Abstract
The extracellular matrix is a dynamic repository harboring instructive cues that embody substantial regulatory dominance over many evolutionarily conserved intracellular activities including proliferation, apoptosis, migration, motility, and autophagy. The matrix also coordinates and parses hierarchical information, such as angiogenesis, tumorigenesis, and immunological responses, typically providing the critical determinants driving each outcome. We provide the first comprehensive review focused on proteoglycan receptors, that is, signaling transmembrane proteins that use secreted proteoglycans as ligands, in addition to their natural ligands. The majority of these receptors belong to an exclusive subset of receptor tyrosine kinases and assorted cell surface receptors that specifically bind, transduce, and modulate fundamental cellular processes following interactions with proteoglycans. The class of small leucine-rich proteoglycans is the most studied so far, and constitutes the best understood example of proteoglycan/receptor interactions. Decorin and biglycan evoke autophagy and immunological responses that deter, suppress, or exacerbate pathological conditions such as tumorigenesis, angiogenesis, and chronic inflammatory disease. Basement membrane-associated heparan sulfate proteoglycans - perlecan, agrin and collagen XVIII - represent a unique cohort and provide proteolytically-cleaved bioactive fragments for modulating cellular behavior. The receptors that bind the genuinely multifactorial and multivalent proteoglycans represent a nexus in understanding basic biological pathways and opens new avenues for therapeutic and pharmacological intervention.
Keywords: decorin, biglycan, perlecan, lumican, agrin, collagen XIV, small leucine rich proteoglycans
Introduction
Instructive cues fundamental for all aspects of multicellular life reside within the ubiquitous and evolutionarily conserved extracellular matrix (ECM). These functional signals range from fully embedded solid phase ligands to soluble mediators that function in a paracrine and/or autocrine fashion by engaging in high-affinity interactions with cell surface receptors1–6. Integrating and parsing bidirectional inputs and outputs allow the ECM to reign as key regulator for maintaining optimal cell and tissue homeostasis7,8. Cells interpret and process this dynamic repository of information through various supramolecular signaling complexes including RTKs, innate immune receptors, and integrins6,9.
The prominent subclasses of matrix constituents responsible for instructive signal transduction include the diverse and multifaceted small leucine-rich proteoglycan (SLRP) gene family and several members of the pericellular heparan sulfate proteoglycans that reside within basement membranes8,10. The original discovery that soluble, monomeric decorin11 is capable of binding EGFR and communicating with the intracellular signaling apparatus via cell surface signaling receptors12 pioneered the paradigm-changing concept that matrix components embody a critical regulatory network5,13–17. Following this initial discovery, decorin emerged as the leading candidate for a matrix-derived repressor of tumorigenic growth with inherent angiostatic and pro-autophagic activities18–20 that wholly manifests from RTK partial agonism. The repertoire of functions ascribed to decorin has since expanded exponentially and now participates in a plethora of diverse processes such as fibrillogenesis and wound healing21–23, keratinocyte function24, allergen-induced asthma25, delayed hypersensitivity26, diabetic nephropathies and renal diseases27,28, skeletal muscle homeostasis29, nurturing hematopoietic stem/progenitor cell niches30, and ensuring proper convergent extension31.
Biglycan, a member of the class I SLRP family with highest homology to decorin, has also been involved in receptor engagement and coordination of intracellular signaling pathways. For example, both circulating biglycan and decorin have been implicated in regulating innate inflammatory responses downstream of TLR2/4 (see below)5,9,32–34. Biomechanically, decorin and biglycan also share overlapping functions in the mechanobiology of tendon structure35–37, and have distinct roles in fetal membrane signaling38. Further, the control of cellular phenotype via ligation of distinct signaling receptors has also been attributed to molecules associated with basement membranes such as perlecan, agrin, and collagen XVIII39. Moreover, some of the basement membrane HSPGs (perlecan, collagen XVIII) are proteolytically processed to liberate a soluble, bioactive fragment capable of engaging cognate receptors40,41.
Although proteoglycans have been previously shown to play a role as endocytic receptors42–44, in this Current Topics, we will evaluate a unique class of signaling receptors that engage and transduce proteoglycan-derived cues. These activities can profoundly impact and reprogram the central processing and integration networks responsible for cell behavior, phenotype, and the development of various pathologies. Therefore, we will critically evaluate the pathways downstream of proteoglycan receptor engagement relevant for tumorigenesis, angiogenesis, autophagy, and immunomodulation.
DECORIN ENGAGES A MULTITUDE OF RTKS FOR PROTRACTED TUMORIGENIC SUPPRESSION
Decorin has emerged as a soluble pan-RTK inhibitor. However, decorin was initially discovered and characterized as a putative collagen binding factor active in competent collagen synthesis, assembly, deposition and fibrillogenesis37,45–48. Decorin also binds multiple matrix components necessary for structural integrity14 and sequesters pleiotropic growth factors, with a predominant tendency to inactivate several TGF-β family members14,18, which consequently suppress downstream TGF-β signaling in an indirect fashion14,18. One receptor-dependent mechanism of TGF-β regulation involves decorin-mediated engagement of the endocytic receptor, lipoprotein receptor-related protein 1 (LRP-1) 49 resulting in PI3K activation and TGF-β modulation via trimeric Smad signaling. Famously and primarily, decorin is a soluble tumor repressor that neutralizes tumorigenic growth and unchecked neovascularization, vis-à-vis RTK-mediated pro-autophagic signaling pathways19. For this reason, decorin has been appropriately designated as “a guardian from the matrix”14. Importantly, the well-established anti-tumorigenic and anti-angiogenic properties borne from the interaction of decorin and receptor (as discussed below) are independent of the covalently attached chondroitin/dermatan sulfate glycosaminoglycan chain50–52. Therefore, this review will focus exclusively on signaling events and cellular responses as mediated by the respective proteoglycan core protein.
EGFR AND MET: IT STARTED WITH A TALE OF TWO RECEPTORS
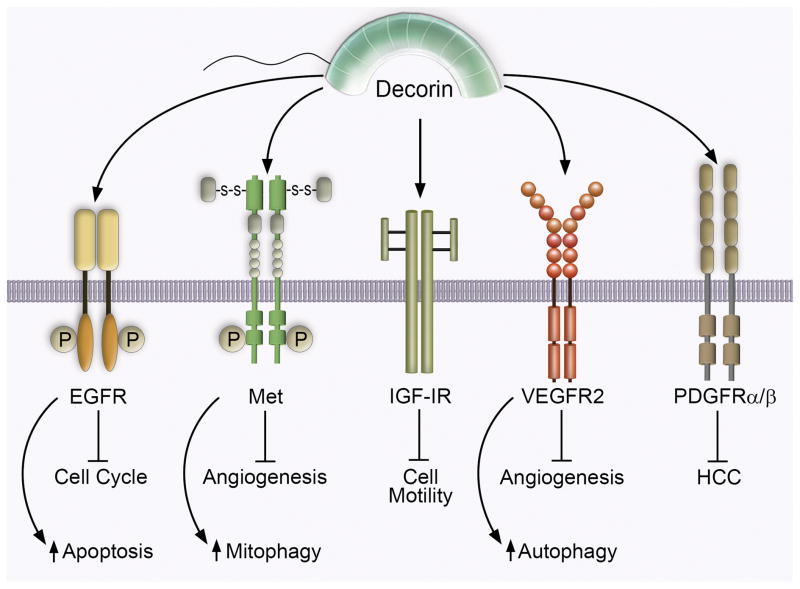
The epidermal growth factor receptor (EGFR) was the original RTK discovered that binds decorin with high affinity12 (Figure 1). Following stimulation of A431 cells, decorin promotes EGFR receptor dimerization, rapid trans-autophosphorylation of the unstructured intracellular tails, increased cytosolic calcium levels53, and evokes EGFR internalization in caveolin-1 positive endosomes14. Presumably, following receptor clearance from the tumor cell surface, the ternary complex of decorin/EGFR/caveolin-1 traffics and ultimately fuses with the lysosomal compartment for receptor complex degradation concomitant with a cessation of EGFR signaling54. Intriguingly, the ligand-binding site of decorin on EGFR partially overlaps with that of EGF, as decorin significantly competes off bound EGF55. Despite this narrow binding cleft shared by EGF and decorin, biologically distinct phenotypes occur insofar as receptor stability, signal intensity, and signal duration. Indeed, EGF sustains the maximal phosphorylation state and signaling capacity of post-internalized EGF:EGFR complexes and permits additional waves of signaling (e.g. bound MAPK, PI3K, PLC-γ1 components) followed by sorting into an endosomal recycling pathway that repopulates the cell surface with active EGFR56. In contrast, after evoking a brief burst of EGFR phosphorylation, declining EGFR cell surface levels (>50% of total), and transient activation of MAPK (ERK1/2), decorin exerts a protracted attenuation of downstream signaling effectors that paradoxically results in cell cycle arrest (induction of the cyclin-dependent kinase inhibitor, p21WAF1) and caspase-3 mediated apoptosis (Figure 1)4,57. Interestingly, depending on the phosphorylation signature decorin evokes, phosphorylated EGFR is required for association with caveolin-1 coated pits58. Unsurprisingly, EGFR is not the only Erb family member by which decorin serves as a direct soluble repressor as ErbB4 is targeted by decorin during scar tissue repair in the central nervous system59.
Figure 1.
Decorin interacts with several receptor tyrosine kinases for the control of fundamental cellular behaviors in normal and malignant circumstances. Schematic representation of cell surface receptor tyrosine kinases occupied by decorin. Biological consequences of binding and resultant signal transduction are outlined below the appropriate receptor. Please refer to the text for additional information.
A recurring hallmark of decorin binding served as the utilitarian means for the identification of Met (known as hepatocyte growth factor or scatter factor receptor) as the primary RTK by which decorin transduces biological information7,60 (Figure 1). In analogy with EGFR, decorin evoked a rapid, but transient burst at a phosphotyrosine residue detectable via a targeted phosphotyrosine RTK array platform60. Moreover, Met was characterized as the central RTK for decorin mediated anti-tumorigenicity and angiostatic properties50,52,60 vis-à-vis higher affinity binding for Met60 as well as via biological, pharmacological, and genetic methodologies52,60. Binary decorin/Met complexes avidly colocalize with caveolin-1 positive endosomes (for proficient lysosomal degradation) whereas pro-tumorigenic HGF/Met complexes associate with clathrin for receptor recycling and additional cycles of signaling50. Consequentially, two potent effector oncogenes downstream of Met signaling, β-catenin and Myc, are targeted for protracted degradation via the 26S proteasome14,50,61. Mechanistically, degradation of Myc - following phosphorylation at Thr58, which is situated within an established degron - permits CDKN1A derepression via loss of the AP4 transcriptional repressor50. Moreover, decorin utilizes Met as the nexus for angiogenic suppression52 in HeLa and MDA-MB-231 cells (Figure 1). Signaling positively through Met, decorin non-canonically suppresses HIF1 expression via transcriptional repression. Compromising HIF-1α expression subsequently decreases VEGFA and MMP2/9 with a concurrent increase of puissant anti-angiogenic effectors such as thrombospondin-1 (TSP-1)(see below) and TIMP-314,18,52. Collectively, decorin subverts the pro-angiogenic signaling network via prolonged attenuation of Met in neoplastic cells.
Similar binding mechanics notwithstanding, decorin may integrate signaling among multiple RTKs expressed by a single cell via the empirically determined binding constants of each receptor, prevailing density of the receptor, and the uniform inclusion of specific structural motifs (e.g. the IgG domain). Moreover, decorin may functionally titrate total receptor levels (via degradation) and thereby deprive signaling clusters of key receptors necessary for competent signal transduction for the development and progression of cancer. This biological process has already been observed via the physical sequestration of EGFR away from EGFR/ErbB1:Her2/Neu/ErbB2 heterodimers7,14. A pertinent example of differential signal integration is the rapid release of TSP-1 from basal breast carcinoma cells62 in a RhoA/ROCK1 dependent manner. Although MDA-MB-231 cells constitutively express EGFR and Met, pharmacological inhibition and RNAi-mediated silencing of Met did not perturb decorin-induced TSP-1 secretion whereas blocking EGFR completely abrogated this effect62 (Figure 1). Functionally, differences may reside in the phosphorylation signatures of the flexible intracellular tails flanking the kinase domain or in the geometrically-constrained structural conformations the receptors adopt following decorin binding.
Recently, a novel mechanism has emerged wherein decorin, acting as a partial Met agonist, induces tumor cell mitophagy (mitochondrial autophagy) as the molecular basis for the observed angiostatic effects in basal breast carcinoma18,19,63. Mitophagic induction may be a general phenomenon as it also occurs in prostate carcinomas transduced with decorin-expressing adenovirus61. Induction of tumor cell mitophagy is entirely dependent on the complex interaction between PGC-1α and mitostatin19,63. Loss of mitostatin, a putative tumor suppressor gene64,65 via RNAi-silencing prevents mitophagy and significantly compromises VEGFA suppression following decorin administration63 (Figure 1). Thus, decorin can negatively regulate two RTKs, EGFR and Met, potent drivers of cancer and angiogenesis and this influence could be due to an endogenous, stromal-derived force to restrain cancer growth and infiltration.
INSULIN-LIKE GROWTH FACTOR RECEPTOR 1 (IGF-IR): THE DECORIN DUALITY
The role of environmental and context-specific signaling is further illustrated with the interaction between decorin and IGF-IR66. An intriguing duality between normal, genomically-stable cells (e.g. endothelial cells)67 and tumor cells (e.g. bladder carcinoma)68 emerges following decorin engagement of IGF-IR (Figure 1). In endothelial cells, decorin triggers levels of IGF-IR phosphorylation comparable to that evoked by IGF-I coincident with downstream Akt signaling via the N-terminus of decorin66,68. Further, decorin is capable of stimulating endothelial cell adhesion and migration of endothelial cells over fibrillar collagen networks in an IGF-IR/α2β1 integrin dependent manner upstream of Rac activation69. Decorin also regulates renal fibrosis by directly engaging IGF-IR present on renal fibroblasts and indirectly by inhibiting the biological activity of CTGF (CCN2)70,71. Moreover, decorin promotes the PI3K/Akt/mTOR pathway in renal cells, which indirectly promotes fibrillin-1 translation, thereby curbing TGF-β bioavailability72–74. Substantially antithetic to the aforementioned role of decorin as a pan-RTK inhibitor, decorin can be a full IGF-IR agonist, analogous to IGF-I, in genomically-stable cells. In this case, soluble decorin exerts positive IGF-IR phosphorylation consistent with improved receptor stabilization and robust downstream effector activity under physiologically-relevant conditions66. In contrast, the net output of IGF-I/IGF-IR signaling in a neoplastic setting promotes urothelial tumor cell motility via Akt/MAPK-dependent paxillin activation75, where decorin can bind IGF-I and associated IR-A ligands76 and the IGF-IR in a region that does not overlap with the IGF-I-binding domain68. Orthodox paradigms regarding the interaction of decorin with cognate receptors no longer apply when discussing the effect of decorin/IGF-IR complexes in a tumorigenic setting66,68. Decorin does not compromise or enhance the IGF-IR phosphorylation state upon binding but allosterically competes and suppresses IGF-I-mediated activation of IGF-IR and downstream Akt/MAPK pathways68. Moreover, prolonged stimulation by decorin neither perturbs receptor stability nor does trigger internalization of the receptor complex with caveolin-1, unlike EGFR and Met, but does trigger degradation of IRS-168. This decorin/IGF-IR interaction is the only known instance whereby decorin does not cause RTK internalization and association with caveosomes. Decorin-evoked negative regulation of IGF-IR culminates in decreased IRS-1 stability that will ultimately prove insufficient for sustainable Akt/MAPK (and paxillin) activity; thereby abrogating IGF-I induced tumor cell motility (Figure 1). It was recently discovered that IGF-I requires a novel kinase involved in urothelial cell motility known as Pyk277. It remains unknown whether Pyk2 is downstream of IGF-IR/IRS-1/Akt/MAPK signaling and whether decorin inactivates this kinase for motility termination. Thus, decorin can exert opposite effects on the IGF-IR system and these effects are dependent on the cell context.
