Abstract
Mitochondria-targeted vitamin E (MVE) is designed to accumulate within mitochondria and is applied to decrease mitochondrial oxidative damage. However, the protective effects of MVE in skin cells have not been identified. We investigated the protective effect of MVE against UVB in dermal fibroblasts and immortalized human keratinocyte cell line (HaCaT). In addition, we studied the wound-healing effect of MVE in animal models. We found that MVE increased the proliferation and survival of fibroblasts at low concentration (i.e., nM ranges). In addition, MVE increased collagen production and downregulated matrix metalloproteinase1. MVE also increased the proliferation and survival of HaCaT cells. UVB increased reactive oxygen species (ROS) production in fibroblasts and HaCaT cells, while MVE decreased ROS production at low concentration. In an animal experiment, MVE accelerated wound healing from laser-induced skin damage. These results collectively suggest that low dose MVE protects skin from UVB irradiation. Therefore, MVE can be developed as a cosmetic raw material.
Keywords: Mitochondria-Targeted vitamin E, UVB protection, Fibroblast, HaCaT, Collagen
INTRODUCTION
Mitochondria are organelles important for both life and death (Newmeyer and Ferguson-Miller, 2003). Mitochondria are major sources of cellular energy and are central to processes resulting in apoptosis induction. The exact mechanism of apoptosis induction in mitochondria is not clarified in detail, but ‘mitocans’ are drugs known to induce cancer cell death by targeting mitochondria (Neuzil et al., 2006; Neuzil et al., 2007). Mitocans destabilize mitochondria, thereby causing cytosolic release of apoptosis modulators (Green and Reed, 1998). The indirect prooxidant activity of these agents in cancer cells is essential for their actions as anticancer agents (Neuzil et al., 2007; Dong et al., 2011). For example, mitochondria-targeted vitamin E (MVE) modulates the expression of mitochondrial DNA transcripts and mitochondrial biogenesis. These changes subsequently result in arrest of cell proliferation (Truksa et al., 2015).
Mitochondria-targeted antioxidants comprise a triphenylphosphonium cation coupled to coenzyme Q or a vitamin E derivative (Smith et al., 2003). These compounds can be safely fed to mice over long periods and are distributed within the heart, brain, liver, and muscle. Mitochondria-targeted bioactive molecules can be administered and used to affect mitochondrial dysfunction in those tissues. MVE is designed to accumulate within mitochondria and to decrease mitochondrial oxidative damage to inhibit a range of human disorders, including neurodegenerative diseases, ischemia-reperfusion injury, and aging-associated dysfunctions (Mao et al., 2010; Rocha et al., 2010; Mao et al., 2011; Ajith and Jayakumar, 2014). Mao et al reported that MVE affected subsarcolemmal mitochondrial density and systemic oxidative stress parameters such as plasma SOD activity and urinary isoprostane concentration (Mao et al., 2011). They also found that oral administration of MVE attenuated hepatic oxidative stress and inhibited fat deposition in mice (Mao et al., 2010).
MVE is concentrated in mitochondria, where it has controversial effects (i.e., antioxidant vs. prooxidant). MVE exhibited protective effects in hepatic cells, but harmful effects in cancer cells. However, the protective effects of MVE in skin cells have not been identified. We investigated the protective effect of MVE against UVB in dermal fibroblasts and immortalized human keratinocyte cell line (HaCaT). In addition, we studied the wound healing effect of MVE in animal experiments.
MATERIALS AND METHODS
Synthesis of MVE
MVE was synthesized as described previously (Mao et al., 2010; Mao et al., 2011). Synthesis of MVE is briefly described here. Trimethylhydroquinone was reacted with myrcene in the presence of (+)-10-camphorsulfonic acid to provide chroman-6-ol, which was protected as the acetate. Oxidative cleavage of the olefin (cat. OsO4, NMO followed by NaIO4), reduction (NaBH4), and deprotection (K2CO3, MeOH) led to the alcohol, which was converted to an iodide. Treatment of the iodide with triphenylphosphine furnished MVE.
Cell culture
Human dermal fibroblasts and spontaneously immortalized keratinocyte cell line (HaCaT) were cultured using DMEM (low, high glucose, Hyclone, Thermo Scientific, Logan, UT, USA) with 10% FBS (Gibco, Invitrogen, Carlsbad, CA, USA), 1% penicillin, and streptomycin (Gibco) at 37°C with 5% CO2 in a humidified atmosphere (Kim et al., 2009). HDF and HaCaT were exchanged in fresh media every two days.
Proliferation assay and viability
HDFs (3×104/well) and HaCaT (4×104/well) were seeded in 6-well plates. After starvation, cells were treated with various concentrations of MVE, and cell proliferation was measured. Cells were then incubated for 48 h and the MTT assay was performed. MTT solution (5 mg/ml in PBS) was added to each well at 1/20 of the media volume, incubated for 2 h, and then supernatant was removed. Dimethyl sulfoxide (DMSO) was then added to dissolve formazan crystals, and the absorbance was measured at 595 nm using an ELISA reader (TECAN, Grodig, Austria).
