Abstract
Mesenchymal stem cells (MSCs) offer significant therapeutic promise for various regenerative therapies. However, MSC-based therapy for injury exhibits low efficacy due to the pathological environment in target tissues and the differences between in vitro and in vivo conditions. To address this issue, we developed adipose-derived MSC spheroids as a novel delivery method to preserve the stem cell microenvironment. MSC spheroids were generated by suspension culture for 3 days, and their sizes increased in a time-dependent manner. After re-attachment of MSC spheroids to the plastic dish, their adhesion capacity and morphology were not altered. MSC spheroids showed enhanced production of hypoxia-induced angiogenic cytokines such as vascular endothelial growth factor (VEGF), stromal cell derived factor (SDF), and hepatocyte growth factor (HGF). In addition, spheroid culture promoted the preservation of extracellular matrix (ECM) components, such as laminin and fibronectin, in a culture time- and spheroid size-dependent manner. Furthermore, phosphorylation of AKT, a cell survival signal, was significantly higher and the expression of pro-apoptotic molecules, poly (ADP ribose) polymerase-1 (PARP-1) and cleaved caspase-3, was markedly lower in the spheroids than in MSCs in monolayers. In the murine hindlimb ischemia model, transplanted MSC spheroids showed better proliferation than MSCs in monolayer. These findings suggest that MSC spheroids promote MSC bioactivities via secretion of angiogenic cytokines, preservation of ECM components, and regulation of apoptotic signals. Therefore, MSC spheroid-based cell therapy may serve as a simple and effective strategy for regenerative medicine.
Keywords: Adipose-derived mesenchymal stem cells, Spheroid, Extracellular matrix, Apoptosis, Cell transplantation, Ischemic disease
INTRODUCTION
Adult stem cells have emerged as a promising source for stem cell-based therapies in regenerative medicine due to their self-renewal capabilities as well as their ability to differentiate into multiple cell lineages. These cells are easily isolated from various tissues, such as the bone marrow (Bruder et al., 1997; Colter et al., 2000), the adipose tissue (Zuk et al., 2001; Zuk et al., 2002), and other sites (Erices et al., 2000; Rodriguez-Lozano et al., 2011) for autologous transplantation. Mesenchymal stem cells (MSCs) possess homing abilities, and can migrate toward injury sites to secrete various soluble factors such as anti-oxidant, anti-apoptotic, and pro-angiogenic cytokines (English et al., 2010; Salem and Thiemermann, 2010; Tolar et al., 2010). Thus, MSCs can be used as a powerful resource in stem cell-based regenerative medicine.
To optimize the functions of MSCs for clinical application, various strategies have been introduced, including genetic modification, cytokine priming, or manipulation of culture conditions. However, despite several potential advantages, stem cell-based therapies show low therapeutic efficacy following engraftment in injured tissues due to poor survival and differentiation efficiency (Zhang et al., 2001; Assmus et al., 2006). It has been suggested that hypoxia and inflammation in injured sites are responsible for the high death rate of the transplanted cells (Hill et al., 2006). It is known that the microenvironment can influence stem cell behaviors such as self-renewal and differentiation via intrinsic and extrinsic signals (Burdick and Vunjak-Novakovic, 2009). Generally, MSCs for transplantation are prepared by traditional two-dimensional (2D) culture and dissociative grafting. However, following several in vitro passages, MSCs prepared by this technique lack sufficient protection against ischemic conditions. The harsh microenvironment leads to a reduction in their replicative ability, colony-forming efficiency, and survival signaling, which results in replicative senescence, loss of self-renewal, and low differentiation capacity (Baer et al., 2010; Park and Patel, 2010). In addition, cells are exposed to harsh proteolytic enzyme treatments when they are harvested from the 2D monolayer culture. This process impedes cell-extracellular matrix interaction, and is partially responsible for the low engraftment of stem cells in vivo (Wang et al., 2009a).
To overcome the limitations of the 2D monolayer culture system, development of three-dimensional (3D) cell culture system has become an important topic in stem cell research. The 3D arrangement of cells increases cell-to-cell interactions, and can better mimic the in vivo microenvironment, as compared with 2D cultures (Haycock, 2011). Mild hypoxic precondition is also established in the inner core of spheroid. This preconditioning induces the cell resistance to hypoxic conditions in the ischemic tissues. In addition, 3D spheroid culture improves ECM organization, production of various growth factors, and gene expression profiles (Cheng et al., 2012; Yoon et al., 2012; Yeh et al., 2014).
