Abstract
Dianthus superbus (D. superbus) is a traditional crude drug used for the treatment of urethritis, carbuncles and carcinomas. The objective of this study was to confirm the cognitive enhancing effect of D. superbus in memory impairment induced mice and to elucidate the possible potential mechanism. Effect of D. superbus on scopolamine induced memory impairment on mice was evaluated using the Morris water maze and passive avoidance tests. We also investigated acetylcholinesterase (AChE) activity and brain-derived neurotropic factor (BDNF) expression in scopolamine-induced mice. HPLC-DAD analysis was performed to identify active compounds in D. superbus. The results revealed that D. superbus attenuated the learning and memory impairment induced by scopolamine. D. superbus also inhibited AChE levels in the hippocampi of the scopolamine-injected mice. Moreover, D. superbus increased BDNF expression in the hippocampus. Eight compounds were identified using HPLC-DAD analysis. The content of 4-hydroxyphenyl acetic acid was higher than contents of other compounds. These results indicated that D. superbus improved memory functioning accompanied by inhibition of AChE and upregulation of BDNF, suggesting that D. superbus may be a useful therapeutic agent for the prevention or treatment of Alzheimer’s disease.
Keywords: Dianthus superbus, Cognitive enhancing effect, Acetylcholinesterase, Brain-derived neurotropic factor
INTRODUCTION
Alzheimer’s disease (AD), the most common cause of dementia and neurodegenerative diseases, is characterized by progressive memory impairment (Crapper and DeBoni, 1978). Risk factors associated with AD include age, genetic inheritance (family history), and environmental factors.
The extracellular deposition of amyloid plaque deposits (Aβ), neurofibrillary tangles (NFT), and neuronal cell death are characteristic pathogenic features of AD (Collerton, 1986).
Cholinergic activity deficiencies have been shown to impair memory and cognitive function via acetylcholine (ACh) dysfunction. Acetylcholinestrase (AChE) is an enzyme that hydrolyzes ACh and increased AChE levels cause a lack of ACh (Coyle et al., 1983; Ballard, 2002).
Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family, regulates cell growth in the central nervous system (CNS). BDNF expression plays a crucial role in learning and memory promotion (Bekinschtein et al., 2008).
Dianthus superbus (D. superbus, Caryophyllaceae) is a traditional herbal medicine for the treatment of numerous conditions, including urethritis, carbuncles, and carcinomas, in China and Korea. Previous studies reported that D. superbus exerted antioxidant, antimicrobial, anticarcinogenic, and anti-inflammatory effects (Yu et al., 2007; Gou et al., 2011; Ding et al., 2013). Additionally, D. superbus suppressed immunoglobulin E (IgE) production and prevented peanut-induced anaphylaxis (Tong et al., 2012; Reid-Adam et al., 2013). D. superbus also stimulated immunosuppressive effects, osteoblastic proliferation, and cytotoxic activity against cancer cells (Lopez-Exposito et al., 2011; Shin et al., 2012; Yu et al., 2012). Studies of D. superbus revealed that the plant consists of dianthosaponins, dianthramide, flavonoid, coumarin, triterpenoid, pyran-type glycoside, and cyclic peptides (Shimizu et al., 1982; Wang et al., 1998; Hsieh et al., 2004; Hsieh et al., 2005; Dahiya et al., 2008; Chen et al., 2010; Luo et al., 2011).
However, the memory-enhancing effect of D. superbus has not been reported. This study uses the Morris water maze and passive avoidance behavior tests to describe the effect of D. superbus on scopolamine-induced memory impairment in the test mice. In addition, we investigated AChE activity and BDNF expression in the hippocampi of the test mice to elucidate the potential mechanism.
MATERIALS AND METHODS
Plant material and chemical materials
D. superbus was purchased from the Kyungdong traditional herbal market (Seoul, Korea) and authenticated by Dr. Young Bae Seo, a professor of the College of Oriental Medicine, Daejeon University, Korea. A voucher specimen (CJ004M) was deposited in the natural products laboratory of Kangwon National University.
Dried D. superbus plants were extracted 3 times with 80% methanol at room temperature by ultrasonication-assisted extraction and, then the extract was evaporated.
Scopolamine and carboxymethylcellulose sodium (CMC) were purchased from Sigma (St Luis, MO, USA). Donepezil was provided by Samjin Pharmaceutical Co., Ltd. (Seoul, Korea).
We isolated (E)-methyl-4-hydroxy-4-(8a-methyl-3-oxodecahydronaphthalen-4a-yl), diosmetin-7-O(2″,6″-di-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside, vanillic acid, 4-hydroxyphenyl acetic acid, 4-methoxyphenyl acetic acid, (E)-4-methoxycinnamic acid, 3-methoxy-4-hydroxyphenylethanol, and methyl hydroferulate from D. superbus and the purities of the eight standard compounds were above 98%.
