Abstract
This study assessed whether premenstrual symptomatology and/or sleep characteristics explain increased luteal phase psychophysiological reactivity to laboratory stressors. We hypothesized that: (1) premenstrual symptoms and sleep characteristics would explain greater luteal versus follicular phase psychophysiological reactivity, (2) symptoms and sleep characteristics would differentially predict psychophysiological reactivity within each cycle phase, and (3) symptoms and sleep characteristics would interact to affect luteal but not follicular reactivity. Freely cycling women (N=87) completed two laboratory sessions, one follicular (cycle days 5–9) and one luteal (days 7–10 post-ovulation). We employed two stressors: one physical (cold pressor task) and the other cognitive in nature (Paced Auditory Serial Addition Task). During testing, electrocardiography monitored heart rate (HR) while a timed and auto-inflatable sphygmomanometer assessed blood pressure (BP). Participants also completed a one-time self-report measure of sleep characteristics and premenstrual symptomatology as well as a measure of state anxiety pre-post stressor. Results revealed greater luteal HR and systolic BP reactivity compared to follicular reactivity (p<0.001 for both analyses), however neither premenstrual symptoms nor sleep characteristics explained this luteal increase. Within cycle analyses revealed that symptoms and sleep characteristics interacted to affect luteal phase state anxiety reactivity (R2=.32, p=.002) with negative affect being associated with more reactivity when sleep hours were low (β=.333, p=.04). Overall, significant relationships existed during the luteal phase only. Findings are discussed in terms of clinical utility and methodological challenges related to performing laboratory stress testing in women.
Keywords: Hemodynamic reactivity, Premenstrual symptomatology, Sleep, Menstrual cycle, Stress, State anxiety, Laboratory stressor, Women
1. Introduction
Research explicating the psychophysiological stress processes in women has identified stress response variations due to the menstrual cycle phase of testing. For example, the hypothalamic–pituitary–adrenal (HPA) axis reactivity, assessed by salivary cortisol responses to laboratory stressors in healthy freely cycling women, is greater during the luteal menstrual cycle phase compared to the follicular menstrual cycle phase (Kajantie and Phillips, 2006; Kirschbaum et al., 1999; Lustyk et al., 2010; Tersman et al., 1991). These findings align with observed increases in psychophysiological reactivity to laboratory stressors during the luteal phase compared to the follicular phase of the menstrual cycle (Lustyk et al., 2010; Sato and Kiuchi, 1995; Tersman, et al., 1991). What remains to be elucidated are the factors that explain the luteal phase increases in stress responses to laboratory stressors and factors that distinguish themselves from affecting follicular phase reactivity.
One potential factor is premenstrual symptomatology. It is well documented that the majority of freely cycling women of reproductive age experience some degree of negative symptomatology during the luteal phase of the menstrual cycle (Campagne and Campagne, 2007; Dell, 2004). Symptoms include affective and somatic changes that vary in severity from molimina, the subclinical level of premenstrual symptomatology experienced by most women (Campagne and Campagne, 2007), to premenstrual dysphoric disorder, the clinical diagnosis assigned to severe premenstrual symptomatology that impairs quality of life and carries considerable disease burden (Halbreich et al., 2003; Lustyk and Gerrish, 2010; Ross and Steiner, 2003).
Studies that systematically investigate the role of premenstrual symptomatology and cycle phase on psychophysiological and neuroendocrine stress responses to a laboratory stressor are scant and those that exist offer discordant findings. For example, Girdler et al. (1993) observed greater peripheral resistance and norepinephrine reactivity in response to a laboratory stressor in women with PMDD compared to healthy control women irrespective of the cycle phase of testing. Conversely, Epperson et al. (2007) found an effect of cycle phase with greater acoustic startle responses during luteal phase testing (defined as days –1 to –7 menses onset) compared to follicular phase testing in women with PMDD. Similarly, Woods et al. (1994) found a significant effect of cycle phase and PMS symptom severity with women meeting criteria for PMS demonstrating greater electromyogram and skin conductance responses to laboratory stressors during the luteal phase of the menstrual cycle (defined as days –1 to –7 menses onset) compared to less symptomatic women. For additional discussion of these and other incongruities see Moline et al. (2003).
In addition to cycle phase and premenstrual symptomatology, another factor that may contribute to stress responses is poor sleep quality. Presently, the relationship of sleep quality to premenstrual symptomatology is not well defined. While the Diagnostic and Statistical Manual of Mental Disorders includes hypersomnia or insomnia among the symptoms of PMDD (APA, 2000), the American College of Obstetricians and Gynecologists’ diagnostic guidelines for PMS do not include sleep disturbances (see Lustyk and Gerrish, 2010 for more discussion). In fact, a clear understanding of cycle phase variation in sleep among women with varying levels of premenstrual symptomatology is lacking. In their review, Shechter and Boivin (2010) summarize research revealing menstrual phase sleep disturbances in women with PMDD and posit that luteal phase decreases in sleep quality and mood could be improved with chronotherapeutic methods aimed at realigning circadian rhythms.
Even less clear are cycle phase sleep characteristics among women with subclinical levels of premenstrual symptomatology and what effects, if any, those characteristics have on stress reactivity. For example, Lee et al. (1990) found that sleep architecture did not change across the menstrual cycle in healthy, freely-cycling women, however, those with premenstrual negative affect had less delta sleep than asymptomatic women. Conversely, Manber and Bootzen (1997) found that sleep latency significantly increased while sleep efficiency and quality significantly decreased during the luteal phase (defined as the six days leading up to menstruation) compared to the follicular phase in healthy freely cycling women. These specific sleep characteristics (i.e., latency, efficiency, and quality) were unrelated to the severity of other premenstrual symptoms. However, there was a significant increase in luteal phase daytime sleepiness reports among women with more severe premenstrual symptomatology. More recently, Schechter et al. (2010) found that circadian variation in slow-wave sleep architecture did not vary by menstrual cycle phase, however, REM sleep was significantly reduced during the luteal phase (defined as days 19–23 post menses). While the authors argue for an interaction between circadian and menstrual cycle influences on REM sleep regulation in women, other premenstrual symptoms were not assessed.
Based on the research cited here, it is reasonable to speculate that many interrelationships among symptoms and sleep characteristics are possible and the combined effects may contribute to stress responses. Given the monetary and time costs of performing repeated polysomnographic testing along with daily symptom monitoring, a reasonable first step towards understanding the interrelationships among premenstrual symptoms, cycle phase of testing, and stress responding is to combine stress testing at two distinct cycle phases (i.e., follicular and luteal) with self-reported measures of sleep quality and premenstrual symptomatology as in the present study.