Research has suggested that the 3D spheroid structure can augment MSC bioactivities and therapeutic efficiency, but the fundamental mechanism underlying the effects of spheroid culture is not well understood, particularly the alterations to cell signal transduction and expression of cytokines and growth factors. In this study, we developed 3D spheroids of adipose-derived MSCs in an attempt to understand the production of cytokines and growth factors, production of ECM components, and cell survival signal cascades in these cells. Moreover, we investigated the proliferative and survival potential of MSC spheroids in a murine hindlimb ischemia model.
MATERIALS AND METHODS
Cell cultures
Human adipose-derived MSCs were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). They were pathogen and mycoplasma free. The supplier certified that the MSCs expressed cell surface markers (CD73 and CD105, but not CD31), and have adipogenic and osteogenic differentiation potential when cultured with specific differentiation media. MSCs were cultured in alpha-minimum essential medium (Gibco BRL, Gaithersburg, MD, USA) supplemented with 10% (v/v) fetal bovine serum (Gibco BRL), 100 U/ml of penicillin, and 100 μg/ml streptomycin. Cells were placed in a humidified incubator at 37°C and 5% CO2.
Generation of MSC spheroids
To generate spheroids, MSCs were cultured in suspension using Ultra-low attachment 6 well plates (Sigma, St. Louis, MO, USA). Cells were cultured in MSC growth media, and placed in a humidified incubator at 37°C and 5% CO2. Spheroids formed at day 3, and they were further cultured for the indicated periods (3, 5, or 7 day) to assess their bioactivities. MSC spheroids were identified and measured using the visual inspection microscope (Olympus, Tokyo, Japan).
Western blot analysis
Monolayer and spheroid MSCs were lysed, and total cellular protein was extracted using RIPA lysis and extraction buffer (Thermo Scientific, Rockford, IL, USA). Cell lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). Membranes were blocked with 5% skim milk, and incubated with primary antibodies against human-specific HIF-1α, vascular endothelial growth factor (VEGF), stromal cell-derived factor (SDF), hepatocyte growth factor (HGF), laminin, fibronectin, phosphor-AKT, AKT, cleaved poly (ADP ribose) polymerase-1 (PARP-1), cleaved caspase-3, and beta-actin (Santa Cruz Biotechnology, Dallas, TX, USA). This was followed by incubation with peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology). Bands were visualized using enhanced chemiluminescence reagents (Amersham Biosciences, Uppsala, Sweden) in a dark room.
Ethics statement
Experiments were performed on 8-week-old male Balb/C nude mice (Biogenomics, Seoul, Korea, http://www.orient.co.kr). Mice were maintained under a 12 h light/dark cycle, in accordance with the regulations of Soonchunhyang University, Seoul Hospital. All procedures were performed in accordance with the policies of the Institutional Animal Care and Use Committee of Soonchunhyang University, Seoul Hospital, Korea (IACUC2013-5).
Spheroid transplantation in a murine hindlimb ischemia model
To induce ischemia and oxidative stress, a hindlimb ischemia model was used as previously described with minor modifications (Limbourg et al., 2009; Han et al., 2015). Ischemia was induced by ligation of the proximal femoral artery and boundary vessels of the mice. No later than 6 h after operation, PBS, monolayer-cultured MSCs, and spheroid-cultured MSCs were injected intramuscularly into the ischemic thigh sites (1×107 cells/60 μl PBS per mouse). Cells were injected into multiple (four) ischemic sites. Three days post-operation, proliferation of transplanted MSCs was investigated via immunohistochemistry.
Immunohistochemistry
Ischemic thigh tissues were removed at 3 days post-operation, and fixed with 4% paraformaldehyde (Affymetrix, Santa Clara, CA, USA). Tissue samples were embedded in paraffin. Immunofluorescent staining was performed using primary antibodies against proliferating cell nuclear antigen (PCNA; Santa Cruz Biotechnology) and human nuclear antigen (HNA; Millipore). Secondary antibodies used were conjugated to Alexa 488 and Alexa 594 (Thermo Scientific). Nuclei were stained with 4̓,6-diaminido-2-phenylindol (DAPI; Vector Laboratories, Burlingame, CA, USA). Immunostained samples were assessed by confocal microscopy (Olympus).
Statistical analysis
All data are expressed as mean ± standard error of the mean (SEM). Statistical significance was assessed with Student’s t test, where differences with p<0.05 were considered significant.