For the measurement of UVB protective effect of MVE, 200 mJ UVB was irradiated for HDFs and 400 mJ for HaCaT cells. After starvation, the cells were treated with various concentrations of MVE in the presence or absence of UVB-irradiation. Then, the MTT assay was performed as described above.
Cellular and mitochondrial ROS generation assay
Cellular ROS generation was measured using 2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA, Molecular Probes, Eugene, OR, USA), as described previously (Hye Kim et al., 2015). Similarly, mitochondrial ROS (mtROS) generation was measured using Mito-Sox (Molecular Probes) (Hye Kim et al., 2015). Cells were seeded in 6-well plates in 0.2% FBS and cultured overnight. After starvation, MVE (100 nM) was added with or without DCF-DA (20 μM) or Mito-Sox (5 μM). Each well was imaged every 10 min for 40 min under standard incubation conditions using an IncuCyteTM ZOOM microscope placed inside an incubator. Image-based analysis of fluorescence intensity was carried out using IncuCyteTM software (Essen Bioscience, MI, USA).
Western blot analysis
Cells (2×105 cells/ml) were seeded in a 60 mm dish and cultured to 80% confluence. After starvation, cells were treated with MVE. Cells were then lysed with 1xRIPA buffer (50 mM Tris-HCl, 0.15 M NaCl, 1 mM EDTA, 1% Triton-X100, pH 7.4, 1% SDS, 50 mM NaF, 1 mM Na3VO4, 5 mM Dithiothreitol, 1 mg/ml Leupeptin, and 1mM phenylmethylsulfonyl fluoride). Sample protein (40 μg) was separated in 10–12% SDS-polyacrylamide gels by electrophoresis. Proteins were transferred to PVDF membranes and incubated with antibodies to collagen (1:1000 rabbit source, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and matrix metalloproteinase 1 (MMP1, 1:1000 rabbit source, Santa Cruz Biotechnology). Membranes were then washed and incubated with horseradish peroxidase-conjugated anti-rabbit IgG antibody (Santa Cruz Biotechnology). Blots were reacted with western reagent (ECL; Millipore Billerica, Ann Arbor, MA, USA) and exposed to X-ray film.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA of HDFs and HaCaT was extracted with Trizol reagent followed by reverse transcription to cDNA. The following oligonucleotides were used as primers: collagen type I (50-TAGGGTCTAGACATGTTCAGCTTTGT-30 and 50-GTGATTGGTGGGATGTCTTCGT-30), MMP-1 (50-AGATGTGGAGTGCCTGATGT-30 and 50-AGCTAGGGTACATCAAAGCC-30), and control GAPDH (50-CGAGATCCCTCCAAAATCAA-30 and 50-TGTGGTCATGAGTCCTCCCA-30). PCR was carried out in a total volume of 30 μl for PCR amplification of cDNA that was reverse-transcribed from the total RNA. After initial denaturation at 95°C for 5 min, amplification was performed in 35 cycles for 30s at 95°C, 20 s at 54°C, and 30 s at 72°C. This was followed by a final extension at 72°C for another 10 min. GAPDH mRNA level was used for sample standardization.
Wound healing experiment
An in vivo experiment was performed on 16-week-old female hairless albino mice (Hos:HR-1). Mice were housed in temperature-controlled, special pathogen-free conditions with a 12 h light/dark cycle. They were allowed to freely access water and standard laboratory food. The Animal Care and Use Committee of Kangbuk Samsung Hospital monitored all animal experiments according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. A fractional CO2 laser (eCO2®, Lutronic Corp, Goyang, Korea) was used to create wounds on the backs of three mice. Mice backs were divided into four equal parts. Each 8×8 mm part was targeted with a 300 tip at 30 W average power and 50 mJ pulse energy for four passes, resulting in 99.2% coverage. After lasing, each area was treated with a DMSO solution of MVE (1, 10, 100 μM concentration). MVE was applied every other day and the longest length and width of each wound was measured.
Statistical analysis
Statistical significance was determined using a Wilcoxon signed-rank test or a Student’s t-test. p<0.05 was considered statistically significant. Statistical analysis was performed using SPSS 18.0 (SPSS, IBM Corp, Armonk, NY, USA).