Thus, the purposes of the current study were to:
determine if premenstrual symptomatology and/or sleep characteristics explain greater luteal versus follicular phase psychophysiological reactivity to a laboratory stressor.
determine if premenstrual symptomatology and/or sleep characteristics differentially predict or interact to affect psychophysiological reactivity to laboratory stressors during the follicular and luteal phases of the menstrual cycle.
We hypothesized that significant between-cycle phase effects would exist. Specifically, we expected both premenstrual symptoms and sleep characteristics to statistically account for the increased luteal phase reactivity. Further, we hypothesized that significant within-cycle phase effects would exist. Specifically, premenstrual symptoms and sleep characteristics would differentially predict responses to the laboratory stressors with symptoms and sleep affecting luteal reactivity but not follicular reactivity. Finally, we hypothesized that symptoms and sleep characteristics would interact to affect luteal phase reactivity but not follicular reactivity.
2. Methods
2.1. Participants
Following the Institutional Review Board approval, participants were recruited through flyer advertisements posted off-campus at various approved locations such as coffee shops, spas, and healthcare clinics. Interested participants were screened over the phone to assess health status and medication use. Participants were free from health problems affecting the heart (e.g., arrhythmia), lungs (e.g., asthma) and nervous system (e.g., seizure disorder) as well as any mental health condition including substance use, eating, mood or psychotic disorders. Participants were not taking psychotropics or any medications known to affect the stress response (e.g., beta blockers). The sample consisted of freely cycling women (N=87) 18–45 years of age (50% between 18 and 21 years, 21% between 22 and 25, and 12% between 26 and 29) with self-reported normal cycle lengths (21–40 days, M=30, SD=4) and who had not nursed a child or experienced a pregnancy or major life stressor (e.g., job loss as verified by the Life Events Questionnaire (Brugha and Cragg, 1990)) within the past 6-months. The majority of the sample reported being physically active (74%) and the average body mass index (BMI) was within normal range (M=22, SD=3). The sample was fairly ethnically diverse with 65% White/Caucasian, 15% Asian/Asian American, 8% Black/African American, 5% Native American/Alaskan, 4% Latino/Hispanic and one participant endorsing “other” for ethnicity. Remuneration was $75.00 for completing all parts of the study, or partial payment was based on level of completion.
2.2. Measures
2.2.1. Demographics and health questionnaire
Participants provided information on their age, ethnicity, physical activity status, and height and weight for calculation of BMI.
2.2.2. Shortened Premenstrual Assessment Form (SPAF)
Developed by Allen et al. (1991), the SPAF was used to assess self-reported premenstrual symptomatology. Participants rated the presence or change in intensity of symptoms typically experienced premenstrually. We chose the SPAF because it assesses change from a base rate, which provides a more sensitive symptom measure than symptom checklists (Halbreich et al., 1982). The 10-items are rated on a 6-point Likert scale ranging from (1) “not present at all or no change from the usual level” to (6) “extreme change, perhaps noticeable even to casual acquaintances.” Scores include an overall summary score ranging from 10 to 60 and three subscale scores for pain, water retention, and affect. Scores on the pain and water retention subscales range from 3 to 18, and for affect, scores range from 4 to 24. The SPAF has been found to have internal consistency (α=.95) with a test–retest coefficient rating of .6 to .7 (Allen et al., 1991). Alpha values in the present study ranged from .81 to .89.
2.2.3. Pittsburg Sleep Quality Index (PSQI)
Developed by Buysse et al. (1988), the PSQI assesses sleep characteristics including quality, disturbance, and the tendency to use sleep medications. Nineteen items are self-rated and five items are rated by a bed partner or roommate (if there is one). Only the self-rated times are included in the scoring which yields seven component scores ranging in value from “0,” indicating “no difficulty,” to “3,” indicating “severe difficulty.” The components are: (1) sleep quality, (2) sleep latency, (3) sleep duration, (4) habitual sleep efficiency, (5) sleep disturbance, (6) use of sleep medications, and (7) daytime dysfunction. When summed, the 7 component scores yield the global severity index (GSI). While the GSI (score ranges from 0 to 21) can be used in clinical contexts to diagnose sleep problems, researchers often find it more meaningful (as well as statistically justified; see: Royston et al., 1995) to allow the measures of sleep duration, latency, and efficiency to remain continuous for analyses rather than binning the data using the suggested coding system. This method was adopted in the present study. Thus, sleep duration is presented in hours spent asleep each night, sleep latency is the time it takes a person to fall asleep and is presented in minutes, and sleep efficiency is the percentage of time spent in bed that was actual sleep time. For the data that were collected in bins, component scores were calculated. These variables included: sleep quality, sleep disturbance, use of sleep medications, and daytime dysfunction.
2.2.4. State-Trait Anxiety Inventory (STAI; Spielberger et al., 1983)
To measure subjective feelings of psychological stress we utilized the state portion of the STAI which is a measure of psychological distress occurring in the present moment. Using a 4-point scale ranging from (1) not at all to (4) very much so, women rated their present moment feelings of tension, upset, and nervousness pre and post stressor. According to Spielberger et al. (1983) the state measure has good internal reliability (alpha=.86–.95) and acceptable test retest reliability (alpha=.16–.62) as is expected with transient emotional states. The STAI-state is routinely used in laboratory stress testing procedures to assess subjective feelings of psychological stress (Lustyk et al., 2010).
2.3. Apparatus
Blood pressure (BP) was measured with an automatic sphygmomanometer (Dinamap 1846: Critikon, Inc., Tampa, FL). Heart rate was continuously monitored via 3-lead electrocardiography (ECG) using the PowerLab data acquisition system (Powerlab 800; ADinstruments, Boulder, CO).
2.4. Stressor tasks
Two tasks were completed, one physical and the other cognitive in nature. The former was the cold pressor task (Cuddy et al., 1966) which involved submerging the non-dominant hand in a warm water (35–37 °C) bath for 4 min, followed by up to 2 min of cold water submersion (1–3 °C) then transferring the hand back to the warm water bath for a final 4 min. The Paced Auditory Serial Addition Task (PASAT; Gronwall, 1977) served as the cognitive stressor and involved four trials of 50 single digit numbers, audibly presented with increasing rapidity at rates of 2.4, 2.0, 1.6, and 1.2 s per digit. Participants were instructed to add the number they heard to the number immediately preceding it rather than keeping a running total. Practice trials confirmed participants’ understanding of the task.
