Abstract
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by social and communication impairments, as well as repetitive and restrictive behaviors. The phenotypic heterogeneity of ASD has made it overwhelmingly difficult to determine the exact etiology and pathophysiology underlying the core symptoms, which are often accompanied by comorbidities such as hyperactivity, seizures, and sensorimotor abnormalities. To our benefit, the advent of animal models has allowed us to assess and test diverse risk factors of ASD, both genetic and environmental, and measure their contribution to the manifestation of autistic symptoms. At a broader scale, rodent models have helped consolidate molecular pathways and unify the neurophysiological mechanisms underlying each one of the various etiologies. This approach will potentially enable the stratification of ASD into clinical, molecular, and neurophenotypic subgroups, further proving their translational utility. It is henceforth paramount to establish a common ground of mechanistic theories from complementing results in preclinical research. In this review, we cluster the ASD animal models into lesion and genetic models and further classify them based on the corresponding environmental, epigenetic and genetic factors. Finally, we summarize the symptoms and neuropathological highlights for each model and make critical comparisons that elucidate their clinical and neurobiological relevance.
Keywords: Autism spectrum disorders, Animal models, Genetic factors, Environmental factors, Clinical relevance
INTRODUCTION
Autism spectrum disorder (ASD) is a prototypical pervasive developmental disorder resulting from abnormal brain development. ASD constitutes two main behavioral symptoms including impairment in social interactions and communication, and restricted, repetitive behaviors, diagnosed at an early age in development (American Psychiatric Association, 2013). The disorder is often accompanied by sensory processing abnormalities (Rogers et al., 2003), sleep problems (Schreck et al., 2004), anxiety and depression (Strang et al., 2012), hyperactivity (Aman and Langworthy, 2000), aggression or self-injurious behaviors (Singh et al., 2006), seizures (Volkmar and Nelson, 1990) and eating or digestive problems (Martins et al., 2008), among others. ASD is incredibly heterogeneous and is commonly comorbid with other psychiatric and neurodevelopmental disorders or syndromes (Leyfer et al., 2006). As one may expect, this condition causes great hardship to affected families, as it may lead to social, occupational and other functional afflictions (American Psychiatric Association, 2013).
Research over the last several decades has identified various risk factors leading to ASD, which can be classified into genetic abnormalities, epigenetic alterations and environmental insults (Gepner and Feron, 2009). Genetic risk factors take the form of monogenic mutations, single nucleotide polymorphism (SNP), and copy number variants (CNVs). Not surprisingly, many of the implicated genes have been associated with modulation of brain development and cortical organization, synapse formation and function, and neurotransmission. On the other hand, environmental insults implicated in ASD comprise prenatal exposure to viral infections or chemicals, including rubella (Chess et al., 1978), cytomegalovirus (Yamashita et al., 2003), thimerosal (Bernard et al., 2002), thalidomide (Stromland et al., 2002) and alcohol (Aronson et al., 1997). Accordingly, it has been shown that both genetic and environmental factors associated with the disorder can directly induce epigenetic disruptions, as is the case of mutations in MeCP2 gene (Amir et al., 1999) and abnormal methylation of the imprinted region of the UBE3A gene (Jiang et al., 2004). Likewise, prenatal valproate (VPA) exposure (Christianson et al., 1994) is thought to mechanistically affect the epigenome at critical developmental stages and lead to ASD. More recently, the effect of increased maternal and paternal age have been linked to ASD risk, mainly by increasing the proportion of copy number variants and de novo mutations in their offspring (Lee and McGrath, 2015).
Based on the identified risk factors and possible etiology of ASD, animal models were created to mimic and understand the pathological mechanisms underlying the behavioral abnormalities of this disorder. Two main types of animal models, the lesion and genetic models thus became apparent. The lesion models include prenatal infection (neonatal Borna disease virus), neonatal amygdala lesion, and prenatal VPA exposure. Thalidomide exposure, which showed different effects between primates and rodents, may not be an appropriate animal model of ASD (Kemper and Bauman, 1993). Genetic models consist of knockout mice of various isolated genes that are thought to be involved in the pathology of both syndromic and non-syndromic ASD such as FMR1 (Fragile X syndrome), NF1 (Neurofibromatosis type 1), TSC1 (Tuberous sclerosis), DHCR7 (Smith-Lemli-Opitz syndrome), MeCP2 (Rett syndrome), SHANK2, CNTNAP2, Eukaryotic translation initiation factor 4E (eIF4E), transgenic mouse targeting Oxytocin, Vasopressin, Reelin, Dishevelled-1, Sert (serotonin transporter), Maoa (monoamine oxidase A), HOXA1, PTEN and Neuroligins.
Although the number of animal models for ASD is rapidly expanding and will continuously increase in upcoming years, systemic efforts to concisely assess their clinical relevance and neurobiological significance are still scarce. In this review, we will tackle the two main classes of ASD animal models, lesion and genetic models, in order to dissect them into specific sub-groups to find out whether the studies of these models have neurobiological relevance to the clinical setting (Table 1).
Table 1.
Neurobiological and behavioral features of ASD animal models in relation to their clinical relevance
| Animal model of ASD | Features of animal model | Clinical behavioral and neurobiological findings | Citations | ||
|---|---|---|---|---|---|
|
| |||||
| Core symptoms of ASD | Other symptoms | Neurobiological defects | |||
| A. Lesion Models | |||||
| Amygdala lesion |
|
|
|
|
aSchumann et al., 2004 bBauman and Kemper, 1985 cKemper and Bauman, 1998 dBarnea-Goraly et al., 2014 eTottenham et al., 2014 fAmaral et al., 2003 gWolterink et al., 2001 hDaenen et al., 2002 iDiergaarde et al., 2005 |
| Cerebellum lesion |
|
|
|
|
aPierce and Courchesne, 2001 bCourchesne et al., 1994 cAllen and Courchesne, 2003 dJoyal et al., 1996 eBobee et al., 2000 fMartin et al., 2010 gWang et al., 2014 |
| mPFC lesion |
|
ND |
|
|
aCarper and Courchesne, 2005; Hazlett et al., 2005 bEslinger et al., 2004 cSchneider and Koch, 2005 |
| Maternal BDV infection |
|
ND |
|
|
aMazina et al., 2015 bHornig et al., 1999 cPletnikov et al., 1999, 2002 dTaieb et al., 2001 |
| Prenatal LPS exposure |
|
|
|
|
aMazina et al., 2015 bJyonouchi et al., 2005 cHava et al., 2006 dWang et al., 2010 eGolan et al., 2005 fRomero et al., 2007 gPatterson, 2009 hLowe et al., 2008 iLante et al., 2008 |
| Prenatal Poly (I:C) exposure |
|
|
|
|
aMazina et al., 2015 bMeyer et al., 2011; cPatterson, 2009 dNaviaux et al., 2013 eShi et al., 2009 |
| Prenatal VPA exposure |
|
|
|
|
aChristianson et al., 1994 bOrnoy, 2009 cMoore et al., 2000 dMoore et al., 2000 eOrnoy, 2009; Kataoka et al., 2013; Kim et al., 2014c fSchneider and Koch, 2005; Schneider et al., 2008; Patterson, 2009 gDufour-Rainfray et al., 2010; hKim et al., 2011 iSpence and Schneider, 2009 jRinaldi et al., 2008 kRinaldi et al., 2007 lMartin and Manzoni, 2014 mKuwagata et al., 2009 nFukuchi et al., 2009 oTyzio et al., 2014 pJung et al., 2008 qGo et al., 2012 rKim et al., 2014b |
| BTBR T+ tf/J mice |
|
ND |
|
ND |
aWahlsten et al., 2003 bBolivar et al., 2007 cAmodeo et al., 2012; McFarlane et al., 2008 dWohr et al., 2011a eBlanchard et al., 2012; fStephenson et al., 2011 |
| B. Genetic Models | |||||
| BDNF−/+mice |
|
|
|
|
aBryn et al., 2015 bPerry et al., 2001 cTsai, 2005 dPardo and Eberhart, 2007 eTaurines et al., 2014 fWeidner et al., 2014 gPapaleo et al., 2011 |
| DHCR7 mutant mice |
|
|
|
|
aIrons et al., 1993 bTierney et al., 2000 cTierney et al., 2006 dMoy et al., 2009 eCorrea-Cerro et al., 2006 fWaage-Baudet et al., 2003 gKorade et al., 2013 |
| EN2−/− mice |
|
|
|
|
aBenayed et al., 2005; bCheh et al., 2006 cKuemerle et al., 2007 dKuemerle et al., 1997 |
| FMR1 knockout mice |
|
|
|
|
aGarber et al., 2008; bHatton et al., 2006 cBernardet and Crusio, 2006 dSilva and Ehninger, 2009 eBrennan et al., 2006 fSpencer et al., 2005; McNaughton et al., 2008 vs Mineur et al., 2006; Liu and Smith, 2009 gIrwin et al., 2000 hNosyreva and Huber, 2006 iBear et al., 2004 jZhang et al., 2009 kHe et al., 2014; Tyzio et al., 2014 lMaccarrone et al., 2010; Jung et al., 2012; Busquets-Garcia et al., 2013; |
| GABRB3 knockout mice |
|
|
|
|
aWagstaff et al., 1991; Nakao et al., 1994; bSamaco et al., 2005 cBlatt et al., 2001 dFatemi et al., 2002 eBuxbaum et al., 2002 fHomanics et al., 1997 gDeLorey et al., 2008 hSinkkonen et al., 2003 |
| MeCP2 knockout mice |
|
|
|
|
aRett, 1966 bChahrour and Zoghbi, 2007 cGuy et al., 2001 dMoretti et al., 2006 eGuy et al., 2011 fMaezawa and Jin, 2010 gBallas et al., 2009 hLarimore et al., 2009 iChao et al., 2010 |
| MAOA deficient mice |
|
|
|
|
aHunter, 2010 bCohen et al., 2003 cHranilovic et al., 2008 dDavis et al., 2008 eCases et al., 1995 fSingh et al., 2013 gPopova et al., 2000 hBortolato et al., 2013 |
| NF1+/− mice | ND |
|
|
|
aRasmussen and Friedman, 2000 bHusi et al., 2000 cSilva et al., 1997 dCosta et al., 2002 eLush et al., 2008 fMbarek et al., 1999; Marui et al., 2004 |
| NLGN1 knockout mice |
|
|
|
|
aYlisaukko-oja et al., 2005 bBlundell et al., 2010 |
| NLGN3 R451C mutant mice |
|
|
|
|
aJamain et al., 2003 bComoletti et al., 2004 cEtherton et al., 2011 dJamain et al., 2008; eRadyushkin et al., 2009 |
| NLGN4 mutant mice |
|
|
|
|
aJamain et al., 2003; bGauthier et al., 2005; cJamain et al., 2008; dRadyushkin et al., 2009 |
| NRXN1α deficient mice |
|
|
|
|
aChing et al., 2010 bEtherton et al., 2009 |
| Oxt deficient mice |
|
|
|
|
aGuastella et al., 2008; Savaskan et al., 2008 bInsel, 2010 cAndari et al., 2010 dHollander et al., 2003 eGuastella et al., 2010 fFerguson et al., 2001; gKavaliers et al., 2003 hWinslow and Insel, 2002 |
| Oxtr-null mice |
|
|
|
|
aJacob et al., 2007; Gregory et al., 2009; Wu et al., 2005; Liu et al., 2010; bLee et al., 2008 cPobbe et al., 2012 dSala et al., 2011 eParker et al., 2014 |
| MAGEL2-deficient mice |
|
|
|
|
aBoccaccio et al., 1999 bSchaaf et al., 2013 cMeziane et al., 2015 dSchaller et al., 2010 |
| PTEN mutant mice |
|
|
|
|
aButler et al., 2005 bKwon et al., 2006 cOgawa et al., 2007 dLuikart et al., 2011 eGregorian et al., 2009; Bonaguidi et al., 2011 |
| Reeler (rl/rl) and mutant (+/rl) mice |
|
|
|
|
aOgnibene et al., 2007 bGillberg, 1998; cMartin, 1981; Goffinet, 1983; Yip et al., 2000; D’Arcangelo, 2005 dPatrylo et al., 2006 eMarrone et al., 2006 fTueting et al., 1999 gSalinger et al., 2003 hPodhorna and Didriksen, 2004 iIafrati et al., 2014 |
| Heterozygous reeler (+/rl) mice | ND |
|
|
|
|
| SERT knockout mice |
|
|
|
|
aHranilovic et al., 2008 bBetancur et al., 2002; Huang et al., 2008 cKalueff et al., 2007 dHolmes et al., 2003; eJiang et al., 2009 fAltamura et al., 2007 gPrasad et al., 2009 hVeenstra-Vanderweele et al., 2009 |
| SHANK1−/− mice |
|
|
|
|
aJiang and Ehlers, 2013 bHung et al., 2008 cWohr et al., 2011b dSilverman et al., 2011 eSato et al., 2012 |
| SHANK2−/− mice |
|
|
|
|
aBerkel et al., 2010; Leblond et al., 2012 bBerkel et al., 2012 cSchmeisser et al., 2012 dWon et al., 2012 |
| SHANK3 mutant mice |
|
|
|
|
aManning et al., 2004 bDurand et al., 2007; cPeca et al., 2011 dGauthier et al., 2010 eYang et al., 2012 fBozdagi et al., 2010 |
| Conditional TSC1 knockout mice |
|
|
|
|
aBolton et al., 2002; Curatolo et al., 2004 bDiMario, 2004; Goorden et al., 2007 cUhlmann et al., 2002 dMeikle et al., 2007 eBateup et al., 2011 fCarson et al., 2012 gTsai et al., 2012 hEhninger et al., 2012 iOnda et al., 2002 jYoung et al., 2010 kChevere-Torres et al., 2012 lReith et al., 2011 mAuerbach et al., 2011 nTang et al., 2014 oLozovaya et al., 2014 |
| Conditional TSC2 knockout mice |
|
|
|
||
| UBE3A mutation |
|
|
|
|
aWagstaff et al., 1991; Nakao et al., 1994; bSmith et al., 2011 cJiang et al., 2010 dMiura et al., 2002 eGreer et al., 2010 fGuffanti et al., 2011 |
| Brattleboro rats |
|
|
|
|
aWassink et al., 2004; Yirmiya et al., 2006 bSchmale et al., 1989 cEngelmann and Landgraf, 1994 dGardiner and Bennett, 1983 eBielsky et al., 2005; Insel, 2010 fWersinger et al., 2004; Caldwell et al., 2008 |
| V1aR knockout mice |
|
|
ND | ||
| V1bR knockout mice |
|
ND | ND | ||
| CNTNAP2−/− mice |
|
|
|
|
aAlarcón et al., 2008; Arking et al., 2008 bPeñagarikano et al., 2011 cWhitehouse et al., 2011 dScott-Van Zeeland et al., 2010 eSampath et al., 2013 |
| eIF4E overexpression mice |
|
ND |
|
|
aSzatmari et al., 2007 bSantini et al., 2013 |
All descriptions with supercript alphabets correspond to their authors in the citation area and these alphabet sets have uniquecitations for each animal model. ND: no data, MIA: maternal immune activation, Italicized sentences-negative, controversial or normal findings, USVs: ultrasonic vocalizations.
LESION MODELS
Anatomical lesion models
Anatomical lesion models have long been used to isolate brain regions involved in the pathologic pathways of a number of neurological disorders. In the case of autism, however, brain damage induced by a gross chemical or electrolytic lesion of specific regions have not been able to even remotely replicate human ASD. The complexity of human development and the heterogeneity that is found in ASD phenotypes could perhaps be two of the main reasons that make the recapitulation of ASD much difficult in this type of postnatal lesion animal models. Moreover, the impairments found in ASD are not likely to be rooted only from a single neural circuit or brain region.
Amygdala:
Early life dysfunction and alterations in the amygdala have long been linked to autistic behaviors (Bachevalier, 1994). Indeed, autistic children have been found to have enlarged amygdala and decreased neuronal cell size within this region, despite increased cell density brain-wide (Kemper and Bauman, 1998). Interestingly, changes in amygdala volume throughout development was directly correlated with initiating communicative eye contact in autistic children (Barnea-Goraly et al., 2014). In addition, other studies found that ASD patients tend to avoid eye contact on facial gaze tasks and showed increased amygdala activity during gaze manipulation along with an increase in subjective threat ratings (Tottenham et al., 2014).
Most established animal models used to assess the contribution of amygdala to autistic behaviors have utilized rats that have undergone direct amygdala lesions or indirect impairment using other etiologic factors. Initial studies from amygdala-lesioned rats (at postnatal day 7) which were subjected to juvenile isolation prior to testing, showed that these animals developed a tendency for stereotyped walking (Wolterink et al., 2001), decreased social play and exploration (Wolterink et al., 2001), impaired social interaction, and decreased spatial learning and memory in the spontaneous alternation task (Diergaarde et al., 2005). However, similar lesions to the amygdala of macaque monkeys failed to affect their social behaviors, and mostly impaired their fear learning and anxiety behaviors (Amaral et al., 2003). These discrepancies are not surprising, as data from humans with ASD show similar inconsistencies.
Accordingly, it seems that ASD-related alterations in amygdala size vary across developmental time-points; some studies have shown that this region is enlarged in children with ASD, whereas it has been found to be smaller in size in adolescents or young adults with the disorder, compared to controls (Schumann et al., 2004). These findings suggest that the involvement of amygdala in ASD is rather complex, making it harder to model both with targeted lesions and in animals. Yet, even if the hallmark social behavior deficits associated with ASD are inconclusive with regards to the amygdala, it is still possible that fear and anxiety phenotypes in ASD are still linked to functional deficits in this brain region (Amaral et al., 2003). Recently, however, another clinical study failed to find a relationship between ASD and amygdala dysfunction, using visual tasks as a measure of impaired social attention (Lee and McGrath, 2015). Thus, further study is needed to clarify and consolidate the results regarding the involvement of amygdala in ASD pathology and to encourage its validity in the modeling of the disorder.
Cerebellum:
Anatomical abnormalities of the cerebellum have been widely observed in human autistic patients (Bauman and Kemper, 1985). Reported abnormalities include hypoplasia of cerebellar vermal lobules VI and VII (Courchesne et al., 1994), which were negatively correlated to increased repetitive behaviors and decreased exploratory behavior in autistic children (Pierce and Courchesne, 2001), results which have been somewhat controversial. Loss of Purkinje cells in the cerebellar vermis and cortex was also reported in human autistic subjects (Allen and Courchesne, 2003). A comprehensive review had been published explaining the pathological involvement of the cerebellum in the development of autism (Fatemi et al., 2012). The review highlighted that in some autistic patients, there are cerebellar abnormalities covering anatomical defects, inflammation, oxidative stress, abnormal neurotransmitters and protein levels, and cerebellum-related motor and cognitive impairments. Moreover, the pathological onset of cerebellar defects in autism is at an early age, which affects the development later in life. Lastly, various risk factors involving genetics, environment, or their combination can affect the development of the cerebral circuitry found in autism (Fatemi et al., 2012).
In rats, lesions in the midline of the cerebellum cause visuomotor defects in the Morris water maze test, despite any spatial memory defects in the spontaneous alternation test (Joyal et al., 1996). Moreover, early postnatal cerebellum lesions have been shown to increase spontaneous motor activity and decrease anxiety-like behavior in rodents (Bobee et al., 2000). Using the lurcher mutation model, which provides a reasonable mouse model of cerebellar defects (Martin et al., 2004), it was shown that the loss of Purkinje cells in mice induced significantly increased repetitive behaviors (Martin et al., 2010). As a whole, these cerebellum lesion studies in rodents confirm a potential role of this structure in producing some of the motor, repetitive and exploratory behavioral deficits or anxiety-like behaviors, observed in autism (Pierce and Courchesne, 2001). This is also consistent with the fact that cerebellar injury at birth is a risk factor for ASD in children (Wang et al., 2014). Indeed, cerebellar damage animal models could be an important tool for isolating the role of the cerebellum in specific autistic symptoms.
Medial prefrontal cortex (mPFC):
Studies have shown that brain overgrowth in the mPFC and dorsolateral PFC is generally pronounced in autistic patients (Carper and Courchesne, 2005; Hazlett et al., 2005). Aside from PFC overgrowth, early PFC damage in humans has also been shown to impair social interaction and cognition (Eslinger et al., 2004). In rats, neonatal mPFC lesions decrease social play, conditioned place-preference associated with social contacts, and social grooming (Schneider and Koch, 2005). Interestingly, similar lesions in adult rats do not seem to affect social interactions as much (Schneider and Koch, 2005), suggesting that these deficits arise early in development. As morphological changes in mPFC have been linked to ASD, the exact neurobiological pathway pertaining to social deficits should be further studied. When doing so, nonetheless, it is important to be aware of the fact that alterations in mPFC function are not unique to ASD, as they also overlap with other neurological conditions.
Maternal infection
Maternal immune activation (MIA) is a proposed risk factor for abnormal fetal brain development leading to neurodevelopmental disorders such as ASD (Mazina et al., 2015). It has been shown that MIA during pregnancy in rodents leads to a dysregulated immune system in offspring and also results in autism-related phenotypes that persist well into adulthood (Patterson, 2011). In more detailed molecular studies, interleukin-6 (IL-6) has been suggested to play a mechanistic role in the transcriptional and behavioral abnormalities of MIA in offspring (Smith et al., 2007). This could parallel some reports from human ASD cases, where mothers of affected individuals display elevated IL-6 levels, as is observed in depression and schizophrenia (Daniels et al., 2008). This overlap with other neuropsychiatric disorders is also to be taken into consideration when using animal models of MIA. Yet, the fact that IL-6 has consistently been found to be increased in ASD brains (Benvenuto et al., 2009) and plasma (Ashwood et al., 2011), makes it a very worthy line of study. Furthermore, recent progress in animal models of MIA have shown promising results, yet further studies will be needed to connect immune dysregulation with ASD pathophysiology (Hsiao et al., 2012). Below, we briefly describe the three most commonly used MIA models of ASD.
Prenatal BDV (Borna Disease Virus) infection was the first virus-induced animal model of ASD (Pletnikov et al., 2002). BDV, a transmissible, progressive and lethal virus that causes encephalomyelitis in horses and sheep, is associated with neurologic impairments and behavioral disorders in humans (Richt et al., 1997). Prenatally BDV-infected rats, which have been commonly used to investigate pathogenic mechanisms (Taieb et al., 2001), show increased stereotypy (Hornig et al., 1999), decreased social play (Pletnikov et al., 2002) and impaired social interactions (Pletnikov et al., 1999), which are clearly ASD-related phenotypes. These BDV-infected rats also display abnormalities in postnatal hippocampus and cerebellum development (Taieb et al., 2001). Although BDV infection shows behavioral changes relevant to autism in offspring of affected animals, its implication and reflection to human condition is still elusive and needs to be studied further for clinical comparison, as no study directly related BDV exposure with autism in humans.
Lipopolysaccharide (LPS) is another immune activator found in the outer membrane of gram-negative bacteria that acts as an endotoxin. It is a highly immunogenic antigen that carries the ability to induce antibody responses in vivo (Skidmore et al., 1975). Although there has been no direct association between LPS and autism, peripheral blood mononuclear cells (PBMCs) of ASD patients seem to produce more TNF-α, IL-1β and IL-6 after LPS exposure, compared to controls (Jyonouchi et al., 2005). Interestingly, prenatally LPS-exposed mice display increased anxiety (Wang et al., 2010), decreased social interactions (Hava et al., 2006) and impaired learning and memory (Golan et al., 2005). In rats, gestational LPS exposure significantly decreases pre-pulse inhibition in male offspring (Romero et al., 2007). Other studies have found that LPS-exposed animals show increased cell density, increased excitability of pyramidal neurons and postsynaptic glutamatergic responses to NMDA-induced synaptic plasticity (Patterson, 2009). These findings from both rat and mouse studies indeed support the possibility that LPS-induced MIA can cause ASD phenotypes in offspring. More importantly, further research should be done in order to determine whether, postmortem autistic brains from individuals with a history of prenatal MIA or infection display some of the anatomical deficits observed in rodents.