RESULTS
MVE protected dermal fibroblasts from UVB
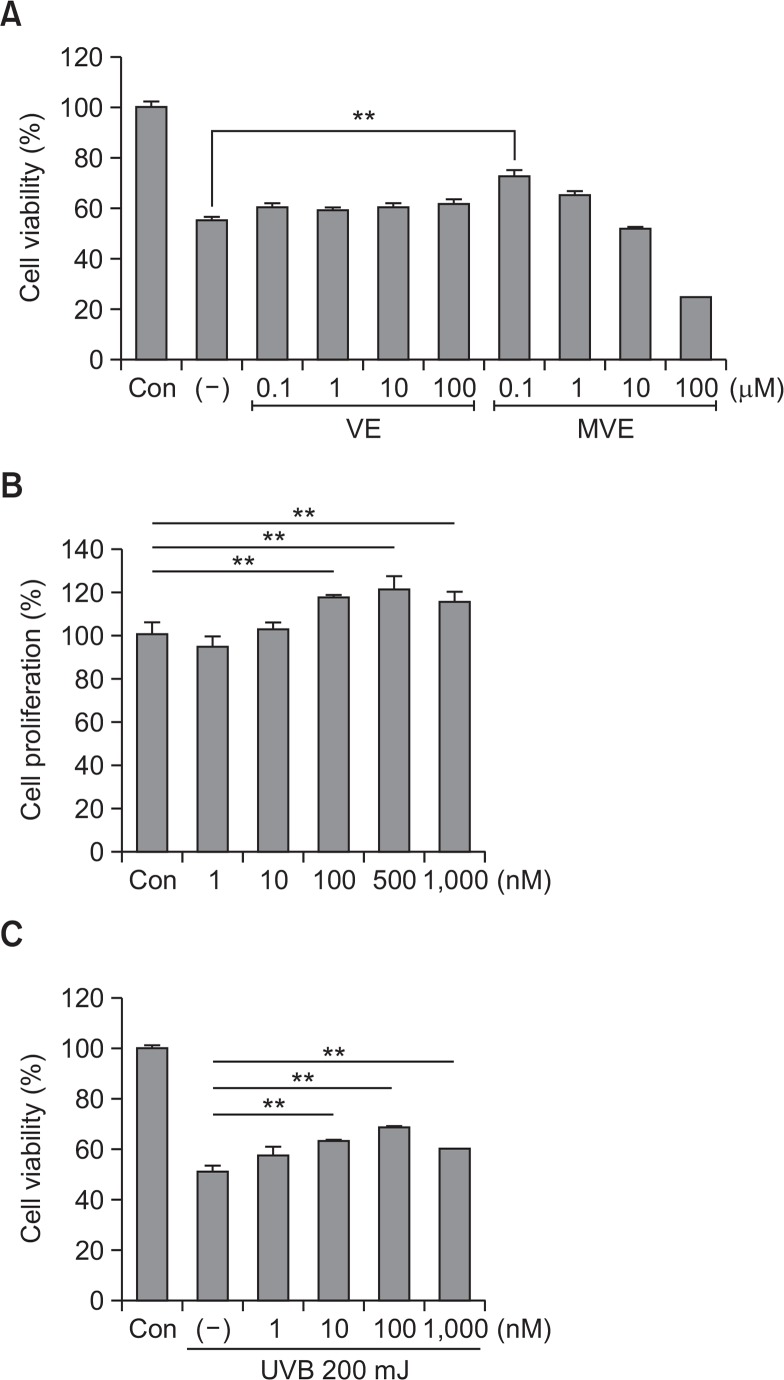
Because vitamin E reportedly protects skin in low μM ranges, the photoprotective effects of vitamin E and MVE were tested in the range of 0.1–100 μM in dermal fibroblasts (Farriol et al., 1994; Offord et al., 2002). Vitamin E slightly increased survival of dermal fibroblasts from UVB irradiation (200 mJ) in a concentration-dependent manner. However, MVE decreased fibroblast survival in the 10–100 μM concentration (Fig. 1A). Therefore, the protective effect of MVE was also studied in nM concentration ranges. As expected, MVE increased fibroblast proliferation in the 100–1000 nM range (Fig. 1B, p<0.01). In addition, MVE protected fibroblasts from UVB in the 10–1000 nM range (Fig. 1C, p<0.01). These results indicate that MVE protects fibroblasts from UVB and is more effective that vitamin E.
Fig. 1.
Mitochondria-targeted vitamin E (MVE) protected dermal fibroblasts from UVB. (A) Vitamin E (VE) slightly increased dermal fibroblast survival with UVB irradiation (200 mJ) in a concentration-dependent manner. However, MVE decreased dermal fibroblast survival at 10∼100 μM concentration (Fig. 1A). (B) MVE also increased fibroblast proliferation at 100–1000 nM concentration (Fig. 1B). (C) MVE protected dermal fibroblasts from UVB at 10–1000 nM concentration. n=3, **p<0.01.
