Abstract
Background and Purpose
Cortical vein thrombosis (CVT) is an uncommon site of involvement in cerebral sinovenous thrombosis (CSVT). Few reports have described pediatric CVT, and none have differentiated its unique attributes. This study assessed the clinical features and radiographic outcome of a cohort of children with CSVT, comparing those with CVT to those without CVT.
Methods
Children diagnosed with CSVT were retrospectively reviewed and separated into two groups based on the presence or absence of cortical vein involvement.
Results
Fifty patients met inclusion criteria, including 12 with CVT. The CVT group was more likely to present with seizure (P=0.0271), altered mental status (P=0.0271), and a family history of clotting disorder (P=0.0477). Acute imaging of the CVT group more commonly demonstrated concurrent superior sagittal sinus thrombosis (P=0.0024), parenchymal hemorrhage (P=0.0141), and restricted diffusion (P<0.0001). At follow up, the CVT group more commonly showed headache, seizure, and/or focal neurologic deficit (P=0.0449), and venous infarction (P=0.0007).
Conclusions
In our cohort, CVT was significantly associated with seizures at presentation, hemorrhage and restricted diffusion on acute imaging, as well as neurologic disability and venous infarction at follow up. Involvement of cortical veins in CSVT is associated with an increased risk of infarction and adverse outcome in children.
Keywords: Cerebral venous thrombosis, pediatric stroke, brain infarction, hemorrhage
INTRODUCTION
While cerebral sinovenous thrombosis (CSVT) in children is rare, with an estimated incidence of 0.67 cases per 100,000 children per year [1], thrombosis of cortical veins is even less common. Cortical vein thrombosis (CVT), either isolated to the cortical veins or coupled with dural sinus thrombosis, is an easily overlooked diagnosis [2,3]. Few reports exist in children with CVT [1,4–7], and none has distinguished the unique features of CVT in the broader category of CSVT. In adults, thrombosis of cortical veins has been associated with increased risk of infarction [3], but little is known about the significance of CVT in children.
Hence, we sought to retrospectively describe the clinical presentation, presumed risk factors, and imaging findings in a cohort of children with CSVT presenting to a single tertiary center; and report the prevalence of CVT in this group. We then compared children with CVT (with or without dural sinus thrombosis) to those with CSVT but without CVT during the same time periods, hypothesizing that cortical vein involvement may portend a worse outcome.
METHODS
From 2006 to 2014, children (<18 years of age at symptom onset) treated at Children’s Hospital Colorado for CSVT in our CSVT clinic were enrolled in a cohort study investigating thromboembolic events. Following approval by the institutional review board (COMIRB 05-0339), informed consent/assent was acquired during visits at our multidisciplinary CSVT clinic in an outpatient setting. Although all inpatients seen by our stroke neurologists were referred to the CSVT clinic, patients not seen in our clinic were not approached for the study.
Demographic and clinical information, including age at diagnosis, sex, race, ethnicity, presenting signs and symptoms, treatment, and family history of clotting disorder, were documented for each patient via chart review at the time of enrollment, and again in August 2014. In addition, we recorded any report of seizure, headache, cranial nerve palsy, hemiparesis, or paresthesia at follow up. Imaging was obtained on a clinical basis and categorized as acute (within 14 days of diagnosis), subacute (6 weeks to 6 months after diagnosis), or chronic (12 months after diagnosis). A pediatric neuroradiologist (N.V.S.) evaluated imaging for venous clot locations, degree of occlusion, and parenchymal abnormality.