Poly I:C is a double-stranded RNA that mimics viral infection and can induce MIA. Offspring of rats that have been injected with Poly I:C during pregnancy display autism-related phenotypes, including increased anxiety, stereotypic-repetitive behaviors (Patterson, 2009; Meyer et al., 2011), decreased social interaction and communication, impaired social preference and decreased sensorimotor coordination (Naviaux et al., 2013) and impaired prepulse inhibition (Patterson, 2009; Meyer et al., 2011). In rat and mice brains exposed to poly I:C has been found a spatially localized deficit in Purkinje cells at the lobule VII of the cerebellum, along with heterotrophic morphology and delayed migration of these cells in lobules VI and VII (Naviaux et al., 2013). In addition, synaptosome abnormalities were also observed, both at structural and chemical levels, including PSD malformation, downregulation of purinergic receptors and reduced phosphorylation of ERK1/2 and CAMKII (Naviaux et al., 2013). However, poly I:C induction in rodents have also been used to model schizophrenia (Kumamaru et al., 2014), so careful interpretation of results should be noted.
Overall, MIA animal models of autism could be a great tool not only in predicting the cause but also in identifying the pathophysiologic pathways involved in the disorder. This is supported by the fact that clinical epidemiologic studies can be the only practical and ethical option to identify disease-causing factors in humans. Although the type of microbial pathogens or immune activators may differ in humans to those used in animal models, the autistic effects of maternal MIA in animal models may somehow explain some pathologic mechanisms involved in human ASD. For more details on this topic, please see the works of Patterson (Patterson, 2011).
Prenatal valproate (VPA) exposure
Valproic acid (VPA) is a commonly used pharmaceutical that relieves seizures, migraine headaches and manic episodes related to bipolar disorder. However, several studies have shown that gestational VPA treatment for a life-threatening epilepsy may cause numerous defects in children, including neural tube defects (Ornoy, 2009), intellectual impairments (Moore et al., 2000) and cognitive-behavioral impairments (Moore et al., 2000), many related to the core symptoms of autism. Moreover, prenatal VPA exposure has often been associated with ASD (Christianson et al., 1994). The VPA animal models of ASD have thus been widely used due to their strong etiological and clinical relevance. Here, we mention notable progress and studies utilizing this model.
To investigate the possible effects of VPA on human embryos and explain its effect on brain development, rats and mice models have been widely used, although treatment dosage and injection timings vary among labs (Kataoka et al., 2013; Kim et al., 2014a). Indeed, prenatal VPA exposure to rodents does induce neural tube defects, abnormal brain mass at birth and behavioral impairments in the offspring (Kataoka et al., 2013; Kim et al., 2014a). Similar to autistic symptoms in human patients, both rats and mice that have been subjected to prenatal VPA exposure show increased stereotypic repetitive behaviors (Schneider et al., 2008), impaired social interactions (Kim et al., 2011), and decreased social preference for social novelty (Kim et al., 2011)
The VPA-exposed animal models also showed some common accompanying phenotypes of ASD such as increased anxiety, impaired reversal learning and fear memory processing, abnormal nesting behaviors (Schneider et al., 2008; Patterson, 2009), and decreased prepulse inhibition (Schneider et al., 2008; Patterson, 2009). In addition, our group and others have recently reported that prenatal VPA exposure at embryonic day 12 (E12) in rats recapitulates autism-related phenotypes, including deficits in social interactions (Kim et al., 2011) and increased seizure susceptibility, as is observed in 1/3 of ASD patients (Spence and Schneider, 2009). Interestingly, these deficits were only observed in male offspring, which is quite striking given that male preponderance is a common feature of neurodevelopmental disorders such as ASD and attention-deficit hyperactivity disorder (ADHD) (Rutter et al., 2003).
Physiologically, the brains of VPA-exposed rats display hyper-connectivity and hyper-plasticity in the mPFC region (Rinaldi et al., 2008), along with an increased NMDA/AMPA ratio (Rinaldi et al., 2007). These animals also show dysregulated LTP and decreased NMDAR-mediated currents in adult mPFC, but not in early postnatal life and adolescence (Martin and Manzoni, 2014). These results demonstrate that synaptic abnormalities in the VPA animal model persist well into adulthood and suggest that an aberrant developmental switch in synaptic function of mPFC could underlie some of the ASD-related pathophysiological mechanisms. In other studies, VPA-exposed rats display abnormal neuronal migration (Kuwagata et al., 2009) aberrant GABAA receptor subunit expression and alterations in benzodiazepines binding, as has been observed in ASD patients (Oblak et al., 2011). Moreover, VPA exposure in rats reduced the expression of glutamate decarboxylase, which catalyzes the decarboxylation of glutamate to GABA and CO2 in young neurons suggesting excitatory/inhibitory imbalance (Fukuchi et al., 2009). In naïve rats, GABA currents are excitatory in early development and undergo an inhibitory switch at birth, presumably through neuromodulation by oxytocin. Such shift is not observed (or is delayed) in both the VPA and fragile X models of autism, resulting in neuronal hyperexcitability and increased glutamatergic neurotransmission (Tyzio et al., 2014). These studies showed that lowering the intracellular chloride concentration by treating the dam with bumetanide produced long-term normalization of electrophysiological properties and behaviors in VPA offspring (Tyzio et al., 2014).
In vitro studies have provided additional evidence supporting the profound effects of early developmental exposure to VPA. First, cultured cells that have been treated with VPA show altered neural progenitor cell properties (Jung et al., 2008) and prolonged neurogenesis (Jung et al., 2008). In addition, VPA-exposed premature neurons and N1E-115 cell lines show enhanced expression of PSD-95 (Kim and Thayer, 2009) and increased neurite outgrowth (Yamauchi et al., 2008), respectively. Finally, studies have demonstrated that VPA treatment in vitro can increase the tissue plasminogen activator (tPA) and decrease plasminogen activator inhibitor-1 (PAI-1) activity in astrocytes but not in neurons (Cho et al., 2013). This led to the increased neurite outgrowth via JNK signaling, which could thus be related to the altered neural development in ASD.
A lot of progress has also been made in terms of the molecular mechanisms underlying the VPA animal model of ASD. For example, we found that the histone deacetylase inhibitor (HDACi) function of VPA prevented the apoptosis of neural progenitor cells (NPC). Mechanistically, VPA reduced the expression level of IκBα and activated the NF-κB signaling pathway, leading to increased expression of the anti-apoptotic protein Bcl-XL (Go et al., 2012). In a follow-up investigation, VPA exposure at E12 enhanced Wnt1 signaling, which activated the GSK-3β/β catenin pathway and lead to increased neurogenesis in the embryonic brain (Go et al., 2012). These processes shed light onto potential molecular pathways that could explain prolonged NPC proliferation and neuron overproduction in ASD.
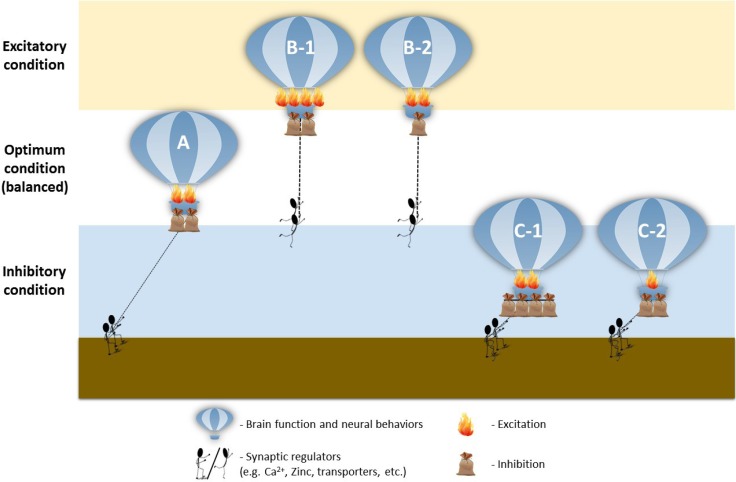
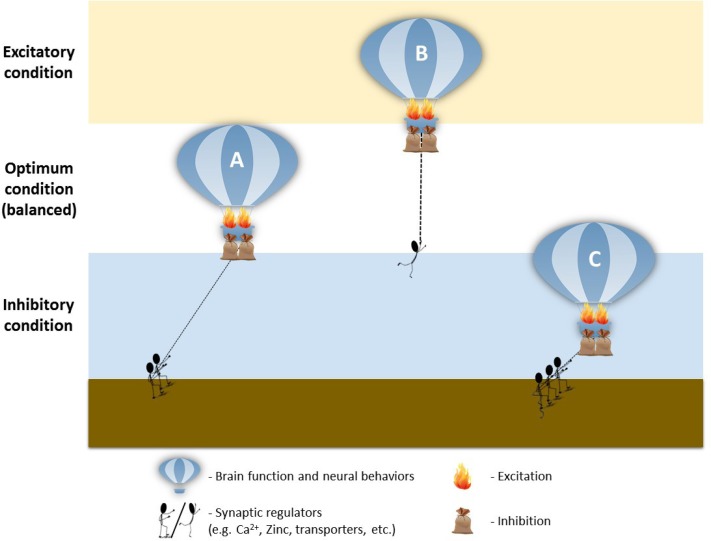
In general, these studies showed that prenatal VPA exposure in rats has profound effects on neurotransmission and leads to excitatory/inhibitory imbalance, reminiscent to the human ASD condition (Kim et al., 2014c). We further discovered that Pax6, a transcription factor that modulates glutamatergic neuronal differentiation, was transiently increased after VPA exposure. This led to the increased sequential expression of additional transcription factors involved in the regulation of glutamatergic differentiation, including Ngn2, Tbr2 and NeuroD1. Ultimately, this series of events directed to the increased glutamatergic neurons in mature brains, marked by increased PSD-95, α-CaMKII, vGluT1 and synaptophysin expression. Conversely, there we found a slight decrease in GABAergic marker Mash1 early in development, followed by a decrease in GAD and Reelin, (Kim et al., 2014c), further highlighting the excitatory/inhibitory ratio shift. This imbalance was concurrent with abnormally elevated kinetic profiles of the glutamatergic NMDA, AMPA and mGluR5 pathways in the PFC of VPA-exposed young rats, through the attenuation of MeCP2 expression (Kim et al., 2014b), suggesting increased excitatory signaling. Intriguingly, Walcott and colleagues found that the increased neuronal excitability in young VPA-exposed rats was gradually corrected to normal levels during the adolescent period, suggesting a delay in neuronal circuit maturation, which prompts further investigation (Walcott et al., 2011).
As can be seen, the VPA model of autism has provided useful insights on potential mechanisms leading to ASD. Consequently, it has been also useful in the search for potential therapeutics for ASD. Our group, for example, used the VPA model to screen for drug treatments targeting known dysregulated pathways and found that NMDA receptor antagonists like MK801 and memantine, as well as the acetylcholinesterase inhibitor donepezil, normalized the social defects in the VPA-exposed animals (Kim et al., 2014a), in addition to bumetanide proposed by Tyzio et al as a therapeutic candidate (Tyzio et al., 2014). Overall, these studies show a great potential for using the VPA animal model of ASD in the mechanistic and therapeutic treatment exploration of ASD.
BTBR T+Itpr3tf/J
The BTBR T+Itpr3tf/J (BTBR) mice are inbred strain mice used as an animal model of ASD, due to its natural traits that resemble ASD-phenotypes. This mouse line is derived from Black and Tan BRachyury inbred strain (BTBR), which carry mutations in at (nonagouti; black and tan), Itpr3tf (inositol 1,4,5- triphosphate receptor 3; tufted), and T (brachyury). Anatomically, BTBR mice exhibit the absence of the corpus callosum and a severe reduction of the hippocampal commis-sure (Wahlsten et al., 2003), as well as high circulating levels of corticosteroid, progesterone, and its 3α,5α-THP metabolite (Frye and Llaneza, 2010). These mice also show altered brain connective tissue, reduced heparan sulfate levels (Blanchard et al., 2012), and reduced adult hippocampal neurogenesis (Stephenson et al., 2011). BTBR mice show decreased social behaviors (Bolivar et al., 2007), defects in ultrasonic vocalizations, (Wohr et al., 2011a), increased stereotyped repetitive behaviors (Amodeo et al., 2012) and repetitive self-grooming (McFarlane et al., 2008). Interestingly, these impairments are alleviated by acute administration of either the mGluR5 antagonist MPEP (Silverman et al., 2010) or the AChE inhibitor, donepezil (Karvat and Kimchi, 2014). Moreover, the SERT blocker fluoxetine enhanced the social interactions in BTBR mice (Chadman, 2011). Indeed, the autism-related phenotypes in this inbred mouse strain could be used as good models for finding therapeutic candidates for broader or idiopathic etiologies and pathophysiologies of ASD.
GENETIC MODELS
Brain-derived neurotrophic factor (BDNF)
BDNF is a secretory protein and a member of the neurotrophic factor family (Binder and Scharfman, 2004), which is widely expressed in the brain and periphery (Murer et al., 2001). BDNF has been proposed as a candidate gene for ASD susceptibility (Pardo and Eberhart, 2007) and plays a key role in the growth and differentiation of new neurons and synapses, as well as in the survival of existing neurons (Huang and Reichardt, 2001). Children with ASD were reported to possess higher BDNF levels in the blood, compared with typically-developing individuals (Bryn et al., 2015). Furthermore, autistic adults have been reported to have increased BDNF levels in the basal forebrain (Perry et al., 2001). These findings, therefore, suggest that over-expression of BDNF at various developmental time points could be associated with ASD and may be an underlying mechanism of brain overgrowth in autistic patients (Tsai, 2005). However, the role of BDNF in ASD etiology remains inconclusive, as recent work demonstrates completely opposite results, finding decreased serum BDNF levels in ASD patients (Taurines et al., 2014). These contradicting outcomes make BDNF a variable and perhaps unreliable biomarker for ASD; yet, it is clear that optimal levels of BDNF are essential for brain development and maintenance of normal brain function.
BDNF function has been extensively studied in mice, mainly through the use of conditional knockouts and mutants. Initial studies in these transgenics found somewhat robust behavioral phenotypes, although they were not initially attributed to autism (MacQueen et al., 2001). One of such phenotypes can be observed in the conditional BDNF knockout mouse, which displays increased locomotor activity in males and depression-like behaviors in BDNF+/− females (Monteggia et al., 2007). Other studies found aggressive behaviors in this model (Chan et al., 2006). The exact mechanism behind these deficits remains elusive, yet some studies suggest that they may be related to alterations in serotonergic signaling (Daws et al., 2007) and 5-HT2A receptor function (Chan et al., 2006).
To this date, only a few researchers have used the BDNF overexpression model (BDNF-tg) to investigate its possible role in ASD pathology. This could mainly be due to the fact that these mice do not display any deficits in social behavior, diminishing its face validity (Weidner et al., 2014). Still, the BDNF overexpression model does recapitulate some ASD-related phenotypes, including high seizure susceptibility in both males and females and other sex-specific phenotypes. For example, male BDNF-tg mice have deficits in marble burying, and display anxiety and depressive-like behaviors (Weidner et al., 2014). Female transgenics, on the other hand, display higher self-grooming, higher anxiety scores but no depression-like behaviors (Papaleo et al., 2011).
Although BDNF overexpression in animals may not well represent a monogenic ASD model, they can still be useful for studying the multifactorial aspects leading to ASD and its comorbidities, such as epilepsy (Weidner et al., 2014). It is also possible that neural substrates such neurotrophins and nerve growth factors, which are connected with BDNF dysregulation, may be cooperatively involved in the ASD pathophysiology.
7-dehydrocholesterol reductase (DHCR7)
DHCR7 is a ubiquitously expressed catalytic enzyme that converts 7-dehydrocholesterol to cholesterol. Defects in DHCR7 function are the main cause of Smith-Lemli-Optiz syndrome (SLOS) (Irons et al., 1993), which is often comorbid with autism (50%) (Tierney et al., 2000). SLOS patients have abnormal behavioral phenotypes including increased hyperactivity, irritability, aggression, insomnia, self-injurious behavior, repetitive and ritualistic behaviors, and impaired communication (Tierney et al., 2000). Interestingly, low cholesterol levels have been observed in children with idiopathic autism (Tierney et al., 2006).
Similar to BDNF homozygous knockouts, DHCR7 null (−/−) mice have high lethality rates at birth (Fitzky et al., 2001); therefore, DHCR7+/− or DHCR7 mutant mice are used as animal models. DHCR7+/− mice show low exploratory activity both in the social preference and open field tests (Moy et al., 2009). DHCR7 mutant mice display increased ventricular size (Correa-Cerro et al., 2006) and abnormalities in the hippocampus and serotonergic neurons (Waage-Baudet et al., 2003). Additionally, the Dhcr7-heterozygous mice show increased response to treatment with a 5-HT2A agonist, as marked by frequent head-twitch, further suggesting the involvement of the serotonergic system in the phenotypes observed in the model (Korade et al., 2013). These data further demonstrate the impact of cholesterol dysregulation on behavior and provide further insights onto a potential ASD mechanism that is often overlooked, as in the case of SLOS patients.
Engrailed-2 (EN2)
EN2 is a homeodomain-containing protein that regulates pattern formation during brain development (Zec et al., 1997). Although not consistently observed (Zhong et al., 2003), it has been suggested that genetic variants of EN2 are associated with ASD (Benayed et al., 2005; Brune et al., 2008), EN2−/− mice were introduced as a potential model of ASD in 2006, and were shown to have behavioral deficits in social play, aggression, spatial memory and motor coordination (Cheh et al., 2006). Furthermore EN2−/− mice display decreased cerebellar size and abnormal foliation patterns, similar to patients with ASD (Kuemerle et al., 2007). In addition, these studies found that major cell types of the olivocerebellar circuit, e.g. Purkinje, were reduced up to 30–40% (Kuemerle et al., 1997). This places the EN2−/− mouse in parallel to the cerebellar lesion model for ASD, as described above. Thus, even though EN2−/− mice do not fully recapitulate all of the structural abnormalities and behavioral phenotypes in ASD, it can somehow provide quality pathophysiologic insights (Kuemerle et al., 2007).
Fragile X mental retardation 1 (FMR1)
The FMR1 gene encodes for fragile X mental retardation protein (FMRP) (Verheij et al., 1993). FMRP is an RNA-binding protein that is commonly expressed in brain, testes, and ovaries. FMRP takes part in local protein synthesis regulation in dendrites, as well as mRNA transport from nucleus to the cytoplasm (Garber et al., 2008). In addition, FMRP plays an essential role in synapse development (Weiler et al., 1997), which is vital for proper neurotransmission, learning and memory, and synaptic plasticity. Mutations in FMR1 take the form of expanded CGG trinucleotide repeats (55–230) in the 5′ gene untranslated region (5′UTR); this halts the production of FMRP and leads to a developmental condition called Fragile X Syndrome (FXS). FXS is characterized by intellectual disabilities, developmental delays, congenital malformations, seizures and autistic-like symptoms (Garber et al., 2008). Indeed, 10–30% of FXS patients were also diagnosed with autism (Hatton et al., 2006).
FMR1 knockout mice have widely been used to investigate the behavioral abnormalities and pathophysiological mechanisms underlying FXS. Interestingly, a number of studies have shown that FMR1 knockout mice display comorbidities with autistic behaviors including decreased social interaction, increased repetitive behaviors, anxiety, hyperactivity (Bernardet and Crusio, 2006), increased seizure susceptibility (Silva and Ehninger, 2009), decreased spatial learning ability, and impaired object recognition (Brennan et al., 2006; Mineur et al., 2006). Nonetheless, some of these results are inconclusive, as McNaughton et al. and Spencer et al. reported that these mice show increased social approach and anxiety (Spencer et al., 2005; McNaughton et al., 2008), whereas Liu and Smith, and Mineur et al. reported decreased social approach and anxiety in FMR1 knockout animals (Mineur et al., 2006; Liu and Smith, 2009). These inconclusive findings might be due to lab-specific technical differences and conditions.
Physiologically, FMR1 knockout mice display increased dendritic spine length (Irwin et al., 2000), decreased mGluR5 expression (Bear et al., 2004) and increased mGluR-dependent LTD (Nosyreva and Huber, 2006), decreased LTP (Zhang et al., 2009), and an imbalance between excitation and inhibition (Silva and Ehninger, 2009). Furthermore, recent studies have demonstrated the involvement of abnormal GABAergic neurotransmission and development in the generation of autistic-related behaviors in FRX mice. For instance, it has been found that the developmental switch in GABA polarity is delayed in these mice (i.e. from depolarizing to hyperpolarizing) and that this might be due to dysregulated intracellular chloride levels, which surely contributes to abnormal brain development and can be implicated in autism pathophysiology (Tyzio et al., 2014).
Some reports have also suggested a dysfunctional endocannabinoid system (ECS) in FXS as a disordered mechanism. The ECS plays a critical role in the regulation of synaptic plasticity, cognition, pain, seizure susceptibility and anxiety (Kano et al., 2009). On the other hand, FMRP modulates mGluR5-mediated signal transduction in glutamatergic synapses, which controls the LTD type of synaptic plasticity. Interestingly, 2-arachidonoyl-sn-glycerol (2-AG), a retrograde endocannabinoid transmitter, mediates mGluR5-dependent LTD in excitatory synapses, in the ventral striatum and PFC (Jung et al., 2012). The FMRP null mice exhibit increased activity of diacylglycerol lipase (DAGL), a limiting enzyme in 2-AG biosynthesis, which disrupts the GABAergic synaptic sensitivity to endocannabinoid mobilization (Maccarrone et al., 2010). Ultimately, this produces enhanced activation of cannabinoid receptors (CB1R and CB2R), which increases synaptic strength and excitation as a result of mGluR5-mediated 2-AG release (Maccarrone et al., 2010). Remarkably, modulating or blocking CB1 and CB2 receptors signaling normalizes 2-AG dysregulation and rescues the cognitive and behavioral abnormalities in FMRP null mice (Busquets-Garcia et al., 2013). These studies show how targeting the ECS and mGluR5 pathways can be of great therapeutic value, which certainly warrant further investigation. Overall, and based on these behavioral and neurological findings, FMR1 knockout animal models provide useful insights on ASD pathology and the involvement of the FMR1 gene or FMRP in the neurobiology of autism.
GABA receptor subunit beta-3 (GABRB3)
The GABRB3 gene encodes for a major subunit of the ligand-gated GABA receptor. The gene itself is located within the 15q11-13 chromosome region. It has long been known that maternal deletion of 15q11-13, which also contains UBE3A, causes Angelman syndrome, which is highly comorbid with ASD (Nakao et al., 1994). Interestingly, down-regulation of UBE3A and GABRB3 result in autism-related phenotypes in mice, similar to those caused by MECP2 mutations in Rett syndrome (described below) (Samaco et al., 2005). The involvement of GABRB3 in modulating inhibitory neurotransmission is further supported by a number of clinical studies, which associate GABRB3 polymorphisms with ASD (Buxbaum et al., 2002). Moreover, individuals with autism often display disruptions in GABAergic biomarkers (Blatt, 2005), including reduced expression of GABAergic receptors (Blatt et al., 2001) and decreased expression of GAD, the enzyme that catalyzes synthesis of GABA (Fatemi et al., 2002)
GABRB3 knockout (−/−) mice display many phenotypes associated with ASD. These include increased neonatal mortality, seizure susceptibility (Homanics et al., 1997), hyperactivity, stereotyped/circling behavior (DeLorey et al., 2008), deficits in learning and memory (DeLorey et al., 1998), and impaired social interactions, nesting ability and exploratory behaviors (DeLorey et al., 2008). Furthermore, GABAA receptors in GABRB3 null mice show a 50% reduction in GABA binding capacity, both in newborns and adults (Sinkkonen et al., 2003). Thus, this model has provided useful insights onto another pathological mechanism underlying Angelman syndrome or potentially ASD. Yet, additional human genetic studies must be done in order to further elucidate the role of GABRB3 in ASD (Tavassoli et al., 2012; Warrier et al., 2013).