Primary antibodies (β-actin and BDNF) and secondary antibodies (goat-anti-rabbit IgG HRP and goat-anti-mouse IgG HRP) were purchased from Santa Cruz Biotechnology, Inc (Dallas, USA).
Animals
Male ICR mice (5 weeks old, 25–30 g) were purchased from Daehan Biolink. Co., Ltd. (Chunkbuk, Korea). The mice were housed in groups of seven per cage at 20 ± 3°C under a 12/12-h light-dark cycle and were fed ad libitum (commercial pellet). All animal experimental procedures in this study were performed in accordance with the guidelines of Kangwon National University IACUC (KIACUC).
Drug administration
The mice were divided into six groups (n=7): control group, scopolamine group, positive control group (donepezil (1 m/kg) treatment), and three D. superbus extract groups (50, 100, and 200 mg/kg of D. superbus treatment). D. superbus extract and donepezil were dissolved in 0.5% CMC solution and were administered orally 90 min before scopolamine treatment. The control group received 0.5% CMC solution. Scopolamine (1 mg/kg) was dissolved in normal saline (0.9% NaCl) and delivered subcutaneously for all groups except the controls (normal saline) prior to the commencement of a 30 min Morris water maze test and a passive avoidance test. The mice were dosed over four consecutive days before undergoing daily trials in the Morris water maze test, but were dosed for only one day (training trial) before the passive avoidance test.
Morris water maze test
The water maze test was performed according to Morris’s description. The water maze is a large circular pool (90 cm diameter, 40 cm height) filled with water (20 ± 1°C) and white milk. The water maze area was divided into four equal quadrants, and a white escape platform (10 cm diameter, 26 cm height) was submerged 1 cm below the surface of the water in the center of one quadrant. The location of the escape platform remained unchanged, and the platform and starting point were never in the same quadrant during the four trial days. All of the properties of the swimming activities of the mice including swim time, distance, and speed were monitored and recorded by a Smart video camera (ver. 2.5.21) linked to a video-tracking system. After a mose found and reached the platform, the time was recorded as the escape latency. Mice were allowed 60 s in the water maze without the platform in a test trial on the first day. The mice were given four trial sessions per day over four consecutive days, and the interval between each trial session was one day. If a mouse could not find the platform within 120 s, the trial session was stopped and the escape latency was recorded as 120 s. On the final day, the platform was removed and the mice were subjected to a probe trial for 60 s. The time spent in the quadrant where the platform was located was recorded to assess the spatial memories of the mice.
Passive avoidance test
The passive avoidance test was carried out using two equally sized compartments (17 cm×12 cm×10 cm) with an electrifiable grid floor and two compartments were separated by a guillotine door. An acquisition trial was performed on the first day. The mouse was initially placed in the light compartment. The door between the two compartments was opened after 40 s. When the mouse moved into the dark compartment, the door automatically closed. At the 24 h mark after the completion of the acquisition trial, the mouse was again placed in the light compartment and again the guillotine door was opened after 40 s. When the mouse moved into the dark compartment, the door automatically closed and an electric foot-shock (0.1 mA/10 g body weight) lasting for 2 s was delivered through the grid floor. After the training trial, the mouse was again placed in the light compartment after another 24 h had passed, and if the mouse did not enter the dark compartment within 180 s of the door being opened, the latency was recorded as 180 s. latency times to dark compartment were measured by using Gemini Avoidance System.
AChE inhibition assay
An AChE inhibition assay was performed according to the method described by Ellman. The brain tissue was immediately removed from the mouse within 30 min after the behavior test. The hippocampi were isolated from the brain tissues of the mice and rapidly homogenized with sodium phosphate buffer. The reaction mixture contained 33 μL of supernatant, 470 μL of phosphate buffer (pH 8.0), 167 μL of 5,5′-dithio-bis (2-nitrobenzoic acid) (DTNB) (3 mM), and 280 μL of acetylcholine iodide (ACh) (1 mM).
Inhibition of AChE enzyme of eight compounds isolated from D. superbus was measured by AChE enzyme. The reaction mixture was consisting of AChE, DTNB and ACh.
AChE activity was measured at 412 nm using a spectrophotometer.
Tissue preparation and western blot analysis
The hippocampus was promptly collected from the mouse’s brain within 30 min after the behavior test, and was homogenized in 200 μl of an ice-cold RIPA buffer containing a protease inhibitor cocktail. The dissolved proteins were centrifuged at 13,000 g for 20 min, and the supernants were stored at −80°C. The supernant containing 40 μg of total protein was separated by 15% SDS-PAGE gel and transferred to a PVDF membrane. After blocking, the membrane was incubated with primary antibodies (1:2000 dilution of β-actin and 1:1000 dilution of BDNF) and secondary antibodies (goat-anti-rabbit IgG HRP 1:2000 for BDNF and goat-anti-mouse IgG HRP 1:2000 for β-actin). Detection was performed using an ECL solution.
