Abstract
Background
The prognosis and management of neuroendocrine carcinoma are largely driven by histologic grade as assessed by mitotic activity. The authors reviewed their institutional experience to determine whether the histologic grade of neuroendocrine carcinoma can differ between primary and metastatic tumors.
Methods
This study examined patients who underwent operative resection of both primary and metastatic foci of neuroendocrine carcinoma. Resected tumors were independently reviewed and categorized as low, intermediate, or high grade as determined by mitotic count.
Results
The authors identified 20 patients with metastatic neuroendocrine carcinoma treated at their institution between 1997 and 2013 for whom complete pathologic review of primary and metastatic tumors was possible. Primary lesions were found in the small intestine (n = 12), pancreas (n = 7), ampulla (n = 1), stomach (n = 1), and rectum (n = 1). The timing of hepatic metastasis was synchronous in 15 cases and metachronous in 5 cases. The histologic grade was concordant between primary and metastatic tumors in 9 cases and discordant in 11 cases. Among the discordant cases, 7 had a higher metastatic grade than primary grade, and 4 had a lower metastatic grade than primary grade. Metachronous presentation was associated with a higher likelihood of grade discordance (p = 0.03). The histologic grade of all metachronous metastases differed from that of the primary tumors.
Conclusion
There is a high prevalence of histologic grade discordance between primary and metastatic foci of neuroendocrine carcinoma, particularly among patients with a metachronous metastatic presentation. Given the importance of histologic grade in disease prognostication and treatment planning, this finding may be informative for the management of patients with metastatic neuroendocrine carcinoma.
Neuroendocrine carcinomas are a relatively rare group of neoplasms arising from neuroendocrine cells in multiple organ systems. Despite a proclivity toward regional and distant metastases, they often exhibit biologic behavior characterized by relatively prolonged survival, even in patients with advanced disease.1–4 This rarity and relative indolence have challenged efforts to identify prognostic variables for stratification of expected survival outcomes for patients with neuroendocrine carcinoma.
For both primary and metastatic neuroendocrine carcinomas, the most informative prognostic variable is histologic differentiation, as assessed by cellular proliferative activity (measured by mitotic figures or Ki-67 immunohistochemical staining).4–7 In general, neuroendocrine carcinomas are classified as well-differentiated carcinomas that include low- and intermediate-grade tumors and poorly differentiated carcinomas that are high grade (G3) and associated with a particularly poor prognosis.
We and others have proposed staging and prognostication systems for gastroenteropancreatic neuroendocrine carcinomas based on cellular proliferative activity that stratify expected survival outcomes for both primary and metastatic neuroendocrine carcinoma.6–13 In this study, we sought to compare the histologic grade of primary and metastatic tumors in patients with synchronous and metachronous metastases and to measure the frequency with which this important prognostic variable changes between primary and metastatic foci of disease.
Methods
We reviewed our institutional database to identify patients with metastatic neuroendocrine carcinoma to the liver who underwent surgical intervention for their metastatic disease at the University of Wisconsin Hospital and Clinics between 1997 and 2013. We restricted our analysis to patients for whom both primary and metastatic foci of disease could be histologically reviewed. Patient demographics and tumor characteristics of both primary and metastatic tumors were reviewed and recorded. Institutional review board approval was obtained before this study was conducted.
Specimens were independently reviewed by a surgical pathologist (M.A.D.), and mitotic figures were counted within the most proliferative areas of each slide. Specifically, all slides were scanned at intermediate to high magnification to identify areas of high proliferative activity. These areas then were evaluated under high magnification (∼0.2 mm2), and mitotic figures were counted in ten ×40 fields within and around these regions of high proliferative activity. For cases in which no mitotic figures were identified on scanning and cases that showed a relatively uniform distribution of mitotic figures, 10 representative fields were selected for evaluation of mitotic rate. Primary and metastatic tumors were categorized as low grade (G1), intermediate grade (G2), or G3 as determined by mitotic count using standard American Joint Committee on Cancer (AJCC) staging criteria as follows: low grade (G1:<2 mitotic figures [MFs] per 10 high-power fields [HPFs]), intermediate grade (G2: 2–20 MFs per 10 HPFs), high grade (G3:>20 MFs per 10 HPFs).14 For cases that had more than one metastatic tumor or more than one grade, the highest grade was selected. Statistical comparisons were performed using Fisher's exact test, the Chi square test, and Kaplan-Meier analysis using SPSS version 18 (IBM, Armonk, NY, USA).
