Abstract
Even though a strong association between inflammation and cancer has been widely accepted, the underlying precise molecular mechanisms are still largely unknown. A complex signaling network between tumor and stromal cells is responsible for the infiltration of inflammatory cells into the cancer micro-environment. Tumor stromal cells such as pancreatic stellate cells (PSCs) and immune cells create a microenvironment that protects cancer cells through a complex interaction, ultimately facilitating their local proliferation and their migration to different sites. Furthermore, PSCs have multiple functions related to local immunity, angiogenesis, inflammation and fibrosis. Recently, many studies have shown that members of the phosphoinositol-3-phosphate kinase (PI3K) family are activated in tumor cells, PSCs and tumor infiltrating inflammatory cells to promote cancer growth. Pro-inflammatory cytokines and chemokines secreted by immune cells and fibroblasts within the tumor environment can activate the PI3K pathway both in cancer and inflammatory cells. In this review, we focus on the central role of the PI3K pathway in regulating the cross-talk between immune/stromal cells and cancer cells. Understanding the role of the PI3K pathway in the development of chronic pancreatitis and cancer is crucial for the discovery of novel and efficacious treatment options.
Keywords: PI3K, pancreatic cancer, chronic pancreatitis, inflammation
Introduction
Pancreatic cancer continues to be one of the most lethal human malignancies with an overall 5-year survival of 6% or below. It is currently the 4th leading cause of cancer-related deaths in the US and its incidence is predicted to rise. Patients are usually diagnosed late in the disease process and often present with local and distant metastases, and only a small percentage of them are candidates for surgical resection. Even among this highly selected group of patients with resectable disease, the 5-year survival in centers of excellence reaches only 25%. In addition, pancreatic cancer is known to be largely resistant to common radio- and chemo-therapy adding to the dismal prognosis for patients1,2.
It is well established that chronic inflammation represents a major risk factor for the development and progression of cancer, including pancreatic cancer. For example, inflammatory bowel disease3, chronic prostatitis4, and chronic obstructive pulmonary disease (COPD)5 represent risk factors for development of cancer in the colon, prostate, and lung, respectively. Chronic pancreatitis, which is characterized by acinar loss, fibrosis, and immune cell infiltration, is the strongest identified risk factor for pancreatic cancer6, 7. Chronic inflammation usually is characterized by a recruitment and infiltration of inflammatory cells into the tissue with an increased production and secretion of chemokines and cytokines. There is a strong interplay between the malignant or premalignant cells and the stromal cells, including inflammatory cells. This inflammatory micro-environment is believed to be a major driver for cancer initiation and/or promotion8. Chronic inflammation also underlies, at least partially, the increased cancer risk by other risk factors, including alcohol abuse, smoking, obesity, and infections. Although today numerous studies link inflammation with cancer, the exact underlying molecular mechanisms and operative cross-talks between malignant and stromal cells are still largely unknown.
During acute and chronic inflammation and tumor development a host of immune cells are recruited to and infiltrate the tissue. The current concept of the role of immune cells during tumor development is called “immunoediting”9 and includes three phases: first, attempt by the immune system to eliminate tumor cells; secondly, the establishment of an equilibrium between tumor cells and the immune system thereby preventing further tumor growth; and thirdly, an escape of a subset of tumor cells from the tumor-suppressive action of the immune system, which leads to development and cancer progression. In this last phase the tumor cells often “hijack” physiological processes of the immune system thereby creating a pro-tumorigenic environment10.
The PI3K signaling cascade is critical in conditions of inflammation and cancer. The PI3K pathway is often activated in both tumor cells and tumor-infiltrating immune cells and is also involved in the cytokine-mediated cross-talk between cancer and inflammatory cells11. Based on the current evidence, targeting the PI3K and its signaling pathway is an intriguing concept for preventing inflammation-associated tumor development. This review will summarize our current understanding about the pancreatitis - pancreatic cancer continuum and will highlight the key role of the PI3K in this process.
Risk factors for pancreatitis and pancreatic cancer
The link between chronic inflammation and cancer is not new: more than 150 years ago, Virchow postulated chronic inflammation as the origin of cancer12. Today, it is well established that immune cells are regularly present in tumors and play a critical role in tumor development and progression13. However, the operative cross-talks between tumor and stromal cells as well as the precise underlying molecular mechanisms remain still largely unknown. Chronic inflammation is also a well-established risk factor for the development of pancreatic cancer and underlies to some extent many of the risk factors for this disease.
Chronic pancreatitis, the strongest identified risk factor for pancreatic cancer, has a high incidence in industrialized countries, ranging from 3.5 to 10 per 100,000 people14. The pathological hallmarks of chronic pancreatitis are inflammation, glandular atrophy, ductal changes, and fibrosis6. There are several hypotheses for the development of chronic pancreatitis. The necrosis-fibrosis hypothesis envisions the development of fibrosis from recurrent acute pancreatitis15. During the development of chronic pancreatitis inflammatory cells, e.g. macrophages, neutrophils, lymphocytes, and mast cells, are recruited into the pancreas leading to fibrosis and inflammation. The infiltrating inflammatory cells create a pro-inflammatory microenvironment by secreting chemokines, cytokines, and growth factors, potentially promoting genetic instability and thereby increasing the risk of malignant transformation16,17. Many risk factors of chronic pancreatitis, including alcohol abuse, cigarette smoking, and obesity also increase the risk of developing pancreatic cancer, thereby highlighting a common underlying pathophysiologic mechanism between these two diseases.
Alcohol abuse, the best characterized risk factor for chronic pancreatitis18, can cause repeated episodes of acute pancreatitis often referred to as acute relapsing pancreatitis. It is accepted today that acute pancreatitis and acute relapsing pancreatitis can develop into chronic pancreatitis19. Takeyama, showed a significant risk of progression to chronic pancreatitis after the first episode of alcohol-related acute pancreatitis20. Recent studies have shown that about 60 g of ethanol (around 5 drinks per day) are needed to significantly increase the risk for developing pancreatitis21. It has been reported that ethanol metabolites damage and kill pancreatic acinar cells; acinar cells metabolize ethanol to ethanol fatty acid ethyl esters (FAEE), which can increase intracellular calcium levels causing acinar cells necrosis22. The resulting necrotic tissue generates a strong inflammatory reaction with recruitment and infiltration of inflammatory cells and the release of chemokines and cytokines. If persistent or recurrent, this process may ultimately lead to chronic inflammation and may promote the generation of fibrosis and development of neoplastic lesions23.
Another risk factor for chronic pancreatitis is cigarette smoking. Studies have shown that N-nitrosamines, which are present in cigarette smoke, are directly secreted into the bile and may stimulate an inflammatory response in the pancreas24. As an alternative mechanism, nicotine metabolites can bind to receptors on the exocrine pancreas, thereby promoting pancreatitis and pancreatic cancer25. Importantly, cigarette smoking increases the risk of pancreatic cancer by 1.5 to 3 fold, depending on the duration and number of cigarette smoked26. Recently, Jang and colleagues showed that the effect of tobacco smoking on increasing the risk of pancreatic cancer may depend on certain genetic variations27. Interestingly, the effects of cigarette smoke are additive to alcohol abuse in increasing the incidence of acute and chronic pancreatitis28.
Many studies identified obesity, in particular visceral adiposity, as another risk factor that promotes the development of acute and chronic pancreatitis, and pancreatic cancer29. Obesity is now recognized as a chronic inflammatory state with increased systemic and tissue levels of cytokines and growth factors30. During the development of obesity, inflammatory cells, e.g. macrophages, are recruited to and infiltrate adipose tissue with subsequent secretion of pro-inflammatory cytokines. The adipose tissue inflammation during obesity is thought to be integral to the development of obesity-associated metabolic diseases31. Besides adipose tissue inflammation, obesity also leads to inflammation in other tissues and organs, including pancreas32. Again, obesity-induced inflammation of the pancreas may create a micro-environment that is conducive to the development of chronic pancreatitis and cancer33. In addition, obesity is often associated with insulin-resistance and frank diabetes mellitus. In that context, an increased risk of developing pancreatic cancer in diabetic patients has been reported34,35.
Hereditary pancreatitis is a rare cause of chronic pancreatitis. The altered genes involved in the pathogenesis are the cationic trypsinogen gene (PRSS1) and the serine protease inhibitor Kazal type 1 (SPINK 1). These genetic mutations can lead to an auto-activation of trypsinogen in the pancreas that leads to necrosis and inflammation36. Importantly, patients with hereditary pancreatitis have a 40–60 fold higher risk to develop pancreatic cancer, and this occurs earlier when individuals are smokers or heavy drinkers37. Noteworthy, patients with hereditary pancreatitis and acute pancreatitis episodes who also drink alcohol and smoke showed a higher risk to progress to recurrent acute pancreatitis, chronic pancreatitis, and finally pancreatic cancer, highlighting the importance of recurrent and chronic inflammation and cancer.