HPLC analysis of D. superbus extract
The HPLC analysis of eight compounds, (E)-methyl-4- hydroxy-4-(8a-methyl-3-oxodecahydronaphthalen-4a-yl), diosmetin-7-O(2″,6″-di-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside, vanillic acid, 4-hydroxyphenyl acetic acid, 4-methoxyphenyl acetic acid, (E)-4-methoxycinnamic acid, 3-methoxy-4-hydroxyphenylethanol, and methyl hydroferulate was performed on Dionex system with LPG 3X00 pump, ACC-3000 auto sampler, column oven, and DAD-3000(RS) diode array UV/VIS detector. Separation was conducted using a Shiseido C18 column (4.6 mmI.D. × 250 mm, 5 μm pore size) at 30°C. The mobile phase consisted of 0.1% trifluoroacetic acid (TFA) aqueous solution (A) and acetonitrile (B). The gradient program was optimized as follows: 15% B at 0–10 min, 15–25% B at 10–15 min, 25–70% B at 15–40 min, 70% B at 40–45 min. The flow rate of mobile phase was at 1.0 ml/min and the injection volume was 20 μL. Compounds was detected was set at 205 and 280 nm of UV wavelength.
Statistical analysis
All results are expressed as mean ± SEM. The mean distance, swimming speed, and time spent in the target quadrant of the probe test in the Morris water maze test and the latency time recorded in the passive avoidance test were statistically analyzed by a one-way analysis of variance (ANOVA), followed by Tukey’s post-hoc test. The escape latency from the Morris water maze test was statistically analyzed by a two-way ANOVA followed by the Newman-Keuls post-hoc test. A value of p<0.05 was considered statistically significant.
RESULTS
D. superbus extract improved spatial memory against scopolamine-induced memory impairment in the Morris water maze test
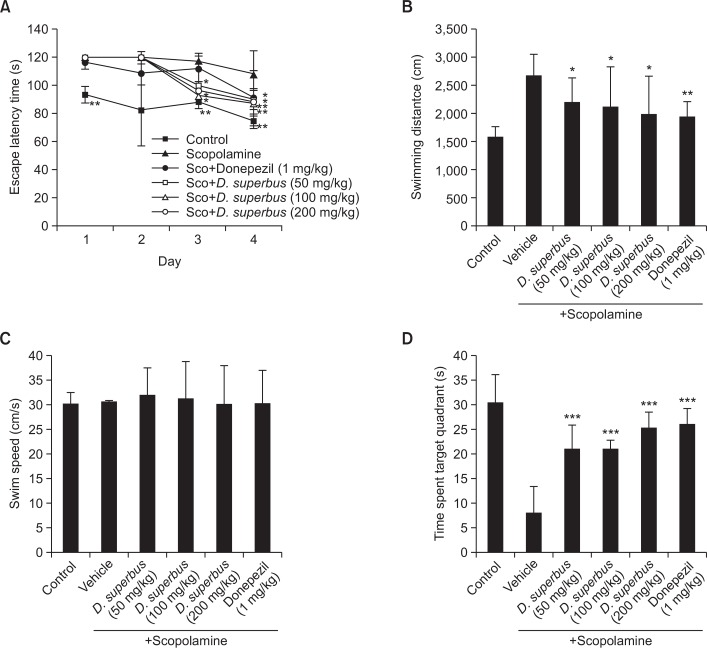
To evaluate the memory-enhancive effect of D. superbus extract, we performed the Morris water maze test which assessed spatial memory and learning. The escape latency during the four trial days is shown in Fig. 1A. The escape latency continually and significantly decreased over the four trial days in the control group. However, the escape latency was longer in the scopolamine-treated group compared with the control group. This result revealed the effect of scopolamine-induced memory impairment. A two-way ANOVA was used to reveal a significant effect from an interaction between treatment [F (5, 154)=9.02, p<0.001] and day [F (3, 154)=8.91, p<0.001] and no significant effect from an interaction between treatment and day [F (15, 154)=0.78, p>0.05] in the Morris water maze test. Scopolamine-induced memory impairment was attenuated in the D. superbus extract-treated group.
Fig. 1.
(A) Effect of D. superbus extract on the escape latency of scopolamine-treated mice in the Morris water maze test. D. superbus extract (50, 100 and 200 mg/kg body weight, P.O) and Donepezil (1 mg/kg body weight, P.O.) are treated 90 min before memory impairment by scopolamine. The escape latency of each group during the training-session trials is presented. (B) Mean distance and (C) swimming speed for finding the platform during 4 days. d) Effect of D. superbus extract in the probe trial. The time spent in the target quadrant during the probe trial is presented. The data are expressed as the mean escape latency ± SD (n=7). (*p<0.05, **p<0.01, and ***p<0.001 versus scopolamine-treated mice). Sco: scopolamine.