Results
Clinicopathologic Characteristics
We identified 20 patients with metastatic neuroendocrine carcinoma treated at our institution between 1997 and 2013 for whom complete pathologic review of primary and metastatic tumors was possible (Table 1). The majority of the patients were female, and the median age at the time of hepatic metastasectomy was 56 years (range 34– 73 years). The most common primary tumor location was the small bowel. The distribution of G1, G2, and G3 histologies was similar between primary and metastatic tumors, and the majority of metastases were synchronous in presentation.
Table 1. Clinicopathologic characteristics.
| n (%) | |
|---|---|
| Median age (years)a | 56 |
| Gender | |
| Female | 14 (70) |
| Male | 6 (30) |
| Primary tumor location | |
| Pancreas | 6 (30) |
| Small intestine | 11 (55) |
| Ampulla | 1 (5) |
| Stomach | 1 (5) |
| Rectum | 1 (5) |
| Primary grade | |
| G1 | 10 (50) |
| G2 | 8 (40) |
| G3 | 2 (10) |
| Metastatic grade | |
| G1 | 7 (35) |
| G2 | 10 (50) |
| G3 | 3 (15) |
| Metastatic presentation | |
| Synchronous | 15 (75) |
| Metachronous | 5 (25) |
At the time of hepatic metastasectomy
Secondary pathologic review of primary and metastatic tumors did not result in any changes in histologic grade from what had been initially reported. However, some of the initial pathology reports did not clearly specify histologic grade. Of 10 cases that had multiple metastases available for secondary pathologic review, two cases demonstrated more than one histologic grade. In these cases, the higher histologic grade was used for statistical analysis. Representative histologic examples are shown in Fig. 1.
Fig. 1.
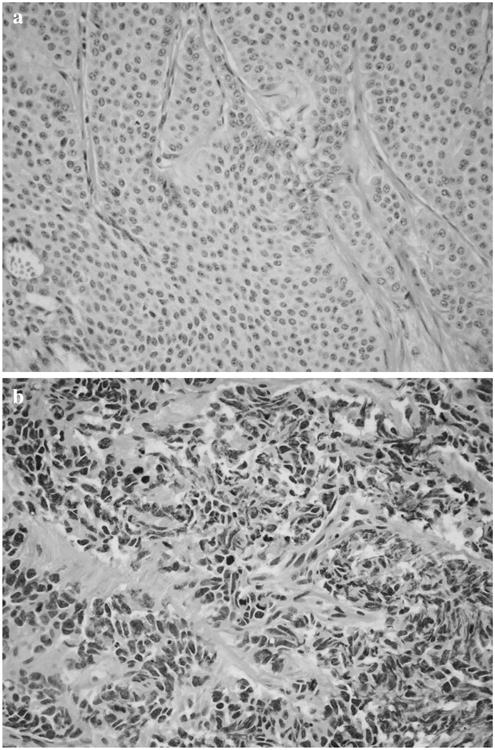
a Representative hematoxylin and eosin (H&E) micrograph of an intermediate-grade (G2) small intestinal primary neuroendocrine carcinoma. b Representative H&E micrograph of a high-grade (G3) hepatic neuroendocrine carcinoma metastasis
Grade Discordance
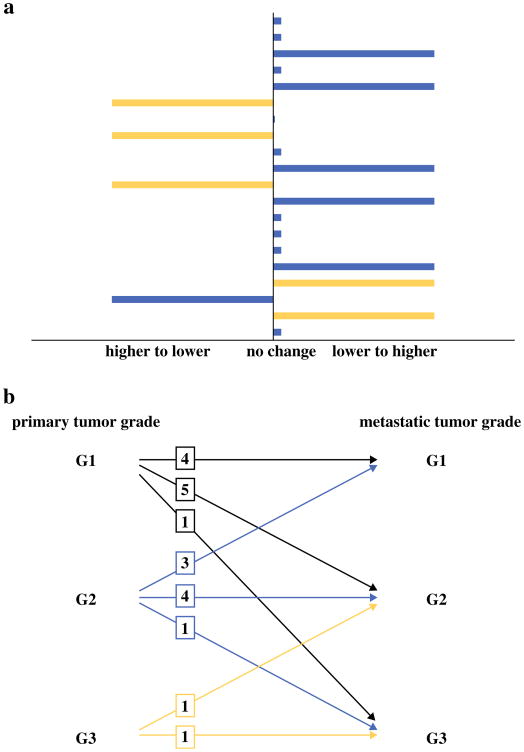
The histologic grades of the primary and metastatic tumors were concordant in nine cases, yielding an overall grade discordance rate of 55 % (Table 2). Among the 11 discordant cases, the grade of the metastasis was higher than the grade of the primary tumor in 7 cases and lower than the grade of the primary tumor in 4 cases. Of the seven cases in which the metastatic grade was higher than the primary grade, five had a grade change of G1 to G2, one had a change from G2 to G3, and one had a change from G1 to G3. Of the four discordant cases in which the metastatic grade was lower than the primary grade, three had a grade change from G2 to G1, and one had a grade change from G3 to G2.
