Abstract
Activation of the PI3K-mTOR pathway via HER2: HER3-mediated signaling in HER2+ breast cancers pose one of the major threats towards the success of trastuzumab. First, trastuzumab cannot perturb survival/proliferative signals following HER2: HER3 heterodimerization in HER2+ tumor cells. Second, trastuzumab treatment has been reported to cause drug-mediated resistance in over 50% of HER2+ breast cancers. We have reported that treatment with an anti-angiogenic drug imparted a significant anti-tumor advantage when combined with trastuzumab plus pertuzumab in the trastuzumab-resistant model of HER2+ breast cancers (PMID: 23959459). The very fact as revealed by our study that an inclusion of anti-angiogenic drug conferred a significant anti-tumor advantage when combined with dual anti-HER2 therapy clearly indicated a critical and indispensable role of angiogenesis in these tumors. Hence, we hypothesized that BEZ235 a dual PI3K/mTOR inhibitor will have an effect on the tumor as well as the angiogenic stromal compartments. In vitro and in vivo efficacy of BEZ235 was determined in HER2+ trastuzumab-sensitive, trastuzumab-resistant and HER2 amplified/PIK3CA mutated cell lines. BEZ235 alone and in combination with trastuzumab was tested on the tumor as well as stromal compartments. AKT-mTOR signal was suppressed following BEZ235 treatment in a concentration and time-dependent manner. AnnexinV, cl-CASPASE3, SURVIVIN and p-FOXO1 indicated that BEZ235-induced cell death occurred predominantly via an apoptotic pathway. Heregulin-induced HIF1α synthesis was also significantly decreased. Oncoprint data (cBioPortal) representing PAM50 Her2 enriched tumors (TCGA, Nature 2012) and Her2-positive breast tumors (TCGA, cell 2015) showed 91.4% genetic alterations and 79.2% genetic alterations in a set of four genes comprised of PIK3CA, ERBB2, VEGFA and HIF1alpha. The co-occurrence of HIF1alpha with VEGFA in PAM50 Her2 enriched tumors (TCGA, Nature 2012) and the co-occurrence of HIF1alpha with VEGFA pair as well as HIF1alpha with PIK3CA pair in Her2-positive breast tumors (TCGA, cell 2015) were found statistically significant. In xenograft models, BEZ235 blocked tumor growth and decreased Ki67, CD31, p-AKT, p-S6RP, p-4EBP1 IHC-expressions. These decreases were more pronounced when BEZ235 was combined with trastuzumab in HER2+/trastuzumab-sensitive, trastuzumab-resistant and HER2+/PIK3CA mutated models. We demonstrated that combined targeting of HER2 and the PI3K-AKT-mTOR pathway is superior to HER2-directed therapy alone. Mechanistically the inhibition of tumor-induced angiogenesis by BEZ235 caused by the down-regulation of PI3K-mTOR-HIF1alpha signaling irrespective of the trastuzumab-sensitivity status of HER2+ breast cancers proving evidence for the first time that the inhibition of angiogenesis is an important component of the anti-tumor efficacy of BEZ235 in HER2 defined breast cancers.
Keywords: Breast cancer, HER2+, PIK3CA mutation, angiogenesis, apoptosis, trastuzumab-sensitive and trastuzumab-resistant
Introduction
Modern cancer treatment focuses on molecular defects of intracellular signal transduction pathways caused by genetic alterations that drive the oncogenesis. One of the most successful examples is the application of trastuzumab, an HER2-specific humanized monoclonal antibody in the treatment of HER2 amplified breast cancer. The original concept behind this idea is derived from the observation that approximately 20-25% of breast cancer patients overexpress HER2 protein due to the amplification of HER2 gene, a disease driving oncogene [1]. Trastuzumab has been reported to have treatment efficacy in HER2+ breast cancers both in the adjuvant and in the advanced disease settings [2-5]. Several large trials showed that the addition of trastuzumab to chemotherapy in early-stage HER2+ breast cancers significantly improved disease-free survival (DFS) and overall survival (OS) [3,4,6-9]. However many HER2 amplified breast cancers exhibit de novo or develop acquired resistance [2,10,11]. Approximately half of the patients with metastatic disease show up-front resistance to trastuzumab-based therapy and the majority of the patients develop progressive disease with one year of treatment initiation [5,12].
Additionally aberrant expression of the PI3K-AKT-mTOR pathway, downstream of HER2, is also known to play a critical role in cancer cell growth, proliferation, angiogenesis and is also a key factor for developing resistance against trastuzumab. The potential mechanism of trastuzumab-based therapy resistance includes increased signaling via the upregulation of the PI3K-AKT-mTOR pathway due to PIK3CA activating mutation or PTEN loss of function, which eliminates the effects of upstream HER2 inhibition [13]. Results obtained from both in vitro and in vivo studies indicate that mutations in the PIK3CA gene [14-17] or loss of PTEN function [15,17-20] confer resistance to trastuzumab. Recently, Jensen and group demonstrated that HER2+ breast cancer patients with PIK3CA mutations or increased PI3K activity had a significantly poorer survival despite adequate treatment with adjuvant chemotherapy and trastuzumab [21]. In the same line, Cizkova et al. reported from patients’ data (n=80 HER2+ patients) that the outcome of HER2+ patients treated with trastuzumab is significantly worse in patients with PIK3CA mutation compared with wild-type tumors (P=0.0063) [22]. Due to the complex nature of feedback regulation and its divergent endpoints, we hypothesized that targeting multiple nodal points of the PI3K-AKT-mTOR pathway may provide better benefit in the clinic. Interestingly, some of this resistance are mediated through other members of the HER family. In addition to the ligand-independent HER2: HER2 homodimerization in the context of overexpression of HER2, a ligand-induced HER2: HER3 heterodimerization has been known to activate downstream proliferative signals via upregulation of the PI3K-mTOR pathway. Thus, the importance of HER3 may be at least partly related to its potential ability to activate the downstream PI3K-AKT-mTOR pathway [23,24]. This upregulation of the PI3K-mTOR pathway can occur under normal expression levels of HER3 protein and can essentially be responsible for the development of trastuzumab resistance due to the inability of the drug to block the ligand-mediated HER2: HER3 heterodimerization in the tumor cells.
It had become clear that the first generation of compounds to block mTOR (rapalogs) had serious limitations, not the least of which was that cancer cells quickly activated a feedback loop when the mTOR pathway was shut down [25]. The kinase domains of the PI3K and mTOR are very similar, so it is reasonable to expect that organic chemical structure active against PI3K would also be active in mTOR. Indeed, the strength of PI3K/mTOR dual inhibitors (e.g. BEZ235) is that they are potent and effectively deprive tumor cells of the feedback loop that limits the efficacy of mTOR inhibitors. Therapeutic targeting of the PI3K pathway with small-molecule inhibitors may have clinical benefit, either as a single agent in the PI3K pathway-addicted cancers or used broadly in combination with other targeted therapies like trastuzumab in HER2 amplified or with tamoxifen or aromatase inhibitor in ER+ breast cancer.
In our recent article, we have demonstrated that treatment with an anti-angiogenic drug imparted a significant anti-tumor advantage when combined with trastuzumab plus pertuzumab in the trastuzumab-resistant model of HER2+ breast cancers [26]. The very fact as revealed by our study that an inclusion of anti-angiogenic drug conferred a significant anti-tumor advantage when combined with dual anti-HER2 therapy clearly indicated a critical and indispensable role of angiogenesis in these tumors. Hence, we argued that BEZ235 a dual PI3K/mTOR inhibitor will have an effect on the tumor as well as the angiogenic stromal compartments.