MVE altered expression of extracellular matrix proteins
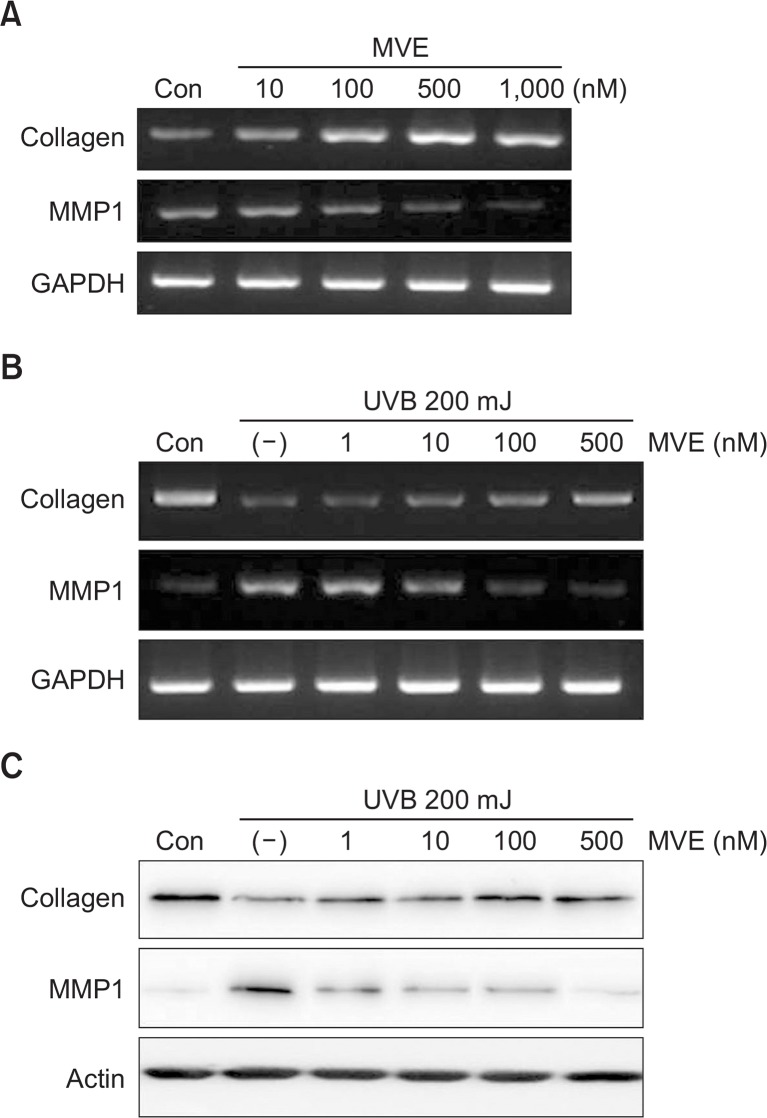
We further examined whether MVE altered the mRNA or protein levels of extracellular matrix (ECM) proteins, such as collagen type I and matrix metalloproteinase 1 (MMP1), in fibroblasts. MVE increased collagen mRNA expression and down-regulated that of MMP1 in dermal fibroblasts (Fig. 2A). The mRNA level of collagen was reduced by UVB irradiation, but attenuated by MVE treatment (Fig. 2B). The mRNA level of MMP1 was increased by UVB, but reduced by MVE treatment (Fig. 2B). In addition, MVE altered the protein levels of collagen and MMP1 in fibroblasts (Fig. 2C).
Fig. 2.
MVE altered expression of extracellular matrix proteins. (A) MVE significantly increased mRNA expression of collagen and down-regulated that of matrix metalloproteinase 1 (MMP1) in dermal fibroblasts. (B) mRNA levels of collagen were reduced by UVB but attenuated by MVE treatment. mRNA levels of MMP1 were induced by UVB but reduced by MVE treatment. (C) MVE altered protein levels of collagen and MMP1 in fibroblasts.
MVE reduced production of reactive oxygen species
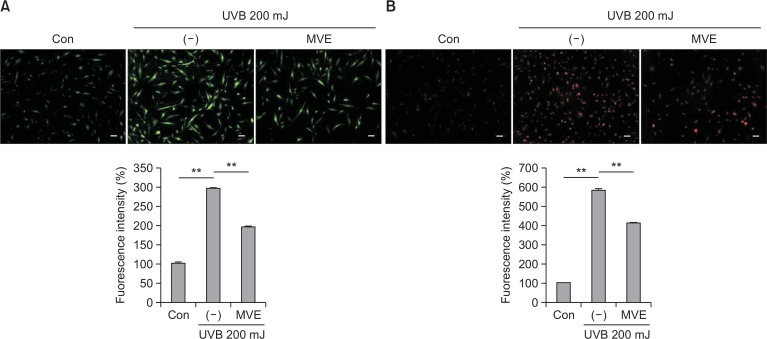
Because UVB reportedly generates reactive oxygen species (ROS) and induced apoptosis in skin, ROS levels were measured after MVE treatment. First, the cytosolic ROS level was measured using DCF-CA (green, Fig. 3A). UVB increased the fluorescent signal intensity of DCF-DA in fibroblasts, while MVE (1000 nM) attenuated the signal intensity of DCF-DA (p<0.01). Mitochondria ROS production was measured using mito-Sox (red, Fig. 3B). UVB increased the fluorescent signal intensity of mito-Sox in fibroblasts, while MVE (1,000 nM) reduced the signal intensity of mito-Sox (p<0.01). These results indicate that MVE protects dermal fibroblasts from UVB via reducing ROS generation.