Table 2. Univariate analysis of clinicopathologic factors associated with grade discordance.
| Grade discordance (%) | p Value | |
|---|---|---|
| Age (years)a | ||
| <56 | 56 | |
| ≥56 | 55 | NS |
| Gender | ||
| Female | 57 | |
| Male | 50 | NS |
| Primary tumor location | ||
| Pancreas | 71 | |
| Other | 46 | NS |
| Primary tumor grade | ||
| G1 | 60 | |
| G2 | 50 | |
| G3 | 50 | NS |
| Metastatic presentation | ||
| Synchronous | 40 | |
| Metachronous | 100 | 0.03 |
NS not significant
At the time of hepatic metastasectomy
In the univariate analysis, the only clinicopathologic variable associated with histologic grade discordance was metachronous presentation (p = 0.01). Indeed, the histologic grade of all the metachronous metastases in our series differed from that of their primary tumors (Fig. 2a). Patients with metachronous metastases were just as likely to present with a lower metastatic grade as a higher metastatic grade than the primary grade (Fig. 2b). None of the variables tested were associated with a likelihood of having a metastatic grade specifically higher than the primary grade (data not shown).
Fig. 2.
a Distribution of histologic grade change from primary to metastatic foci of neuroendocrine carcinoma. Blue bars denote patients who presented with synchronous hepatic metastases, and yellow bars denote patients who presented with metachronous hepatic metastases. b Patterns of histologic grade changes from primary to metastatic foci of neuroendocrine carcinoma. Boxes indicate the number of patients who exhibited each pattern of grade change
Survival
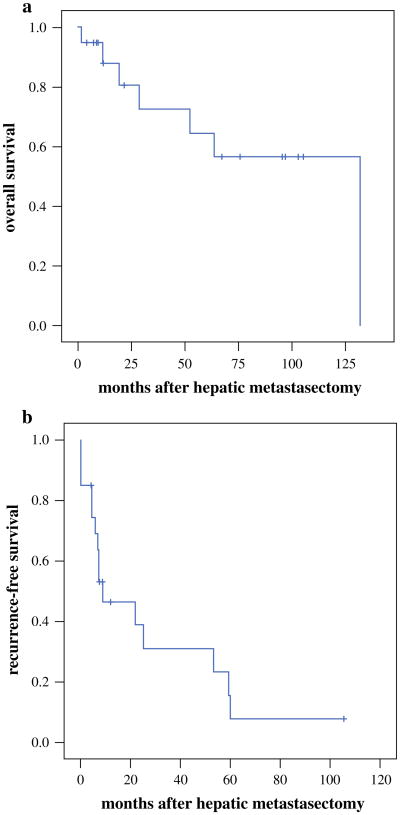
The median follow-up period for surviving patients was 67 months (5.6 years) from the time of primary tumor resection and 45 months (3.8 years) from the time of hepatic metastasectomy. Kaplan-Meier estimates of median overall survival and post metastasectomy recurrence-free survival were respectively 251 months (20.9 years) and 20 months (1.7 years) from the time of primary tumor resection and respectively 131 months (10.9 years) and 9 months (0.8 years) from the time of hepatic metastasectomy (Fig. 3). No statistically significant differences in overall or recurrence-free survival were observed between patients whose metastatic grade was lower, higher, or unchanged compared with the primary grade (data not shown).