VASCULAR ENDOTHELIAL GROWTH FACTOR RECEPTOR 2 (VEGFR2): A NEW ERA FOR SLRPS
A unique high-resolution transcriptomic platform capable of differentiating species-specific transcripts from engrafted H. sapiens orthotopic tumors (MDA-MB-231) from the recipient M. musculus microenvironment has changed our understanding of decorin (and thus SLRP) biology51. These analyses revealed an exclusive subset of genes that are differentially regulated only within the tumor stroma. Among these genes, Peg3 emerged as a highly favored candidate51. Utilizing endothelial cells (MDEC and HUVEC) as a proxy for the mouse tumor stroma, we found that Peg3 was intimately involved in orchestrating decorin-evoked endothelial cell autophagy18–20,78 under nutrient enriched conditions. Further analysis revealed a strict dependence on the primary endothelial cell RTK, VEGFR2, as the mechanism for decorin-mediated autophagy and angiostasis78 (Figure 1). In a similar mechanism, decorin bioactivity requires competent RTK signaling as inhibition of VEGFR2 with the small molecule inhibitor SU11274 or genetic depletion of the receptor, abrogates Peg3 induction and subsequently prevents an increase in Beclin 1 and LC378. Therefore, decorin acts as a partial VEGFR2 agonist, whose predicted ligand-binding domain partially overlaps with that of VEGFA, the natural ligand of VEGFR220,78. Proficient autophagic stimulation relies on decorin/VEGFR2 interactions and subsequent downstream AMPKα activation (at Thr172)19,79,80. Critically, AMPKα serves as the chief energy sensing kinase responsible for autophagic initiation by inhibiting the anti-autophagic mTOR pathway81. This represents the first report that AMPKα can be activated by an upstream RTK and that it is stimulated in a manner commensurate with canonical autophagic stimuli (e.g. amino acid withdrawal and nutrient deprivation, rapamycin, or Torin-1)79. Moreover, the magnitude of autophagic induction attained with decorin is comparable to traditional methods of inducing autophagy. Therefore, decorin can be considered a soluble pro-autophagic effector that requires and binds two distinct pro-autophagic receptors, i.e., Met for tumor cell mitophagy and VEGFR2 for endothelial cell autophagy. In both instances, their tyrosine kinase activity is necessary for downstream biological function.
PLATELET DERIVED GROWTH FACTOR RECEPTOR (PDGFR): DECORIN IS A GENUINE PAN-RTK INHIBITOR
A prime example of the pervasive and widespread effect of decorin mediated RTK antagonism lies with the identification of platelet derived growth factor receptor α/β (PDGFRα/β) as a key target for combatting tumorigenesis82. Utilization of two different chemically-induced models of hepatocellular carcinoma (HCC) in either a wild-type or Dcn−/− background82, has identified several RTK targets. The screen revealed that when decorin is globally deleted, many RTKs become hyperactive, i.e., an increase in the phospho-Tyr signal, even under basal conditions82. From this screen, PDGFRα/β has emerged as a high-affinity interacting partner for decorin and proved a critical avenue by which decorin suppresses HCC development and progression82,83 (Figure 1). Thus, a genetic background lacking an important signaling SLRP causes a constitutive activation of several RTKs, a function not attributable to the collagen-binding role of decorin during development and tissue homeostasis84. The constitutive activation of RTKs in the absence of decorin, thus, provides a mechanistic explanation for a permissive role of decorin in tumorigenesis as shown in other genetic cancer models85–87.
INCITING INFLAMMATION
A second, closely-related class I SLRP, biglycan, shares greater than 65% homology with decorin in both human and mouse genes6,8. Biglycan contains two covalently-attached glycosaminoglycan chains, hence earning the eponym of biglycan6. Functionally, biglycan sequesters and modulates the activity of several TGF-β superfamily members including TGFβ/Smad288,89, and BMP-490. In addition, biglycan modulates the Wnt/β-catenin signaling axis91 (see below). Biglycan also binds and potentiates VEGFA signaling during fracture healing under normal physiological conditions92. Indeed, decorin may also be pro-angiogenic in certain physiological settings14. Non-overlapping functions do exist between biglycan and decorin insofar as biglycan is a critical regulator of skeletal bone growth6,16,93 and cardiac remodeling following myocardial infarction94. However, biglycan and decorin have functional commonality in the realm of regulating innate immunological responses.
BIGLYCAN LINKS SOLUBLE MATRIX WITH INNATE IMMUNE RESPONSES VIA TLR2/4 AND P2X SIGNALING
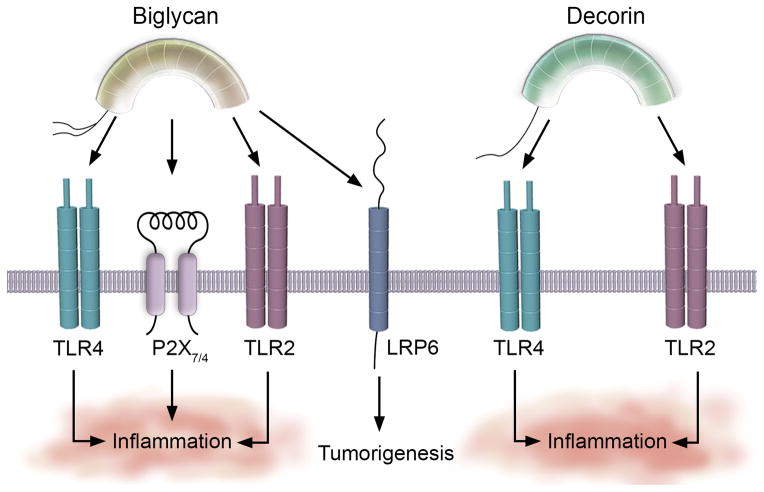
A groundbreaking discovery has been leading a revolution in further understanding biglycan biology6. Biglycan acts as an endogenous agonist for the innate immune receptors, Toll-like receptor 2 and 4 (TLR2/4) expressed on the surface of macrophages95–97 (Figure 2), and can aggravate ischemic acute renal injury28. As decorin is a soluble tumor repressor, biglycan has been identified as a soluble signaling molecule, a so-called “danger signal”6,98,99 that interfaces with the innate immune system33,74 following sepsis or ischemic injury. De novo synthesized and secreted by circulating macrophages34,97, biglycan engages TLR2/4. This initiates a pro-inflammatory cascade that converges on NF-κB and evokes the synthesis and development of mature IL-1β96, and release of TNF-α and IL-6. Mechanistically, biglycan promotes receptor clustering and cooperativity of TLR2/4 with the purinergic P2X7/P2X4 receptors (Figure 2)5,9,96. This affords rapid generation of reactive oxygen species that is directly involved in activating the NLRP3/ASC inflammasome96. Biglycan-mediated activation of the NLRP3/ASC inflammasome induces caspase-1-dependent cleavage of pro-IL-1β into mature IL-1β with subsequent secretion. Two feed-forward loops become established whereby biglycan promotes expression of NLRP3 and IL1B transcripts96 and, in turn, IL-1β and IL-6 can promote BGN expression and synthesis. Moreover, biglycan mediated signaling via the MyD88/TRIF34 arm downstream of TLR2/4 in kidneys result in CCL2 and CCL5 synthesis for the recruitment of macrophages and T-lymphocytes, respectively34,99,100. The CXCL class chemokines (e.g. CXCL1, CXCL2 and CXCL13) are subsequently released for macrophage34,95 and B-lymphocyte conscription in murine lupus nephritis101. Biglycan, as a newly-discovered DAMP, fundamentally connects soluble mediators derived from the matrix with regulating and inducing robust innate immune responses.
Figure 2.
Biglycan and decorin bind innate immune system receptors for immunoregulation and tumorigenesis. The innate immune receptors TLR2, TLR4 and the purinergic P2X7/4 provide novel signaling circuits through which biglycan and decorin bind for regulating the innate immune system and cultivating a pro-inflammatory environment in sepsis and tumorigenesis. Biglycan also binds LRP6 for tumor promotion. Please refer to the text for more information.
These observations have been elegantly confirmed in vivo6,8,9. Biglycan-deficient mice respond significantly less vigorously (e.g. decreased levels of active caspase-1 and lower titers of mature IL-1β) when challenged with inflammatory renal injury or lipopolysaccharide96. Physiologically, less IL-1β is found in the circulation, kidneys, and lungs96. Moreover, in a mouse model of ischemic acute kidney injury where biglycan was over-expressed, appreciably increased plasma and renal levels of TNF-α, CXCL1, CCL-2, and CCL-5 were found concomitant with increased frequency of infiltrating macrophages, neutrophils, and T-lymphocytes97. Overall, this resulted in considerably worse renal function and paints biglycan as a key mediator of inflammatory renal disease28. Conversely, the impairment of renal function is markedly ameliorated in the compound knockout mouse lacking TLR2/4 indicating a crucial pathological role for the biglycan/TLR2/4 interaction in vivo (Figure 2)97.
Biglycan also promotes a pro-inflammatory milieu within the lungs74. Under septic conditions, biglycan levels are substantially increased within cells infiltrating the pulmonary parenchyma95. In parallel with renal injury, biglycan deficiency dampens the immune cell population from breaching the lung tissue and results in less pulmonary damage95. Functionally, diminutive amounts of active caspase-1 and lower amounts of mature IL-1β were found within the lungs96.
LOW-DENSITY LIPOPROTEIN RECEPTOR-RELATED PROTEIN 6 (LRP6) IS A TUMOR RECEPTOR FOR BIGLYCAN
Not unforeseeable, and consistent with the abovementioned biological interactions (such as modulating Wnt signaling), biglycan is poised as a potent regulator of tumorigenesis and angiogenesis. Several solid malignancies are known to over-express biglycan which could contribute to tumor motility and even drug resistance102. The sources of biglycan in cancer currently remain elusive. However, it is known that TGF-β can induce biglycan from stromal fibroblasts102. Moreover, infiltrating immune cells (macrophages, neutrophils) constitutively secrete IL-1β and IL-6 under malignant conditions, and this will mechanistically support the feed-forward loop via TLR2/4 signaling.
Biglycan represents a functional dyad for the documented tumorigenic effects. Anti-proliferative effects of biglycan stem from a study in which cells transformed with the Her2/Neu oncogene secrete decreased levels of biglycan in a PKC/CREB-dependent manner103. RNAi-mediated silencing of biglycan in the Her2/Neu transformed fibroblasts augments growth rate and migration103, suggesting that biglycan can stymie the malignant phenotype conveyed by the Her2/Neu oncogene. However, biglycan can also compromise tissue architecture and integrity by promoting tumorigenesis via enhanced Wnt/β-catenin signaling91. Biglycan participates in a tripartite complex involving Wnt3a and the LRP6 (low-density lipoprotein receptor-related protein 6) co-receptor91 (Figure 2). Biglycan enhances LRP6 phosphorylation as cells deficient in biglycan have reduced cell surface retention of Wnt3a and decreased LRP6 phosphorylation91. Collectively, this trimeric complex is sufficient for the canonical induction of β-catenin/TCF target genes and also transactivates the RUNX2 transcriptional complex in osteoprogenitor cells91. Finally, biglycan may also promote angiogenesis via enhanced VEGFA signal potentiation92 and/or via the formation of reactive oxygen species downstream from TLR2/4-dependent signaling as a consequence of the NLRP3/ASC activation pathway (Figure 2).
DECORIN FOSTERS A PRO-INFLAMMATORY SIGNATURE VIA TLR2/4
Several lines of evidence have converged on the concept that decorin may also be involved in regulating immunological responses in a manner congruent with biglycan5,104. Decorin suppresses TGF-β, thereby repressing the macrophage phenotype via p27Kip1 and p21WAF1 induction105, promotes synthesis of the potent chemoattractant MCP-1106, and potentiates IFN-γ for allergen-induced inflammation107. In a mechanistic equivalence with biglycan function, LPS-induced sepsis greatly induces decorin expression in the plasma as well as in the perivascular regions and in bronchial epithelial cells32, whereas decorin deficiency attenuates the pro-inflammatory state induced by LPS32.
In a manner analogous to its relative, decorin binds TLR2/4 with high affinity on the surface of macrophages (Figure 2). Receptor binding initiates a pro-inflammatory cascade transduced by the MAPKs, ERK1/2 and the SAPK, and by p38 for the synthesis and secretion of TNF-α and IL-12p7032. Decorin also induces PDCD4 (programmed cell death 4), a translational repressor that post-transcriptionally suppresses the anti-inflammatory modulator, IL-1032. During active TGF-β signaling, microRNA-21 (miR-21) is in abundance and decreases levels of PDCD4, thereby permitting high levels of IL-10. Therefore, during LPS-induced sepsis, decorin levels increase and signal via TLR2/4 for TNF-α and IL-12p70 production, while concurrently blocking TGF-β from accessing the TGFβR. The sequestration of TGF-β by decorin circumvents miR-21-mediated repression of PDCD4, thereby enabling PDCD4 to translationally repress IL-1032. The total outcome of this intricate regulatory system advocates for the differential synthesis of pro-inflammatory immunomodulators while concurrently suppressing anti-inflammatory molecules and licenses decorin as a proinflammatory proteoglycan (Figure 2). This mechanism also operates within the tumor microenvironment and creates an inflammatory milieu that combats tumorigenic growth of established tumors via the differential regulation of PDCD4 and miR-21 and the secretion of TNF-α and IL-12p7032. Over-expressing decorin via adenovirus in vivo has provided robust evidence concerning the link among increased decorin abundance, the release of pro-inflammatory regulators, and the significant decrease in tumorigenicity32.