Taken together, several factors are known to increase the risk of pancreatic cancer. Chronic inflammation with infiltration of inflammatory cells into the pancreas and subsequent secretion of numerous cytokines, thereby creating an inflammatory micro-environment, is thought to be an important mechanism of pancreatic cancer development and underlies most, if not all, of the risk factors for chronic pancreatitis and pancreatic cancer.
Pancreatic inflammation: the path to pancreatic cancer
Many factors can cause an acute inflammation in the pancreas (acute pancreatitis) that if persistent or recurrent can lead to the development of chronic pancreatitis with disturbances in exocrine pancreas function, the formation of desmoplasia, and an increased risk of pancreatic cancer. Several cell populations play a central role in acute and chronic pancreatic injury and inflammation, including pancreatic stellate cells, inflammatory cells, i.e. macrophages, and pancreatic acinar cells.
Pancreatic stellate cells (PSC) are myofibroblast-like cells in the pancreas, which are currently known to be responsible for the formation of the desmoplastic reaction in chronic pancreatitis and pancreatic cancer38. In healthy subjects with normal pancreatic tissue, PSCs are quiescent39 while after pancreatic inflammation or injury they are activated and transformed into myofibroblast-like cells, which characteristically express alpha-smooth muscle actin (alpha-SMA)38. Once activated PSCs start to proliferate, migrate, produce extracellular matrix (ECM) and, importantly, display a pro-inflammatory phenotype, releasing chemokines, cytokines, and growth factors such as IL-1, IL-6, IL-8, TNF-alpha, TGF-beta, VEGF, and PDGF; all these factors can attract other inflammatory cells into the pancreas, thereby perpetuating the inflammatory reaction40. PSCs also express and secrete metalloproteinases, e.g. MMP-9, MMP-13, MMP-2, highlighting their role in the formation and modulation of the pancreatic stroma during the development of chronic pancreatitis and pancreatic cancer41. Thus, PSCs can create a microenvironment that favors cancer cells growth, survival and migration42. It has also been reported that PSCs can exhibit a macrophage-like phenotype, due to their capacity to phagocytize necrotic debris and foreign elements, similarly to Kupffer cells in the liver43,44. In addition, PSCs can promote angiogenesis by producing vascular endothelial growth factor (VEGF)45. A strong correlation between high levels of VEGF, angiogenesis, and fibrosis has been demonstrated in chronic pancreatitis and pancreatic cancer46.
Among inflammatory cells, macrophages are considered to play a central and important role in chronic pancreatitis and pancreatic cancer. As discussed above, many factors contribute to an inflammatory and pro-carcinogenic environment in the pancreas with the recruitment and infiltration of inflammatory cells, including macrophages. Signals in the tumor environment lead to maturation of two types of macrophages: tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs)47. TAMs are the predominant leukocytes in solid tumors48: they produce cytokines and growth factors that can promote tumor cell growth49. MDSCs are activated immature cells with morphological and functional heterogeneous characteristics that play a key role in cancer immune evasion. Recently, Khaled and colleagues found high levels of MDSCs in the blood of patients with pancreatic cancer50. Thus, TAMs and MDSCs can be recruited by signals from the tumor and other inflammatory cells and secrete cytokines that may promote cancer progression and metastasis51. Similar to Th1 and Th2 lymphocytes, macrophages can be divided into two principal subtypes: M1 macrophages that produce cytokines with inhibitory function on cell proliferation and M2 macrophages that release cytokines that promote cell proliferation. It has been shown that pro-tumorigenic M2 macrophages can be recruited by signals from the tumor and other inflammatory cells52.
Besides pancreatic stellate and inflammatory cells, pancreatic acinar cells are critical in the development of chronic pancreatitis and cancer53. Pancreatic acinar cells are the main component of the exocrine pancreas, constituting about 80% of the gland. After pancreatic injury, acinar cells can secrete cytokines, chemokines, and other pro-inflammatory molecules54. Acinar cell necrosis during pancreatitis can attract immune cells, which in turn produce pro-inflammatory molecules. The extrinsic factors lead to oxidative stress with the formation of reactive oxygen species (ROS) and reactive nitrogen species (RNS). ROS and RNS can promote DNA mutations and other genetic alterations. In addition, these reactive molecules can lead to protein and lipid modifications and damage that can contribute to the development of chronic pancreatitis55. Interestingly, certain immune cells, e.g. MDSCs, can also produce ROS56. The role of acinar cell in pancreatic cancer development is less recognized. Some authors demonstrated that chronic pancreatitis promotes the development of PanIN lesions and pancreatic cancer in mice that express an oncogenic Kras in pancreatic acinar cells57. Furthermore, acinar cells have been recently demonstrated to be the cells of origin for pancreatic cancer in at least mouse models of the disease58.
Taken together, several different cell populations play a central and important role during acute and chronic pancreatic inflammation. Injury to pancreatic acinar cells, activation of stellate cells, and recruitment and activation of inflammatory cells, i.e. macrophages, contribute and sustain an inflammatory environment in the pancreas, which ultimately can increase the risk for developing pancreatic cancer.
PI3K in chronic pancreatitis and pancreatic cancer
Many inflammatory molecules that are present and important during pancreatic inflammation and cancer activate the PI3K signaling pathway. In addition, activation of PI3K has been shown to be critical for inflammation and cancer development59. Furthermore, common mutations found in pancreatic cancer can activate PI3K, highlighting the central role of this signaling molecule.
PI3K belongs to the family of lipid kinases that phosphorylate the 3′-hydroxyl group of phosphoinositides60. This family of enzymes includes eight mammalian isoforms clustered in three classes (I–III) based on different structure and substrate selection. The class I PI3K has been divided in two subsets, IA and IB. PI3K IA is comprised of p110 alpha, p110 beta, p110 delta catalytic subunit, and the regulatory subunit p85. The only member of the class IB is p110 gamma61. The importance of PI3K in cancer in general has been described in many recent reviews62. P110 gamma is the principal isoform in leukocytes and it plays a crucial role in immunity by regulating cell proliferation, maturation and motility of neutrophils, macrophages, mast cells, natural killer cells and CD8+ T cells63,64. The p110 gamma isoform is essential for the activation and migration of macrophages and granulocytes in response to chemokines and cytokines released from cancer cells65. Upon activation, macrophages and neutrophilic granulocytes produce chemokines and cytokines that attract other immune cells, e.g. T- and mast cells to the inflammatory site66. Interestingly, Edling and colleagues demonstrated that p110 gamma is overexpressed in pancreatic ducts of patients with pancreatic cancer and chronic pancreatitis, suggesting an important role of this PI3K isoform in regulating proliferation and motility both in immune and tumor cells67.
Among tumors, pancreatic cancer has one of the highest rates of genetic mutations and the most frequent (>90%) is the K-Ras mutation68. In addition, K-Ras mutations can also be found in patients with chronic pancreatitis69. Oncogenic RAS often leads to a pathological downstream activation of the PI3K pathway70. This process, in the presence of an inflammatory environment, leads to an over-activation of intracellular signals that promote permanent inflammation and consequently genetic mutations that can lead to development and progression of cancer. Another frequent genetic alteration in pancreatic cancer is the loss of PTEN, either by mutation or deletion71. PTEN is a tumor suppressor gene that dephosphorylates PIP3, the principal product of PI3K, into PIP2 thereby counteracting the PI3K pathway activation72. The decreased PTEN expression and activity result in an over-activation of PI3K pathway, which has been showed in both chronic pancreatitis and pancreatic cancer73.
Persistent overproduction of chemokines and cytokines, e.g. IL-1β, is critical for the development of chronic pancreatitis. Inflammatory cytokines can stimulate the expression of other inflammatory mediators, e.g. cyclooxygenase-2 (COX-2). Some authors showed that high levels of COX-2 are present both in pancreatitis and pancreatic cancer74. COX-2 is the inducible isoform of cyclooxygenases, enzymes responsible for the synthesis of prostaglandins, which are potent players in inflammation. COX-2 generated prostaglandins, which bind to their respective G-protein-coupled receptors, e.g. EP1-4 for PGE2, may, in turn, activate the PI3K pathway75. Activation of the PI3K pathway leads to several phenotypic responses, including activation of cell survival programs (by expression of anti-apoptotic molecules, e.g. bcl-2), cell proliferation (by induction of cell cycle proteins), angiogenesis (via production of angiogenic factors, e.g. VEGF), and modulation of cellular metabolism (through activation of downstream mTORC1). High levels of COX-2 have been demonstrated in macrophages in patients with chronic pancreatitis and also in patients with pancreatic cancer and pancreatic intraepithelial neoplasia (PanIN), highlighting a possible role of COX-2 in linking chronic pancreatitis and pancreatic cancer76. This hypothesis is supported by the finding that inhibition of COX-2 leads to reduced fibrosis, inflammation and tumor lesions77. In addition, several growth factors, e.g. IGF, PDGF, and EGF that are present in chronic pancreatitis can activate RAS, which in turn can activate PI3K. This leads to the activation of the serine/threonine kinases AKT1, AKT2, and AKT3. AKT has many substrates, such as Bad, caspase 9, mTOR, GSK3 beta, and tuberin, which are involved in the regulation of cell proliferation, survival, metabolism, angiogenesis, and motility78.