Fig. 3.
a Overall survival estimates for the entire cohort of patients from the time of hepatic metastasectomy. b Recurrence-free survival estimates for the entire cohort of patients from the time of hepatic metastasectomy
Discussion
In the management of neuroendocrine neoplasms, the prognostic importance of histologic grade as assessed by cellular proliferative activity has been well established.5–14 The AJCC grading system classifies neuroendocrine tumors as G1, G2, and G3 based on mitotic count, Ki67 staining positivity, or both.14 Our previous work strongly suggests that this pathologic classification schema retains prognostic significance when applied to both primary and metastatic tumors, suggesting that histologic assessment can inform surgical treatment planning for patients with both localized and metastatic disease.6,7,13 For example, surgical therapy is routinely avoided in favor of systemic chemotherapy for patients presenting with biopsy-proven, high-grade neuroendocrine carcinoma. To date, whether and how often the histologic grade varies between primary and metastatic foci of disease in the same patient are unclear.
In this study, we observed a high (55 %) prevalence of histologic grade discordance between primary and metastatic tumors. The relatively small number of patients in our series prevented exhaustive analyses of the biologic underpinnings and prognostic impact of this phenomenon. However, we observed a very strong correlation between timing of metastatic presentation and grade discordance. Whereas the prevalence of primary versus metastatic grade discordance was 40 % among the patients with synchronous metastases, 100 % of the patients who presented with metachronous metastases exhibited grade discordance. The fact that metastatic tumors were not of uniformly higher grade than primary tumors argues against the potential mechanistic explanation that more aggressive clones of primary cancer cells are more likely to disseminate and establish metastases.
In contrast to the bidirectional grade changes we observed between primary and metastatic tumors, Zen and Heaton15 recently reported four cases of neuroendocrine carcinoma grade discordance, all of which involved an increase in grade category from primary to metastatic tumor. The current study was limited by the small sample size. However, our findings substantiate a very recent and similar analysis from the Odette Cancer Centre in Toronto. In this study, Singh et al.16 identified a relatively high prevalence of intertumor variability in the Ki-67 staining index among patients with multiple neuroendocrine tumor biopsies. They also observed that 14 (36 %) of 39 patients with biopsies of both primary and metastatic tumor sites were noted to have discordant grade classifications. Notably, the histologic data used in this analysis were based largely on core biopsy specimens because only five of these patients had undergone operative tumor resection. Another recent and very similar analysis from Hammersmith Hospital in London identified a similar prevalence in grade discordance of 35.3 %.17
Intratumoral heterogeneity is well recognized as a common feature of neuroendocrine carcinoma.18 Therefore, a potential underrepresentation of intertumoral grade heterogeneity by core biopsy specimen analysis may explain why our analysis of resected tumors identified a higher prevalence of grade discordance. In conjunction with these previous studies, our study's reliance on comprehensive pathologic analysis of resected tumors (vs needle biopsies) strongly reinforces the suggestion that the biologic behavior of neuroendocrine carcinoma may be polyclonal in nature.
Our limited sample size prevented definitive evaluation of the prognostic significance of changes in histologic grade between primary and metastatic tumors. However, given the prognostic importance of histologic grade for both primary and metastatic neuroendocrine carcinoma, we conclude that our study potentially implies that biopsies of both primary and metastatic tumors may have clinical utility in some clinical circumstances. For example, for patients with a history of a resected low-grade pancreatic neuroendocrine carcinoma presenting with potentially resectable hepatic metastases, it may be advisable to consider percutaneous biopsy to exclude the possibility of high-grade metastatic disease. Similarly, consideration may need to be given to serial biopsies for patients exhibiting a change in the clinical growth pattern of multifocal or recurrent tumors. In addition, we found more than one metastatic grade in 2 of 10 cases with multiple metastases available for secondary pathologic review. This prevalence of heterogeneity suggests that careful pathologic analysis of all metastatic foci should be undertaken for patients with multifocal metastatic disease. Further investigation with larger data sets will help to validate these potential recommendations.
Footnotes
Presented at the Society of Surgical Oncology Annual Cancer Symposium in Phoenix, Arizona, March, 2014.
There are no conflicts of interest.