In the treated group on the third and fourth days, 50, 100, and 200 mg/kg of D. superbus extract significantly shortened the escape latency compared with the scopolamine-treated group (p<0.05). Moreover, the escape latency was also significantly shortened for the donepezil-treated group on the fourth day. As shown in Fig. 1B, D. superbus extract significantly shortened the increased distance that the scopolamine-treated group swam to reach the platform during 4 days (p<0.05).
In the probe test, the time spent in the target quadrant is shown in Fig. 1D. The control group spent a longer duration in the target quadrant than the scopolamine-treated group. The D. superbus extract-treated group spent a significantly shorter time in the target quadrant than the scopolamine-treated group. Moreover, the effect of 200 mg/kg of D. superbus extract in the probe trial was similar to that of donepezil.
The mean swimming speed of the mice in the water maze test was not significantly different among all groups (Fig. 1C), suggesting that the effects of scopolamine, donepezil, and D. superbus extract were not due to the locomotor activity of the mice.
D. superbus extract attenuated scopolamine-induced memory impairment in the passive avoidance test
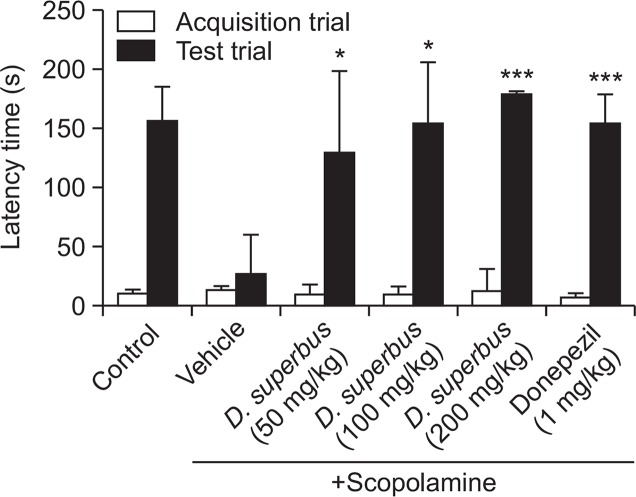
The effect of D. superbus extract on emotional learning and memory was evaluated in the passive avoidance test. The latency of the scopolamine-treated group was significantly shorter than that of the control group in the test trial (Fig. 2). Treatment with 50, 100, and 200 mg/kg D. superbus extract significantly attenuated scopolamine-induced memory impairment. In addition, D. superbus extract (200 mg/kg)-treated mice exhibited a longer latency than the donepezil-treated group.
Fig. 2.
Effect of D. superbus extract on scopolamine-induced memory impairment in the passive avoidance test. The latency time to enter the dark compartment was recorded. The data are expressed as mean latency time (s) ± SD (n=7) *p<0.05, ***p<0.001 compared with the scopolamine group.
There was no significant difference in the latency time between the groups during the acquisition trial of the passive avoidance test. However, a significant difference in latency time was observed in the test trial. This result indicated that D. superbus extract attenuated scopolamine-induced memory impairment without affecting the locomotor and exploratory activity of the mice.
D. superbus extract inhibited AChE activity in the hippocampus
The effect of D. superbus extract on AChE activity in the hippocampus was investigated. Treatment with scopolamine increased AChE activity compared to that of the control group, whereas D. superbus extract significantly inhibited AChE activity in a dose-dependent manner (p<0.05) (Fig. 3).
Fig. 3.
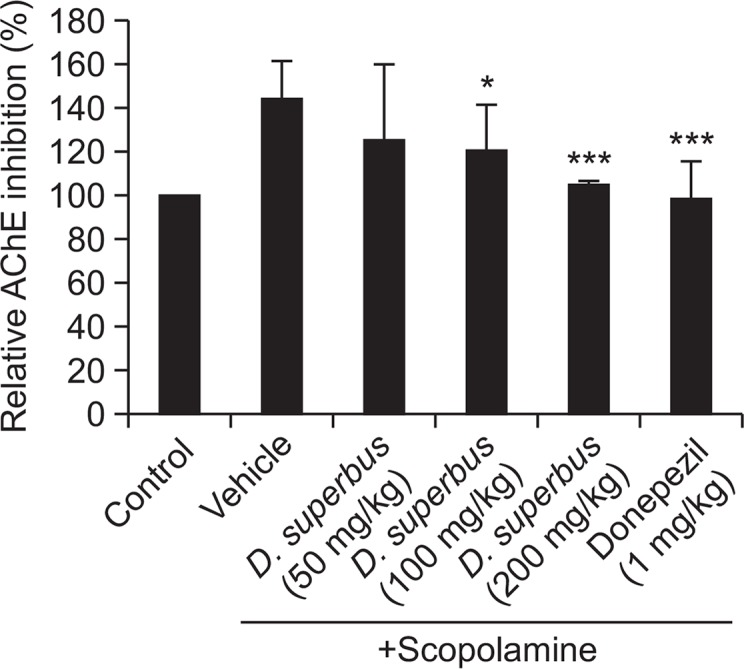
Effect of D. superbus extract on acetylcholinesterase (AChE) activity in the hippocampi of the mice. Data represent the mean ± SD. *p<0.05, ***p<0.001 compared with the Scopolamine-treated group (n=3).
