Abstract
We examined whether wogonin (WO) improved hippocampal neuronal activity, behavioral alterations and cognitive impairment, in rats induced by administration of trimethyltin (TMT), an organotin compound that is neurotoxic to these animals. The ability of WO to improve cognitive efficacy in the TMT-induced neurodegenerative rats was investigated using a passive avoidance test, and the Morris water maze test, and using immunohistochemistry to detect components of the acetylcholinergic system, brain-derived neurotrophic factor (BDNF), and cAMP-response element-binding protein (CREB) expression. Rats injected with TMT showed impairments in learning and memory and daily administration of WO improved memory function, and reduced aggressive behavior. Administration of WO significantly alleviated the TMT-induced loss of cholinergic immunoreactivity and restored the hippocampal expression levels of BDNF and CREB proteins and their encoding mRNAs to normal levels. These findings suggest that WO might be useful as a new therapy for treatment of various neurodegenerative diseases.
Keywords: Wogonin, Trimethyltin, Memory, Cholinergic neurons, cAMP-response element-binding protein
INTRODUCTION
Current exposure of human populations to a wide variety of chemicals has generated concern about the potential neurotoxicities of both new and existing compounds (Geloso et al., 2011). Trimethyltin (TMT) is an organotin compound that exerts potent neurotoxic effects (Kim et al., 2013). TMT is regarded as particularly useful in studies of the response to brain injury because it causes a distinct pattern of brain degeneration (Yang et al., 2012). In particular, intoxication with TMT produces significant and selective neuronal degeneration triggering behavioral alterations and cognitive deficits in humans. These effects include increased levels of disorientation, confusion, memory deficits, aggressiveness, and seizures (Geloso et al., 2011). In the rat, TMT administration produces the so-called “TMT syndrome,” characterized by tremor, hyperactivity, aggression, seizure susceptibility, and impairments in learning and cognitive function (Kaur et al., 2013a; Kaur and Nehru, 2013b). TMT-induced neurodegeneration is characterized by massive neuronal death and neuroinflammation, principally in the limbic system and especially the hippocampus, accompanied by reactive gliosis, epilepsy, and marked neurobehavioral alterations (Brabeck et al., 2002; Geloso et al., 2002; Geloso et al., 2004). These behavioral changes are associated with impairment of neurotransmitter systems including those involving acetylcholine (Nishimura et al., 2001). TMT administration to rats impairs the performance of hippocampus-dependent behaviors, including learning acquisition in water maze tasks, creation of working and reference memories in radial maze tasks and the passive avoidance test (PAT), locomotor activity which increases, and self-grooming which is disrupted (Koda et al., 2008; Park et al., 2012). In particular, the TMT-intoxicated rat model is an attractive model for study of degenerative diseases such as Alzheimer’s disease (AD), which is the most common cause of dementia (Koda et al., 2008).
Some studies have found that traditional herbs are both attractive health foods and useful source materials for drug development (Zhao, 2009). Also, natural products derived from plant extracts are currently used as alternative or complementary therapies for AD or dementia. Such materials improve memory, cognitive functioning, and memory-related adverse symptoms, because the materials exhibit antioxidant activities and target many proteins (Zhao, 2009). However, scientific evidence regarding the pharmacological effectiveness and mechanisms remains sparse.
Wogonin (5,7-dihydroxy-8-methoxyflavone), which originates from the roots of the medicinal herb Scutellaria baicalensis, is a biologically active compound (Lee et al., 2011). In both in vitro and in vivo studies, wogonin (WO) has reported to have antioxidant, antiinflammatory, and neuroprotective effects (Cho and Lee, 2004). WO inhibits lipopolysaccharide (LPS)-induced production of nitric oxide (NO), prostaglandin E2 and pro-inflammatory cytokines in immune cells like macrophages and microglial cells (Chen et al., 2015). WO also alleviates inflammatory processes by decreasing the expression of inducible cyclooxygenase-2 and monocyte chemoattractant protein-1 (MCP-1), a crucial factor for early inflammatory responses (Piao et al., 2008).
Although a brief report of the anti-inflammatory activity and neuroprotective effects of WO has appeared, it is currently unclear whether the therapeutic efficacy of WO in improving learning and memory after induction of neuronal damage by a single dose of TMT involves effects on cholinergic neurons, brain-derived neurotrophic factor (BDNF), and/or cAMP-response element-binding protein (CREB) (Viviani et al., 2005). It is appropriate to study WO further to better understand the mechanisms of action underlying the WO-caused improvements in learning and memory noted in TMT-treated animal models (Park et al., 2011).
The aim of the present study was to evaluate the therapeutic efficacy of WO on TMT-induced behavioral alterations (hyperactivity and aggression) and cognitive impairment (memory loss and learning impairment) and to determine the mechanism underlying the beneficial effects using an animal model in combination with appropriate behavioral and neurobiological methods. To this end, WO was evaluated in terms of efficacy in alleviating spatial learning and memory deficits in rats given a single dose of TMT. The PAT and the Morris water maze (MWM) test were administered. Using immunohistochemistry and RT-PCR, the effects of WO on the activities of cholinergic neurons, expression of BDNF and CREB, and neural activity in the hippocampus were also investigated.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley (SD) rats weighing 260–280 g were purchased from Samtako Animal Co (Seoul, Korea). The animals were housed in individual cages under light-controlled conditions (12/12-h light/dark cycle; lights on at 7:00 a.m., lights off at 7:00 p.m.) under controlled temperature (22 ± 2°C) and humidity (55 ± 15%). Food and water were made available ad libitum. The rats were housed in a limited access rodent facility with up to five rats per polycarbonate cage. All the experiments were approved by the Kyung Hee University Institutional Animal Care and Use Committee for the use of Human or Animal Subjects or that procedures are in compliance with at least the Declaration of Helsinki for Human subjects, or the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 85-23), revised in 1996. The animals were allowed to acclimatize themselves for at least 7 days prior the experimentation. The effects were made to minimize the number and suffering of animals.
Experimental design
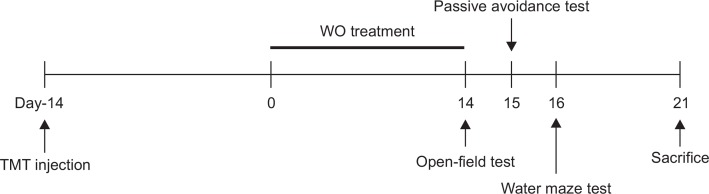
This study was designed to explore the efficacy of WO administration for healing TMT-induced memory impairment in an animal model using behavioral and neurobiological methodologies. To develop learning and memory deficits in the brain, the rats received a single intraperitoneal (i.p.) injection of trimethyltin chloride (TMT, 8.0 mg/kg, body weight, Sigma-Aldrich Chemical Co., St Louis, MO, USA) dissolved in physiological saline solution; then they were returned to their home cages. The rats were randomly into six groups of six or seven individuals each as follows: vehicle saline-injected group, instead of TMT (0.9% NaCl, i.p., SAL group; n=6); TMT-injected and vehicle-injected group (TMT group as a negative control; n=7); TMT-injected plus 10 mg/kg WO-treated group (WO10 group; n=6); TMT-injected plus 20 mg/kg WO-treated group (WO20 group; n=6); TMT-injected plus 50 mg/kg WO-treated group (WO50 group; n=6); and TMT-injected plus 40 mg/kg ibuprofen-treated (IBU group as a positive control; n=6). Ibuprofen (Sigma-Aldrich Chemical Co.), a centrally acting cholinesterase inhibitor, was used as a positive control. The WO and the IBU were dissolved in 0.9% physiological saline before use and administrated intraperitoneally in a volume of 10 mL/ kg for a period of 14 days. The SAL group and TMT group also received saline instead of WO as a vehicle control in an equal volume. All drugs were freshly prepared right before each experiment. All rats sequentially performed the PAT on the 29th day after the TMT injection, and the MWM test on the 30th day after the TMT injection. After the behavioral testing, rats were sacrificed and brain tissues were immediately collected for experiments or stored at −70°C for later use. The entire experimental schedule of all drug administration and behavioral examinations are shown in Fig. 1.