2.5. Procedure
Participants completed two stress testing sessions: one during their follicular menstrual cycle phase (cycle days 5–9) and the other during their luteal phase (days 7–10 post-confirmed ovulation). The latter cycle phase was chosen to target the symptomatic luteal phase. Briefly, the American College of Obstetricians and Gynecologists’ guidelines for diagnosis of PMS (ACOG, 2000) and the American Psychiatric Association’s guidelines for diagnosis of PMDD (APA, 2000) require symptom assessment during the 5 to 7 days prior to menses to capture symptom peaks which may occur at that time (Stoddard et al., 2007) and remit with menses onset or the start of the follicular phase (APA, 2000). Research from our labs have shown that the premenses week (days 1 to 7 prior to menses onset) was a robust estimate of the time frame during which ovarian steroids influenced stress reactivity (Woods et al., 1994) and days 7–10 post-ovulation yielded psychophysiological stress reactivity patterns distinct from the follicular phase (Lustyk et al., 2010). As the 7–10 day post-ovulation luteal window also captures the symptom peak period (Stoddard et al., 2007) that same luteal window was used in the present study.
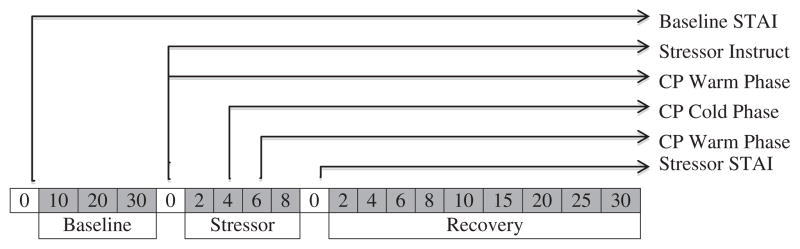
Forty-three women completed the cold-pressor task during their follicular phase followed by the PASAT during the luteal phase; and 44 women completed the tasks in opposite order. Both sessions occurred between 1:00–3:00 p.m., following 24 h of abstinence from alcohol/tobacco use (note: sample self-identified as non-smokers) or medication use including over-the-counter medications or supplements. Participants ate or drank nothing but water an hour before testing and did not engage in vigorous exercise in the morning of testing. These exclusion criteria were verbally confirmed in the morning of testing. At the first testing session, participants provided informed consent and completed the demographic and health questionnaire. Testing sessions consisted of 30 min of baseline, the 10-min stressor task (i.e., cold pressor or PASAT), and 30 min of post-task recovery. ECG was continuously monitored throughout the testing session while BP was taken at 5-min intervals during baseline and recovery and 2-min intervals during the stressor task. The STAI-state was completed just prior to baseline and again immediately post-stressor. A timing schematic indicating data collection time points is provided in Fig. 1.
Fig. 1.
Stress testing protocol. Note: Schematic depiction of the laboratory stress testing session. Each time block represents a time point where blood pressure was administered (e.g. blood pressure was taken at 10, 20, and 30 min during baseline) and heart rate was continuously monitored. STAI = Speilberger Trait Anxiety Inventory-State Measure, Stressor = PASAT or cold pressor, CP = cold pressor.
Following the follicular testing session, participants were given a home ovulation test kit with instructions on when and how to use the kit to confirm ovulation. Participants called the laboratory when their ovulation test was positive to schedule the second, luteal phase testing session. The luteal phase testing session was identical to the follicular session except for the stressor task, which was counterbalanced across the cycle phase. Following the luteal phase testing session, participants were given a take-home packet of questionnaires containing the SPAF and PSQI and instructed to complete them within three days of the luteal test and return them to the researchers via mail. Neither the SPAF nor the PSQI was completed during the follicular phase. Participants were paid $75 upon receipt of the questionnaire packet and confirmation of day one of the cycle following the luteal test. This latter data point was used to confirm that ovulation occurred 14±2 days prior to menses onset, providing further support for the luteal testing window.
3. Data analytic strategy
Data were analyzed using the Statistical Package for the Social Sciences (SPSS Version 18.0). Paired t-tests assessed differences in mean HR, BP, and state anxiety reactivity scores by cycle phase. To determine if premenstrual symptomatology or sleep characteristics explained the cycle dependent effects on psychophysiological reactivity to the laboratory stressor, hierarchical regression was implemented. The effects of the menstrual cycle phase of testing and stressor type on psychophysiological and neuroendocrine responses are detailed in Lustyk et al. (2010). In this secondary analysis we assessed, for the first time, whether sleep characteristics and/or premenstrual symptomatology can account for cycle phase specific psychophysiological reactivity to the laboratory stressors. Reactivity scores were generated using the maximum stressor score minus the average baseline value. Previously, we demonstrated non-significant effects of stressor type on hemodynamic responses (Lustyk et al., 2010). To confirm that stressor type did not significantly impact the present analyses, all regressions were run with the stressor type entered in block one. As we previously observed with RM-ANOVA, all effects of stressor type on physiological reactivity were non-significant, therefore our results are reported here with the stressor type omitted from those models. Again, models assessing effects on psychological stress, (i.e., state anxiety) demonstrated a significant effect of stressor type and as such, those statistics are included here. In instances where interactions among predictors were assessed, predictor variables were mean centered to reduce the effects of multicolinearity (Aiken and West, 1991).
4. Results
4.1. Premenstrual symptoms and sleep characteristics
Table 1 reports the means, standard deviations, reported ranges and possible ranges of the premenstrual symptoms and sleep variables. There were no significant effects of stressor type on symptoms or sleep characteristics. Women who performed the PASAT during the luteal phase (n=43) reported similar symptomatology (mean PMSR summary score=30.1) and sleep problems (mean global PSQI score=5.9) to those who performed the cold pressor during the luteal phase (n=44; mean PMSR summary score=30.3, p=.93; mean global PSQI score= 6.3, p=.47). All subscale analyses were similarly non-significant (p values ranging from .25 to .95 for symptom subscales and .08 to .89 for sleep subscales).
Table 1.