Here we describe the preclinical antitumor efficacy of an orally available dual PI3K/mTOR inhibitor BEZ235 either as a single agent or in combination with trastuzumab. The activity of the PI3K-AKT-mTOR pathway inhibitors in combination with classical anticancer agents has proved highly effective in several experimental models [27-29]. Combining drugs targeting different nodal points of the same pathway is likely to be more effective in cells that are addicted to that pathway. We show BEZ235 inhibits PI3K-AKT-mTOR signaling, leading to inhibition of cancer cell viability in diagnostically defined HER2+ breast cancer cells characterized by trastuzumab-sensitive, trastuzumab-resistant or PIK3CA activating mutation. Flow cytometric analysis revealed an accumulation of cells in the G1 phase with concomitant loss of S phase. Analysis of annexin V-positive cells and cleaved CASPASE3 expression indicated that BEZ235-induced cell death through an apoptosis-dependent pathway. Interestingly heregulin-induced HIF1α synthesis, an important angiogenic modulator in breast cancer cells was also significantly decreased following the treatment of BEZ235. Furthermore, we show that BEZ235 is efficacious as a single agent or in combination with trastuzumab in all three xenograft (trastuzumab-sensitive, trastuzumab-resistant or HER2+/PIK3CA mutated) models investigated along with suppression of tumor-induced angiogenesis by decreased expression of CD31, p-VEGFR positive cells and decreased expression of phosphorylation of AKT and S6 ribosomal protein. Our preclinical studies provide a strong mechanistic rationale for clinical development of BEZ235 in the treatment of HER2+ breast cancer, particularly in trastuzumab-resistant breast cancer patients.
Materials and methods
Cell culture
BT474 and SKBR3 human breast cancer cells were obtained from the American Type Culture Collection (ATCC) and BT474HerR, a trastuzumab-resistant derivative obtained by serial passage in the presence of increasing concentrations of trastuzumab (100 µg/ml final maintenance dose), was kindly provided by Dr. Mark Pegram (Stanford University, CA). HER2 overexpressed BT474 and trastuzumab-resistant BT474HerR breast cancer cells were cultured in DMEM supplemented with 10% fetal bovine serum, 1% HEPES (Cellgro, Hemdon, VA) with 100 units/ml penicillin and streptomycin (Cellgro, Hemdon, VA) at 37°C in a humidified atmosphere containing 5% CO2. Trastuzumab-resistant cells were maintained with 100 µg/ml of trastuzumab (Genentech Incorporation, San Francisco, CA). HER2 overexpressing SKBR3 cells were cultured in McCoys 5A (Cellgro, Hemdon, VA) supplemented with 10% fetal bovine serum and 100 units/ml penicillin and streptomycin. HER2+/PIK3CA mutated HCC1954 and UACC893 breast cancer cells were also obtained from ATCC. HCC1954 and UACC893 cells were cultured in RPMI1640 and Leibovitz’s L-15 media (procured from ATCC) respectively at 37°C in a humidified atmosphere containing 5% CO2. Both the media were supplemented with 10% FBS with 100 units/ml penicillin and streptomycin.
Antibodies and reagents
We sincerely thank Novartis for providing BEZ235 and RAD001. Trastuzumab was purchased from Mckesson Pharmacy (Richmond, VA). Antibodies used include phospho-AKT (Ser473, Thr308), AKT, phospho-P70S6K, P70S6K, phospho-S6 ribosomal protein (Ser235/236), S6 ribosomal protein, phospho-4EBP1 (Thr37/46), 4EBP1, phospho-FOXO1, FOXO1, cleaved CASPASE3 (Asp175), phospho-ERK (T202/Y204), ERK and total HER2 were obtained from Cell Signaling Technology (Danvers, MA). A beta-ACTIN antibody was obtained from Sigma (St. Louis, MO). PAK-1 PBD agarose (for RAC1 assay reagent) for pull-down of activated RAC1 and mouse monoclonal antibody against RAC1 were procured from Upstate Biotechnology/Millipore (Temecula, CA). Mouse monoclonal antibody against HIF1α was bought from Becton Dickinson (Bedford, MA). HRP-tagged anti-rabbit IgG, anti-mouse IgG and Chemiluminescence Kit were from Amersham Pharmacia Biotech (Uppsala, Sweden). Recombinant human heregulin-β1 and fibronectin were purchased from Peprotech Inc. (Rocky Hill, NJ) and Becton Dickinson (Bedford, MA) respectively. Matrigel for tumor cell inoculation was purchased from BD laboratories. All other chemicals were purchased from Sigma (St. Louis, MO) unless otherwise stated.
Cell viability assay
Cells were plated in 96-well flat-bottomed plates and cultured for 24 hours in serum containing medium before exposure to a various concentration of drugs (either alone or combination) for 48 hours. Thereafter, a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution was added to each well. After 4 hours of incubation at 37°C, cell solubilization solution (SDS-HCL) was added. Cells were incubated overnight at 37°C and then the optical density was measured at 570 nm using an iMAX microplate reader (BioRad).
3D ON-TOP colony assay
This assay was carried out to examine the effect of BEZ235 on clonogenic growth of HER2+ breast cancer cells. The assay was standardized with little modification from that originally described by Lee et al. [30]. Pictures of the live colonies were taken using an Olympus DP72 digital camera.
Biochemical analysis
Evaluation of the effect of dual PI3K/mTOR inhibitor BEZ235 on the AKT-mTOR pathway activity: 10 cm2 dishes were seeded with five million cells in a volume of 10 ml complete medium followed by incubation at 37°C under 5% CO2 overnight (~16 hours). Cells were treated with indicated concentration of BEZ235 or RAD001 for the time indicated. At the end of the treatment, cells were washed with cold PBS and solubilized with lysis buffer [50 mmol/L Tris-HCL (pH7.6), 150 mmol/L NaCl, 100 mmol/L NaF, 1 mmol/L EDTA, 1 mmol/L EGTA, 0.05% NP40, 1% aprotinin, 0.01 mg/mL leupeptin, and 0.08 mmol/L-phenyl methyl sulfonyl fluoride] for Western blots. For immunoblots, equal amounts of proteins were separated by SDS-PAGE, proteins were transferred to nitrocellulose membrane using Criterion system and protocol from Bio-Rad (Hercules, CA). For HIF1α accumulation study, cells were serum starved for 20 hours in medium lacking FBS and then exposed to heregulin for indicated time in presence or absence of indicated concentration of BEZ235. The whole cell extracts were fractioned by SDS-PAGE, transferred to nitrocellulose membrane, and analyzed with anti-HIF1α mouse monoclonal antibody [31].
GST-fusion protein pull-down assay
The GST-fusion proteins, corresponding to the human PAK1 p21 binding domain (PBD, residues 67-150) were expressed in E. coli. The final protein products were bound to glutathione agarose in liquid suspension, 300 µg of PAK1-PBD in 20 mM PBS, pH7.4, containing 50% glycerol. The 70-80% confluent cells were kept in serum starvation for 18 hours before integrin engagement in the presence of heregulin (fibronectin-coated plates) for different time periods. Stimulated cells were lysed with extraction buffer (25 mM HEPES pH7.5, 150 mM NaCl, 1% Igepol CA630, 10 MgCl2, 1 mM EDTA, 10% glycerol, 10 µg/ml leupeptin, 10 µg/ml aprotinin, 1 mM NaF, and 1 mM Na-orthovanadate). Following centrifugation, 10 µl PAK1-PBD (1 µg/ml) was added per lysate sample and incubate for 45 minutes at 4°C with gentle rocking. Agarose beads were collected (by pulse centrifugation for 10 seconds at 14,000 rpm), washed in extraction buffer, and resuspended in 30 µl Laemmli sample buffer to resolve protein by 15% SDS-PAGE. The membrane was probed with anti-RAC1 monoclonal antibody [32,33].
Data analysis using c-BioPortal
We used cBioportal to find out the percentage of alterations of a set of four genes and co-occurrence between gene pairs within this gene set in two data sets of HER2+ breast cancers. In cBioPortal, OncoPrints are generated for visualizing the alteration of a set of four genes as well as pathway alterations across a set of cases, and for visually identifying trends, such as trends in mutual exclusivity or co-occurrence between gene pairs within a gene set as mentioned earlier [24,34]. Individual genes are represented as rows, and individual cases or patients are represented as columns. We studied the alterations in gene set of PIK3CA, ERBB2, VEGFA and HIF1alpha (amplification, gain, deep deletion, shallow deletion and missense mutation) in Breast Invasive Carcinoma (TCGA, Nature 2012) case set and in Breast Invasive Carcinoma (TCGA, Cell 2015) case sets using c-BioPortal (Figure 6H, 6I). The details of the co-occurrence have been described in the figure legends. cBioPortal data is subjected to scheduled updates.
Figure 6.