Fig. 1.
Experimental schedule of lesion generation, WO administration, and behavioral tests in rats. WO: Wogonin; TMT: trimethyltin.
Assessment of aggression
The aggression scores were measured by the scoring method of Kaur et al. (2013a). The aggression response was observed in freely moving rats. The events were observed during a 30 min stimulation period. The scores are as follows: Score 1: remains calm when approached and grasped; Score 2: shies from hand when grasped; Score 3: avoids hand by running, struggles when captured, or both; and Score 4: leaps, struggles and bites when captured.
Open field test
Prior to water maze testing, the rats were individually housed in a rectangular container that was made of black polyethylene (60×60×30 cm) to provide the best contrast to the white rats in a dimly lit room equipped with a video camera above the center of the room, and their locomotor activities (animal’s movements) were then measured. The locomotor activity indicated by the speed and distance of movements was monitored by a computerized video-tracking system using the SMART program (Panlab Co., Barcelona, Spain). Tests were performed in the breeding room from 8:30 to 16:00 on the 28th day after starting TMT injection. The animals were allowed to adapt to the container for 5 min before testing to acclimatize to the new environment. The individual rats were placed in the middle of the chamber for each trail. After 5 min adaptation, the distance they traveled in the container was recorded for another 5 min. The locomotor activity was expressed in centimeters. The floor surface of each chamber was thoroughly cleaned with 70% ethanol between tests. The number of rearing events of the rats was also recorded in order to analyze locomotor activity in the open field test (OFT). The rearing was defined as the number of times the rat stood upright on its hind limbs.
Passive avoidance test
The test was basically performed according to the step-through method. The Gemini Avoidance System (SD Instruments., San Diego, CA, USA) was used for this experiment. Basically, the step-through passive avoidance apparatus (PAA) consists of a tilting floor acrylic box divided into two-compartments, a lightened compartment connected to a darkened compartment, by an automatic guillotine door and a control unit generating electric shock (Behbood Pardaz Co., Ghaem, Iran). The electric shock can be delivered to the grid floor, made of stainless steel rods (3 mm diameter) spaced 1 cm apart, in both compartments. First, the rats were give trials to acquisition test in the apparatus. In the training session, a rat was placed in a lightened compartment of the PAA facing away from the entrance to the dark compartment, and then the guillotine door was opened. Because of intrinsic preference to the dark environment, the rat immediately entered the dark compartment and the door was closed. During the acquisition test, the latency time before entry into the dark compartment was recorded for each rat. After 30 min, the rats were placed in the lightened compartment once again. After entering the dark compartment, the guillotine door was closed, and subsequently a mild electrical shock (0.5 mA) was applied for 3s. The retention test was started 24 h after the acquisition trial for training. The rat was again placed in the lightened compartment and the guillotine door was opened. In the retention test, the rat was placed in the PAA as previously described and the time required for the rat to enter the dark compartment was measured for a maximum period of 3 min as in the same method with the acquisition test. The rats that did not enter the dark compartment within this period received a latency time of 180 s.
Morris water maze test
The MWM test was performed in a small circular pool (2.0 m in diameter and 0.35 m deep) made of polypropylene and internally painted white. The pool was half-filled with water to a depth of 30 cm. The water in the pool was made opaque by adding 1 kg skim milk powder and continuously maintained at 22 ± 2°C. The pool was divided into four quadrants of equal area. During the MWM test, an escape platform (15 cm in diameter) was located in one of the four sections of the pool, being hidden 1.5 cm below the water surface and approximately 50 cm away from the sidewalls. Several visual cues were placed around the pool in plain sight of the animals. A digital camera was mounted to the ceiling straight above the center of the pool and was connected to a computerized recording system equipped with a tracking program (S-MART: Panlab Co., Barcelona, Spain), which permitted on- and off-line automated tracking of the paths taken by the rats. The MWM test was initiated on the 30st day after the WO and TMT administration commenced. The animals received three trials per day. The rats were trained to find the hidden platform, whi ch remained in a fixed location throughout the test. The trials lasted for a maximum of 180 s, and the escape latency was expressed by the swimming time to find the submerged platform in the pool. The animals were tested with three trials per day for 5 days, and they received a 60-s probe trial on the sixth day. Finding the platform was defined as staying on it for at least 4 s before the acquisition time of 180 s ended. When the rat failed to find the platform in the limited time in first trial of hidden platform test, the rats should be placed on the platform for 20 s and assigned a latency of 180 s. Between one trial and the next, the water in the pool was stirred to remove olfactory traces of previous swim patterns. The entire schedule proceeded for 6 days and each animal had three trials for training per day with 30–40 min inter-trial interval. For the probe trial, a rat was placed in the quadrant located diagonally from the target quadrant and allowed to swim to the quadrant from which the escape platform had been removed for a maximum of 60 s. The probe trial was expressed by the ratio of the time spent (or the distance traveled) in searching for the platform in the target quadrant to the total duration spent swimming in the pool.
Histology-Cresyl violet-staining
After behavioral testing was completed, three rats in each groups were deeply anesthetized with sodium pentobarbital (80 mg/kg, by intraperitoneal injection) and perfused through the ascending aorta with normal saline (0.9%) followed by 300 ml (per rat) of 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS). The brains were removed in a randomized order, post-fixed in the same fixative over-night, and cryoprotected with 20% sucrose solution in 0.1 M PBS at 4°C. Coronal sections 30 μm thick were serially cut through the hippocampus using a cryostat (Leica CM1850; Leica Microsystems Ltd., Nussloch, Germany), which were processed histochemically as free-floating sections. For cresyl violet (Nissl) staining, the sections were mounted on gelatine-coated microscopic slides and allowed to air-dry for 10 min. The mounted sections were rehydrated in distilled water, and submerged in 0.2% cresyl violet solution (ICN biomedicals, Aurora, USA) for about 5 min until the desired depth of staining was achieved. The sections were rinsed in dH2O and dehydrated in graded series of ethanol, immersed in xylene, mounted in DPX (BDH Laboratory Supplies, Poole, Dorset, UK) and cover slipped, along the antero-posterior hippocampal axis, in order to determine levels of cell loss as well as the morphology of the hippocampus. Images were captured using the DP2-BSW imaging system (Olympus, CA, USA) and processed using Adobe Photoshop (Adobe Systems, Inc., San Jose, CA, USA). For measuring the hippocampal cells, the cells of the hippocampal CA1 area were counted at 100× magnification using a microscope rectangle grid measuring 200×200 microns, anatomically localized in at least three different hippocampal sections per rat brain according to the stereotactic rat brain atlas of Paxinos and Watson (1986).