Premenstrual symptoms and sleep characteristics.
| Variable | Mean (SD) | Reported range | Possible range |
| PMSR summary score | 30.18 (9.4) | 12–51 | 10–60 |
| Affect | 12.80 (4.5) | 4–24 | 4–24 |
| Water retention | 8.24 (3.4) | 3–16 | 3–18 |
| Pain | 9.14 (3.4) | 3–17 | 3–18 |
| Global PSQI score | 6.13 (2.5) | 2–13 | 0–21 |
| Sleep hours | 7.08 (1.0) | 4–10 | – |
| Sleep latency (min) | 18.33 (17.6) | 1–120 | – |
| Sleep efficiency | 91.20 (9.9) | 59–118 | – |
| Component scores | |||
| Quality | .89 (.68) | 0–3 | 0–3 |
| Disturbance | 2.14 (.47) | 1–3 | 0–3 |
| Medication | .20 (.55) | 0–3 | 0–3 |
| Daytime dysfunction | 1.17 (.58) | 0–2 | 0–3 |
Note. Descriptive statistics and possible score ranges for the Shortened Premenstrual Assessment Form and the Pittsburg Sleep Quality Inventory (PSQI) administered via take-home packet following the luteal phase testing session. Habitual sleep efficiency was calculated with the following equation: (number of hours slept/number of hours spent in bed)×100. SD = standard deviation; PMSR = premenstrual symptom reports.
Bivariate correlations were run between each of the variables tested in the hierarchical models. Using the Bonferroni correction for 11 correlations (alpha=.005), none of the sleep characteristics proved to be significantly related to premenstrual symptoms. Thus, regression models were tested without concern of significant multicolinearity among the predictors.
4.2. Between cycle analyses
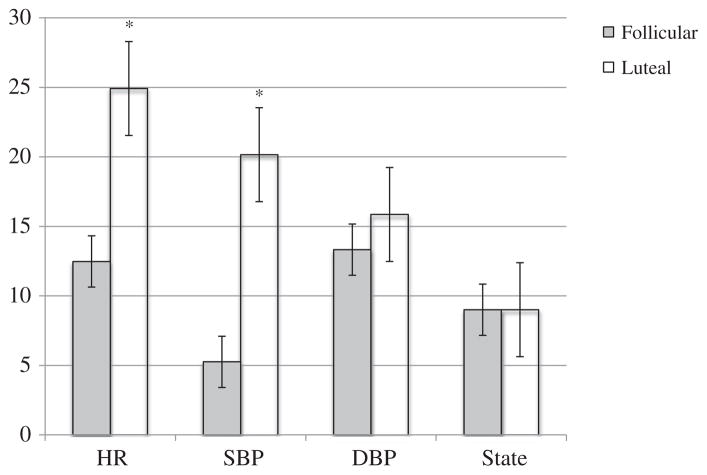
To test our first hypothesis, regression assessed whether premenstrual symptoms and/or sleep characteristics explained the luteal phase increases in psychophysiological reactivity to the stressor. A delta score reflecting the magnitude of change from follicular to luteal phase of testing was calculated for HR, blood pressure and state anxiety using the formula: Δ =luteal reactivity–follicular reactivity. Reactivity scores by cycle phase are depicted in Fig. 2.
Fig. 2.
Mean reactivity scores by cycle phase. Note. Mean changes in physiological and psychological stress reactivity were calculated by subtracting the average baseline value from the maximum stressor value. HR = heart rate and values are reported in beats per minute; SBP = systolic blood pressure and values are reported in mm Hg; DBP = diastolic blood pressure is reported in mm Hg; state = state anxiety which serves as a measure of psychological stress and values reflect Likert scale ratings. Follicular = follicular cycle testing and luteal = luteal cycle testing. Y-axis values vary according to the variable depicted. Significant t-test results are indicated by *p<.05.
As shown in Fig. 2, only HR and SBP demonstrated a significant change across the cycle (HR t (86)=9.21, p<0.001; SBP t (86)=10.865, p<0.001). Thus, to address our first hypothesis, we tested predictive models for HR and SBP only. Hierarchical regression modeling revealed non-significant effects of stressor type on physiological reactivity (HR R2=.00, p=.85; SBP R2=.01, p=.28), therefore our results are reported here collapsing across the stressor type. Table 2 shows the results from hierarchical regression modeling predicting the delta score for HR and SBP from premenstrual symptoms (model 1) and sleep characteristics (model 2). Neither of these models were statistically significant, indicating that neither combination of symptoms or sleep characteristics explained the increased luteal phase reactivity in HR or SBP.
Table 2.
Predicting physiological stress reactivity (N=87).
| Heart rate reactivity
|
Systolic blood pressure
|
Diastolic blood pressure
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Follicular
|
Luteal
|
Follicular
|
Luteal
|
Follicular
|
Luteal
|
|||||||
| β | R2 | β | R2 | β | R2 | β | R2 | β | R2 | β | R2 | |
| Shortened Premenstrual Assessment Form | ||||||||||||
| Premenstrual affect score | .01 | −.37 | −.01 | −.14 | .15 | −.14 | ||||||
| Premenstrual water retention score | .18 | −.03 | −.13 | −.31* | .01 | .04 | ||||||
| Premenstrual pain score | .10 | .19 | .08 | .21 | .03 | .04 | ||||||
| Model 1 | .07 | .03 | .01 | .10* | .03 | .01 | ||||||
| Pittsburgh Sleep Quality Index | ||||||||||||
| Subjective sleep quality | .03 | .09 | .27 | −.10 | .19 | .33* | ||||||
| Minutes to fall asleep | −.04 | −.06 | −.14 | .12 | .03 | .08 | ||||||
| Hours spent asleep | −.07 | −.16 | −.17 | −.21 | .03 | −.02 | ||||||
| Habitual sleep efficiency | −.09 | −.41** | .04 | .09 | .08 | .01 | ||||||
| Sleep disturbance | .08 | .15 | .01 | .07 | −.25 | −.19 | ||||||
| Use of sleep medication | −.001 | −.20* | .01 | .07 | .16 | −.09 | ||||||
| Daytime dysfunction | −.01 | −.04 | .02 | −.14 | .004 | −.02 | ||||||
| Model 2 | .03 | .31** | .10 | .05 | .12 | .06 | ||||||
| Total | .09 | .33** | .11 | .15 | .15 | .08 | ||||||
Note. Hierarchical regression models showing the effects of premenstrual symptoms and sleep characteristics on physiological stress reactivity during the follicular and luteal phases of the menstrual cycle. Model statistics reflect R2 change for addition of the variables listed above it to the model.
p<.05.
p<.01.
