Abstract
Emerging evidence indicates that the interactions between tumor cells and the bone microenvironment have a crucial role in the pathogenesis of bone metastasis and that they can influence tumor cell dissemination, quiescence and tumor growth in the bone. The vasculature is known to be critical for primary tumor growth, and anti-angiogenesis drugs are approved for the treatment of certain tumor types. The role of the vasculature in bone metastasis is less well known, but recent evidence shows that blood vessels in the bone are a key component of the local microenvironment for the tumor cells and contribute to the different consecutive phases of bone metastasis. A better insight in the importance of the vasculature for bone metastasis may help develop novel treatment modalities that either slow down tumor growth or, preferably, prevent or cure bone metastasis.
Introduction
Malignant cells have acquired mutations that lead to uncontrolled proliferation and the ability to migrate and leave the site of origin to disseminate via the circulation and then engraft and colonize distant organs. Breast and prostate tumor cells preferentially colonize the bone, causing numerous symptoms and a high morbidity.1 Current therapies mainly target the later stages of bone metastasis, and thereby slow down the progression of the disease, but they are not curative. A possible approach to improve the therapeutic outcome is to target the initiation and development of bone metastases, but this strategy requires interfering with the initial stages of metastasis, including survival, quiescence, migration and proliferation of metastatic tumor cells, followed later by colonization of the bone environment.2 The intimate association of tumor cells with cells from the microenvironment is considered to be important during the early stages of metastasis, and, the vasculature is a key component of this environment, as it facilitates metastatic dissemination and supports tumor growth in later stages via the delivery of oxygen, nutrients and growth factors.3
In the adult, the vasculature is mature and relatively stable, but blood vessels can be reactivated by proangiogenic factors produced by hypoxic cells, a situation that typically occurs during tumor growth.4 In response to the proangiogenic factors, new blood vessels start to develop from pre-existing ones (that is, angiogenesis), and this involves sprouting, migration and proliferation of endothelial cells.5 Even though tumor angiogenesis recapitulates many of the events of normal blood vessel development, some differences exist. Indeed, in contrast to normal vessels, the tumor vasculature is highly disorganized, and, the vessel walls are leaky due to the presence of endothelial fenestrae and transcellular holes, thereby facilitating spreading of tumor cells and limiting the delivery of chemotoxic agents.6
In this review, we provide an overview of the significance of blood vessels for driving metastatic spreading to the bone and their role during metastatic outgrowth. Moreover, we critically evaluate the antimetastatic character of treatment modalities that target the tumor vasculature and discuss novel anti-angiogenic therapies and innovative techniques to study the role of the vasculature in bone metastases.
Vasculature characteristics may guide tumor cell dissemination to the bone microenvironment
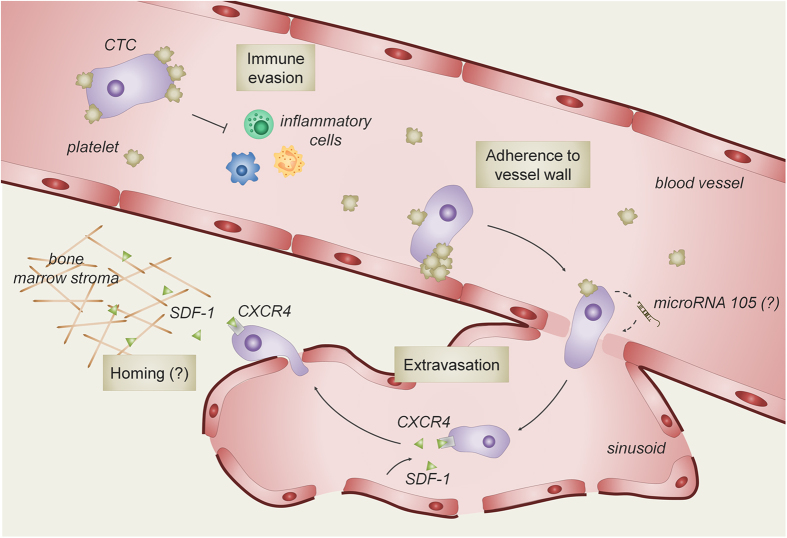
When cancer cells enter the circulation, the majority (>99%) of these circulating tumor cells (CTCs) are quickly eliminated by cells of the immune system or through apoptotic cell death induced by local shear stress or loss of adherence (that is, anoikis).7,8 The few cells (<0.01%) that are able to survive in the circulation are believed to be associated with platelets.9 These platelets aggregate around the CTCs and form a physical barrier that protects the CTCs from immunological detection and apoptosis. In addition, aggregated tumor cells and platelets form a clump in the blood stream, leading to reduced blood flow and thus facilitating adhesion of the tumor cells to the capillary network of specific tissues.