Immunohistochemistry
For immunohistochemical studies, the primary antibodies against the following specific antigen were used: choline acetyltransferase (ChAT; rabbit polyclonal ChAT, 1:2000 dilution, Cambridge Research Biochemicals Co., Bellingham, UK), aectylcholinesterase (AchE; goat polyclonal AchE, 1:2000 dilution: Santa Cruz Biotechnology Inc., CA, USA), BDNF (rabbit polyclonal BDNF, 1:200 dilution, Cell signaling, Boston, MA, USA) and CREB (rabbit polyclonal CREB, 1:250 dilution, Cell signaling, Boston, MA, USA). Briefly, the sections were incubated with primary antiserum in PBST (PBS plus 0.3% Triton X-100) for 72 h at 4°C. The sections were incubated for 120 min at room temperature with secondary antibody. The secondary antibodies were obtained from Vector Laboratories Co. (Burlingame, CA, USA) and diluted 1:200 in PBST containing 2% normal serum. To visualize immunoreactivity, the sections were incubated for 90 min in avidin-biotin-peroxidase complex (ABC) reagent (Vectastain Elite ABC kit; Vector Labs. Co., Burlingame, CA, USA), and incubated in a solution containing 3,3′-diaminobenzidine (DAB; Sigma-Aldrich Chemical Co., St. Louis, MO, USA) and 0.01% H2O2 for 1 min. Finally, the tissues were washed in PBS, followed by a brief rinse in distilled water, and mounted individually onto slides. Images were captured using the DP2-BSW imaging system (Olympus, CA, USA) and processed using Adobe Photoshop (Adobe Systems, Inc., San Jose, CA, USA). The sections were viewed at 100× magnification, and the numbers of ChAT, AchE, BDNF and CREB labeled cells was quantified in the hippocampus. ChAT-, AchE-, BDNF- and CREB-labeled cells were counted by an observer blinded to the experimental groups. Counting the immunopositive cells were performed within the square (200×200 μm2), anatomically localized in at least three different hippocampal sections per rat brain according to the stereotactic rat brain atlas of Paxinos and Watson (1986). The counted sections were randomly chosen from equal levels of serial sections along the rostral-caudal axis. The stained cells for which intensities to a defined value above the background were only considered as immunopositive cells. Distinct brown spots indicating ChAT-, AchE-, BDNF- and CREB-immunopositive cells was observed in the cytoplasms and in the membranes of cone-shaped cells in the hippocampus. The differences of brightness and contrast among raw images were not adjusted, in order to exclude any possibility of subjective selection of the immunoreactive cells.
Total RNA preparation and RT-PCR analysis
The expression levels of BDNF and CREB mRNAs were determined by reverse transcription-polymerase chain reaction (RT-PCR). The brain hippocampus was isolated from three rats per group. After decapitation, the brain was quickly removed and stored at −80°C until use. The total RNA was prepared from the brain tissue using a TRIzol® reagent (Invitrogen Co., Carlsbad, CA, USA) according to the supplier’s instructions. Complementary DNA was first synthesized from total RNA using reverse transcriptase (Takara Co., Shiga, Japan). PCR was performed using a PTC-100 programmable thermal controller (MJ Research, Inc., Watertown, MA, USA). The operating conditions were as follows: for glyceraldehydes-3-phosphate dehydrogenase (GAPDH), 30 cycles of denaturation at 95°C for 30 sec, annealing at 58°C for 30 sec, and extension at 72°C for 30 sec; for BDNF, 27 cycles of denaturation at 95°C for 30 sec, annealing at 57°C for 30 sec, and extension at 72°C for 30 sec.; for CREB, 27 cycles of denaturation at 95°C for 30 sec, annealing at 51°C for 30 sec, and extension at 72°C for 30 sec. All primers were designed using published mRNA sequences of those cytokines and a primer designing software, Primer 3, offered by the Whitehead Institute for Biomedical Research (Cambridge, MA, USA; http://primer3.wi.mit.edu). The following sequences were used: for GAPDH (409 bp), (forward) 5′-ATC CCA TCA CCA TCT TCC AG-3′ and (reverse) 5′-CCT GCT TCA CCA CCT TCT TG-3′; for BDNF (153 bp), (forward) 5′-CAG GGG CAT AGA CAA AAG-3′ and (reverse) 5′-CTT CCC CTT TTA ATG GTC-3′; for CREB (183 bp), (forward) 5′-TAC CCA GGG AGG AGC AAT AC-3′ and (reverse) 5′-GAG GCA GCT TGA ACA ACA AC-3′. The PCR products were separated on 1.2% agarose gels and stained with ethidium bromide. The density of each band was quantified using an image-analyzing system (i-MaxTM, Core-Bio System Co., Seoul, Korea). The expression levels were compared each other by calculating the relative density of the target band, such as BDNF and CREB, to that of GAPDH.
Statistical analysis
All measurements were performed by an independent investigator blinded to the experimental conditions. The results in figures are expressed as the mean ± standard error of the means (SEM). Differences within or between normally distributed data were analyzed by analysis of variance (ANOVA) using SPSS (Version 13.0; SPSS, Inc., Chicago, IL, USA) followed by Tukey’s post hoc test. A p-value of less than 0.05 was regarded as statistically significant.
RESULTS
Effect of WO on TMT-induced aggression in an open field apparatus
Aggression scores were evaluated in the rats using an open-field apparatus (Fig. 2). These results indicated that the rats with TMT injection significantly increased their aggression scores, compared with those in the SAL group (p<0.05). This suggests that the rats treated with TMT, subsequently produced aggressive behavior, which has been closely associated with brain dysfunction or spontaneous seizures in the open field apparatus. However, the WO-treated rats (20 or 50 mg/kg) demonstrated significantly decreased aggression scores compared with those in the TMT group (p<0.05). The WO-treated rats showed a marked decrease in behavioral alteration for aggression for 14 days, indicating that treatment with WO restored aggressive behavior to a normal level and accordingly alleviated the TMT-induced impairment in the rats.
Fig. 2.
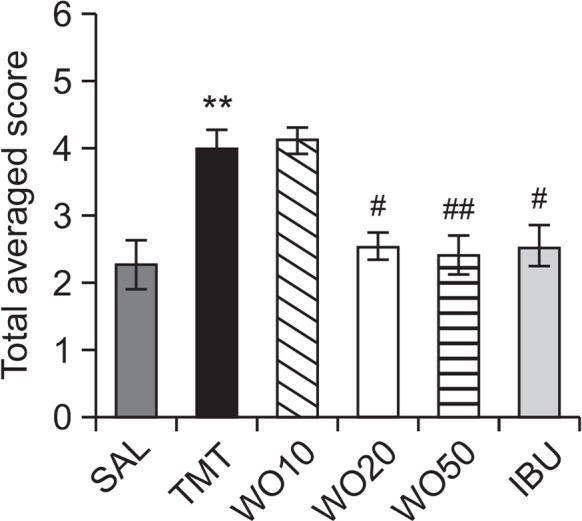
Effects of WO administration on total average score in the open-field apparatus during TMT injection. **p<0.01 vs. the SAL group; #p<0.05 and ##p<0.01 vs. the TMT group.