We determined effect of eight compounds isolated from D. superbus using a spectrophotometric method, as described by Ellman et al. Among 8 compounds, 4-hydroxyphenyl acetic acid (IC50=2024.29 ± 0.13 μM) has exhibited AChE activity inhibition and other compounds not showed inhibitory effect.
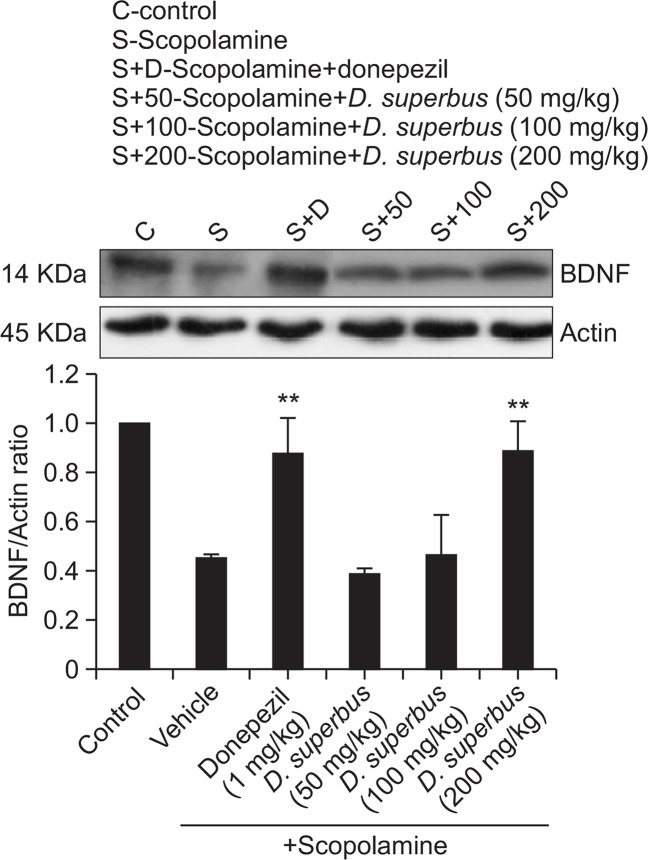
D. superbus extract increased the BDNF level in the hippocampus
BDNF plays a critical role in neuronal plasticity for the long-term memory of certain neurons in the CNS. As shown in Fig. 4, we observed that the expression of BDNF was decreased after exposure to scopolamine. BDNF levels in the hippocampus were significantly increased in D. superbus extract (200 mg/kg)-treated mice compared with scopolamine-treated mice.
Fig. 4.
The effect of D. superbus extract on BDNF expression in the hippocampi of the mice using a western blot analysis. Data represent the mean ± SD. **p<0.01 compared with the Scopolamine-treated group (n=3).
Identification of compounds of D. superbus extract
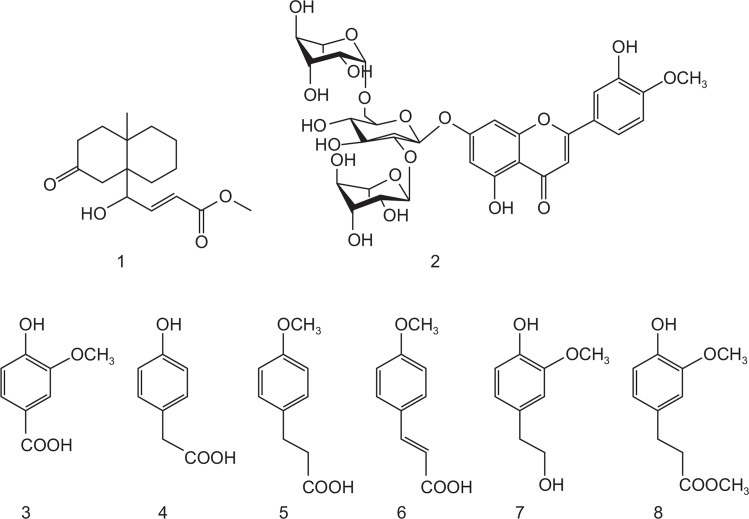
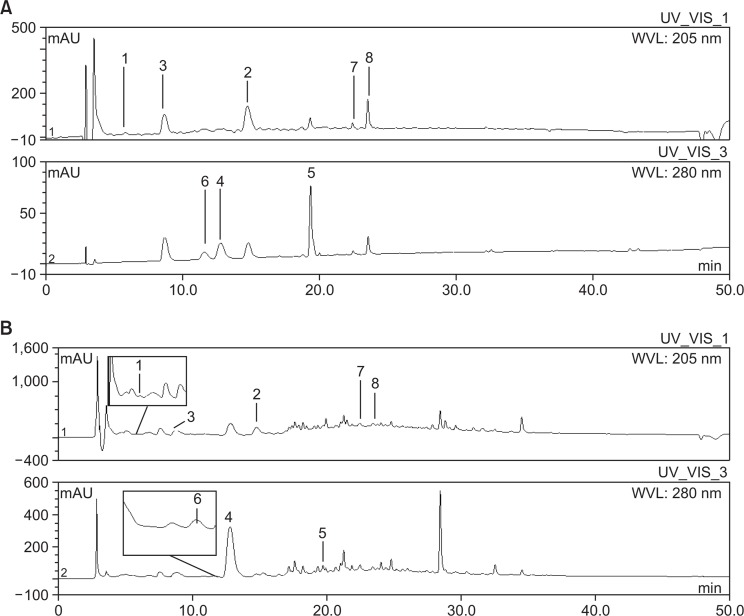
Eight compounds, (E)-methyl-4-hydroxy-4-(8a-methyl-3-oxode cahydronaphthalen-4a-yl), diosmetin-7-O(2″,6″-di-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside, vanillic acid, 4-hydroxyphenyl acetic acid, 4-methoxyphenyl acetic acid, (E)-4-methoxycinnamic acid, 3-methoxy-4-hydroxyphenylethanol, and methyl hydroferulate were identified in the D. superbus extract by HPLC-DAD analysis (Fig. 5). HPLC chromatogram of compounds and D. superbus extract is shown Fig. 6. The content of 4-hydroxyphenyl acetic acid was 84.12 ± 0.08 μg/mg which was higher than other compounds. On the other hand, 4-methoxypheny acetic acid (1.27 ± 0.02 μg/mg) and methyl hydroferulate (1.58 ± 0.09 μg/mg) were found in the lowest content in the D. superbus extract (Table 1).
Fig. 5.
Chemical structures of eight compounds. (E)-methyl-4-hydroxy-4-(8a-methyl-3-oxodecahydronaphthalen-4a-yl) [1], diosmetin-7-O(2″,6″-di-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside [2], vanillic acid [3], 4-hydroxyphenyl acetic acid [4], 4-methoxyphenyl acetic acid [5], (E)-4-methoxycinnamic acid [6], 3-methoxy-4-hydroxyphenylethanol [7], and methyl hydroferulate [8].
