the findings of this study support the notion that the significance of these imaging findings is incompletely understood.
99mTc MDP SPECT/CT bone scan and MRI with fat suppression should not be viewed as substitute examinations for facet joint evaluation in clinical practice or research.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Gaskin DJ, Richard P. Institute of Medicine Committee on Advancing Pain Research, Care, and Education. Washington DC: National Academies Press (US); 2011. [Accessed May 7, 2015]. Relieving pain in America: a blueprint for transforming prevention, care, education, and research: Appendix C the economic costs of pain in the United States. Available at: http://www.ncbi.nlm.nih.gov/books/NBK92521/?report=classic. [Google Scholar]
- 2.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. http://dx.doi.org/10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DePalma MJ, Ketchum JM, Saullo T. What is the source of chronic low back pain and does age play a role? Pain Med. 2011;12:223–233. doi: 10.1111/j.1526-4637.2010.01045.x. http://dx.doi.org/10.1111/j.1526-4637.2010.01045.x. [DOI] [PubMed] [Google Scholar]
- 4.Bogduk N. Degenerative joint disease of the spine. Radiol Clin North Am. 2012;50:613–628. doi: 10.1016/j.rcl.2012.04.012. http://dx.doi.org/10.1016/j.rcl.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Pneumaticos SG, Chatziioannou SN, Hipp JA, Moore WH, Esses SI. Low back pain: prediction of short-term outcome of facet joint injection with bone scintigraphy. Radiology. 2006;238:693–698. doi: 10.1148/radiol.2382041930. http://dx.doi.org/10.1148/radiol.2382041930. [DOI] [PubMed] [Google Scholar]
- 6.Dolan AL, Ryan PJ, Arden NK, et al. The value of SPECT scans in identifying back pain likely to benefit from facet joint injection. Br J Rheumatol. 1996;35:1269–1273. doi: 10.1093/rheumatology/35.12.1269. http://dx.doi.org/10.1093/rheumatology/35.12.1269. [DOI] [PubMed] [Google Scholar]
- 7.Bogduk N. ISIS practice guidelines for spinal diagnostic and treatment procedures. 2nd ed. San Francisco: International Spine Intervention Society; 2004. pp. 559–563. [Google Scholar]
- 8.Czervionke LF, Fenton DS. Fat-saturated MR imaging in the detection of inflammatory facet arthropathy (facet synovitis) in the lumbar spine. Pain Med. 2000;9:400–406. doi: 10.1111/j.1526-4637.2007.00313.x. http://dx.doi.org/10.1111/j.1526-4637.2007.00313.x. [DOI] [PubMed] [Google Scholar]
- 9.Lehman VT, Wood CP, Hunt CH, et al. Facet joint signal change on MRI at levels of acute/subacute lumbar compression fractures. AJNR Am J Neuroradiol. 2013;34:1468–1473. doi: 10.3174/ajnr.A3449. http://dx.doi.org/10.3174/ajnr.A3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sieper J, Rudwaleit M, Baraliakos X, et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis. 2009;68(Suppl 2):1–44. doi: 10.1136/ard.2008.104018. http://dx.doi.org/10.1136/ard.2008.104018. [DOI] [PubMed] [Google Scholar]
- 11.Friedrich KM, Nemec S, Peloschek P, Pinker K, Weber M, Trattnig S. The prevalence of lumbar facet joint edema in patients with low back pain. Skeletal Radiol. 2007;36:755–760. doi: 10.1007/s00256-007-0293-7. http://dx.doi.org/10.1007/s00256-007-0293-7. [DOI] [PubMed] [Google Scholar]