Methyl CpG binding protein 2 (MeCP2)
MeCP2 acts mainly as a transcriptional repressor by binding to methyl groups in CpG islands of DNA (Yasui et al., 2007), although its role in activating gene transcription has also been observed (Chahrour et al., 2008). In mammals, regulation of genetic transcription by Mecp2 has been shown to be crucial for the modulation of chromatin at critical developmental time points. Human mutations in MeCP2 cause Rett syndrome, a progressive X-linked neurodevelopmental disorder that causes mental retardation and a number of developmental deficits in females (Rett, 1966); it is thought that homozygous mutations in males result in lethality in utero (Rett, 1966). Historically, Rett syndrome had been initially categorized as a subtype of autism, yet more recent DSM-V criteria separate it from ASD (American Psychiatric Association, 2013).
MeCP2 knockout mice show increased anxiety (Chahrour and Zoghbi, 2007), decreased motor coordination (Guy et al., 2001), impaired social interactions, impaired long-term social memory, decreased nest-building ability, and impaired learning and memory (Moretti et al., 2006; Chahrour and Zoghbi, 2007). It has also been shown that neurons of MeCP2-null mice have increased gene transcription, likely mediated through enhanced histone acetylation (Guy et al., 2011). This is also accompanied by neurotoxicity due to excessive glutamate release from microglia (Maezawa and Jin, 2010). Moreover, MeCP2-null astrocytes are unable to support normal dendritic morphology in wild-type hippocampal neurons (Ballas et al., 2009). In addition, MeCP2-null neurons have abnormal dendritic and axonal development (Larimore et al., 2009), and MeCP2-deficient GABAergic neurons show reduced inhibitory quantal size and decreased expression of GAD (Chao et al., 2010). These findings from the MeCP2 knockout model converge with the VPA animal model, which also shows a decreased MeCP2 expression in a male-specific manner, leading to increased glutamatergic neurotransmission (Kim et al., 2014b). Collectively, this highlights the potential pathophysiological role of epigenetic dysregulation on genetic determinants of ASD.
Monoamine oxidase A (MAOA)
MAOA and its neighboring gene are regulators of the mitochondrial enzyme MAO, responsible for the oxidative deamination of monoamine neurotransmitters such as dopamine and norepinephrine. Mutations in MAOA cause Brunner syndrome and have been linked to antisocial behaviors, low IQ, impulsiveness and violent behaviors (Hunter, 2010). In addition, the alleles regulating the levels of MAOA have been correlated with autism in humans (Cohen et al., 2003), which is mainly driven my maternally inherited mutations in male off-spring (Cohen et al., 2011). Moreover, clinical studies have found that variations in the number of MAOA-upstream variable number of tandem repeats (uVNTR) are associated with ASD hyperserotonemia (Hranilovic et al., 2008) and cortical enlargement (Davis et al., 2008).
Similar to the human phenotype, MAOA-deficient mice display aggressive behaviors (Cases et al., 1995), increased fear, aberrant eye-blink conditioning (Singh et al., 2013) and hyper-responsiveness to acoustic stimuli (Popova et al., 2000). In accordance with ASD, this animal model has social and communication impairments, repetitive behaviors, behavioral rigidity and motor abnormalities (Bortolato et al., 2013). These mice also display increased hippocampal LTP and NMDAR expression, thinning of the corpus callosum, increased dendritic arborizations in pyramidal neurons of the PFC, and disrupted microarchitecture of the cerebellum (Singh et al., 2013). As expected, these mice also show increased brain serotonin, dopamine and norepinephrine levels (Bortolato et al., 2013; Singh et al., 2013), which might be directly related to the manifestation of anxiety and aggressive behaviors in MAOA-deficient mice, albeit these phenotypes have not been well characterized in humans (Cohen et al., 2003). This remarkable recapitulation of the core deficits of ASD symptoms, as well as the clinically similar neuropathologic phenotypes in MAOA-deficient mice, makes them a plausible model wherein potential therapeutic agents could be tested, especially with monoamines as the main target.
Neurofibromin 1 (NF1)
NF1, also called neurofibromatosis-related protein, is a gene that functions as a tumor suppressor in the nervous system and plays a role in controlling the Ras signaling pathway (Cichowski and Jacks, 2001). NF1 is part of the NMDA receptor complex (Husi et al., 2000), which is suggested to underlie mental retardation and learning deficits in humans (Husi et al., 2000). In accordance with its proposed function, mutations in NF1 result in a life-shortening condition known as Neurofibromatosis (Rasmussen and Friedman, 2000), an autosomal dominant disorder characterized by cognitive and language deficits, poor motor skills and peripheral nerve tumors (Silva et al., 1997).
Interestingly, NF1 heterozygous (+/−) mice show deficits in spatial learning in the Morris water maze (Costa et al., 2002; Silva et al., 1997), delayed acquisition of motor skills and impaired fear conditioning (Costa et al., 2001). In addition, cortical neurons and astrocytes of NF1 mutant mice fail to form cortical barrels in somatosensory cortex (Lush et al., 2008). In human studies, however, NF1 polymorphisms and overexpression of the gene, not deficiency, have been associated with autism (Mbarek et al., 1999; Marui et al., 2004) Thus, further study of NF1 polymorphisms in animal models, as opposed to knock-outs or knock-downs, should be conducted in the future.
Neuroligin (NLGN) family
The NLGN family of genes encode for neuroligin proteins, which are cell adhesion molecules required for synaptic function (Sudhof, 2008). NLGN is found in the postsynaptic membrane and mediates synapse transmission between neurons (Jamain et al., 2008). Mutations of NLGN3 and NLGN4 are associated with X-linked intellectual disability, seizures, and autism (Jamain et al., 2003) In addition, an in-depth molecular genetic analysis of the NLGN family found an association between non-functional polymorphisms and ASD in the Finnish population. This study concluded that neuroligin mutations may cause autism only in rare cases and that variations in neuroligin alleles are not a major risk factor for autism (Ylisaukko-oja et al., 2005). Nonetheless, the role of several neuroligin subunits and their association with ASD have been studied in mice.
NLGN1:
NLGN1 encodes for a group of neuronal membrane-bound proteins which are involved in CNS synapse development. NLGN1 knockout mice display impaired spatial memory and repetitive/stereotyped grooming (Blundell et al., 2010). The latter is thought to be related to reduced NMDA/AMPA ratios in cortico-striatal synapses (Blundell et al., 2010). More studies are needed in order to provide a clinical link between the autism-related phenotypes found in NLGN1 knockout mice.
NLGN3:
Arg451Cys (R451C) mutation of NLGN3 has been associated with autism in humans (Comoletti et al., 2004). NLGN3 mutant mice carrying the R451C mutation have been long used as a model of ASD. These mice have impaired social interactions and enhanced spatial learning, as well as enhanced synaptic inhibition in the somatosensory cortex (Etherton et al., 2011). However, the Etherton et al. study also showed an increased excitatory transmission within the hippocampal region of NLGN3 (R451C) mutant mice, but not in NLGN3 KO mice. This study concluded that NLGN3 is differentially involved in modulation of excitatory and inhibitory synaptic neurotransmission in a brain region-specific manner (Etherton et al., 2011). NLGN3 mutant mice also showed deficits in ultrasonic vocalizations, impaired preference for social novelty (Jamain et al., 2008) and altered olfactory function (Radyushkin et al., 2009). However, there were no changes in time spent engaged in social interaction, pre-pulse inhibition and seizure propensity in NLGN3 mutant mice, as compared with their wild-type controls (Radyushkin et al., 2009). Thus, the NLGN3 mutant mice may only partly model autistic features and not the global ASD condition.
NLGN4:
Mutations in NLGN4 have been associated with X-linked mental retardation, autism (Jamain et al., 2003), and other neurodevelopmental conditions comorbid with ASD, although it has been suggested that the contribution of NLGN4 mutations to ASD is very small (Gauthier et al., 2005). Yet, NLGN4 deficient mice, display impairments in social interactions and social memory (Jamain et al., 2008), along with reduced brain volume (Jamain et al., 2008). However, these mice did not display deficits in repetitive behaviors, exploratory activity, anxiety, and learning and memory (Jamain et al., 2008). This shows that, although NLGN4 and the rest of the NLGN subunits may not consistently represent a very strong cause for ASD, they may still contribute to the complex, connecting pathways of ASD pathophysiology.
Neurexin 1 (NRXN1)
NRXN1 is a pre-synaptic membrane cell adhesion molecule and a receptor which mediates the synaptic interaction between neurons (Li et al., 2006), mainly through interactions with neuroligins. Phenotypes of individuals with NRXN1 deletion vary, and include mental retardation, language delay, schizophrenia (The International Schizophrenia Consortium, 2008; Walsh et al., 2008; Need et al., 2009), nicotine dependence (Nussbaum et al., 2008) and ASD (Ching et al., 2010). Behavioral testing of NRXN1α deficient mice have shown deficits in pre-pulse inhibition and nesting ability, along with increased grooming activity, and enhanced motor learning on the rota-rod test (Etherton et al., 2009), despite the absence of obvious social defects (Etherton et al., 2009). Physiologically, these mice also display alterations in excitatory synaptic transmission. As a whole, the neurexin-1 model is somewhat complex in terms of using it for the study of ASD, as many of the observed phenotypes overlap or could be associated with other disorders.
Oxytocin (OXT), oxytocin receptor (OXTR), and MAGEL2
Oxt encodes the protein precursor of oxytocin and neurophysin 1. Oxytocin is a neuromodulating hormone produced by the posterior pituitary gland. It stimulates uterine muscle contraction during childbirth and stimulates lactation. Moreover, it is known to participate in various cognitive, adaptive, cardiovascular, excretory and complex sexual functions. Recent studies have also found that oxytocin has a key role in social recognition and social interactions (Guastella et al., 2008; Savaskan et al., 2008). Such findings have produced an increasing interest in the study of oxytocin and its involvement in ASD (Gregory et al., 2009). Research in humans have found that oxytocin plasma levels are reduced in autistic children (Insel, 2010) and that intranasal administration of the hormone to autistic patients enhances their social interactions (Andari et al., 2010), reduces repetitive behaviors (Hollander et al., 2003) and improves emotional recognition (Guastella et al., 2010). Moreover, in VPA prenatal exposure and FXS animal models of autism, prenatal oxytocin treatment can rescue autistic-like behaviors in offspring, which further demonstrates the pathophysiologic involvement and therapeutic potential of this hormone in ASD (Tyzio et al., 2014).
In mice, Oxt deficiency induces impairments in social recognition (Ferguson et al., 2001) and decreased social odor memory in females (Kavaliers et al., 2003), despite normal social approach and decreased aggression (Ferguson et al., 2001; Winslow and Insel, 2002). Interestingly, oxytocin administration directly into the amygdala region has been shown to enhance social recognition in Oxt knockout mice (Winslow and Insel, 2002), similar to what is observed when oxytocin is administered to autistic individuals (Andari et al., 2010).
In recent studies, common polymorphisms in the oxytocin receptor gene (Oxtr) have also revealed an association with ASD (Jacob et al., 2007; Gregory et al., 2009). Moreover, a number of Oxtr SNPs were associated with autism in various ethnic populations, encouraging further exploration to define its role in ASD (Wu et al., 2005; Jacob et al., 2007; Liu et al., 2010). However, a study discouraged the association of common Oxtr variation with autism in mixed Caucasian populations (Tansey et al., 2010) but later meta-analysis studies with one of the largest population ever investigated for Oxtr polymorphisms, showed positive correlations with autism (LoParo and Waldman, 2015). Additional studies further support these later findings by finding a positive correlation between Oxtr polymorphisms and social recognition skills, thus suggesting Oxtr SNPs as predictors of social impairments in both typically developing individuals and in children with ASD (Parker et al., 2014).
Accordingly, conditional Oxtr knockout mice, display deficits in social memory and defective ultrasonic vocalizations (Lee et al., 2008), but are devoid of stereotyped/repetitive behavior phenotypes (Pobbe et al., 2012). In addition, Oxtr-null mice display increased aggression and high seizure susceptibility (Sala et al., 2011). These mice also have a decreased ratio of GABAergic presynapses to the total number of presynapses in hippocampal neurons (Sala et al., 2011), suggesting that alterations in the oxytocin system or aberrant oxytocin receptor function can have profound effects in the overall excitatory/inhibitory balance in the brain.
Additional evidence supporting the role of oxytocin in ASD comes from studies using the Magel2 mouse model. MAGEL2 is a paternally imprinted gene that has been recently identified as an autism risk gene and has been associated with Prader-Willi Syndrome (PWS) (Boccaccio et al., 1999; Schaaf et al., 2013). PWS results from large deletions in chromosome 15q11-q13 and is characterized by intellectual disabilities, repetitive behaviors, and hyperphagia-induced obesity. Interestingly, individuals with PWS have high comorbidity with ASD (>30%) (Dykens et al., 2011). In congruence with ASD, the Magel2-deficient mice showed abnormalities in social recognition and interaction, as well as learning difficulties in adults (Meziane et al., 2015). More importantly, at birth, these mice have reduced production of oxytocin in the hypothalamus, which causes decreased suckling behavior and leads to a 50% mortality rate (Schaller et al., 2010). Interestingly, a single postnatal injection or a one-week-long administration of oxytocin after birth improved suckling, prevented mortality as well as the development of behavioral impairments in adulthood (Schaller et al., 2010; Meziane et al., 2015), suggesting a mechanistic link between oxytocin and Magel2. Thus, additional studies exploring the relationship between Magel2 and oxytocin will be beneficial for supporting the potential of oxytocin as a therapeutic treatment for ASD.
Phosphatase and tensin homolog (PTEN)
PTEN is a tumor suppressor protein involved in cell cycle arrest and apoptosis through negative regulation of the AKT/PKB signaling pathway (Chu and Tarnawski, 2004). A number of genetic variants in PTEN have been observed in ASD patients with macrocephalic phenotypes (Butler et al., 2005), which stimulated the study of a PTEN transgenic mouse model. Given that PTEN-null mice die during embryogenesis (Di Cristofano et al., 1998), conditional knockout mice are used to investigate the role of PTEN in development and autism pathogenesis. PTEN mutant mice display abnormal social interactions, hyperactivity, excessive responses to external stimuli and decreased prepulse inhibition (Ogawa et al., 2007); they also develop macrocephaly and neuronal hypertrophy in the CNS, similar to human patients (Kwon et al., 2006). A recent study suggested that PTEN deficiency in vivo increases the net excitatory drive onto granule neurons, enlarges the neuronal size, and increases the density of dendritic spines (Luikart et al., 2011). Further studies have shown that deletion of PTEN leads to increased cell proliferation (Gregorian et al., 2009; Bonaguidi et al., 2011). These findings all agree with the role of PTEN as a regulator of neural stem cell proliferation and lineage specification (Zhou and Parada, 2012). Lastly, PTEN may also interact and synergize with other signaling pathways, such as the PI3K/AKT and TSC/mTORC1 pathway, that contribute to the complex pathogenesis of the global ASD condition (Zhou and Parada, 2012).
Reelin (RELN)
Reelin is a secreted extracellular matrix (ECM) protein that is important for ECM development and plays an essential role in the migration and proper positioning of cortical neurons (D’Arcangelo, 2005). In adult brains, Reelin is actively involved in synaptic regulation, formation of dendrites and modulation of cognitive function (Rogers et al., 2011). In the hippocampus, Reelin accumulation is essential for NMDA subunit receptor maturation and NR2B surface mobility, which ultimately leads to mature excitatory synapses (Groc et al., 2007). In addition to NMDA receptor regulation in cortical neurons, Reelin mediates tyrosine phosphorylation and increases calcium influx, which is physiologically involved in learning and memory (Chen et al., 2005). Down-regulation of Reelin in cortical GABAergic interneurons has been frequently observed in schizophrenia, bipolar disorders and autism (Ognibene et al., 2007). Furthermore, mutations in 7q22-23, consisting of longer triplet repeats in the 5′UTR of RELN gene locus, have been observed in autistic patients (Gillberg, 1998; Yan et al., 2000).
Mice used to model the effects of RELN mutations, known as Reeler (rl/rl) mice, display cortical disorganization in various brain regions, including cortex, cerebellum, hippocampus, subcortical regions, and spinal cord (Martin, 1981; Goffinet, 1983; Yip et al., 2000; D’Arcangelo, 2005). Decreased density of striatal GABAergic interneurons were also found (Marrone et al., 2006). Behaviorally, Reeler mice showed increased seizure susceptibility (Patrylo et al., 2006) and decreased ultrasonic vocalizations (Ognibene et al., 2007).
In previous reports, heterozygous Reeler mutant (+/rl) mice had shown indistinguishable features in anatomy (Stanfield and Cowan, 1979) and behavior (Muroga et al., 1982) from normal (+/+) mice. However, later studies reported that +/rl mice displayed abnormalities in anatomical (Smalheiser et al., 2000; Liu et al., 2001) and behavioral (Tueting et al., 1999) phenotypes, resembling those of human schizophrenia patients. Reeler mutant mice also showed deficits in pre-pulse inhibition and decreased exploration in the elevated plus maze test (Tueting et al., 1999). Other studies, such as those of Salinger et al., reported that +/rl mice have normal social aggressive behaviors (Salinger et al., 2003), whereas Podhoma et al found no behavioral abnormalities in the model (Podhorna and Didriksen, 2004). Recently, Iafrati and colleagues developed the juvenile reelin-haploinsufficient heterozygous reeler mice (HRM), which exhibited a reduction in dendritic spine density and abnormal LTP in the prefrontal cortex, as well as deficits in fear memory formation (Iafrati et al., 2014). Overall, studies in Reeler mice have resulted in conflicting and inconsistent findings of behavioral phenotypes. Whether +/rl mice are suitable as animal models for schizophrenia and/or autism requires further investigation.
Serotonin transporter (SERT, SLC6A4)
The serotonin transporter (5-HTT, SERT) removes serotonin from the synaptic cleft back into the presynaptic terminal and has a general role in the termination and recycling of serotonin during neurotransmission. Hyperserotonemia is one of the most consistent findings in ASD patients (Hranilovic et al., 2008). However, there is conflicting evidence regarding the involvement of SERT in ASD, especially from a genetics standpoint. Only one study found a significant association between SERT polymorphism (SLC6A4 variants) and autism hyperserotonemia, which failed to replicate in other studies (Betancur et al., 2002; Huang and Santangelo, 2008). It is also difficult to determine whether hyperserotonemia in autism is related to serotonin activity and re-uptake (Prasad et al., 2009).
In preclinical studies, the 5-HTT knockout mice showed decreased exploratory behavior, increased anxiety-like behaviors (Holmes et al., 2003), elevated sensitivity to stress (Jiang et al., 2009) and reduced social interactions (Kalueff et al., 2007). In the brains of 5-HTT knockout mice, altered cortical thickness and cell density (Altamura et al., 2007) as well as altered hypothalamic-pituitary-adrenal (HPA) axis signaling (Jiang et al., 2009) were observed. Genetic variations in the SERT gene, including Gly56Ala, Ile425Leu, Ile425Val, Phe-465Leu, Leu550Val, and Lys605Asn, enhance the serotonin re-uptake activity of SERT proteins (Prasad et al., 2009). Currently, no strong association between SERT polymorphisms and ASD has been found, despite the consistent occurrence of hyperserotonemia in autism; thus, the involvement of SERT variation is yet to be established. As a result, further research is needed both at the clinical and animal model level. It is important nonetheless, to acknowledge the fact that SERT animal models may not be uniquely reflecting autism-related phenotypes, as there may be some association between the gene and other disorders, such as obsessive-compulsive disorder (Veenstra-Vanderweele et al., 2009). Thus, careful interpretation of results when using this animal model is needed.
SH3 and multiple ankyrin repeat domains protein (SHANK)
SHANKs are postsynaptic scaffold proteins that interact with neurotransmitter receptors, ion channels, and other membrane proteins. SHANKs play a key role in synapse formation and dendritic spine maturation during brain development. Shank genes have long been implicated in ASD and Shank dysregulation supports the synaptic dysfunction hypothesis in ASD pathophysiology (Jiang and Ehlers, 2013). Nevertheless, the molecular diversity of SHANK genes and their heterogeneity in both the human and mouse genome poses a great challenge in using Shank mutant models, as described below.
SHANK1:
Hung and colleagues reported that SHANK1−/− mice showed increased anxiety-like behavior, impaired contextual fear memory and enhanced spatial learning (Hung et al., 2008). Another study also reported reduced ultrasonic vocalizations and decreased scent marking behaviors in SHANK1−/− mice (Wohr et al., 2011b). However, Silverman and colleagues observed that although null mutant mice showed some degree of motor disability and anxiety, they did not display other autism-related deficits, especially in terms of reciprocal social interactions (Silverman et al., 2011). This study, therefore, raised the notion that SHANK1−/− mice may not be appropriate for modeling autism-related social deficits, but could be useful in understanding alterations in motor function. Further neurobiological studies showed that SHANK1−/− mice displayed alterations in the composition of postsynaptic density proteins, reduced size of dendritic spines and weaker basal synaptic transmission (Hung et al., 2008). The involvement of SHANK1 gene in autism etiology has not been ruled out since a previous study found a male-heritable SHANK1 microdeletion in ASD patients (Sato et al., 2012).
SHANK2:
Mutations in the SHANK2 gene have been reported in ASD patients (Berkel et al., 2010; Leblond et al., 2012). Furthermore, heritable SHANK2 variants, particularly T1127M and R462X, are known to affect spine volume and result in smaller SHANK2 cluster sizes (Berkel et al., 2012). Rodent overexpression of the R462X variant results in a more severe phenotype of defective dendritic branching and decreased postsynaptic clustering (Berkel et al., 2012). In animal models, ProSAP1/Shank2−/− mutant mice displayed fewer dendritic spines and lower basal synaptic transmission along with increased NMDA-mediated excitatory currents (Schmeisser et al., 2012). These mutant mice display autism-related behavioral phenotypes, including repetitive grooming, hyperactivity, and impaired vocal and social behaviors (Schmeisser et al., 2012). Won et al. confirmed and further found that Shank2−/− mutant mice exhibited decreased social interactions, impaired ultrasonic vocalizations, and repetitive jumping behavior. However, in contrast to the previous study, these mutant mice showed decreased NMDA receptor function (Won et al., 2012). Based on these mechanistic findings, Won et al.’s study also tested therapeutic candidates for ASD and found that D-cycloserine (a partial agonist of NMDA receptor) normalized the function of NMDAR and enhanced social interactions in Shank2−/− mutant mice (Won et al., 2012). It is likely that the opposing NMDA function findings in these studies are related to the fact that they use different exon mutation sites in each model, which could result in slightly different protein disruptions. Yet, and as a whole, these results provide useful insights in the importance of maintaining a normal range of NMDA function in the brain, as both over- and under- regulation of NMDA transmission could result in abnormal behavioral phenotypes.
SHANK3:
Mutations in SHANK3, such as those observed in microdeletions of 22q13, have been implicated in ASD etiology. Recent studies reported that SHANK3 genes are lost or rearranged in ASD patients and are associated with developmental delays, dysmorphic features and autistic behaviors (Manning et al., 2004). Moreover, heterozygous mutations of SHANK3 may cause ASD in a gene-dosage-dependent manner (Durand et al., 2007). SHANK3 mutant mice have been recently proposed as a model of ASD and have provided great insights on the pathological mechanisms that could underlie the disorder. For example, SHANK3B−/− mice display repetitive grooming and deficits in social interactions, along with corticostriatal circuit alterations and striatal synaptic defects (Peca et al., 2011). In another study, these mice showed deficits in glutamatergic synaptic transmission and hippocampal LTP, yet only displayed mild social deficits in juvenile but not adult age (Yang et al., 2012). In addition, increased self-grooming, decreased ultrasonic vocalizations, and decreased reversal learning were observed only in some cohorts, suggesting variable phenotypic severity in SHANK3 mutant mice (Yang et al., 2012). Yet another investigation revealed decreased excitatory postsynaptic currents (EPSCs) in pyramidal neurons of the hippocampal CA1 region, highlighting a reduced basal neurotransmission in these animals in an AMPAR-mediated manner (Bozdagi et al., 2010). The GluR1-immunoreactive puncta of the stratum radiatum was also quantitatively reduced, along with impaired LTP but not LTD (Bozdagi et al., 2010). Behaviorally, these mice displayed reduced social sniffing and ultrasonic vocalizations in the presence of a female mouse (Bozdagi et al., 2010).