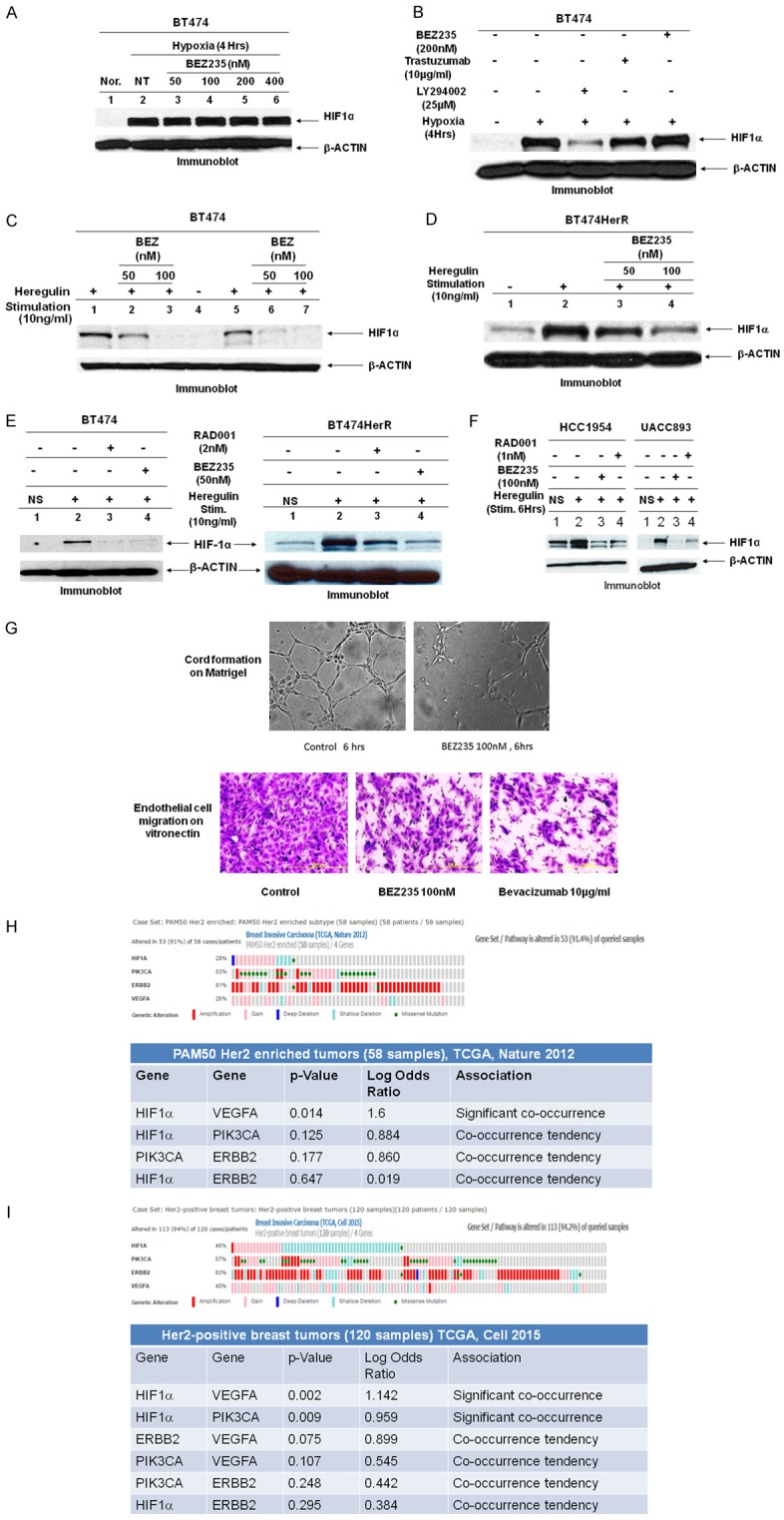
Involvement of PI3K, AKT, and mTOR in signaling from HER2 to HIF1α: (A) Hypoxia-induced HIF1α stabilization is not blocked by PI3K/mTOR dual inhibitor, BEZ235, Nor: normoxia. (B) Effects of kinase inhibitors (BEZ235 or LY294002) or trastuzumab on hypoxia-induced HIF1α stabilization. (C, D) Effects of heregulin-induced HIF1α expression in trastuzumab-sensitive (BT474) (C) and trastuzumab-resistant (BT474HerR) (D) breast tumor cells were abrogated by BEZ235. Lanes 1 & 5 are heregulin stimulation for 6 and 12 hours respectively in (C). Similarly lane 2 is heregulin stimulation for 6 hours in (D). Lanes 2, 3, 6, 7 in (C) and lanes 3, 4 in (D) are preincubated with BEZ235 (indicated concentrations of drug). Lane 4 in (C) and lane 1 in (D) are no stimulation condition. Data suggest that heregulin stimulates HIF1α expression and heregulin-induced HIF1α expression is more in resistance cells. BEZ235 dose-dependently attenuates heregulin-induced HIF1α expression in both these cell lines. (E) Serum-starved cells (BT474 & BT474HerR), was pretreated for 1 hour with vehicle or inhibitor (dual PI3K/mTOR inhibitor, BEZ235 or mTOR1 inhibitor, RAD001) then exposed to no treatment or 10 ng/ml of heregulin for 6 hours prior to HIF1α immunoblot assay of whole cell lysates. (F) Similar experiments were performed with HER2+/PIK3CA mutated cells (HCC1954 & UACC893). Data show that treatment with BEZ235 or RAD001 was associated with decreased HIF1α expression following heregulin stimulation. Furthermore, compare to RAD001, dual PI3K/mTOR inhibitor, BEZ235 was more effective for the inhibition of HIF1α expression/synthesis. (G) Integrin-directed endothelial cell migration is one of the critical steps for tumor-induced angiogenesis. Our data show that BEZ235 inhibits HUVEC cells migration on vitronectin (αvβ3/αvβ5) (lower panel). Bevacizumab was used as a positive control. BEZ235 at 100 nM concentration significantly abrogates endothelial cells tube formation on Matrigel (upper panel). These data indicate that unlike LY294002 (in B lane 3), BEZ235 cannot control hypoxia-mediated HIF1α stabilization; rather a BEZ235 dose-dependently regulates HIF1α expression level following heregulin stimulation. Taken together (expression of HIF1α, endothelial cell migration, and tube formation on Matrigel) our data clearly indicate that BEZ235 effectively controls tumor-induced angiogenesis. (H) Oncoprint data represents samples from two published sets (cBioPortal). The queried samples represent PAM50 HER2 enriched tumors (58 samples; Breast Invasive Carcinoma; TCGA, Nature 2012). A set of four genes comprised of PIK3CA, ERBB2, VEGFA and HIF1alpha was found to be altered in 53 (91.4%) of queried samples. The type of genetic alterations included in both sets is amplification, gain, deep deletion, shallow deletion and a missense mutation. The table of co-occurrent alterations is presented below. Out of four gene pairs with co-occurrent alterations in PAM50 Her2 enriched tumors (58 samples), TCGA, Nature 2012, the co-occurrence between HIF1alpha and VEGF was found statistically significant. p value was derived from Fisher Exact Test. Log Odds Ratio quantifies how strongly the presence or absence of alterations in gene A are associated with the presence or absence of alterations in gene B in the selected tumors, PAM50 Her2 enriched tumors (58 samples; Breast Invasive Carcinoma; TCGA, Nature 2012). Log Odds Ratio>0: Association towards co-occurrence p-Value<0.05: Significant association. The table was generated using cBioPortal. cBioPortal data is subjected to scheduled updates. (I) The second queried samples represent HER2-positive breast tumors (120 samples; Breast Invasive Carcinoma; TCGA, cell 2015). A set of four genes comprised of PIK3CA, ERBB2, VEGFA and HIF1alpha was found to be altered in 95 (79.2%) of queried samples. The type of genetic alterations included in both sets is amplification, gain, deep deletion, shallow deletion and a missense mutation. Oncoprint data represents samples from two published sets (cBioPortal). The table of co-occurrent alterations is presented below. Out of six gene pairs with co-occurrent alterations, of the HER2-positive breast tumors (120 samples) TCGA, Cell 2015 the co-occurrence between HIF1alpha and VEGF pair and HIF1alpha and PIK3CA pair were found statistically significant. p value was derived from Fisher Exact Test. Log Odds Ratio quantifies how strongly the presence or absence of alterations in gene A are associated with the presence or absence of alterations in gene B in the selected tumors, Her2-positive breast tumors (120 samples; Breast Invasive Carcinoma; TCGA, cell 2015). Log Odds Ratio>0: Association towards co-occurrence p-Value<0.05: Significant association. The table was generated using cBioPortal. cBioPortal data is subjected to scheduled updates.