RESULTS
Formation of MSC spheroids in suspension cultures
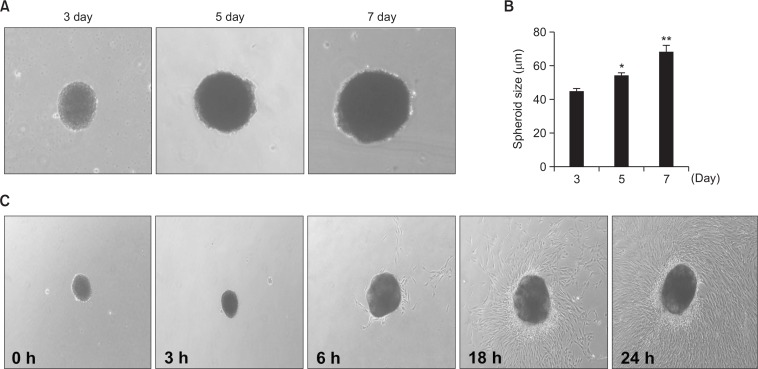
To generate MSC spheroids in order to mimic the in vivo microenvironment, including cell-to-cell interactions and ECM organization, single MSCs dissociated from the monolayer culture were placed in suspension cultures in ultra-low attachment dishes. After an incubation period of 3 days, single MSCs spontaneously assembled into spheroids. MSC spheroids cultured for three days had an average diameter of approximately 40 μm (Fig. 1A, B). Morphologically, they appeared spherical or slightly oblong. To determine whether sizes of MSC spheroids increase in a time-dependent manner, MSC spheroids were cultured for a prolonged period (3, 5, and 7 days). As was expected, the average diameter of MSC spheroids increased with time (Fig. 1A, B). To determine whether the generation of spheroids induced adhesion, migration, and cytological changes in MSCs, MSC spheroids were re-cultured as a 2D monolayer culture in attachment dishes. While they reattached to the surface of dishes, cell adhesion, migration, and morphology were preserved in the MSC spheroids (Fig. 1C).
Fig. 1.
Formation of three-dimensional MSC spheroids and morphological analysis for adhesion capacity. (A) Phase contrast microscopy showing the time course of adipose-derived MSC spheroid formation over 7 days (magnification ×40). (B) Sizes of spheroids generated by adipose-derived MSCs for 3, 5, and 7 days. Values represent means ± SEM. *p<0.05 and **p<0.01 vs. MSC spheroids cultured for 3 days. (C) Assessment of adhesion and morphology of MSC spheroids at 0, 3, 6, 18, and 24 h in vitro.
Production of angiogenic cytokines from MSC spheroids
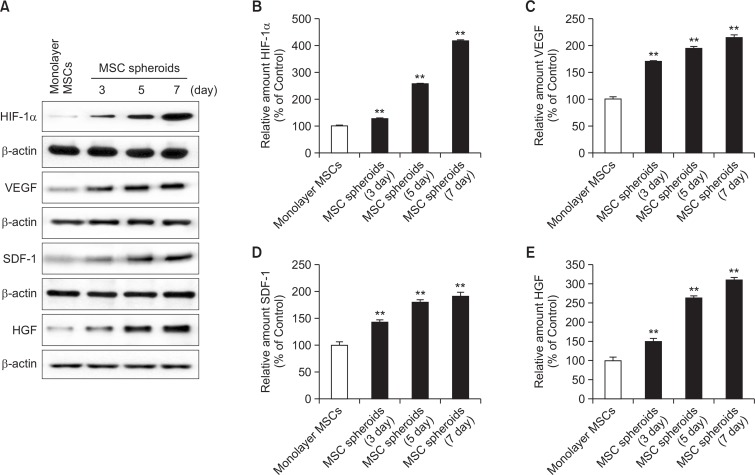
To assess the expression of angiogenic cytokines in MSC spheroids, protein expression of VEGF, SDF, and HGF were investigated in monolayer MSCs and spheroid MSCs (incubated for 3, 5, and 7 days). Western blot assays indicated that the expression of angiogenic cytokines in spheroid-cultured MSCs increased a time-dependent manner and was significantly higher than that in other MSCs following culture for seven days (Fig. 2).
Fig. 2.
Expression of hypoxia-induced angiogenic cytokines in MSC spheroids. (A) Western blot analysis for hypoxic inducible factor-1α (HIF-1α), vascular endothelial growth factor (VEGF), stromal cell derived factor (SDF), and hepatocyte growth factor (HGF) in monolayer MSCs and MSC spheroids cultured for 3, 5, and 7 days. (B-E) Relative expression of HIF-1α, VEGF, SDF, and HGF normalized to that of β-actin. Values represent means ± SEM. **p<0.01 vs. monolayer MSCs.