Although the Shank3 mouse model data is very compelling, human studies still suggest that not all genetic mutations and alterations in SHANK3 directly lead to ASD, thus careful interpretations should be given. This was suggested by a clinical study, where a child with autism showed a rare genetic variant in Shank3, consisting of a 1-bp insertion in exon 11; although this mutation was of high penetrance, it was not attributed a strong etiological relationship to the ASD phenotype (Kolevzon et al., 2011). Lastly, it is important to note that the phenotypic consequences of Shank3 dysfunction can be rather complex and require careful interpretation since similar mutations can be associated to both ASD and schizophrenia (Gauthier et al., 2010).
Tuberous sclerosis complex protein (TSC) 1 or 2
Mutations of TSC1 or TSC2 cause the Tuberous sclerosis complex (TSC) disorder. TSC1 encodes hamartin and TSC2 encodes tuberin. TSC1/TSC2 act as tumor growth suppressors and are involved in cell proliferation and differentiation. TSC patients have a high prevalence of autism, ranging from 20 to 60% (Bolton et al., 2002; Curatolo et al., 2004). TSC1/TSC2 mutations are associated with neurological deficits including cognitive dysfunction, epilepsy, and autism (DiMario, 2004; Goorden et al., 2007).
Mutant mice that lack TSC1 in astrocytes showed significant brain pathologies and seizure vulnerability (Uhlmann et al., 2002). Furthermore, neuronal loss of TSC1 induced cortical hyperexcitability and seizure susceptibility in mice (Meikle et al., 2007). Sparse deletion of TSC1 in CA1 hippocampal neurons led to enhanced AMPAR and NMDAR-mediated EPSCs, as well as an increase in spontaneous EPSC frequency, and absent mGluR-LTD in the hippocampus (Bateup et al., 2011). TSC1 conditional knockout in neural progenitor cells resulted in increased brain size and elevated mToRC1 signaling, as well as decreased mToRC2 signaling in mice (Carson et al., 2011). More importantly, hetero- or homozygous loss of TSC in mice induced abnormal social interactions, repetitive behaviors, and impaired vocalizations, coupled with decreased Purkinje neurons (Tsai et al., 2012). Overall, these studies suggest that conditional or complete TSC1 deficiency leads to an elevation in glutamatergic or excitatory synaptic activity, and once again implies dysregulation of mToR signaling in the pathophysiology of ASD.
Interestingly, TSC2w/− mice showed no significant brain pathology (Onda et al., 2002), yet a recent study suggested that heterozygous TSC (+/−) mutant mice with maternal immune activation by poly I:C leads to impaired social interactions in offspring (Ehninger et al., 2012). In other studies, TSC+/− mice displayed alterations in ultrasonic vocalizations (Young et al., 2010) while mice expressing a dominant negative form of tuberin showed impaired social interactions (Chevere-Torres et al., 2012). Moreover, selective deletion of TSC2 in Purkinje cells increased Purkinje cell size and induced ER oxidative stress, which eventually led to cellular apoptosis in these neurons (Reith et al., 2011). Interestingly, such phenotypes were rescued by treatment with the mToRC1 inhibitor rapamycin (Reith et al., 2011). Concurrent with these studies, Tsc2+/− mice exhibit mToR-dependent upregulation of GluN2C-containing NMDARs, and display seizure symptoms early in life (Lozovaya et al., 2014) and deficient mGluR-LTD in the hippocampus (Auerbach et al., 2011). More recently, Tang et al. showed that Tsc2+/− mice have impaired social behaviors, in which their brain exhibit upregulated mToR activity and abnormal post-natal dendritic pruning mediated by inactivation of normal autophagy (Tang et al., 2014).
Although there is a considerable prevalence of ASD in TSC, and mouse models show compelling evidence supporting the role of TSC1/TSC2 in autism etiology, the exact neurobiological mechanism by which this occurs is still poorly understood (Numis et al., 2011). Some would suggest persistent seizures or epilepsy in early development as the main driver of autistic behavioral phenotypes in TSC (Curatolo et al., 2004; Numis et al., 2011). Yet, it is worthy of noting that gene-environment interactions (e.g. maternal immune activation) could play a major role in precipitating the development autism symptomatology and could provide novel implications for ASD pathophysiology and etiology (Ehninger et al., 2012). In this way, animal models of TSC could help uncover unknown biological pathways associated with ASD, especially in terms of how early disruptions in brain activity (e.g. seizures), genes, and environmental factors lead to the disorder.
Ubiquitin-protein ligase E3A (UBE3A)
UBE3A gene encodes the E6AP ubiquitin-protein ligase (E6AP) protein, which mediates ubiquitin-dependent protein degradation. As mentioned above, mutations of UBE3A and GABRB3, both located in chromosome 15q11-13, cause Angelman syndrome (AS) (Wagstaff et al., 1991). UBE3A regulates excitatory synapse development by ubiquitin-dependent degradation of Arc, a synaptic protein that promotes AMPA receptor internalization (Greer et al., 2010). Interestingly, preclinical studies have found that increased gene dosage of UBE3A leads to reduced glutamate synaptic transmission in mice and result in autism-related behaviors (Smith et al., 2011). Furthermore, maternal deletion of UBE3A increases the emission of ultrasonic vocalizations (Jiang et al., 2010) and seizure susceptibility in offspring mice (Miura et al., 2002). On the other hand, UBE3A knockout in mice leads to Arc accumulation in neurons, which weakens synaptic function through the excessive internalization of AMPA receptors (Greer et al., 2010). These findings could mean that UBE3A mutations, more specifically implicated in AS, may also have a specific role in ASD etiology. This is supported by a clinical study in Italian patients with autism, which found a potential role of UBE3A in ASD pathogenesis (Guffanti et al., 2011). Another clinical study highlighted the phenotypic overlap between AS and ASD, as autistic phenotypes were observed in some children with AS (Peters et al., 2004). Indeed, the UBE3A mouse models present good candidates for studying ASD pathogenesis, as the gene seems to partake in complex signaling pathways that exacerbate autism symptomatology in syndromes such as AS.
Arginine Vasopressin (AVP) and its receptor (AVPR1A)
AVP is a pituitary hormone that mainly functions as an antidiuretic by stimulating water reabsorption in the collecting ducts of nephrons. It also plays a contributive role in peripheral vasoconstriction, smooth muscle contraction during parturition and lactation, cognition, tolerance, adaptation, cardiovascular regulation and complex sexual and maternal behaviors; indeed, it functions very similarly to oxytocin. The AVP receptor, AVPR1A, belongs to the G-protein coupled receptor family (along with V2R and OXT receptors) and works by activating the phosphatidylinositol-calcium second messenger system. When activated, AVPR1A leads to cell proliferation and contraction, glycogenolysis, the release of coagulation factors and platelet aggregation. Interestingly, the AVPR1A gene has been associated with ASD (Wassink et al., 2004; Yirmiya et al., 2006).
With regards to ASD, the study of the Brattleboro rat could reveal useful insights on the effect of AVP alterations in producing autism-related phenotypes. The Brattleboro rat is an inbred strain that does not synthesize vasopressin due to a homozygous frameshift mutation in the AVP gene (Schmale et al., 1989). This rat strain was found to have decreased social recognition (Engelmann and Landgraf, 1994) and no cardiovascular response to social isolation (Gardiner and Bennett, 1983). Similarly, vasopressin receptor 1a (V1aR) knockout mice also show defects in social recognition, social interactions, anxiety (Bielsky et al., 2005; Insel, 2010), social aggression, social motivation, social memory (Wersinger et al., 2004; Caldwell et al., 2008), and ultrasonic vocalizations (Scattoni et al., 2008). Interestingly, studies in humans have found a significant association between autism and heritable genetic defects in AVPR1a (Wassink et al., 2004), including transmission disequilibrium within the AVR intronic microsatellite region (Yirmiya et al., 2006). These findings, together with the known role of AVPR1a in social skill formation, suggest a link between vasopressin dysregulation and development of autism, thus warranting the use of AVP mouse models for the elucidation of ASD-related mechanistic insights.
Contactin-associated protein-like 2 (CNTNAP2)
The CNTNAP2 gene encodes contactin associated protein-like 2 (Caspr2) protein, which is part of the neurexin family and, therefore, plays a key role in cell adhesion. During the early nervous system development, Caspr2 mediates neuronglia interactions and helps in localizing potassium channels in developing axons (Poliak et al., 1999). The transcription factor forkhead box protein P2 (FOXP2), involved in speech and language development, regulates and directly binds to CNTNAP2 (Fisher and Scharff, 2009). Recessive-truncating mutations in CNTNAP2 cause Cortical Dysplasia Focal Epilepsy (CDFE) syndrome, which is highly comorbid with ASD (70%) (Strauss et al., 2006). Additionally, many other mutations within the CNTNAP2 gene have been identified in a number of ASD patients (Alarcón et al., 2008; Arking et al., 2008).
Interestingly, Cntnap2−/− mice exhibit the three core symptoms of ASD, including decreased social interactions, reduced vocalizations, and repetitive behaviors, as well as hyperactivity and epileptic seizures (Peñagarikano et al., 2011). Moreover, these knockout mice show abnormal neuronal migration and decreased neuronal synchrony, concurrent with a reduction in the total number of GABAergic interneurons (Peñagarikano et al., 2011). This study found that risperidone treatment rescued the repetitive behaviors of these mice. More recently, the same group found that the oxytocin system is defective in these mice and that both acute and chronic administration of the hormone rescued the social behavioral phenotypes (Peñagarikano et al., 2015). In other recent studies, Cntnap2−/− mice display impairments in auditory processing, and their hippocampus show reduction of evoked IPSC in the perisomatic part of the CA1 region while the excitatory inputs were barely affected (Truong et al., 2015). These findings further elucidate the roles of Cntnap2 gene in language development and synaptic function which are commonly affected in ASD.
In the Australian population common genetic variants in the CNTNAP2 gene were found, one of which was carried by a boy with autism and speech delays, confirming the gene’s involvement in language development and ASD (Whitehouse et al., 2011). Another study found that polymorphisms in CNTNAP2 were correlated with autism and impaired frontal lobe connectivity (Scott-Van Zeeland et al., 2010). In a population study of Chinese families with autism, a number CNTNAP2 SNPs were identified and significantly correlated with increased ASD risk (Li et al., 2010). However, not all CNTNAP2 variants are necessarily implicated in ASD, which may limit and complicate the use of knockout mice (Sampath et al., 2013). Nevertheless, the known and important roles of CNTNAP2 in brain and language development may help in elucidating the pathophysiologic mechanisms of ASD in a subset of patients.
Eukaryotic translation initiation factor 4E (eIF4E)
The eIF4E gene is found in the chromosome 4q locus and encodes a component of the eukaryotic translation initiation factor 4F complex. The encoded protein recognizes the 7-methylguanosine cap structure found at the 5′ end of mRNA. eIF4E acts as a downstream effector in the mToR, PTEN and FMRP pathways and functions as a promoter of translational initiation of target mRNAs such as neuroligins, by recruiting ribosomes to mRNA. Patients with ASD have been found to carry variants within the eIF4E gene promoter and mToR-mediated eIF4A hyperactivation has been observed in autistic FXS patients (Szatmari et al., 2007; Hoeffer et al., 2013).
In mice with direct eIF4E overexpression, or indirect over-expression through knockout of eIF4E repressor 4E-BP2 (eukaryotic translation initiation factor 4E-binding protein 2), impaired social interactions, communication deficits, and repetitive behaviors were observed (Gkogkas et al., 2013; Santini et al., 2013). In line with these autistic behaviors, these mice also display synaptic abnormalities in the mPFC, striatum and hippocampus, including increased excitatory to inhibitory ratios and an increase in eIF4E-dependent neuroligins expression (Gkogkas et al., 2013). These alterations were partly due to an increase in cap-dependent translation (Santini et al., 2013), which could be a promising pathophysiological target with implications for therapeutic treatment of ASD. The clear causal relationship between a known genetic defect and direct neurobehavioral abnormalities validates the use of the eIF4E animal model and makes it of high clinical relevance. In addition, eIF4E’s causal relationship to synaptic development and plasticity in mice supports the involvement of excitation/inhibition imbalance in ASD.
DISCUSSION
Frequency of use and general applicability of ASD animal models
A number of ASD animal models have been established, proposed and utilized to investigate the pathways of abnormal development leading to ASD. Notably, prenatal VPA exposure and monogenetic defects in FMR1, MeCP2, NLGNs, and Oxt have been most substantially studied. Moreover, the maternal immune activation (MIA) model and the prenatal VPA exposure model have both consistently recapitulated general autistic symptoms and phenotypes. Particularly, VPA exposure produced various autistic symptoms and phenotypes in rodents, such as macrocephaly, seizure susceptibility, GABAergic defects, and male-specificity. In the genetic models, knockout of FMR1 or GABRB3 leads to impaired social interactions, repetitive behaviors, increased excitatory neurotransmission, and seizure susceptibility. However, only a handful of studies on the neurobiological defects of GABRB3 knockout mice has been reported. These genetic models were inspired by studies that showed a strong association between the genes and ASD. Recently, SHANK3 mutant mice have emerged as a compelling model of ASD, and most studies have focused in understanding the underlying synaptic abnormalities. Indeed, animal models have played an essential role in the molecular and neurobiological exploration of various autism etiologies and have led to the testing and discovery of many therapeutic candidates. These studies highlight the incredible utility of animal models, despite the clear evolutionary separation and evident differences in brain structure, function, and complexity.
Imbalance between excitation and inhibition in ASD animal models
The brain of ASD patients show specific and notable features that include increased prevalence of macrocephaly (Fidler et al., 2000; Hardan et al., 2001; Gillberg and de Souza, 2002), reduced GABAergic signaling (Blatt et al., 2001; Oblak et al., 2011) and increased glutamatergic signaling (Shinohe et al., 2006). Moreover, it has also been reported that ASD patients have abnormalities in cortical minicolumn organization (Casanova et al., 2002) and synaptic development (Geschwind and Levitt, 2007; Hutsler and Zhang, 2010). In addition, some of the most prominent anatomical features of autistic brains include increased number of neurons (Casanova et al., 2006), reduced number of Purkinje cells in the cerebellum (Rout and Dhossche, 2008) and reduced GABAergic neurons and markers (Fatemi et al., 2009). These features are reminiscent of imbalanced excitation and inhibition in the autistic brain, which has been recently proposed as a major cause of ASD symptomatology. Along these lines, a number of studies have suggested that an increase in the ratio of excitatory versus inhibitory neurotransmission is consistent across various autism etiologies (Dani et al., 2005). For example, the expression of GABAA receptor subunits (Samaco et al., 2005) and GAD proteins (Fatemi et al., 2002) are decreased in the brain of autistic patients. Disrupted inhibitory architecture in the brain of ASD patients were also reported (Casanova et al., 2003).
Converging evidence supporting the role of imbalanced excitatory/inhibitory neurotransmission in ASD has also been found in a number of animal models. For example, prenatal VPA exposed-rats have increased excitation and hyperconnectivity in the brain, as well as NMDA receptor over-expression (Rinaldi et al., 2007, 2008). Moreover, the prenatal LPS-exposed models show pyramidal neuron hyperexcitability, increased postsynaptic glutamatergic activity, and reduced NMDA-induced synaptic plasticity (Lante et al., 2008; Patterson, 2009). In addition, reduction of mGluR5 expression in the brain of FMR1 knockout mice was also associated with an increased excitatory/inhibitory ratio (Silva and Ehninger, 2009). MeCP2 knockout mice, on the other hand, showed defects in GABAergic neuron function, as demonstrated by reduced inhibitory quantal size and decreased GAD expression (Chao et al., 2010). In addition, these mice display excessive glutamate release by microglia (Maezawa and Jin, 2010). Interestingly, in the VPA prenatal exposure animal model, MeCP2 expression was attenuated and glutamatergic transmission was increased, reflected by the upregulation of NMDA, AMPA and mGlu receptors and postsynaptic proteins (Kim et al., 2014b). In the Oxtr-null mice, a decreased ratio of GABAergic synapse versus the total presynapse was observed (Sala et al., 2011), while, in PTEN mutant mice, the net excitatory drive was increased (Luikart et al., 2011). In Reeler mice, decreased density of striatal GABAergic interneurons was shown (Marrone et al., 2006). More recently, eIF4E’s causal role in synaptic development and plasticity has provided additional evidence supporting the E/I imbalance hypothesis of ASD pathogenesis (Santini et al., 2013). Both human and animal studies have demonstrated that the overall anatomical sites and neuro-transmission phenotypes related to ASD, especially in terms of E/I imbalance, could vary depending on the etiologic factors involved. Many of the studies included in this review suggest either “too much excitation” or “too little inhibition” and vice versa, as the culprits for the altered E/I ratios in the animal models, which mostly result in common, although not identical, behavioral phenotypes. By carefully comparing the data obtained from the various animal models of ASD, we can obtain an idea of how and why the seemingly diverse neurobiological changes caused by each etiologic factor induce similar behavioral phenotypes. Furthermore, the data gathered from all of these models may help us find multiple levels of convergence in terms of signaling pathways, receptor and neurotransmitter systems, and specific neuronal circuits involved in the pathophysiologic mechanisms of ASD.
Alterations in the function of postsynaptic proteins, which mediate synapse formation and maturation, can be also notably related to the defects in excitatory neurotransmission. For example, genetic abnormalities in neuroligins and neurexins result in decreased excitatory activity and altered NMDA/AMPA ratios in multiple animal models (Etherton et al., 2009; Blundell et al., 2010). In addition, defects in the SHANK genes also led to impaired NMDA receptor function and mGluR5-dependent synaptic transmission (Verpelli et al., 2011). This suggests that the study of a subset of these animal models can provide useful information about the specific implications of synaptic dysregulation in the pathophysiology of ASD.
Altogether, our current knowledge from animal models, in combination with clinical findings in ASD, suggests that aberrations in E/I activity are likely involved in the disorder pathogenesis. This leads to the conclusion that optimal levels of excitation and inhibition are essential in the maintenance of proper synaptic function and prevent the precipitation of aberrant behavioral phenotypes (Fig. 1). As schematically represented in our hot air balloon conceptualization, maintaining the optimal range of neurobehavioral activity requires adequate balance of excitation (as represented by the hot air regulated by the activity of gas burners) and inhibition (as represented by weights regulated by sand bags); alterations in either direction will cause a drift into pathologic neurobehavioral phenotypes. One important aspect of this model is the role that synaptic activity modulators have in the overall E/I state, as they become key determinants of whether or not autistic behaviors arise, in a way that might involve the meta-plastic regulation of LTP and LTD (Abraham, 2008; Turrigiano, 2012). For examples, the innate properties of neural circuits, as well as the homeostatic maintenance of intracellular calcium concentration and the expression level of other neuromodulators, may differentially determine the final behavioral outcome. These, together with an interplay between genetic and/or environmental factors, will ultimately regulate and determine synapse quantity and structure (Fig. 2 as represented by persons grabbing the safety line of the hot air balloon). This model also predicts that stimuli affecting the long-term activity and expression of synaptic modulators may result in long-term hyper- or hypo-sensitivity, and is analogous to how gene x gene or gene x environmental interactions contribute to specific phenotypes (see below).
Fig. 1.
Excitatory/Inhibitory Imbalance in ASD. (A) Normal/optimum condition (balanced excitation, inhibition and synaptic regulation). (B) Hyper-excitatory condition due to increased excitation from a variety of genetic and/or environmental factors (B-1, i.e. FMR1, MeCP2, NLGN3, PTEN, SAHNK2 and PTEN genetic knockout/mutations; LPS and VPA prenatal exposures) or decreased-inhibitory regulators (B-2, i.e. CNTNAP2, GABRB3, MeCP2, RELN genetic knockout/mutations; prenatal VPA exposure) affecting synaptic strength; synaptic regulators could be normal. (C) Hyper-inhibitory condition due to increased inhibition from genetic or environmental factors (C-1, for example, NLGN3 mutation) and decreased excitation inducers (C-2, i.e. SHANK2 & UBE3A genetic knockout/mutations).
Fig. 2.
Altered synaptic regulators in ASD leading to E-I imbalance. (A) Normal/optimum condition (balanced excitation, inhibition, and synaptic regulation). (B) Hyper-excitatory condition due to altered synaptic regulators. Even with the normal synaptic structure and numbers, dysregulation of synaptic modulators such as altered intracellular calcium level either by genetic or environmental factors may render the brain to more excitable states. (C) Hyper-inhibitory condition due to altered synaptic regulators, for example, reduced intracellular calcium level. With the altered synaptic regulators function, otherwise harmless weak stimuli (either genetic or environmental) may contribute to the manifestation of autistic phenotypes, which explains various types of gene (environmental) x gene (environmental) interaction. Alternatively, innate differences in the synaptic modulator functions between male and female may explain the gender-skewed prevalence of ASD.
Opposing neurotransmission profiles with similar behavior symptoms in animal models
As can be appreciated from the numerous studies described above, most of them found similar behavioral phenotypes in their models, yet somewhat opposing neurotransmission profiles. One good example is the Shank2−/− mutant mice, where Schmeisser et al. (2012) found heightened NMDAR excitatory currents in the model, whereas Won et al. (2012) discovered a decreased NMDA receptor function. Nevertheless, both studies found repetitive grooming, hyperactivity, impaired vocalizations and social behaviors in Shank2−/− mutant mice. A similar phenomenon was also observed in models showing abnormalities in the serotonergic system. For example, rats exposed to prenatal VPA displayed low serotonin levels in the hippocampus at postnatal day 50 (Dufour-Rainfray et al., 2010) as well as the abnormal migration of 5-HT+ neurons (Kuwagata et al., 2009). BDNF−/+ and DHCR7 mutant mice further showed serotonergic transmission defects (Rios et al., 2006; Daws et al., 2007). On the other hand, MAOA deficient mice displayed elevated levels of serotonin and other monoamines in the brain (Bortolato et al., 2013; Singh et al., 2013). Interestingly, all of these models express similar aggressive and social deficit behaviors (although varied in locomotor activity), despite the opposing serotonin phenotypes. As such, this demonstrates an obvious variability across the various animal models, where opposing neurophysiological phenotypes can overlap with common behavioral symptoms. These conflicting results can perhaps be resolved by employing a more careful comparison of the various animal models and the clinical presentation within each corresponding etiological subgroup, be it at the molecular, behavioral, and physiological level. Nonetheless, this might be a challenging task given the heterogeneity of ASD. This, therefore, calls for a more individualized approach in the treatment of ASD patients. This approach will also be beneficial for future patient stratification and subgroup classification, which may aid in devising a group or patient-specific treatment based on specific autistic features and range in the spectrum.
Interactions between environment and genetics
Although a number of genetic models have been reported for ASD, most, if not all of them, only share a part of the core symptoms of the disorder. Moreover, single gene knockout models usually correspond to specific syndromes that can be distinguished from ASD. Thus, although ASD-like symptoms occur in a number of single-gene disorders such as tuberous sclerosis, Angelman syndrome, phenylketonuria, Joubert syndrome, Mőbius syndrome and fragile X syndrome, more than 90% of ASD cases are not related to any of these (Geschwind, 2011). Indeed, the etiological heterogeneity of ASD further hinders the identification of causative genes (Bill and Geschwind, 2009). Twin studies showed that ASD is highly heritable, with monozygotic twins showing 60–90% concordance and dizygotic twins showing <5% concordance (Steffenburg et al., 1989; Bailey et al., 1995). However, recent studies have shown that monozygotic twins do not always display complete heritability (100% concordance) and attribute this in part to environmental factors, which could either aggravate or even protect against ASD (Croen et al., 2005; Hallmayer et al., 2011). Moreover, it was suggested that at least 40% of ASD cases are likely spawned by environmental causes (Hertz-Picciotto et al., 2006). Based on these reports, the role of the environment should not be ignored as it might have a substantial impact in the development of ASD (Bill and Geschwind, 2009). In addition, the prevalence of ASD has remarkably increased over the years, (Hertz-Picciotto et al., 2006) from 4–5 per 10,000 births in 1990s (Fombonne, 1999) to 4–6 per 1000 births in early 2000s (Chakrabarti and Fombonne, 2005) and to 14.7 per 1000 children (US) in 2010 (Autism and Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators, 2014). It is thought that, perhaps, environmental factors could be contributing to the rise in ASD prevalence over time. One good example that used animal models to explore this hypothesis was the work of Ehninger et al., as described above, which demonstrated that MIA (through poly I:C treatment) in TSC mutant mice potentiated the social impairments in the offspring (Ehninger et al., 2012). Indeed, this experimental design provides new insights to potentially harmonize the involvement of genetics and environment in the development of ASD. Along with the increasing awareness about the co-contributions of environmental and genetic factors in the development and rise of ASD diagnoses (Arndt et al., 2005; Bello, 2007; Kolevzon et al., 2007), other animal models suitable for the study of gene-environmental interactions (G×E) in ASD are eagerly anticipated. In this regard, our group is now actively investigating the effects of combined environmental (VPA exposure) and genetic mutations in animal brain development and autism-related phenotypes.