Fig. 3.
MVE reduced production of reactive oxygen species (ROS). (A) Cytosolic ROS level was measured using DCF-CA (green). UVB increased the fluorescent signal intensity of DCF-DA in fibroblasts, while MVE (1000 nM) attenuated the signal intensity of DCF-DA. (B) Mitochondria ROS production was measured using mito-Sox (red). Likewise, UVB increased the fluorescent signal intensity of mito-Sox in fibroblasts, while MVE (1000 nM) reduced the signal intensity of mito-Sox. Scale bar = 100 μm, n=3, **p<0.01.
MVE protected HaCaT cells from UVB
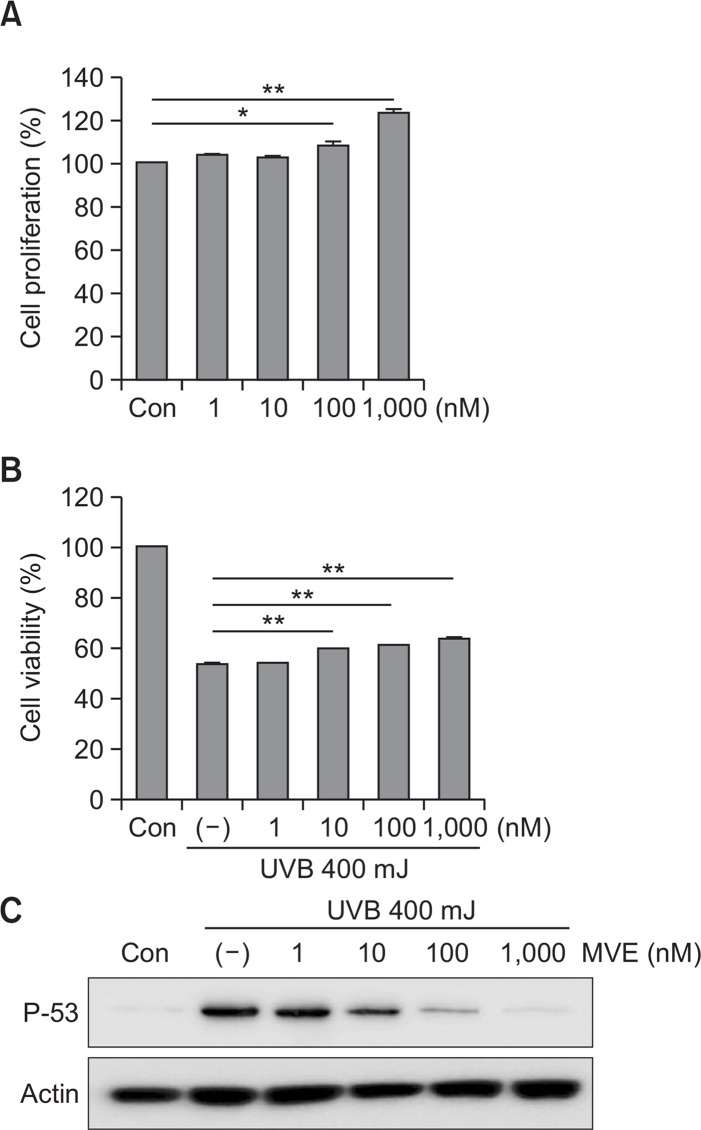
The protective effects of MVE in epidermal skin cells were also investigated using the HaCaT cell line. MVE significantly increased proliferation of HaCaT cells in a dose-dependent manner (Fig. 4A, p<0.05). In addition, MVE increased the UVB-reduced cell viability (Fig. 4B, p<0.01). UVB induced p53 protein levels in HaCaT cells, while MVE reduced p53 levels in HaCaT cells.
Fig. 4.
MVE protected HaCaT cells from UVB. (A) MVE significantly increased HaCaT cell proliferation in a dose-dependent manner. (B) MVE increased UVB-reduced cell viability. (C) UVB induced p53 protein levels in HaCaT cells, while MVE attenuated UVB-induced p53 levels in HaCaT cells. n=3, *p<0.05, **p<0.01.