- 12.Feinstein AR, Cicchetti DV. High agreement but low kappa: I. The problems of two paradoxes. J Clin Epidemiol. 1990;43:543–549. doi: 10.1016/0895-4356(90)90158-l. http://dx.doi.org/10.1016/0895-4356(90)90158-L. [DOI] [PubMed] [Google Scholar]
- 13.Byrt T, Bishop J, Carlin JB. Bias, prevalence and kappa. J Clin Epidemiol. 1993;46:423–429. doi: 10.1016/0895-4356(93)90018-v. http://dx.doi.org/10.1016/0895-4356(93)90018-V. [DOI] [PubMed] [Google Scholar]
- 14.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. http://dx.doi.org/10.2307/2529310. [PubMed] [Google Scholar]
- 15.Fleiss JL, Levin B, Paik MC. Statistical methods for rates and proportions. 3rd ed. Hoboken NJ: John Wiley & Sons; 2003. p. 607. http://dx.doi.org/10.1002/0471445428. [Google Scholar]
- 16.Kim KA, Wang MY. Magnetic resonance images-based morphologic predictors of single photon emission computed tomography-positive facet arthropathy in patients with axial back pain. Neurosurgery. 2006;59:147–156. doi: 10.1227/01.NEU.0000219956.58725.6F. http://dx.doi.org/10.1227/01.NEU.0000219956.58725.6F. [DOI] [PubMed] [Google Scholar]
- 17.Campbell RSD, Grainger AJ, Hide AJ, Papastefanou S, Greenough CG. Juvenile spondylolysis: a comparative analysis of CT, SPECT and MRI. Skeletal Radiol. 2005;34:63–73. doi: 10.1007/s00256-004-0878-3. http://dx.doi.org/10.1007/s00256-004-0878-3. [DOI] [PubMed] [Google Scholar]
- 18.Fogelman I. Skeletal uptake of diphosphonate: a review. Eur J Nucl Med. 1980;5:473–476. doi: 10.1007/BF00252034. http://dx.doi.org/10.1007/BF00252034. [DOI] [PubMed] [Google Scholar]
- 19.Christensen SB. Localization of bone-seeking agents in developing, experimentally induced osteoarthritis in the knee joint of the rabbit. Scand J Rheumatol. 1983;12:343–349. doi: 10.3109/03009748309099738. http://dx.doi.org/10.3109/03009748309099738. [DOI] [PubMed] [Google Scholar]
- 20.Rahme R, Moussa R. The modic vertebral endplate and marrow changes: pathologic significance and relation to low back pain and segmental instability of the lumbar spine. AJNR Am J Neuroradiol. 2008;29:838–842. doi: 10.3174/ajnr.A0925. http://dx.doi.org/10.3174/ajnr.A0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehman VT, Murphy RC, Kaufmann TJ, et al. Frequency of discordance between facet joint activity on technetium Tc99m methylene diphosphonate SPECT/CT and selection for percutaneous treatment at a large multispecialty institution. AJNR Am J Neuroradiol. 2014;35:609–614. doi: 10.3174/ajnr.A3731. http://dx.doi.org/10.3174/ajnr.A3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manchikanti L, Manchukonda R, Pampati V, Damron KS, McManus CD. Prevalence of facet joint pain in chronic low back pain in postsurgical patients by controlled comparative local anesthetic blocks. Arch Phys Med Rehabil. 2007;88:449–455. doi: 10.1016/j.apmr.2007.01.015. http://dx.doi.org/10.1016/j.apmr.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Dansie DM, Luetmer PH, Lane JI, Thielen KR, Wald JT, Kallmes DF. MRI findings after successful vertebroplasty. AJNR Am J Neuroradiol. 2005;26:1595–1600. [PMC free article] [PubMed] [Google Scholar]
- 24.Lehman VT, Murphy RC, Maus TP. 99mTc-MDP SPECT/CT of the spine and sacrum at a multispecialty institution: clinical use, findings, and impact on patient management. Nucl Med Commun. 2013;34:1097–1106. doi: 10.1097/MNM.0b013e328364bfa6. http://dx.doi.org/10.1097/MNM.0b013e328364bfa6. [DOI] [PubMed] [Google Scholar]