The morphology and structure of blood vessels differs between organs and may thus influence organ-specific extravasation of tumor cells.10 The blood vessels in the metaphysis of the long bones are characterized by the constitutive expression of adhesive proteins, including P-selectin and E-selectin, intercellular adhesion molecule A and vascular adhesion molecule 1, which promote the interaction with tumor cells and facilitate their adhesion8 (Figure 1). In contrast to the bone marrow vasculature, endothelial cells constituting the blood vessels in other tissues only express these molecules when stimulated by inflammatory cytokines.8 Moreover, the vascular system in the bone metaphysis consists of voluminous sinusoids, likely resulting in reduced blood flow, and the endothelial cell layer is highly fenestrated.11,12 These particular characteristics of the bone marrow vasculature are thought to facilitate the docking of CTCs to the endothelial cells and help the extravasation out of the vasculature into the bone marrow stroma. In addition, tumor-derived factors may change the structure of the endothelial cell layer. Indeed, recent studies show that the secretion of microRNA-105 (miR-105) and angiogenic factors by breast tumor cells downregulates the expression of the tight junction proteins in the endothelial monolayer barrier of the lungs and the brain, thereby facilitating the extravasation of the CTCs out of the blood vessels.13 A similar phenomenon may occur during bone metastasis but is probably less important, as the fenestrated sinusoids in the bone marrow may already support the migration of tumor cells across the bone marrow endothelium.10,14,15
Figure 1.
Blood vessels guide tumor cells to the bone microenvironment. After they break free from the primary tumor, most cancer cells that enter the circulation are quickly eliminated. A small portion of the circulating tumor cells (CTCs) survives in the blood stream by interacting with platelets, which protects tumor cells from apoptosis caused by local shear stress and immunological reaction. Moreover, clumping of CTCs with platelets reduces blood flow and facilitates adhesion of the tumor cells to the blood vessel wall. CTCs leave the circulation and enter the local bone marrow via transmigration through the endothelial barrier, in part mediated by the secretion of tumor cell-specific factors (for example, microRNA-105). Moreover, local high levels of stromal cell-derived growth factor 1 (SDF-1) derived from endothelial cells and cells from the bone marrow stroma attract C-X-C receptor 4 (CXCR4) expressing CTCs to the local microenvironment.
The endothelial cells of the bone marrow vasculature not only differ from other blood vessels in their expression of adhesion receptors but also in growth factor production. Indeed, endothelial cells of bone marrow blood vessels express high levels of stromal cell-derived growth factor 1 (SDF-1) in specific local areas,16 and this factor attracts metastatic tumor cells that express high levels of the SDF-1 receptor, that is C-X-C chemokine receptor 4 (CXCR4).17,18,19 This specialized endothelium thus defines a unique microenvironment in the bone marrow that guides CTCs for extravasation and engraftment around these vessels. In agreement herewith, genetic profiling of tumor cells showed that breast tumor cells with a high propensity to form bone metastases express high levels of CXCR4.20
To conclude, the vasculature seems to guide tumor cells to the bone by the means of its structure, adhesive proteins and growth factor production. Interestingly, most of these bone-targeting mechanisms are normally used by hematopoietic stem cells to reside and occupy their specific niche in the bone marrow, but these pathways are hijacked by metastatic tumor cells during the development of bone metastasis.21,22
The vasculature mediates quiescence but also reactivation of disseminated tumor cells in the bone marrow
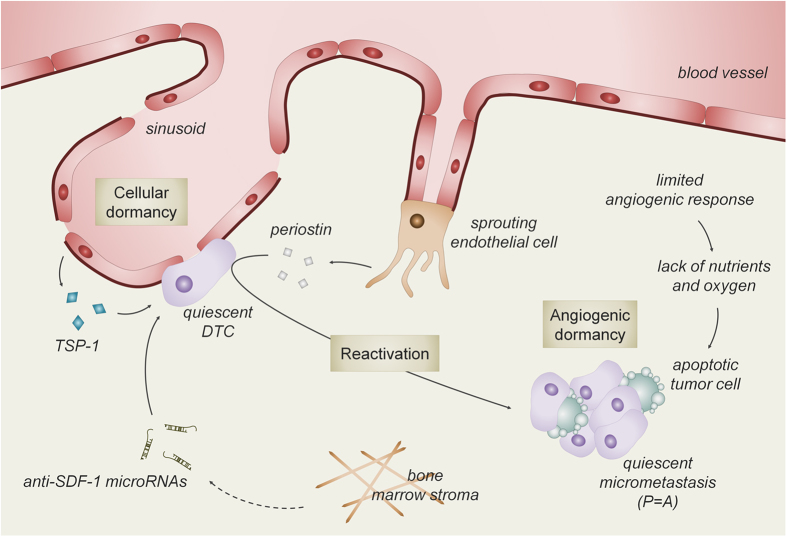
When the disseminated tumor cells (DTCs) have infiltrated into the bone marrow, they rarely progress immediately to an overt metastatic tumor. Indeed, the local environment is often very different from the primary tumor site and thus hostile for the DTCs, thereby hindering instant tumor outgrowth. Despite this unreceptive environment, DTCs can survive and remain in a dormant state as single cells or micrometastases.10 This state of metastatic latency, which is defined as the time between primary tumor diagnosis and clinically detectable metastatic relapse, can take up to several years for breast cancer DTCs. Exactly how DTC dormancy is regulated remains largely unknown but likely includes cellular dormancy, angiogenic dormancy and immunosurveillance.23,24