Collectively, these studies reveal entirely new roles for the circulating, soluble forms of biglycan and decorin in regulating and evoking protracted innate immune responses. Therefore, the importance of matrix-derived factors that would otherwise not be considered in the realm of immunology is furthered underscored by the range of homeostatic, physiologically-relevant, and pathobiological processes these dynamic proteoglycans often contribute, coordinate, and stop.
BASEMENT MEMBRANE HEPARAN SULFATE PROTEOGLYCANS INTEGRATE SIGNALING OVER MULTIPLE RECEPTORS
The three best characterized basement membrane HSPGs (perlecan, agrin, and collagen XVIII) are functionally and structurally diverse participants that contribute nanostructural architecture for tissue stability, homeostasis, and tether the pericellular matrix to the cell surfaces6,40. Primarily harboring heparan sulfate (HS) glycosaminoglycan chains, these HSPGs serve as sinks for multiple cytokines and growth factors6. Further, these elongated and multimodal molecules can act as co-receptors via HS-mediated presentation of growth factors in the proper orientation within three-dimensional space for optimal interactions with the cognate signaling receptor, e.g. FGF2/FGFR2108. Despite maintaining structural integrity as a network in basement membranes and presenting growth factors, perlecan, agrin, and collagen XVIII also signal independently of bound cytokines2,109. Indeed, each member can bind distinct and multiple cell surface receptors in a variety of tissues and microenvironments (Figure 3). Moreover, aberrant expression contributes significantly during pathobiological processes.
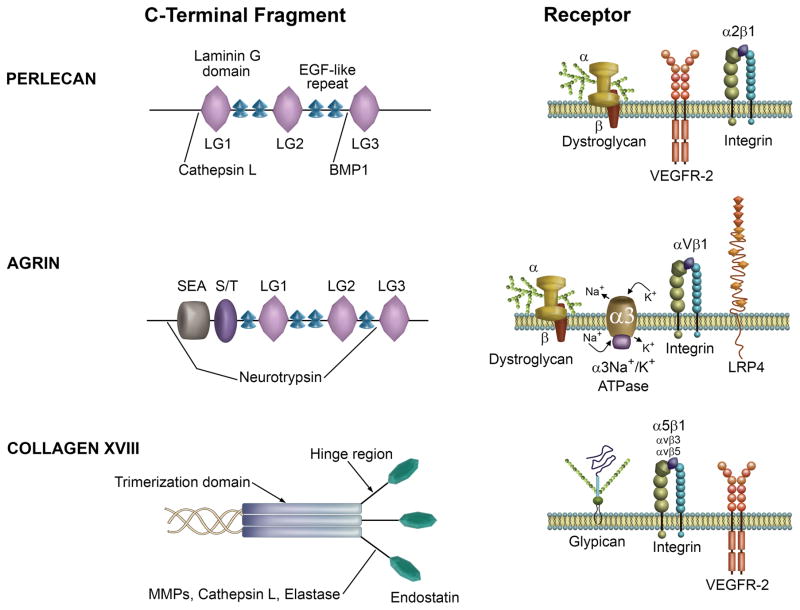
Figure 3.
Basement membrane heparan sulfate proteoglycans ligate multiple receptors for effector function in a variety of cells and tissues. Graphical description of the C-terminal domains of the major basement membrane HSPGs demonstrating modular organization and architecture (left) and a summary of the primary receptors engaged (right). Figure adapted from Iozzo et al 2.
PERLECAN RECEPTORS: A DUAL RECEPTOR ANTAGONISM
Perlecan is a gigantic (470 kDa protein core), multimodular HSPG, comprising five distinct protein modules, that exemplifies angiogenic bivalency by concealing pro- and anti-angiogenic properties within the same molecule2,110,111. Perlecan contributes positively by binding HS-interacting angiokines such as FGF2/7/18112, VEGFA, PDGF, and progranulin113, and operates at multiple levels throughout developmental angiogenesis by modulating the VEGFA/VEGFR2 axis and α2β1 integrin function114,115. Perlecan can also exert biomechanical anti-thrombotic properties116–119. Perlecan-null mice embryos are embryonic lethal and exhibit pericardial hemorrhage and deficits of the major cardiac vessels120,121. Seemingly, morpholino-mediated knockdown of perlecan in zebrafish shows lack of angiogenesis and skeletal muscle defects122. In solid tumors, perlecan also contributes positively as a pro-angiogenic store of potent angiokines for rampant neovascularization via potentiated FGF2 and VEGFA signaling resulting in unchecked tumorigenic growth and spreading2.
As a genuine diametric opposite, the most C-terminal domain of perlecan, known as endorepellin, confers potent inhibitory properties upon endothelial cells by specifically repelling (hence the eponym) migration and blunting capillary morphogenesis123 (Figure 3). Exposure of recombinant endorepellin to stationary endothelial cells, both micro- and macro-vascular, disrupts the actin cytoskeleton and decreases β-actin levels downstream of the α2β1 integrin124,125 thereby providing a mechanism for its ability to block endothelial cell migration. Structurally, endorepellin recapitulates the modular architecture of the parent molecule by containing three laminin-like globular domains (LG1, LG2, LG3) each interspersed by tandem EGF-like motifs (Figure 3). Proteolytic cleavage of endorepellin from the parent perlecan molecule occurs following release of active cathepsin L from dying endothelial cells N-terminal to LG119,126. However, in mast cells, evidence exists for a co-transcriptional mechanism involving alternative splicing of the primary perlecan mRNA transcript that ultimately yields functional endorepellin for regulating angiogenesis and wound healing127.
Upon liberation, soluble endorepellin engages in a novel form of suppression known as “dual receptor antagonism” and functions as a molecular bridge by ligating the α2β1 integrin and VEGFR2128 for angiostasis. Simultaneous interaction of both receptors conveys high sensitivity, cell type specificity, and wholly nullifies angiogenic responses in vitro128 and in vivo by specific targeting of the tumor vasculature via the α2β1 integrin129,130, the only cell type that expresses both receptors. Moreover, the BMP-1/Tolloid protease can release the bioactive fragment, LG3131, whose three-dimensional crystal structure has been solved132. The concept of “dual receptor antagonism” is also exhibited by other proteolytically processed matrix components that yield soluble effectors (e.g. endostatin from collagen XVIII, see below) for transducing and activating signaling programs fundamental for cellular behavior.
Physically, endorepellin interacts with VEGFR2 (between IgG domains 3–5 with an empirically derived Kd of 2.8 nM and in a region that overlaps with VEGFA binding) via the N-terminal LG1/2 domains while recruiting and binding the α2β1 integrin with the C-terminal LG3 module via the α2βI domain133. Mechanistically, this tethering brings the SHP-1 phosphatase, which is putatively bound to the cytoplasmic tail of the α2 integrin subunit, into functional proximity with the intracellular tails of VEGFR2 and executes rapid VEGFR2 dephosphorylation, inactivation, and ternary complex internalization128,134,135 consisting of endorepellin, VEGFR2 and the α2β1 integrin. Importantly, SHP-1 catalyzes dephosphorylation of Tyr1175 of VEGFR2136, a critical docking site for Shb and PLC-γ (see below)135. Biologically, dual receptor antagonism permits actin dissolution via the α2β1 integrin, and angiogenic suppression via the down-regulation of VEGFR2110. Downstream of simultaneous VEGFR2/α2β1 binding and attenuation, multiple pro-angiogenic signaling pathways chiefly emanating from VEGFR2 are significantly compromised135. As discussed, endorepellin promotes active dephosphorylation, via SHP-1, of Tyr1175 of VEGFR2 thereby disrupting the recruitment, coupling, and activation of the angiogenic PI3K/Akt signaling axis. Impaired Akt (Ser473) signaling downstream of VEGFR2 manifests as blunted activation of PDK (Ser241), eNOS (Ser1177), and mTOR (Ser2448) despite the overabundance of exogenous VEGFA, suggesting that endorepellin competently and allosterically abrogates pro-VEGFA signaling135. The attenuation of mTOR signaling negatively impacted HIF-1α expression (a major downstream effector of mTOR) and activity in an oxygen-independent manner and, unbeknownst at the time, would be involved in a much more sublime manner (see below). Endorepellin also prevents PLC-γ/VEGFR2 docking (via diminished P-Tyr1175), resulting in the protracted attenuation of the PKC/JNK/AP1 signaling arm with concomitant attenuation of calcineurin activity and subsequent cytosolic retention of NFAT135. This dual receptor antagonism suppresses key pathways for VEGFA expression and secretion.
Recently, a novel finding has expanded our understanding of the angiostatic function of endorepellin19. Endorepellin evokes endothelial cell autophagy in a VEGFR2- and Peg3-dependent manner under nutrient-enriched conditions137 characterized by dually positive (e.g. Beclin 1/LC3) autophagosomes and the dynamic regulation of p62/SQSTM1. Perhaps the previous discovery of mTOR suppression (mTOR is staunchly anti-autophagic138) was prescient for endorepellin-evoked autophagy. Partial agonism is a recurring theme with soluble matrix constituents as endorepellin requires VEGFR2 signaling in much the same manner that decorin requires VEGFR2 kinase activity for Peg3-dependent autophagy137. Intriguingly, truncated endorepellin consisting of only LG1/2 was sufficient for Peg3, Beclin 1, and LC3 induction and autophagosome formation that subsequently incorporates these pro-autophagic mediators19,137. Endorepellin solely requires an interaction with VEGFR2 for endothelial cell autophagy; the LG3/α2β1 interaction appears dispensable and perhaps even inhibitive137 (Figure 3). Functionally, distinct signaling pathways are either activated or attenuated following dual receptor antagonism and thereby holds physiological relevance. A molecular dissection of endorepellin revealed a profound dependence on LG1/2 for autophagic induction and angiogenic suppression; however, inhibiting cell motility relies heavily on the α2β1-mediated arm. Therefore, we postulate that endorepellin-mediated prolonged autophagic induction may underlie its ability to suppress angiogenesis.
Clinically, endorepellin is released under physiological conditions139, whereas LG3 is a candidate serological biomarker that has been amply identified under many pathological conditions, including breast cancer and allograft rejection140–145. Moreover, LG3 possesses intrinsic angiostatic abilities via calcium regulation131 through the α2β1 integrin. Additional binding partners of endorepellin include the α/β-dystroglycan complex whereby perlecan146 and agrin147 (see below) are interacting partners148 (Figure 3). Specifically, the proximal LG1/2 modules of endorepellin contain high-affinity binding sites for α/β-dystroglycan binding in maintaining skeletal muscle integrity2 and may have a role in the development of ameloblastoma149. Disrupting perlecan:α/β-dystroglycan interactions compromises basement membrane stability150. Recently, it was discovered that perlecan is recruited to the nodes of Ranvier and participates in rapid neural conduction151. Dystroglycan selectively recruits perlecan as a novel component of the nodal matrix and is involved in nodogenesis via gliomedin clustering151 (Figure 3). Overall, these findings propose a novel role for protein core fragments of basement membrane HSPGs in concurrently affecting cell adhesion and pro-angiogenic signaling receptors. The convergence of signaling towards a pro-autophagic pathway could exacerbate the growth of vascular cells and thus contributes to the angiostatic properties of endorepellin and perhaps other bioactive protein modules such as endostatin (see below).
AGRIN: A SYNAPTIC PROTEOGLYCAN THAT ENGAGES A DIVERSE ARRAY OF RECEPTORS
Structurally, agrin appears similar to the aforementioned proteoglycan, perlecan. Agrin is a multimodular HSPG with up to three HS that are apparently dispensable for function152. Agrin protein core has an additional complexity conveyed by alternative splicing of the AGRN mRNA153. Substitution of the N-terminal domain of agrin via an alternatively-spliced mRNA converts agrin into a type-II transmembrane proteoglycan154 and can modulate Fyn and MAPK signaling pathways155. Chiefly involved in forming and maintaining homeostasis of neural and neuromuscular synapses156, agrin can also modulate neurite and motor neuron outgrowth via FGF2 binding157,158, and retinal development159. In combination with perlecan, agrin promotes oral squamous cell carcinoma160, and also accumulates in HCC161 with dynamic expression in cholangiocarcinoma162.
Agrin can be proteolytically cleaved by MMPs 163 and by the serine protease neurotrypsin immediately upstream of the SEA domain and distally between LG2 and LG3164,165 (Figure 3). Processing generates 110-, 90-, and 22-kDa fragments of agrin 2. Intriguingly, the processed fragments flanked by the neurotrypsin sites bear striking resemblance to the C-terminal perlecan fragment, endorepellin (Figure 3, see above section), which also harbor the majority of the interaction sites (similar to perlecan and collagen XVIII, see below). Intact or proteolytically-processed agrin interacts with several receptors with the caveat that specific splice variants of agrin bind specific cell surface receptors2,166,167. Agrin is an avid binding partner of the critical cell adhesion glycoprotein α/β-dystroglycan in muscle and non-muscle tissues alike147. Mutations that disrupt or result in inappropriate glycosylation of the membrane-localized α/β-dystroglycan complex have been implicated in a broad spectrum (ranging from mild to severe) of muscular dystrophies. The formation of the α/β-dystroglycan heterodimer represents the fundamental component responsible for linking extracellular matrix constituents (e.g. perlecan, agrin, laminin) with dystrophin. These interactions primarily occur via carbohydrate moieties appended to the α/β-dystroglycan core complex by LARGE, which is necessary for proper α/β-dystroglycan function168. This interaction is obligatory for agrin-mediated clustering of acetylcholinesterase at neuromuscular junctions169 in conjunction with perlecan. Recently, agrin-binding dystroglycan has been implicated in promoting synaptic plasticity and specialized GABAergic synapses170. Further, α/β-dystroglycan exhibits strong affinity with a large stretch of the C-terminal portion of agrin that has been alternatively spliced in a manner that excludes the Y and Z inserts171. These inserts, if present, negatively regulate association of agrin with α/β-dystroglycan171. Agrin also interacts with the α/β-dystroglycan receptor in the formation of immunological synapses with lymphocytes and aids in activation172 as well as maintaining monocyte cell survival downstream in an α-dystroglycan dependent manner173.