Effect of WO on TMT-induced motor function and hyperactivity in the open field test
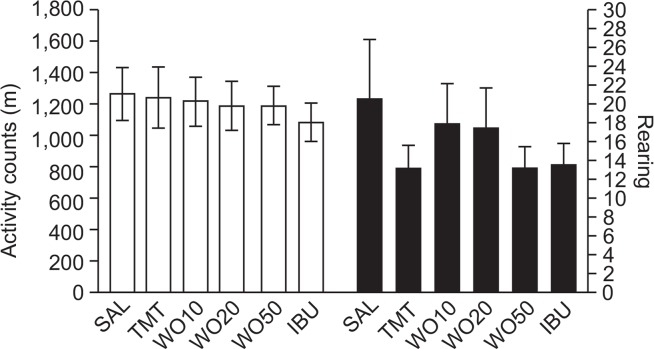
Open field activity was used to evaluate locomotor activity and hyperactivity in the rats (Fig. 3). The results indicated no effect on the locomotor activities (motor function) or total rearing activity (hyperactivity) of the rats in all groups on the open-field test (OFT). Because no significant difference in locomotor activity was observed among groups in the OFT, the observed impairments in memory in the rats receiving TMT injections were not attributable to differences in locomotion activity. Rats may also show water avoidance stress when confronted with a MWM test. However, our results suggest that rats in all groups displayed no anxiolytic-like behaviors in the OFT after a pretest stress exposure in the MWM test. This indicates that administration of WO50 did not affect active responses or psychomotor function as measured by the rats’ performance in the MWM test. An OFT was also performed to rule out any confounding motor impairment that can influence outcomes in many behavioral tests. No significant individual differences in motor function behavior were observed between groups, suggesting that the administration of WO50 had no effect on sensorimotor performance. According to the next figure, the changes in behavioral performance in the MWM task were likely due to improved memory, rather than differences in sensorimotor function, motor output, or limb flexibility.
Fig. 3.
Effects of WO administration on activity counts of locomotor activity and total number of rearing in the open-field test during TMT injection.
Effect of WO on TMT-induced step-through latency deficit in the passive avoidance test
To determine whether WO promotes the recovery of memory dysfunction, WO was administered to rats with TMT-induced impairment of memory, and their memory and cognitive functions were examined by the PAT (Fig. 4). It was confirmed, through acquisition trials without an electric challenge, that the rats in all groups had no physiological defect (i.e., motor function) or intrinsic cognitive impairment. During the acquisition trials, indicated by latencies for entering the dark compartment, there was no significant difference among the groups. After the acquisition trials, the effect of WO on the retention latency was examined 24 h after applying electric shock in the dark box in the PAT. It was seen that the rats in the WO50 group had significantly increased latencies to enter the dark compartment for retention compared with those in the TMT group (p<0.05). This indicated that the TMT injection impaired long-term memory and that treatment with WO significantly attenuated the TMT-induced memory deficit in the PAT. It also indicated that the restoration of memory function in the WO50 group was close to that in the IBU group.
Fig. 4.
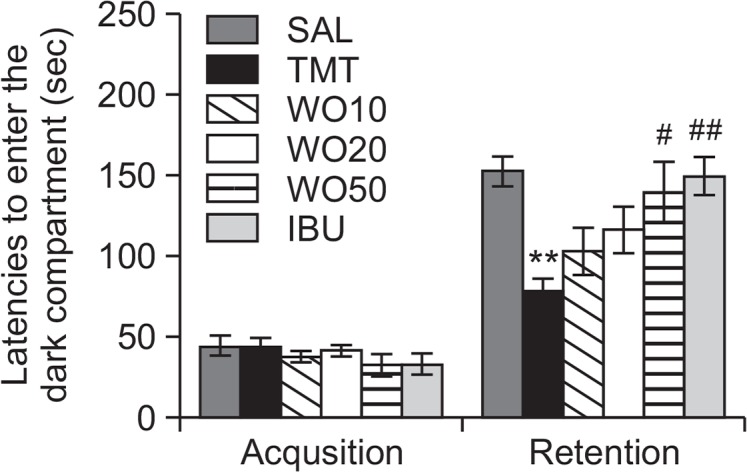
Effects of WO administration on the latency to enter the dark compartment during the acquisition trial and retention test in the passive avoidance test. **p<0.01 vs. the SAL group; #p<0.05 and ##p<0.01 vs. the TMT group.
Effect of WO on TMT-induced spatial memory impairment in the water maze test
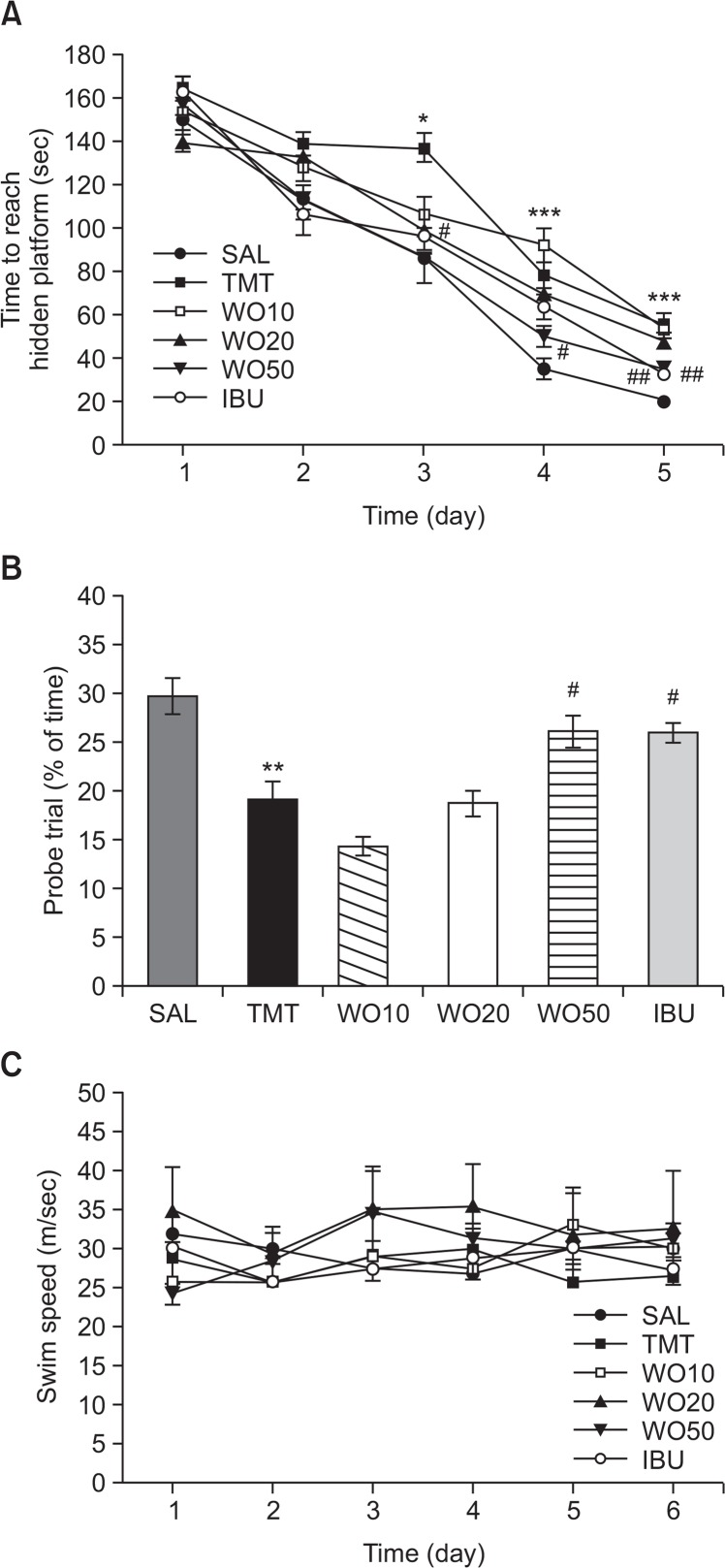