Fig. 6.
HPLC Chromatograms of eight standard compounds from D. superbus (A) and D. superbus extract (B). (E)-methyl-4-hydroxy-4-(8amethyl-3-oxodecahydronaphthalen-4a-yl) [1], diosmetin-7-O(2″,6″-di-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside [2], vanillic acid [3], 4-hydroxyphenyl acetic acid [4], 4-methoxyphenyl acetic acid [5], (E)-4-methoxycinnamic acid [6], 3-methoxy-4-hydroxyphenylethanol [7], and methyl hydroferulate [8].
Table 1.
Retention time and contents of 8 compounds in D. superbus. (E)-Methyl-4-hydroxy-4-(8a-methyl-3-oxodecahydronaphthalen-4a-yl)but-2-enoate (1), chrysoeriol-5-methylether-7-O(2″,6″-di-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside (2), vanillic acid (3), 4-hydroxy-phenyl acetic acid (4), 4-methoxyphenyl acetic acid (5), (E)-4-methoxycinnamic acid (6), 3-methoxy-4-hydroxyphenylethanol (7), and methyl hydroferulate (8).
| Compounds | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Retention time (S) | 5.830 | 14.750 | 8.713 | 12.767 | 19.967 | 11.600 | 22.413 | 23.530 |
| Content (μg/mg) | 14.17 ± 0.76 | 7.69 ± 0.00 | 5.32 ± 0.04 | 84.12 ± 0.08 | 1.27 ± 0.02 | 3.51 ± 0.54 | 0.81 ± 0.00 | 1.58 ± 0.09 |
DISCUSSION
The present study demonstrated for the first time that D. superbus extract ameliorated learning and memory deficits induced by scopolamine in the Morris water maze and passive avoidance tests. Scopolamine, a nonselective and competitive muscarinic cholinergic receptor antagonist, blocks the cholinergic signaling and impairs learning and memory including long-term and short-term memory (Flood and Cherkin, 1986). The scopolamine model was widely used in mouse-behavior tests that assessed cognition.
The Morris water maze test is designed to assess spatial learning and memory (Morris, 1984), and has been linked to long-term potentiation (LTP). Spatial learning and memory were investigated through repeated trial tests over four days, and the time spent in the target quadrant in the probe test indicated LTP. D. superbus extract attenuated scopolamine-induced memory impairment in the Morris water maze test. Administration of D. superbus extract exhibited a similar effect compared with that of the donepezil (positive control)-treated group.