CONCLUSION AND FUTURE DIRECTION
While the prevalence of ASD has increased over the years, the number of proposed etiologic factors including genetic, epigenetic and environmental factors has also grown. Moreover, theories about the pathophysiologic mechanisms and pathways underlying the disorder have greatly diversified. As much as it is beneficial and essential to dig deeper into the neurobiological events underlying the phenotypes in ASD, animal studies require careful interpretation, especially due to the increasing evidence of ASD comorbidity and phenotype overlap with other disorders. Animal models provide an important role in completing the puzzle of how each etiologic factor contributes to ASD pathophysiology. It is evident that every specific etiologic condition being modeled in ASD holds a uniquely different set of behavioral and neurobiological phenotypes. This nonetheless, will likely foment the development of therapeutic strategies targeted towards the specific symptoms and neuronal abnormalities observed. Scientists and medical practitioners should, therefore, collaborate and create a database or open resource for mining the possible environmental or genetic causes that can be assessed in every autistic family and individual, in combination with potential case-specific therapeutic treatments. From there, more applicable and effective therapies could be given to autistic patients with a clearly identified etiology. Patient- and etiology-specific approaches may be the ultimate solution to treat the complex and heterogeneous disorder that is ASD.
Acknowledgments
The authors declare no conflict of interests. This work was supported by the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (No. A120029), and Mid-career Researcher Program (2014R1A2A2A01003079) through the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST), and supported by NSF-GRFP DGE-1144087 and UCLA Pre-doctoral Training Program in Neurogenetics (NIMH-T32).
REFERENCES
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Alarcón M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, Sebat J, Wigler M, Martin CL, Ledbetter DH, Nelson SF, Cantor RM, Geschwind DH. Linkage, Association, and Gene-Expression Analyses Identify CNTNAP2 as an Autism-Susceptibility Gene. Am J Hum Genet. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G, Courchesne E. Differential effects of developmental cerebellar abnormality on cognitive and motor functions in the cerebellum: an fMRI study of autism. Am. J. Psychiatry. 2003;160:262–273. doi: 10.1176/appi.ajp.160.2.262. [DOI] [PubMed] [Google Scholar]
- Altamura C, Dell’Acqua ML, Moessner R, Murphy DL, Lesch KP, Persico AM. Altered neocortical cell density and layer thickness in serotonin transporter knockout mice: a quantitation study. Cereb. Cortex. 2007;17:1394–1401. doi: 10.1093/cercor/bhl051. [DOI] [PubMed] [Google Scholar]
- Aman MG, Langworthy KS. Pharmacotherapy for hyperactivity in children with autism and other pervasive developmental disorders. J Autism Dev Disord. 2000;30:451–459. doi: 10.1023/A:1005559725475. [DOI] [PubMed] [Google Scholar]
- Amaral D, Bauman M, Schumann CM. The amygdala and autism: implications from non-human primate studies. Genes Brain Behav. 2003;2:295–302. doi: 10.1034/j.1601-183X.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. American Psychiatric Publishing; 2013. [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Amodeo DA, Jones JH, Sweeney JA, Ragozzino ME. Differences in BTBR T+ tf/J and C57BL/6J mice on probabilistic reversal learning and stereotyped behaviors. Behav Brain Res. 2012;227:64–72. doi: 10.1016/j.bbr.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci USA. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arking DE, Cutler DJ, Brune CW, Teslovich TM, West K, Ikeda M, Rea A, Guy M, Lin S, Cook EH, Chakravarti A. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am J Hum Genet. 2008;82:160–164. doi: 10.1016/j.ajhg.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt TL, Stodgell CJ, Rodier PM. The teratology of autism. Int J Dev Neurosci. 2005;23:189–199. doi: 10.1016/j.ijdevneu.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Aronson M, Hagberg B, Gillberg C. Attention deficits and autistic spectrum problems in children exposed to alcohol during gestation: a follow-up study. Dev Med Child Neurol. 1997;39:583–587. doi: 10.1111/j.1469-8749.1997.tb07493.x. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480:63–68. doi: 10.1038/nature10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators Prevalence of autism spectrum disorder among children aged 8 years-autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63:1–21. [PubMed] [Google Scholar]
- Bachevalier J. Medial temporal lobe structures and autism: a review of clinical and experimental findings. Neuropsychologia. 1994;32:627–648. doi: 10.1016/0028-3932(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25:63–77. doi: 10.1017/S0033291700028099. [DOI] [PubMed] [Google Scholar]
- Ballas N, Lioy DT, Grunseich C, Mandel G. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci. 2009;12:311–317. doi: 10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Frazier TW, Piacenza L, Minshew NJ, Keshavan MS, Reiss AL, Hardan AY. A preliminary longitudinal volumetric MRI study of amygdala and hippocampal volumes in autism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2014;48:124–128. doi: 10.1016/j.pnpbp.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateup HS, Takasaki KT, Saulnier JL, Denefrio CL, Sabatini BL. Loss of Tsc1 in vivo impairs hippocampal mGluRLTD and increases excitatory synaptic function. J Neurosci. 2011;31:8862–8869. doi: 10.1523/JNEUROSCI.1617-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman M, Kemper TL. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35:866–874. doi: 10.1212/WNL.35.6.866. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Bello SC. Autism and environmental influences: review and commentary. Rev. Environ. Health. 2007;22:139–156. doi: 10.1515/REVEH.2007.22.2.139. [DOI] [PubMed] [Google Scholar]
- Benayed R, Gharani N, Rossman I, Mancuso V, Lazar G, Kamdar S, Bruse SE, Tischfield S, Smith BJ, Zimmerman RA, Dicicco-Bloom E, Brzustowicz LM, Millonig JH. Support for the homeobox transcription factor gene ENGRAILED 2 as an autism spectrum disorder susceptibility locus. Am J Hum Genet. 2005;77:851–868. doi: 10.1086/497705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenuto A, Moavero R, Alessandrelli R, Manzi B, Curatolo P. Syndromic autism: causes and pathogenetic pathways. World J Pediatr. 2009;5:169–176. doi: 10.1007/s12519-009-0033-2. [DOI] [PubMed] [Google Scholar]
- Berkel S, Marshall CR, Weiss B, Howe J, Roeth R, Moog U, Endris V, Roberts W, Szatmari P, Pinto D, Bonin M, Riess A, Engels H, Sprengel R, Scherer SW, Rappold GA. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat Genet. 2010;42:489–491. doi: 10.1038/ng.589. [DOI] [PubMed] [Google Scholar]
- Berkel S, Tang W, Treviño M, Vogt M, Obenhaus HA, Gass P, Scherer SW, Sprengel R, Schratt G, Rappold GA. Inherited and de novo SHANK2 variants associated with autism spectrum disorder impair neuronal morphogenesis and physiology. Hum Mol Genet. 2012;21:344–357. doi: 10.1093/hmg/ddr470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard S, Enayati A, Roger H, Binstock T, Redwood L. The role of mercury in the pathogenesis of autism. Mol. Psychiatry. 2002;7 Suppl(2):S42–S43. doi: 10.1038/sj.mp.4001177. [DOI] [PubMed] [Google Scholar]
- Bernardet M, Crusio WE. Fmr1 KO mice as a possible model of autistic features. Sci World J. 2006;6:1164–1176. doi: 10.1100/tsw.2006.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur C, Corbex M, Spielewoy C, Philippe A, Laplanche JL, Launay JM, Gillberg C, Mouren-Siméoni MC, Hamon M, Giros B, Nosten-Bertrand M, Leboyer M. Serotonin transporter gene polymorphisms and hyperserotonemia in autistic disorder. Mol. Psychiatry. 2002;7:67–71. doi: 10.1038/sj.mp.4000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Bill BR, Geschwind DH. Genetic advances in autism: heterogeneity and convergence on shared pathways. Curr Opin Genet Dev. 2009;19:271–278. doi: 10.1016/j.gde.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Defensor EB, Meyza KZ, Pobbe RL, Pearson BL, Bolivar VJ, Blanchard RJ. BTBR T+tf/J mice: autism-relevant behaviors and reduced fractone-associated heparan sulfate. Neurosci Biobehav Rev. 2012;36:285–296. doi: 10.1016/j.neubiorev.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt GJ. GABAergic cerebellar system in autism: a neuropathological and developmental perspective. Int Rev Neurobiol. 2005;71:167–178. doi: 10.1016/S0074-7742(05)71007-2. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Fitzgerald CM, Guptill JT, Booker AB, Kemper TL, Bauman ML. Density and distribution of hippocampal neurotransmitter receptors in autism: an autoradiographic study. J Autism Dev Disord. 2001;31:537–543. doi: 10.1023/A:1013238809666. [DOI] [PubMed] [Google Scholar]
- Blundell J, Blaiss CA, Etherton MR, Espinosa F, Tabuchi K, Walz C, Bolliger MF, Sudhof TC, Powell CM. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J Neurosci. 2010;30:2115–2129. doi: 10.1523/JNEUROSCI.4517-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobee S, Mariette E, Tremblay-Leveau H, Caston J. Effects of early midline cerebellar lesion on cognitive and emotional functions in the rat. Behav Brain Res. 2000;112:107–117. doi: 10.1016/S0166-4328(00)00166-2. [DOI] [PubMed] [Google Scholar]
- Boccaccio I, Glatt-Deeley H, Watrin F, Roeckel N, Lalande M, Muscatelli F. The human MAGEL2 gene and its mouse homologue are paternally expressed and mapped to the Prader-Willi region. Hum Mol Genet. 1999;8:2497–2505. doi: 10.1093/hmg/8.13.2497. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton PF, Park RJ, Higgins JN, Griffiths PD, Pickles A. Neuro-epileptic determinants of autism spectrum disorders in tuberous sclerosis complex. Brain. 2002;125:1247–1255. doi: 10.1093/brain/awf124. [DOI] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Godar SC, Alzghoul L, Zhang J, Darling RD, Simpson KL, Bini V, Chen K, Wellman CL, Lin RC, Shih JC. Monoamine oxidase A and A/B knockout mice display autistic-like features. Int J Neuropsychopharmacol. 2013;16:869–888. doi: 10.1017/S1461145712000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O, Sakurai T, Papapetrou D, Wang X, Dickstein DL, Takahashi N, Kajiwara Y, Yang M, Katz AM, Scattoni ML, Harris MJ, Saxena R, Silverman JL, Crawley JN, Zhou Q, Hof PR, Buxbaum JD. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol. Autism. 2010;1:15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan FX, Albeck DS, Paylor R. Fmr1 knockout mice are impaired in a leverpress escape/avoidance task. Genes Brain Behav. 2006;5:467–471. doi: 10.1111/j.1601-183X.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- Brune CW, Korvatska E, Allen-Brady K, Cook EH, Jr, Dawson G, Devlin B, Estes A, Hennelly M, Hyman SL, McMahon WM, Munson J, Rodier PM, Schellenberg GD, Stodgell CJ, Coon H. Heterogeneous association between engrailed-2 and autism in the CPEA network. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008;147B:187–193. doi: 10.1002/ajmg.b.30585. [DOI] [PubMed] [Google Scholar]
- Bryn V, Halvorsen B, Ueland T, Isaksen J, Kolkova K, Ravn K, Skjeldal O. Brain derived neurotrophic factor (BDNF) and autism spectrum disorders (ASD) in childhood. Eur J Paediatr Neurol. 2015;19:411–414. doi: 10.1016/j.ejpn.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Busquets-Garcia A, Gomis-González M, Guegan T, Agustín-Pavón C, Pastor A, Mato S, Pérez-Samartín A, Matute C, de la Torre R, Dierssen M, Maldonado R, Ozaita A. Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nat Med. 2013;19:603–607. doi: 10.1038/nm.3127. [DOI] [PubMed] [Google Scholar]
- Butler MG, Dasouki MJ, Zhou XP, Talebizadeh Z, Brown M, Takahashi TN, Miles JH, Wang CH, Stratton R, Pilarski R, Eng C. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet. 2005;42:318–321. doi: 10.1136/jmg.2004.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum JD, Silverman JM, Smith CJ, Greenberg DA, Kilifarski M, Reichert J, Cook EH, Jr, Fang Y, Song CY, Vitale R. Association between a GABRB3 polymorphism and autism. Mol. Psychiatry. 2002;7:311–316. doi: 10.1038/sj.mp.4001011. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Wersinger SR, Young WS., 3rd The role of the vasopressin 1b receptor in aggression and other social behaviours. Prog Brain Res. 2008;170:65–72. doi: 10.1016/S0079-6123(08)00406-8. [DOI] [PubMed] [Google Scholar]
- Carper RA, Courchesne E. Localized enlargement of the frontal cortex in early autism. Biol. Psychiatry. 2005;57:126–133. doi: 10.1016/j.biopsych.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Carson RP, Van Nielen DL, Winzenburger PA, Ess KC. Neuronal and glia abnormalities in Tsc1-deficient forebrain and partial rescue by rapamycin. Neurobiol Dis. 2012;45:369–380. doi: 10.1016/j.nbd.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden D, Gomez J. Disruption in the inhibitory architecture of the cell minicolumn: implications for autisim. Neuroscientist. 2003;9:496–507. doi: 10.1177/1073858403253552. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Brown C. Clinical and macroscopic correlates of minicolumnar pathology in autism. J Child Neurol. 2002;17:692–695. doi: 10.1177/088307380201700908. [DOI] [PubMed] [Google Scholar]
- Casanova MF, van Kooten IA, Switala AE, van Engeland H, Heinsen H, Steinbusch HW, Hof PR, Trippe J, Stone J, Schmitz C. Minicolumnar abnormalities in autism. Acta Neuropathol. 2006;112:287–303. doi: 10.1007/s00401-006-0085-5. [DOI] [PubMed] [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Muller U, Aguet M, Babinet C, Shih JC, De Maeyer E. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadman KK. Fluoxetine but not risperidone increases sociability in the BTBR mouse model of autism. Pharmacol Biochem Behav. 2011;97:586–594. doi: 10.1016/j.pbb.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children: confirmation of high prevalence. Am. J. Psychiatry. 2005;162:1133–1141. doi: 10.1176/appi.ajp.162.6.1133. [DOI] [PubMed] [Google Scholar]
- Chan JP, Unger TJ, Byrnes J, Rios M. Examination of behavioral deficits triggered by targeting Bdnf in fetal or postnatal brains of mice. Neuroscience. 2006;142:49–58. doi: 10.1016/j.neuroscience.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, Gong S, Lu HC, Heintz N, Ekker M, Rubenstein JL, Noebels JL, Rosenmund C, Zoghbi HY. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheh MA, Millonig JH, Roselli LM, Ming X, Jacobsen E, Kamdar S, Wagner GC. En2 knockout mice display neurobehavioral and neurochemical alterations relevant to autism spectrum disorder. Brain Res. 2006;1116:166–176. doi: 10.1016/j.brainres.2006.07.086. [DOI] [PubMed] [Google Scholar]
- Chen Y, Beffert U, Ertunc M, Tang TS, Kavalali ET, Bezprozvanny I, Herz J. Reelin modulates NMDA receptor activity in cortical neurons. J Neurosci. 2005;25:8209–8216. doi: 10.1523/JNEUROSCI.1951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess S, Fernandez P, Korn S. Behavioral consequences of congenital rubella. J Pediatr. 1978;93:699–703. doi: 10.1016/S0022-3476(78)80921-4. [DOI] [PubMed] [Google Scholar]
- Chevere-Torres I, Maki JM, Santini E, Klann E. Impaired social interactions and motor learning skills in tuberous sclerosis complex model mice expressing a dominant/negative form of tuberin. Neurobiol Dis. 2012;45:156–164. doi: 10.1016/j.nbd.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching MS, Shen Y, Tan WH, Jeste SS, Morrow EM, Chen X, Mukaddes NM, Yoo SY, Hanson E, Hundley R, Austin C, Becker RE, Berry GT, Driscoll K, Engle EC, Friedman S, Gusella JF, Hisama FM, Irons MB, Lafiosca T, LeClair E, Miller DT, Neessen M, Picker JD, Rappaport L, Rooney CM, Sarco DP, Stoler JM, Walsh CA, Wolff RR, Zhang T, Nasir RH, Wu BL. Deletions of NRXN1 (neurexin-1) predispose to a wide spectrum of developmental disorders. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2010;153B:937–947. doi: 10.1002/ajmg.b.31063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KS, Kwon KJ, Choi CS, Jeon SJ, Kim KC, Park JH, Ko HM, Lee SH, Cheong JH, Ryu JH, Han SH, Shin CY. Valproic acid induces astrocyte-dependent neu-rite outgrowth from cultured rat primary cortical neuron via modulation of tPA/PAI-1 activity. Glia. 2013;61:694–709. doi: 10.1002/glia.22463. [DOI] [PubMed] [Google Scholar]
- Christianson AL, Chesler N, Kromberg JG. Fetal valproate syndrome: clinical and neuro-developmental features in two sibling pairs. Dev Med Child Neurol. 1994;36:361–369. doi: 10.1111/j.1469-8749.1994.tb11858.x. [DOI] [PubMed] [Google Scholar]
- Chu EC, Tarnawski AS. PTEN regulatory functions in tumor suppression and cell biology. Med Sci Monit. 2004;10:RA235–RA241. [PubMed] [Google Scholar]
- Cichowski K, Jacks T. NF1 tumor suppressor gene function: narrowing the GAP. Cell. 2001;104:593–604. doi: 10.1016/S0092-8674(01)00245-8. [DOI] [PubMed] [Google Scholar]
- Cohen I, Liu X, Lewis M, Chudley A, Forster-Gibson C, Gonzalez M, Jenkins E, Brown W, Holden J. Autism severity is associated with child and maternal MAOA genotypes. Clin Genet. 2011;79:355–362. doi: 10.1111/j.1399-0004.2010.01471.x. [DOI] [PubMed] [Google Scholar]
- Cohen IL, Liu X, Schutz C, White BN, Jenkins EC, Brown WT, Holden JJ. Association of autism severity with a monoamine oxidase A functional polymorphism. Clin Genet. 2003;64:190–197. doi: 10.1034/j.1399-0004.2003.00115.x. [DOI] [PubMed] [Google Scholar]
- Comoletti D, De Jaco A, Jennings LL, Flynn RE, Gaietta G, Tsigelny I, Ellisman MH, Taylor P. The Arg451Cysneuroligin-3 mutation associated with autism reveals a defect in protein processing. J Neurosci. 2004;24:4889–4893. doi: 10.1523/JNEUROSCI.0468-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Cerro LS, Wassif CA, Kratz L, Miller GF, Munasinghe JP, Grinberg A, Fliesler SJ, Porter FD. Development and characterization of a hypomorphic Smith-Lemli-Opitz syndrome mouse model and efficacy of simvastatin therapy. Hum Mol Genet. 2006;15:839–851. doi: 10.1093/hmg/ddl003. [DOI] [PubMed] [Google Scholar]
- Costa RM, Federov NB, Kogan JH, Murphy GG, Stern J, Ohno M, Kucherlapati R, Jacks T, Silva AJ. Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature. 2002;415:526–530. doi: 10.1038/nature711. [DOI] [PubMed] [Google Scholar]
- Costa RM, Yang T, Huynh DP, Pulst SM, Viskochil DH, Silva AJ, Brannan CI. Learning deficits, but normal development and tumor predisposition, in mice lacking exon 23a of Nf1. Nat Genet. 2001;27:399–405. doi: 10.1038/86898. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Saitoh O, Yeung-Courchesne R, Press GA, Lincoln AJ, Haas RH, Schreibman L. Abnormality of cerebellar vermian lobules VI and VII in patients with infantile autism: identification of hypoplastic and hyperplastic subgroups with MR imaging. AJR Am J Roentgenol. 1994;162:123–130. doi: 10.2214/ajr.162.1.8273650. [DOI] [PubMed] [Google Scholar]
- Croen LA, Grether JK, Yoshida CK, Odouli R, Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study. Arch Pediatr Adolesc Med. 2005;159:151–157. doi: 10.1001/archpedi.159.2.151. [DOI] [PubMed] [Google Scholar]
- Curatolo P, Porfirio MC, Manzi B, Seri S. Autism in tuberous sclerosis. Eur J Paediatr Neurol. 2004;8:327–332. doi: 10.1016/j.ejpn.2004.08.005. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G. The reeler mouse: anatomy of a mutant. Int Rev Neurobiol. 2005;71:383–417. doi: 10.1016/S0074-7742(05)71016-3. [DOI] [PubMed] [Google Scholar]
- Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, Nelson SB. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci USA. 2005;102:12560–12565. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels JL, Forssen U, Hultman CM, Cnattingius S, Savitz DA, Feychting M, Sparen P. Parental psychiatric disorders associated with autism spectrum disorders in the offspring. Pediatrics. 2008;121:e1357–e1362. doi: 10.1542/peds.2007-2296. [DOI] [PubMed] [Google Scholar]
- Davis LK, Hazlett HC, Librant AL, Nopoulos P, Sheffield VC, Piven J, Wassink TH. Cortical enlargement in autism is associated with a functional VNTR in the monoamine oxidase A gene. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008;147B:1145–1151. doi: 10.1002/ajmg.b.30738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC, Munn JL, Valdez MF, Frosto-Burke T, Hensler JG. Serotonin transporter function, but not expression, is dependent on brain-derived neurotrophic factor (BDNF): in vivo studies in BDNF-deficient mice. J Neurochem. 2007;101:641–651. doi: 10.1111/j.1471-4159.2006.04392.x. [DOI] [PubMed] [Google Scholar]
- DeLorey TM, Handforth A, Anagnostaras SG, Homanics GE, Minassian BA, Asatourian A, Fanselow MS, Delgado-Escueta A, Ellison GD, Olsen RW. Mice lacking the beta3 subunit of the GABAA receptor have the epilepsy phenotype and many of the behavioral characteristics of Angelman syndrome. J Neurosci. 1998;18:8505–8514. doi: 10.1523/JNEUROSCI.18-20-08505.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorey TM, Sahbaie P, Hashemi E, Homanics GE, Clark JD. Gabrb3 gene deficient mice exhibit impaired social and exploratory behaviors, deficits in non-selective attention and hypoplasia of cerebellar vermal lobules: a potential model of autism spectrum disorder. Behav Brain Res. 2008;187:207–220. doi: 10.1016/j.bbr.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Gerrits MA, Brouwers JP, van Ree JM. Early amygdala damage disrupts performance on medial prefrontal cortex-related tasks but spares spatial learning and memory in the rat. Neuroscience. 2005;130:581–590. doi: 10.1016/j.neuroscience.2004.09.022. [DOI] [PubMed] [Google Scholar]
- DiMario FJ., Jr Brain abnormalities in tuberous sclerosis complex. J Child Neurol. 2004;19:650–657. doi: 10.1177/08830738040190090401. [DOI] [PubMed] [Google Scholar]