A second receptor for agrin has been identified as the α3Na+/K+ ATPase, which functions primarily as a neuronal ion pump for maintaining proper membrane potential174 (Figure 3). Agrin binding α3Na+/K+ ATPase (interestingly, resting neuronal synapses harbor a small percentage of agrin/α3Na+/K+ ATPase complexes) on pre- and post-synaptic neurons inhibits ion pump activity and results in a net loss of membrane polarization and a corresponding increase in neuronal action potential174. Since cardiac tissue expresses both agrin and the α3Na+/K+ ATPase, it is more probable that agrin has a role in cardiac pathology (e.g. congestive heart failure) via the pro-inotropic effects of agrin-mediated α3Na+/K+ ATPase modulation174. A third receptor, the αVβ1 integrin, binds the LG2 domain of agrin and aids in proper synaptic localization of agrin (Figure 3)175. The αVβ1 integrin was discovered in a screen for interacting agrin partners, which revealed that αVβ1 binds the second of three LG (LG2) domains. Further, agrin also contains a distinct α1 interaction motif in the last EGF (EGF4) repeat175. The interaction of agrin with neurons is seemingly integrin and divalent cation (Mg2+) dependent as EDTA and monoclonal blocking antibodies directed against these sites substantially abrogated neuronal adhesion to agrin175. Moreover, αVβ1 modulates the ability of agrin to appropriately cluster AChR on the surface of myotubes at neuromuscular junctions, orchestrates cation-coordination for proper cell-matrix adhesion, and may also fine-tune agrin-directed neurite outgrowth176.
A fourth receptor, LRP4 (low-density lipoprotein receptor-related protein 4) is the agrin receptor responsible for MuSK (muscle-specific receptor tyrosine kinase) phosphorylation that permits appropriate AChR clustering at the neuromuscular junction177 (Figure 3). Unlike binding α/β-dystroglycan, the Z-insert and the C-most terminal LG domain (LG3) are sufficient for LRP4 binding and downstream activation of MuSK177. Using SILAC quantitative proteomics, a role of the agrin/LRP4/MuSK signaling axis has been determined as a driving oncogenic force in the development of hepatocellular carcinoma178.
Unfortunately, the downstream signaling effectors for the agrin receptors remain elusive and very poorly understood for this versatile proteoglycan. Deciphering the downstream apparati will open novel therapeutic avenues for mitigating agrin pathologies (e.g. muscular dystrophies). However, despite this lack of basic signal transduction knowledge, the agrin LG3 domain has been used as a biomarker, akin with the LG3 domain of perlecan as a serological marker, as a detection method of prematurely ruptured fetal membranes179.
COLLAGEN XVIII: A UNIQUE HSPG THAT HARBORS ENDOSTATIN
Collagen XVIII is a unique HSPG180 that contains ten interrupted collagenous domains flanked by non-collagenous regions localized at the N- and C-termini2. Collagen XVIII, member of the multiplexin gene family181, is a homotrimer comprising three identical α1 chains, encoded by the COL18A1 gene. It also harbors consensus sites for the attachment of HS chains182. Despite the multifactorial nature of this particular proteoglycan and the nearly global expression pattern of Col18a1 within vascular basement membranes, mice lacking collagen XVIII are not embryonic lethal and are fertile183 suggesting it does not play a role in developmental vasculogenesis or angiogenesis. However, genetic ablation of Col18a1 disrupts the structural integrity of the choroid plexus basement membrane and results in hydrocephaly184. Lack of Col18a1 also causes hypertriglyceridemia in mice and humans185 given modulation of LDL complexes within the subendothelial matrix via endostatin (see below)186. Collagen XVIII is required for proper eye development2,183, vision, and retinal pigment function187. Moreover, collagen XVIII may have very specific and context-dependent roles for regulating angiogenesis188 and tumor growth183. However, during HCC development, collagen XVIII expression is decreased189.
Despite not having a large role in developmental angiogenesis and seemingly not observing any enhanced tumorigenic growth upon genetic inactivation, collagen XVIII harbors a potent anti-angiogenic inhibitor, endostatin190–192 that completely neutralizes tumorigenic growth in various models191,193. As mentioned above, collagen XVIII is composed of three identical α1 chains. Each chain has an NC1 (non-collagenous 1) region that includes the trimerization domain, various sites sensitive to MMPs194, cathepsin L195, and elastase2,196, a hinge region, and the most C-terminal endostatin domain (Figure 3)197–199. Endostatin orchestrates anti-angiogenic activities in a zinc-dependent manner200,201.
Mechanistically, endostatin binds multiple endothelial cell-specific integrins (e.g. α5β1, αVβ3, αVβ5)202,203, and exhibits low affinity interactions for glypicans204. In analogy to the bioactivity of endorepellin, endostatin also binds VEGFR2205 (Figure 3) and can suppress VEGFA and Myc expression199,206. Ligation of endostatin with the cognate receptor results in a total hijacking of the malignant gene expression programs and counteracts tumorigenicity by reprogramming the responsive cells that ultimately interfere with endothelial cell migration and survival. As an additional mechanism of endothelial cell regulation, endostatin is one of a newly emergent class of proteoglycans that regulates autophagy19,207 in an α5β1-dependent manner207, resulting in apoptosis208. Indeed, loss of Beclin 1 exacerbates hypoxia-driven angiogenesis209 while the induction of autophagy, in turn, inhibits tumorigenesis in a Beclin 1-dependent manner 210. Therefore, endostatin mediated autophagy, via Beclin 1 function, may ameliorate hypoxia-driven angiogenesis in an autophagy-dependent manner.
Collectively, these studies stress an important concept, that is, fragments of basement membrane HSPGs, which are located close to the plasma membrane, are in constant dialogue with signaling receptors and their liberation from a matrix-bound state during tissue remodeling would have a strong effect on cell behavior.
CONCLUSIONS
Our aggregate knowledge concerning the underlying fundamental molecular and cellular mechanisms governed by interactions between soluble matrix constituents and cell surface receptors is growing at an exponential rate. Since the initial pioneering discovery that a considerable fraction of decorin is soluble and engages EGFR via high-affinity interactions and compromises oncogenic intracellular signaling, numerous similar paradigms have emerged as functional explanations for a variety of biological phenotypes6. The most recent example is lumican, a class II SLRP implicated in cancer and angiogenesis211, which promotes wound healing via direct ALK5 (TGFβ receptor 1) binding and activation212. By integrating signaling among multiple receptors, where each receptor is differentially expressed, cognizant of the cell- and tissue-specific resident microenvironment, and the empirically computed differential binding constants, licenses decorin (and related SLRPs) with an ability to affect a wide variety of processes. The perceived promiscuity of decorin is an inherent and critical facet of decorin (and related SLRPs) biology. We should point out that Myc is capable of regulating 1,500 genes and this ability is widely accepted in the literature. We propose that some matrix proteoglycans, because of their structure endowed with leucine-rich repeats, are designed to interact with other proteins and several RTKs, many of which contain Ig-like repeats (VEGFR2, etc.) or leucine-rich repeats (TLR2/4). Canonically, decorin has been viewed as an obligate antagonistic ligand for the EGFR and Met for tumorigenic and angiogenic suppression16,50. With the new dawn that decorin evokes endothelial cell autophagy and tumor cell mitophagy by interacting with VEGFR2 and Met, respectively, the effector landscape has shifted and decorin seemingly acts as a partial agonist for autophagic-mediated tumorigenic and angiogenic abrogation14,18,80. Mounting evidence suggests the requirement of the tyrosine kinase domain for proficient signal transduction and autophagic and mitophagic induction and ensuing angiostasis. Moreover, the case of IGF-IR epitomizes the concept of context- and environmental-dependent signaling in normal, genomically stable cells when compared to transformed cells.
Decorin has long been heralded as a pan-RTK inhibitor that potently opposes tumorigenesis and angiogenesis in a multitude of solid tumors. However, in analogy with biglycan, decorin engages the innate immune receptors TLR2/4 and evokes a proinflammatory phenotype. Indeed, biglycan and decorin have been implicated in regulating immune responses during ischemic acute renal diseases, fibrosis, and tumorigenesis9. Indeed, generating a pro-inflammatory milieu within the tumor stroma appears as an additional layer of activity for the tumoricidal functions of decorin. Interestingly, transcriptome wide analysis of basal breast carcinoma tumor xenografts following systemic administration of decorin protein core has shown a suppression of multiple immunoregulatory genes within the tumor stroma51. Conversely, soluble biglycan signaling via LRP6 potentiates canonical Wnt signaling and may contribute to enhance cancer growth and progression via β-catenin-driven tumorigenesis.
Lastly, the pericellular matrix, responsible for organizing and maintaining the basement membrane structure and reliability in a multitude of tissues, directly participates in regulating centrally conserved cellular processes such as synaptogenesis, nodogenesis, autophagy80, angiogenesis, and tumorigenesis. Perlecan, agrin, and collagen XVIII are unique insofar as being multifunctional effectors that are proteolytically processed and yield modular effector units2. These soluble fragments can bind multiple receptors as diverse as integrins, glypicans, and RTKs for exercising their inherent biological functions across an array of tissues and maladies. Commanding a comprehensive understanding of the intricacies of proteoglycan signaling via this specialized, but ever-expanding, community of proteoglycan receptors will permit more personalized and targeted therapeutic options that will effectively combat our most insidious and devastating diseases.
Acknowledgments
Funding Source Statement: The original research was supported in part by National Institutes of Health Grants RO1 CA39481, RO1 CA47282 and RO1 CA164462 (R.V.I.), and by grants from the German Research Council SFB 815, project A5, SFB 1039, project B2, and Excellence Cluster ECCPS (L.S.).
We thank all the past and present members of our laboratories and apologize for not referencing many valuable contributors because of space limitation.
ABBREVIATIONS
- SLRP
small leucine-rich proteoglycan
- RTK
receptor tyrosine kinase
- HUVEC
human umbilical vein endothelial cells
- ECM
extracellular matrix
- HS
heparan sulfate
- HSPG
HS proteoglycan
- EGF
epidermal growth factor
- EGFR
EGF receptor
- IGF-I
insulin like growth factor I
- IGF-IR
IGF-I receptor
- VEGFA
vascular endothelial cell growth factor A
- VEGFR2
VEGF receptor 2
- PDGFR
platelet-derived growth factor receptor
- TLR
toll-like receptor
- LPS
lipopolysaccharide
- PDCD4
programmed cell death 4
- MAPK
mitogen activated protein kinase
- LRP6
Low-density lipoprotein receptor-related protein 6
- ERK1/2
extracellular regulated kinase 1/2
- SAPK
stress activated protein kinase
- mTOR
mammalian target of rapamycin
- PI3K
phosphoinositidyl-3-kinase
- HIF-1α
hypoxia inducible factor-1α
- NFAT
nuclear factor of activated T-cell
- NFκB
nuclear factor kappa B
- MMP
matrix metalloproteinase
- TSP-1
Thrombospondin-1
- TIMP-3
tissue inhibitor of metalloproteinase-3
- AMPKα
AMP-activated protein kinase α subunit
- DAMP
damage associated molecular patterns
- MCP-1
monocyte chemoattractant protein-1
- SHP-1
Src homology region 2 domain-containing phosphatase-1
- LRP4
Low-density lipoprotein receptor-related protein 4
- MuSK
muscle-specific receptor tyrosine kinase
- AChR
acetylcholinesterase receptor
- HCC
hepato-cellular carcinoma
- LRP-1
lipoprotein receptor-related protein 1
References
- 1.Hynes RO. Cell-matrix adhesion in vascular development. J Thromb Haemost. 2007;5(Suppl 1):32–40. doi: 10.1111/j.1538-7836.2007.02569.x. [DOI] [PubMed] [Google Scholar]
- 2.Iozzo RV, Zoeller JJ, Nyström A. Basement membrane proteoglycans: Modulators par excellence of cancer growth and angiogenesis. Mol Cells. 2009;27:503–513. doi: 10.1007/s10059-009-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iozzo RV, Sanderson RD. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J Cell Mol Med. 2011;15:1013–1031. doi: 10.1111/j.1582-4934.2010.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldoni S, Iozzo RV. Tumor microenvironment: Modulation by decorin and related molecules harboring leucine-rich tandem motifs. Int J Cancer. 2008;123:2473–2479. doi: 10.1002/ijc.23930. [DOI] [PubMed] [Google Scholar]
- 5.Merline R, Nastase MV, Iozzo RV, Schaefer L. Small Leucine-rich proteoglycans: multifunctional signaling effectors. In: Karamanos N, editor. Extracellular Matrix: Pathobiology and signaling. Berlin: Walter de Gruytier Gmbh and Co; 2012. pp. 185–196. [Google Scholar]
- 6.Iozzo RV, Schaefer L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iozzo RV, Goldoni S, Berendsen A, Young MF. Small leucine-rich proteoglycans. In: Mecham RP, editor. Extracellular Matrix: An overview. Springer; 2011. pp. 197–231. [Google Scholar]