The passive avoidance test is a fear-motivated avoidance test to evaluate long-term memory, or reference memory (O’Keefe and Nadel, 1978). The test is employed to describe the way in which the animal learns to avoid an aversive stimulus (electronic foot-shock). In the passive avoidance test, the treatment of D. superbus extract ameliorated the scopolamine-induced memory deficit and may be associated with long-term memory improvement.
ACh is an important neurotransmitter for learning and memory. In the CNS, decreasing ACh levels were responsible for memory deficits in neurodegenerative diseases; thus, a high level of AChE reduced ACh levels, as observed in the brains of AD patients (Lewis and Shute, 1964; Blokland, 1995). Recently, AD treatment focused on the inhibition of AChE activity. AChE inhibitors, including donepezil, galantamine, and tacrine, can attenuate memory impairment by inhibiting the destruction of ACh (McGleenon et al., 1999; Ballard, 2002).
Scopolamine causes memory dysfunction in brain tissue by increasing AChE activity, so we therefore investigated the AChE-inhibitory effect of D. superbus extract. In the AChE activity assay, D. superbus extract inhibited AChE activity in the hippocampus in a dose-dependent manner.
These results indicated that D. superbus extract ameliorated the scopolamine-induced memory deficit by increasing cholinergic activity through the inhibition of AChE activity.
By activating neurotrophin, the BDNF plays an essential role in memory processes and contributes to LTP formation at the hippocampal and cortical synapse (Panja and Bramham, 2014). The brain of a patient with AD shows down-regulation of BDNF gene expression. BDNF expression can be increased by neuronal activity and regulates various forms of synaptic plasticity (Bramham and Messaoudi, 2005).
We investigated the effect of D. superbus extract on BDNF expression in scopolamine-treated mice using a western blot analysis. Scopolamine decreased BDNF expression in mice. We confirmed that D. superbus extract reversed the scopolamine-induced reduction of BDNF expression in the hippocampi of the mice. These results indicated that the cognitive-enhancing effect of D. superbus extract in the behavioral tests was correlated with activation of the BDNF.
We analyzed eight compounds, (E)-methyl-4-hydroxy-4-(8amethyl-3-oxodecahydronaphthalen-4a-yl), diosmetin-7-O(2″, 6″-di-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside, vanillic acid, 4-hydroxyphenyl acetic acid, 4-methoxyphenyl acetic acid, (E)-4-methoxycinnamic acid, 3-methoxy-4-hydroxyphenylethanol, and methyl hydroferulate from D. superbus to confirm bioactivity compounds of D. superbus extract. 4-hydroxyphenyl acetic acid content was high in the D. superbus extract compared with other compounds. We determined effect of 4-hydroxyphenyl acetic acid on AChE activity. 4-hydroxyphenyl acetic acid may have contributed to AChE activity inhibition of D. superbus.
In conclusion, we found that D. superbus extract ameliorated scopolamine-induced memory deficits in mice in the Morris water maze and passive avoidance tests. D. superbus extract also inhibited AChE activity and improved BDNF expression. The present study suggests that D. superbus extract may be a potential therapeutic agent in preventive AD treatment; however, further study should be conducted to understand the mechanism of the cognitive-enhancing effect of D. superbus extract.
Acknowledgments
This research was supported by a Basic Science Research Program grant from the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0005149).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- Ballard CG. Advances in the treatment of Alzheimer’s disease: benefits of dual cholinesterase inhibition. Eur Neurol. 2002;47:64–70. doi: 10.1159/000047952. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Izquierdo I, Medina JH. BDNF and memory formation and storage. Neuroscientist. 2008;14:147–156. doi: 10.1177/1073858407305850. [DOI] [PubMed] [Google Scholar]
- Blokland A. Acetylcholine: A neurotransmitter for learning and memory? Brain Res Brain Res Rev. 1995;21:285–300. doi: 10.1016/0165-0173(95)00016-X. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Chen X, Luo JG, Kong LY. Two new triterpenoid saponins from Dianthus superbus L. J Asian Nat Prod Res. 2010;12:458–463. doi: 10.1080/10286020.2010.493326. [DOI] [PubMed] [Google Scholar]