PI3K also regulates the expression of uPA (urokinase-type plasminogen activator), a serine protease that is secreted from leukocytes, macrophages, fibroblasts, and cancer cells. Normally, uPA is over-produced in inflammatory cells and in inflamed tissues79,80. Once bound to its receptor uPAR, the uPA/uPAR complex cleaves plasminogen to plasmin. In turn, plasmin promotes fibrinolysis and the degradation of ECM that is associated with the release of growth factors and other proteases, which in turn can activate the PI3K pathway81. In pancreatic cancer as well as in other tumors, there is an abnormal expression of uPA/uPAR that promotes cancer cells survival, angiogenesis, invasion, and migration. uPA/uPAR plays also a key role in the tumor microenvironment being expressed in tumor-associated inflammatory cells and stromal cells. Noteworthy, tumor-associated macrophages are more attracted to the inflammatory environment surrounding cancer cells when they expressed higher levels of uPAR82. Thus, inflammation and overexpression of PI3K can stimulate the uPA/uPAR system that, in turn, causes a release of growth factor and proteases that can promote the inflammatory environment.
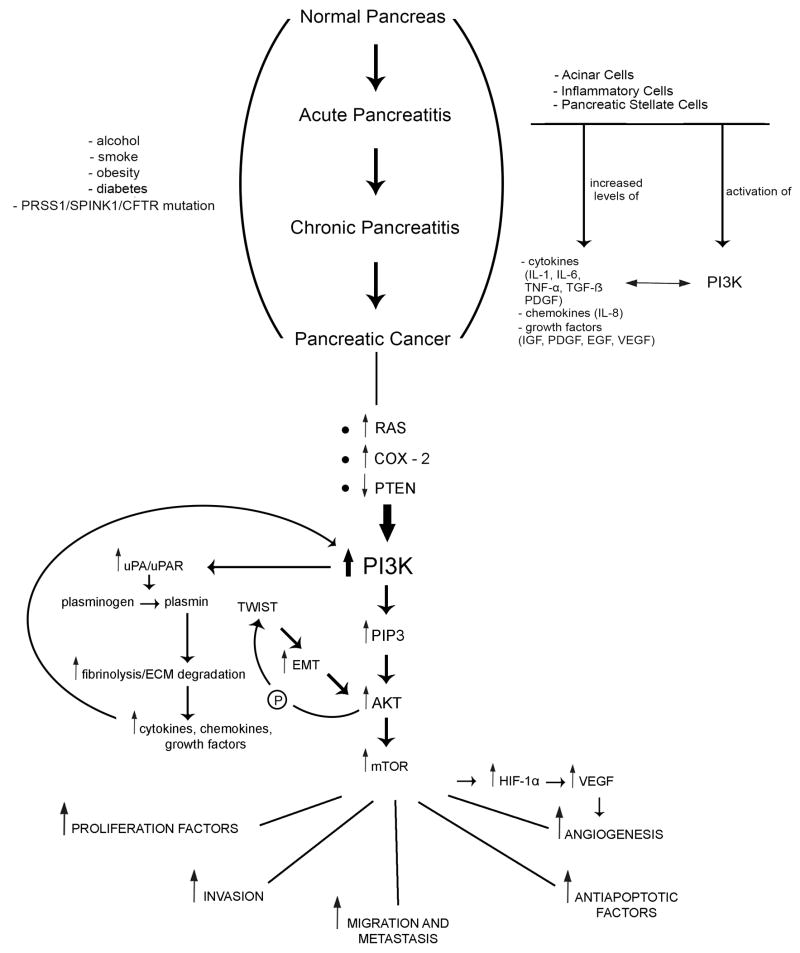
Taken together, genetic alterations and inflammatory molecules that are present and secreted in chronic pancreatitis (inflammation) and pancreatic cancer can activate the PI3K signaling pathway, which in turn can stimulate the inflammatory process and tumor development further (Table 1, Figure 1). Since PI3K is activated in both inflammatory and neoplastic diseases of the pancreas, it represents an intriguing therapeutic and preventive target for the pancreatic inflammation – pancreatic cancer progression.
Table 1.
PI3K-related molecular factors in chronic pancreatitis and pancreatic cancer
| Description of the genetic/cellular factors | Expression/activity in chronic pancreatitis | Expression/activity in pancreatic cancer | Ref. |
|---|---|---|---|
|
PI3K
Regulates the transcription of many anti-apoptotic factors (Bcl2), proliferation factors (c-myc, cyclin D1 and again COX-2) and angiogenesis factors (VEGF). The isoform p110 gamma is expressed in leukocytes and it controls the immune cells proliferation, maturation and motility |
Increased activity | Increased activity | 73, 74, 77 |
|
K-Ras Is an oncogene that determines the transcription of membrane-bound called RAS, which is crucial protein for the transmission of signals from extracellular to intracellular environment. This protein controls positively PI3K activity. |
Overexpressed | Overexpressed | 79, 135 |
|
PTEN Is a tumor suppressor gene that dephosphorylates PIP3, the main second messenger of PI3K. PTEN thus negatively regulates PI3K activity. |
Down-regulated expression | Down-regulated expression | 82, 83 |
|
AKT Is a serine/threonine kinase activated by PIP3 that regulates cell proliferation, survival and motility |
Increased activity | Increased activity | 135, 144, 144 |
|
mTOR Is a serine/threonine kinase activated by AKT that controls cell growth, proliferation and transcription |
Increased activity | Increased activity | 83 |
|
COX-2 Is a gene coding for an enzyme that synthesizes prostaglandins. It has a crucial role as inflammation inducer. COX2 increases activation of PI3K pathway. |
Overexpressed | Overexpressed | 85, 86 |
|
uPA/uPAR Is a serine protease regulated by PI3K and it is secreted from leukocytes, macrophages, fibroblasts and cancer cells. It promotes an overproduction of cytokines, chemokines and growth factor that stimulate, in turn, PI3K |
Increased activity | Increased activity | 89, 90, 91, 92 |
Figure 1.
The effects of alcohol, smoke, obesity, diabetes and hereditary factors on the development and progression of recurrent acute pancreatitis, chronic pancreatitis and pancreatic cancer through increased levels of inflammatory molecules mediated by the PI3K pathway.
PI3K: a common pathway in multiple cell lineages
Several cell populations are critically involved in the development of chronic pancreatitis and the progression to pancreatic cancer. The PI3K pathway has been shown to play a central and critical role in various cell lineages during this development and is involved in multiple cellular processes, such as proliferation, growth, survival, metabolism, and migration (Table 2).
Table 2.
PI3K: a common pathway in different cells in chronic pancreatitis and pancreatic cancer
| Cell type | PI3K function | Chronic pancreatitis | Pancreatic cancer | Author, year |
|---|---|---|---|---|
| Pancreatic Stellate Cells (PSCs) | Activation; chemotaxis; migration in the damaged area; proliferation; increases collagen synthesis and fibrosis | Activated | Activated | 94, 95, 96, 97, 98 |
| Acinar cells | Trypsinogen activation, necrosis, transcription of pro-inflammatory factors; controls protein synthesis and metabolism; mitogenic and anti-apoptotic factor | Damaged | De-differentiated | 101, 102, 103 |
| Macrophages | Differentiation in different subtypes; survival, adhesion, motility | Present +++ |
Present +++ |
111, 112,113, 115, 116 |
| Neutrophils | Growth, survival, phagocytosis, firm adhesion, diapedesis, chemotaxis | Present +++ |
Present + |
119, 120, 121 |
| Mast Cells | Differentiation, degranulation, cytokines production, chemotaxis, adhesion, cellular growth and survival | Present ++ |
Present ++ |
122, 123, 124 |
| Lymphocytes T CD8+ | Activation, chemotaxis, development, proliferation | Present ++ |
Present ++ |
125, 126, 127 |
| Natural Killer Cells (NK) | Development, cellular function, chemotaxis, cytokines production | Present ++ |
Present + |
130, 131, 133, 134 |
PI3K and pancreatic stellate cells
Pancreatic stellate cells play a central role in the formation of the desmoplastic reaction by depositing extracellular matrix (ECM) proteins, in the promotion of angiogenesis, and in the local pancreatic immune response83. Activated pancreatic stellate cells can express cytokines, chemokines, and various growth factors that promote and sustain a persistent and chronic inflammation. Some authors showed that PDGF is one of the principal mitogens for PSCs. PDGF is produced after pancreatic injury and during inflammation by mononuclear cells, macrophages, and platelets. PDGF induces PI3K pathway activation in PSCs, which leads to their enhanced migration and proliferation. This mechanism is fundamental for the formation of the pancreatic fibrosis84,85. Besides TGF-β86, CCK (cholecystokinin) and gastrin have also been shown to activate PSCs and stimulate collagen production. Interestingly, patients with chronic pancreatitis often have elevated levels of CCK87. After binding of CCK to their respective receptors on PSCs, the PI3K pathway is activated, which increases collagen synthesis and fibrosis. Noteworthy, PI3K activity in PSCs has also been implicated to promote the development of pancreatic cancer. Indeed, pancreatic cancer produces pro-mitogenic and pro-fibrotic factors like TGF-β and PDGF that activate PI3K in PSCs. In turn, PSCs release factors such as PDGF, IGF-1, and matrix metalloproteinases that can activate the PI3K pathway in cancer cells promoting tumor growth, survival, metastasis and resistance to chemotherapy88. Thus, this mechanism creates a positive loop between PSCs and pancreatic cancer cells.