MVE reduced production of reactive oxygen species
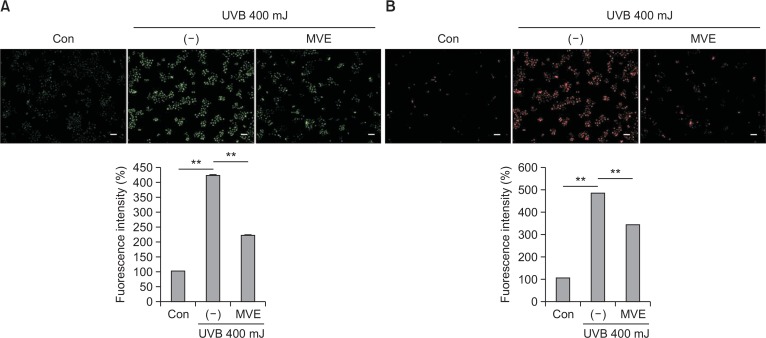
ROS levels were also measured after MVE treatment in HaCaT calls. The cytosolic ROS level was measured using DCF-CA. UVB increased the fluorescent signal intensity of DCF-DA in HaCaT cells, while MVE (1000 nM) attenuated the signal intensity of DCF-DA (Fig. 5A, p<0.01). Mitochondrial ROS production was also measured using mito-Sox. UVB increased the fluorescent signal intensity of mito-Sox in HaCaT cells, while MVE (1000 nM) reduced the signal intensity of mito-Sox (Fig. 5B, p<0.01). These results indicate that MVE protects dermal fibroblasts from UVB via reducing ROS generation.
Fig. 5.
MVE reduced production of ROS. (A) Cytosolic ROS level was measured using DCF-CA (green). UVB increased the fluorescent signal intensity of DCF-DA in HaCaT cells, while MVE (1000 nM) attenuated the signal intensity of DCF-DA. (B) Mitochondria ROS production was measured using mito-Sox (red). UVB increased the fluorescent signal intensity of mito-Sox in HaCaT cells, while MVE (1000 nM) reduced the signal intensity of mito-Sox. Scale bar=100 μm, n=3, **p<0.01.
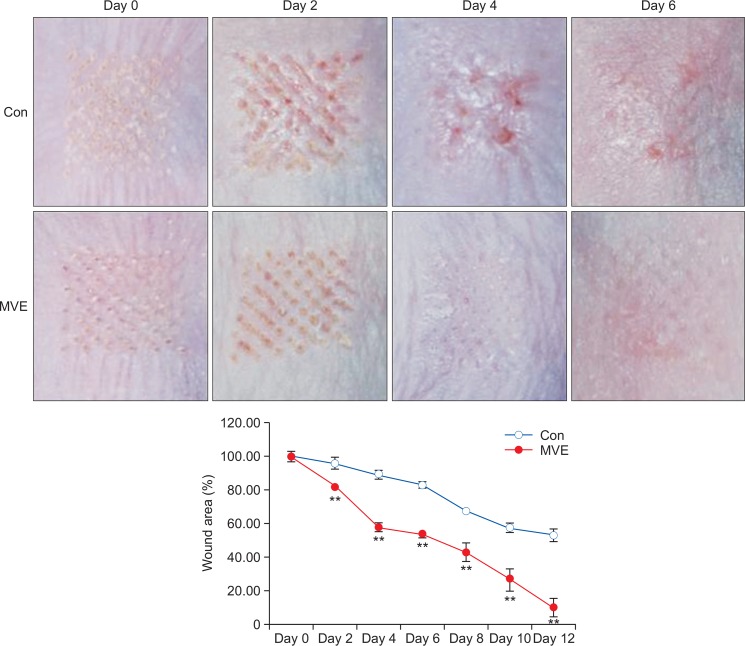
MVE accelerated wound healing
Because MVE showed protective effects in vitro, the wound healing effects of MVE were further examined in animal experiments. After lasing, wounds were treated with MVE. Although 1 and 10 μM MVE did not have an affect (data not shown), topical application of 100 μM MVE enhanced wound healing of laser burns (Fig. 6). In addition, the wound area was significantly reduced with 100 μM MVE treatment (Fig. 6, p<0.01).
Fig. 6.
MVE accelerated wound healing. After lasing, wounds were treated with MVE every other day. Topical application of 100 μM MVE enhanced wound healing of laser burns. Wound area was significantly reduced by 100 μM MVE treatment. All values are presented as mean ± STD. n=3, **p<0.01.
DISCUSSION
The present study investigated the protective effects of MVE against UVB in dermal fibroblasts and epidermal HaCaT cells. In addition, the wound-healing effects of MVE were studied in animal models. MVE increased the proliferation and survival of fibroblasts at low concentration (i.e., nM ranges). In addition, MVE increased collagen production and downregulated MMP1 expression. MVE also increased the proliferation and survival of HaCaT cells. UVB increased ROS production in fibroblasts and HaCaT cells, while MVE decreased ROS production in these cells. In an animal experiment, MVE accelerated wound healing from laser-induced skin damage. These results collectively suggest that low dose MVE protects skin from UVB irradiation. Therefore, MVE can be used as a cosmetic raw material.