- 8.Schaefer L. Proteoglycans, key regulators of cell-matrix dynamics. Matrix Biol. 2014;35:1–2. doi: 10.1016/j.matbio.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Anders HJ, Schaefer L. Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J Am Soc Nephrol. 2014;25:1387–1400. doi: 10.1681/ASN.2014010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bilandzic M, Wang Y, Ahmed N, Luwor RB, Zhu HJ, Findlay JK, Stenvers KL. Betaglycan blocks metastatic behaviors in human granulosa cell tumors by suppressing NFkappaB-mediated induction of MMP2. Cancer Lett. 2014;354:107–114. doi: 10.1016/j.canlet.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 11.Goldoni S, Owens RT, McQuillan DJ, Shriver Z, Sasisekharan R, Birk DE, Campbell S, Iozzo RV. Biologically active decorin is a monomer in solution. J Biol Chem. 2004;279:6606–6612. doi: 10.1074/jbc.M310342200. [DOI] [PubMed] [Google Scholar]
- 12.Iozzo RV, Moscatello D, McQuillan DJ, Eichstetter I. Decorin is a biological ligand for the epidermal growth factor receptor. J Biol Chem. 1999;274:4489–4492. doi: 10.1074/jbc.274.8.4489. [DOI] [PubMed] [Google Scholar]
- 13.Theocharis AD, Tzanakakis G, Karamanos NK. Proteoglycans in health and disease: Novel proteoglycan roles in malignancy and their pharmacological targeting. FEBS J. 2010;277:3904–3923. doi: 10.1111/j.1742-4658.2010.07800.x. [DOI] [PubMed] [Google Scholar]
- 14.Neill T, Schaefer L, Iozzo RV. Decorin, a guardian from the matrix. Am J Pathol. 2012;181:380–387. doi: 10.1016/j.ajpath.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem. 2008;283:21305–21309. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iozzo RV, Schaefer L. Proteoglycans in health and disease: Novel regulatory signaling mechanisms evoked by the small leucine-rich proteoglycans. FEBS J. 2010;277:3864–3875. doi: 10.1111/j.1742-4658.2010.07797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iozzo RV, Karamanos N. Proteoglycans in health and disease: emerging concepts and future directions. FEBS J. 2010;277:3863. doi: 10.1111/j.1742-4658.2010.07796.x. [DOI] [PubMed] [Google Scholar]
- 18.Neill T, Schaefer L, Iozzo RV. An oncosuppressive role for decorin. Mol Cell Oncol. 2015;2:e975645. doi: 10.4161/23723556.2014.975645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neill T, Schaefer L, Iozzo RV. Instructive roles of extracellular matrix on autophagy. Am J Pathol. 2014;184:2146–2153. doi: 10.1016/j.ajpath.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neill T, Torres AT, Buraschi S, Iozzo RV. Decorin has an appetite for endothelial cell autophagy. Autophagy. 2013;9:1626–1628. doi: 10.4161/auto.25881. [DOI] [PubMed] [Google Scholar]
- 21.Reese SP, Underwood CJ, Weiss JA. Effects of decorin proteoglycan on fibrillogenesis, ultrastructure, and mechanics of type I collagen gels. Matrix Biol. 2013;32:414–423. doi: 10.1016/j.matbio.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Järveläinen H, Puolakkainen P, Pakkanen S, Brown EL, Höök M, Iozzo RV, Sage H, Wight TN. A role for decorin in cutaneous wound healing and angiogenesis. Wound Rep Reg. 2006;14:443–452. doi: 10.1111/j.1743-6109.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- 23.Baghy K, Iozzo RV, Kovalszky I. Decorin-TGFβ axis in hepatic fibrosis and cirrhosis. J Histochem Cytochem. 2012;60:262–268. doi: 10.1369/0022155412438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikolovska K, Renke JK, Jungmann O, Grobe K, Iozzo RV, Zamfir AD, Seidler DG. A decorin-deficient matrix affects skin chondroitin/dermatan sulfate levels and keratinocyte function. Matrix Biol. 2014;35:91–102. doi: 10.1016/j.matbio.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchica CL, Pinelli V, Borges M, Zummer J, Narayanan V, Iozzo RV, Ludwig MS. A role for decorin in a murine model of allergen-induced asthma. Am J Physiol Lung Cell Mol Physiol. 2011;300:863–873. doi: 10.1152/ajplung.00300.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seidler DG, Mohamed NA, Bocian C, Stadtmann A, Hermann S, Schäfers K, Schäfers M, Iozzo RV, Zarbock A, Götte M. The role for decorin in delayed-type hypersensitivity. J Immunol. 2011;187:6108–6199. doi: 10.4049/jimmunol.1100373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merline R, Lazaroski S, Babelova A, Tsalastra-Greul W, Pfeilschifter J, Schluter KD, Gunther A, Iozzo RV, Schaefer RM, Schaefer L. Decorin deficiency in diabetic mice: aggravation of nephropathy due to overexpression of profibrotic factors, enhanced apoptosis and mononuclear cell infiltration. J Physiol Pharmacol. 2009;60(suppl 4):5–13. [PMC free article] [PubMed] [Google Scholar]
- 28.Hsieh LT, Nastase MV, Zeng-Brouwers J, Iozzo RV, Schaefer L. Soluble biglycan as a biomarker of inflammatory renal diseases. Int J Biochem Cell Biol. 2014;54C:223–235. doi: 10.1016/j.biocel.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandan E, Gutierrez J. Role of skeletal muscle proteoglycans during myogenesis. Matrix Biol. 2013;32:289–297. doi: 10.1016/j.matbio.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Ichii M, Frank MB, Iozzo RV, Kincade PW. The canonical Wnt pathway shapes niches supportive of hematopoietic stem/progenitor cells. Blood. 2012;119:1683–1692. doi: 10.1182/blood-2011-07-369199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zoeller JJ, Pimtong W, Corby H, Goldoni S, Iozzo AE, Owens RT, Ho SY, Iozzo RV. A central role for decorin during vertebrate convergent extension. J Biol Chem. 2009;284:11728–11737. doi: 10.1074/jbc.M808991200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merline R, Moreth K, Beckmann J, Nastase MV, Zeng-Brouwers J, Tralhão JG, Lemarchand P, Pfeilschifter J, Schaefer RM, Iozzo RV, Schaefer L. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and microRNA-21. Sci Signal. 2011;4:ra75. doi: 10.1126/scisignal.2001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frey T, Schroeder N, Manon-Jensen T, Iozzo RV, Schaefer L. Biological interplay between proteoglycans and their innate immune receptors in inflammation. FEBS J. 2013;280:2165–2179. doi: 10.1111/febs.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng-Brouwers J, Beckmann J, Nastase MV, Iozzo RV, Schaefer L. De novo expression of circulating biglycan evokes an innate inflammatory tissue response via MyD88/TRIF pathways. Matrix Biol. 2014;35:132–142. doi: 10.1016/j.matbio.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunkman AA, Buckley MR, Mienaltowski MJ, Adams SM, Thomas SJ, Kumar A, Beason DP, Iozzo RV, Birk DE, Soslowsky LJ. The injury response of aged tendons in the absence of biglycan and decorin. Matrix Biol. 2014;35:232–238. doi: 10.1016/j.matbio.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunkman AA, Buckley MR, Mienaltowski MJ, Adams SM, Thomas SJ, Satchell L, Kumar A, Pathmanathan L, Beason DP, Iozzo RV, Birk DE, Soslowsky LJ. Decorin expression is important for age-related changes in tendon structure and mechanical properties. Matrix Biol. 2013;32:3–13. doi: 10.1016/j.matbio.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen SC, Young MF, Chakravarti S, Birk DE. Interclass small leucine-rich repeat proteoglycan interactions regulate collagen fibrillogenesis and corneal stromal assembly. Matrix Biol. 2014;35:103–111. doi: 10.1016/j.matbio.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Z, Horgan CE, Carr O, Owens RT, Iozzo RV, Lechner BE. Biglycan and decorin differentially regulate signaling in the fetal membranes. Matrix Biol. 2014;35:266–275. doi: 10.1016/j.matbio.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Listrat A, Meunier B, Gueugneau M, Coudy-Gandilhon C, Combaret L, Taillandier D, Polge C, Attaix D, Lethias C, Lee K, Goh KL, Bechet D. Apoptosis in capillary endothelial cells in ageing skeletal muscle. Aging Cell. 2014;13:254–262. doi: 10.1111/acel.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iozzo RV. Basement membrane proteoglycans: from cellar to ceiling. Nat Rev Mol Cell Biol. 2005;6:646–656. doi: 10.1038/nrm1702. [DOI] [PubMed] [Google Scholar]
- 41.Whitelock JM, Melrose J, Iozzo RV. Diverse cell signaling events modulated by perlecan. Biochemistry. 2008;47:11174–11183. doi: 10.1021/bi8013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuki IV, Kuhn KM, Lomazov IR, Rothman VL, Tuszynski GP, Iozzo RV, Swenson TL, Fisher EA, Williams KJ. The syndecan family of proteoglycans. Novel receptors mediating internalization of atherogenic lipoproteins in vitro. J Clin Invest. 1997;100:1611–1622. doi: 10.1172/JCI119685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fuki I, Iozzo RV, Williams KJ. Perlecan heparan sulfate proteoglycan. A novel receptor that mediates a distinct pathway for ligand catabolism. J Biol Chem. 2000;275:25742–25750. doi: 10.1074/jbc.M909173199. [DOI] [PubMed] [Google Scholar]
- 44.Christianson HC, Belting M. Heparan sulfate proteoglycan as a cell-surface endocytosis receptor. Matrix Biol. 2014;35:51–55. doi: 10.1016/j.matbio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 2006;98:1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- 46.Zhang G, Chen S, Goldoni S, Calder BW, Simpson HC, Owens RT, McQuillan DJ, Young MF, Iozzo RV, Birk DE. Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan. J Biol Chem. 2009;284:8888–8897. doi: 10.1074/jbc.M806590200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen S, Sun M, Meng X, Iozzo RV, Kao WWY, Birk DE. Pathophysiological mechanisms of autosomal dominant congenital stromal corneal dystrophy. C-terminal-truncated decorin results in abnormal matrix assembly and altered expression of small leucine-rich proteoglycans. Am J Pathol. 2011;179:2409–2419. doi: 10.1016/j.ajpath.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen S, Birk DE. The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly. FEBS J. 2013;280:2120–2137. doi: 10.1111/febs.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cabello-Verrugio C, Brandan E. A novel modulatory mechanism of transforming growth factor-β signaling through decorin and LRP-1. J Biol Chem. 2007;282:18842–18850. doi: 10.1074/jbc.M700243200. [DOI] [PubMed] [Google Scholar]
- 50.Buraschi S, Pal N, Tyler-Rubinstein N, Owens RT, Neill T, Iozzo RV. Decorin antagonizes Met receptor activity and downregulates β-catenin and Myc levels. J Biol Chem. 2010;285:42075–42085. doi: 10.1074/jbc.M110.172841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buraschi S, Neill T, Owens RT, Iniguez LA, Purkins G, Vadigepalli R, Evans B, Schaefer L, Peiper SC, Wang Z, Iozzo RV. Decorin protein core affects the global gene expression profile of the tumor microenvironment in a triple-negative orthotopic breast carcinoma xenograft model. PLoS ONE. 2012;7:e45559. doi: 10.1371/journal.pone.0045559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neill T, Painter H, Buraschi S, Owens RT, Lisanti MP, Schaefer L, Iozzo RV. Decorin antagonizes the angiogenic network. Concurrent inhibition of Met, hypoxia inducible factor-1α and vascular endothelial growth factor A and induction of thrombospondin-1 and TIMP3. J Biol Chem. 2012;287:5492–5506. doi: 10.1074/jbc.M111.283499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel S, Santra M, McQuillan DJ, Iozzo RV, Thomas AP. Decorin activates the epidermal growth factor receptor and elevates cytosolic Ca2+ in A431 cells. J Biol Chem. 1998;273:3121–3124. doi: 10.1074/jbc.273.6.3121. [DOI] [PubMed] [Google Scholar]
- 54.Zhu JX, Goldoni S, Bix G, Owens RA, McQuillan D, Reed CC, Iozzo RV. Decorin evokes protracted internalization and degradation of the EGF receptor via caveolar endocytosis. J Biol Chem. 2005;280:32468–32479. doi: 10.1074/jbc.M503833200. [DOI] [PubMed] [Google Scholar]
- 55.Santra M, Reed CC, Iozzo RV. Decorin binds to a narrow region of the epidermal growth factor (EGF) receptor, partially overlapping with but distinct from the EGF-binding epitope. J Biol Chem. 2002;277:35671–35681. doi: 10.1074/jbc.M205317200. [DOI] [PubMed] [Google Scholar]
- 56.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seidler DG, Goldoni S, Agnew C, Cardi C, Thakur ML, Owens RA, McQuillan DJ, Iozzo RV. Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase-3 activation. J Biol Chem. 2006;281:26408–26418. doi: 10.1074/jbc.M602853200. [DOI] [PubMed] [Google Scholar]
- 58.Abulrob A, Giuseppin S, Andrade MF, McDermid A, Moreno M, Stanimirovic D. Interactions of EGFR and caveolin-1 in human glioblastoma cells: evidence that tyrosine phosphorylation regulates EGFR association with caveolae. Oncogene. 2004;23:6967–6979. doi: 10.1038/sj.onc.1207911. [DOI] [PubMed] [Google Scholar]