PI3K and pancreatic acinar cells
Some studies have demonstrated a crucial role of the PI3K pathway in the regulation of Ca2+ signaling in pancreatic acinar cells. In particular, PI3K acts in two principal ways in regulating Ca2+ signaling: by regulating bile acid-induced Ca2+ responses and by acting directly on the sarcoendoplasmic reticulum Ca2+ ATPase (SERCA). Bile acids and PI3K cause an inhibition of SERCA that consequently leads to Ca2+ release from endoplasmic reticulum stores89,90. An increase in intracellular Ca2+ in pancreatic acinar cells has been shown to induce cell death, lead to trypsinogen activation, and induce the activation of pro-inflammatory transcription factors such as NF-kB, ultimately promoting pancreatitis91,92. PI3K activity in pancreatic acinar cells has been implicated in protein synthesis, cellular metabolism and as a mitogenic and anti-apoptotic factor93. Recent reports have described that at least in animal models PanINs develop from acinar cells via acinar cell de-differentiation and acinar to ductal metaplasia94. The transformation from acinar cells into pre-cancerous and cancerous cells is at least partially governed by genetic alterations of the K-Ras oncogene with downstream PI3K activation95,96. Interestingly, macrophages have been demonstrated to contribute in promoting acinar cell de-differentiation97.
PI3K and macrophages
Macrophages are thought to play an important and central role in the development of chronic pancreatitis and pancreatic cancer98. There is currently great interest in studying the role of macrophages and the conversion of M0/M1 macrophages into pro-tumorigenic M2 macrophages. Some authors have described how PI3K can convert macrophages into the immunosuppressive and pro-tumorigenic M2 type99,100. In macrophages the PI3K pathway is involved in cellular survival, adhesion, and motility101–104. Macrophages are present in chronic pancreatitis and pancreatic cancer and have been shown to play a fundamental role in pancreatic inflammation and tumor progression105,106.
PI3K and neutrophils
Neutrophils are an important component of the innate immune system. They are recruited to sites of acute inflammation and into the tumor microenvironment. They are capable of inducing tumor growth and invasion through the production of proteases and reactive oxygen species (ROS). On the other hand, neutrophils have also been shown to be noxious for cancer cells107. Neutrophils are commonly present in chronic pancreatitis but are rarely found in pancreatic cancer108. PI3K plays a crucial role for neutrophil growth, survival, phagocytosis, adhesion, diapedesis, and chemotaxis109–111.
PI3K and mast cells
Mast cells are fundamental in the initiation of an inflammatory response. Mast cells are recruited and activated during inflammation. They produce cytokines and chemokines, thereby maintaining the inflammatory reaction. Mast cells have been shown to promote pancreatic cancer growth and progression by releasing pro-inflammatory and pro-angiogenic factors112. The PI3K pathway plays a key role in regulating mast cell differentiation, degranulation, cytokine production, chemotaxis, adhesion, cellular growth, and survival, at least partially through regulation of intracellular calcium levels113,114.
PI3K in CD8+ T Cells and Natural Killer Cells
T-Lymphocytes are the principal cells of acquired immunity. Several studies showed that PI3K is a crucial pathway involved in T cell activation, chemotaxis, development, and proliferation115–117. CD8+ T lymphocytes are the principal T cell subtype present in chronic pancreatitis and in the tumor stroma118,119. Some authors highlighted also the crucial role of the PI3K pathway in natural killer cells (NK), as a key factor for cellular development, function, chemotaxis, and cytokine production120. The exact role of NK cells in pancreatic cancer is complicated and multifaceted. It has been shown that NK cells are capable of promoting tumor cell lysis121. On the other hand, evidence suggests that although NK cells are recruited into the cancer, signals from the tumor microenvironment suppresses NK cell cytotoxicity while increasing their cytokine production (split anergy)122. It has been reported that NK cells are increased in the pancreatic tissue of patients with chronic pancreatitis123 and decreased in pancreatic cancer124.
PI3K in pancreatic cancer cells
The central role of the PI3K pathway in pancreatic cancer cells in regulating critical cancer cell phenotypic processes is well documented125,126. Activation of PI3K has been linked to proliferation127, inhibition of apoptosis, survival128, invasion, migration, metastasis11, angiogenesis129, and altered tumor cell metabolism130. The PI3K pathway regulates G1-phase progression in cancer cells thereby promoting the S-phase of the cell cycle131, and contributes to an increase in pancreatic cancer cell size132; PI3K favors an anaerobic environment through activation of glycolysis, thus creating a hypoxic state which may promote tumor growth and survival133. The PI3K pathway plays also a crucial role in angiogenesis and metastasis. It has been shown that epithelial-mesenchymal transition (EMT) is a critical step in the dissemination of (pancreatic) cancer cells. EMT is regulated by several transcription factors, e.g. twist. Reports have described an interplay between transcriptional regulators of EMT, like twist, and PI3K and downstream Akt134,135. The PI3K pathway is also involved in the promotion of tumor angiogenesis by regulating hypoxia-inducible factor (HIF)-1 alpha. HIF-1 alpha induces the transcription of VEGF, which plays a fundamental role in promoting angiogenesis129,136,137.
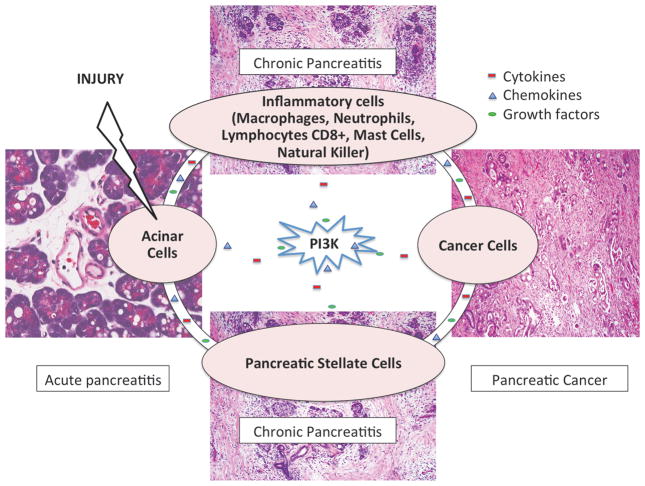
Taken together, numerous reports described a central role of PI3K in various cell populations in the pancreas during the development of pancreatic inflammation (pancreatitis) and cancer. A complex reciprocal cross-talk exists between stromal, inflammatory, and pancreatic cells potentially leading to chronic pancreatitis. This resulting inflammatory micro-environment is conducive to pancreatic tumor development. Pancreatic pre-cancer and cancer cells in turn communicate with other cell types in the pancreatic micro-environment to sustain cell survival and further tumor growth. The crosstalk between various cell populations is maintained by molecules secreted by various cell types into the pancreatic stroma, which can activate PI3K and downstream events in an autocrine and/or paracrine manner. Activation of PI3K in turn can lead to the production of further inflammatory molecules, creating a complex communication network (Figure 2). Due to its central role in pancreatic inflammation and cancer, PI3K is an intriguing molecular target for cancer prevention and therapy. Targeting PI3K may reduce the risk of developing (inflammation-associated) pancreatic cancer and decrease tumor growth by affecting fibrosis, inflammation, immune responses, and cancer cell growth. The importance of PI3K as a molecular target in pancreatic cancer is reflected by ongoing clinical trials using various combination regimens (Table 3). In addition, PI3K is also one of the main targets of nutriceuticals underlying their health promoting effects138.
Figure 2.
Several cell types in the pancreatic microenvironment, including macrophages, stellate cells, contribute to the development of acute pancreatitis, chronic pancreatitis, and pancreatic cancer. PI3K plays a central role in mediating the communication between all these cell types.
Table 3.