- Dufour-Rainfray D, Vourc’h P, Le Guisquet AM, Garreau L, Ternant D, Bodard S, Jaumain E, Gulhan Z, Belzung C, Andres CR, Chalon S, Guilloteau D. Behavior and serotonergic disorders in rats exposed prenatally to valproate: a model for autism. Neurosci Lett. 2010;470:55–59. doi: 10.1016/j.neulet.2009.12.054. [DOI] [PubMed] [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gillberg IC, Anckarsater H, Sponheim E, Goubran-Botros H, Delorme R, Chabane N, Mouren-Simeoni MC, de Mas P, Bieth E, Roge B, Heron D, Burglen L, Gillberg C, Leboyer M, Bourgeron T. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39:25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykens EM, Lee E, Roof E. Prader-Willi syndrome and autism spectrum disorders: an evolving story. J Neurodev Disord. 2011;3:225–237. doi: 10.1007/s11689-011-9092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Sano Y, de Vries PJ, Dies K, Franz D, Geschwind DH, Kaur M, Lee YS, Li W, Lowe JK, Nakagawa JA, Sahin M, Smith K, Whittemore V, Silva AJ. Gestational immune activation and Tsc2 haploinsufficiency cooperate to disrupt fetal survival and may perturb social behavior in adult mice. Mol. Psychiatry. 2012;17:62–70. doi: 10.1038/mp.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M, Landgraf R. Microdialysis administration of vasopressin into the septum improves social recognition in Brattleboro rats. Physiol Behav. 1994;55:145–149. doi: 10.1016/0031-9384(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Flaherty-Craig CV, Benton AL. Developmental outcomes after early prefrontal cortex damage. Brain Cogn. 2004;55:84–103. doi: 10.1016/S0278-2626(03)00281-1. [DOI] [PubMed] [Google Scholar]
- Etherton M, Foldy C, Sharma M, Tabuchi K, Liu X, Shamloo M, Malenka RC, Sudhof TC. Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function. Proc Natl Acad Sci USA. 2011;108:13764–13769. doi: 10.1073/pnas.1111093108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton MR, Blaiss CA, Powell CM, Sudhof TC. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc Natl Acad Sci USA. 2009;106:17998–18003. doi: 10.1073/pnas.0910297106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ, Chauhan A, Chauhan V, Dager SR, Dickson PE, Estes AM, Goldowitz D, Heck DH, Kemper TL, King BH, Martin LA, Millen KJ, Mittleman G, Mosconi MW, Persico AM, Sweeney JA, Webb SJ, Welsh JP. Consensus paper: pathological role of the cerebellum in autism. Cerebellum. 2012;11:777–807. doi: 10.1007/s12311-012-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol. Psychiatry. 2002;52:805–810. doi: 10.1016/S0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Thuras PD. GABA(A) receptor downregulation in brains of subjects with autism. J Autism Dev Disord. 2009;39:223–230. doi: 10.1007/s10803-008-0646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler DJ, Bailey JN, Smalley SL. Macrocephaly in autism and other pervasive developmental disorders. Dev Med Child Neurol. 2000;42:737–740. doi: 10.1017/S0012162200001365. [DOI] [PubMed] [Google Scholar]
- Fisher SE, Scharff C. FOXP2 as a molecular window into speech and language. Trends Genet. 2009;25:166–177. doi: 10.1016/j.tig.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Fitzky BU, Moebius FF, Asaoka H, Waage-Baudet H, Xu L, Xu G, Maeda N, Kluckman K, Hiller S, Yu H, Batta AK, Shefer S, Chen T, Salen G, Sulik K, Simoni RD, Ness GC, Glossmann H, Patel SB, Tint GS. 7-Dehydrocholesterol-dependent proteolysis of HMG-CoA reductase suppresses sterol biosynthesis in a mouse model of Smith-Lemli-Opitz/RSH syndrome. J Clin Invest. 2001;108:905–915. doi: 10.1172/JCI200112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fombonne E. The epidemiology of autism: a review. Psychol Med. 1999;29:769–786. doi: 10.1017/S0033291799008508. [DOI] [PubMed] [Google Scholar]
- Frye CA, Llaneza DC. Corticosteroid and neurosteroid dysregulation in an animal model of autism, BTBR mice. Physiol Behav. 2010;100:264–267. doi: 10.1016/j.physbeh.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi M, Nii T, Ishimaru N, Minamino A, Hara D, Takasaki I, Tabuchi A, Tsuda M. Valproic acid induces up- or down-regulation of gene expression responsible for the neuronal excitation and inhibition in rat cortical neurons through its epigenetic actions. Neurosci Res. 2009;65:35–43. doi: 10.1016/j.neures.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Garber KB, Visootsak J, Warren ST. Fragile X syndrome. Eur J Hum Genet. 2008;16:666–672. doi: 10.1038/ejhg.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SM, Bennett T. The cardiovascular and renal responses to short-term isolation in Brattleboro rats. Clin Sci. 1983;64:377–382. doi: 10.1042/cs0640377. [DOI] [PubMed] [Google Scholar]
- Gauthier J, Bonnel A, St-Onge J, Karemera L, Laurent S, Mottron L, Fombonne E, Joober R, Rouleau GA. NLGN3/NLGN4 gene mutations are not responsible for autism in the Quebec population. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005;132B:74–75. doi: 10.1002/ajmg.b.30066. [DOI] [PubMed] [Google Scholar]
- Gauthier J, Champagne N, Lafrenière RG, Xiong L, Spiegelman D, Brustein E, Lapointe M, Peng H, Côté M, Noreau A, Hamdan FF, Addington AM, Rapoport JL, Delisi LE, Krebs MO, Joober R, Fathalli F, Mouaffak F, Haghighi AP, Néri C, Dubé MP, Samuels ME, Marineau C, Stone EA, Awadalla P, Barker PA, Carbonetto S, Drapeau P, Rouleau GA. De novo mutations in the gene encoding the synaptic scaffolding protein SHANK3 in patients ascertained for schizophrenia. Proc Natl Acad Sci USA. 2010;107:7863–7868. doi: 10.1073/pnas.0906232107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepner B, Feron F. Autism: a world changing too fast for a mis-wired brain? Neurosci Biobehav Rev. 2009;33:1227–1242. doi: 10.1016/j.neubiorev.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Geschwind DH. Genetics of autism spectrum disorders. Trends Cogn Sci. 2011;15:409–416. doi: 10.1016/j.tics.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Gillberg C. Chromosomal disorders and autism. J Autism Dev Disord. 1998;28:415–425. doi: 10.1023/A:1026004505764. [DOI] [PubMed] [Google Scholar]
- Gillberg C, de Souza L. Head circumference in autism, Asperger syndrome, and ADHD: a comparative study. Dev Med Child Neurol. 2002;44:296–300. doi: 10.1111/j.1469-8749.2002.tb00814.x. [DOI] [PubMed] [Google Scholar]
- Gkogkas CG, Khoutorsky A, Ran I, Rampakakis E, Nevarko T, Weatherill DB, Vasuta C, Yee S, Truitt M, Dallaire P, Major F, Lasko P, Ruggero D, Nader K, Lacaille JC, Sonenberg N. Autism-related deficits via dysregulated eIF4E-dependent translational control. Nature. 2013;493:371–377. doi: 10.1038/nature11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go HS, Kim KC, Choi CS, Jeon SJ, Kwon KJ, Han S.-H, Lee J, Cheong JH, Ryu JH, Kim C.-H, Ko KH, Shin CY. Prenatal exposure to valproic acid increases the neural progenitor cell pool and induces macrocephaly in rat brain via a mechanism involving the GSK-3β/β-catenin pathway. Neuropharmacology. 2012;63:1028–1041. doi: 10.1016/j.neuropharm.2012.07.028. [DOI] [PubMed] [Google Scholar]
- Goffinet AM. The embryonic development of the inferior olivary complex in normal and reeler (rlORL) mutant mice. J Comp Neurol. 1983;219:10–24. doi: 10.1002/cne.902190103. [DOI] [PubMed] [Google Scholar]
- Golan HM, Lev V, Hallak M, Sorokin Y, Huleihel M. Specific neurodevelopmental damage in mice offspring following maternal inflammation during pregnancy. Neuropharmacology. 2005;48:903–917. doi: 10.1016/j.neuropharm.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Goorden SM, van Woerden GM, van der Weerd L, Cheadle JP, Elgersma Y. Cognitive deficits in Tsc1+/− mice in the absence of cerebral lesions and seizures. Ann Neurol. 2007;62:648–655. doi: 10.1002/ana.21317. [DOI] [PubMed] [Google Scholar]
- Greer PL, Hanayama R, Bloodgood BL, Mardinly AR, Lipton DM, Flavell SW, Kim TK, Griffith EC, Waldon Z, Maehr R, Ploegh HL, Chowdhury S, Worley PF, Steen J, Greenberg ME. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell. 2010;140:704–716. doi: 10.1016/j.cell.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorian C, Nakashima J, Le Belle J, Ohab J, Kim R, Liu A, Smith KB, Groszer M, Garcia AD, Sofroniew MV, Carmichael ST, Kornblum HI, Liu X, Wu H. Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis. J Neurosci. 2009;29:1874–1886. doi: 10.1523/JNEUROSCI.3095-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory SG, Connelly JJ, Towers AJ, Johnson J, Biscocho D, Markunas CA, Lintas C, Abramson RK, Wright HH, Ellis P, Langford CF, Worley G, Delong GR, Murphy SK, Cuccaro ML, Persico A, Pericak-Vance MA. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med. 2009;7:62. doi: 10.1186/1741-7015-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Choquet D, Stephenson FA, Verrier D, Manzoni OJ, Chavis P. NMDA receptor surface trafficking and synaptic subunit composition are developmentally regulated by the extracellular matrix protein Reelin. J Neurosci. 2007;27:10165–10175. doi: 10.1523/JNEUROSCI.1772-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol. Psychiatry. 2010;67:692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biol. Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Guffanti G, Lievers LS, Bonati MT, Marchi M, Geronazzo L, Nardocci N, Estienne M, Larizza L, Macciardi F, Russo S. Role of UBE3A and ATP10A genes in autism susceptibility region 15q11-q13 in an Italian population: A positive replication for UBE3A. Psychiatry Res. 2011;185:33–38. doi: 10.1016/j.psychres.2010.04.057. [DOI] [PubMed] [Google Scholar]
- Guy J, Cheval H, Selfridge J, Bird A. The Role of MeCP2 in the Brain. Annu Rev Cell Dev Biol. 2011;27:631–652. doi: 10.1146/annurev-cellbio-092910-154121. [DOI] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Miller J, Fedele A, Collins J, Smith K, Lotspeich L, Croen LA, Ozonoff S, Lajonchere C, Grether JK, Risch N. Genetic Heritability and Shared Environmental Factors Among Twin Pairs With Autism. Arch. Gen. Psychiatry. 2011;68:1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardan AY, Minshew NJ, Mallikarjuhn M, Keshavan MS. Brain volume in autism. J Child Neurol. 2001;16:421–424. doi: 10.1177/088307380101600607. [DOI] [PubMed] [Google Scholar]
- Hatton DD, Sideris J, Skinner M, Mankowski J, Bailey DB, Jr, Roberts J, Mirrett P. Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. Am. J. Med. Genet. A. 2006;140A:1804–1813. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- Hava G, Vered L, Yael M, Mordechai H, Mahoud H. Alterations in behavior in adult offspring mice following maternal inflammation during pregnancy. Dev Psychobiol. 2006;48:162–168. doi: 10.1002/dev.20116. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Gilmore J, Piven J. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch. Gen. Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect. 2006;114:1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Santini E, Ma T, Arnold EC, Whelan AM, Wong H, Pierre P, Pelletier J, Klann E. Multiple components of eIF4F are required for protein synthesis-dependent hippocampal long-term potentiation. J Neurophysiol. 2013;109:68–76. doi: 10.1152/jn.00342.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, Aronowitz BR, Mosovich S. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger’s disorders. Neuropsychopharmacology. 2003;28:193–198. doi: 10.1038/sj.npp.1300021. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL. Mice lacking the serotonin transporter exhibit 5-HT(1A) receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology. 2003;28:2077–2088. doi: 10.1038/sj.npp.1300266. [DOI] [PubMed] [Google Scholar]
- Homanics GE, DeLorey TM, Firestone LL, Quinlan JJ, Handforth A, Harrison NL, Krasowski MD, Rick CE, Korpi ER, Makela R, Brilliant MH, Hagiwara N, Ferguson C, Snyder K, Olsen RW. Mice devoid of gamma-aminobutyrate type A receptor beta3 subunit have epilepsy, cleft palate, and hypersensitive behavior. Proc Natl Acad Sci USA. 1997;94:4143–4148. doi: 10.1073/pnas.94.8.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornig M, Weissenbock H, Horscroft N, Lipkin WI. An infection-based model of neurodevelopmental damage. Proc Natl Acad Sci USA. 1999;96:12102–12107. doi: 10.1073/pnas.96.21.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hranilovic D, Novak R, Babic M, Novokmet M, Bujas-Petkovic Z, Jernej B. Hyperserotonemia in autism: the potential role of 5HT-related gene variants. Coll. Antropol. 2008;32(Suppl 1):75–80. [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc Natl Acad Sci USA. 2012;109:12776–12781. doi: 10.1073/pnas.1202556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CH, Santangelo SL. Autism and serotonin transporter gene polymorphisms: A systematic review and meta-analysis. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008;147B:903–913. doi: 10.1002/ajmg.b.30720. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung AY, Futai K, Sala C, Valtschanoff JG, Ryu J, Woodworth MA, Kidd FL, Sung CC, Miyakawa T, Bear MF, Weinberg RJ, Sheng M. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J Neurosci. 2008;28:1697–1708. doi: 10.1523/JNEUROSCI.3032-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter P. The psycho gene. EMBO Rep. 2010;11:667–669. doi: 10.1038/embor.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci. 2000;3:661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- Hutsler JJ, Zhang H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010;1309:83–94. doi: 10.1016/j.brainres.2009.09.120. [DOI] [PubMed] [Google Scholar]
- Iafrati J, Orejarena M, Lassalle O, Bouamrane L, Gonzalez-Campo C, Chavis P. Reelin, an extracellular matrix protein linked to early onset psychiatric diseases, drives postnatal development of the prefrontal cortex via GluN2B-NMDARs and the mTOR pathway. Mol. Psychiatry. 2014;19:417–426. doi: 10.1038/mp.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65:768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons M, Elias ER, Salen G, Tint GS, Batta AK. Defective cholesterol biosynthesis in Smith-Lemli-Opitz syndrome. Lancet. 1993;341:1414. doi: 10.1016/0140-6736(93)90983-N. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Galvez R, Greenough WT. Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb. Cortex. 2000;10:1038–1044. doi: 10.1093/cercor/10.10.1038. [DOI] [PubMed] [Google Scholar]
- Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH., Jr Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci Lett. 2007;417:6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, Frahm J, Fischer J, Bourgeron T, Ehrenreich H, Brose N. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci USA. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Wang J, Luo T, Li Q. Impaired hypothalamic-pituitary-adrenal axis and its feedback regulation in serotonin transporter knockout mice. Psychoneuroendocrinology. 2009;34:317–331. doi: 10.1016/j.psyneuen.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YH, Ehlers MD. Modeling Autism by SHANK Gene Mutations in Mice. Neuron. 2013;78:8–27. doi: 10.1016/j.neuron.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YH, Pan Y, Zhu L, Landa L, Yoo J, Spencer C, Lorenzo I, Brilliant M, Noebels J, Beaudet AL. Altered ultrasonic vocalization and impaired learning and memory in Angelman syndrome mouse model with a large maternal deletion from Ube3a to Gabrb3. PLoS One. 2010;5:e12278. doi: 10.1371/journal.pone.0012278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YH, Sahoo T, Michaelis RC, Bercovich D, Bressler J, Kashork CD, Liu Q, Shaffer LG, Schroer RJ, Stockton DW, Spielman RS, Stevenson RE, Beaudet AL. A mixed epigenetic/genetic model for oligogenic inheritance of autism with a limited role for UBE3A. Am. J. Med. Genet. A. 2004;131:1–10. doi: 10.1002/ajmg.a.30297. [DOI] [PubMed] [Google Scholar]
- Joyal CC, Meyer C, Jacquart G, Mahler P, Caston J, Lalonde R. Effects of midline and lateral cerebellar lesions on motor coordination and spatial orientation. Brain Res. 1996;739:1–11. doi: 10.1016/S0006-8993(96)00333-2. [DOI] [PubMed] [Google Scholar]
- Jung GA, Yoon JY, Moon BS, Yang DH, Kim HY, Lee SH, Bryja V, Arenas E, Choi KY. Valproic acid induces differentiation and inhibition of proliferation in neural progenitor cells via the beta-catenin-Ras-ERK-p21Cip/WAF1 pathway. BMC Cell Biol. 2008;9:66. doi: 10.1186/1471-2121-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K.-M, Sepers M, Henstridge CM, Lassalle O, Neuhofer D, Martin H, Ginger M, Frick A, DiPatrizio NV, Mackie K, Katona I, Piomelli D, Manzoni OJ. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat Commun. 2012;3:1080. doi: 10.1038/ncomms2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyonouchi H, Geng L, Ruby A, Zimmerman-Bier B. Dysregulated innate immune responses in young children with autism spectrum disorders: their relationship to gastrointestinal symptoms and dietary intervention. Neuropsychobiology. 2005;51:77–85. doi: 10.1159/000084164. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Fox MA, Gallagher PS, Murphy DL. Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice. Genes Brain Behav. 2007;6:389–400. doi: 10.1111/j.1601-183X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Karvat G, Kimchi T. Acetylcholine elevation relieves cognitive rigidity and social deficiency in a mouse model of autism. Neuropsychopharmacology. 2014;39:831–840. doi: 10.1038/npp.2013.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka S, Takuma K, Hara Y, Maeda Y, Ago Y, Matsuda T. Autism-like behaviours with transient histone hyperacetylation in mice treated prenatally with valproic acid. Int J Neuropsychopharmacol. 2013;16:91–103. doi: 10.1017/S1461145711001714. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Colwell DD, Choleris E, Agmo A, Muglia LJ, Ogawa S, Pfaff DW. Impaired discrimination of and aversion to parasitized male odors by female oxytocin knockout mice. Genes Brain Behav. 2003;2:220–230. doi: 10.1034/j.1601-183X.2003.00021.x. [DOI] [PubMed] [Google Scholar]
- Kemper TL, Bauman M. Neuropathology of infantile autism. J Neuropathol Exp Neurol. 1998;57:645–652. doi: 10.1097/00005072-199807000-00001. [DOI] [PubMed] [Google Scholar]
- Kemper TL, Bauman ML. The contribution of neuropathologic studies to the understanding of autism. Neurol Clin. 1993;11:175–187. [PubMed] [Google Scholar]
- Kim HJ, Thayer SA. Lithium increases synapse formation between hippocampal neurons by depleting phosphoinositides. Mol Pharmacol. 2009;75:1021–1030. doi: 10.1124/mol.108.052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Seung H, Kwon KJ, Ko MJ, Lee EJ, Oh HA, Choi CS, Kim KC, Gonzales EL, You JS, Choi DH, Lee J, Han SH, Yang SM, Cheong JH, Shin CY, Bahn GH. Subchronic treatment of donepezil rescues impaired social, hyperactive, and stereotypic behavior in valproic acid-induced animal model of autism. PLoS One. 2014a;9:e104927. doi: 10.1371/journal.pone.0104927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KC, Choi CS, Kim J.-W, Han S.-H, Cheong JH, Ryu JH, Shin CY. MeCP2 Modulates Sex Differences in the Postsynaptic Development of the Valproate Animal Model of Autism. Mol Neurobiol. 2014b:1–17. doi: 10.1007/s12035-014-8987-z. [DOI] [PubMed] [Google Scholar]
- Kim KC, Kim P, Go HS, Choi CS, Yang SI, Cheong JH, Shin CY, Ko KH. The critical period of valproate exposure to induce autistic symptoms in Sprague-Dawley rats. Toxicol Lett. 2011;201:137–142. doi: 10.1016/j.toxlet.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Kim KC, Lee D.-K, Go HS, Kim P, Choi CS, Kim J.-W, Jeon SJ, Song M.-R, Shin CY. Pax6-dependent cortical glutamatergic neuronal differentiation regulates autism-like behavior in prenatally valproic acid-exposed rat offspring. Mol Neurobiol. 2014c;49:512–528. doi: 10.1007/s12035-013-8535-2. [DOI] [PubMed] [Google Scholar]
- Kolevzon A, Cai G, Soorya L, Takahashi N, Grodberg D, Kajiwara Y, Willner JP, Tryfon A, Buxbaum JD. Analysis of a purported SHANK3 mutation in a boy with autism: Clinical impact of rare variant research in neurodevelopmental disabilities. Brain Res. 2011;1380:98–105. doi: 10.1016/j.brainres.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Kolevzon A, Gross R, Reichenberg A. Prenatal and perinatal risk factors for autism: a review and integration of findings. Arch Pediatr Adolesc Med. 2007;161:326–333. doi: 10.1001/archpedi.161.4.326. [DOI] [PubMed] [Google Scholar]
- Korade Z, Folkes OM, Harrison FE. Behavioral and serotonergic response changes in the Dhcr7-HET mouse model of Smith-Lemli-Opitz syndrome. Pharmacol Biochem Behav. 2013;106:101–108. doi: 10.1016/j.pbb.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Kuemerle B, Gulden F, Cherosky N, Williams E, Herrup K. The mouse Engrailed genes: a window into autism. Behav Brain Res. 2007;176:121–132. doi: 10.1016/j.bbr.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuemerle B, Zanjani H, Joyner A, Herrup K. Pattern deformities and cell loss in Engrailed-2 mutant mice suggest two separate patterning events during cerebellar development. J Neurosci. 1997;17:7881–7889. doi: 10.1523/JNEUROSCI.17-20-07881.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamaru E, Egashira Y, Takenaka R, Takamori S. Valproic acid selectively suppresses the formation of inhibitory synapses in cultured cortical neurons. Neurosci Lett. 2014;569:142–147. doi: 10.1016/j.neulet.2014.03.066. [DOI] [PubMed] [Google Scholar]
- Kuwagata M, Ogawa T, Shioda S, Nagata T. Observation of fetal brain in a rat valproate-induced autism model: a developmental neurotoxicity study. Int J Dev Neurosci. 2009;27:399–405. doi: 10.1016/j.ijdevneu.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lante F, Meunier J, Guiramand J, De Jesus Ferreira MC, Cambonie G, Aimar R, Cohen-Solal C, Maurice T, Vignes M, Barbanel G. Late N-acetylcysteine treatment prevents the deficits induced in the offspring of dams exposed to an immune stress during gestation. Hippocampus. 2008;18:602–609. doi: 10.1002/hipo.20421. [DOI] [PubMed] [Google Scholar]