Augmentation of ECM preservation in MSC spheroids
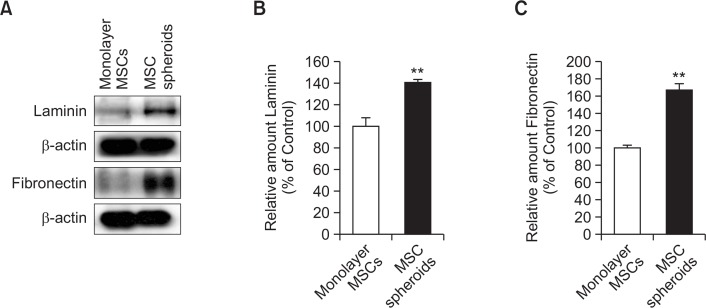
Anoikis is apoptosis induced by the loss of interaction between the cell and the ECM. To investigate ECM preservation in MSC spheroids, ECM components, including laminin and fibronectin, in monolayer MSCs and MSC spheroids were measured by western blot analysis. MSC spheroids demonstrated better preservation of laminin and fibronectin than did monolayer MSCs (Fig. 3).
Fig. 3.
Expression of extracellular matrix (ECM) components in MSC spheroids. (A) Western blot analysis for ECM components, including laminin and fibronectin in monolayer MSCs and MSC spheroids cultured for 7 days. (B, C) Relative expression of laminin and fibronectin normalized to that of β-actin. Values represent means ± SEM. **p<0.01 vs. monolayer MSCs.
Inhibition of anoikis in MSC spheroids
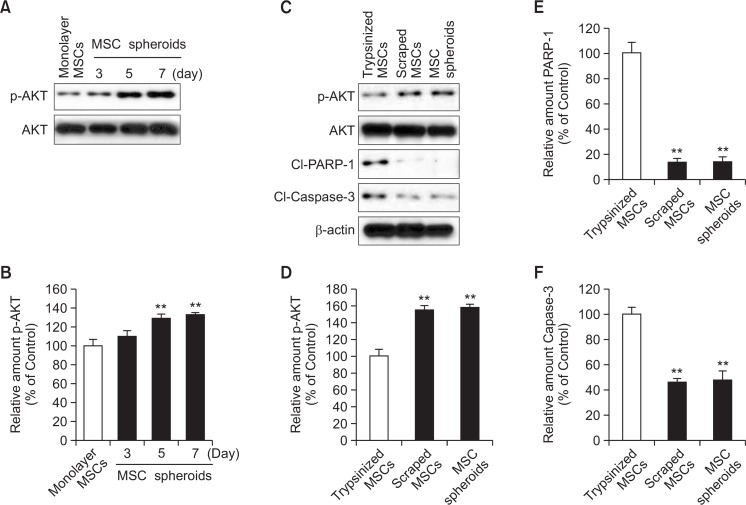
To explore the inhibitory effect of spheroid culture on anoikis, AKT phosphorylation, which is associated with cell survival signal pathway, was assessed by western blot analysis. AKT phosphorylation was significantly higher in MSC spheroids cultured for seven days than in monolayer MSCs and MSC spheroids cultured for three days (Fig. 4A, B). To confirm that anoikis was indeed induced by a lack of cell-to-ECM interactions in MSCs, phosphorylation of AKT and expression of pro-apoptotic factors, including PARP-1 and caspase-3, were investigated in trypsinized MSCs, scraped MSCs, and MSC spheroids cultured for seven days. Phosphorylation of AKT was significantly up-regulated and expression of pro-apoptotic factors was significantly down-regulated in MSC spheroids, as compared with those in trypsinized MSCs (Fig. 4C–F). The extent of AKT phosphorylation and expression of pro-apoptotic proteins were similar in both MSC spheroids and scraped MSCs with preserved ECM components.
Fig. 4.
Apoptosis-associated signals in MSCs spheroids. (A) Western blot analysis for phosphorylation of AKT in monolayer MSCs and MSC spheroids cultured for 3, 5, and 7 days. (B) Relative expression of phosphorylated AKT normalized to total-AKT expression. Values represent means ± SEM. **p<0.01 vs. monolayer MSCs. (C) Western blot analysis for AKT phosphorylation and expression of cleaved PARP-1 and cleaved caspase-3 in trypsinized MSCs, scraped MSCs, and MSC spheroids cultured for 7 days. (D–F) Relative expression of phosphorylated AKT was normalized to total-AKT expression; Expression of cleaved PARP-1 and cleaved caspase-3 was normalized to that of β-actin. Values represent means ± SEM. **p<0.01 vs. trypsinized MSCs.