- 59.Minor KH, Bournat JC, Toscano N, Giger RJ, Davies SJA. Decorin, erythroblastic leukaemia viral oncogene homologue B4 and signal transducer and activator of transcription 3 regulation of semaphorin 3A in central nervous system scar tissue. Brain. 2011;134:1140–1155. doi: 10.1093/brain/awq304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goldoni S, Humphries A, Nyström A, Sattar S, Owens RT, McQuillan DJ, Ireton K, Iozzo RV. Decorin is a novel antagonistic ligand of the Met receptor. J Cell Biol. 2009;185:743–754. doi: 10.1083/jcb.200901129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu W, Neill T, Yang Y, Hu Z, Cleveland E, Wu Y, Hutten R, Xiao X, Stock SR, Shevrin D, Kaul K, Brendler C, Iozzo RV, Seth P. The systemic delivery of an oncolytic adenovirus expressing decorin inhibits bone metastasis in a mouse model of human prostate cancer. Gene Therapy. 2015;22:31–40. doi: 10.1038/gt.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neill T, Jones HR, Crane-Smith Z, Owens RT, Schaefer L, Iozzo RV. Decorin induces rapid secretion of thrombospondin-1 in basal breast carcinoma cells via inhibition of Ras homolog gene family, member A/Rho-associated coiled-coil containing protein kinase 1. FEBS J. 2013;280:2353–2368. doi: 10.1111/febs.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neill T, Torres A, Buraschi S, Owens RT, Hoek J, Baffa R, Iozzo RV. Decorin induces mitophagy in breast carcinoma cells via peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) and mitostatin. J Biol Chem. 2014;289:4952–4968. doi: 10.1074/jbc.M113.512566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller JK, Shattuck D, Ingalla EQ, Yen L, Borowsky AD, Young LJT, Cardiff RD, Carraway KL, III, Sweeney C. Suppression of the negative regulator LRIG1 contributes to ErbB2 overexpression in breast cancer. Cancer Res. 2008;68:8286–8294. doi: 10.1158/0008-5472.CAN-07-6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fassan M, D’Arca D, Letko J, Vecchione A, Gardiman MP, McCue P, Wildemore B, Rugge M, Shupp-Byrne D, Gomella LG, Morrione A, Iozzo RV, Baffa R. Mitostatin is down-regulated in human prostate cancer and suppresses the invasive phenotype of prostate cancer cells. PLoS ONE. 2011;6:e19771. doi: 10.1371/journal.pone.0019771. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Morrione A, Neill T, Iozzo RV. Dichotomy of decorin activity on the insulin-like growth factor-I system. FEBS J. 2013;280:2138–2149. doi: 10.1111/febs.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schönherr E, Sunderkötter C, Iozzo RV, Schaefer L. Decorin, a novel player in the insulin-like growth factor system. J Biol Chem. 2005;280:15767–15772. doi: 10.1074/jbc.M500451200. [DOI] [PubMed] [Google Scholar]
- 68.Iozzo RV, Buraschi S, Genua M, Xu SQ, Solomides CC, Peiper SC, Gomella LG, Owens RT, Morrione A. Decorin antagonizes IGF receptor I (IGF-IR) function by interfering with IGF-IR activity and attenuating downstream signaling. J Biol Chem. 2011;286:34712–34721. doi: 10.1074/jbc.M111.262766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fiedler LR, Schönherr E, Waddington R, Niland S, Seidler DG, Aeschlimann D, Eble JA. Decorin regulates endothelial cell motility on collagen I through activation of Insulin-like growth factor I receptor and modulation of α2β1 integrin activity. J Biol Chem. 2008;283:17406–17415. doi: 10.1074/jbc.M710025200. [DOI] [PubMed] [Google Scholar]
- 70.Brandan E, Cabello-Verrugio C, Vial C. Novel regulatory mechanisms for the proteoglycans decorin and biglycan during muscle formation and muscular dystrophy. Matrix Biol. 2008;27:700–708. doi: 10.1016/j.matbio.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 71.Vial C, Gutierrez J, Santander C, Cabrera D, Brandan E. Decorin interacts with connective tissue growth factor (CTGF)/CCN2 by LRR12 inhibiting its biological activity. J Biol Chem. 2011;286:24242–24252. doi: 10.1074/jbc.M110.189365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schaefer L, Mihalik D, Babelova A, Krzyzankova M, Grone HJ, Iozzo RV, Young MF, Seidler DG, Lin G, Reinhardt D, Schaefer RM. Regulation of fibrillin-1 by biglycan and decorin is important for tissue preservation in the kidney during pressure-induced injury. Am J Pathol. 2004;165:383–396. doi: 10.1016/S0002-9440(10)63305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schaefer L, Tsalastra W, Babelova A, Baliova M, Minnerup J, Sorokin L, Gröne HJ, Reinhardt DP, Pfeilschifter J, Iozzo RV, Schaefer RM. Decorin-mediated regulation of fibrillin-1 in the kidney involves the insulin-like growth factor-1 receptor and mammalian target of rapamycin. Am J Pathol. 2007;170:301–315. doi: 10.2353/ajpath.2007.060497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moreth K, Iozzo RV, Schaefer L. Small leucine-rich proteoglycans orchestrate receptor crosstalk during inflammation. Cell Cycle. 2012;11:2084–2091. doi: 10.4161/cc.20316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Metalli D, Lovat F, Tripodi F, Genua M, Xu SQ, Spinelli M, Alberghina L, Vanoni M, Baffa R, Gomella LG, Iozzo RV, Morrione A. The insulin-like growth factor receptor I promotes motility and invasion of bladder cancer cells through Akt- and mitogen-activated protein kinase-dependent activation of paxillin. Am J Pathol. 2010;176:2997–3006. doi: 10.2353/ajpath.2010.090904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morcavallo A, Buraschi S, Xu SQ, Belfiore A, Schaefer L, Iozzo RV, Morrione A. Decorin differentially modulates the activity of insulin receptor isoform A ligands. Matrix Biol. 2014;35:82–90. doi: 10.1016/j.matbio.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Genua M, Xu SQ, Buraschi S, Peiper SC, Gomella LG, Belfiore A, Iozzo RV, Morrione A. Prolyne-rich tyrosine kinase 2 (Pyk2) regulates IGF-I-induced cell motility and invasion of urothelial carcinoma cells. PLoS ONE. 2012;7:e40148. doi: 10.1371/journal.pone.0040148. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Buraschi S, Neill T, Goyal A, Poluzzi C, Smythies J, Owens RT, Schaefer L, Torres A, Iozzo RV. Decorin causes autophagy in endothelial cells via Peg3. Proc Natl Acad Sci USA. 2013;110:E2582–E2591. doi: 10.1073/pnas.1305732110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goyal A, Neill T, Owens RT, Schaefer L, Iozzo RV. Decorin activates AMPK, an energy sensor kinase, to induce autophagy in endothelial cells. Matrix Biol. 2014;34:46–54. doi: 10.1016/j.matbio.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nussenzweig SC, Verma S, Finkel T. The role of autophagy in vascular biology. Circ Res. 2015;116:480–488. doi: 10.1161/CIRCRESAHA.116.303805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phopshorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Horvath Z, Kovalszky I, Fullar A, Kiss K, Schaff Z, Iozzo RV, Baghy K. Decorin deficiency promotes hepatic carcinogenesis. Matrix Biol. 2014;35:194–205. doi: 10.1016/j.matbio.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baghy K, Horváth Z, Regõs E, Kiss K, Schaff Z, Iozzo RV, Kovalszky I. Decorin interferes with platelet-derived growth factor receptor signaling in experimental hepatocarcinogenesis. FEBS J. 2013;280:2150–2164. doi: 10.1111/febs.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iozzo RV. Proteoglycans and neoplasia. Cancer Metastasis Rev. 1988;7:39–50. doi: 10.1007/BF00048277. [DOI] [PubMed] [Google Scholar]
- 85.Iozzo RV, Chakrani F, Perrotti D, McQuillan DJ, Skorski T, Calabretta B, Eichstetter I. Cooperative action of germline mutations in decorin and p53 accelerates lymphoma tumorigenesis. Proc Natl Acad Sci USA. 1999;96:3092–3097. doi: 10.1073/pnas.96.6.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bi X, Tong C, Dokendorff A, Banroft L, Gallagher L, Guzman-Hartman G, Iozzo RV, Augenlicht LH, Yang W. Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation. Carcinogenesis. 2008;29:1435–1440. doi: 10.1093/carcin/bgn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bi X, Pohl NM, Yang GR, Gou Y, Guzman G, Kajdacsy-Balla A, Iozzo RV, Yang W. Decorin-mediated inhibition of colorectal cancer growth and migration is associated with E-cadherin in vitro and in mice. Carcinogenesis. 2012;33:326–330. doi: 10.1093/carcin/bgr293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hildebrand A, Romaris M, Rasmussen LM, Heinegård D, Twardzik DR, Border WA, Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor β. Biochem J. 1994;302:527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Melchior-Becker A, Dai G, Ding Z, Schafer L, Schrader J, Young MF, Fischer JW. Deficiency of biglycan causes cardiac fibroblasts to differentiate into a myofibroblast phenotype. J Biol Chem. 2011;286:17365–17375. doi: 10.1074/jbc.M110.192682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moreno M, Muñoz R, Aroca F, Labarca M, Brandan E, Larraín J. Biglycan is a new extracellular component of the chordin-BMP4 signaling pathway. EMBO J. 2005;24:1397–1405. doi: 10.1038/sj.emboj.7600615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Berendsen AD, Fisher LW, Kilts TM, Owens RT, Robey PG, Gutkind JS, Young MF. Modulation of canonical Wnt signaling by the extracellular matrix component biglycan. Proc Natl Acad Sci USA. 2011;108:17022–17027. doi: 10.1073/pnas.1110629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Berendsen AD, Pinnow EL, Maeda A, Brown AC, McCartney-Francis N, Kram V, Owens RT, Robey PG, Holmbeck K, de Castro LF, Kilts TM, Young MF. Biglycan modulates angiogenesis and bone formation during fracture healing. Matrix Biol. 2014;35:223–231. doi: 10.1016/j.matbio.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nikitovic D, Aggelidakis J, Young MF, Iozzo RV, Karamanos NK, Tzanakakis GN. The biology of small leucine-rich proteoglycans in bone pathophysiology. J Biol Chem. 2012;287:33926–33933. doi: 10.1074/jbc.R112.379602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Westermann D, Mersmann J, Melchior A, Freudenberger T, Petrik C, Schaefer L, Lüllmann-Rauch R, Lettau O, Jacoby C, Schrader J, Brand-Herrmann SM, Young MF, Schultheiss HP, Levkau B, Baba HA, Unger T, Zacharowski K, Tschöpe C, Fischer JW. Biglycan is required for adaptive remodeling after myocardial infarction. Circulation. 2008;117:1269–1276. doi: 10.1161/CIRCULATIONAHA.107.714147. [DOI] [PubMed] [Google Scholar]
- 95.Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Götte M, Malle E, Schaefer RM, Gröne HJ. The matrix component biglycan is proinflammatory and signals through toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115:2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Babelova A, Moreth K, Tsalastra-Greul W, Zeng-Brouwers J, Eickelberg O, Young MF, Bruckner P, Pfeilschifter J, Schaefer RM, Gröne HJ, Schaefer L. Biglycan, a danger signal that activates the NLRP3 inflammasome via Toll-like and P2X receptors. J Biol Chem. 2009;284:24035–24048. doi: 10.1074/jbc.M109.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moreth K, Frey H, Hubo M, Zeng-Brouwers J, Nastase MV, Hsieh LT, Haceni R, Pfeilschifter J, Iozzo RV, Schaefer L. Biglycan-triggered TLR-2- and TLR-4-signaling exacerbates the pathophysiology of ischemic acute kidney injury. Matrix Biol. 2014;35:143–151. doi: 10.1016/j.matbio.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schaefer L, Iozzo RV. Small leucine-rich proteoglycans, at the crossroad of cancer growth and inflammation. Curr Opin Genet Dev. 2012;22:56–57. doi: 10.1016/j.gde.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 99.Schaefer L. Extracellular matrix molecules: endogenous danger signals as new drug targets in kidney diseases. Curr Opin Pharmacol. 2010;10:185–190. doi: 10.1016/j.coph.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 100.Schaefer L. Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem. 2014;289:35237–35245. doi: 10.1074/jbc.R114.619304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moreth K, Brodbeck R, Babelova A, Gretz N, Spieker T, Zeng-Brouwers J, Pfeilschifter J, Young MF, Schaefer RM, Schaefer L. The proteoglycan biglycan regulates expression of the B cell chemoattractant CXCL13 and aggravates murine lupus nephritis. J Clin Invest. 2010;120:4251–4272. doi: 10.1172/JCI42213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Theocharis AD, Skandalis SS, Neill T, Multhaupt HA, Hubo M, Frey H, Gopal S, Gomes A, Afratis N, Lim HC, Couchman JR, Filmus J, Ralph DS, Schaefer L, Iozzo RV, Karamanos NK. Insights into the key roles of proteoglycans in breast cancer biology and translational medicine. Biochim Biophys Acta. 2015;1855:276–300. doi: 10.1016/j.bbcan.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Recktenwald CV, Leisz S, Steven A, Mimura K, Müller A, Wulfänger J, Kiessling R, Seliger B. HER-2/neu-mediated dosn-regulation of biglycan associated with altered growth properties. J Biol Chem. 2012;287:24320–24329. doi: 10.1074/jbc.M111.334425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Merline R, Schaefer RM, Schaefer L. The matricellular functions of small leucine-rich proteoglycans (SLRPs) J Cell Commun Signal. 2009 doi: 10.1007/s12079-009-0066-2. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xaus J, Comalada M, Cardó M, Valledor AF, Celada A. Decorin inhibits macrophage colony-stimulating factor proliferation of macrophages and enhances cell survival through induction of p27Kip1 and p21Waf1. Blood. 2001;98:2124–2133. doi: 10.1182/blood.v98.7.2124. [DOI] [PubMed] [Google Scholar]
- 106.Köninger J, Giese NA, Bartel M, di Mola FF, Berberat PO, di Sebastiano P, Giese T, Büchler MW, Friess H. The ECM proteoglycan decorin links desmoplasia and inflammation in chronic pancreatitis. J Clin Pathol. 2012;59:21–27. doi: 10.1136/jcp.2004.023135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bocian C, Urbanowitz AK, Owens RT, Iozzo RV, Gotte M, Seidler DG. Decorin potentiates interferon-gamma activity in a model of allergic inflammation. J Biol Chem. 2013;288:12699–12711. doi: 10.1074/jbc.M112.419366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smith SML, West LA, Govindraj P, Zhang X, Ornitz DM, Hassell JR. Heparan and chondroitin sulfate on growth plate perlecan mediate binding and delivery of FGF-2 to FGF receptors. Matrix Biol. 2007;26:175–184. doi: 10.1016/j.matbio.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 109.Lord MS, Chuang CY, Melrose J, Davies MJ, Iozzo RV, Whitelock JM. The role of vascular-derived perlecan in modulating cell adhesion, proliferation and growth factor signaling. Matrix Biol. 2014;35:112–122. doi: 10.1016/j.matbio.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Willis CD, Schaefer L, Iozzo RV. The biology of perlecan and its bioactive modules. In: Karamanos NK, editor. Extracellular Matrix: Pathobiology and Signaling. Berlin: Walter de Gruyter GmbH & Co. KG; 2012. pp. 171–184. [Google Scholar]