Recently, Ghajar et al.25 introduced novel mechanistic insights in the process of cellular dormancy. Quiescent breast cancer DTCs are observed in close intimacy with the stable microvasculature of the bone marrow. These endothelial cells secrete thrombospondin 1, which induces tumor cell quiescence (Figure 2). When angiogenesis is stimulated, by still uncharacterized triggers, endothelial cells start sprouting and limit the secretion of thrombospondin 1 but induce the expression of molecules like transforming growth factor-β1, periostin and fibronectin. These factors stimulate the DTCs to get out of their quiescent state and start colonizing the bone. In addition, the adhesion of DTCs to fibronectin in the bone marrow can be enhanced by local growth factor production. Indeed, we could show that the expression in the bone microenvironment of placental growth factor (PlGF), a pleiotropic and angiogenic growth factor, promotes the adhesion of breast tumor cells to fibronectin during the initial stages of bone metastasis.26 Blocking specifically the action of stromal-derived PlGF, using anti-mouse-PlGF antibodies, resulted in decreased bone metastasis. Belonging to the same family as PlGF, vascular endothelial growth factor (VEGF) is one of the most potent angiogenic growth factors4 and regulates angiogenic processes at several stages during bone development.27,28 The role of VEGF during the early stages of bone metastases seems, however, limited. Indeed, inhibition of VEGF production in tumor cells together with blocking VEGF signaling from the start of the arrival of DTCs, that is, as a preventive strategy, reduced but did not totally block the development of bone osteolytic lesions.29 Moreover, compared with the curative protocol, when treatment was started after the development of bone metastases, the preventive strategy was only marginally superior, suggesting that this strategy mainly targeted bone metastasis progression and not the initial stages of bone metastasis development.
Figure 2.
The bone marrow vasculature mediates tumor cell quiescence and reactivation. When disseminated tumor cells (DTCs) arrive in the bone marrow microenvironment they encounter an unreceptive environment, which hinders immediate overt tumor growth. DTCs survive in the hostile environment in close intimacy with the stable bone microvasculature which induces tumor cell cycle arrest, mediated by thrombospondin 1 (TSP-1) and anti-SDF-1 microRNA (that is, cellular dormancy). On the other hand, sprouting endothelial cells produce factors, like periostin, that drive the quiescent DTCs out of their dormant state and stimulate proliferation (P), resulting in a micrometastatic tumor. When micrometastases reach a certain size that cannot be further supported by the normal tissue vasculature, the supply of nutrients and oxygen will decrease, hereby inducing tumor cell apoptosis (A). Collectively, tumor cell apoptosis balances proliferation (P=A) and limits the development of an overt metastatic tumor.
Thus, these data indicate that endothelial cells form niches that support cellular dormancy and may in this respect resemble the role of endothelial cells to regulate hematopoietic stem cell quiescence. Several factors are being identified that control the endothelial–hematopoietic stem cell niche including E-selectin, stem cell factor and SDF-1.18,30 Interestingly, the local regulation of SDF-1 levels has also been linked to DTC dormancy. A recent study shows that bone marrow stromal cells contribute to quiescence of DTCs by secreting miRs targeting SDF-1 expression. These anti-SDF-1 miRs are transported from the stromal cells to the breast tumor cells via gap junctions and can then induce tumor cell cycle arrest.31 However, the question whether endothelial cells are the source of these miRs or that rather other bone marrow stromal cells, such as mesenchymal (stem) cells and immune cells, produce these miRs, needs still to be answered.
In conclusion, the bone vasculature appears to have a dual role during metastatic outgrowth, as it regulates dormancy of DTCs during the initial stages of metastasis but may also trigger tumor cell proliferation and colonization of the bone environment.