We acknowledge the c-BioPortal for Cancer Genomics site (http://cbioportal.org) which provides a Web resource for exploring, visualizing, and analyzing multidimensional cancer genomics data. The portal reduces molecular profiling data from cancer tissues and cell lines into readily understandable genetic, epigenetic, gene expression and proteomic events (Gao and group 2013, Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the c-BioPortal, Sci. Signal., 2 April, Vol. 6, Issue 269, p. pl1) [35]. We acknowledge works of Cerami and group (The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discovery. May 2012 2; 401) [36] and works of Gao and group (Integrative analysis of complex cancer genomics and clinical profiles using the c-BioPortal. Sci. Signal. 6, pl1, 2013). We also acknowledge the TCGA Research Network for generating TCGA datasets.
Cell migration assays
Transwell assay
Migration assay was performed toward fibronectin (haptotaxis) using a transwell with polycarbonate membrane from Costar (diameter 6.5 mm, pore size 8 μm; Costar Corp., Cambridge, MA). The underside of the membrane to which cells migrate was coated with 20 µg/ml fibronectin in PBS for 1 hour at 37°C. Surfaces were subsequently blocked with heat-denatured BSA. Cells were added at the concentration of 2×105 cells/well (in 100 µl of media containing 0.1% serum) into the upper chamber of the well with or without 10 ng/ml of heregulin and incubated for 4 hours in presence or absence of BEZ235 at indicated concentrations. For negative control, the wells were coated on both sides with fibronectin and cells were added in presence or absence of heregulin. The total numbers of cells on the fibronectin-coated side were quantified as described previously [33,37,38].
Scratch assay
Cells were cultured on 12 well plates coated with 20 µg/ml of fibronectin. Scratches were created in a line across the plates by scraping with a sterile 200 µl standard pipette tip. The scratched monolayers were then washed twice with serum-free media to remove all cell debris and incubated with media containing 0.1% serum and 10 ng/ml of heregulin in presence or absence of BEZ235. Photomicrographs were taken at 0 hours and 24 hours. Quantitative analysis of the scratch was determined by measuring the scratch area covered by the migrated cells. Less covered area means less migrated cells [39].
Cell cycle analysis and apoptosis assays
Cells were trypsinized, fixed, and permeabilized in cold 70% ethanol. The cells were then washed with RPMI (phenol red free with 1% FBS), and stained with 15 mg/mL propidium iodide (PI; Sigma) and 10 mg/ml RNaseA (Sigma) on ice for 30 minutes, and analyzed by flow cytometry (Accuri C6). To detect apoptosis, cells were EDTA released and resuspended in AnnexinV binding buffer containing 4 mmol/l CaCl2, 3% Annexin V-PE (BD Pharmingen) and 3% 7-AAD. The mixture was incubated at room temperature for 15 minutes, and cells were analyzed by flow cytometry (Accuri C6) [31].
Animals for in vivo studies
Athymic female mice (NCr, 20-25 g) were used for all in vivo tumor growth inhibition studies. Mice were purchased from Taconic and housed on a 12-hour light/dark cycle with food and water ad libitum under specific pathogen-free conditions, in accordance with the guidelines of the Association for the Assessment and Accreditation of Laboratory Animal Care, International. All in vivo studies were carried out under approved institutional experimental animal care and use protocols.
Tumor implantation
BT474, BT474HerR and HCC1954 breast cancer cell lines were harvested from mid-log phase cultures using trypsin-EDTA (Invitrogen Inc.). Five million cells in 100 µl PBS/matrigel (1:1 mixture of DMEM and matrigel) were injected subcutaneously into the right flank of each mouse in order to generate tumor xenografts. Tumor growth was monitored twice per week for external measurements using Vernier calipers. Tumor volume was calculated using the formula V = (A × B 2)/2 where A and B represent length and width of the tumor, respectively [26].
Treatment of mice with trastuzumab and BEZ235
Treatment was initiated when tumors in all mice in each experiment ranged in size ~200 mm3 and were distributed into groups of no less than 8 animals/group ensuring each group had equivalent mean tumor volumes prior to initiating dosing for antitumor efficacy studies. Control-1 mice received human myeloma IgG1 antibody (Calbiochem-Novabiochem, La Jolla, CA) 10 mg/kg twice-weekly by i.p. injection and control-2 mice received NMP (N-Methylpyrrolidone)/polyethyleneglycol 300 (10/90 v/v). Trastuzumab (diluted in sterile PBS; 10 mg/kg) was administered twice per week. BEZ235 was formulated at concentrations (45 mg/kg) in NMP to achieve the indicated dosages and was administrated every alternate day for 4 weeks with 100 µl via oral gavage [26]. Tumor volumes were determined by digital calipers using the formula (L × W × W)/2 and expressed as mean relative tumor volume (mm3). Tumor sizes and body weights were recorded twice weekly over the course of the study. No untoward effects were noted in mice treated with trastuzumab, BEZ235, combination of trastuzumab plus BEZ235 or vehicles.
Pharmacodynamic studies
The pharmacodynamic (PD) biomarkers were measured to ascertain whether BEZ235 blocked PI3K-AKT-mTOR signaling in xenograft tumors. For the PD studies, tumors were analyzed for the markers shown at the end of the treatment period. For marker analysis, immunohistochemistry (IHC) was performed on the paraffin-embedded tumor tissue sections using Ki67, CD31, phosphorylated vascular endothelial growth factor receptor (p-VEGFR), phospho-AKT (Ser473), and phospho-S6RP antibodies [31].
Statistical analysis
All in vitro experiments were performed independently, at least, three times in triplicates. Statistical analyzes were carried out using Microsoft Excel software. All numerical data are expressed as means ± SD between triplicate experiments. Significant differences were analyzed using Student’s t-test and two-tailed distribution. Data were considered to be statistically significant if P<.001. Student’s t-test is used to evaluate differences observed between treated groups and vehicle treated controls. In vivo data are presented as means ± SE from 8 mice/group and were analyzed by unpaired two-tailed Student’s t-test. A p-value of p<0.05 was considered statistically significant.
Results
BEZ235 inhibits proliferation of HER2 overexpressing breast cancer cells
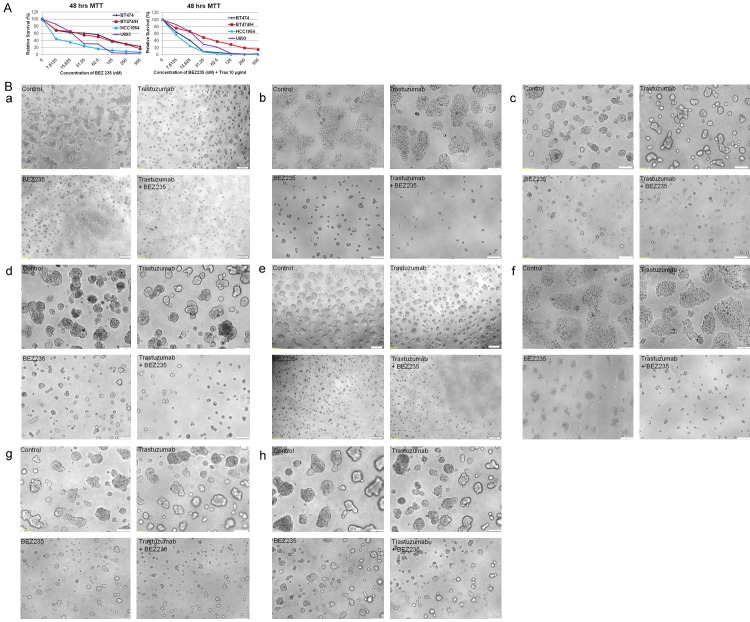
BT474 (trastuzumab-sensitive), BT474HerR (trastuzumab-resistant), HCC1954 and UACC893 (both PIK3CA mutated) were treated with increasing concentrations of BEZ235 ranging from zero to 500 nM for 48 hours. Cell viability was determined by MTT assay (Figure 1A). Cell viability/proliferation was inhibited by BEZ235 in a dose-dependent manner, with BT474-sensitive and BT474-resistant cells demonstrating almost similar responses with IC50 values of approximately 80 nM. Importantly BEZ235 was more effective in PIK3CA mutated cells (~10-30 nM) and PIK3CA mutation is often found (~30-40%) in HER2+ breast cancer patients. The PI3K pathway activation has been suggested to negatively influence towards the response to anti-HER2 therapies including trastuzumab therapy in HER2-positive breast cancer patients.