- Larimore JL, Chapleau CA, Kudo S, Theibert A, Percy AK, Pozzo-Miller L. Bdnf overexpression in hippocampal neurons prevents dendritic atrophy caused by Rett-associated MECP2 mutations. Neurobiol Dis. 2009;34:199–211. doi: 10.1016/j.nbd.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond CS, Heinrich J, Delorme R, Proepper C, Betancur C, Huguet G, Konyukh M, Chaste P, Ey E, Rastam M, Anckars?ter H, Nygren G, Gillberg IC, Melke J, Toro R, Regnault B, Fauchereau F, Mercati O, Lemi?re N, Skuse D, Poot M, Holt R, Monaco AP, Järvelä I, Kantojärvi K, Vanhala R, Curran S, Collier DA, Bolton P, Chiocchetti A, Klauck SM, Poustka F, Freitag CM, Waltes R, Kopp M, Duketis E, Bacchelli E, Minopoli F, Ruta L, Battaglia A, Mazzone L, Maestrini E, Sequeira AF, Oliveira B, Vicente A, Oliveira G, Pinto D, Scherer SW, Zelenika D, Delepine M, Lathrop M, Bonneau D, Guinchat V, Devillard F, Assouline B, Mouren MC, Leboyer M, Gillberg C, Boeckers TM, Bourgeron T. Genetic and functional analyses of SHANK2 mutations suggest a multiple hit model of autism spectrum disorders. PLoS Genet. 2012;8:e1002521. doi: 10.1371/journal.pgen.1002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BK, McGrath JJ. Advancing parental age and autism: multifactorial pathways. Trends Mol Med. 2015;21:118–125. doi: 10.1016/j.molmed.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Caldwell HK, Macbeth AH, Young WS., 3rd Behavioural studies using temporal and spatial inactivation of the oxytocin receptor. Prog Brain Res. 2008;170:73–77. doi: 10.1016/S0079-6123(08)00407-X. [DOI] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, Tager-Flusberg H, Lainhart JE. Comorbid psychiatric disorders in children with autism: Interview development and rates of disorders. J Autism Dev Disord. 2006;36:849–861. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- Li X, Hu Z, He Y, Xiong Z, Long Z, Peng Y, Bu F, Ling J, Xun G, Mo X. Association analysis of CNTNAP2 polymorphisms with autism in the Chinese Han population. Psychiatr Genet. 2010;20:113–117. doi: 10.1097/YPG.0b013e32833a216f. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang J, Cao Z, Wu J, Shi Y. Solution structure of GOPC PDZ domain and its interaction with the C-terminal motif of neuroligin. Protein Sci. 2006;15:2149–2158. doi: 10.1110/ps.062087506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WS, Pesold C, Rodriguez MA, Carboni G, Auta J, Lacor P, Larson J, Condie BG, Guidotti A, Costa E. Down-regulation of dendritic spine and glutamic acid decarboxylase 67 expressions in the reelin haploinsufficient heterozygous reeler mouse. Proc Natl Acad Sci USA. 2001;98:3477–3482. doi: 10.1073/pnas.051614698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Kawamura Y, Shimada T, Otowa T, Koishi S, Sugiyama T, Nishida H, Hashimoto O, Nakagami R, Tochigi M, Umekage T, Kano Y, Miyagawa T, Kato N, Tokunaga K, Sasaki T. Association of the oxytocin receptor (OXTR) gene polymorphisms with autism spectrum disorder (ASD) in the Japanese population. J Hum Genet. 2010;55:137–141. doi: 10.1038/jhg.2009.140. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Smith CB. Dissociation of social and nonsocial anxiety in a mouse model of fragile X syndrome. Neurosci Lett. 2009;454:62–66. doi: 10.1016/j.neulet.2009.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoParo D, Waldman ID. The oxytocin receptor gene (OXTR) is associated with autism spectrum disorder: a meta-analysis. Mol. Psychiatry. 2015;20:640–646. doi: 10.1038/mp.2014.77. [DOI] [PubMed] [Google Scholar]
- Lozovaya N, Gataullina S, Tsintsadze T, Tsintsadze V, Pallesi-Pocachard E, Minlebaev M, Goriounova NA, Buhler E, Watrin F, Shityakov S, Becker AJ, Bordey A, Milh M, Scavarda D, Bulteau C, Dorfmuller G, Delalande O, Represa A, Cardoso C, Dulac O, Ben-Ari Y, Burnashev N. Selective suppression of excessive GluN2C expression rescues early epilepsy in a tuberous sclerosis murine model. Nat Commun. 2014;5:4563. doi: 10.1038/ncomms5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luikart BW, Schnell E, Washburn EK, Bensen AL, Tovar KR, Westbrook GL. Pten knockdown in vivo increases excitatory drive onto dentate granule cells. J Neurosci. 2011;31:4345–4354. doi: 10.1523/JNEUROSCI.0061-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lush ME, Li Y, Kwon CH, Chen J, Parada LF. Neurofibromin is required for barrel formation in the mouse somatosensory cortex. J Neurosci. 2008;28:1580–1587. doi: 10.1523/JNEUROSCI.5236-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Rossi S, Bari M, De Chiara V, Rapino C, Musella A, Bernardi G, Bagni C, Centonze D. Abnormal mGlu 5 receptor/endocannabinoid coupling in mice lacking FMRP and BC1 RNA. Neuropsychopharmacology. 2010;35:1500–1509. doi: 10.1038/npp.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen GM, Ramakrishnan K, Croll SD, Siuciak JA, Yu G, Young LT, Fahnestock M. Performance of heterozygous brain-derived neurotrophic factor knockout mice on behavioral analogues of anxiety, nociception, and depression. Behav Neurosci. 2001;115:1145–1153. doi: 10.1037/0735-7044.115.5.1145. [DOI] [PubMed] [Google Scholar]
- Maezawa I, Jin LW. Rett syndrome microglia damage dendrites and synapses by the elevated release of glutamate. J Neurosci. 2010;30:5346–5356. doi: 10.1523/JNEUROSCI.5966-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning MA, Cassidy SB, Clericuzio C, Cherry AM, Schwartz S, Hudgins L, Enns GM, Hoyme HE. Terminal 22q deletion syndrome: a newly recognized cause of speech and language disability in the autism spectrum. Pediatrics. 2004;114:451–457. doi: 10.1542/peds.114.2.451. [DOI] [PubMed] [Google Scholar]
- Marrone MC, Marinelli S, Biamonte F, Keller F, Sgobio CA, Ammassari-Teule M, Bernardi G, Mercuri NB. Altered cortico-striatal synaptic plasticity and related behavioural impairments in reeler mice. Eur J Neurosci. 2006;24:2061–2070. doi: 10.1111/j.1460-9568.2006.05083.x. [DOI] [PubMed] [Google Scholar]
- Martin HG, Manzoni OJ. Late onset deficits in synaptic plasticity in the valproic acid rat model of autism. Front Cell Neurosci. 2014;8:23. doi: 10.3389/fncel.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LA, Escher T, Goldowitz D, Mittleman G. A relationship between cerebellar Purkinje cells and spatial working memory demonstrated in a lurcher/chimera mouse model system. Genes Brain Behav. 2004;3:158–166. doi: 10.1111/j.1601-183x.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- Martin LA, Goldowitz D, Mittleman G. Repetitive behavior and increased activity in mice with Purkinje cell loss: a model for understanding the role of cerebellar pathology in autism. Eur J Neurosci. 2010;31:544–555. doi: 10.1111/j.1460-9568.2009.07073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MR. Morphology of the cochlear nucleus of the normal and reeler mutant mouse. J Comp Neurol. 1981;197:141–152. doi: 10.1002/cne.901970111. [DOI] [PubMed] [Google Scholar]
- Martins Y, Young RL, Robson DC. Feeding and eating behaviors in children with autism and typically developing children. J Autism Dev Disord. 2008;38:1878–1887. doi: 10.1007/s10803-008-0583-5. [DOI] [PubMed] [Google Scholar]
- Marui T, Hashimoto O, Nanba E, Kato C, Tochigi M, Umekage T, Ishijima M, Kohda K, Kato N, Sasaki T. Association between the neurofibromatosis-1 (NF1) locus and autism in the Japanese population. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2004;131B:43–47. doi: 10.1002/ajmg.b.20119. [DOI] [PubMed] [Google Scholar]
- Mazina V, Gerdts J, Trinh S, Ankenman K, Ward T, Dennis MY, Girirajan S, Eichler EE, Bernier R. Epigenetics of autism-related impairment: copy number variation and maternal infection. J Dev Behav Pediatr. 2015;36:61–67. doi: 10.1097/DBP.0000000000000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbarek O, Marouillat S, Martineau J, Barthelemy C, Muh JP, Andres C. Association study of the NF1 gene and autistic disorder. Am J Med Genet. 1999;88:729–732. doi: 10.1002/(SICI)1096-8628(19991215)88:6<729::AID-AJMG26>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- McNaughton CH, Moon J, Strawderman MS, Maclean KN, Evans J, Strupp BJ. Evidence for social anxiety and impaired social cognition in a mouse model of fragile X syndrome. Behav Neurosci. 2008;122:293–300. doi: 10.1037/0735-7044.122.2.293. [DOI] [PubMed] [Google Scholar]
- Meikle L, Talos DM, Onda H, Pollizzi K, Rotenberg A, Sahin M, Jensen FE, Kwiatkowski DJ. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci. 2007;27:5546–5558. doi: 10.1523/JNEUROSCI.5540-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Dammann O. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr Res. 2011;69:26R–33R. doi: 10.1203/PDR.0b013e318212c196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meziane H, Schaller F, Bauer S, Villard C, Matarazzo V, Riet F, Guillon G, Lafitte D, Desarmenien MG, Tauber M, Muscatelli F. An Early Postnatal Oxytocin Treatment Prevents Social and Learning Deficits in Adult Mice Deficient for Magel2, a Gene Involved in Prader-Willi Syndrome and Autism. Biol. Psychiatry. 2015;78:85–94. doi: 10.1016/j.biopsych.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Huynh LX, Crusio WE. Social behavior deficits in the Fmr1 mutant mouse. Behav Brain Res. 2006;168:172–175. doi: 10.1016/j.bbr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Miura K, Kishino T, Li E, Webber H, Dikkes P, Holmes GL, Wagstaff J. Neurobehavioral and electroencephalographic abnormalities in Ube3a maternal-deficient mice. Neurobiol Dis. 2002;9:149–159. doi: 10.1006/nbdi.2001.0463. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parada LF, Nestler EJ. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol. Psychiatry. 2007;61:187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Moore SJ, Turnpenny P, Quinn A, Glover S, Lloyd DJ, Montgomery T, Dean JC. A clinical study of 57 children with fetal anticonvulsant syndromes. J Med Genet. 2000;37:489–497. doi: 10.1136/jmg.37.7.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti P, Levenson JM, Battaglia F, Atkinson R, Teague R, Antalffy B, Armstrong D, Arancio O, Sweatt JD, Zoghbi HY. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J Neurosci. 2006;26:319–327. doi: 10.1523/JNEUROSCI.2623-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Grossman AW, Murphy DL, D’Ercole AJ, Crawley JN, Magnuson TR, Lauder JM. Social approach in genetically engineered mouse lines relevant to autism. Genes Brain Behav. 2009;8:129–142. doi: 10.1111/j.1601-183X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer’s disease and Parkinson’s disease. Prog Neurobiol. 2001;63:71–124. doi: 10.1016/S0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- Muroga T, Adachi K, Konagaya M, Takayanagi T, Sobue I. Effects of thyrotropin releasing hormone on cerebellar mutant mice--a kinesiological comparison between rolling mouse Nagoya, weaver and reeler. Jpn J Med. 1982;21:101–108. doi: 10.2169/internalmedicine1962.21.101. [DOI] [PubMed] [Google Scholar]
- Nakao M, Sutcliffe JS, Durtschi B, Mutirangura A, Ledbetter DH, Beaudet AL. Imprinting analysis of three genes in the Prader-Willi/Angelman region: SNRPN, E6-associated protein, and PAR-2 (D15S225E) Hum Mol Genet. 1994;3:309–315. doi: 10.1093/hmg/3.2.309. [DOI] [PubMed] [Google Scholar]
- Naviaux RK, Zolkipli Z, Wang L, Nakayama T, Naviaux JC, Le TP, Schuchbauer MA, Rogac M, Tang Q, Dugan LL, Powell SB. Antipurinergic therapy corrects the autism-like features in the poly(IC) mouse model. PLoS One. 2013;8:e57380. doi: 10.1371/journal.pone.0057380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Need AC, Ge D, Weale ME, Maia J, Feng S, Heinzen EL, Shianna KV, Yoon W, Kasperaviciute D, Gennarelli M, Strittmatter WJ, Bonvicini C, Rossi G, Jayathilake K, Cola PA, McEvoy JP, Keefe RS, Fisher EM, St Jean PL, Giegling I, Hartmann AM, Moller HJ, Ruppert A, Fraser G, Crombie C, Middleton LT, St Clair D, Roses AD, Muglia P, Francks C, Rujescu D, Meltzer HY, Goldstein DB. A genome-wide investigation of SNPs and CNVs in schizophrenia. PLoS Genet. 2009;5:e1000373. doi: 10.1371/journal.pgen.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J Neurophysiol. 2006;95:3291–3295. doi: 10.1152/jn.01316.2005. [DOI] [PubMed] [Google Scholar]
- Numis A, Major P, Montenegro M, Muzykewicz D, Pulsifer M, Thiele E. Identification of risk factors for autism spectrum disorders in tuberous sclerosis complex. Neurology. 2011;76:981–987. doi: 10.1212/WNL.0b013e3182104347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum J, Xu Q, Payne TJ, Ma JZ, Huang W, Gelernter J, Li MD. Significant association of the neurexin-1 gene (NRXN1) with nicotine dependence in European- and African-American smokers. Hum Mol Genet. 2008;17:1569–1577. doi: 10.1093/hmg/ddn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oblak AL, Gibbs TT, Blatt GJ. Reduced GABAA receptors and benzodiazepine binding sites in the posterior cingulate cortex and fusiform gyrus in autism. Brain Res. 2011;1380:218–228. doi: 10.1016/j.brainres.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Kwon CH, Zhou J, Koovakkattu D, Parada LF, Sinton CM. A seizure-prone phenotype is associated with altered free-running rhythm in Pten mutant mice. Brain Res. 2007;1168:112–123. doi: 10.1016/j.brainres.2007.06.074. [DOI] [PubMed] [Google Scholar]
- Ognibene E, Adriani W, Macri S, Laviola G. Neurobehavioural disorders in the infant reeler mouse model: interaction of genetic vulnerability and consequences of maternal separation. Behav Brain Res. 2007;177:142–149. doi: 10.1016/j.bbr.2006.10.027. [DOI] [PubMed] [Google Scholar]
- Onda H, Crino PB, Zhang H, Murphey RD, Rastelli L, Gould Rothberg BE, Kwiatkowski DJ. Tsc2 null murine neuroepithelial cells are a model for human tuber giant cells, and show activation of an mTOR pathway. Mol Cell Neurosci. 2002;21:561–574. doi: 10.1006/mcne.2002.1184. [DOI] [PubMed] [Google Scholar]
- Ornoy A. Valproic acid in pregnancy: how much are we endangering the embryo and fetus? Reprod Toxicol. 2009;28:1–10. doi: 10.1016/j.reprotox.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Silverman JL, Aney J, Tian Q, Barkan CL, Chadman KK, Crawley JN. Working memory deficits, increased anxiety-like traits, and seizure susceptibility in BDNF overexpressing mice. Learn Mem. 2011;18:534–544. doi: 10.1101/lm.2213711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo CA, Eberhart CG. The neurobiology of autism. Brain Pathol. 2007;17:434–447. doi: 10.1111/j.1750-3639.2007.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Garner JP, Libove RA, Hyde SA, Hornbeak KB, Carson DS, Liao CP, Phillips JM, Hallmayer JF, Hardan AY. Plasma oxytocin concentrations and OXTR polymorphisms predict social impairments in children with and without autism spectrum disorder. Proc Natl Acad Sci USA. 2014;111:12258–12263. doi: 10.1073/pnas.1402236111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrylo PR, Browning RA, Cranick S. Reeler homozygous mice exhibit enhanced susceptibility to epileptiform activity. Epilepsia. 2006;47:257–266. doi: 10.1111/j.1528-1167.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Maternal infection and immune involvement in autism. Trends Mol Med. 2011;17:389–394. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peca J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola CD, Fu Z, Feng G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A, Golshani P, Trachtenberg JT, Peles E, Geschwind DH. Absence of CNTNAP2 Leads to Epilepsy, Neuronal Migration Abnormalities, and Core Autism-Related Deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñagarikano O, Lazaro MT, Lu XH, Gordon A, Dong H, Lam HA, Peles E, Maidment NT, Murphy NP, Yang XW, Golshani P, Geschwind DH. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med. 2015;7:271ra8. doi: 10.1126/scitranslmed.3010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry EK, Lee ML, Martin-Ruiz CM, Court JA, Volsen SG, Merrit J, Folly E, Iversen PE, Bauman ML, Perry RH, Wenk GL. Cholinergic activity in autism: abnormalities in the cerebral cortex and basal forebrain. Am. J. Psychiatry. 2001;158:1058–1066. doi: 10.1176/appi.ajp.158.7.1058. [DOI] [PubMed] [Google Scholar]
- Peters S, Beaudet A, Madduri N, Bacino C. Autism in Angelman syndrome: implications for autism research. Clin Genet. 2004;66:530–536. doi: 10.1111/j.1399-0004.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- Pierce K, Courchesne E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol. Psychiatry. 2001;49:655–664. doi: 10.1016/S0006-3223(00)01008-8. [DOI] [PubMed] [Google Scholar]
- Pletnikov MV, Moran TH, Carbone KM. Borna disease virus infection of the neonatal rat: developmental brain injury model of autism spectrum disorders. Front Biosci. 2002;7:d593–d607. doi: 10.2741/pletnik. [DOI] [PubMed] [Google Scholar]
- Pletnikov MV, Rubin SA, Vasudevan K, Moran TH, Carbone KM. Developmental brain injury associated with abnormal play behavior in neonatally Borna disease virus-infected Lewis rats: a model of autism. Behav Brain Res. 1999;100:43–50. doi: 10.1016/S0166-4328(98)00111-9. [DOI] [PubMed] [Google Scholar]
- Pobbe RL, Pearson BL, Defensor EB, Bolivar VJ, Young WS, 3rd, Lee HJ, Blanchard DC, Blanchard RJ. Oxytocin receptor knockout mice display deficits in the expression of autism-related behaviors. Horm Behav. 2012;61:436–444. doi: 10.1016/j.yhbeh.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podhorna J, Didriksen M. The heterozygous reeler mouse: behavioural phenotype. Behav Brain Res. 2004;153:43–54. doi: 10.1016/j.bbr.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Poliak S, Gollan L, Martinez R, Custer A, Einheber S, Salzer JL, Trimmer JS, Shrager P, Peles E. Caspr2, a New Member of the Neurexin Superfamily, Is Localized at the Juxtaparanodes of Myelinated Axons and Associates with K+ Channels. Neuron. 1999;24:1037–1047. doi: 10.1016/S0896-6273(00)81049-1. [DOI] [PubMed] [Google Scholar]
- Popova NK, Vishnivetskaya GB, Ivanova EA, Skrinskaya JA, Seif I. Altered behavior and alcohol tolerance in transgenic mice lacking MAO A: a comparison with effects of MAO A inhibitor clorgyline. Pharmacol Biochem Behav. 2000;67:719–727. doi: 10.1016/S0091-3057(00)00417-2. [DOI] [PubMed] [Google Scholar]
- Prasad HC, Steiner JA, Sutcliffe JS, Blakely RD. Enhanced activity of human serotonin transporter variants associated with autism. Philos Trans R Soc Lond, B, Biol Sci. 2009;364:163–173. doi: 10.1098/rstb.2008.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radyushkin K, Hammerschmidt K, Boretius S, Varoqueaux F, El-Kordi A, Ronnenberg A, Winter D, Frahm J, Fischer J, Brose N, Ehrenreich H. Neuroligin-3-deficient mice: model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav. 2009;8:416–425. doi: 10.1111/j.1601-183X.2009.00487.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Friedman JM. NF1 gene and neurofibromatosis 1. Am J Epidemiol. 2000;151:33–40. doi: 10.1093/oxfordjournals.aje.a010118. [DOI] [PubMed] [Google Scholar]
- Reith RM, Way S, McKenna J, 3rd, Haines K, Gambello MJ. Loss of the tuberous sclerosis complex protein tuberin causes Purkinje cell degeneration. Neurobiol Dis. 2011;43:113–122. doi: 10.1016/j.nbd.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rett A. [On a unusual brain atrophy syndrome in hyperammonemia in childhood] Wien Med Wochenschr. 1966;116:723–726. [PubMed] [Google Scholar]
- Richt JA, Pfeuffer I, Christ M, Frese K, Bechter K, Herzog S. Borna disease virus infection in animals and humans. Emerging Infect Dis. 1997;3:343–352. doi: 10.3201/eid0303.970311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi T, Kulangara K, Antoniello K, Markram H. Elevated NMDA receptor levels and enhanced postsynaptic long-term potentiation induced by prenatal exposure to valproic acid. Proc Natl Acad Sci USA. 2007;104:13501–13506. doi: 10.1073/pnas.0704391104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi T, Perrodin C, Markram H. Hyper-connectivity and hyper-plasticity in the medial prefrontal cortex in the valproic Acid animal model of autism. Front. Neural Circuits. 2008;2:4. doi: 10.3389/neuro.04.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios M, Lambe EK, Liu R, Teillon S, Liu J, Akbarian S, Roffler-Tarlov S, Jaenisch R, Aghajanian GK. Severe deficits in 5-HT2A -mediated neurotransmission in BDNF conditional mutant mice. J Neurobiol. 2006;66:408–420. doi: 10.1002/neu.20233. [DOI] [PubMed] [Google Scholar]
- Rogers JT, Rusiana I, Trotter J, Zhao L, Donaldson E, Pak DT, Babus LW, Peters M, Banko JL, Chavis P, Rebeck GW, Hoe HS, Weeber EJ. Reelin supplementation enhances cognitive ability, synaptic plasticity, and dendritic spine density. Learn Mem. 2011;18:558–564. doi: 10.1101/lm.2153511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn S, Wehner E. Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. J Autism Dev Disord. 2003;33:631–642. doi: 10.1023/B:JADD.0000006000.38991.a7. [DOI] [PubMed] [Google Scholar]
- Romero E, Ali C, Molina-Holgado E, Castellano B, Guaza C, Borrell J. Neurobehavioral and immunological consequences of prenatal immune activation in rats. Influence of antipsychotics. Neuropsychopharmacology. 2007;32:1791–1804. doi: 10.1038/sj.npp.1301292. [DOI] [PubMed] [Google Scholar]
- Rout UK, Dhossche DM. A pathogenetic model of autism involving Purkinje cell loss through anti-GAD antibodies. Med. Hypotheses. 2008;71:218–221. doi: 10.1016/j.mehy.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Rutter M, Caspi A, Moffitt TE. Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies. J. Child Psychol. Psychiatry. 2003;44:1092–1115. doi: 10.1111/1469-7610.00194. [DOI] [PubMed] [Google Scholar]
- Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, Finardi A, Donzelli A, Pattini L, Rubino T, Parolaro D, Nishimori K, Parenti M, Chini B. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol. Psychiatry. 2011;69:875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Salinger WL, Ladrow P, Wheeler C. Behavioral phenotype of the reeler mutant mouse: effects of RELN gene dosage and social isolation. Behav Neurosci. 2003;117:1257–1275. doi: 10.1037/0735-7044.117.6.1257. [DOI] [PubMed] [Google Scholar]
- Samaco RC, Hogart A, LaSalle JM. Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Hum Mol Genet. 2005;14:483–492. doi: 10.1093/hmg/ddi045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath S, Bhat S, Gupta S, O’Connor A, West AB, Arking DE, Chakravarti A. Defining the Contribution of CNTNAP2 to Autism Susceptibility. PLoS One. 2013;8:e77906. doi: 10.1371/journal.pone.0077906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Huynh TN, MacAskill AF, Carter AG, Pierre P, Ruggero D, Kaphzan H, Klann E. Exaggerated translation causes synaptic and behavioural aberrations associated with autism. Nature. 2013;493:411–415. doi: 10.1038/nature11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato D, Lionel AC, Leblond CS, Prasad A, Pinto D, Walker S, O’Connor I, Russell C, Drmic IE, Hamdan FF, Michaud JL, Endris V, Roeth R, Delorme R, Huguet G, Leboyer M, Rastam M, Gillberg C, Lathrop M, Stavropoulos DJ, Anagnostou E, Weksberg R, Fombonne E, Zwaigenbaum L, Fernandez BA, Roberts W, Rappold GA, Marshall CR, Bourgeron T, Szatmari P, Scherer SW. SHANK1 Deletions in Males with Autism Spectrum Disorder. Am J Hum Genet. 2012;90:879–887. doi: 10.1016/j.ajhg.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaskan E, Ehrhardt R, Schulz A, Walter M, Schachinger H. Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology. 2008;33:368–374. doi: 10.1016/j.psyneuen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Scattoni ML, McFarlane HG, Zhodzishsky V, Caldwell HK, Young WS, Ricceri L, Crawley JN. Reduced ultrasonic vocalizations in vasopressin 1b knockout mice. Behav Brain Res. 2008;187:371–378. doi: 10.1016/j.bbr.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf CP, Gonzalez-Garay ML, Xia F, Potocki L, Gripp KW, Zhang B, Peters BA, McElwain MA, Drmanac R, Beaudet AL, Caskey CT, Yang Y. Truncating mutations of MAGEL2 cause Prader-Willi phenotypes and autism. Nat Genet. 2013;45:1405–1408. doi: 10.1038/ng.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller F, Watrin F, Sturny R, Massacrier A, Szepetowski P, Muscatelli F. A single postnatal injection of oxytocin rescues the lethal feeding behaviour in mouse newborns deficient for the imprinted Magel2 gene. Hum Mol Genet. 2010;19:4895–4905. doi: 10.1093/hmg/ddq424. [DOI] [PubMed] [Google Scholar]