Need of angiogenesis for oxygen and nutrient delivery during metastatic outgrowth
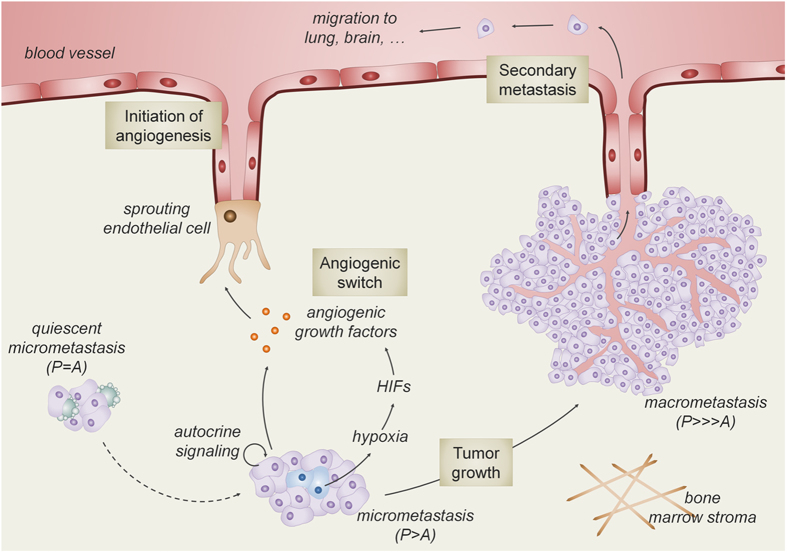
Once DTCs emerge from cellular dormancy and start to proliferate to form micrometastases, they may encounter another type of dormancy. Angiogenic dormancy has generally been considered as the failure to induce the angiogenic switch to sustain the growth of micrometastases. Generally, when micrometastases reach a certain size that cannot be further supported by the normal tissue vasculature, tumor cell nutrition and oxygenation will decrease drastically.23,32,33 Consequently, tumor cell death will balance proliferation and thus prevents tumor growth and the development of clinically manifest metastases.34 Tumor cells can respond to these stressful conditions and induce adaptive mechanisms to survive. The local drop in oxygen levels activates hypoxia-inducible factor (HIF), a transcription factor that induces the expression of proangiogenic factors, including VEGF, PlGF, platelet-derived growth factor and fibroblast growth factor.35 These growth factors, produced by the tumor cells, induce angiogenesis by a process called ‘the angiogenic switch'.32,36,37 This hypoxia-induced process observed in primary tumors also contributes to the growth of bone metastases (Figure 3). Indeed, inhibiting HIF signaling in breast tumor cells decreases the number of osteolytic lesions, which was accompanied by reduced VEGF expression and blood vessel density.38 The HIF-mediated vascular response thus facilitates further metastatic outgrowth but may also allow tumor cells to subsequently metastasize to other organs, including lung, brain and colon.
Figure 3.
Blood vessels drive macrometastatic tumor growth and secondary colonization. Through still unknown mechanisms, tumor cell proliferation is stimulated and outpaces apoptosis (P>A) in the micrometastatic tumor. This progression is possible when blood vessel ingrowth is promoted (that is, angiogenic switch). A major driver of this process is the production of angiogenic growth factors by the hypoxic tumor cells, resulting from activation of hypoxia-inducible factor (HIF) signaling. In addition to HIF-dependent stimulation of angiogenesis, tumor cell intrinsic signaling increases the production of angiogenic growth factors, further enhancing metastatic outgrowth and secondary metastasis to distant organs.
Not only tumor cell hypoxia but also tumor-derived growth factors may stimulate the production of angiogenic factors by tumor or bone cells and contribute to the angiogenic switch. Parathyroid hormone-related peptide (PTHrP) is an important factor in the feed-forward cycle of osteolytic lesions. PTHrP is secreted by tumor cells and indirectly stimulates osteoclast formation and thereby increases bone destruction.15 In addition to regulating bone resorption, recent data show that PTHrP also stimulates angiogenesis in an autocrine manner by increasing VEGF production by the metastatic breast cancer cells.39 This suggests a supportive role for PTHrP during tumor growth and colonization of the bone microenvironment by regulating blood vessel growth.
On the basis of these VEGF expression data, interfering with VEGF signaling seems an interesting strategy to target bone metastasis. Preclinical studies show that angiogenesis and osteolysis are reduced when monotherapy with a VEGF-neutralizing antibody (Bevacizumab) or VEGF receptor tyrosine kinase inhibitors (RTKIs, such as Sunitinib, Sorafenib) was used in a rat breast cancer metastasis model.40 The positive effect of this monotherapy was not confirmed in another study that highlighted the importance of combining Bevacizumab and Vatalanib (a RTKI) to significantly reduce the number and size of the osteolytic lesions. These findings indicate that efficient inhibition of the progression of bone metastasis requires the targeting of VEGF as well as its receptor.29 A possible explanation for the lower effectiveness of anti-angiogenic monotherapy is the observation that several other proangiogenic factors, like fibroblast growth factor and platelet-derived growth factor, are released during cancer-induced bone remodeling or tumor hypoxia and thus can compensate for the VEGF blockade.29 On the other hand, targeting VEGF and/or its receptor in prostate cancer-mediated osteoblastic metastasis resulted in decreased tumor burden and osteoblastic activity.41,42 A possible explanation for the positive effect in prostate cancer metastasis is the osteoblastic nature of the bone metastases and that VEGF stimulates osteoblast differentiation in vitro, suggesting a direct effect on osteoblasts. However, how VEGF stimulates osteoblast differentiation in vivo is still not fully elucidated. Indeed, a recent study suggests that VEGF controls osteoblastogenesis via intracrine VEGF signaling, as conditional deletion of VEGF in osteoblastic progenitor cells resulted in decreased osteoblast differentiation by reducing Runx2 expression, whereas the addition of extracellular VEGF or antibodies against VEGF had no effect on differentiation.43 Other explanations for the beneficial effect of anti-VEGF therapy in prostate cancer-mediated osteoblastic metastasis41 include an anti-angiogenic effect, as metastatic tumor growth was reduced, and a decrease in blood vessel density may indirectly influence osteoblast characteristics. On the other hand, osteoblast- or tumor-derived VEGF may also promote osteoclast differentiation by a paracrine mechanism44 and may thereby increase bone remodeling. Whether anti-VEGF therapy in vivo will affect, besides the vasculature, also bone cell properties in a direct and angiogenesis-independent way is not known yet, but hypothetically it may decrease osteoclastogenesis by interfering with the paracrine signaling, and may have little effect on osteoblast differentiation, given the intracrine signaling of VEGF in osteoblast differentiation.