Figure 1.
Effect of BEZ235 and BEZ235 plus trastuzumab on proliferation assays: BT474, BT474HR, UACC893 and HCC1954 cells exhibit differential susceptibility to trastuzumab but are nearly equally sensitive to BEZ235. HCC1954 (HER2+, PIK3CAmutant [H1047R]) cell was particularly sensitive to this effect. Dose-response curves were obtained by MTT assay (A) after 48 hrs exposures of these four cell lines to BEZ235 (left panel) and the combination of Trastuzumab (10 µg/ml fixed dose) plus different doses of BEZ235 (right panel). Data clearly show that BEZ235 is highly effective in all four cell lines irrespective of PIK3CA mutation or not. (B) Representative clonogenic survival of HER2+/trastuzumab-sensitive (BT474) and HER2+/trastuzumab-resistant (BT474HerR) breast tumor cells were grown in the presence of trastuzumab (10 µg/ml) or BEZ235 (50 nM) or trastuzumab plus BEZ235. BT474 and BT474HerR cell lines were incubated with BEZ235, trastuzumab or a combination of trastuzumab plus BEZ235 for 48 hours and subsequently allowed to grow into colonies for both 96 hours and 7 days. Data clearly show as a single agent BEZ235 significantly inhibits cells growth as indicated by 2D and 3D assays at both 96 hours and 7 days. a: BT474, 2D assay at 96 hours, b: BT474, 2D assay at 7 days, c: BT474, 3D-ON-TOP clonogenic assay at 96 hours, d: BT474, 3D-ON-TOP clonogenic assay at 7 days, e: BT474HerR, 2D assay at 96 hours, f: BT474HerR, 2D assay at 7 days, g: BT474HerR, 3D-ON-TOP clonogenic assay at 96 hours, h: BT474HerR, 3D-ON-TOP clonogenic assay at 7 days. More importantly, the additive effect of the BEZ235 plus trastuzumab combination achieves a more powerful inhibition of clonogenic growth (both 2D and 3D-ON-TOP assays) than either agent in isolation.
In addition to cell viability assays, we examined the effect of a combination treatment of BEZ235 plus trastuzumab in 2D and 3D-ON-TOP clonogenic assays (Figure 1B). Trastuzumab-sensitive (BT474) and trastuzumab-resistant (BT4743HerR) cells were placed on matrigel-coated plates with BEZ235, trastuzumab or a combination of both drugs and 3D colonies were photomicrographed at the end of 96 hours and 7 days. Trastuzumab showed little to no inhibition of colony growth (in trastuzumab-resistant cells) (Figure 1Be-h), while BEZ235 alone caused 70-80% growth inhibition in both sensitive and resistant cell lines (Figure 1B). Combined trastuzumab plus BEZ235 achieved maximum inhibition (>95%) of 2D or 3D-ON-TOP colony growth than BEZ235 alone in both cell lines (Figure 1B). Thus, BEZ235 appears to increase trastuzumab-mediated growth inhibition in HER2 overexpressing breast cancer cells including trastuzumab-resistant and PIK3CA mutated conditions (data not shown).
BEZ235-mediaited alterations of PI3K and MAPK pathways
To investigate potential molecular markers of response to BEZ235, we examined the signaling components of PI3K-mTOR signaling network including RAS-MAPK in cell lines representing trastuzumab-sensitive (BT474, SKBR3), trastuzumab-resistant (BT474HerR) and activating mutation of PIK3CA (HCC1954 and UACC893). In each cell line treated with BEZ235 (indicated concentration and time), phosphorylation of downstream markers of the PI3K-AKT-mTOR pathway was reduced (Figure 2). These included signaling markers downstream of PI3K, such as phospho-AKT (Thr308), which is phosphorylated by PDK1, biomarkers downstream of mTORC2 such as phospho-AKT (Ser473) and biomarkers downstream of mTORC1 such as phospho-P70S6K, phospho-S6 ribosomal protein (S6RP) and p-4EBP1 (Thr 37/46 and Thr70) (Figure 2A-G). We also assessed phospho-ERK (a downstream marker of the RAS-MAPK pathway) in BEZ235 treated cells and observed a reduction of this marker (in BT474, BT474HR and HCC1954) to very high upregulation of this marker (in UACC893) in different cell lines (Figure 2H upper and lower panels). High expression of HER3 has been demonstrated in UACC893 cells and has been linked to resistance against trastuzumab and lapatinib [40]. It is possible that higher HER3 expression may be responsible for high activation of phospho-ERK following the treatment of BEZ235 for 3 hours through GRB2-SOS-RAS signaling. Conversely, RAD001-induced phospho-ERK was observed in all four HER2-positive cell lines (Figure 2H). Other studies have demonstrated that inhibition of mTORC1 leads to mitogen-activated protein kinase (MAPK) pathway activation through a PI3K-dependent feedback loop [41].
Figure 2.
Effect of BEZ235 and comparison on the effects of BEZ235 and RAD001 in HER2+ breast cancer cells: Western blot showing BEZ235 time/dose-dependently blocked activation of AKT and its downstream effectors in total lysates from HER2+ [trastuzumab-sensitive BT474 (A) & SKBR3 (B)], trastuzumab-resistant [BT474HerR (C)] breast cancer cells. Moreover, time course experiments revealed that long-term exposure to low concentration of BEZ235 resulted in an increase in p-AKT levels. Such an effect on p-AKT was completely abolished in cells incubated at higher concentrations (100 and 200 nM) of the inhibitor (see lane 8, 9 and 11, 12 in all three figures (A-C), BEZ235. Differential effects of BEZ235 versus RAD001 on intracellular signaling: BT474 (D), BT474HerRR (E), HER2+/PIK3CA mutated HCC1954 (F) and UACC893 (G) cells were treated with BEZ235 (50 nM) or RAD001 (1 and 2 nM) for different time periods (1 hr, 6 hrs, 24 hrs, and 48 hrs) and then lysed and analyzed by Western blots. Data show that both drugs completely blocked p-P70S6K, p-S6RP but only BEZ235 consistently blocked phosphorylation of AKT (Ser 474 and Thr 308) and 4EBP1. As expected RAD001-induced AKT activation was observed. Data also showed that a combination of BEZ235 plus RAD001 at sub-optimal concentration might have an additive effect on the PI3K-AKT-mTOR pathway molecules (lane 5, 9, 13 & 17 in D, E). The effect of BEZ235 was independent of the mutation profile of the cell cultures used. (H) PI3K pathway inhibition and ERK phosphorylation in HER2+ or HER2+/PIK3CA mutated breast cancer cells: Immunoblots of BT474, BT474HR, HCC1954 and UACC893 for indicated time points with different PI3K pathway inhibitors (dual PI3K/mTOR inhibitor, BEZ235 or mTOR1 inhibitor, RAD001) at the indicated concentration (nM). Our data show that mTOR1 inhibition induces ERK phosphorylation in all 4 cell lines. Importantly, BEZ235 at 50 nM concentration blocked phosphorylation ERK except UACC 893 cell lines where phosphorylation of ERK is upregulated following the treatment of BEZ235 (for the indicated time points). It has been known that level of HER3 is high in UACC cell line and it is resistant against trastuzumab and lapatinib (Slamon DJ 2010 Mol Can Ther 9: 1489).
Furthermore, BEZ235 dose-dependently blocked ligand-induced AKT activation in both trastuzumab-sensitive (BT474) and trastuzumab-resistant (BT474HerR) cell lines (Figure 3A lane 5, 6 and 12, 13). Importantly inhibition was more potent when trastuzumab was combined with low dosage of BEZ235 (25 nM) in both sensitive and resistant cells (Figure 3A lane 7 and 14) following heregulin stimulation and this combination was equally effective in basal condition (Figure 3Ba lane 5 in both BT474 and BT474HerR cells and Figure 3Bb lane 5 in SKBR3 cells). More importantly, this combination was highly effective in HER2+/PIK3CA mutated HCC1954 cells (Figure 2F lane 8 and 9).
Figure 3.