4.3. Within cycle analyses
To determine if premenstrual symptomatology and/or sleep characteristics differentially predicted psychophysiological reactivity to the laboratory stressors during each phase of the menstrual cycle, hierarchical models were tested as before only with within cycle phase reactivity scores (i.e., luteal or follicular reactivity) as the criterion. Results of these analyses are shown in Table 2. Again, non-significant effects of stressor type on physiological reactivity were observed (follicular HR R2=.02, p=.20; luteal HR R2=.003, p=.61; follicular SBP R2=.002, p=.69; luteal SBP R2=.002, p=.28; follicular DBP R2=.01, p=.50; luteal DBP R2=.02, p=.24), therefore our results are reported here with stressor type omitted from these models. Significant predictive models were observed with HR and SBP during the luteal phase only. Specifically, the model containing premenstrual symptoms and SBP reactivity was statistically significant as was the model containing sleep characteristics and HR reactivity. While none of the models involving DBP reactivity were statistically significant, diastolic blood pressure was positively related to sleep quality such that higher scores (which reflect poorer sleep quality) were related to higher diastolic blood pressure reactivity during the luteal phase.
The inverse relationships between sleep characteristics and HR reactivity align with our hypotheses. However, as determined at screening and prior to testing, participants were not taking sleep medication during the study, thus the significant relationship with sleep medication use is somewhat unexpected. Women who endorsed a tendency towards the use of sleep medication showed more luteal phase HR reactivity than those who endorsed less of a tendency to medicate. Thus, rather than reflecting an effect of sleep medication per se, this relationship likely reflects a tendency towards medication use.
Contrary to physiological reactivity findings across the cycle phase, state anxiety reactivity was affected by stressor type. Table 3 shows the results from hierarchical regression modeling predicting follicular and luteal state anxiety reactivity from stressor type (model 1), premenstrual symptoms (model 2) and sleep characteristics (model 3). Negative premenstrual affect was positively related to luteal state anxiety reactivity such that greater anxiety reactivity was observed in those with more affective symptomatology. Hours spent asleep was inversely related to luteal state anxiety reactivity such that less anxiety reactivity was observed in those who got more hours of sleep at night. These relationships did not appear during follicular phase testing.
Table 3.
Predicting psychological stress reactivity (N=87).
| State anxiety
|
|||||
|---|---|---|---|---|---|
| Follicular
|
Luteal
|
||||
| β | R | β | R | ||
| Stressor | .68** | −.43** | |||
| Model 1 | .46** | .18** | |||
| β | R2 | β | R2 | ||
| Shortened Premenstrual Assessment Form | |||||
| Premenstrual affect score | −.04 | .20* | |||
| Premenstrual water retention score | −.04 | −.12 | |||
| Premenstrual pain score | .11 | .09 | |||
| Model 2 | .01 | .04 | |||
| Pittsburgh Sleep Quality Index | |||||
| Subjective sleep quality | −.03 | .19 | |||
| Minutes to fall asleep | .15 | −.06 | |||
| Hours spent asleep | .08 | −.34** | |||
| Habitual sleep efficiency | .03 | .13 | |||
| Sleep disturbance | .08 | −.13 | |||
| Use of sleep medication | −.02 | −.01 | |||
| Daytime dysfunction | −.04 | −.09 | |||
| Model 3 | .04 | .13 | |||
| Total | .51 | .35 | |||
Note. Hierarchical regression models showing the effects of premenstrual symptoms and sleep characteristics on psychological stress reactivity during the follicular and luteal phases of the menstrual cycle while controlling for stressor type. Model statistics reflect R2 change for addition of the variables listed above it to the model.
p<.05.
p<.01.
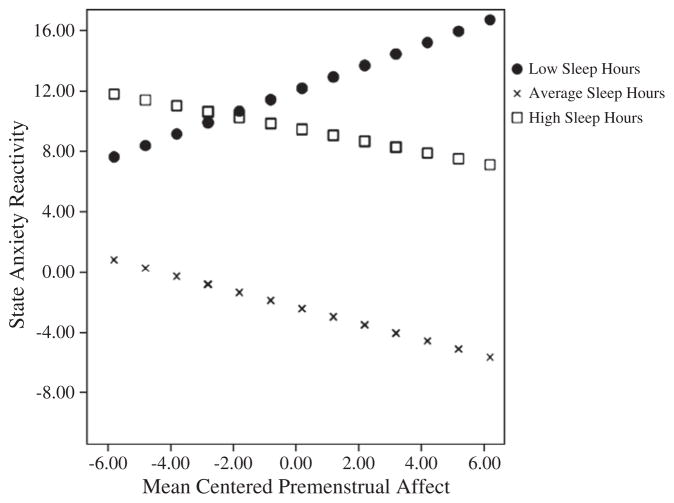
To assess whether premenstrual symptoms and sleep characteristics interacted to affect stress reactivity during the luteal phase, multiple regressions with interaction probing were performed in accordance with the method of Aiken and West (1991). Only one significant interaction emerged between premenstrual affect and hours spent asleep on luteal phase state anxiety reactivity in response to the PASAT. These relationships are depicted in Fig. 3. The model with the interaction was statistically significant, R2=.32, F (3, 43)=6.15, p=.002, accounting for an additional 1% of variance above the model without the interaction, R2=.25, F (2, 43)=6.79, p=.003. While this statistically significant model points to a moderating effect of hours spent asleep on the relationship between premenstrual affect and state anxiety reactivity, the cross-product beta only tended towards significance (cross-product β=−.54; t (43)= −1.98, p=.055). Thus, the significant model indicates that the slopes for the levels of the moderator (i.e., hours spent asleep) significantly differ, however, the increments of variance accounted for by including the interaction in the model is small: R2Δ=.07. To further probe this effect, we performed simple slope analyses in accordance with the methods of Aiken and West (1991). For the moderator (hours spent asleep), separate variables were created that were ±1 standard deviations from the mean resulting in high and low levels of the moderator. These values are shown in Fig. 3 as high sleep hours and low sleep hours, respectively. These variables were then included in separate regression models with the interaction term. As can be seen in Fig. 3, results revealed that low sleep hours augmented the effect of premenstrual negative affect on state anxiety reactivity in response to the PASAT (β=.333, p=.04). Conversely, both high and average sleep hours were associated with inverse relationships between premenstrual negative affect and state anxiety reactivity albeit statistically non-significant (β average sleep=.57 p=.07; β high sleep=−.171 p=.58). In other words, the impact of negative affect on stressor-induced state anxiety reactivity was greatest in women who endorsed the least number of hours of sleep per night. However, the effects of negative affect on stressor-induced state anxiety reactivity was not made significantly better with reportedly more hours of sleep per night but the effect of average sleep was nearly significant.
Fig. 3.