The effect of WO (10, 20 and 50 mg/kg) treatment on swimming to reach the submerged platform in the MWM test is shown in Fig. 5. Rats in the SAL group rapidly learned the location of the submerged hidden platform and reached it within 25 s on day 5 of the trials. The TMT group showed marked retardation in escape latency during all the trial sessions, probably due to memory deficits resulting from TMT-induced impairment of learning and memory. The escape latency differed among the groups when the results were averaged over all the session (Fig. 5A). The TMT group showed worse performance than the normal group (p<0.05 on the day 3, p<0.001 on the days 4 and 5). The analysis of escape latency revealed that rats in the WO50 group had significantly reduced swimming latency compared with those in the TMT group (p<0.05 on days 3 and 4, p<0.01 on day 5). To investigate the effect on the spatial memory of rats, performance in the probe trial on day 6 was examined by analyzing the percentages of time spent swimming to the expected position of the platform, to investigate the effect on spatial memory (Fig. 5B). The swimming times were reduced in the rats that swam directly and without confusion to the target area where the platform had been located. The rats with TMT injection showed severe impairment of spatial performance in the MWM test (p<0.01). The rats in the 50 mg/kg WO-treated group spent more time around the platform area than did those in the TMT group (p<0.05). This indicated that the swimming latency in rats receiving the WO was higher, compared with the TMT-induced deficit in learning and memory of the rats used as controls. Thus, WO-treated rats showed a significant amelioration in the memory retention test because they spent more time in the quadrant where the platform was formerly located and swam over the former location of the platform more frequently. The TMT group was not significantly different from the other groups in mean swimming speed, as calculated by dividing the total swim distance by latency (p=0.764; Fig. 5C). Based on these results, rats treated with 50 mg/kg WO showed greater improvement in acquisition during the hidden platform trial and, accordingly, reached the platform quicker than the TMT-treated rats. The results also indicated that the swimming latency of the TMT-induced rats receiving 50 mg/kg WO was similar to that of rats receiving 40 mg/kg IBU.
Fig. 5.
Effects of WO administration on time to escape (latency) from water during acquisition trials using a submerged platform (A), on the percentages of time in a probe trial without a platform (B), and swim speed (C) in the Morris water maze test. *p<0.05, **p<0.01 and ***p<0.001 vs. the SAL group; #p<0.05 and ##p<0.01 vs. the TMT group.
Effect of WO on TMT-induced neuronal cell loss in the hippocampus
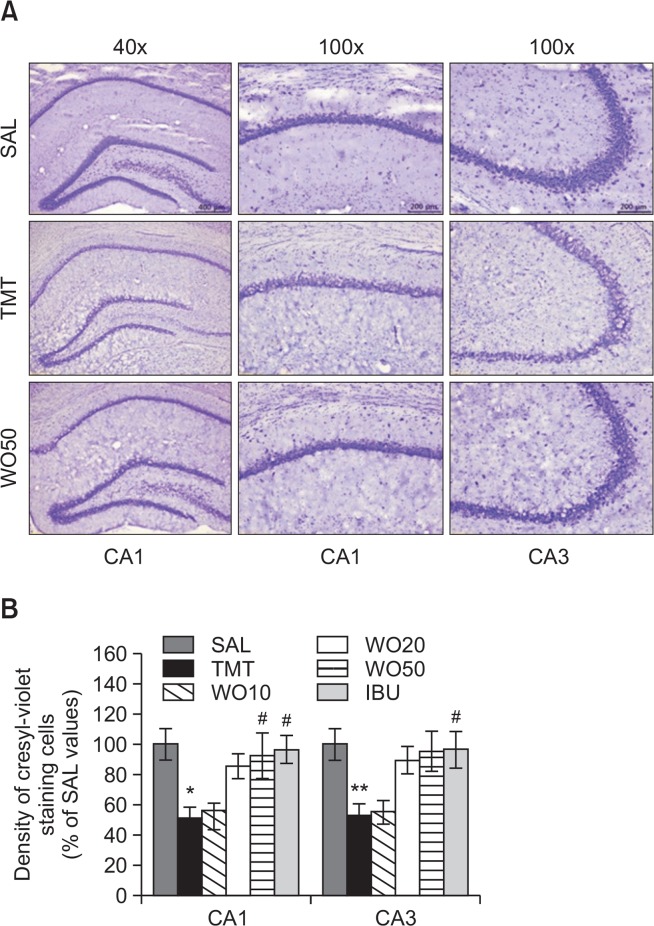
Cresyl-violet staining analysis also provided supporting evidence that administration of WO attenuated the TMT-induced decrease of healthy neurons with a normal morphological appearances in the hippocampus (Fig. 6A). Post-hoc comparisons indicated that the neuronal loss in the hippocampal granule cell layer, CA1, and the CA3 regions in the TMT group was significantly higher than that of the SAL group (granule cell layer or CA1, p<0.05 and CA3, p<0.01; Fig. 6B). The atrophy in the hippocampus was obvious in the TMT group. Examination of cresyl violet-stained sections confirmed that TMT administration induced histological changes similar to those observed previously (Ishikura et al., 2002). However, in the WO50 group, TMT-induced neuronal loss in the hippocampal CA1 or CA3 area was significantly prevented versus the TMT group (p<0.05). The histological results demonstrated that neuronal loss in the hippocampus in rats receiving 50 mg/kg WO was similar to that in rats receiving 40 mg/kg IBU.
Fig. 6.
Effects of WO on TMT-induced neuronal cell damage in the hippocampus. The density of hippocampal CA1 cell of cresyl violet-stained sections was measured within a 100 (100 um gird over the areas at 400 (magnification. Representative photographs and the relative percentage values are indicated in (A) and (B), respectively. *p<0.05, **p<0.01 vs. the SAL group; #p<0.05 vs. the TMT group.