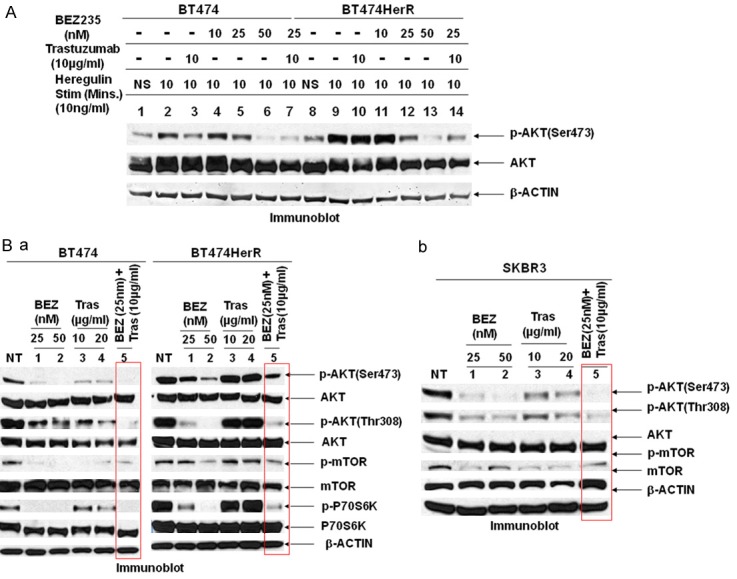
(A) BEZ235 interrupts heregulin-induced PI3K-AKT signaling: Under the basal condition in serum free medium (overnight serum starved, NS), the PI3K signaling was assessed by the p-AKT level. On ligand (heregulin) stimulation p-AKT was upregulated in both sensitive (BT474) and trastuzumab-resistant (BT474HerR) cell lines (lane 2 and 9). The activity of AKT in both the cell lines was significantly inhibited by BEZ235 at 25 and 50 nM concentration (lane 5, 6 for sensitive cell line and lane 12, 13 in resistant cell line), suggesting its effectiveness on blocking the PI3K-AKT signal pathway in HER2+ breast cancer cells. Moreover, this inhibition is more when trastuzumab (10 µg/ml) was treated along with BEZ235 (25 nM). BEZ235 has no effect on AKT activation at 10 nM concentration (lane 4 and 11). Expression of the corresponding total target protein (AKT) and β-ACTIN was used as loading control. (B) Combination of BEZ235 along with trastuzumab more efficiently blocked the PI3K-AKT-mTOR pathway: Western blot showing combination of BEZ235 as low as 25 nM concentration plus trastuzumab at 10 µg/ml is significantly blocked AKT (both Ser473 and Thr308), and its downstream mTOR and P70S6 Kinase activations compare to either BEZ235 or trastuzumab alone (lane 5 in BT474, BT474HerR (a), SKBR3 (b) and lane 8 & 9 in HCC1954 cells, see Figure 2F). Additionally, this combination is more effective than the combination of RAD001 and trastuzumab in terms of pathway inactivation (compare lane 8 & 9 with lane 10 & 11, Figure 2F). Data show that combination is more effective compared to either drug alone and, more importantly, the combination is also effective against trastuzumab-resistant and PIK3CA mutated cell line.
RAD001 enhances the effectiveness of BEZ235 to inhibit mTOR signaling in HER2+ breast cancer cells
Recently several groups have reported that the combination of RAD001 and BEZ235 has synergistic antitumor activity in non-small cell lung cancer and hepatocellular cancer [42,43]. We have now studied whether the combination of BEZ235 and RAD001 exerts augmented anti-PI3K-AKT-mTOR signaling activity in HER2+ cells. Interestingly we found that the combination of low concentrations of BEZ235 and RAD001 was much more potent than each single agent in both BT474 and BT474HerR cells (Figure 2D and 2E lane 17).
BEZ235 induces cell cycle arrest and apoptosis in HER2+ breast cancer cell lines
Consistent with the anti-proliferative and anti-clonogenic effects of BEZ235 (Figure 1A, 1B), the proportion of cells in the G1 phase of the cell cycle was subsequently increased in all three cell lines (BT474, BT474HerR and HCC1954) with a concomitant decrease of S phase following the treatment with BEZ235 (Figure 4A). We further analyzed the potential of BEZ235 to induce apoptosis in HER2+ breast cancer cells. We assessed AnnexinV staining, an early marker of apoptosis induction by flow cytometry (Figure 4B) in addition to biochemical markers of both apoptosis and survival (cleaved Caspase3 and SURVIVIN) by Western blotting in trastuzumab sensitive (Figure 4Ca), trastuzumab-resistant (Figure 4Cb) and PIK3CA mutated/HER2+ cell lines (Figure 4Cc). To better understand and define the role of PI3K-AKT-mTOR on induction of apoptosis in HER2+ cells, the effect of the mTORC1 allosteric inhibitor RAD001 was compared with BEZ235. In contrast to BEZ235, RAD001 at doses which successfully blocked the phosphorylation of p70S6K, pS6RP and p4EBP1 (three downstream effectors molecules of mTOR) was substantially less effective at inducing apoptosis in all HER2+ cell lines except the trastuzumab-resistant cell line (Figure 4B), suggesting that the activity of BEZ235 could be attributed to its PI3K-inhibiting property. To ensure that the cleaved CASPASE3 expression is irreversible proportionate with phosphorylation status of AKT, we ran a Western blot with a same lysate of BT474HerR following the treatment of BEZ235 at indicated concentrations and time. As expected less AKT phosphorylation was observed and this abrogation of the AKT activity was exactly matching with high expression of cleaved CASPASE3 (compare Figure 4Cb with 4Cd).
Figure 4.
Effect of BEZ235 on cell cycle progression, apoptosis in HER2 amplified trastuzumab-sensitive, trastuzumab-resistant and PIK3CA mutated breast cancer cells: Effect of three doses of BEZ 235 (100, 200 and 500 nM) on cell cycle distribution (A) in BT474, BT474HerR and HCC1954. Cells were treated as indicated for 24 hours. Cells were released and ethanol fixed before staining with propidium iodide for analysis by flow cytometry. (B) Effect of BEZ235 (100, 200 and 500 nM) on apoptotic (early) response analyzed by annexin V/7AAD staining in BT474, BT474HerR and HCC1954. Cells were treated for 48 hours, released, rinsed, and placed in the annexin V binding buffer. Cells were labeled with annexin V-PE and 7AAD for analysis. Error bars represent SEM from triplicates. Interestingly, RAD001 has a very insignificant response to the early phase of apoptosis in HER2+ cells when compared with BEZ235. (C) BEZ235 induces apoptosis through induction of cleaved CASPASE3 and inhibition of the expression of SURVIVIN: BT474 (a), BT474HerR (b) and HCC1954 (c) cell lines were incubated for different time periods (6, 24, 48 and 72 hours) with the indicated amount of BEZ235. Data showed that cleaved CASPASE3 expression was more at higher concentration of BEZ235. Furthermore, the expression was more in trastuzumab-resistant cells (lane 6 and 8 in Cb) might be due to addiction of the PI3K-AKT-mTOR pathway in resistant cells. Data also showed that SURVIVIN expression was more in resistant cells and SURVIVIN expression was significantly inhibited following the treatment of BEZ235 in trastuzumab-sensitive (a), trastuzumab-resistant (b) and PIK3CA mutant (c) cells. (Cd) Levels of p-AKT(Ser473) expression following the treatment of BEZ235 was reciprocally correlated (both time and dose) with the expression levels of cleaved CASPASE3. Data suggest that BEZ235 increases apoptosis through the AKT-SURVIVIN-CASPASE3 pathway. (Da) Effects of BEZ235 on the expression of p-FOXO1 and its target gene: BT474 and BT474HerR cells were treated with BEZ235 (100 nM) for 24, 48 and 72 hrs. Cells were harvested to measure the expression of phosphorylation (Ser256) of FOXO1 and one of its transcriptional target genes, p27. Data suggest BEZ235 induced dephosphorylation of FOXO1, leads to upregulation of p27 which is the transcriptional target gene of FOXO1 and upregulated p27 may be (one of the key factors) responsible for induction of cell cycle arrest. (Db) FOXO1 plays a pivotal role in tumor growth suppression by inducing growth arrest and apoptosis: Loss of function of FOXO1 protein due to phosphorylation and proteasomal degradation has been implicated in cell transformation and malignancy. It has been known that SKP2 interacts with ubiquitinates, and promotes the degradation of FOXO1. This effect of SKP2 requires AKT-specific phosphorylation of FOXO1 and translocation of phosphorylated FOXO1 (inactive form) from nucleus to cytosol. Activation of FOXO1 by upstream inhibition of the PI3K-AKT pathway, results in upregulation of p27kip, cyclin-dependent kinase inhibitor and downregulation of cyclin D, thereby increasing apoptosis (please see the cartoon).