Enhanced proliferation of MSCs in ischemic tissue by transplanted spheroids
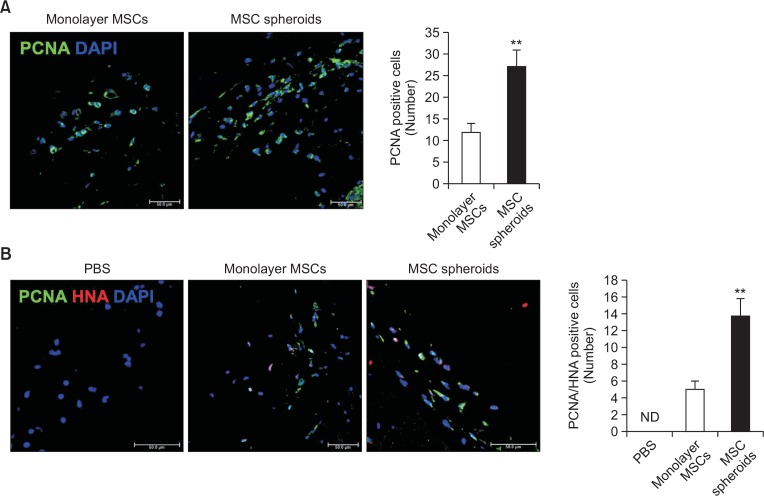
To determine whether MSC spheroids improve cell proliferation in mouse ischemic limb sites, proliferation of monolayer MSCs and MSC spheroids in ischemic tissues were analyzed using a mice hindlimb ischemia model. At post-operative day three, immunofluorescent staining was performed to examine the expression of the proliferation marker PCNA in ischemic tissues transplanted with monolayer MSCs or MSC spheroids. Expression of PCNA was higher in ischemic tissues transplanted with MSC spheroids than in tissues transplanted with monolayer MSCs (Fig. 5A). Furthermore, immunofluorescent staining for PCNA and HNA in ischemic tissues also confirmed that proliferation of MSCs was significantly higher in tissues transplanted with MSC spheroids than in tissues transplanted with monolayer MSCs (Fig. 5B).
Fig. 5.
Proliferation of MSC spheroids in vivo. (A) Immunofluorescent staining of proliferating cell nuclear antigen (PCNA; green) in murine ischemic limb sites 3 days after transplantation of monolayer MSCs and MSC spheroid cultured for 7 days (scale bar=50 μm). Bar graph shows the number of PCNA-positive cells 3 days after transplantation. Values represent means ± SEM. **p<0.01 vs. monolayer MSCs. (B) Immunofluorescent staining of PCNA (green) and human nucleic antigen (HNA; red) in murine ischemic limb site at 3 days after transplantation of monolayer MSCs and MSC spheroids cultured for 7 days (scale bar=50 μm). Bar graph shows the number of PCNA and HNA double-positive cells 3 days after transplantation. Values represent means ± SEM. **p<0.01 vs. monolayer MSCs.
DISCUSSION
MSCs are extensively used as a source of cells for a wide variety of diseases, including graft-versus-host disease (Le Blanc et al., 2008), type 1 diabetes (Fiorina et al., 2009), and inflammatory bowel disease (Gonzalez et al., 2009). MSCs derived from adult tissues contain a heterogeneous subset of stromal cells that retain the capacity to adhere to plastic dishes, form colonies, differentiate into multiple lineages, modulate immune responses, and reduce inflammation (da Silva Meirelles et al., 2006). MSCs can be isolated from the stromal fraction of various postnatal tissues. Although they are usually extracted from the bone marrow, adipose tissue is the most attractive source for clinical applications. Adipose tissue is more easily accessible than the bone marrow, and the extraction process is less invasive. In addition, bone marrow-derived MSCs are a rare population, and their numbers decline with age and health status. Moreover, applications using embryonic stem cells and induced pluripotent stem cells are limited by ethical considerations and unforeseen immaturity, respectively (Thomson et al., 1998; Hyun et al., 2007). Therefore, human adipose-derived MSCs represent an attractive source for stem cell-based therapies in several ischemic diseases, including myocardial infarction, stroke, and limb ischemia due to their biological and practical advantages.