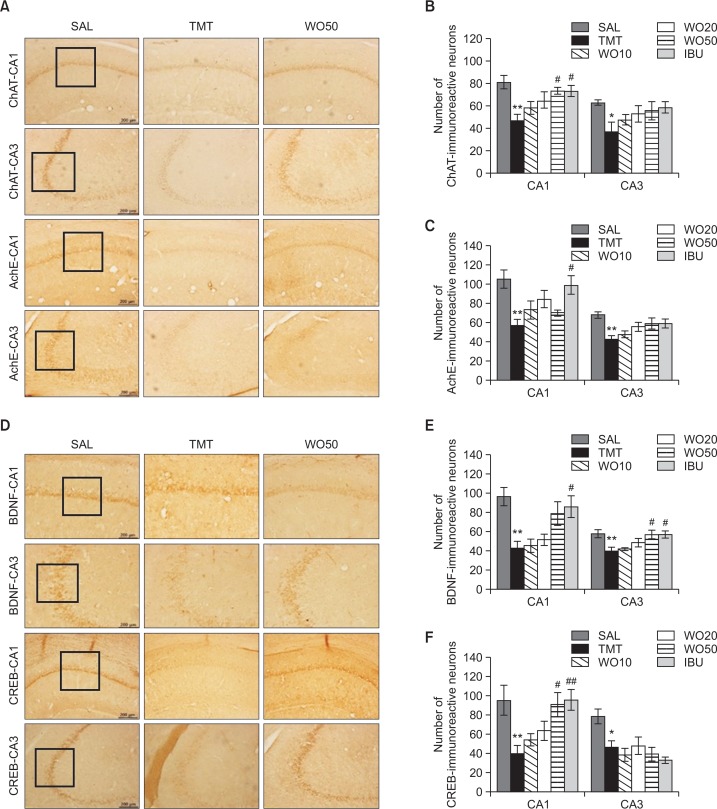
Effect of WO on TMT-induced immunohistochemical changes in ChAT, AchE, BDNF and CREB in the hippocampus
Following the behavioral tasks, brain tissue samples from the rats were analyzed using immunohistochemistry to investigate the effect of WO administration on neuronal loss associated with TMT-induced memory impairment. The results per section of the evaluations of the ChAT and AchE immunoreactive cells from the different hippocampal cholinergic neuron areas are shown in Fig. 7A. Post-hoc comparisons indicated that the ChAT activity in the hippocampus of the TMT group was significantly lower than that of the SAL group (p<0.01 in the CA1 and p<0.05 in the CA3; Fig. 7B). The number of ChAT-immunoreactive neurons in the WO50 group was significantly increased in the CA1 of the hippocampal region, compared with those of the TMT group (p<0.05). Similarly, the number of AchE-immunopositive neuronal cells in the rat hippocampus in the TMT group was significantly decreased compared with those in the SAL group (p<0.01 in the CA1 and CA3; Fig. 7C). However, the AchE-reactive neuronal activity in the hippocampus that was also associated with TMT-induced memory impairment was not significantly restored in the WO50 group, compared with the TMT group, but there was a slight trend toward an interaction effect on the expression of AchE in the hippocampus (p=0.080). The results also indicated that the number of ChAT-reactive neuronal cells in the hippocampus in rats receiving 50 mg/kg WO was similar to that in rats receiving 40 mg/kg IBU.
Fig. 7.
Effect of WO on the percentage (± SEM) values of the mean number of choline acetyltransferase (ChAT)-stained neurons, acetylcholinesterase (AchE)-stained neurons, brain-derived neurotrophic factor (BDNF)-stained neurons, and cAMP-response element-binding protein (CREB)-stained neurons in different hippocampal area after the Morris water maze task. Representative photographs and the relative percentage values are indicated in (A, B, C, D, E and F), respectively. *p<0.05, **p<0.01 vs. the SAL group; #p<0.05 and ##p<0.01 vs. the TMT group.
Additionally, the results of the evaluations of BDNF and CREB immunoreactive cells per section from the different hippocampal areas are shown in Fig. 7D. The number of BDNF-immunopositive neuronal cells in the rat hippocampus in the TMT group was significantly decreased compared with those in the SAL group (p<0.01 in the CA1 and CA3; Fig. 7E). The number of BDNF-immunoreactive neurons in the WO50 group was significantly increased in the CA3 hippocampal region, compared with the TMT group (p<0.05). Also, the number of CREB-immunopositive neuronal cells in the hippocampus in the TMT group was significantly decreased compared with those in the SAL group (p<0.05 in the CA1 and p<0.01 in the CA3; Fig. 7F). The CREB-reactive neuronal activity in the CA1 of the hippocampus associated with TMT-induced memory impairment was significantly restored in the WO50 group compared with the TMT group (p<0.05). The results also indicated that the number of CREB-reactive neuronal cells in the hippocampus in rats receiving 50 mg/kg WO was similar to that in rats receiving 40 mg/kg IBU.
Effect of WO on TMT-induced expression of BDNF and CREB mRNAs in the hippocampus
The effect of WO administration on the expression levels of BDNF and CREB mRNAs in rats with TMT-induced hippocampal lesions was investigated using RT-PCR (Fig. 8). Hippocampal expression of BDNF mRNA in the TMT group was significantly decreased compared with that in the SAL group (p<0.05). The decreased expression of BDNF mRNA in the TMT group was significantly restored in the WO50 group (p<0.05). Hippocampal expression of CREB mRNA in the TMT group was also significantly decreased compared with that in the SAL group (p<0.05). The decreased expression of CREB mRNA in the TMT group was significantly restored in the WO50 group (p<0.05). This also indicated that the expression of CREB mRNA in the hippocampus in rats receiving 50 mg/kg WO was similar to that in rats receiving 40 mg/kg IBU.
Fig. 8.
Effects of WO administration on the expression of brain-derived neurotrophic factor (BDNF) and cAMP-response element-binding protein (CREB) mRNAs in rats following TMT-induced hippocampal impairment. PCR bands on agarose gel and their relative intensities are indicated in (A) and (B), respectively. The expression levels of BDNF and CREB mRNAs were normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA as an internal control. *p<0.05 vs. the SAL group; #p<0.05 vs. the TMT group.
DISCUSSION
Our findings demonstrated that TMT-induced memory impairment resulted in severe performance deficits in tests of cognitive functioning as well as corresponding signs of neurodegeneration in the brain, including decreased BDNF and CREB expression in the hippocampus. Our results showed that administration of WO significantly improved learning and memory on the PAT, and decreased the escape latency and increased the number of platform crossing in the MWM test in a rat model of dementia or AD. Administration of WO also produced increased cholinergic enzyme activity, BDNF and CREB immunoreactivty, and increased BDNF and CREB mRNA expression in the hippocampus associated with TMT-induced memory impairment in male rats. Thus, these studies suggest that the effects of the administration of WO might be attributable to changes in behavioral task-related pathways that are modulated by the cholinergic system. Further, coactivation of the cholinergic system and the BDNF-CREB pathway in the hippocampus might influence learning and memory processes, which may in turn lead to development of novel therapeutics, based on administration of WO to treat neurode-generative diseases.
The PAT is generally used to evaluate treatments for impairments in the three stages of memory: learning acquisition, memory retention, and memory retrieval (Frautschy et al., 2001). The present study showed that TMT injection significantly shortened the step-through latency of the retention trial and severely impaired long-term memory in the PAT (Park et al., 2012). Our results also indicated that administration of WO significantly prolonged the step-through latency, and attenuated TMT-induced memory deficit in the PAT, demonstrating that the central cholinergic neuronal system and activation of BDNF and CREB, play an important role in learning acquisition (Viviani et al., 2005). Thus, these results suggested that the anti-AD effect of the WO on TMT-induced memory impairment may be related to the cholinergic nervous system and activation of BDNF and CREB (Viviani et al., 2005).