It has been reported earlier that overexpression of HER2 leads to activation of the PI3K-AKT pathway and subsequent inactivation of FOXO1 (phosphorylation of FOXO1 at Ser 256) in HER2 overexpressing breast tumor cells [44]. FOXO family of transcription factors consisting of FOXO1, FOXO3a, FOXO4, and FOXO6, are direct phosphorylation targets of the protein kinase AKT [45,46]. Wu et al. demonstrated [44] that inactivation of AKT led to increased expression of FOXO1 which transcriptionally activated p27 and finally inhibited cell proliferation (see the cartoon in Figure 4Db). In the same line, our data also showed that FOXO1 was activated following the treatment of BEZ235 (by inhibiting its phosphorylation at serine 256) and consequently FOXO1-mediated transcriptional upregulation of downstream p27 was observed in both BT474 and BT474HerR cells (Figure 4Da). Phospho-27kip1 ability to block cell cycle progression in different solid tumors has also been demonstrated previously [44,47].
Taken together our data clearly demonstrated that PI3K and AKT inhibition are key molecules of this pathway for induction of cell cycle arrest and apoptosis by BEZ235 treatment, and mTORC2 (and its immediate target effector i.e. serine phosphorylated AKT) more than mTORC1 is probably involved in this event.
The PI3K-AKT-mTOR signaling pathway controls heregulin-induced HER2+ breast cancer cell migration and RAC1 activation on fibronectin
One of the major threats for breast cancer death is metastasis, which accounts for >90% of breast cancer deaths [48]. Integrin-mediated cell migration is one of the essential steps for metastasis. Here we examined the effect of dual PI3K/mTOR inhibitor BEZ235 on heregulin-induced, integrin directed migration of HER2+ breast cancer cells. Treating the PIK3CA mutated/HER2+ (HCC1954) or inactivating mutation of PTEN/HER2+ (HCC1569) cells with BEZ235 significantly attenuated the migration of HER2+ cells on fibronectin (either by scratch assay or by transwell haptotaxis assay). In contrast, RAD001 had no to moderate activity on integrin-directed migratory propensity in both cell lines (Figure 5A).
Figure 5.
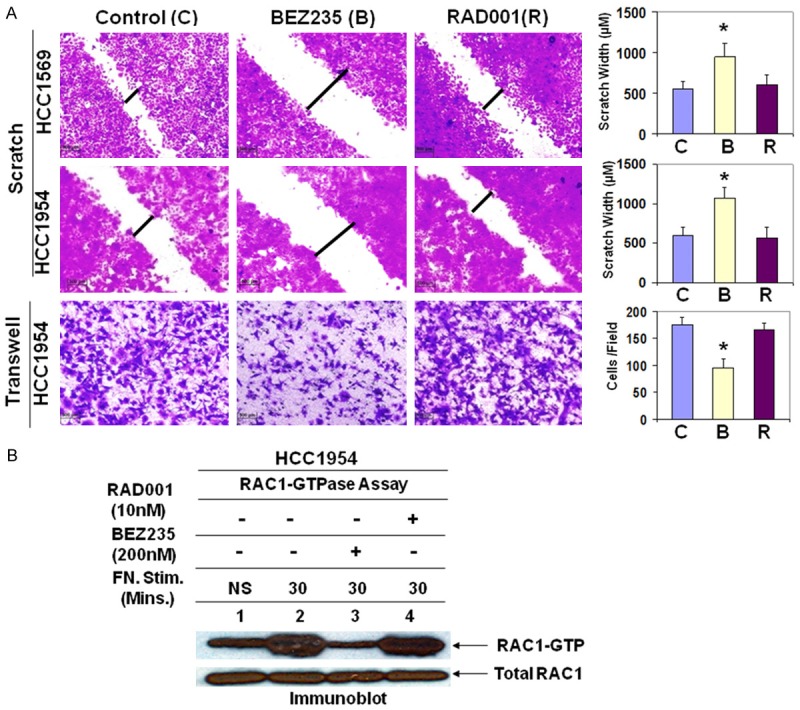
Blocking of the PI3K-AKT pathway inhibited HER2+ cell migration and decreased RAC1 activation: A. Wound healing assay. A confluent monolayer of cells (HER2+/PIK3CA mutated, HCC1954 and HER2+/PTEN inactivation mutation, HCC1569) was scratched using a sterile pipette tip. At 24 hours after treatment, migrated cells into the scratched area were photomicrographed and measured the scratch area covered by the migrated cells. Transwell assay was carried out with HCC1954 cells following the treatment of BEZ235 (100 nM) or RAD001 (5 nM) (bottom panel). Photomicrograph and counting of cells that migrate through the filter to the fibronectin coated surface after crystal violet staining were showed in the bar diagram (*<0.001, n=3). BEZ235-mediated abrogation of integrin (α4β1/α5β1) -induced HER2+ cell migration was significantly well-defined than an mTOR1 inhibitor, RAD001 in both cells and in both assay types. B. Integrin (α4β1/α5β1)-induced RAC1 activation was significantly attenuated following the treatment of dual PI3K/mTOR inhibitor, BEZ235 but not by RAD001. The block of RAC1 activation has been correlated with an observed reduction in cell migration.
The roles of RAC1 GTPases are widely implicated in metastasis. RAC1 transduces signals from integrins, growth factor receptors, and G-protein-coupled receptors (GPCRs) and controls a number of essential cellular functions including motility. Recently, it has been reported by other that PAK1 (Ser/Thr kinase) is an immediate downstream of RAC1 and is an integral component of growth factor signaling networks (including HER2) and its cellular functions fundamental to tumorigenesis [49]. Consistent with the known role for Rho family small GTPases in cell migration, fibronectin engagement caused a time-dependent increase in RAC1 activation in HCC1954 cells in the presence of heregulin (10 ng/ml) (Figure 5B lane 2). Since RAC1 activation was pronounced at 30 minutes following fibronectin engagement, the effect of BEZ235 was evaluated at 30 minutes as compared to the non-treated cells after fibronectin-attachment. Figure 5B shows that fibronectin-dependent RAC1 activation was significantly attenuated in HCC1954 cells. Similar to migration experiments RAD001 had no effect on integrin-induced RAC1 activation (Figure 5B lane 4). These results are directly correlated with the capacity of the PI3K-AKT-mTOR pathway to control heregulin-induced HER2+ breast cancer cell migration on fibronectin. Overall these data clearly indicate that BEZ235 controls metastatic property of HER2+ breast cancer cells.
Antitumor efficacy of BEZ235, trastuzumab or combination in HER2+ mouse xenograft models
Plasticity and redundancy of the signaling network require a complete inhibition of signaling pathways with combinatorial therapies. Aberrant activation of the PI3K-AKT-mTOR pathway has been shown to correlate with a diminished response to HER2 directed therapies [50]. Approximately 40% of HER2+ breast cancers harbor an activating mutation of PIK3CA [51], consistent with the notion that these two oncogenes have non-overlapping functions and may cooperate to promote tumorigenesis [52]. Activating mutations in the PI3K-AKT-mTOR pathway are present in the majority of breast cancers and, therefore, are one of the major areas of drug development and clinical trials. Pathway mutations have been proposed as predictive biomarkers for the efficacy of the PI3K pathway-targeted therapies. However, the precise contribution of distinct PI3K pathway mutations to drug sensitivity is unknown.