Ongoing Clinical Trials in Patients with Pancreatic Cancer with Drugs Targeting the PI3K Pathway
| NCT Number | Title | Phase | Drug targeting the PI3K pathway |
|---|---|---|---|
| NCT01155453 | A Study to Investigate Safety, Pharmacokinetics (PK) and Pharmacodynamics (PD) of BKM120 Plus GSK1120212 in Selected Advanced Solid Tumor Patients | I | BKM120 |
| NCT01363232 | Safety, Pharmacokinetics and Pharmacodynamics of BKM120 Plus MEK162 in Selected Advanced Solid Tumor Patients | I | BKM120 |
| NCT01576666 | Phase Ib, Dose Escalation Study of Oral LDE225 in Combination With BKM120 in Patients With Advanced Solid Tumors | I | BKM120 |
| NCT01096199 | A Study of TS-1, Cisplatin (CDDP) and RAD001 (Everolimus) | I | Everolimus |
| NCT01077986 | Everolimus, Cetuximab and Capecitabine in Patients With Metastatic Pancreatic Cancer | I–II | Everolimus |
| NCT01096199 | A Study of TS-1, Cisplatin (CDDP) and RAD001 (Everolimus) | I | Everolimus |
| NCT00499486 | Sirolimus in Treating Patients With Advanced Pancreatic Cancer | II | Sirolimus |
| NCT00075647 | CCI-779 in Treating Patients With Locally Advanced or Metastatic Pancreatic Cancer | II | Temsirolimus |
| NCT01210911 | Metformin Combined With Chemotherapy for Pancreatic Cancer | II | Metformin |
| NCT01347866 | Clinical Study Of PI3K/mTOR Inhibitors In Combination With An Oral MEK Inhibitor Or Irinotecan In Patients With Advanced Cancer | I | PF-05212384 |
| NCT01449058 | A Phase Ib Study of MEK162 Plus BYL719 in Adult Patients With Selected Advanced Solid Tumors | I–II | BYL719 |
| NCT00777699 | Safety Study of XL765 (SAR245409) in Combination With Erlotinib in Adults With Solid Tumors | I | XL765 |
| NCT00692640 | Safety Study of XL147 (SAR245408) in Combination With Erlotinib in Adults With Solid Tumors | I | XL147 |
Exosomes and PI3K pathway
Interestingly, a bidirectional crosstalk between inflammatory and cancer cells can cause a surrounding pro-tumorigenic microenvironment. Some authors showed that also release and exchange of secreted extracellular vesicles (EVs) can play a critical role in the intercellular crosstalk between tumor and immune cells 139. Exosomes are 50–150 nm CD9-positive nanovescicles, acting as natural shuttles of RNA and cargo of proteins, lipids and carbohydrates 140. Recently, some authors demonstrated that exosomes play a fundamental role in the intercellular signaling, transfecting different molecules like IGF, EGF, VEGF, IL-6, leptin and insulin that can activate PI3K pathway141.
Exosomes can be released from PSCs to other PSCs, transfecting connective tissue factor (CCN2) and microRNA-21 (miR-21) that lead to collagen production, playing thus a fundamental role in the development of chronic pancreatitis142. miR-21 is also up-regulated in several solid neoplasia, including pancreatic cancer143 and inhibits PTEN expression, inducing in this way a PI3K pathway activation144. Tumor cells secrete more exosomes compared to normal cells and the amount of exosomes increases as the disease advances145,146. It was showed that through exosomes, pancreatic cancer cells can secrete several factors, like EGFR, leading to an up-regulation of PI3K in other cells147. Similarly, cancer cells can transfer cytokines, chemokines and growth factors to distant cells, promoting tumor metastasis148.
Conclusion
Several risk factors can increase the development of chronic pancreatitis and pancreatic cancer. Inflammation is a critical mechanism underlying these risk factors. Pancreatic inflammation is characterized by a recruitment and infiltration of inflammatory cells that secrete cytokines, chemokines, and growth factors. Persistence of this inflammatory environment increases the risk of genetic instability and alterations promoting the development of tumors. There is plenty of evidence that the PI3K pathway is a critical signaling module in inflammatory pancreatic diseases, including chronic pancreatitis and pancreatic cancer. Numerous reports described activation of PI3K in stromal, immune, and pancreatic cancer cells. PI3K plays a key role in tumor-associated immune responses, tumor cell growth, survival, proliferation, angiogenesis and dissemination. Importantly, cytokines, chemokines, and growth factors represent a “common language”, by which inflammatory and cancer cells can cross talk, thereby creating a positive feedback loop. In fact, many inflammatory molecules present in chronic pancreatic inflammation and cancer can activate the PI3K pathway. Thus, PI3K represents a common intracellular signaling pathway stimulated in pancreatic inflammatory and neoplastic diseases. Because of its central role in inflammatory and neoplastic pancreatic disease, PI3K is an intriguing target for therapy and prevention of pancreatic cancer. Several clinical trials are currently underway to evaluate targeting PI3K in pancreatic cancer patients.
Acknowledgments
Funding: the study was partly supported by P50 AA11999, P01 CA163200, P01 DK098108, P01AT3960 grants, and Department of Veterans Affairs.
Footnotes
Conflict of interest disclosure: The authors declare no conflict of interest
References
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Al Haddad AH, Adrian TE. Challenges and future directions in therapeutics for pancreatic ductal adenocarcinoma. Expert Opin Investig Drugs. 2014;23:1499–1515. doi: 10.1517/13543784.2014.933206. [DOI] [PubMed] [Google Scholar]
- 3.Rubin DC, Shaker A, Levin MS. Chronic intestinal inflammation: inflammatory bowel disease and colitis-associated colon cancer. Front Immunol. 2012;3:107. doi: 10.3389/fimmu.2012.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakai Y, Nonomura N. Inflammation and prostate carcinogenesis. Int J Urol. 2013;20:150–160. doi: 10.1111/j.1442-2042.2012.03101.x. [DOI] [PubMed] [Google Scholar]
- 5.Sekine Y, Hata A, Koh E, et al. Lung carcinogenesis from chronic obstructive pulmonary disease: characteristics of lung cancer from COPD and contribution of signal transducers and lung stem cells in the inflammatory microenvironment. Gen Thorac Cardiovasc Surg. 2014;62:415–421. doi: 10.1007/s11748-014-0386-x. [DOI] [PubMed] [Google Scholar]
- 6.Liao Z, Jin G, Cai D, et al. Guidelines: diagnosis and therapy for chronic pancreatitis. J Interv Gastroenterol. 2013;3:133–136. [Google Scholar]
- 7.Raimondi S, Lowenfels AB, Morselli-Labate AM, et al. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol. 2010;24:349–358. doi: 10.1016/j.bpg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 10.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 11.Kolsch V, Charest PG, Firtel RA. The regulation of cell motility and chemotaxis by phospholipid signaling. J Cell Sci. 2008;121:551–559. doi: 10.1242/jcs.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010;29:309–316. doi: 10.1007/s10555-010-9223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colotta F, Allavena P, Sica A, et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 14.Witt H, Apte MV, Keim V, et al. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology. 2007;132:1557–1573. doi: 10.1053/j.gastro.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Stevens T, Conwell DL, Zuccaro G. Pathogenesis of chronic pancreatitis: an evidence-based review of past theories and recent developments. Am J Gastroenterol. 2004;99:2256–2270. doi: 10.1111/j.1572-0241.2004.40694.x. [DOI] [PubMed] [Google Scholar]
- 16.Malka D, Hammel P, Maire F, et al. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut. 2002;51:849–852. doi: 10.1136/gut.51.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinho AV, Chantrill L, Rooman I. Chronic pancreatitis: a path to pancreatic cancer. Cancer Lett. 2014;345:203–209. doi: 10.1016/j.canlet.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Gukovsky I, Lugea A, Shahsahebi M, et al. A rat model reproducing key pathological responses of alcoholic chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2008;294:G68–79. doi: 10.1152/ajpgi.00006.2007. [DOI] [PubMed] [Google Scholar]
- 19.Lankisch PG, Breuer N, Bruns A, et al. Natural history of acute pancreatitis: a long-term population-based study. Am J Gastroenterol. 2009;104:2797–2805. doi: 10.1038/ajg.2009.405. quiz 2806. [DOI] [PubMed] [Google Scholar]
- 20.Takeyama Y. Long-term prognosis of acute pancreatitis in Japan. Clin Gastroenterol Hepatol. 2009;7:S15–17. doi: 10.1016/j.cgh.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 21.Yadav D, Hawes RH, Brand RE, et al. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med. 2009;169:1035–1045. doi: 10.1001/archinternmed.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Criddle DN, Raraty MG, Neoptolemos JP, et al. Ethanol toxicity in pancreatic acinar cells: mediation by nonoxidative fatty acid metabolites. Proc Natl Acad Sci U S A. 2004;101:10738–10743. doi: 10.1073/pnas.0403431101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandol SJ, Saluja AK, Imrie CW, et al. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127–1151. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 24.Harnack LJ, Anderson KE, Zheng W, et al. Smoking, alcohol, coffee, and tea intake and incidence of cancer of the exocrine pancreas: the Iowa Women’s Health Study. Cancer Epidemiol Biomarkers Prev. 1997;6:1081–1086. [PubMed] [Google Scholar]