Besides growth factors, adhesion proteins on endothelial cells, like integrins, are also involved in tumor-associated angiogenesis.45 Within the large family of integrins, the αvβ3 integrin seems especially interesting with respect to bone metastasis.46,47 Indeed, the αvβ3 integrin is critical for tumor angiogenesis as it sustains the newly formed blood vessels and promotes their maturation.45 In addition, αvβ3 integrin is also important for osteoclast activity, and drugs blocking integrin activity are reported to increase bone mineral density in postmenopausal women.48 Finally, αvβ3 integrin may contribute to bone colonization by tumor cells overexpressing this integrin.49 Recent studies showed that cilengitide, an inhibitor of αvβ3 and αvβ5 integrin, decreased vessel density and changed the vessel phenotype in breast tumor bone metastasis already at an early stage whereas it changed tumor cell metabolism, as well as decreased bone resorption in a later stage.50,51,52 Thus, targeting signaling pathways that are not only important for angiogenesis but also for osteoclast activity as well as tumor cell behavior may provide an interesting approach.
Taken together, these data show that, like in primary tumors and metastases to other organs, the growth of bone metastases is coordinated by a complex interplay between tumor cells, tissue-specific cells and the vasculature. Although the specifics of the angiogenic processes during bone metastasis remain to be elucidated, targeting angiogenesis is suggested to represent an interesting way to deregulate the cellular cross-talk during tumor growth and inhibit progression of bone metastases, but further investigation is necessary to prove this hypothesis.
Anti-angiogenic therapy: reduction or normalization of the vasculature?
Given the crucial role of VEGF and its receptor in angiogenesis, blocking this signaling pathway was considered to decrease angiogenesis and/or destroy existing vessels, hereby starving the tumor from its nutrients. Currently, the US Food and Drug Administration has approved the use of Bevacizumab in combination with chemotherapy or cytokine therapy for various late-stage advanced metastatic cancers including metastatic breast cancer. However, phase-3 clinical trials show that the addition of Bevacizumab to chemotherapy only modestly improves progression-free survival in metastatic breast cancer.53 Bevacizumab even failed to improve outcomes in combination with adjuvant chemotherapy in early triple-negative breast cancer.54
These observations and the data obtained in preclinical models (see previous section) suggest that we likely should focus on the development of additional or alternative anti-angiogenic treatment strategies to improve clinical outcome. In this regard, the development of reliable biomarkers to monitor resistance to anti-angiogenic agents could select responsive patients and thus increase therapeutic efficacy.55 Indeed, it has recently been found that polymorphisms in the VEGF gene were associated with the type of response to anti-VEGF treatment.56 Moreover, conceptually distinct strategies to block metastatic spread should also be considered. Targeting blood vessel normalization rather than inhibition of blood vessel formation or destruction of existing blood vessels may be a valuable alternative. Indeed, tumors generally overexpress angiogenic factors, and, the normal balance between angiogenic activators and inhibitors is thus disturbed, resulting in a blood vessel network that is abnormal in almost all aspects of structure and function, eg. tortuous, irregular branching, chaotic network, reduced perfusion and leaky.57 These abnormal vessels not only result in inadequate nutrient delivery but also in insufficient drug delivery and provide a gateway for DTCs, hereby fueling disease progression. These observations may explain the transient and often limited effect of anti-VEGF therapy, as this strategy often induces vessel destruction, resulting in more hypoxia and angiogenic factor secretion, as well as poor delivery of chemotherapeutics. On the basis of these findings, vessel normalization rather than vessel reduction seems an interesting and a relevant approach. More recent studies, using genetically modified mice (PHD2, Ang2 and Rgs5)58,59,60,61 or blocking miRs (miR-105),13 provide evidence for the beneficial effects of promoting vascular normalization in a non-transient way, resulting in reduced metastasis and improved efficacy of chemotherapy and immune therapy.62,63 These observations suggest that vessel normalization may be a good alternative in treating metastasis.
Despite the many remaining questions, vasculature-targeting therapy is considered to be a promising strategy in the treatment of (bone) metastasis but likely requires a better identification of patients who may benefit from this type of therapy and strategies inducing vessel normalization may be warranted.
New models to study the role of the vasculature in bone metastases
To further unravel the complex role of the vasculature in the arrival, quiescence, activation and growth of tumor cells in the bone, the development of new techniques and models is crucial. As the metastatic process is determined by complex cellular and molecular interactions between tumor and host, studies in an in vivo setting are preferred, certainly when considering angiogenic processes. Ideally, noninvasive imaging techniques in preclinical models are used in this setting, allowing long-term follow-up of functional and structural parameters.