- Collerton D. Cholinergic function and intellectual decline in Alzheimer’s disease. Neuroscience. 1986;19:1–28. doi: 10.1016/0306-4522(86)90002-3. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Price DL, DeLong MR. Alzheimer’s disease: a disorder of cortical cholinergic innervation. Science. 1983;219:1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- Crapper DR, DeBoni U. Brain aging and Alzheimer’s disease. Can Psychiatr Assoc J. 1978;23:229–233. doi: 10.1177/070674377802300406. [DOI] [PubMed] [Google Scholar]
- Dahiya R. Synthesis and biological activity of a cyclic hexapeptide from Dianthus superbus. Chem Pap. 2008;62:527–535. doi: 10.2478/s11696-008-0052-9. [DOI] [Google Scholar]
- Ding C, Zhang W, Li J, Lei J, Yu J. Cytotoxic constituents of ethyl acetate fraction from Dianthus superbus. Nat Prod Res. 2013;27:1691–1694. doi: 10.1080/14786419.2012.763127. [DOI] [PubMed] [Google Scholar]
- Flood JF, Cherkin A. Scopolamine effects on memory retention in mice: a model of dementia? Behav Neural Biol. 1986;45:169–184. doi: 10.1016/S0163-1047(86)90750-8. [DOI] [PubMed] [Google Scholar]
- Gou J, Zou Y, Ahn J. Enhancement of antioxidant and antimicrobial activities of Dianthus superbus, Polygonum aviculare, Sophora flavescens, and Lygodium japonicum by pressure-assisted water extraction. Food Sci Biotechnol. 2011;20:283–287. doi: 10.1007/s10068-011-0040-7. [DOI] [Google Scholar]
- Hsieh PW, Chang FR, Wu CC, Wu KY, Li CM, Chen SL, Wu YC. New cytotoxic cyclic peptides and dianthramide from Dianthus superbus. J Nat Prod. 2004;67:1522–1527. doi: 10.1021/np040036v. [DOI] [PubMed] [Google Scholar]
- Hsieh PW, Chang FR, Wu CC, Li CM, Wu KY, Chen SL. Longicalycinin A, a new cytotoxic cyclic peptide from Dianthus superbus var. longicalycinus (MAXIM.) WILL. Chem Pharm Bull. 2005;53:336–338. doi: 10.1248/cpb.53.336. [DOI] [PubMed] [Google Scholar]
- Lewis PR, Shute CC, Silver A. Confirmation from choline acetylase analyses of a massive cholinergic innervation to the rat hippocampus. J Physiol. 1967;191:215–224. doi: 10.1113/jphysiol.1967.sp008246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Exposito I, Castillo A, Yang N, Liang B, Li XM. Chinese herbal extracts of Rubia cordifolia and Dianthus superbus suppress IgE production and prevent peanut-induced anaphylaxis. Chin Med. 2011;16:35–44. doi: 10.1186/1749-8546-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo JG, Chen XC, Kong LY. Three new triterpenoid saponins from Dianthus superbus. Chem Pharm Bull. 2011;59:518–521. doi: 10.1248/cpb.59.518. [DOI] [PubMed] [Google Scholar]
- McGleenon BM, Dynan KB, Passmore AP. Acetylcholinesterase inhibitors in Alzheimer’s disease. Br J Clin Pharmacol. 1999;48:471–480. doi: 10.1046/j.1365-2125.1999.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. Clarendon Press; Oxford: 1978. [Google Scholar]
- Reid-Adam J, Yang N, Song Y, Cravedi P, Li XM, Heeger P. Immunosuppressive effects of the traditional Chinese herb qu mai on human alloreactive T cells. Am J Transplant. 2013;13:1159–1167. doi: 10.1111/ajt.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panja D, Bramham CR. BDNF mechanisms in late LTP formation: A synthesis and breakdown. Neuropharmacology. 2014;76:664–676. doi: 10.1016/j.neuropharm.2013.06.024. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Hayashi T, Shimizu K, Morita N. A pyran-type glycoside from Dianthus superbus var. longicalycinus. Phytochemistry. 1982;21:245–247. doi: 10.1016/0031-9422(82)80063-0. [DOI] [Google Scholar]
- Shin IS, Lee MY, Ha H, Jeon WY, Seo CS, Shin HK. Dianthus superbus fructus suppresses airway inflammation by downregulating of inducible nitric oxide synthase in an ovalbumin-induced murine model of asthma. J. Inflamm. (Lond) 2012;9:41–49. doi: 10.1186/1476-9255-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Luo JG, Wang R, Wang XB, Kong LY. New cyclic peptides with osteoblastic proliferative activity from Dianthus superbus. Bioorg Med Chem Lett. 2012;22:1908–1911. doi: 10.1016/j.bmcl.2012.01.058. [DOI] [PubMed] [Google Scholar]
- Wang YC, Tan NH, Zhou J, Wu H. Cyclopeptides from Dianthus superbus. Phytochemistry. 1998;49:1453–1456. doi: 10.1016/S0031-9422(97)00857-1. [DOI] [Google Scholar]
- Yu JO, Liao ZX, Lei JC, Hu XM. Antioxidant and cytotoxic activities of various fractions of ethanol extract of Dianthus superbus. Food Chem. 2007;104:1215–1219. doi: 10.1016/j.foodchem.2007.01.039. [DOI] [Google Scholar]
- Yu JQ, Yin Y, Lei JC, Zhang XQ, Chen W, Ding CL, Wu S, He XY, Liu YW, Zou GL. Activation of apoptosis by ethyl acetate fraction of ethanol extract of Dianthus superbus in HepG2 cell line. Cancer Epidemiol. 2012;36:e40–e45. doi: 10.1016/j.canep.2011.09.004. [DOI] [PubMed] [Google Scholar]