To confirm the effect of WO on other types of memory, we performed the MWM test to evaluate spatial learning. The MWM test is a hippocampus-dependent memory task that is frequently used for examining cognitive deficits and demonstrating permanent spatial learning ability and reference memory in rodents (Koda et al., 2008; Park et al., 2011). Animals encode spatial working information during the learning step, which then serves to guide future memory retrieval (Park et al., 2012). The finding that the memory deficits demonstrated by the TMT-treated rats in a MWM task were more pronounced than those in the SAL group were consistent with findings from previous studies (Park et al., 2011). These results indicated that the chronic administration of TMT to rat brains slowed escape latency in the MWM test, demonstrating deficits in spatial learning ability and reference memory (Park et al., 2012; Kaur and Nehru, 2013b). The escape latency score in the spatial probe test of the MWM test is considered to reflect long-term spatial memory ability (Koda et al., 2008; Park et al., 2012). During the trial sessions in the MWM, administration of WO resulted in a significant reduction in escape latency, enhanced cognitive performance, and amelioration of the memory deficits associated with TMT.
We also showed that there was regional selectivity of neuronal death following TMT. It is generally known that the loss of pyramidal cells in CA3 and, although less prominent, in CA1 of the hippocampus is observed after TMT exposure. Many studies reported that a low dose (5 mg/kg) of TMT induces selective loss of pyramidal cells in CA3 in the rat hippocampus and that the cell damage extends to CA1 when the TMT dose is increased (Gasparova et al., 2012). These changes may be due to TMT-induced cell losses, or in part as direct consequence of cognitive impairment. This peculiarity supports the usefulness of the TMT model as a tool to study chronic neurodegeneration, because it mimics the characteristic patterns seen in a series of human neurodegenerative diseases. Thus, the CA1 region of the hippocampus is known to be selectively vulnerable to AD or temporal lobe epilepsy (Ishikura et al., 2002). Our results suggest that WO improved both aspects of spatial learning capability and short-term working memory and prevented neuronal loss in the hippocampal CA1 and CA3 regions resulting from TMT application.
The expression of ChAT and CREB-immunopositive neuronal cells in the rat hippocampus following administration of WO was associated with restored memory impairment-related cholinergic function, and activated CREB expression. The damage caused by TMT-induced reductions in cholinergic activity has been hypothesized to play a role in reducing hippocampal volume, which has often been associated with progression of memory decay during AD (Kim et al., 2009). The central cholinergic neuronal system likely plays an important role in learning acquisition, spatial learning ability and synaptic plasticity (Giacobini, 2002; Kotani et al., 2006). WO improved cholinergic neurons in the hippocampus and continuously induced increases in ChAT and AchE activities, which eventually resulted in recovery of the entire cholinergic circulation pathway (Giacobini, 2002). According to the cholinergic hypothesis, memory impairment in patients with senile dementia is due to selective and irreversible deficits in cholinergic function or alternations in hippocampal functioning in the brain (Kotani et al., 2006). The expression and activation of ChAT and AchE regulate the dynamic concentration of ACh in the cholinergic synapses in the AD brain (Giacobini, 2002). In the present study, we observed cholinergic dysfunction in rat amnesia induced by TMT in vivo. The memory dysfunction caused by TMT-induced cholinergic dysfunction has been hypothesized to play an important role in the pathogenesis of degenerative changes and cognitive impairments (Kim et al., 2009; Kaur et al., 2013a). Thus, we propose that the beneficial effects of WO in ameliorating memory impairment could be related to an increase in central cholinergic functioning.
Despite its actions on neuronal cell survival and differentiation, the synaptic plasticity of neurons, and the prevention of neurodegeneration, recent experimental evidence has strongly supported the role of BDNF in the pathogenesis of memory deficits and learning and memory processes (Vaynman et al., 2003). CREB dysfunction disrupts hippocampus-dependent memory formation, and it has been suggested that CREB is required for memory stability (Park et al., 2012). Thus, BDNF transcriptional activity, upregulated by CREB, may also play an important role in adaptive neuronal responses underlying learning and memory function (Vaynman et al., 2003). To identify other WO-related mechanisms of memory improvement, the effects of WO on BDNF and CREB expression, believed to be key molecules for formation of memories were investigated. TMT-induced memory deficits induced significant reductions in BDNF and CREB mRNA expression in the hippocampus and resulted in poor performance on hippocampus-dependent tasks (Vaynman et al., 2003). It is proposed that the administration of WO significantly prevented the reduction of BDNF and CREB expression induced by the administration of TMT, which leads to memory deficits. These findings suggest that the beneficial effects of WO are medicated by the increase in BDNF expression via the CREB signaling pathway and may be related to an increase in neuronal functioning and performance in memory tasks.
We also demonstrated significant decreases in BDNF and CREB mRNA expression in the rat hippocampal tissues following TMT-induced memory deficits and showed that administration of WO restored BDNF and CREB mRNAs expression levels. This study suggests a close correlation between proteins and genes in the reduced expression of BDNF and CREB in the hippocampus. Administration of WO significantly attenuated the symptoms of TMT-induced dementia, as indicated by improvement of cognitive function in the behavioral tests, improved cholinergic function, and activated BDNF and CREB expression (Vaynman et al., 2003; Viviani et al., 2005).
In conclusion, WO has potential as a therapeutic for treating AD-type dementia. The present study demonstrates that, in a rat model of progressive memory deficits in neurodegenerative disease, TMT induced impaired neuronal function as well as associated memory and cognitive deficits. This was evidenced by performance on the PAT and MWM test, and by protein and gene expression analyses. However, the administration of WO attenuated this TMT-induced destruction, as indicated by improved cognitive functioning during behavioral tests. Additionally, improved cholinergic function, and increased expression of BDNF and CREB were observed. These results showed that WO significantly counteracted the hippocampal neuronal impairment and memory dysfunction caused by administration of TMT to rats. WO may improve cognitive function via regulation of the CREB signaling pathway and the cholinergic system of the hippocampus. Thus, WO may be a useful agent in preventing neuronal impairment such as that observed in the progression of memory deficits associated with neurodegenerative diseases. It could be useful as a supplement in health foods and as a drug for the prevention of AD-type dementia.
Acknowledgments
This research was supported by a Grant from the National Research Foundation of Korea funded by the Korean government (MEST)(2013R1A1A2063051).