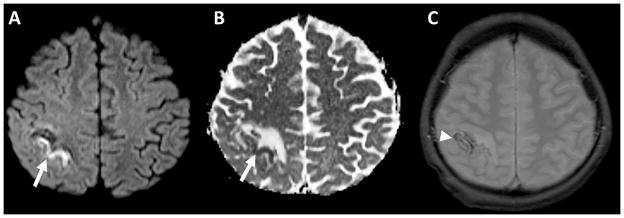
CSVT was diagnosed by identifying dural venous sinus filling defects on post-contrast MRI or CT, or by a lack of segmental dural sinus flow-related enhancement on non-contrast time-of-flight MR venography. CVT was diagnosed by identifying cord-like hypointensity corresponding to cortical veins on gradient echo images [8], or abnormal cord-like density on non-contrast head CT with corresponding non-enhancement on CT venography (Figure 1). Parenchymal abnormalities on acute imaging, including restricted diffusion or hemorrhage (Figure 2), were recorded. Subacute and/or chronic images, when available, were evaluated for clot resolution and venous infarction, as evidenced by parenchymal T2 hyperintensity.
Figure 1.
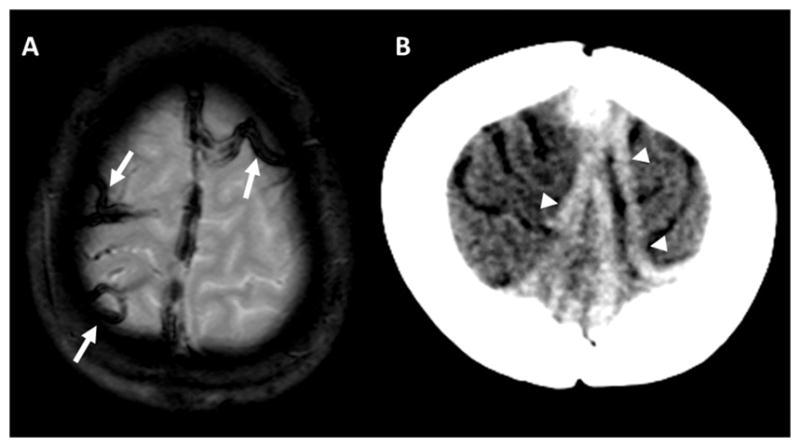
A) Gradient echo image demonstrates thickened, cord-like areas of hypointensity over the vertex, compatible with thrombosed cortical veins (arrows). B) Non-contrast head CT in a different patient demonstrates abnormal, cord-like densities over the vertex extending to the superior sagittal sinus, also compatible with CVT (arrowheads).
Figure 2.
Diffusion-weighted image A) and apparent diffusion coefficient map B) demonstrates a gyriform focus of restricted diffusion (arrows) surrounded by increased diffusivity, characteristic of acute venous ischemia. C) Gradient echo images in the same patient demonstrate foci of susceptibility compatible with hemorrhage (arrowhead).
Patients with cortical vein involvement (CVT group) were compared to patients with CSVT but without CVT (non-CVT group) using Fisher’s exact test for categorical data and Student’s t-test for continuous data, with P<0.05 considered significant.
RESULTS
Fifty patients with CSVT were identified, including seven neonates and 43 children. CSVT was diagnosed on acute imaging by MRI in 43 patients and by CT venography alone in seven. Our cohort included 42/50 patients with subacute imaging, and 13/50 with chronic imaging. The most common locations of thrombosis were the right transverse sinus (58%) and right sigmoid sinus (52%), although most patients demonstrated obstruction of multiple sites (Table 1).
Table 1.