- 25.Edderkaoui M, Thrower E. Smoking and Pancreatic Disease. J Cancer Ther. 2013;4:34–40. doi: 10.4236/jct.2013.410A005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wittel UA, Momi N, Seifert G, et al. The pathobiological impact of cigarette smoke on pancreatic cancer development (review) Int J Oncol. 2012;41:5–14. doi: 10.3892/ijo.2012.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jang JH, Cotterchio M, Borgida A, et al. Interaction of polymorphisms in mitotic regulator genes with cigarette smoking and pancreatic cancer risk. Mol Carcinog. 2013;52(Suppl 1):E103–109. doi: 10.1002/mc.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yadav D, Whitcomb DC. The role of alcohol and smoking in pancreatitis. Nat Rev Gastroenterol Hepatol. 2010;7:131–145. doi: 10.1038/nrgastro.2010.6. [DOI] [PubMed] [Google Scholar]
- 29.Vongsuvanh R, George J, Qiao L, et al. Visceral adiposity in gastrointestinal and hepatic carcinogenesis. Cancer Lett. 2013;330:1–10. doi: 10.1016/j.canlet.2012.11.038. [DOI] [PubMed] [Google Scholar]
- 30.Tilg H, Moschen AR. Mechanisms behind the link between obesity and gastrointestinal cancers. Best Pract Res Clin Gastroenterol. 2014;28:599–610. doi: 10.1016/j.bpg.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Walker GE, Marzullo P, Ricotti R, et al. The pathophysiology of abdominal adipose tissue depots in health and disease. Horm Mol Biol Clin Investig. 2014;19:57–74. doi: 10.1515/hmbci-2014-0023. [DOI] [PubMed] [Google Scholar]
- 32.Gukovsky I, Li N, Todoric J, et al. Inflammation, autophagy, and obesity: common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1199–1209. e1194. doi: 10.1053/j.gastro.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwan HY, Chao X, Su T, et al. Dietary lipids and adipocytes: potential therapeutic targets in cancers. J Nutr Biochem. 2015;26:303–311. doi: 10.1016/j.jnutbio.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Cui Y, Andersen DK. Diabetes and pancreatic cancer. Endocr Relat Cancer. 2012;19:F9–F26. doi: 10.1530/ERC-12-0105. [DOI] [PubMed] [Google Scholar]
- 35.Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an update. Dig Dis. 2010;28:645–656. doi: 10.1159/000320068. [DOI] [PubMed] [Google Scholar]
- 36.Witt H, Becker M. Genetics of chronic pancreatitis. J Pediatr Gastroenterol Nutr. 2002;34:125–136. doi: 10.1097/00005176-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Chu D, Kohlmann W, Adler DG. Identification and screening of individuals at increased risk for pancreatic cancer with emphasis on known environmental and genetic factors and hereditary syndromes. JOP. 2010;11:203–212. [PubMed] [Google Scholar]
- 38.Omary MB, Lugea A, Lowe AW, et al. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117:50–59. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaster R. Molecular regulation of pancreatic stellate cell function. Mol Cancer. 2004;3:26. doi: 10.1186/1476-4598-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mews P, Phillips P, Fahmy R, et al. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut. 2002;50:535–541. doi: 10.1136/gut.50.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneiderhan W, Diaz F, Fundel M, et al. Pancreatic stellate cells are an important source of MMP-2 in human pancreatic cancer and accelerate tumor progression in a murine xenograft model and CAM assay. J Cell Sci. 2007;120:512–519. doi: 10.1242/jcs.03347. [DOI] [PubMed] [Google Scholar]
- 42.Apte MV, Xu Z, Pothula S, et al. Pancreatic cancer: The microenvironment needs attention too! Pancreatology. 2015;15:S32–38. doi: 10.1016/j.pan.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 43.Shimizu K, Kobayashi M, Tahara J, et al. Cytokines and peroxisome proliferator-activated receptor gamma ligand regulate phagocytosis by pancreatic stellate cells. Gastroenterology. 2005;128:2105–2118. doi: 10.1053/j.gastro.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 44.Buchholz M, Kestler HA, Holzmann K, et al. Transcriptome analysis of human hepatic and pancreatic stellate cells: organ-specific variations of a common transcriptional phenotype. J Mol Med (Berl) 2005;83:795–805. doi: 10.1007/s00109-005-0680-2. [DOI] [PubMed] [Google Scholar]
- 45.Man K, Ng KT, Lo CM, et al. Ischemia-reperfusion of small liver remnant promotes liver tumor growth and metastases--activation of cell invasion and migration pathways. Liver Transpl. 2007;13:1669–1677. doi: 10.1002/lt.21193. [DOI] [PubMed] [Google Scholar]
- 46.Kuehn R, Lelkes PI, Bloechle C, et al. Angiogenesis, angiogenic growth factors, and cell adhesion molecules are upregulated in chronic pancreatic diseases: angiogenesis in chronic pancreatitis and in pancreatic cancer. Pancreas. 1999;18:96–103. doi: 10.1097/00006676-199901000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Inman KS, Francis AA, Murray NR. Complex role for the immune system in initiation and progression of pancreatic cancer. World J Gastroenterol. 2014;20:11160–11181. doi: 10.3748/wjg.v20.i32.11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 49.Solinas G, Schiarea S, Liguori M, et al. Tumor-conditioned macrophages secrete migration-stimulating factor: a new marker for M2-polarization, influencing tumor cell motility. J Immunol. 2010;185:642–652. doi: 10.4049/jimmunol.1000413. [DOI] [PubMed] [Google Scholar]
- 50.Khaled YS, Ammori BJ, Elkord E. Increased levels of granulocytic myeloid-derived suppressor cells in peripheral blood and tumour tissue of pancreatic cancer patients. J Immunol Res. 2014;2014:879897. doi: 10.1155/2014/879897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qian B, Deng Y, Im JH, et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One. 2009;4:e6562. doi: 10.1371/journal.pone.0006562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rooman I, Real FX. Pancreatic ductal adenocarcinoma and acinar cells: a matter of differentiation and development? Gut. 2012;61:449–458. doi: 10.1136/gut.2010.235804. [DOI] [PubMed] [Google Scholar]
- 54.Pandol SJ, Raraty M. Pathobiology of alcoholic pancreatitis. Pancreatology. 2007;7:105–114. doi: 10.1159/000104235. [DOI] [PubMed] [Google Scholar]
- 55.Leung PS, Chan YC. Role of oxidative stress in pancreatic inflammation. Antioxid Redox Signal. 2009;11:135–165. doi: 10.1089/ars.2008.2109. [DOI] [PubMed] [Google Scholar]
- 56.Kusmartsev S, Nefedova Y, Yoder D, et al. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 57.Guerra C, Schuhmacher AJ, Canamero M, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 58.Kopp JL, von Figura G, Mayes E, et al. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22:737–750. doi: 10.1016/j.ccr.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Costa C, Hirsch E. More than just kinases: the scaffolding function of PI3K. Curr Top Microbiol Immunol. 2010;346:171–181. doi: 10.1007/82_2010_57. [DOI] [PubMed] [Google Scholar]
- 60.Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 61.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 62.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hirsch E, Ciraolo E, Franco I, et al. PI3K in cancer-stroma interactions: bad in seed and ugly in soil. Oncogene. 2014;33:3083–3090. doi: 10.1038/onc.2013.265. [DOI] [PubMed] [Google Scholar]
- 64.Rommel C, Camps M, Ji H. PI3K delta and PI3K gamma: partners in crime in inflammation in rheumatoid arthritis and beyond? Nat Rev Immunol. 2007;7:191–201. doi: 10.1038/nri2036. [DOI] [PubMed] [Google Scholar]
- 65.Gonzalez-Garcia A, Sanchez-Ruiz J, Flores JM, et al. Phosphatidylinositol 3-kinase gamma inhibition ameliorates inflammation and tumor growth in a model of colitis-associated cancer. Gastroenterology. 2010;138:1374–1383. doi: 10.1053/j.gastro.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 66.Bono MR, Elgueta R, Sauma D, et al. The essential role of chemokines in the selective regulation of lymphocyte homing. Cytokine Growth Factor Rev. 2007;18:33–43. doi: 10.1016/j.cytogfr.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 67.Edling CE, Selvaggi F, Buus R, et al. Key role of phosphoinositide 3-kinase class IB in pancreatic cancer. Clin Cancer Res. 2010;16:4928–4937. doi: 10.1158/1078-0432.CCR-10-1210. [DOI] [PubMed] [Google Scholar]
- 68.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:2140–2141. doi: 10.1056/NEJMc1412266. [DOI] [PubMed] [Google Scholar]