Despite these advantages, the retention of transplanted MSCs at injured sites is usually limited due to the harsh conditions of the diseased target tissues, such as ischemia, hypoxia, poor supply of nutrients, and inflammatory reactions from host cells. To address this issue, several studies tried to transplant genetically modified MSCs, pretreated MSCs with cytokines, and MSCs preconditioned under hypoxia in order to enhance graft preservation and therapeutic efficacy (Li et al., 2007; Hahn et al., 2008; Rosova et al., 2008). However, these efforts were unable to solve problems such as lack of cell-cell interactions, disruption of the natural niche conditions, and apoptosis during the preparation steps (Grossmann, 2002). Recently, a series of studies demonstrated that MSC spheroids can be used as a novel delivery system to improve graft survival (Potapova et al., 2008; Cochrane et al., 2010). Spheroid culture enhanced the expression of stem cell-related genes, multipotent differentiation capacity, secretion of angiogenic, anti-apoptotic, and anti-inflammatory molecules, up-regulation of hypoxia-adaptive signals, and preservation of ECM components (Wang et al., 2009b; Bartosh et al., 2010; Li et al., 2010; Ylostalo et al., 2012; Tseng and Hsu, 2014; Yamaguchi et al., 2014). Although several studies have shown that MSC spheroids have enhanced bioactivities, these studies only confirmed the beneficial effects in short-term culture (generally 1–3 days), and the functional improvements in MSC spheroids due to culture duration and spheroid size are still unknown. In the present study, we investigated long-term three-dimensional spheroid culture of MSCs until 7 days and demonstrated that spheroid culture increased the expression of angiogenic cytokines such as VEGF, SDF, and HGF, as well as ECM components such as laminin and fibronectin, in adipose-derived MSCs in a culture time- and spheroid-size-dependent manner. Moreover, augmentation of angiogenic cytokines and preservation of ECM components led to inhibition of apoptosis and increase in cell proliferation.
Following a seven-day incubation period, adipose-derived MSC spheroids showed maximal expression of HIF-1α and hypoxia-induced angiogenic cytokines including VEGF, SDF, and HGF. Previous studies have demonstrated that hypoxic conditions trigger the expression of hypoxia-responsive genes involved in migration and angiogenesis, such as VEGF, SDF-1α, and CXCR4 (Chacko et al., 2010; Peterson et al., 2011). In addition, human adipose-derived stromal cell spheroids stimulated the secretion of VEGF, FGF2, and HGF (Bhang et al., 2011). These factors are key angiogenic cytokines and are induced by hypoxic conditions (Rehman et al., 2004). Our findings show that formation of spheroids induced mild hypoxic conditions, resulting in the expression of hypoxia-induced survival factors such as HIF-1α and SDF, as well as angiogenic factors such as VEGF and HGF.
We also demonstrated that the expression of pro-apoptotic proteins was inhibited and cell survival signals were increased in MSCs in spheroids. In the present study, MSC spheroids showed significantly increased expression of ECM molecules, such as laminin and fibronectin, as compared with monolayer MSCs. In contrast to the general 2D culture system, spheroid cultures augment cell-to-cell contact and cell-to-ECM interactions. This environment highly influences the bioactivities and fate of cells by regulating cell-to-cell or ECM signal transduction, resulting in enhanced cell survival. We demonstrated augmented AKT phosphorylation, a cell survival signal, in MSCs in spheroids. These MSCs also showed decreased expression of cleaved PARP-1 and cleaved caspase-3, which are pro-apoptotic molecules. One reason for apoptosis is the loss of ECM components from cells, also known as anoikis (Frisch and Screaton, 2001). Our data show that survival signals are significantly enhanced in MSC spheroids, as compared with trypsinized MSCs and MSC spheroids that were similar to scraped MSCs. In particular, this effect was maximal after seven days of culture. In addition, we transplanted MSC spheroids in a murine hindlimb ischemia model. Proliferation of MSCs was markedly increased in transplanted spheroid MSCs as compared with that in monolayer MSCs. A previous study demonstrated in a myocardial infarction model that human umbilical cord blood-derived MSC spheroids show enhanced proliferative activity (Lee et al., 2012). Human adipose-derived stromal cell spheroids also showed enhanced survival ratio in a murine hindlimb ischemia model (Bhang et al., 2011). Our results suggest that spheroid culture augmented the survival and proliferative capacity of MSCs via regulation of ECM and expression of survival proteins.
Taken together, our findings show that 3D spheroid culture improved the secretion of angiogenic factors, expression of ECM molecules, activation of survival signals, and inhibition of apoptotic proteins in MSCs, resulting in enhanced proliferative ability of transplanted cells in vivo. Moreover, we report, for the first time, that these benefits are correlated to the duration of culture and the size of spheroids. These findings indicate that use of adipose-derived MSC spheroids may be an efficient and suitable strategy for clinical application in regenerative medicine.