Recently, a novel bone chamber implant model (also known as femur window) was developed by Hansen-Algenstaedt et al.64 This chamber consists of a circular titanium device containing a transparent window that is inserted into the femoral cortex of a mouse, allowing microscopic visualization of the bone marrow space, while preserving the structural and mechanical properties of the bone. After induction of bone metastasis with fluorescent tumor cells, the interplay between the tumor cells, bone cells and microcirculation can be observed at a high spatial resolution using intravital multi-photon confocal microscopy in combination with specific fluorescent labeling agents. This study showed that the tumor vasculature consisted of an irregular network with variation in vessel diameter and loop formations compared with the physiologic vasculature of cancellous bone.
To better characterize the morphology and functionality of the vasculature during bone metastasis, conventional two-dimensional (fluorescent) imaging techniques, including histology, can be complemented by three-dimensional techniques that try to capture the full trajectories of the blood vessels inside tissues. One of these techniques is volumetric computed tomography, which can also be applied for the analysis of the bone and tumor vasculature, when used in combination with a contrast agent.65 Therefore, establishment of both two-dimensional and three-dimensional imaging techniques will likely be essential to fully elucidate the role of the vasculature during bone metastasis.
In addition to the need for novel imaging modalities, models that mimic as close as possible the human microenvironment are highly valuable. To this end, researchers have developed methods for growing humanized bone ossicles subcutaneously in mice by combining human bone marrow stromal cells and calcium phosphate-based carrier structures. Recently, it was shown that human breast and prostate cancer cells efficiently metastasize to these ossicles, making this a relevant model for the study of human bone metastasis.66,67 Additional incorporation of human endothelial cells in the ossicles furthermore leads to a human-mouse chimeric vasculature, which may in the future help in understanding the specifics of the human endothelial compartment during bone metastasis.68,69 In addition, genetic modification of the implanted endothelial cells may represent a straightforward approach in unraveling the molecular aspects of tumor cell extravasation and angiogenic dormancy in the bone marrow.
Conclusion
Increasing evidence shows that the vasculature is crucial during the multistep process of bone metastasis, but many questions about the specific roles of the vasculature, especially during the initial stages of bone metastasis, remain. Insight in these aspects is needed before effective and safe anti-angiogenic therapies can be developed as part of the treatment of bone metastases.
Acknowledgments
This work was supported by grants from Stichting tegen Kanker (F17) and Fund for Scientific Research (FWO; G.0835.11, G.0A72.13). NvG is funded by BOF-KU Leuven GOA project 3M120209. SS is a fellow from the Agency for Innovation by Science and Technology in Flanders (IWT).
Footnotes
The authors declare no conflict of interest.
References
- Walkington L, Coleman RE. Advances in management of bone disease in breast cancer. Bone 2011; 48: 80–87. [DOI] [PubMed] [Google Scholar]
- Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer 2004; 4: 448–456. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012; 21: 309–322. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000; 407: 249–257. [DOI] [PubMed] [Google Scholar]
- Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 2007; 8: 464–478. [DOI] [PubMed] [Google Scholar]
- De Bock K, Mazzone M, Carmeliet P. Antiangiogenic therapy, hypoxia, and metastasis: risky liaisons, or not? Nat Rev Clin Oncol 2011; 8: 393–404. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. The biology of cancer metastasis. Semin Cancer Biol 2011; 21: 71. [DOI] [PubMed] [Google Scholar]
- Glinsky VV. Intravascular cell-to-cell adhesive interactions and bone metastasis. Cancer Metastasis Rev 2006; 25: 531–540. [DOI] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer 2009; 9: 239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 2009; 9: 274–284. [DOI] [PubMed] [Google Scholar]
- Mazo IB, von Andrian UH. Adhesion and homing of blood-borne cells in bone marrow microvessels. J Leukoc Biol 1999; 66: 25–32. [DOI] [PubMed] [Google Scholar]
- Mastro AM, Gay CV, Welch DR. The skeleton as a unique environment for breast cancer cells. Clin Exp Metastasis 2003; 20: 275–284. [DOI] [PubMed] [Google Scholar]
- Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014; 25: 501–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S, Cui J, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol 2004; 167: 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002; 2: 584–593. [DOI] [PubMed] [Google Scholar]
- Kaplan RN, Psaila B, Lyden D. Bone marrow cells in the 'pre-metastatic niche': within bone and beyond. Cancer Metastasis Rev 2006; 25: 521–529. [DOI] [PubMed] [Google Scholar]