- 69.Ji B, Tsou L, Wang H, et al. Ras activity levels control the development of pancreatic diseases. Gastroenterology. 2009;137:1072–1082. 1082 e1071–1076. doi: 10.1053/j.gastro.2009.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Castellano E, Downward J. Role of RAS in the regulation of PI 3-kinase. Curr Top Microbiol Immunol. 2010;346:143–169. doi: 10.1007/82_2010_56. [DOI] [PubMed] [Google Scholar]
- 71.Ying H, Elpek KG, Vinjamoori A, et al. PTEN is a major tumor suppressor in pancreatic ductal adenocarcinoma and regulates an NF-kappaB-cytokine network. Cancer Discov. 2011;1:158–169. doi: 10.1158/2159-8290.CD-11-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 73.Bellizzi AM, Bloomston M, Zhou XP, et al. The mTOR pathway is frequently activated in pancreatic ductal adenocarcinoma and chronic pancreatitis. Appl Immunohistochem Mol Morphol. 2010;18:442–447. doi: 10.1097/PAI.0b013e3181de115b. [DOI] [PubMed] [Google Scholar]
- 74.Farrow B, Evers BM. Inflammation and the development of pancreatic cancer. Surg Oncol. 2002;10:153–169. doi: 10.1016/s0960-7404(02)00015-4. [DOI] [PubMed] [Google Scholar]
- 75.Tougeron D, Sha D, Manthravadi S, et al. Aspirin and colorectal cancer: back to the future. Clin Cancer Res. 2014;20:1087–1094. doi: 10.1158/1078-0432.CCR-13-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Albazaz R, Verbeke CS, Rahman SH, et al. Cyclooxygenase-2 expression associated with severity of PanIN lesions: a possible link between chronic pancreatitis and pancreatic cancer. Pancreatology. 2005;5:361–369. doi: 10.1159/000086536. [DOI] [PubMed] [Google Scholar]
- 77.Reding T, Bimmler D, Perren A, et al. A selective COX-2 inhibitor suppresses chronic pancreatitis in an animal model (WBN/Kob rats): significant reduction of macrophage infiltration and fibrosis. Gut. 2006;55:1165–1173. doi: 10.1136/gut.2005.077925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 79.Whitley BR, Beaulieu LM, Carter JC, et al. Phosphatidylinositol 3-kinase/Akt regulates the balance between plasminogen activator inhibitor-1 and urokinase to promote migration of SKOV-3 ovarian cancer cells. Gynecol Oncol. 2007;104:470–479. doi: 10.1016/j.ygyno.2006.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laufs S, Schumacher J, Allgayer H. Urokinase-receptor (u-PAR): an essential player in multiple games of cancer: a review on its role in tumor progression, invasion, metastasis, proliferation/dormancy, clinical outcome and minimal residual disease. Cell Cycle. 2006;5:1760–1771. doi: 10.4161/cc.5.16.2994. [DOI] [PubMed] [Google Scholar]
- 81.Mazar AP, Ahn RW, O’Halloran TV. Development of novel therapeutics targeting the urokinase plasminogen activator receptor (uPAR) and their translation toward the clinic. Curr Pharm Des. 2011;17:1970–1978. doi: 10.2174/138161211796718152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hildenbrand R, Gandhari M, Stroebel P, et al. The urokinase-system--role of cell proliferation and apoptosis. Histol Histopathol. 2008;23:227–236. doi: 10.14670/HH-23.227. [DOI] [PubMed] [Google Scholar]
- 83.Pandol S, Edderkaoui M, Gukovsky I, et al. Desmoplasia of pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2009;7:S44–47. doi: 10.1016/j.cgh.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Masamune A, Kikuta K, Satoh M, et al. Differential roles of signaling pathways for proliferation and migration of rat pancreatic stellate cells. Tohoku J Exp Med. 2003;199:69–84. doi: 10.1620/tjem.199.69. [DOI] [PubMed] [Google Scholar]
- 85.McCarroll JA, Phillips PA, Kumar RK, et al. Pancreatic stellate cell migration: role of the phosphatidylinositol 3-kinase(PI3-kinase) pathway. Biochem Pharmacol. 2004;67:1215–1225. doi: 10.1016/j.bcp.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 86.Kordes C, Brookmann S, Haussinger D, et al. Differential and synergistic effects of platelet-derived growth factor-BB and transforming growth factor-beta1 on activated pancreatic stellate cells. Pancreas. 2005;31:156–167. doi: 10.1097/01.mpa.0000168222.05591.a0. [DOI] [PubMed] [Google Scholar]
- 87.Berna MJ, Seiz O, Nast JF, et al. CCK1 and CCK2 receptors are expressed on pancreatic stellate cells and induce collagen production. J Biol Chem. 2010;285:38905–38914. doi: 10.1074/jbc.M110.125534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Duner S, Lopatko Lindman J, Ansari D, et al. Pancreatic cancer: the role of pancreatic stellate cells in tumor progression. Pancreatology. 2010;10:673–681. doi: 10.1159/000320711. [DOI] [PubMed] [Google Scholar]
- 89.Fischer L, Gukovskaya AS, Penninger JM, et al. Phosphatidylinositol 3-kinase facilitates bile acid-induced Ca(2+) responses in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G875–886. doi: 10.1152/ajpgi.00558.2005. [DOI] [PubMed] [Google Scholar]
- 90.Gukovsky I, Cheng JH, Nam KJ, et al. Phosphatidylinositide 3-kinase gamma regulates key pathologic responses to cholecystokinin in pancreatic acinar cells. Gastroenterology. 2004;126:554–566. doi: 10.1053/j.gastro.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 91.Kim JY, Kim KH, Lee JA, et al. Transporter-mediated bile acid uptake causes Ca2+-dependent cell death in rat pancreatic acinar cells. Gastroenterology. 2002;122:1941–1953. doi: 10.1053/gast.2002.33617. [DOI] [PubMed] [Google Scholar]
- 92.Kruger B, Albrecht E, Lerch MM. The role of intracellular calcium signaling in premature protease activation and the onset of pancreatitis. Am J Pathol. 2000;157:43–50. doi: 10.1016/S0002-9440(10)64515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Williams JA, Sans MD, Tashiro M, et al. Cholecystokinin activates a variety of intracellular signal transduction mechanisms in rodent pancreatic acinar cells. Pharmacol Toxicol. 2002;91:297–303. doi: 10.1034/j.1600-0773.2002.910606.x. [DOI] [PubMed] [Google Scholar]
- 94.Zhu L, Shi G, Schmidt CM, et al. Acinar cells contribute to the molecular heterogeneity of pancreatic intraepithelial neoplasia. Am J Pathol. 2007;171:263–273. doi: 10.2353/ajpath.2007.061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu CY, Carpenter ES, Takeuchi KK, et al. PI3K Regulation of RAC1 Is Required for KRAS-Induced Pancreatic Tumorigenesis in Mice. Gastroenterology. 2014;147:1405–1416. e1407. doi: 10.1053/j.gastro.2014.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hermann PC, Sancho P, Canamero M, et al. Nicotine Promotes Initiation and Progression of KRAS-Induced Pancreatic Cancer via Gata6-Dependent Dedifferentiation of Acinar Cells in Mice. Gastroenterology. 2014;147:1119–1133. e1114. doi: 10.1053/j.gastro.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 97.Folias AE, Penaranda C, Su AL, et al. Aberrant innate immune activation following tissue injury impairs pancreatic regeneration. PLoS One. 2014;9:e102125. doi: 10.1371/journal.pone.0102125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Helm O, Mennrich R, Petrick D, et al. Comparative characterization of stroma cells and ductal epithelium in chronic pancreatitis and pancreatic ductal adenocarcinoma. PLoS One. 2014;9:e94357. doi: 10.1371/journal.pone.0094357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xie S, Chen M, Yan B, et al. Identification of a role for the PI3K/AKT/mTOR signaling pathway in innate immune cells. PLoS One. 2014;9:e94496. doi: 10.1371/journal.pone.0094496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sly LM, Ho V, Antignano F, et al. The role of SHIP in macrophages. Front Biosci. 2007;12:2836–2848. doi: 10.2741/2276. [DOI] [PubMed] [Google Scholar]
- 101.Arranz A, Doxaki C, Vergadi E, et al. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc Natl Acad Sci U S A. 2012;109:9517–9522. doi: 10.1073/pnas.1119038109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu F, Kang Y, Zhang H, et al. Akt1-mediated regulation of macrophage polarization in a murine model of Staphylococcus aureus pulmonary infection. J Infect Dis. 2013;208:528–538. doi: 10.1093/infdis/jit177. [DOI] [PubMed] [Google Scholar]
- 103.Liu H, Perlman H, Pagliari LJ, et al. Constitutively activated Akt-1 is vital for the survival of human monocyte-differentiated macrophages. Role of Mcl-1, independent of nuclear factor (NF)-kappaB, Bad, or caspase activation. J Exp Med. 2001;194:113–126. doi: 10.1084/jem.194.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Busca A, Saxena M, Iqbal S, et al. PI3K/Akt regulates survival during differentiation of human macrophages by maintaining NF-kappaB-dependent expression of antiapoptotic Bcl-xL. J Leukoc Biol. 2014;96:1011–1022. doi: 10.1189/jlb.1A0414-212R. [DOI] [PubMed] [Google Scholar]
- 105.Goecke H, Forssmann U, Uguccioni M, et al. Macrophages infiltrating the tissue in chronic pancreatitis express the chemokine receptor CCR5. Surgery. 2000;128:806–814. doi: 10.1067/msy.2000.108613. [DOI] [PubMed] [Google Scholar]