- 111.Farach-Carson MC, Warren CR, Harrington DA, Carson DD. Border patrol:Insights into the unique role of perlecan/heparan sulfate proteoglycan 2 at cell and tissue borders. Matrix Biol. 2014;34:64–79. doi: 10.1016/j.matbio.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chuang CY, Lord MS, Melrose J, Rees MD, Knox SM, Freeman C, Iozzo RV, Whitelock J. Heparan sulfate-dependent signaling of fibroblast growth growth factor 18 by chondrocyte-derived perlecan. Biochemistry. 2010;49:5524–5532. doi: 10.1021/bi1005199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gonzalez EM, Mongiat M, Slater SJ, Baffa R, Iozzo RV. A novel interaction between perlecan protein core and progranulin: Potential effects on tumor growth. J Biol Chem. 2003;278:38113–38116. doi: 10.1074/jbc.C300310200. [DOI] [PubMed] [Google Scholar]
- 114.Zoeller JJ, Whitelock J, Iozzo RV. Perlecan regulates developmental angiogenesis by modulating the VEGF-VEGFR2 axis. Matrix Biol. 2009;28:284–291. doi: 10.1016/j.matbio.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.San Antonio JD, Zoeller JJ, Habursky K, Turner K, Pimtong W, Burrows M, Choi S, Basra S, Bennett JS, DeGrado WF, Iozzo RV. A key role for the integrinα2β1 in experimental and developmental angiogenesis. Am J Pathol. 2009;175:1338–1347. doi: 10.2353/ajpath.2009.090234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wilusz RE, DeFrate LE, Guilak F. A biomechanical role for perlecan in the pericellular matrix of articular cartilage. Matrix Biol. 2012;31:320–327. doi: 10.1016/j.matbio.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wilusz RE, Sanchez-Adams J, Guilak F. The structure and function of the pericellular matrix of articular cartilage. Matrix Biol. 2014;39:25–32. doi: 10.1016/j.matbio.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baker AB, Ettenson DS, Jonas M, Nugent MA, Iozzo RV, Edelman ER. Endothelial cells provide feedback control for vascular remodeling through a mechanosensitive autocrine TGF-β signaling pathway. Circ Res. 2008;103:289–297. doi: 10.1161/CIRCRESAHA.108.179465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nugent MA, Nugent HM, Iozzo RV, Sanchack K, Edelman ER. Perlecan is required to inhibit thrombosis after deep vascular injury and contributes to endothelial cell-mediated inhibition of intimal hyperplasia. Proc Natl Acad Sci USA. 2000;97:6722–6727. doi: 10.1073/pnas.97.12.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Costell M, Gustafsson E, Aszódi A, Mörgelin M, Bloch W, Hunziker E, Addicks K, Timpl R, Fässler R. Perlecan maintains the integrity of cartilage and some basement membranes. J Cell Biol. 1999;147:1109–1122. doi: 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Arikawa-Hirasawa E, Watanabe E, Takami H, Hassell JR, Yamada Y. Perlecan is essential for cartilage and cephalic development. Nat Genet. 1999;23:354–358. doi: 10.1038/15537. [DOI] [PubMed] [Google Scholar]
- 122.Zoeller JJ, McQuillan A, Whitelock J, Ho SY, Iozzo RV. A central function for perlecan in skeletal muscle and cardiovascular development. J Cell Biol. 2008;181:381–394. doi: 10.1083/jcb.200708022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mongiat M, Sweeney S, San Antonio JD, Fu J, Iozzo RV. Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan. J Biol Chem. 2003;278:4238–4249. doi: 10.1074/jbc.M210445200. [DOI] [PubMed] [Google Scholar]
- 124.Bix G, Fu J, Gonzalez E, Macro L, Barker A, Campbell S, Zutter MM, Santoro SA, Kim JK, Höök M, Reed CC, Iozzo RV. Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through the α2β1 integrin. J Cell Biol. 2004;166:97–109. doi: 10.1083/jcb.200401150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zoeller JJ, Iozzo RV. Proteomic profiling of endorepellin angiostatic activity on human endothelial cells. Proteome Sci. 2008;6:7. doi: 10.1186/1477-5956-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cailhier JF, Sirois I, Raymond MA, Lepage S, Laplante P, Brassard N, Prat A, Iozzo RV, Pshezhetsky AV, Hebért MJ. Caspase-3 activation triggers extracellular release of cathepsin L and endorepellin proteolysis. J Biol Chem. 2008;283:27220–27229. doi: 10.1074/jbc.M801164200. [DOI] [PubMed] [Google Scholar]
- 127.Jung M, Lord MS, Cheng B, Lyons JG, Alkhouri H, Hughes JM, McCarthy SJ, Iozzo RV, Whitelock JM. Mast cells produce novel shorter forms of perlecan that contain functional endorepellin: A role in angiogenesis and wound healing. J Biol Chem. 2013;288:3289–3304. doi: 10.1074/jbc.M112.387811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Goyal A, Pal N, Concannon M, Paulk M, Doran M, Poluzzi C, Sekiguchi K, Whitelock JM, Neill T, Iozzo RV. Endorepellin, the angiostatic module of perlecan, interacts with both the α2β1 integrin and vascular endothelial growth factor receptor 2 (VEGFR2) J Biol Chem. 2011;286:25947–25962. doi: 10.1074/jbc.M111.243626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bix G, Castello R, Burrows M, Zoeller JJ, Weech M, Iozzo RA, Cardi C, Thakur MT, Barker CA, Camphausen KC, Iozzo RV. Endorepellin in vivo: targeting the tumor vasculature and retarding cancer growth and metabolism. J Natl Cancer Inst. 2006;98:1634–1646. doi: 10.1093/jnci/djj441. [DOI] [PubMed] [Google Scholar]
- 130.Bix G, Iozzo RV. Novel interactions of perlecan: Unraveling perlecan’s role in angiogenesis. Microsc Res. 2008;71:339–348. doi: 10.1002/jemt.20562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gonzalez EM, Reed CC, Bix G, Fu J, Zhang Y, Gopalakrishnan B, Greenspan DS, Iozzo RV. BMP-1/Tolloid-like metalloproteases process endorepellin, the angiostatic C-terminal fragment of perlecan. J Biol Chem. 2005;280:7080–7087. doi: 10.1074/jbc.M409841200. [DOI] [PubMed] [Google Scholar]
- 132.Le BV, Kim H, Choi J, Kim JH, Hahn MJ, Lee C, Kim KK, Hwang HY. Crystal Structure of the LG3 domain of endorepellin, an angiogenesis inhibitor. J Mol Biol. 2011;414:231–242. doi: 10.1016/j.jmb.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 133.Willis CD, Poluzzi C, Mongiat M, Iozzo RV. Endorepellin laminin-like globular repeat 1/2 domains bind Ig3-5 of vascular endothelial growth factor(VEGF) receptor 2 and block pro-angiogenic signaling by VEGFA in endothelial cells. FEBS J. 2013;280:2271–2294. doi: 10.1111/febs.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nyström A, Shaik ZP, Gullberg D, Krieg T, Eckes B, Zent R, Pozzi A, Iozzo RV. Role of tyrosine phosphatase SHP-1 in the mechanism of endorepellin angiostatic activity. Blood. 2009;114:4897–4906. doi: 10.1182/blood-2009-02-207134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Goyal A, Poluzzi C, Willis AC, Smythies J, Shellard A, Neill T, Iozzo RV. Endorepellin affects angiogenesis by antagonizing diverse VEGFR2- evoked signaling pathways: transcriptional repression of HIF-1α and VEGFA and concurrent inhibition of NFAT1 activation. J Biol Chem. 2012;287:43543–43556. doi: 10.1074/jbc.M112.401786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bhattacharya R, Kwon J, Wang E, Mukherjee P, Mukhopadhyay D. Src homology 2 (SH2) domain containing protein tyrosine phosphatase-1 (SHP-1) dephosphorylates VEGF receptor-2 and attenuates endothelial DNA synthesis, but not migration. J Mol Signal. 2008;3:8. doi: 10.1186/1750-2187-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Poluzzi C, Casulli J, Goyal A, Mercer TJ, Neill T, Iozzo RV. Endorepellin evokes autophagy in endothelial cells. J Biol Chem. 2014;289:16114–16128. doi: 10.1074/jbc.M114.556530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Alers S, Löffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: Crosstalk, shortcuts, and feedbacks. Mol Cell Biol. 2012;32:2–11. doi: 10.1128/MCB.06159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.West L, Govindraj P, Koob TJ, Hassell JR. Changes in perlecan during chondrocyte differentiation in the fetal bovine rib growth plate. J Orthop Res. 2006;24:1317–1326. doi: 10.1002/jor.20160. [DOI] [PubMed] [Google Scholar]
- 140.Chang JW, Kang UB, Kim DH, Yi JK, Lee JW, Noh DY, Lee C, Yu MH. Identification of circulating endorepellin LG3 fragment: Potential use as a serological biomarker for breast cancer. Proteomics Clin Appl. 2008;2:23–32. doi: 10.1002/prca.200780049. [DOI] [PubMed] [Google Scholar]
- 141.Parker TJ, Sampson DL, Broszczak D, Chng YL, Carter SL, Leavesley DI, Parker AW, Upton Z. A fragment of the LG3 peptide of endorepellin is present in the urine of physically active mining workers: a potential marker of physical activity. PLoS ONE. 2012;7:e33714. doi: 10.1371/journal.pone.0033714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Saini MG, Bix GJ. Oxygen-glucose deprivation (OGD) and interleukin-1 (IL-1) differentially modulate cathepsin B/L mediated generation of neuroprotective perlecan LG3 by neurons. Brain Res. 2012;1438:65–74. doi: 10.1016/j.brainres.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Saini MG, Pinteaux E, Lee B, Bix GJ. Oxygen-glucose deprivation and interleukin-1α trigger the release of perlecan LG3 by cells of neurovascular unit. J Neurochem. 2011;119:760–771. doi: 10.1111/j.1471-4159.2011.07484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Soulez M, Pilon EA, Dieudé M, Cardinal H, Brassard N, Qi S, Wu SJ, Durocher Y, Madore F, Perreault C, Hébert MJ. The perlecan fragment LG3 is a novel regulator of obliterative remodeling associated with allograft vascular rejection. Circ Res. 2012;110:94–104. doi: 10.1161/CIRCRESAHA.111.250431. [DOI] [PubMed] [Google Scholar]
- 145.Surin B, Sachon E, Rougier JP, Steverlynck C, Garreau C, Lelongt B, Ronco P, Piedagnel R. LG3 fragment of endorepellin is a possible biomarker of severity in lgA nephropathy. Proteomics. 2013;13:142–152. doi: 10.1002/pmic.201200267. [DOI] [PubMed] [Google Scholar]
- 146.Henry MD, Campbell KP. Dystroglycan inside and out. Curr Opin Cell Biol. 1999;11:602–607. doi: 10.1016/s0955-0674(99)00024-1. [DOI] [PubMed] [Google Scholar]
- 147.Gesemann M, Brancaccio A, Schumacher B, Ruegg MA. Agrin is a high-affinity binding protein of dystroglycan in non-muscle tissue. J Biol Chem. 1998;273:600–605. doi: 10.1074/jbc.273.1.600. [DOI] [PubMed] [Google Scholar]
- 148.Hohenester E, Tisi D, Talts JF, Timpl R. The crystal structure of a laminin G-like module reveals the molecular basis of α-dystroglycan binding to laminins, perlecan, and agrin. Mol Cell. 1999;4:783–792. doi: 10.1016/s1097-2765(00)80388-3. [DOI] [PubMed] [Google Scholar]
- 149.Ida-Yonemochi H, Ahsan MS, Saku T. Differential expression profiles between α-dystroglycan and integrin β1 in ameloblastoma: two possible perlecan signalling pathways for cellular growth and differentiation. Histopathology. 2011;58:234–245. doi: 10.1111/j.1365-2559.2010.03732.x. [DOI] [PubMed] [Google Scholar]
- 150.Kanagawa M, Michele DE, Satz JS, Barresi R, Kusano H, Sasaki T, Timpl R, Henry MD, Campbell KP. Disruption of perlecan binding and matrix assembly by post-translational or genetic disruption of dystroglycan function. FEBS Lett. 2005;579:4792–4796. doi: 10.1016/j.febslet.2005.07.059. [DOI] [PubMed] [Google Scholar]
- 151.Colombelli C, Palmisano M, Eshed-Eisenbach Y, Zambroni D, Pavoni E, Ferri C, Saccucci S, Nicole S, Soininen R, McKee KK, Yurchenco PD, Peles E, Wrabetz L, Feltri ML. Perlecan is recruited by dystroglycan to nodes of Ranvier and binds the clustering molecule gliomedin. J Cell Biol. 2015;208:313–329. doi: 10.1083/jcb.201403111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lin S, Maj M, Bezakova G, Magyar JP, Brenner HR, Ruegg MA. Muscle-wide secretion of a miniaturized form of neural agrin rescues focal neuromuscular innervation in agrin mutant mice. Proc Natl Acad Sci USA. 2008;105:11406–11411. doi: 10.1073/pnas.0801683105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Stetefeld J, Alexandrescu AT, Maciejewski MW, Jenny M, Rathgeb-Szabo K, Schulthess T, Landwehr R, Frank S, Ruegg MA, Kammerer RA. Modulation of agrin function by alternative splicing and Ca2+ binding. Structure. 2004;12:503–515. doi: 10.1016/j.str.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 154.Neumann FR, Bittcher G, Annies M, Schumacher B, Kröger S, Ruegg MA. An alternative amino-terminus expressed in the central nervous system converts agrin to a type II transmembrane protein. Mol Cell Neurosci. 2001;17:208–225. doi: 10.1006/mcne.2000.0932. [DOI] [PubMed] [Google Scholar]
- 155.Ramseger R, White R, Kröger S. Transmembrane form agrin-induced process formation requires lipid rafts and the activation of Fyn and MAPK. J Biol Chem. 2009;284:7697–7705. doi: 10.1074/jbc.M806719200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bezakova G, Rüegg MA. New insights into the roles of agrin. Nature Rev Mol Cell Biol. 2003;4:295–308. doi: 10.1038/nrm1074. [DOI] [PubMed] [Google Scholar]
- 157.Kim MJ, Liu IH, Song Y, Lee JA, Halfter W, Balice-Gordon RJ, Linney E, Cole GJ. Agrin is required for posterior development and motor axon outgrowth and branching in embryonic zebrafish. Glycobiology. 2007;17:231–247. doi: 10.1093/glycob/cwl069. [DOI] [PubMed] [Google Scholar]
- 158.Kim MJ, Cotman SL, Halfter W, Cole G. The heparan sulfate proteoglycan agrin modulates neurite outgrowth mediated by FGF-2. Neurobiol. 2003;55:261–277. doi: 10.1002/neu.10213. [DOI] [PubMed] [Google Scholar]
- 159.Liu IH, Zhang C, Kim JM, Cole GJ. Retina development in zebrafish requires the heparan sulfate proteoglycan agrin. Develop Neurobiol. 2008;68:877–898. doi: 10.1002/dneu.20625. [DOI] [PubMed] [Google Scholar]