Regression of luteal state anxiety reactivity to the PASAT on mean centered pre-menstrual affect at values of hours spent asleep. Note. The effects of self-reported pre-menstrual negative affect on state anxiety reactivity during the PASAT are marked by sleep hours. The effect of negative premenstrual affect on state anxiety reactivity is only significant for those that endorsed low sleep hours (β low sleep=.333, p=.04; β average sleep=.57 p=.07; β high sleep=−.171 p=.58).
5. Discussion
5.1. Summary
In this study we found that hemodynamic responses to laboratory stressors were greater during the luteal than follicular menstrual cycle phases in women. However, between cycle analyses revealed that the magnitude of increase (assessed via delta scores) was not accounted for by either premenstrual symptoms or sleep characteristics. Conversely, within-cycle analyses revealed that premenstrual symptoms and sleep characteristics explained luteal phase stress responses but not follicular phase stress responses. Moreover, low sleep hours augmented the effects of negative premenstrual symptoms on stressor-induced state anxiety during the luteal phase only. As such, our hypotheses were partially supported. First, neither premenstrual symptoms nor sleep characteristics explained the change in hemodynamic responses from the follicular phase to the luteal phase of the cycle. While we expected negative symptoms and sleep disturbance to affect a woman’s vulnerability to stress reactivity and thus statistically explain the luteal phase increase in reactivity, our findings suggest that other factors are operating to affect the observed luteal increase in hemodynamic reactivity. One obvious possibility is the changing hormonal milieu during the premenstruum. We tested women during days 7–10 post-ovulation, which corresponds with the initial and continued drop-off in progesterone in an approximate 28-day cycle. Withdrawal from progesterone may result in anxiety (Lovick, 2008), water retention (Rosenfeld et al., 2008), and sleep changes (Baker et al., 2007), suggesting that progesterone withdrawal or metabolic components of progesterone such as allopregnenalone (Genazzani et al., 1998) may have affected symptoms, sleep characteristics, and luteal stress reactivity independently. Moreover, the magnitude of increase from the follicular to luteal phase (assessed via delta scores) may be a function of some underlying mechanism relating to the hormonal milieu not captured by symptoms or sleep characteristics and thus neither variable can predict the magnitude of increase.
Conversely, in support of our second hypothesis, within-cycle analyses revealed significant relationships among symptoms, sleep, and stress variables only during the luteal phase. Two models were statistically significant: premenstrual symptoms predicted luteal SBP reactivity (i.e., change from baseline to stressor) and sleep characteristics predicted luteal HR reactivity. With regard to the latter, women with greater sleep efficiency (i.e., it took less time for them to fall asleep once in bed) showed less luteal HR reactivity than women with poor sleep efficiency. In light of the reactivity hypothesis, which posits that heightened hemodynamic reactivity to laboratory stressors is predictive of cardiovascular disease (Krantz and Manuck, 1984), our observation that increased reactivity was a luteal phase phenomenon may have clinical significance and warrants further study.
Another sleep characteristic that was related to luteal HR reactivity was sleep medication use as assessed by the PSQI. This inverse relationship was unexpected and it is difficult to explain. All women were screened for pharmacotherapy and supplement use including drugs and other substance use that would affect the stress response such as sleep medications. Participants were also instructed to avoid taking medication the night before testing. The variable, sleep medication use, is a composite variable where a higher score indicates a greater tendency to use prescribed or over the counter medication to facilitate sleep. Thus, it could rightly be described as a trait variable reflecting a person’s likelihood to self-medicate rather than an effect of medication per se. Given our observations, additional research testing the effects of self-regulatory choice behavior on hemodynamic and psychological responses to laboratory stressors in women seems warranted.
In the statistically significant model involving premenstrual symptoms and SBP, water retention was inversely related to SBP reactivity during luteal testing. One possible explanation is that greater water retention during the luteal phase may reduce the upper range of vascular response producing a kind of ceiling effect on blood pressure. However, this restricted range would likely be reflected in elevated baseline blood pressure, which was not observed. Moreover, as there were no cycle phase differences between mean baseline blood pressures nor a significant relationship between water retention and blood pressure during the follicular phase, the luteal phase SBP and water retention relationship is difficult to understand. Additional research is needed to tease out potential neuroendocrine effects on SBP during the luteal phase including possible effects of antidiuretic hormone, aldosterone, and/or the renin angiotensin system.
While neither the premenstrual symptoms nor sleep characteristic models with DBP were statistically significant, sleep quality was positively related to DBP reactivity during luteal phase testing. Specifically, greater DBP reactivity was associated with poorer sleep quality. This is consistent with previous studies that demonstrate that poor sleep is related to increased blood pressure (e.g., Franzen et al., 2011).
The only model in which sleep characteristics and premenstrual symptoms interacted to affect stressor-induced responses involved state anxiety reactivity during the luteal phase of testing with the PASAT. The interaction accounted for a small amount of variance (i.e., 1%) over and above the independent predictors which was explained by the augmented relationship between low sleep hours and negative premenstrual affect on state anxiety reactivity. While greater sleep hours failed to significantly moderate the relationship between negative premenstrual affect and state anxiety reactivity, it is noteworthy that the effect of average sleep hours on this relationship approached significance (p=.07). Even more noteworthy is the fact that anxiety reactivity was zero or less in women endorsing higher negative premenstrual affect and average sleep hours. Conversely, both low and high sleep hours were associated with much higher state anxiety reactivity across the various levels of negative premenstrual affect. These findings point to a kind of “Goldilocks” relationship among premenstrual affect, sleep, and state anxiety reactivity. That is, the relationship between negative premenstrual affect and state anxiety reactivity may be improved with getting just the right amount of sleep each night, not too much and not too little.