- 106.Sica A, Larghi P, Mancino A, et al. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349–355. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 107.Houghton AM. The paradox of tumor-associated neutrophils: fueling tumor growth with cytotoxic substances. Cell Cycle. 2010;9:1732–1737. doi: 10.4161/cc.9.9.11297. [DOI] [PubMed] [Google Scholar]
- 108.Reid MD, Basturk O, Thirabanjasak D, et al. Tumor-infiltrating neutrophils in pancreatic neoplasia. Mod Pathol. 2011;24:1612–1619. doi: 10.1038/modpathol.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hawkins PT, Stephens LR, Suire S, et al. PI3K signaling in neutrophils. Curr Top Microbiol Immunol. 2010;346:183–202. doi: 10.1007/82_2010_40. [DOI] [PubMed] [Google Scholar]
- 110.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 111.Pinho V, Russo RC, Amaral FA, et al. Tissue- and stimulus-dependent role of phosphatidylinositol 3-kinase isoforms for neutrophil recruitment induced by chemoattractants in vivo. J Immunol. 2007;179:7891–7898. doi: 10.4049/jimmunol.179.11.7891. [DOI] [PubMed] [Google Scholar]
- 112.Wachsmann MB, Pop LM, Vitetta ES. Pancreatic ductal adenocarcinoma: a review of immunologic aspects. J Investig Med. 2012;60:643–663. doi: 10.231/JIM.0b013e31824a4d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim MS, Radinger M, Gilfillan AM. The multiple roles of phosphoinositide 3-kinase in mast cell biology. Trends Immunol. 2008;29:493–501. doi: 10.1016/j.it.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fukao T, Terauchi Y, Kadowaki T, et al. Role of phosphoinositide 3-kinase signaling in mast cells: new insights from knockout mouse studies. J Mol Med (Berl) 2003;81:524–535. doi: 10.1007/s00109-003-0475-2. [DOI] [PubMed] [Google Scholar]
- 115.Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3:317–330. doi: 10.1038/nri1056. [DOI] [PubMed] [Google Scholar]
- 116.Oak JS, Matheu MP, Parker I, et al. Lymphocyte cell motility: the twisting, turning tale of phosphoinositide 3-kinase. Biochem Soc Trans. 2007;35:1109–1113. doi: 10.1042/BST0351109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.So L, Fruman DA. PI3K signalling in B- and T-lymphocytes: new developments and therapeutic advances. Biochem J. 2012;442:465–481. doi: 10.1042/BJ20112092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Emmrich J, Weber I, Nausch M, et al. Immunohistochemical characterization of the pancreatic cellular infiltrate in normal pancreas, chronic pancreatitis and pancreatic carcinoma. Digestion. 1998;59:192–198. doi: 10.1159/000007488. [DOI] [PubMed] [Google Scholar]
- 119.Emmrich J, Sparmann G, Hopt U, et al. Typing of leukocytes in pancreatic tissue surrounding human pancreatic carcinoma. Ann N Y Acad Sci. 1999;880:171–174. doi: 10.1111/j.1749-6632.1999.tb09520.x. [DOI] [PubMed] [Google Scholar]
- 120.Gumbleton M, Kerr WG. Role of inositol phospholipid signaling in natural killer cell biology. Front Immunol. 2013;4:47. doi: 10.3389/fimmu.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kitayama J, Atomi Y, Nagawa H, et al. Functional analysis of TCR gamma delta+ T cells in tumour-infiltrating lymphocytes (TIL) of human pancreatic cancer. Clin Exp Immunol. 1993;93:442–447. doi: 10.1111/j.1365-2249.1993.tb08198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jewett A, Tseng HC. Tumor induced inactivation of natural killer cell cytotoxic function; implication in growth, expansion and differentiation of cancer stem cells. J Cancer. 2011;2:443–457. doi: 10.7150/jca.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hunger RE, Mueller C, Z’Graggen K, et al. Cytotoxic cells are activated in cellular infiltrates of alcoholic chronic pancreatitis. Gastroenterology. 1997;112:1656–1663. doi: 10.1016/s0016-5085(97)70048-9. [DOI] [PubMed] [Google Scholar]
- 124.Clark CE, Hingorani SR, Mick R, et al. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–9527. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 125.Ferro R, Falasca M. Emerging role of the KRAS-PDK1 axis in pancreatic cancer. World J Gastroenterol. 2014;20:10752–10757. doi: 10.3748/wjg.v20.i31.10752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lim KH, Counter CM. Reduction in the requirement of oncogenic Ras signaling to activation of PI3K/AKT pathway during tumor maintenance. Cancer Cell. 2005;8:381–392. doi: 10.1016/j.ccr.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 127.Perugini RA, McDade TP, Vittimberga FJ, Jr, et al. Pancreatic cancer cell proliferation is phosphatidylinositol 3-kinase dependent. J Surg Res. 2000;90:39–44. doi: 10.1006/jsre.2000.5833. [DOI] [PubMed] [Google Scholar]
- 128.Semba S, Moriya T, Kimura W, et al. Phosphorylated Akt/PKB controls cell growth and apoptosis in intraductal papillary-mucinous tumor and invasive ductal adenocarcinoma of the pancreas. Pancreas. 2003;26:250–257. doi: 10.1097/00006676-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 129.Ma J, Sawai H, Ochi N, et al. PTEN regulates angiogenesis through PI3K/Akt/VEGF signaling pathway in human pancreatic cancer cells. Mol Cell Biochem. 2009;331:161–171. doi: 10.1007/s11010-009-0154-x. [DOI] [PubMed] [Google Scholar]
- 130.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 131.Schild C, Wirth M, Reichert M, et al. PI3K signaling maintains c-myc expression to regulate transcription of E2F1 in pancreatic cancer cells. Mol Carcinog. 2009;48:1149–1158. doi: 10.1002/mc.20569. [DOI] [PubMed] [Google Scholar]
- 132.Agbunag C, Bar-Sagi D. Oncogenic K-ras drives cell cycle progression and phenotypic conversion of primary pancreatic duct epithelial cells. Cancer Res. 2004;64:5659–5663. doi: 10.1158/0008-5472.CAN-04-0807. [DOI] [PubMed] [Google Scholar]
- 133.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 134.Yamamoto S, Tomita Y, Hoshida Y, et al. Prognostic significance of activated Akt expression in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10:2846–2850. doi: 10.1158/1078-0432.ccr-02-1441. [DOI] [PubMed] [Google Scholar]
- 135.Cheng GZ, Zhang W, Wang LH. Regulation of cancer cell survival, migration, and invasion by Twist: AKT2 comes to interplay. Cancer Res. 2008;68:957–960. doi: 10.1158/0008-5472.CAN-07-5067. [DOI] [PubMed] [Google Scholar]
- 136.Xia C, Meng Q, Cao Z, et al. Regulation of angiogenesis and tumor growth by p110 alpha and AKT1 via VEGF expression. J Cell Physiol. 2006;209:56–66. doi: 10.1002/jcp.20707. [DOI] [PubMed] [Google Scholar]
- 137.Graupera M, Guillermet-Guibert J, Foukas LC, et al. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature. 2008;453:662–666. doi: 10.1038/nature06892. [DOI] [PubMed] [Google Scholar]
- 138.Li Y, Go VL, Sarkar FH. The Role of Nutraceuticals in Pancreatic Cancer Prevention and Therapy: Targeting Cellular Signaling, MicroRNAs, and Epigenome. Pancreas. 2015;44:1–10. doi: 10.1097/MPA.0000000000000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.D’Souza-Schorey C, Clancy JW. Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes Dev. 2012;26:1287–1299. doi: 10.1101/gad.192351.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thery C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15. doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gangoda L, Boukouris S, Liem M, et al. Extracellular vesicles including exosomes are mediators of signal transduction: are they protective or pathogenic? Proteomics. 2015;15:260–271. doi: 10.1002/pmic.201400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Charrier A, Chen R, Chen L, et al. Connective tissue growth factor (CCN2) and microRNA-21 are components of a positive feedback loop in pancreatic stellate cells (PSC) during chronic pancreatitis and are exported in PSC-derived exosomes. J Cell Commun Signal. 2014;8:147–156. doi: 10.1007/s12079-014-0220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Iorio MV, Ferracin M, Liu CG, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 144.Sicard F, Gayral M, Lulka H, et al. Targeting miR-21 for the therapy of pancreatic cancer. Mol Ther. 2013;21:986–994. doi: 10.1038/mt.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Peinado H, Aleckovic M, Lavotshkin S, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ghosh AK, Secreto CR, Knox TR, et al. Circulating microvesicles in B-cell chronic lymphocytic leukemia can stimulate marrow stromal cells: implications for disease progression. Blood. 2010;115:1755–1764. doi: 10.1182/blood-2009-09-242719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Adamczyk KA, Klein-Scory S, Tehrani MM, et al. Characterization of soluble and exosomal forms of the EGFR released from pancreatic cancer cells. Life Sci. 2011;89:304–312. doi: 10.1016/j.lfs.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 148.Suetsugu A, Honma K, Saji S, et al. Imaging exosome transfer from breast cancer cells to stroma at metastatic sites in orthotopic nude-mouse models. Adv Drug Deliv Rev. 2013;65:383–390. doi: 10.1016/j.addr.2012.08.007. [DOI] [PubMed] [Google Scholar]