REFERENCES
- Brabeck C, Michetti F, Geloso MC, Corvino V, Goezalan F, Meyermann R, Schluesener HJ. Expression of EMAP-II by activated monocytes/microglial cells in different regions of the rat hippocampus after trimethyltin-induced brain damage. Exp Neurol. 2002;177:341–346. doi: 10.1006/exnr.2002.7985. [DOI] [PubMed] [Google Scholar]
- Chen S, Xiong J, Zhan Y, Liu W, Wang X. Wogonin inhibits LPS-induced inflammatory responses in rat dorsal root ganglion neurons via inhibiting TLR4-MyD88-TAK1-mediated NF-κB and MAPK signaling pathway. Cell Mol Neurobiol. 2015;35:523–531. doi: 10.1007/s10571-014-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J, Lee HK. Wogonin inhibits excitotoxic and oxidative neuronal damage in primary cultured rat cortical cells. Eur J Pharmacol. 2004;485:105–110. doi: 10.1016/j.ejphar.2003.11.064. [DOI] [PubMed] [Google Scholar]
- Frautschy SA, Hu W, Kim P, Miller SA, Chu T, Harris-White ME, Cole GM. Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol. Aging. 2001;22:993–1005. doi: 10.1016/S0197-4580(01)00300-1. [DOI] [PubMed] [Google Scholar]
- Gasparova Z, Janega P, Stara V, Ujhazy E. Early and late stage of neurodegeneration induced by trimethyltin in hippocampus and cortex of male Wistar rats. Neuroendocrinol Lett. 2012;33:689–696. [PubMed] [Google Scholar]
- Geloso MC, Corvino V, Cavallo V, Toesca A, Guadagni E, Passalacqua R, Michetti F. Expression of astrocytic nestin in the rat hippocampus during trimethyltin-induced neurodegeneration. Neurosci Lett. 2004;357:103–106. doi: 10.1016/j.neulet.2003.11.076. [DOI] [PubMed] [Google Scholar]
- Geloso MC, Corvino V, Michetti F. Trimethyltin-induced hippocampal degeneration as a tool to investigate neurodegenerative processes. Neurochem Int. 2011;58:729–738. doi: 10.1016/j.neuint.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Geloso MC, Vercelli A, Corvino V, Repici M, Boca M, Haglid K, Zelano G, Michetti F. Cyclooxygenase-2 and caspase 3 expression in trimethyltin-induced apoptosis in the mouse hippocampus. Exp Neurol. 2002;175:152–160. doi: 10.1006/exnr.2002.7866. [DOI] [PubMed] [Google Scholar]
- Giacobini E. Long term stabilizing effect of cholinesterase inhibitors in the therapy of Alzheimer’s disease. J. Neural Transm. Suppl. 2002. pp. 181–187. [DOI] [PubMed]
- Ishikura N, Tsunashima K, Watanabe K, Nishimura T, Minabe Y, Kato N. Neuropeptide Y and somatostatin participate differently in the seizure-generating mechanisms following trimethyltin-induced hippocampal damage. Neurosci Res. 2002;44:237–248. doi: 10.1016/S0168-0102(02)00132-3. [DOI] [PubMed] [Google Scholar]
- Kaur S, Chhabra R, Nehru B. Ginkgo biloba extract attenuates hippocampal neuronal loss and cognitive dysfunction resulting from trimethyltin in mice. Phytomedicine. 2013a;20:178–186. doi: 10.1016/j.phymed.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Kaur S, Nehru B. Alteration in glutathione homeostasis and oxidative stress during the sequelae of trimethyltin syndrome in rat brain. Biol Trace Elem Res. 2013b;153:299–308. doi: 10.1007/s12011-013-9676-x. [DOI] [PubMed] [Google Scholar]
- Kim JK, Bae H, Kim MJ, Choi SJ, Cho HY, Hwang HJ, Kim YJ, Lim ST, Kim EK, Kim HK, Kim BY, Shin DH. Inhibitory effect of Poncirus trifoliate on acetylcholinesterase and attenuating activity against trimethyltin-induced learning and memory impairment. Biosci Biotechnol Biochem. 2009;73:1105–1112. doi: 10.1271/bbb.80859. [DOI] [PubMed] [Google Scholar]
- Kim BK, Tran HY, Shin EJ, Lee C, Chung YH, Jeong JH, Bach JH, Kim WK, Park DH, Saito K, Nabeshima T, Kim HC. IL-6 attenuates trimethyltin-induced cognitive dysfunction via activation of JAK2/STAT3, M1 mAChR and ERK signaling network. Cell Signal. 2013;25:1348–1360. doi: 10.1016/j.cellsig.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Koda T, Kuroda Y, Imai H. Protective effect of rutin against spatial memory impairment induced by trimethyltin in rats. Nutr Res. 2008;28:629–634. doi: 10.1016/j.nutres.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Kotani S, Yamauchi T, Teramoto T, Oqura H. Pharmacological evidence of cholinergic involvement in adult hippocampal neurogenesis in rats. Neuroscience. 2006;142:505–514. doi: 10.1016/j.neuroscience.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Lee YM, Cheng PY, Chen SY, Chung MT, Sheu JR. Wogonin suppresses arrhythmias, inflammatory responses, and apoptosis induced by myocardial ischemia/reperfusion in rats. J Cardiovasc Pharmacol. 2011;58:133–142. doi: 10.1097/FJC.0b013e31821a5078. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Schwarzer C, Furtinger S, Imai H, Kato N, Sperk G. Changes in the GABA-ergic system induced by trimethyltin application in the rat. Brain Res Mol Brain Res. 2001;97:1–6. doi: 10.1016/S0169-328X(01)00278-9. [DOI] [PubMed] [Google Scholar]
- Park HJ, Shim HS, Ahn YH, Kim KS, Park KJ, Choi WK, Ha HC, Kang JI, Kim TS, Yeo IH, Kim JS, Shim I. Tremella fuciformis enhances the neurite outgrowth of PC12 cells and restores trimethyltin-induced impairment of memory in rats via activation of CREB transcription and cholinergic systems. Behav Brain Res. 2012;229:82–90. doi: 10.1016/j.bbr.2011.11.017. [DOI] [PubMed] [Google Scholar]
- Park HJ, Shim HS, Choi WK, Kim KS, Bae H, Shim I. Neuroprotective Effect of Lucium chinense Fruit on Trimethyltin-Induced Learning and Memory Deficits in the Rats. Exp Neurobiol. 2011;20:137–143. doi: 10.5607/en.2011.20.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York, U.S.A: 1986. pp. 54–85. [Google Scholar]
- Piao HZ, Choi IY, Park JS, Kim HS, Cheong JH, Son KH, Jeon SJ, Ko KH, Kim WK. Wogonin inhibits microglial cell migration via suppression of nuclear factor-kappa B activity. Int Immunopharmacol. 2008;8:1658–1662. doi: 10.1016/j.intimp.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122:647–657. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Corsini E, Villa P, Ghezzi P, Garau A, Galli CL, Marinovich M. Erythropoietin protects primary hippocampal neurons increasing the expression of brain-derived neurotrophic factor. J Neurochem. 2005;93:412–421. doi: 10.1111/j.1471-4159.2005.03033.x. [DOI] [PubMed] [Google Scholar]
- Yang M, Kim J, Kim T, Kim SH, Kim JC, Kim J, Takayama C, Hayashi A, Joo HG, Shin T, Moon C. Possible involvement of galectin-3 in microglial activation in the hippocampus with trimethyltin treatment. Neurochem Int. 2012;61:955–962. doi: 10.1016/j.neuint.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Zhao B. Natural antioxidants protect neurons in Alzheimer’s disease and Parkinson’s disease. Neurochem Res. 2009;34:630–638. doi: 10.1007/s11064-008-9900-9. [DOI] [PubMed] [Google Scholar]