- 160.Kawahara R, Granato DC, Carnielli CM, Cervigne NK, Oliveria CE, Martinez CA, Yokoo S, Fonseca FP, Lopes M, Santos-Silva AR, Graner E, Coletta RD, Paes Leme AF. Agrin and perlecan mediate tumorigenic processes in oral squamous cell carcinoma. PLoS One. 2014;9:e115004. doi: 10.1371/journal.pone.0115004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tátrai P, Dudás J, Batmunkh E, Máthé M, Zalatnai A, Schaff Z, Ramadori G, Kovalszky I. Agrin, a novel basement membrane component in human rat and liver, accumulates in cirrhosis and hepatocellular carcinoma. Lab Invest. 2006;86:1149–1160. doi: 10.1038/labinvest.3700475. [DOI] [PubMed] [Google Scholar]
- 162.Batmunkh E, Tátrai P, Szabó E, Lódi C, Holczbauer A, Páska C, Kupcsulik P, Kiss A, Schaff Z, Kovalszky I. Comparison of the expression of agrin, a basement membrane heparan sulfate proteoglycan, in cholangiocarcinoma and hepatocellular carcinoma. Human Pathol. 2007;38:1508–1515. doi: 10.1016/j.humpath.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 163.Patel TR, Butler G, McFarlane A, Xie I, Overall CM, Stetefeld J. Site specific cleavage mediated by MMPs regulates function of agrin. PLoS ONE. 2012;7:e43669. doi: 10.1371/journal.pone.0043669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Stephan A, Mateos JM, Kozlov SV, Cinelli P, Kistler AD, Hettwer S, Rülicke T, Streit P, Kunz B, Sonderegger P. Neurotrypsin cleaves agrin locally at the synapse. FASEB J. 2008;22:1861–1873. doi: 10.1096/fj.07-100008. [DOI] [PubMed] [Google Scholar]
- 165.Reif R, Sales S, Hettwer S, Dreier B, Gisler C, Wölfel J, Lüscher D, Zurlinden A, Stephan A, Ahmed S, Baici A, Ledermann B, Kunz B, Sonderegger P. Specific cleavage of agrin by neurotrypsin, a synaptic protease linked to mental retardation. FASEB J. 2007;21:3468–3478. doi: 10.1096/fj.07-8800com. [DOI] [PubMed] [Google Scholar]
- 166.O’Toole JJ, Deyst KA, Bowe MA, Nastuk MA, McKechnie BA, Fallon JA. Alternative splicing of agrin regulates its binding to heparin, α-dystroglycan, and the cell surface. Proc Natl Acad Sci USA. 1996;93:7369–7374. doi: 10.1073/pnas.93.14.7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yamada H, Denzer AJ, Hori H, Tanaka T, Anderson LVB, Fujita S, Fukuta-Oh H, Shimizu T, Ruegg MA, Matsumura K. Dystroglycan is a dual receptor for agrin and laminin-2 in schwann cell membrane. J Biol Chem. 1996;271:23418–23423. doi: 10.1074/jbc.271.38.23418. [DOI] [PubMed] [Google Scholar]
- 168.Godfrey C, Foley AR, Clement E, Muntoni F. Dystroglycanopathies: coming into focus. Curr Opin Genet Dev. 2011;21:278–285. doi: 10.1016/j.gde.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 169.Peng HB, Xie H, Rossi SG, Rotundo RL. Acetylcholinesterase clustering at the neuromuscular junction involves perlecan and dystroglycan. J Cell Biol. 1999;145:911–921. doi: 10.1083/jcb.145.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pribiag H, Peng H, Shah WA, Stellwagen D, Carbonetto S. Dystroglycan mediates homeostatic synaptic plasticity at GABAergic synapses. Proc Natl Acad Sci USA. 2014;111:6810–6815. doi: 10.1073/pnas.1321774111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Scotton P, Bleckmann D, Stebler M, Sciandra F, Brancaccio A, Meier T, Stetefeld J, Ruegg MA. Activation of muscle-specific receptor tyrosine kinase and binding to dystroglycan are regulated by alternative mRNA splicing of agrin. J Biol Chem. 2006;281:36835–36845. doi: 10.1074/jbc.M607887200. [DOI] [PubMed] [Google Scholar]
- 172.Zhang J, Wang Y, Chu Y, Su L, Gong Y, Zhang R, Xiong S. Agrin is involved in lymphocytes activation that is mediated by α-dystroglycan. FASEB J. 2006;20:50–58. doi: 10.1096/fj.04-3303com. [DOI] [PubMed] [Google Scholar]
- 173.Mazzon C, Anselmo A, Soldani C, Cibella J, Ploia C, Moalli F, Burden SJ, Dustin ML, Sarukhan A, Viola A. Agrin is required for survival and function of monocytic cells. Blood. 2012;119:5502–5511. doi: 10.1182/blood-2011-09-382812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hilgenberg LGW, Su H, Gu H, O’Dowd DK, Smith MA. α3Na+/K+-ATPase is a neuronal receptor for agrin. Cell. 2006;125:359–369. doi: 10.1016/j.cell.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 175.Burgess RW, Dickman DK, Nunez L, Glass DJ, Sanes JR. Mapping sites responsible for interactions of agrin with neurons. J Neurochem. 2002;83:271–284. doi: 10.1046/j.1471-4159.2002.01102.x. [DOI] [PubMed] [Google Scholar]
- 176.Martin PT, Sanes JR. Integrins mediate adhesion to agrin and modulate agrin signaling. Development. 1997;124:3909–3917. doi: 10.1242/dev.124.19.3909. [DOI] [PubMed] [Google Scholar]
- 177.Kim N, Stiegler AL, Cameron TO, Hallock PT, Gomez AM, Huang JH, Hubbard SR, Dustin ML, Burden SJ. Lrp4 is a receptor for agrin and forms a complex with MuSK. Cell. 2008;135:334–342. doi: 10.1016/j.cell.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chakraborty S, Lakshmanan M, Swa HL, Chen J, Zhang X, Ong YS, Loo LS, Akincilar SC, Gunaratne J, Tergaonkar V, Hui KM, Hong W. An oncogenic role of Agrin in regulating focal adhesion integrity in hepatocellular carcinoma. Nat Commun. 2015;6:6184. doi: 10.1038/ncomms7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vuadens F, Benay C, Crettaz D, Gallot D, Sapin V, Schneider P, Binevenut WV, Lémery D, Quadroni M, Dastugue B, Tissot JD. Identification of biologic markers of the premature rupture of fetal membranes: proteomic approach. Proteomics. 2003;3:1521–1525. doi: 10.1002/pmic.200300455. [DOI] [PubMed] [Google Scholar]
- 180.Halfter W, Schurer B. A new heparan sulfate proteoglycan in the extracellular matrix of the developing chick embryo. Exp Cell Res. 1994;214:285–296. doi: 10.1006/excr.1994.1260. [DOI] [PubMed] [Google Scholar]
- 181.Marneros AG, Olsen BR. Physiological role of collagen XVIII and endostatin. FASEB J. 2005;19:716–728. doi: 10.1096/fj.04-2134rev. [DOI] [PubMed] [Google Scholar]
- 182.Dong S, Cole GJ, Halfter W. Expression of collagen XVIII and localization of its glycosaminoglycan attachment sites. J Biol Chem. 2003;278:1700–1707. doi: 10.1074/jbc.M209276200. [DOI] [PubMed] [Google Scholar]
- 183.Fukai N, Eklund L, Marneros AG, Oh SP, Keene DR, Tamarkin L, Niemelä M, Ilves M, Li E, Pihlajaniemi T, Olsen BR. Lack of collagen XVIII/endostatin results in eye abnormalities. EMBO J. 2002;21:1535–1544. doi: 10.1093/emboj/21.7.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Utriainen A, Sormunen R, Kettunen M, Carvalhaes LS, Sajanti E, Eklund L, Kauppinen R, Kitten GT, Pihlajaniemi T. Structurally altered basement membranes and hydrocephalus in a type XVIII collagen deficient mouse line. Human Mol Gen. 2004;13:2089–2099. doi: 10.1093/hmg/ddh213. [DOI] [PubMed] [Google Scholar]
- 185.Bishop JR, Passos-Bueno MR, Fong L, Stanford KI, Gonzales JC, Yeh E, Young SG, Bensadoun A, Wizemann H, Esko JD, Moulton KS. Deletion of basement membrane heparan sulfate proteoglycan type XVIII collagen causes hypertriglyceridemia in mice and humans. PLoS ONE. 2011;5:e13919. doi: 10.1371/journal.pone.0013919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zeng X, Chen J, Miller YI, Javaherian K, Moulton KS. Endostatin binds biglycan and LDL and interferes with LDL retention to the subendothelial matrix during atherosclerosis. J Lipid Res. 2005;46:1849–1859. doi: 10.1194/jlr.M500241-JLR200. [DOI] [PubMed] [Google Scholar]
- 187.Marneros AG, Keene DR, Hansen U, Fukai N, Moulton K, Goletz PL, Moiseyev G, Pawlyk BS, Halfter W, Dong S, Shibata M, Li T, Crouch RK, Bruckner P, Olsen BR. Collagen XVIII/endostatin is essential for vision and retinal pigment epithelial function. EMBO J. 2004;23:89–99. doi: 10.1038/sj.emboj.7600014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Moulton KS, Olsen BR, Sonn S, Fukai N, Zurakowski D, Zeng X. Loss of collagen XVIII enhances neovascularization and vascular permeability in atherosclerosis. Circulation. 2004;110:1330–1336. doi: 10.1161/01.CIR.0000140720.79015.3C. [DOI] [PubMed] [Google Scholar]
- 189.Musso O, Rehn M, Théret N, Turlin B, Bioulac-Sage P, Lotrian D, Campion JP, Pihlajaniemi T, Clément B. Tumor progression is associated with a significant decrease in the expression of the endostatin precursor collagen XVIII in human hepatocellular carcinomas. Cancer Res. 2001;61:45–49. [PubMed] [Google Scholar]
- 190.Zatterstrom UK, Felbor U, Fukai N, Olsen BR. Collagen XVIII/endostatin structure and functional role in angiogenesis. Cell Struct Funct. 2000;25:97–101. doi: 10.1247/csf.25.97. [DOI] [PubMed] [Google Scholar]
- 191.O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 192.Cho H, Kim WJ, Lee YM, Kin YM, Kwon YG, Park YS, Choi EY, Kim KW. N-/C-terminal deleted mutant of human endostatin efficiently acts as an anti-angiogenic and anti-tumorigenic agent. Onc Reports. 2004;11:191–195. [PubMed] [Google Scholar]
- 193.Parangi S, O’Reilly M, Christofori G, Holmgren L, Grosfeld J, Folkman J, Hanahan D. Antiangiogenic therapy of transgenic mice impairs de novo tumor growth. Proc Natl Acad Sci USA. 1996;93:2002–2007. doi: 10.1073/pnas.93.5.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ferreras M, Felbor U, Lenhard T, Olsen BR, Delaisse J. Generation and degradation of human endostatin proteins by various proteinases. FEBS Lett. 2000;486:247–251. doi: 10.1016/s0014-5793(00)02249-3. [DOI] [PubMed] [Google Scholar]
- 195.Felbor U, Dreier L, Bryant RAR, Ploegh HL, Olsen BR, Mothes W. Secreted cathepsin L generates endostatin from collagen XVIII. EMBO J. 2000;19:1187–1194. doi: 10.1093/emboj/19.6.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wen W, Moses MA, Wiederschain D, Arbiser JL, Folkman J. The generation of endostatin is mediated by elastase. Cancer Res. 1999;59:6052–6056. [PubMed] [Google Scholar]
- 197.Folkman J. Is angiogenesis an organizing principle in biology and medicine? J Pediatr Surg. 2007;42:1–11. doi: 10.1016/j.jpedsurg.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 198.Kuo CJ, LaMontagne KR, Garcia-Cardena G, Ackley BD, Kalman D, Park S, Christofferson R, Kamihara J, Ding YH, Lo KM, Gillies S, Folkman J, Mulligan RC, Javaherian K. Oligomerization-dependent regulation of motility and morphogenesis by the collagen XVIII NC1/endostatin domain. J Cell Biol. 2001;152:1233–1246. doi: 10.1083/jcb.152.6.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Abdollahi A, Hahnfeldt P, Maercker C, Gröne HJ, Debus J, Ansorge W, Folkman J, Hlatky L, Huber PE. Endostatin’s antioangiogenic signaling network. Mol Cell. 2004;13:649–663. doi: 10.1016/s1097-2765(04)00102-9. [DOI] [PubMed] [Google Scholar]
- 200.Boehm T, O’Reilly MS, Keough K, Shiloach J, Shapiro R, Folkman J. Zinc-binding of endostatin is essential for its antiangiogenic activity. Biochemical & Biophysical Research Communications. 1998;252:190–194. doi: 10.1006/bbrc.1998.9617. [DOI] [PubMed] [Google Scholar]
- 201.Tjin Tham Sjin RM, Satchi-Fainaro R, Birsner AE, Ramanujam VM, Folkman J, Javaherian K. A 27-amino-acid synthetic peptide corresponding to the NH2-terminal zinc-binding domain of endostatin is responsible for its antitumor activity. Cancer Res. 2005;65:3656–3663. doi: 10.1158/0008-5472.CAN-04-1833. [DOI] [PubMed] [Google Scholar]
- 202.Rehn M, Veikkola T, Kukk-Valdre E, Nakamura H, Ilmonen M, Lombardo CR, Pihlajaniemi T, Alitalo K, Vuori K. Interaction of endostatin with integrins implicated in angiogenesis. Proc Natl Acad Sci USA. 2001;98:1024–1029. doi: 10.1073/pnas.031564998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sudhakar A, Sugimoto H, Yang C, Lively J, Zeisberg M, Kalluri R. Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by αvβ3 and α5β1 integrins. Proc Natl Acad Sci USA. 2003;100:4766–4771. doi: 10.1073/pnas.0730882100. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 204.Karumanchi SA, Jha V, Ramchandran R, Karihaloo A, Tsiokas L, Chan B, Dhanabai M, Hanai JC, Venkataraman G, Shriver Z, Keiser N, Kalluri R, Zeng H, Mukhopadhyay D, Chen RL, Lander AD, Hagihara K, Yamaguchi Y, Sasisekarharan R, Cantley L, Sukhatme VP. Cell surface glypicans are low-affinity endostatin receptors. Mol Cell. 2001;7:811–822. doi: 10.1016/s1097-2765(01)00225-8. [DOI] [PubMed] [Google Scholar]
- 205.Kim YM, Hwang S, Kim YM, Pyun BJ, Kim TY, Lee ST, Gho YS, Kwon YG. Endostatin blocks vascular endothelial growth factor-mediated signaling via direct interaction with KDR/Flk-1. J Biol Chem. 2002;277:27872–27879. doi: 10.1074/jbc.M202771200. [DOI] [PubMed] [Google Scholar]
- 206.Shichiri M, Hirata Y. Antiangiogenesis signals by endostatin. FASEB J. 2001;15:1044–1053. doi: 10.1096/fj.99-1083com. [DOI] [PubMed] [Google Scholar]
- 207.Nguyen TMB, Subramanian IV, Xiao X, Ghosh G, Nguyen P, Kelekar A, Ramakrishnan S. Endostatin induces autophagy in endothelial cells by modulating Beclin 1 and β-catenin levels. J Cell Mol Med. 2009;13:3687–3698. doi: 10.1111/j.1582-4934.2009.00722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chau YP, Lin JY, Chen JHC, Tai MH. Endostatin induces autophagic cell death in EAhy926 human endothelial cells. Histol Histopathol. 2003;18:715–726. doi: 10.14670/HH-18.715. [DOI] [PubMed] [Google Scholar]
- 209.Lee SJ, Kim HP, Jin Y, Choi AMK, Ryter SW. Beclin 1 deficiency is associated with increased hypoxia-induced angiogenesis. Autophagy. 2011;7:829–839. doi: 10.4161/auto.7.8.15598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 211.Nikitovic D, Papoutsidakis A, Karamanos N, Tzanakasis GN. Lumican affects tumor cell functions, tumor-ECM interactions, angiogenesis and inflammatory response. Matrix Biol. 2014;35:206–214. doi: 10.1016/j.matbio.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 212.Yamanaka O, Yuan Y, Coulson-Thomas VJ, Gesteira TF, Call MK, Zhang Y, Zhang J, Chang SH, Xie C, Liu CY, Saika S, Jester JV, Kao WW. Lumican binds ALK5 to promote epithelium wound healing. PLoS One. 2013;8:e82730. doi: 10.1371/journal.pone.0082730. [DOI] [PMC free article] [PubMed] [Google Scholar]