- Lu X, Yan CH, Yuan M, Wei Y, Hu G, Kang Y. In vivo dynamics and distinct functions of hypoxia in primary tumor growth and organotropic metastasis of breast cancer. Cancer Res 2010; 70: 3905–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 2013; 495: 231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipkins DA, Wei X, Wu JW, Runnels JM, Cote D, Means TK et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 2005; 435: 969–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 2003; 3: 537–549. [DOI] [PubMed] [Google Scholar]
- Shiozawa Y, Pienta KJ, Taichman RS. Hematopoietic stem cell niche is a potential therapeutic target for bone metastatic tumors. Clin Cancer Res 2011; 17: 5553–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol 2006; 6: 93–106. [DOI] [PubMed] [Google Scholar]
- Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer 2007; 7: 834–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedley BD, Chambers AF. Tumor dormancy and metastasis. Adv Cancer Res 2009; 102: 67–101. [DOI] [PubMed] [Google Scholar]
- Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol 2013; 15: 807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenegrachts L, Maes C, Torrekens S, Van Looveren R, Mazzone M, Guise TA et al. Anti-placental growth factor reduces bone metastasis by blocking tumor cell engraftment and osteoclast differentiation. Cancer Res 2010; 70: 6537–6547. [DOI] [PubMed] [Google Scholar]
- Maes C, Coenegrachts L, Stockmans I, Daci E, Luttun A, Petryk A et al. Placental growth factor mediates mesenchymal cell development, cartilage turnover, and bone remodeling during fracture repair. J Clin Invest 2006; 116: 1230–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes C, Carmeliet P, Moermans K, Stockmans I, Smets N, Collen D et al. Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mech Dev 2002; 111: 61–73. [DOI] [PubMed] [Google Scholar]
- Bachelier R, Confavreux CB, Peyruchaud O, Croset M, Goehrig D, van der Pluijm G et al. Combination of anti-angiogenic therapies reduces osteolysis and tumor burden in experimental breast cancer bone metastasis. Int J Cancer 2014; 135: 1319–1329. [DOI] [PubMed] [Google Scholar]
- Winkler IG, Barbier V, Nowlan B, Jacobsen RN, Forristal CE, Patton JT et al. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat Med 2012; 18: 1651–1657. [DOI] [PubMed] [Google Scholar]
- Lim PK, Bliss SA, Patel SA, Taborga M, Dave MA, Gregory LA et al. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res 2011; 71: 1550–1560. [DOI] [PubMed] [Google Scholar]
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer 2003; 3: 401–410. [DOI] [PubMed] [Google Scholar]
- Vajkoczy P, Farhadi M, Gaumann A, Heidenreich R, Erber R, Wunder A et al. Microtumor growth initiates angiogenic sprouting with simultaneous expression of VEGF, VEGF receptor-2, and angiopoietin-2. J Clin Invest 2002; 109: 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer 2014; 14: 611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med 2003; 9: 677–684. [DOI] [PubMed] [Google Scholar]
- Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science 2008; 319: 195–198. [DOI] [PubMed] [Google Scholar]
- Gao D, Nolan D, McDonnell K, Vahdat L, Benezra R, Altorki N et al. Bone marrow-derived endothelial progenitor cells contribute to the angiogenic switch in tumor growth and metastatic progression. Biochim Biophys Acta 2009; 1796: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LK, Mohammad KS, Fournier PG, McKenna CR, Davis HW, Niewolna M et al. Hypoxia and TGF-beta drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PLoS One 2009; 4: e6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isowa S, Shimo T, Ibaragi S, Kurio N, Okui T, Matsubara K et al. PTHrP regulates angiogenesis and bone resorption via VEGF expression. Anticancer Res 2010; 30: 2755–2767. [PubMed] [Google Scholar]
- Bauerle T, Hilbig H, Bartling S, Kiessling F, Kersten A, Schmitt-Graff A et al. Bevacizumab inhibits breast cancer-induced osteolysis, surrounding soft tissue metastasis, and angiogenesis in rats as visualized by VCT and MRI. Neoplasia 2008; 10: 511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa Y, Dai J, Zhang J, Keller JM, Nor J, Yao Z et al. Vascular endothelial growth factor contributes to prostate cancer-mediated osteoblastic activity. Cancer Res 2005; 65: 10921–10929. [DOI] [PubMed] [Google Scholar]
- Roberts E, Cossigny DA, Quan GM. The role of vascular endothelial growth factor in metastatic prostate cancer to the skeleton. Prostate Cancer 2013; 2013: 418340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Berendsen AD, Jia S, Lotinun S, Baron R, Ferrara N et al. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J Clin Invest 2012; 122: 3101–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niida S, Kaku M, Amano H, Yoshida H, Kataoka H, Nishikawa S et al. Vascular endothelial growth factor can substitute for macrophage colony-stimulating factor in the support of osteoclastic bone resorption. J Exp Med 1999; 190: 293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G et al. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 1994; 79: 1157–1164. [DOI] [PubMed] [Google Scholar]