Vitamin E is a group of compounds that include both tocopherols and tocotrienols. As a fat-soluble antioxidant, vitamin E inhibits the production of ROS when fat undergoes oxidation (Herrera and Barbas, 2001; Packer et al., 2001). As an antioxidant, vitamin E acts as a peroxyl radical scavenger, preventing the propagation of free radicals in tissues. Hydrogen donors such as vitamin C then reduce vitamin E to return to its reduced state (Traber and Stevens, 2011). There is substantial evidence that vitamin E protects dermal fibroblasts and epidermal keratinocytes from UVB, and vitamin E has been used to protect skin from UVB (Placzek et al., 2005; Song and Liu, 2005; Shibata et al., 2010; Burns et al., 2013). For example, α-tocotrienol protected HaCaT keratinocytes from UVB induced inflammation (Shibata et al., 2010). The antioxidants ascorbic acid and D-alpha-tocopherol prevented UVB-induced DNA damage in human epidermis (Placzek et al., 2005). Vitamin E and its derivatives are included as anti-oxidants in many sunscreens and lotions currently on the market. (Song and Liu, 2005; Burns et al., 2013). MVE can also be added to sunscreens and lotions to prevent photo damage.
MVE is designed to accumulate within mitochondria, and it is applied to decrease mitochondrial oxidative damage. Therefore, MVE can be used to protect normal cells from oxidative damage. Mao et al reported that MVE reduced systemic oxidative stress parameters such as plasma SOD activity. In addition, MVE attenuated hepatic oxidative stress and inhibited fat deposition in mice (Mao et al., 2010; Mao et al., 2011). However, there are controversial reports that MVE increased ROS generation in cancer cells and acts as a prooxidant to suppress the proliferation of cancer cells (Neuzil et al., 2007; Dong et al., 2011). In the present study, MVE showed a dual mode of actions. At low concentrations (<1 μM), MVE protected dermal fibroblasts and epidermal HaCaT cells from UVB via scavenging ROS production (Fig. 1A). However, MVE inhibited the survival of dermal fibroblasts at high concentrations (>1 μM). Conversely, vitamin E slightly increased fibroblast survival with UVB at relatively high concentrations (μM range). Although we did not further clarify the mechanism of action, MVE acts as an antioxidant at low concentrations and as a prooxidant at high concentrations in normal cells.
In summary, low concentration MVE protected dermal fibroblasts and epidermal HaCaT cells from UVB irradiation by scavenging ROS in these cells. MVE also increased collagen production and decreased MMP1 expression. In an animal experiment, MVE accelerated the healing of laser-induced burns. Therefore, MVE can be developed and used for cosmetic raw materials.
Acknowledgments
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HN14C0084), and by a grant of Yonsei University (2014-22-0178).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest and have received no funding for this manuscript.
REFERENCES
- Ajith TA, Jayakumar TG. Mitochondria-targeted agents: Future perspectives of mitochondrial pharmaceutics in cardiovascular diseases. World J Cardiol. 2014;6:1091–1099. doi: 10.4330/wjc.v6.i10.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns EM, Tober KL, Riggenbach JA, Kusewitt DF, Young GS, Oberyszyn TM. Differential effects of topical vitamin E and C E Ferulic(R) treatments on ultraviolet light B-induced cutaneous tumor development in Skh-1 mice. PLoS ONE. 2013;8:e63809. doi: 10.1371/journal.pone.0063809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong LF, Jameson VJ, Tilly D, Cerny J, Mahdavian E, Marin-Hernandez A, Hernandez-Esquivel L, Rodriguez-Enriquez S, Stursa J, Witting PK, Stantic B, Rohlena J, Truksa J, Kluckova K, Dyason JC, Ledvina M, Salvatore BA, Moreno-Sanchez R, Coster MJ, Ralph SJ, Smith RA, Neuzil J. Mitochondrial targeting of vitamin E succinate enhances its pro-apoptotic and anti-cancer activity via mitochondrial complex II. J Biol Chem. 2011;286:3717–3728. doi: 10.1074/jbc.M110.186643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farriol M, Mourelle M, Schwartz S. Effect of vitamin C and vitamin E analog on aged fibroblasts. Rev Esp Fisiol. 1994;50:253–257. [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Herrera E, Barbas C. Vitamin E: action, metabolism and perspectives. J Physiol Biochem. 2001;57:43–56. doi: 10.1007/BF03179812. [DOI] [PubMed] [Google Scholar]