Imaging Characteristics
| All (n=50) | CVT (n=12) | Non-CVT (n=38) | P value | |
|---|---|---|---|---|
| Acute sites of thrombosis, n (%) | ||||
| Superior Sagittal Sinus | 20 (40) | 10 (83) | 10 (26) | 0.0024 |
| Right Transverse Sinus | 29 (58) | 7 (58) | 22 (57) | 1.0000 |
| Left Transverse Sinus | 13 (26) | 1 (8) | 12 (31) | 0.1466 |
| Straight Sinus/Deep | 7 (14) | 2 (16) | 5 (13) | 1.0000 |
| Right Sigmoid Sinus | 26 (52) | 5 (41) | 21 (55) | 0.5144 |
| Left Sigmoid Sinus | 14 (28) | 2 (16) | 12 (31) | 0.4682 |
| Cavernous Sinus | 2 (4) | 0 (0) | 2 (5) | 1.0000 |
| Right Internal Jugular Vein | 21 (42) | 5 (41) | 16 (42) | 1.0000 |
| Left Internal Jugular Vein | 12 (24) | 2 (16) | 10 (26) | 0.7046 |
| Cortical Veins | 12 (24) | 12 (100) | 0 (0) | - |
| Vein of Labbé | 4 (33) | 4 (33) | - | - |
| Vein of Trolard | 8 (67) | 8 (67) | - | - |
| OSCV | 8 (67) | 8 (67) | - | - |
| Parenchymal hemorrhage, n (%) | 11 (22) | 6 (50) | 5 (13) | 0.0141 |
| Acute restricted diffusion, n (%)* | 12/43 (28) | 9/12 (75) | 3/31 (10) | <0.0001 |
| Venous infarction, n (%) | 10 (20) | 7 (58) | 3 (7) | 0.0007 |
OSCV=other superior cerebral veins
43/50 patients underwent acute MRI scan
Cortical vein involvement was identified in 12 patients (10/12 had CVT with coexistent dural sinus thrombosis). The vein of Trolard and other superior cerebral veins draining into the superior sagittal sinus were thrombosed in 10/12 patients, and the vein of Labbé was thrombosed in 4/12. The associated dural venous sinuses were fully obstructed in all 10 CVT cases with coexistent dural venous sinus thrombosis (Tables I and II in the online-only Data Supplement).
Compared to the non-CVT group, the CVT group was more likely to have parenchymal hemorrhage (P=0.0141), acute restricted diffusion (P<0.0001), venous infarction (P=0.0007), and concurrent superior sagittal sinus thrombosis (P=0.0024). The five non-CVT patients with parenchymal hemorrhage were all neonates. Foci of restricted diffusion, hemorrhage, and infarct were always located in venous territory drained by the corresponding thrombosed veins.
In cases where follow-up imaging was available, complete or near-complete resolution of thrombosis, i.e. recanalization, occurred in 79% of patients by the subacute period and 75% by the chronic period, without significant difference between the CVT and non-CVT groups.
Common presenting signs and symptoms in the cohort included headache (64%) and focal neurologic deficit (34%). The CVT group more frequently presented with seizure (P=0.0271), altered mental status (P=0.0271), and family history of clotting disorder (P=0.0477; Tables III and IV in the online-only Data Supplement).
All patients were discharged home in stable condition. During the subacute follow-up period, the CVT group was more likely to present with headache, seizure, and/or focal neurologic deficit (P=0.0449). Two patients died within one year of diagnosis.
The majority of patients in the cohort were anticoagulated at diagnosis with either heparin (84%) or warfarin (4%). Anticoagulation was withheld when intracranial hemorrhage was suspected. Two patients received catheter-directed tPA followed by heparin, and one received aspirin. Treatment was relatively homogeneous between the two groups (Table V in the online-only Data Supplement).
DISCUSSION
To our knowledge, this is the first study describing the unique differences in presentation and radiographic outcome between children with CSVT involving cortical veins (CVT group) and those without cortical vein involvement (non-CVT group). Despite the rarity of reported pediatric CVT, nearly a quarter of our CSVT cohort had cortical vein involvement.
The CVT group in our cohort was strongly associated with restricted diffusion, parenchymal hemorrhage, and venous infarction. These findings are similar to those previously reported in the adult CVT literature. In a cohort of patients with isolated CVT, of whom the vast majority were adults, 81% of patients had an associated parenchymal brain lesion [7]. In another study, thrombosis of cortical veins was associated with an increased risk of infarction, while thrombosis of large sinuses was comparably well tolerated [3]. Collectively, these data suggest that CVT is correlated with the presence of parenchymal lesions, although additional factors, such as age at thrombosis, may be contributory.