Acknowledgments
This study was supported by the Soonchunhyang University Research Fund and a National Research Foundation grant funded by the Korean government (NRF-2011-0009610) and a grant from the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI14C2253). The funders had no role in the study design, data collection or analysis, the decision to publish, or preparation of the manuscript.
REFERENCES
- Assmus B, Honold J, Schächinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- Baer PC, Griesche N, Luttmann W, Schubert R, Luttmann A, Geiger H. Human adipose-derived mesenchymal stem cells in vitro: evaluation of an optimal expansion medium preserving stemness. Cytotherapy. 2010;12:96–106. doi: 10.3109/14653240903377045. [DOI] [PubMed] [Google Scholar]
- Bartosh TJ, Ylöstalo JH, Mohammadipoor A, Bazhanov N, Coble K, Claypool K, Lee RH, Choi H, Prockop DJ. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci USA. 2010;107:13724–13729. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhang SH, Cho SW, La WG, Lee TJ, Yang HS, Sun AY, Baek SH, Rhie JW, Kim BS. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials. 2011;32:2734–2747. doi: 10.1016/j.biomaterials.2010.12.035. [DOI] [PubMed] [Google Scholar]
- Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–294. doi: 10.1002/(SICI)1097-4644(199702)64:2<278::AID-JCB11>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Burdick JA, Vunjak-Novakovic G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng. Part A. 2009;15:205–219. doi: 10.1089/ten.tea.2008.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko SM, Ahmed S, Selvendiran K, Kuppusamy ML, Khan M, Kuppusamy P. Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am J Physiol Cell Physiol. 2010;299:C1562–C1570. doi: 10.1152/ajpcell.00221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NC, Wang S, Young TH. The influence of spheroid formation of human adipose-derived stem cells on chitosan films on stemness and differentiation capabilities. Biomaterials. 2012;33:1748–1758. doi: 10.1016/j.biomaterials.2011.11.049. [DOI] [PubMed] [Google Scholar]
- Cochrane DJ, Stannard SR, Firth EC, Rittweger J. Comparing muscle temperature during static and dynamic squatting with and without whole-body vibration. Clin. Physiol. Funct. Imaging. 2010;30:223–229. doi: 10.1111/j.1475-097X.2010.00931.x. [DOI] [PubMed] [Google Scholar]
- Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci USA. 2000;97:3213–3218. doi: 10.1073/pnas.97.7.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- English K, French A, Wood KJ. Mesenchymal stromal cells: facilitators of successful transplantation? Cell Stem Cell. 2010;7:431–442. doi: 10.1016/j.stem.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- Fiorina P, Jurewicz M, Augello A, Vergani A, Dada S, La Rosa S, Selig M, Godwin J, Law K, Placidi C, Smith RN, Capella C, Rodig S, Adra CN, Atkinson M, Sayegh MH, Abdi R. Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol. 2009;183:993–1004. doi: 10.4049/jimmunol.0900803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–562. doi: 10.1016/S0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–989. doi: 10.1053/j.gastro.2008.11.041. [DOI] [PubMed] [Google Scholar]
- Grossmann J. Molecular mechanisms of “detachment-induced apoptosis--Anoikis”. Apoptosis. 2002;7:247–260. doi: 10.1023/A:1015312119693. [DOI] [PubMed] [Google Scholar]
- Hahn JY, Cho HJ, Kang HJ, Kim TS, Kim MH, Chung JH, Bae JW, Oh BH, Park YB, Kim HS. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol. 2008;51:933–943. doi: 10.1016/j.jacc.2007.11.040. [DOI] [PubMed] [Google Scholar]
- Han YS, Lee JH, Jung JS, Noh H, Baek MJ, Ryu JM, Yoon YM, Han HJ, Lee SH. Fucoidan protects mesenchymal stem cells against oxidative stress and enhances vascular regeneration in a murine hindlimb ischemia model. Int J Cardiol. 2015;198:187–195. doi: 10.1016/j.ijcard.2015.06.070. [DOI] [PubMed] [Google Scholar]
- Haycock JW. 3D cell culture: a review of current approaches and techniques. Methods Mol Biol. 2011;695:1–15. doi: 10.1007/978-1-60761-984-0_1. [DOI] [PubMed] [Google Scholar]