- Hye Kim J, Gyu Park S, Kim WK, Song SU, Sung JH. Functional regulation of adipose-derived stem cells by PDGF-D. Stem Cells. 2015;33:542–556. doi: 10.1002/stem.1865. [DOI] [PubMed] [Google Scholar]
- Kim WS, Park BS, Park SH, Kim HK, Sung JH. Antiwrinkle effect of adipose-derived stem cell: activation of dermal fibroblast by secretory factors. J Dermatol Sci. 2009;53:96–102. doi: 10.1016/j.jdermsci.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Mao G, Kraus GA, Kim I, Spurlock ME, Bailey TB, Beitz DC. Effect of a mitochondria-targeted vitamin E derivative on mitochondrial alteration and systemic oxidative stress in mice. Br J Nutr. 2011;106:87–95. doi: 10.1017/S0007114510005830. [DOI] [PubMed] [Google Scholar]
- Mao G, Kraus GA, Kim I, Spurlock ME, Bailey TB, Zhang Q, Beitz DC. A mitochondria-targeted vitamin E derivative decreases hepatic oxidative stress and inhibits fat deposition in mice. J Nutr. 2010;140:1425–1431. doi: 10.3945/jn.110.121715. [DOI] [PubMed] [Google Scholar]
- Neuzil J, Tomasetti M, Zhao Y, Dong LF, Birringer M, Wang XF, Low P, Wu K, Salvatore BA, Ralph SJ. Vitamin E analogs, a novel group of “mitocans,” as anticancer agents: the importance of being redox-silent. Mol Pharmacol. 2007;71:1185–1199. doi: 10.1124/mol.106.030122. [DOI] [PubMed] [Google Scholar]
- Neuzil J, Wang XF, Dong LF, Low P, Ralph SJ. Molecular mechanism of ‘mitocan’-induced apoptosis in cancer cells epitomizes the multiple roles of reactive oxygen species and Bcl-2 family proteins. FEBS Lett. 2006;580:5125–5129. doi: 10.1016/j.febslet.2006.05.072. [DOI] [PubMed] [Google Scholar]
- Newmeyer DD, Ferguson-Miller S. Mitochondria: releasing power for life and unleashing the machineries of death. Cell. 2003;112:481–490. doi: 10.1016/S0092-8674(03)00116-8. [DOI] [PubMed] [Google Scholar]
- Offord EA, Gautier JC, Avanti O, Scaletta C, Runge F, Kramer K, Applegate LA. Photoprotective potential of lycopene, beta-carotene, vitamin E, vitamin C and carnosic acid in UVA-irradiated human skin fibroblasts. Free Radic Biol Med. 2002;32:1293–1303. doi: 10.1016/S0891-5849(02)00831-6. [DOI] [PubMed] [Google Scholar]
- Packer L, Weber SU, Rimbach G. Molecular aspects of alpha-tocotrienol antioxidant action and cell signalling. J Nutr. 2001;131:369S–373S. doi: 10.1093/jn/131.2.369S. [DOI] [PubMed] [Google Scholar]
- Placzek M, Gaube S, Kerkmann U, Gilbertz KP, Herzinger T, Haen E, Przybilla B. Ultraviolet B-induced DNA damage in human epidermis is modified by the antioxidants ascorbic acid and D-alpha-tocopherol. J Invest Dermatol. 2005;124:304–307. doi: 10.1111/j.0022-202X.2004.23560.x. [DOI] [PubMed] [Google Scholar]
- Rocha M, Apostolova N, Hernandez-Mijares A, Herance R, Victor VM. Oxidative stress and endothelial dysfunction in cardiovascular disease: mitochondria-targeted therapeutics. Curr Med Chem. 2010;17:3827–3841. doi: 10.2174/092986710793205444. [DOI] [PubMed] [Google Scholar]
- Shibata A, Nakagawa K, Kawakami Y, Tsuzuki T, Miyazawa T. Suppression of gamma-tocotrienol on UVB induced inflammation in HaCaT keratinocytes and HR-1 hairless mice via inflammatory mediators multiple signaling. J Agric Food Chem. 2010;58:7013–7020. doi: 10.1021/jf100691g. [DOI] [PubMed] [Google Scholar]
- Smith RA, Porteous CM, Gane AM, Murphy MP. Delivery of bioactive molecules to mitochondria in vivo. Proc Nat Acad Sci USA. 2003;100:5407–5412. doi: 10.1073/pnas.0931245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Liu S. A new healthy sunscreen system for human: solid lipid nanoparticles as carrier for 3,4,5-trimethoxybenzoylchitin and the improvement by adding Vitamin E. Int J Biol Macromol. 2005;36:116–119. doi: 10.1016/j.ijbiomac.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Traber MG, Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med. 2011;51:1000–1013. doi: 10.1016/j.freeradbiomed.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truksa J, Dong LF, Rohlena J, Stursa J, Vondrusova M, Goodwin J, Nguyen M, Kluckova K, Rychtarcikova Z, Lettlova S, Spacilova J, Stapelberg M, Zoratti M, Neuzil J. Mitochondrially targeted vitamin e succinate modulates expression of mitochondrial DNA transcripts and mitochondrial biogenesis. Antioxid Redox Signal. 2015;22:883–900. doi: 10.1089/ars.2013.5594. [DOI] [PubMed] [Google Scholar]