- Stucci S, Tucci M, Passarelli A, Silvestris F. Abeta integrin: pathogenetic role in osteotropic tumors. Crit Rev Oncol Hematol 2015; . [DOI] [PubMed] [Google Scholar]
- Schneider JG, Amend SR, Weilbaecher KN. Integrins and bone metastasis: integrating tumor cell and stromal cell interactions. Bone 2011; 48: 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MG, Cerchio K, Stoch SA, Gottesdiener K, Wu M, Recker R. Effect of L-000845704, an alphaVbeta3 integrin antagonist, on markers of bone turnover and bone mineral density in postmenopausal osteoporotic women. J Clin Endocrinol Metab 2005; 90: 2022–2028. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Bachelier R, Treilleux I, Pujuguet P, Peyruchaud O, Baron R et al. Tumor alphavbeta3 integrin is a therapeutic target for breast cancer bone metastases. Cancer Res 2007; 67: 5821–5830. [DOI] [PubMed] [Google Scholar]
- Bauerle T, Komljenovic D, Merz M, Berger MR, Goodman SL, Semmler W. Cilengitide inhibits progression of experimental breast cancer bone metastases as imaged noninvasively using VCT, MRI and DCE-MRI in a longitudinal in vivo study. Int J Cancer 2011; 128: 2453–2462. [DOI] [PubMed] [Google Scholar]
- Bretschi M, Merz M, Komljenovic D, Berger MR, Semmler W, Bauerle T. Cilengitide inhibits metastatic bone colonization in a nude rat model. Oncol Rep 2011; 26: 843–851. [DOI] [PubMed] [Google Scholar]
- Bretschi M, Cheng C, Witt H, Dimitrakopoulou-Strauss A, Strauss LG, Semmler W et al. Cilengitide affects tumor compartment, vascularization and microenvironment in experimental bone metastases as shown by longitudinal (1)(8)F-FDG PET and gene expression analysis. J Cancer Res Clin Oncol 2013; 139: 573–583. [DOI] [PubMed] [Google Scholar]
- Ocana A, Amir E, Vera F, Eisenhauer EA, Tannock IF. Addition of bevacizumab to chemotherapy for treatment of solid tumors: similar results but different conclusions. J Clin Oncol 2011; 29: 254–256. [DOI] [PubMed] [Google Scholar]
- Cameron D, Brown J, Dent R, Jackisch C, Mackey J, Pivot X et al. Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): primary results of a randomised, phase 3 trial. Lancet Oncol 2013; 14: 933–942. [DOI] [PubMed] [Google Scholar]
- Jain RK. Lessons from multidisciplinary translational trials on anti-angiogenic therapy of cancer. Nat Rev Cancer 2008; 8: 309–316. [DOI] [PubMed] [Google Scholar]
- Schneider BP, Wang M, Radovich M, Sledge GW, Badve S, Thor A et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol 2008; 26: 4672–4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev 2005; 15: 102–111. [DOI] [PubMed] [Google Scholar]
- Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 2009; 136: 839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite de Oliveira R, Deschoemaeker S, Henze AT, Debackere K, Finisguerra V, Takeda Y et al. Gene-targeting of Phd2 improves tumor response to chemotherapy and prevents side-toxicity. Cancer Cell 2012; 22: 263–277. [DOI] [PubMed] [Google Scholar]
- Hamzah J, Jugold M, Kiessling F, Rigby P, Manzur M, Marti HH et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature 2008; 453: 410–414. [DOI] [PubMed] [Google Scholar]
- Falcon BL, Hashizume H, Koumoutsakos P, Chou J, Bready JV, Coxon A et al. Contrasting actions of selective inhibitors of angiopoietin-1 and angiopoietin-2 on the normalization of tumor blood vessels. Am J Pathol 2009; 175: 2159–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov 2011; 10: 417–427. [DOI] [PubMed] [Google Scholar]
- Maes H, Kuchnio A, Peric A, Moens S, Nys K, De Bock K et al. Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell 2014; 26: 190–206. [DOI] [PubMed] [Google Scholar]
- Hansen-Algenstaedt N, Schaefer C, Wolfram L, Joscheck C, Schroeder M, Algenstaedt P et al. Femur window--a new approach to microcirculation of living bone in situ. J Orthop Res 2005; 23: 1073–1082. [DOI] [PubMed] [Google Scholar]
- Nyangoga H, Mercier P, Libouban H, Basle MF, Chappard D. Three-dimensional characterization of the vascular bed in bone metastasis of the rat by microcomputed tomography (MicroCT). PLoS One 2011; 6: e17336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzapfel BM, Wagner F, Loessner D, Holzapfel NP, Thibaudeau L, Crawford R et al. Species-specific homing mechanisms of human prostate cancer metastasis in tissue engineered bone. Biomaterials 2014; 35: 4108–4115. [DOI] [PubMed] [Google Scholar]
- Thibaudeau L, Taubenberger AV, Holzapfel BM, Quent VM, Fuehrmann T, Hesami P et al. A tissue-engineered humanized xenograft model of human breast cancer metastasis to bone. Dis Model Mech 2014; 7: 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gastel N, Torrekens S, Roberts SJ, Moermans K, Schrooten J, Carmeliet P et al. Engineering vascularized bone: osteogenic and proangiogenic potential of murine periosteal cells. Stem Cells 2012; 30: 2460–2471. [DOI] [PubMed] [Google Scholar]
- Tsigkou O, Pomerantseva I, Spencer JA, Redondo PA, Hart AR, O'Doherty E et al. Engineered vascularized bone grafts. Proc Natl Acad Sci USA 2010; 107: 3311–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]