5.2. Limitations
Limitations to this study exist. First, both premenstrual symptoms and sleep characteristics were assessed retrospectively using self-report methods. Repeated assessments over the menstrual cycle with polysomnography may have been more sensitive to nuances in characteristics undetected by self-report while allowing for the assessment of sleep architecture. Whether changes in sleep architecture interact with premenstrual symptoms to affect laboratory stress responses across cycle phases requires further study. Additionally, assessing sleep characteristics the night before testing may have offered some insights into the observed stress responses. With regard to our measure of premenstrual symptomatology, prospective monitoring of symptom changes using daily diary methods may have provided a more accurate assessment of symptom severity. Yet, polysomnography and daily symptom monitoring are costly for both participant (in terms of time investment) and researcher, thus decreasing their feasibility. Additionally, Okamura et al. (2010) found that short sleepers displayed different psychobiological responses to stress than those who slept 6 to 8 h a night, but the present study did not include many individuals who slept less than 5 h. Therefore, the results of this study may not generalize to a sample that shows clinical levels of sleep disturbance. Also, in order to capture one healthy cycle governed by the same set of endocrine tissues across that cycle, we did not randomize cycle phase start (see Lustyk et al., 2010 for details). While this may have introduced uncontrolled ordering effects, our reactivity results do not argue for practice or habituation effects. Because we counterbalanced stressors, participants experienced a novel protocol at each testing session. Finally, due to cost limitations daily blood levels of estrogen and progesterone along with cortisol responses across the entire stress testing protocol were not assessed. Daily quantification of ovarian steroid levels would have provided the most accurate method for documenting time of cycle and assuring the absence of a luteal phase defect. The latter can result in atypical hormone release patterns and cycle length variation beyond that dictated by the follicular phase. Our method of counting back from day one of the cycle following luteal testing provided information on luteal cycle length but did not confirm proper corpus luteum function. As such, there is the possibility that luteal testing occurred for some women when the hormonal milieu differed from the expected plummeting pattern. Combining daily blood draws with ovulation testing would allow for a more accurate confirmation of cycle timing and cycle length as well as characterize the hormonal milieu. Moreover, assessing cortisol responses, both reactivity to and recovery from the stressors, would allow for the assessment of the hypothalamic–pituitary–adrenal axis activity which is influenced by estrogen (Kirschbaum et al., 1999). Given that cortisol can affect sympathetically mediated stress responses once they occur (McEwen and Stellar, 1993), assessing cortisol influences in models of reactivity and recovery during the different cycle phases would extend the present findings. Future research should consider these limitations, and continue to probe the relationship between stress reactivity and menstrual cycle phase as well as sleep characteristics and premenstrual symptoms.
5.3. Conclusions
The present study revealed that sleep characteristics and premenstrual symptoms independently predicted hemodynamic reactivity to laboratory stressors in women tested during the luteal menstrual cycle phase. Conversely, sleep characteristics and symptoms interacted to affect luteal phase psychological stress reactivity (i.e., state anxiety) to the cognitive stressor with negative affect being associated with more reactivity when sleep hours were low. Neither sleep characteristics nor premenstrual symptoms predicted follicular phase reactivity in any form. These findings support prior findings that demonstrate cycle-phase differences in laboratory stress responses; additionally, presenting evidence suggesting that both sleep and symptoms affect luteal phase responses. From a methodological perspective, the absence of relationships during the follicular phase suggests that the follicular window may serve as an ideal and easily identified timeframe for assessing stress in women. This is a timely finding given that a recent report from the IOM (Institute of Medicine, IOM, 2010) found that women are still largely underrepresented in clinical trials positing complications due to menstrual cycle influences on outcome measures as one contributing factor. The IOM further reported that when women are included in clinical trials, data analyses often fail to take gender differences or cycle phase of assessment into account thus hindering scientific advancement. Our results suggest, for stress testing, that an easy control of cycle phase effects can be accomplished by testing women during the follicular phase. Given that neither sleep nor premenstrual symptoms explained the luteal phase increases in hemodynamic responses to the laboratory stressor, future research should continue to explore what variables may contribute to this increase.
References
- Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Sage; Newbury Park, CA: 1991. [Google Scholar]
- Allen SS, McBride CM, Pirie PL. The Shortened Premenstrual Assessment Form. The Journal of Reproductive Medicine. 1991;36:769–772. [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists. Clinical management guidelines for obstetrician–gynecologists: premenstrual syndrome. ACOG Practice Bulletin. 2000;15:1–8. [Google Scholar]
- Baker FC, Kahan TL, Trinder J, Colrain IM. Sleep quality and the sleep electroencephalogram in women with severe premenstrual syndrome. Sleep. 2007;30:1283–1291. doi: 10.1093/sleep/30.10.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugha TS, Cragg D. The list of threatening life experiences: the reliability and validity of a brief life events questionnaire. Acta Psychiatrica Scandinavica. 1990;82:77–81. doi: 10.1111/j.1600-0447.1990.tb01360.x. http://dx.doi.org/10.1111/j.1600-0447.1990.tb01360.x. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1988;28:193–213. doi: 10.1016/0165-1781(89)90047-4. http://dx.doi.org/10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Campagne DM, Campagne G. The premenstrual syndrome revisited. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2007;130:4–17. doi: 10.1016/j.ejogrb.2006.06.020. http://dx.doi.org/10.1016/j.ejogrb.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Cuddy RP, Smulyan H, Keighley JF, Markanson JR, Eich RH. Hemodynamic and catecholamine changes during a standard cold pressor test. American Heart Journal. 1966;71:446–454. doi: 10.1016/0002-8703(66)90209-2. http://dx.doi.org/10.1016/0002-8703(66)90209-2. [DOI] [PubMed] [Google Scholar]