- Schmale H, Borowiak B, Holtgreve-Grez H, Richter D. Impact of altered protein structures on the intracellular traffic of a mutated vasopressin precursor from Brattleboro rats. Eur J Biochem. 1989;182:621–627. doi: 10.1111/j.1432-1033.1989.tb14871.x. [DOI] [PubMed] [Google Scholar]
- Schmeisser MJ, Ey E, Wegener S, Bockmann J, Stempel AV, Kuebler A, Janssen A.-L, Udvardi PT, Shiban E, Spilker C, Balschun D, Skryabin BV, Dieck S. t, Smalla KH, Montag D, Leblond CS, Faure P, Torquet N, Le Sourd AM, Toro R, Grabrucker AM, Shoichet SA, Schmitz D, Kreutz MR, Bourgeron T, Gundelfinger ED, Boeckers TM. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature. 2012;486:256–260. doi: 10.1038/nature11015. [DOI] [PubMed] [Google Scholar]
- Schneider M, Koch M. Deficient social and play behavior in juvenile and adult rats after neonatal cortical lesion: effects of chronic pubertal cannabinoid treatment. Neuropsychopharmacology. 2005;30:944–957. doi: 10.1038/sj.npp.1300634. [DOI] [PubMed] [Google Scholar]
- Schneider T, Roman A, Basta-Kaim A, Kubera M, Budziszewska B, Schneider K, Przewlocki R. Gender-specific behavioral and immunological alterations in an animal model of autism induced by prenatal exposure to valproic acid. Psychoneuroendocrinology. 2008;33:728–740. doi: 10.1016/j.psyneuen.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Schreck KA, Mulick JA, Smith AF. Sleep problems as possible predictors of intensified symptoms of autism. Res Dev Disabil. 2004;25:57–66. doi: 10.1016/j.ridd.2003.04.007. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, Lammers CR, Reiss AL, Amaral DG. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24:6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, Abrahams BS, Alvarez-Retuerto AI, Sonnenblick LI, Rudie JD, Ghahremani D, Mumford JA, Poldrack RA, Dapretto M, Geschwind DH, Bookheimer SY. Altered functional connectivity in frontal lobe circuits is associated with variation in the autism risk gene CNTNAP2. Sci Transl Med. 2010;2:56ra80. doi: 10.1126/scitranslmed.3001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohe A, Hashimoto K, Nakamura K, Tsujii M, Iwata Y, Tsuchiya KJ, Sekine Y, Suda S, Suzuki K, Sugihara G, Matsuzaki H, Minabe Y, Sugiyama T, Kawai M, Iyo M, Takei N, Mori N. Increased serum levels of glutamate in adult patients with autism. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30:1472–1477. doi: 10.1016/j.pnpbp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Ehninger D. Adult reversal of cognitive phenotypes in neurodevelopmental disorders. J Neurodev Disord. 2009;1:150–157. doi: 10.1007/s11689-009-9018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Frankland PW, Marowitz Z, Friedman E, Laszlo GS, Cioffi D, Jacks T, Bourtchuladze R. A mouse model for the learning and memory deficits associated with neurofibromatosis type I. Nat Genet. 1997;15:281–284. doi: 10.1038/ng0397-281. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010;35:976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Turner SM, Barkan CL, Tolu SS, Saxena R, Hung AY, Sheng M, Crawley JN. Sociability and motor functions in Shank1 mutant mice. Brain Res. 2011;1380:120–137. doi: 10.1016/j.brainres.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh C, Bortolato M, Bali N, Godar SC, Scott AL, Chen K, Thompson RF, Shih JC. Cognitive abnormalities and hippocampal alterations in monoamine oxidase A and B knockout mice. Proc Natl Acad Sci USA. 2013;110:12816–12821. doi: 10.1073/pnas.1308037110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NN, Lancioni GE, Winton AS, Fisher BC, Wahler RG, Mcaleavey K, Singh J, Sabaawi M. Mindful parenting decreases aggression, noncompliance, and self-injury in children with autism. J Emot Behav Disord. 2006;14:169–177. doi: 10.1177/10634266060140030401. [DOI] [Google Scholar]
- Sinkkonen ST, Homanics GE, Korpi ER. Mouse models of Angelman syndrome, a neurodevelopmental disorder, display different brain regional GABA(A) receptor alterations. Neurosci Lett. 2003;340:205–208. doi: 10.1016/S0304-3940(03)00123-X. [DOI] [PubMed] [Google Scholar]
- Skidmore BJ, Chiller JM, Morrison DC, Weigle WO. Immunologic properties of bacterial lipopolysaccharide (LPS): correlation between the mitogenic, adjuvant, and immunogenic activities. J Immunol. 1975;114:770–775. [PubMed] [Google Scholar]
- Smalheiser NR, Costa E, Guidotti A, Impagnatiello F, Auta J, Lacor P, Kriho V, Pappas GD. Expression of reelin in adult mammalian blood, liver, pituitary pars intermedia, and adrenal chromaffin cells. Proc Natl Acad Sci USA. 2000;97:1281–1286. doi: 10.1073/pnas.97.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Zhou YD, Zhang G, Jin Z, Stoppel DC, Anderson MP. Increased gene dosage of Ube3a results in autism traits and decreased glutamate synaptic transmission in mice. Sci Transl Med. 2011;3:103ra97. doi: 10.1126/scitranslmed.3002627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence SJ, Schneider MT. The role of epilepsy and epileptiform EEGs in autism spectrum disorders. Pediatr Res. 2009;65:599–606. doi: 10.1203/PDR.0b013e31819e7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer CM, Alekseyenko O, Serysheva E, Yuva-Paylor LA, Paylor R. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes Brain Behav. 2005;4:420–430. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- Stanfield BB, Cowan WM. The morphology of the hippocampus and dentate gyrus in normal and reeler mice. J Comp Neurol. 1979;185:393–422. doi: 10.1002/cne.901850302. [DOI] [PubMed] [Google Scholar]
- Steffenburg S, Gillberg C, Hellgren L, Andersson L, Gillberg IC, Jakobsson G, Bohman M. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J. Child Psychol. Psychiatry. 1989;30:405–416. doi: 10.1111/j.1469-7610.1989.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Stephenson DT, O’Neill SM, Narayan S, Tiwari A, Arnold E, Samaroo HD, Du F, Ring RH, Campbell B, Pletcher M, Vaidya VA, Morton D. Histopathologic characterization of the BTBR mouse model of autistic-like behavior reveals selective changes in neurodevelopmental proteins and adult hippocampal neurogenesis. Mol. Autism. 2011;2:7. doi: 10.1186/2040-2392-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang JF, Kenworthy L, Daniolos P, Case L, Wills MC, Martin A, Wallace GL. Depression and Anxiety Symptoms in Children and Adolescents with Autism Spectrum Disorders without Intellectual Disability. Res Autism Spectr Disord. 2012;6:406–412. doi: 10.1016/j.rasd.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss KA, Puffenberger EG, Huentelman MJ, Gottlieb S, Dobrin SE, Parod JM, Stephan DA, Morton DH. Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. N Engl J Med. 2006;354:1370–1377. doi: 10.1056/NEJMoa052773. [DOI] [PubMed] [Google Scholar]
- Stromland K, Philipson E, Andersson Gronlund M. Off-spring of male and female parents with thalidomide embryopathy: birth defects and functional anomalies. Teratology. 2002;66:115–121. doi: 10.1002/tera.10083. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari P, Maziade M, Zwaigenbaum L, Merette C, Roy MA, Joober R, Palmour R. Informative phenotypes for genetic studies of psychiatric disorders. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007;144B:581–588. doi: 10.1002/ajmg.b.30426. [DOI] [PubMed] [Google Scholar]
- Taieb O, Baleyte JM, Mazet P, Fillet AM. Borna disease virus and psychiatry. Eur. Psychiatry. 2001;16:3–10. doi: 10.1016/S0924-9338(00)00529-0. [DOI] [PubMed] [Google Scholar]
- Tang G, Gudsnuk K, Kuo S-H, Cotrina ML, Rosoklija G, Sosunov A, Sonders MS, Kanter E, Castagna C, Yamamoto A, Yue Z, Arancio O, Peterson BS, Champagne F, Dwork AJ, Goldman J, Sulzer D. Loss of mTOR-Dependent Macroautophagy Causes Autistic-like Synaptic Pruning Deficits. Neuron. 2014;83:1131–1143. doi: 10.1016/j.neuron.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey KE, Brookes KJ, Hill MJ, Cochrane LE, Gill M, Skuse D, Correia C, Vicente A, Kent L, Gallagher L, Anney RJ. Oxytocin receptor (OXTR) does not play a major role in the aetiology of autism: genetic and molecular studies. Neurosci Lett. 2010;474:163–167. doi: 10.1016/j.neulet.2010.03.035. [DOI] [PubMed] [Google Scholar]
- Taurines R, Segura M, Schecklmann M, Albantakis L, Grunblatt E, Walitza S, Jans T, Lyttwin B, Haberhausen M, Theisen FM, Martin B, Briegel W, Thome J, Schwenck C, Romanos M, Gerlach M. Altered peripheral BDNF mRNA expression and BDNF protein concentrations in blood of children and adolescents with autism spectrum disorder. J. Neural Transm. (Vienna) 2014;121:1117–1128. doi: 10.1007/s00702-014-1162-x. [DOI] [PubMed] [Google Scholar]
- Tavassoli T, Auyeung B, Murphy LC, Baron-Cohen S, Chakrabarti B. Variation in the autism candidate gene GABRB3 modulates tactile sensitivity in typically developing children. Mol. Autism. 2012;3:6. doi: 10.1186/2040-2392-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The International Schizophrenia Consortium Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455:237–241. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney E, Bukelis I, Thompson RE, Ahmed K, Aneja A, Kratz L, Kelley RI. Abnormalities of cholesterol metabolism in autism spectrum disorders. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006;141B:666–668. doi: 10.1002/ajmg.b.30368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney E, Nwokoro NA, Kelley RI. Behavioral phenotype of RSH/Smith-Lemli-Opitz syndrome. Ment Retard Dev Disabil Res Rev. 2000;6:131–134. doi: 10.1002/1098-2779(2000)6:2<131::AID-MRDD7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hertzig ME, Gillespie-Lynch K, Gilhooly T, Millner AJ, Casey B. Elevated amygdala response to faces and gaze aversion in autism spectrum disorder. Soc Cogn Affect Neurosci. 2014;9:106–117. doi: 10.1093/scan/nst050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong DT, Rendall AR, Castelluccio BC, Eigsti IM, Fitch RH. Auditory processing and morphological anomalies in medial geniculate nucleus of Cntnap2 mutant mice. Behav Neurosci. 2015;129:731–743. doi: 10.1037/bne0000096. [DOI] [PubMed] [Google Scholar]
- Tsai PT, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, Steinberg J, Crawley JN, Regehr WG, Sahin M. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 2012;488:647–651. doi: 10.1038/nature11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SJ. Is autism caused by early hyperactivity of brain-derived neurotrophic factor? Med. Hypotheses. 2005;65:79–82. doi: 10.1016/j.mehy.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Tueting P, Costa E, Dwivedi Y, Guidotti A, Impagnatiello F, Manev R, Pesold C. The phenotypic characteristics of heterozygous reeler mouse. Neuroreport. 1999;10:1329–1334. doi: 10.1097/00001756-199904260-00032. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb Perspect Biol. 2012;4:a005736. doi: 10.1101/cshperspect.a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzio R, Nardou R, Ferrari DC, Tsintsadze T, Shahrokhi A, Eftekhari S, Khalilov I, Tsintsadze V, Brouchoud C, Chazal G, Lemonnier E, Lozovaya N, Burnashev N, Ben-Ari Y. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science. 2014;343:675–679. doi: 10.1126/science.1247190. [DOI] [PubMed] [Google Scholar]
- Uhlmann EJ, Wong M, Baldwin RL, Bajenaru ML, Onda H, Kwiatkowski DJ, Yamada K, Gutmann DH. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol. 2002;52:285–296. doi: 10.1002/ana.10283. [DOI] [PubMed] [Google Scholar]
- Veenstra-Vanderweele J, Jessen TN, Thompson BJ, Carter M, Prasad HC, Steiner JA, Sutcliffe JS, Blakely RD. Modeling rare gene variation to gain insight into the oldest biomarker in autism: construction of the serotonin transporter Gly56Ala knock-in mouse. J Neurodev Disord. 2009;1:158–171. doi: 10.1007/s11689-009-9020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheij C, Bakker CE, de Graaff E, Keulemans J, Willemsen R, Verkerk AJ, Galjaard H, Reuser AJ, Hoogeveen AT, Oostra BA. Characterization and localization of the FMR-1 gene product associated with fragile X syndrome. Nature. 1993;363:722–724. doi: 10.1038/363722a0. [DOI] [PubMed] [Google Scholar]
- Verpelli C, Dvoretskova E, Vicidomini C, Rossi F, Chiappalone M, Schoen M, Di Stefano B, Mantegazza R, Broccoli V, Bockers TM, Dityatev A, Sala C. Importance of Shank3 protein in regulating metabotropic glutamate receptor 5 (mGluR5) expression and signaling at synapses. J Biol Chem. 2011;286:34839–34850. doi: 10.1074/jbc.M111.258384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar FR, Nelson DS. Seizure Disorders in Autism. J. Am. Acad. Child Adolesc Psychiatry. 1990;29:127–129. doi: 10.1097/00004583-199001000-00020. [DOI] [PubMed] [Google Scholar]
- Waage-Baudet H, Lauder JM, Dehart DB, Kluckman K, Hiller S, Tint GS, Sulik KK. Abnormal serotonergic development in a mouse model for the Smith-Lemli-Opitz syndrome: implications for autism. Int J Dev Neurosci. 2003;21:451–459. doi: 10.1016/j.ijdevneu.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Wagstaff J, Knoll JH, Fleming J, Kirkness EF, Martin-Gallardo A, Greenberg F, Graham JM, Jr, Menninger J, Ward D, Venter JC, Lalande M. Localization of the gene encoding the GABAA receptor beta 3 subunit to the Angelman/Prader-Willi region of human chromosome 15. Am J Hum Genet. 1991;49:330–337. [PMC free article] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, Crabbe JC. Survey of 21 inbred mouse strains in two laboratories reveals that BTBR T/+ tf/tf has severely reduced hippocampal commissure and absent corpus callosum. Brain Res. 2003;971:47–54. doi: 10.1016/S0006-8993(03)02354-0. [DOI] [PubMed] [Google Scholar]
- Walcott EC, Higgins EA, Desai NS. Synaptic and intrinsic balancing during postnatal development in rat pups exposed to valproic acid in utero. J Neurosci. 2011;31:13097–13109. doi: 10.1523/JNEUROSCI.1341-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Nord AS, Kusenda M, Malhotra D, Bhandari A, Stray SM, Rippey CF, Roccanova P, Makarov V, Lakshmi B, Findling RL, Sikich L, Stromberg T, Merriman B, Gogtay N, Butler P, Eckstrand K, Noory L, Gochman P, Long R, Chen Z, Davis S, Baker C, Eichler EE, Meltzer PS, Nelson SF, Singleton AB, Lee MK, Rapoport JL, King MC, Sebat J. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Wang H, Meng XH, Ning H, Zhao XF, Wang Q, Liu P, Zhang H, Zhang C, Chen GH, Xu DX. Age- and gender-dependent impairments of neurobehaviors in mice whose mothers were exposed to lipopolysaccharide during pregnancy. Toxicol Lett. 2010;192:245–251. doi: 10.1016/j.toxlet.2009.10.030. [DOI] [PubMed] [Google Scholar]
- Wang SS, Kloth AD, Badura A. The Cerebellum, Sensitive Periods, and Autism. Neuron. 2014;83:518–532. doi: 10.1016/j.neuron.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrier V, Baron-Cohen S, Chakrabarti B. Genetic variation in GABRB3 is associated with Asperger syndrome and multiple endophenotypes relevant to autism. Mol. Autism. 2013;4:48. doi: 10.1186/2040-2392-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassink TH, Piven J, Vieland VJ, Pietila J, Goedken RJ, Folstein SE, Sheffield VC. Examination of AVPR1a as an autism susceptibility gene. Mol. Psychiatry. 2004;9:968–972. doi: 10.1038/sj.mp.4001503. [DOI] [PubMed] [Google Scholar]
- Weidner KL, Buenaventura DF, Chadman KK. Mice over-expressing BDNF in forebrain neurons develop an altered behavioral phenotype with age. Behav Brain Res. 2014;268:222–228. doi: 10.1016/j.bbr.2014.04.025. [DOI] [PubMed] [Google Scholar]
- Weiler IJ, Irwin SA, Klintsova AY, Spencer CM, Brazelton AD, Miyashiro K, Comery TA, Patel B, Eberwine J, Greenough WT. Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc Natl Acad Sci USA. 1997;94:5395–5400. doi: 10.1073/pnas.94.10.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger SR, Kelliher KR, Zufall F, Lolait SJ, O’Carroll AM, Young WS., 3rd Social motivation is reduced in vasopressin 1b receptor null mice despite normal performance in an olfactory discrimination task. Horm Behav. 2004;46:638–645. doi: 10.1016/j.yhbeh.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Whitehouse AJ, Bishop DV, Ang Q, Pennell CE, Fisher SE. CNTNAP2 variants affect early language development in the general population. Genes Brain and Behav. 2011;10:451–456. doi: 10.1111/j.1601-183X.2011.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. The social deficits of the oxytocin knockout mouse. Neuropeptides. 2002;36:221–229. doi: 10.1054/npep.2002.0909. [DOI] [PubMed] [Google Scholar]
- Wohr M, Roullet FI, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011a;10:35–43. doi: 10.1111/j.1601-183X.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohr M, Roullet FI, Hung AY, Sheng M, Crawley JN. Communication impairments in mice lacking Shank1: reduced levels of ultrasonic vocalizations and scent marking behavior. PLoS One. 2011b;6:e20631. doi: 10.1371/journal.pone.0020631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolterink G, Daenen LE, Dubbeldam S, Gerrits MA, van Rijn R, Kruse CG, Van Der Heijden JA, Van Ree JM. Early amygdala damage in the rat as a model for neurodevelopmental psychopathological disorders. Eur Neuropsychopharmacol. 2001;11:51–59. doi: 10.1016/S0924-977X(00)00138-3. [DOI] [PubMed] [Google Scholar]
- Won H, Lee HR, Gee HY, Mah W, Kim JI, Lee J, Ha S, Chung C, Jung ES, Cho YS, Park SG, Lee JS, Lee K, Kim D, Bae YC, Kaang BK, Lee MG, Kim E. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature. 2012;486:261–265. doi: 10.1038/nature11208. [DOI] [PubMed] [Google Scholar]
- Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, Gong X, Zhang Y, Yang X, Zhang D. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biol. Psychiatry. 2005;58:74–77. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Fujimoto C, Nakajima E, Isagai T, Matsuishi T. Possible association between congenital cytomegalovirus infection and autistic disorder. J Autism Dev Disord. 2003;33:455–459. doi: 10.1023/A:1025023131029. [DOI] [PubMed] [Google Scholar]
- Yamauchi J, Miyamoto Y, Kusakawa S, Torii T, Mizutani R, Sanbe A, Nakajima H, Kiyokawa N, Tanoue A. Neurofibromatosis 2 tumor suppressor, the gene induced by valproic acid, mediates neurite outgrowth through interaction with paxillin. Exp Cell Res. 2008;314:2279–2288. doi: 10.1016/j.yexcr.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Yan WL, Guan XY, Green ED, Nicolson R, Yap TK, Zhang J, Jacobsen LK, Krasnewich DM, Kumra S, Lenane MC, Gochman P, Damschroder-Williams PJ, Esterling LE, Long RT, Martin BM, Sidransky E, Rapoport JL, Ginns EI. Childhood-onset schizophrenia/autistic disorder and t(1;7) reciprocal translocation: identification of a BAC contig spanning the translocation breakpoint at 7q21. Am J Med Genet. 2000;96:749–753. doi: 10.1002/1096-8628(20001204)96:6<749::AID-AJMG10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Yang M, Bozdagi O, Scattoni ML, Wöhr M, Roullet FI, Katz AM, Abrams DN, Kalikhman D, Simon H, Woldeyohannes L, Zhang JY, Harris MJ, Saxena R, Silverman JL, Buxbaum JD, Crawley JN. Reduced excitatory neurotransmission and mild autism-relevant phenotypes in adolescent Shank3 null mutant mice. J Neurosci. 2012;32:6525–6541. doi: 10.1523/JNEUROSCI.6107-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui DH, Peddada S, Bieda MC, Vallero RO, Hogart A, Nagarajan RP, Thatcher KN, Farnham PJ, Lasalle JM. Integrated epigenomic analyses of neuronal MeCP2 reveal a role for long-range interaction with active genes. Proc Natl Acad Sci USA. 2007;104:19416–19421. doi: 10.1073/pnas.0707442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip JW, Yip YP, Nakajima K, Capriotti C. Reelin controls position of autonomic neurons in the spinal cord. Proc Natl Acad Sci USA. 2000;97:8612–8616. doi: 10.1073/pnas.150040497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya N, Rosenberg C, Levi S, Salomon S, Shulman C, Nemanov L, Dina C, Ebstein RP. Association between the arginine vasopressin 1a receptor (AVPR1a) gene and autism in a family-based study: mediation by socialization skills. Mol. Psychiatry. 2006;11:488–494. doi: 10.1038/sj.mp.4001812. [DOI] [PubMed] [Google Scholar]
- Ylisaukko-oja T, Rehnström K, Auranen M, Vanhala R, Alen R, Kempas E, Ellonen P, Turunen JA, Makkonen I, Riikonen R, Nieminen-von Wendt T, von Wendt L, Peltonen L, Järvelä I. Analysis of four neuroligin genes as candidates for autism. Eur J Hum Genet. 2005;13:1285–1292. doi: 10.1038/sj.ejhg.5201474. [DOI] [PubMed] [Google Scholar]
- Young DM, Schenk AK, Yang SB, Jan YN, Jan LY. Altered ultrasonic vocalizations in a tuberous sclerosis mouse model of autism. Proc Natl Acad Sci USA. 2010;107:11074–11079. doi: 10.1073/pnas.1005620107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zec N, Rowitch DH, Bitgood MJ, Kinney HC. Expression of the homeobox-containing genes EN1 and EN2 in human fetal midgestational medulla and cerebellum. J Neuropathol Exp Neurol. 1997;56:236–242. doi: 10.1097/00005072-199703000-00002. [DOI] [PubMed] [Google Scholar]
- Zhang J, Hou L, Klann E, Nelson DL. Altered hippocampal synaptic plasticity in the FMR1 gene family knockout mouse models. J Neurophysiol. 2009;101:2572–2580. doi: 10.1152/jn.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Serajee FJ, Nabi R, Huq AH. No association between the EN2 gene and autistic disorder. J Med Genet. 2003;40:e4. doi: 10.1136/jmg.40.1.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Parada LF. PTEN signaling in autism spectrum disorders. Curr Opin Neurobiol. 2012;22:873–879. doi: 10.1016/j.conb.2012.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.