- Hill E, Boontheekul T, Mooney DJ. Regulating activation of transplanted cells controls tissue regeneration. Proc Natl Acad Sci USA. 2006;103:2494–2499. doi: 10.1073/pnas.0506004103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun I, Hochedlinger K, Jaenisch R, Yamanaka S. New advances in iPS cell research do not obviate the need for human embryonic stem cells. Cell Stem Cell. 2007;1:367–368. doi: 10.1016/j.stem.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Park SJ, Kang SK, Kim GH, Kang HJ, Lee SW, Jeon HB, Kim HS. Spherical bullet formation via E-cadherin promotes therapeutic potency of mesenchymal stem cells derived from human umbilical cord blood for myocardial infarction. Mol Ther. 2012;20:1424–1433. doi: 10.1038/mt.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TS, Cheng K, Lee ST, Matsushita S, Davis D, Malliaras K, Zhang Y, Matsushita N, Smith RR, Marbán E. Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell-matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells. 2010;28:2088–2098. doi: 10.1002/stem.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, Furlani D, Piechaczek C, Moebius JM, Lützow K, Lendlein A, Stamm C, Li RK, Steinhoff G. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007;25:2118–2127. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- Limbourg A, Korff T, Napp LC, Schaper W, Drexler H, Limbourg FP. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat Protoc. 2009;4:1737–1746. doi: 10.1038/nprot.2009.185. [DOI] [PubMed] [Google Scholar]
- Park E, Patel AN. Changes in the expression pattern of mesenchymal and pluripotent markers in human adipose-derived stem cells. Cell Biol Int. 2010;34:979–984. doi: 10.1042/CBI20100124. [DOI] [PubMed] [Google Scholar]
- Peterson KM, Aly A, Lerman A, Lerman LO, Rodriguez-Porcel M. Improved survival of mesenchymal stromal cell after hypoxia preconditioning: role of oxidative stress. Life Sci. 2011;88:65–73. doi: 10.1016/j.lfs.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapova IA, Brink PR, Cohen IS, Doronin SV. Culturing of human mesenchymal stem cells as three-dimensional aggregates induces functional expression of CXCR4 that regulates adhesion to endothelial cells. J Biol Chem. 2008;283:13100–13107. doi: 10.1074/jbc.M800184200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Lozano FJ, Bueno C, Insausti CL, Meseguer L, Ramírez MC, Blanquer M, Marín N, Martínez S, Moraleda JM. Mesenchymal stem cells derived from dental tissues. Int Endod J. 2011;44:800–806. doi: 10.1111/j.1365-2591.2011.01877.x. [DOI] [PubMed] [Google Scholar]
- Rosová I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tolar J, Le Blanc K, Keating A, Blazar BR. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells. 2010;28:1446–1455. doi: 10.1002/stem.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng TC, Hsu SH. Substrate-mediated nanoparticle/gene delivery to MSC spheroids and their applications in peripheral nerve regeneration. Biomaterials. 2014;35:2630–2641. doi: 10.1016/j.biomaterials.2013.12.021. [DOI] [PubMed] [Google Scholar]
- Wang CC, Chen CH, Hwang SM, Lin WW, Huang CH, Lee WY, Chang Y, Sung HW. Spherically symmetric mesenchymal stromal cell bodies inherent with endogenous extracellular matrices for cellular cardiomyoplasty. Stem Cells. 2009a;27:724–732. doi: 10.1634/stemcells.2008-0944. [DOI] [PubMed] [Google Scholar]
- Wang W, Itaka K, Ohba S, Nishiyama N, Chung UI, Yamasaki Y, Kataoka K. 3D spheroid culture system on micropatterned substrates for improved differentiation efficiency of multipotent mesenchymal stem cells. Biomaterials. 2009b;30:2705–2715. doi: 10.1016/j.biomaterials.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Ohno J, Sato A, Kido H, Fukushima T. Mesenchymal stem cell spheroids exhibit enhanced in-vitro and in-vivo osteoregenerative potential. BMC Biotechnol. 2014;14:105. doi: 10.1186/s12896-014-0105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh HY, Liu BH, Sieber M, Hsu SH. Substrate-dependent gene regulation of self-assembled human MSC spheroids on chitosan membranes. BMC Genomics. 2014;15:10. doi: 10.1186/1471-2164-15-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylöstalo JH, Bartosh TJ, Coble K, Prockop DJ. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells. 2012;30:2283–2296. doi: 10.1002/stem.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HH, Bhang SH, Shin JY, Shin J, Kim BS. Enhanced cartilage formation via three-dimensional cell engineering of human adipose-derived stem cells. Tissue Eng. Part A. 2012;18:1949–1956. doi: 10.1089/ten.tea.2011.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]