- Dell DL. Premenstrual syndrome, premenstrual dysphoric disorder, and premenstrual exacerbation of another disorder. Clinical Obstetrics and Gynecology. 2004;47:568–575. doi: 10.1097/01.grf.0000135298.39050.b3. http://dx.doi.org/10.1097/01.grf.0000135298.39050.b3. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Pittman B, Czarkowski KA, Stiklus S, Krystal JH, Grillon C. Luteal-phase accentuation of acoustic startle response in women with premenstrual dysphoric disorder. Neuropsychopharmacology. 2007;32:2190–2198. doi: 10.1038/sj.npp.1301351. http://dx.doi.org/10.1038/sj.npp.1301351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen PL, Gianaros PJ, Marsland AL, Hall MH, Siegle GJ, Dahl RE, Buysse DJ. Cardiovascular reactivity to acute psychological stress following sleep deprivation. Psychosomatic Medicine. 2011;73:679–682. doi: 10.1097/PSY.0b013e31822ff440. http://dx.doi.org/10.1097/PSY.0b013e31822ff440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genazzani AR, Petraglia F, Bernadi F, Cassarossa C, Salvestroni C, Tonetti A, Nappi RE, Luisi S, Palumbo M, Purdy RH, Luisi M. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. Journal of Clinical Endocrinology and Metabolism. 1998;83:2099–2103. doi: 10.1210/jcem.83.6.4905. http://dx.doi.org/10.1210/jc.83.6.2099. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Pedersen CA, Stern RA, Light KC. Menstrual cycle and premenstrual syndrome: modifiers of cardiovascular reactivity in women. Health Psychology. 1993;12:180–192. doi: 10.1037//0278-6133.12.3.180. http://dx.doi.org/10.1037//0278-6133.12.3.180. [DOI] [PubMed] [Google Scholar]
- Gronwall DM. Paced auditory serial-addition task: a measure of recovery from concussion. Perceptual and Motor Skills. 1977;44:367–373. doi: 10.2466/pms.1977.44.2.367. http://dx.doi.org/10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Endicott J, Schatch S. Premenstrual syndromes: a new instrument for their assessment. Journal of Psychiatric Evaluation and Treatment. 1982;4:161–164. [Google Scholar]
- Halbreich U, Borenstein J, Pearlstein T, Kahn LS. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD) Psychoneuroendocrinology. 2003;281 doi: 10.1016/s0306-4530(03)00098-2. http://dx.doi.org/10.1016/S0306-4530(03)00098-2. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (IOM) Women’s Health Research: Progress, Pitfalls and Promise. Institute of Medicine; Washington, DC: 2010. [Google Scholar]
- Kajantie E, Phillips DIW. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. http://dx.doi.org/10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer N, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus–pituitary–adrenal axis. Psychosomatic Medicine. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Krantz DS, Manuck SB. Acute psychophysiologic reactivity and risk of cardiovascular disease: a review and methodologic critique. Psychological Bulliton. 1984;96:435–464. http://dx.doi.org/10.1037//0033-2909.96.3.435. [PubMed] [Google Scholar]
- Lee KA, Shaver JF, Giblin EC, Woods NF. Sleep patterns related to menstrual cycle phase and premenstrual affective symptoms. Sleep. 1990;13:403–409. [PubMed] [Google Scholar]
- Lovick TA. Plasticity of GABAA receptor subunit expression during the oestrus cycle of the rat: implications for premenstrual syndrome. Experimental Physiology. 2008;91:655–660. doi: 10.1113/expphysiol.2005.032342. http://dx.doi.org/10.1113/expphysiol.2005.032342. [DOI] [PubMed] [Google Scholar]
- Lustyk MKB, Gerrish WG. Premenstrual syndrome and premenstrual dysphoric disorder: issues of quality of life, stress, and exercise. In: Preedy V, Watson RR, editors. Handbook of Disease Burdens and Quality of life Measures Part 3,3.1. Springer; Heidelberg: 2010. pp. 1951–1975. http://dx.doi.org/10.1007/978-0-387-78665-0_115. [Google Scholar]
- Lustyk MKB, Olson KC, Gerrish WG, Holder A, Widman L. Psychophysiological and neuroendocrine responses to laboratory stressors in women: implications of menstrual cycle phase and stressor type. Biological Psychology. 2010;82:84–92. doi: 10.1016/j.biopsycho.2009.11.003. http://dx.doi.org/10.1016/j.biopsycho.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Manber R, Bootzen RR. Sleep and the menstrual cycle. Journal of Health Psychology. 1997;16:209–214. doi: 10.1037//0278-6133.16.3.209. http://dx.doi.org/10.1053/smrv.2001.0228. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual: mechanisms leading to disease. Archives of Internal Medicine. 1993;153:2093–2101. [PubMed] [Google Scholar]
- Moline ML, Broch L, Zak R, Gross V. Sleep in women across the life cycle from adulthood through menopause. Sleep Medicine Reviews. 2003;7:155–177. doi: 10.1053/smrv.2001.0228. http://dx.doi.org/10.1053/smrv.2001.0228. [DOI] [PubMed] [Google Scholar]
- Okamura H, Tsuda A, Yajima J, Mark H, Horiuchi S, Toyoshima N, Matsuishi T. Short sleeping time and psychobiological responses to acute stress. International Journal of Psychophysiology. 2010;78:209–214. doi: 10.1016/j.ijpsycho.2010.07.010. http://dx.doi.org/10.1016/j.ijpsycho.2010.07.010. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Author; Washington, DC: 2000. text rev. [Google Scholar]
- Rosenfeld R, Livne D, Bevo O, Dayan L, Milloul V, Lavi S, Jacob G. Hormonal and volume dysregulation in women with premenstrual syndrome. Hypertension. 2008;51:1225–1230. doi: 10.1161/HYPERTENSIONAHA.107.107136. http://dx.doi.org/10.1161/hypertensionaha.107.107136. [DOI] [PubMed] [Google Scholar]
- Ross LE, Steiner M. Therapeutic patents for the treatment of premenstrual syndrome and premenstrual dysphoric disorder: historical perspectives and future directions. Expert Opinion on Therapeutic Patents. 2003;13:1491–1499. http://dx.doi.org/10.1517/13543776.13.10.1491. [Google Scholar]
- Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Statistics in Medicine. 1995;25:127–145. doi: 10.1002/sim.2331. http://dx.doi.org/10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- Sato N, Kiuchi K. Adrenergic responsiveness is reduced, while baseline cardiac function is preserved in old adult conscious monkeys. American Journal of Physiology. 1995;269:H1664. doi: 10.1152/ajpheart.1995.269.5.H1664. [DOI] [PubMed] [Google Scholar]
- Schechter A, Varin F, Biovin D. Circadian variation of sleep during the follicular and luteal phases of the menstrual cycle. Sleep. 2010;5:647–656. doi: 10.1093/sleep/33.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter A, Boivin DB. Sleep, hormones, and circadian rhythms throughout the menstrual cycle in healthy women and women with premenstrual dysphoric disorder. International Journal of Endocrinology. 2010 doi: 10.1155/2010/259345. http://dx.doi.org/10.1155/2010/259345. [DOI] [PMC free article] [PubMed]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Stoddard JL, Dent CW, Shames L, Bernstein L. Exercise training effects on premenstrual distress and ovarian steroid hormones. European Journal of Applied Physiology. 2007;99(1):27–37. doi: 10.1007/s00421-006-0313-7. http://dx.doi.org/10.1007/s00421-006-0313-7. [DOI] [PubMed] [Google Scholar]
- Tersman Z, Collins A, Eneroth P. Cardiovascular responses to psychological and physiological stressors during the menstrual cycle. Psychosomatic Medicine. 1991;53:185–197. doi: 10.1097/00006842-199103000-00008. [DOI] [PubMed] [Google Scholar]
- Woods NF, Lentz MJ, Mitchell ES, Kogan H. Arousal and stress response across the menstrual cycle in women with three perimenstrual symptom patterns. Research in Nursing and Health. 1994;17:99–110. doi: 10.1002/nur.4770170205. http://dx.doi.org/10.1002/nur.4770170205. [DOI] [PubMed] [Google Scholar]