Disclosure The contents of this report do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- 1.Busch RA, Cho CS. Pancreatic neuroendocrine neoplasms. In: Zyromski NJ, editor. Handbook of hepato-pancreato-biliary surgery Wolters. Kluwer; Philadelphia: 2015. pp. 62–75. [Google Scholar]
- 2.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 3.Landry CS, Scoggins CR, McMasters KM, Martin RC., II Management of hepatic metastasis of gastrointestinal carcinoid tumors. J Surg Oncol. 2008;97:253–8. doi: 10.1002/jso.20957. [DOI] [PubMed] [Google Scholar]
- 4.Ramage JK, Ahmed A, Ardill J, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumors. Gut. 2012;561:6–32. doi: 10.1136/gutjnl-2011-300831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hochwald SN, Zee S, Conlon KC, Colleoni R, Louie O, Brennan MF, Klimstra DS. Prognostic factors in pancreatic endocrine neoplasms: an analysis of 136 cases with a proposal for low-grade and intermediate-grade groups. J Clin Oncol. 2002;20:2633–42. doi: 10.1200/JCO.2002.10.030. [DOI] [PubMed] [Google Scholar]
- 6.Cho CS, Labow DM, Tang L, et al. Histologic grade is correlated with outcome after resection of hepatic neuroendocrine neoplasms. Cancer. 2008;113:126–34. doi: 10.1002/cncr.23523. [DOI] [PubMed] [Google Scholar]
- 7.Ballian N, Loeffler AG, Rajamanickam V, Norstedt PA, Weber SM, Cho CS. A simplified prognostic system for resected pancreatic neuroendocrine neoplasms. HPB. 2009;11:422–8. doi: 10.1111/j.1477-2574.2009.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rindi G, D'Adda T, Froio E, Fellagra G, Bordi C. Prognostic factors in gastrointestinal endocrine tumors. Endocr Pathol. 2007;18:145–9. doi: 10.1007/s12022-007-0020-x. [DOI] [PubMed] [Google Scholar]
- 9.Ferrone CR, Tang LH, Tomlinson J, et al. Determining prognosis in patients with pancreatic endocrine neoplasms: can the WHO classification system be simplified? J Clin Oncol. 2007;25:5609–15. doi: 10.1200/JCO.2007.12.9809. [DOI] [PubMed] [Google Scholar]
- 10.Pape UF, Jann H, Muller-Nordhorn J, et al. Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer. 2008;113:256–65. doi: 10.1002/cncr.23549. [DOI] [PubMed] [Google Scholar]
- 11.Strosberg J, Nasir A, Coppola D, Wick M, Kvols L. Correlation between grade and prognosis in metastatic gastroenteropancreatic neuroendocrine tumors. Hum Pathol. 2009;40:1262–8. doi: 10.1016/j.humpath.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Bosman F, Carneiro F, Hruban R, et al. WHO classification of tumours of the digestive system. Lyon: IARC Press; 2010. [Google Scholar]
- 13.Martin RC, Kooby DA, Weber SM, et al. Analysis of 6747 pancreatic neuroendocrine tumors for a proposed staging system. J Gastrointest Surg. 2011;15:175–83. doi: 10.1007/s11605-010-1380-y. [DOI] [PubMed] [Google Scholar]
- 14.American Joint Committee on Cancer. Neuroendocrine tumors. In: Edge SB, editor. AJCC cancer staging manual. 7th. Philadelphia: Lippincott-Raven; 2010. pp. 181–90. [Google Scholar]
- 15.Zen Y, Heaton N. Elevated Ki-67 labeling index in “synchronous liver metastases” of well-differentiated enteropancreatic neuroendocrine tumor. Pathol Int. 2013;63:532–8. doi: 10.1111/pin.12108. [DOI] [PubMed] [Google Scholar]
- 16.Singh S, Hallet J, Rowsell C, Law CHL. Variability of Ki67 labeling index in multiple neuroendocrine tumors specimens over the course of the disease. Eur J Surg Oncol. 2014;40:1517–22. doi: 10.1016/j.ejso.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Miller HC, Drymousis P, Flora R, Goldin R, Spalding D, Frilling A. Role of Ki-67 proliferation index in the assessment of patients with neuroendocrine neoplasias regarding the stage of disease. World J Surg. 2014;38:1353–61. doi: 10.1007/s00268-014-2451-0. [DOI] [PubMed] [Google Scholar]
- 18.Yang Z, Tang LH, Klimstra DS. Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: implications for prognostic stratification. Am J Surg Pathol. 2011;35:853–60. doi: 10.1097/PAS.0b013e31821a0696. [DOI] [PubMed] [Google Scholar]