Despite treatment and eventual recanalization, many children in our CSVT cohort displayed neurologic disability at follow up. The patients with cortical vein involvement were at greater risk for continued neurologic disability, corroborating their adverse imaging findings. The majority of the CVT group experienced recanalization between six weeks to six months, yet these patients were significantly more likely to have headache, seizure and/or focal neurologic deficit than those in the non-CVT group during the same time interval, suggesting that thrombosis of cortical veins may be associated with neurologic disability even after the clot resolves. While intriguing, our findings need to be validated in a larger CSVT cohort aimed at prospectively examining functional outcomes. Further work on the clinical-radiographic evolution of venous infarction in the developing brain is also warranted.
Limitations of this study included its small sample size, retrospective design, and lack of comprehensive follow-up imaging. Improvements in neuroimaging quality that occurred over the eight-year study period could have impacted the incidence of CSVT and CVT diagnoses. Furthermore, the incidence of CVT could have been underestimated because seven children only underwent CT venography acutely, which is likely less sensitive than MRI for cortical vein involvement. We also acknowledge that there may have been a selection bias towards enrolling patients with more significant symptoms, as patients were recruited in a tertiary care setting.
In summary, we investigated a cohort of children with CSVT and found that those with involvement of the cortical veins were more likely to have seizures at presentation, hemorrhage and restricted diffusion on acute imaging, as well as venous infarction and neurologic disability at follow up. Clinicians and radiologists evaluating children with acute CSVT should assess for cortical vein involvement and be aware of its influence on presentation and potential brain injury.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This research was supported by the American Stroke Association/Bugher Foundation Stroke Collaborative Research Center (Grant 14BFSC17540000), NIH/National Center for Advancing Translational Sciences Colorado Clinical & Translational Sciences Institute (Grant UL1 TR001082), the American Heart Association Predoctoral Fellowship to Z.R. (Award 15PRE25550151), and the Maternal and Child Health Bureau (MCHB) 340B program at the Mountain States Hemophilia and Thrombosis Center.
Footnotes
DISCLOSURES
None.
References
- 1.deVeber G, Andrew M, Adams C, Bjornson B, Booth F, Buckley DJ, et al. Cerebral sinovenous thrombosis in children. New England Journal of Medicine. 2001;345:417–423. doi: 10.1056/NEJM200108093450604. [DOI] [PubMed] [Google Scholar]
- 2.Bousser MG. Cerebral venous thrombosis: diagnosis and management. Journal of Neurology. 2000;247:252–258. doi: 10.1007/s004150050579. [DOI] [PubMed] [Google Scholar]
- 3.Bergui M, Bradac G, Daniele D. Brain lesions due to cerebral venous thrombosis do not correlate with sinus involvement. Neuroradiology. 1999;41:419–424. doi: 10.1007/s002340050775. [DOI] [PubMed] [Google Scholar]
- 4.Sebire G, Tabarki B, Saunders DE, Leroy I, Liesner R, Saint-Martin C, et al. Cerebral venous sinus thrombosis in children: Risk factors, presentation, diagnosis and outcome. Brain. 2005;128:477–489. doi: 10.1093/brain/awh412. [DOI] [PubMed] [Google Scholar]
- 5.Wasay M, Dai AI, Ansari M, Shaikh Z, Roach ES. Cerebral venous sinus thrombosis in children: A multicenter cohort from the United States. Journal of Child Neurology. 2008;23:26–31. doi: 10.1177/0883073807307976. [DOI] [PubMed] [Google Scholar]
- 6.Kenet G, Kirkham F, Niederstadt T, Heinecke A, Saunders D, Stoll M, et al. Risk factors for recurrent venous thromboembolism in the European collaborative paediatric database on cerebral venous thrombosis: A multicentre cohort study. The Lancet Neurology. 2007;6:595–603. doi: 10.1016/S1474-4422(07)70131-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coutinho JM, Gerritsma JJ, Zuurbier SM, Stam J. Isolated cortical vein thrombosis: Systematic review of case reports and case series. Stroke. 2014;45:1836–1838. doi: 10.1161/STROKEAHA.113.004414. [DOI] [PubMed] [Google Scholar]
- 8.Leach JL, Strub WM, Gaskill-Shipley MF. Cerebral venous thrombus signal intensity and susceptibility effects on gradient recalled-echo MR imaging. AJNR. 2007;28:940–945. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.