Several studies suggest that activation of the PI3K pathway and/or activating mutations of PIK3CA confer resistance to anti-HER2 therapy including trastuzumab [16,50,53], however, confirmation of a causal relationship between the PI3K-AKT-mTOR pathway and resistance to trastuzumab in the clinic is not clear yet. Here we sought to examine whether the combination of a dual PI3K/mTOR inhibitor BEZ235 along with trastuzumab may effectively override the PI3K pathway-mediated trastuzumab resistance in mouse xenograft models. The in vitro sensitivity profile of BEZ235 alone and in combination with trastuzumab was recapitulated in in vivo in BT474 (Figure 7Aa) and BT474HerR xenograft models (Figure 7Ba). We also confirmed the efficacy of the combination in a more clinically relevant disease model of HER2+ breast cancer, HCC1954 which harbors activating mutation of PIK3CA (Figure 7Ca). In our resistant model systems (BT474HerR and HCC1954), treatment with BEZ235 was more effective than trastuzumab in achieving the inhibition and the additive effect of the BEZ235 plus trastuzumab combination achieved a more powerful blockade of the pathway than the either drug in isolation (Figure 7B-D), suggesting that inhibition of both oncogenes is required to inhibit tumor growth.
Figure 7.
Efficacy of BEZ235 alone and in combination with trastuzumab in HER2+/trastuzumab-sensitive, BT474 (A), HER2+/trastuzumab-resistant, BT474HerR (B) and HER2+/PIK3CA mutated, HCC1954 (C) human tumor xenograft models: A pilot study was conducted with HCC1954 cells to determine 1) the number of cells required to inject for the establishment of tumors and their maintenance in animals throughout the period of drug administration and 2) the maximum tolerable dose of drugs in animals with tumor burden. The number of cells injected was adjusted on the basis of the tolerable tumor burden in untreated animals (following IACUC guidelines). On the basis of the results of the pilot study, cells were injected in matrigel subcutaneously into the flank of immunocompromised female nude (nu/nu) mice. Established xenograft tumors were treated with BEZ235 (45 mg/kg, oral, every other day) alone and in combination with trastuzumab (10 mg/kg i.p., twice weekly for 3 weeks). The table [(lower panel of (Aa), (Ba) & (Ca)] shows the change in volumes of xenograft tumors and body weights of the mice in response to drug combinations. PD data of BT474 tumor (Ab), BT474HerR (Bb) and HCC1954 (Cb) for cell proliferation marker (Ki67), tumor-induced angiogenic markers (CD31 & pVEGFR and cell signaling markers (pAKT & pS6RP) were presented. (D) A dual PI3K/mTOR inhibitor (BEZ235) plus trastuzumab showed in vivo efficacy in mouse xenograft models. Quantification of the mean changes (relative values) in tumor volume of xenografts after 3 weeks treatment, negative values indicate tumor regression.
Several reports in the literature suggest that HER2 requires HIF1α for mammary tumor growth [54] and PTEN or inhibitors of PI3K or mTOR can block angiogenesis via HIF1α stabilization or synthesis [55-57]. The mechanisms for anti-angiogenic activity of pan-PI3K inhibitors are not completely clear but seem to be involved in coordinating regulation of proangiogenic and anti-angiogenic effectors, such as thrombospondin-1, HIF1α, VEGF, and others [58,59]. The PTEN-PI3K-AKT-mTOR pathway has been shown to regulate the transcription factor HIF1α, a control point for the hypoxic induction of VEGF [57,59,60]. Hence, it might be expected that this dual targeted inhibitor BEZ235 will display anti-angiogenic activity. To investigate the effect of BEZ235 on angiogenesis, we evaluated its effects on the hypoxic stabilization of HIF1α and growth factor-mediated HIF1α synthesis under hypoxic conditions in BT474 and BT474HerR cells. As shown in Figure 6A, 6B the results showed that unlike other pan-PI3K inhibitors (e.g. SF1126, LY294003) BEZ235 failed to block HIF1α accumulation in the presence of hypoxia but exerted its effect via dose-dependent blockade of HIF1α synthesis following heregulin stimulation in both cell lines (Figure 6C and 6D). Similarly, BEZ235 also abrogated heregulin-induced HIF1α synthesis in HER2+/PIK3CA mutated HCC1954 and UACC893 cells (Figure 6F). Interestingly, BEZ235 mediated abrogation of HIF1α synthesis was more when compared with RAD001 in all four cell lines (Figure 6E and 6F). Our in vitro data show that BEZ235 abrogated vitronectin-directed migration and capillary-like tube formation on matrigel of endothelial cell (HUVEC) (Figure 6G). We observed that PIK3CA, ERBB2, VEGFA and HIF1alpha genes were altered in from Her2-positive tumors and PAM50 Her2-enriched tumors from breast cancer patients as documented in cBioPortal. Oncoprint data from two published data sets in the cBioPortal representing PAM50 Her2 enriched tumors (TCGA, Nature 2012) (Figure 6H) and Her2-positive breast tumors (TCGA, cell 2015) (Figure 6I) showed 91.4% genetic alterations and 79.2% genetic alterations in a set of four genes comprised of PIK3CA, ERBB2, VEGFA and HIF1alpha. The co-occurrence between HIF1alpha and VEGFA in PAM50 Her2 enriched tumors (TCGA, Nature 2012) (Figure 6H) was found statistically significant. The co-occurrence between HIF1alpha and VEGFA pair and HIF1alpha and PIK3CA pair in Her2-positive breast tumors (TCGA, cell 2015) (Figure 6I) were found statistically significant. Furthermore, we evaluated the effects of BEZ235 on the capacity of BT474, BT474HerR and HCC1954 tumor cells to recruit a blood supply in vivo during treatments with BEZ235 at 45 mg/kg. BEZ235 treated tumors showed a significant decrease in microvessel density (MVD) (positive for CD31 and positive for p-VEGFR IHC staining) (Figure 7Ab, 7Bb and 7Cb) suggesting that this treatment had substantial tumor-induced anti-angiogenic properties in vivo. The anti-angiogenic activity of BEZ235 correlates with a block in the HIF1α-VEGF signaling in the tumor cell.
To further investigate the mechanism of action of BEZ235 alone or its combination with trastuzumab in tumor growth inhibition, expression levels of proliferative marker, (Ki67), and PI3K-AKT-mTOR signaling pathway-specific PD markers (p-AKT and p-S6RP) were determined by IHC from the FFPE sections of the tumors from animals from each arm of the xenograft studies. High expression of Ki67 has been associated with poorer outcomes and measurement of Ki67 index pre- and post-therapy provided an accurate surrogate for responsiveness of breast cancer to the treatment [61]. PD studies showed a decrease in the Ki67, p-AKT and p-S6RP expression in tumors from mice treated with BEZ235 in combination with trastuzumab as compared to the control (Figure 7Ab, 7Bb and 7Cb). These results in agreement with the in vitro observations demonstrated that BEZ235 in combination with trastuzumab was an efficacious anti-tumor drug combination in HER2+/trastuzumab-resistant as well as HER2+/PIK3CA mutated breast tumor models.
Discussion
The results of our studies suggest that combined targeting of HER2 and the PI3K-AKT-mTOR pathway is superior to HER2-directed therapy alone. Notably, BEZ235 is also active in breast cancer cells with acquired resistance to trastuzumab. We have shown that targeting the PI3K-AKT-mTOR signaling pathway has antitumor activity in combination with anti-HER2 therapy (using trastuzumab). This is clinically significant, as resistance to trastuzumab therapy is a common clinical problem that limits the survival of patients with HER2 amplified breast cancers. As agents targeting PI3K/mTOR, such as BEZ235 or GDC-0980 are currently undergoing clinical investigation, these data would support the combination of the PI3K-AKT-mTOR pathway targeted therapy with trastuzumab in HER2+ breast cancer.
The involvement of PI3K-mTOR signaling in breast cancers has been established. We have recently demonstrated that an inhibition of this pathway is necessary for the anti-tumor effect in subtypes of breast cancers [31,62]. The PI3K-mTOR pathway is frequently dysregulated and is causally related to the development of resistance to targeted/cytotoxic agents in HER2+ breast cancers. Although multiple agents targeting the PI3K-mTOR pathway are either approved for clinical use or are being currently evaluated in clinical trials, the functional superiority of a combination of the anti-HER2 agent with a targeted inhibitor of the PI3K-mTOR pathway has not been established yet in HER2+ breast cancers. Recently the biomarker study of the BOLERO3 trial indicated that patients with low PTEN levels as well as patients with high pS6 levels had a greater benefit (PFS) when everolimus was added to trastuzumab. Mechanistically our data clearly showed that BEZ235 significantly abrogated heregulin induced HER2: HER3 hetero-dimer-mediated PI3K-AKT-mTOR signaling as well as perturbed mTOR-mediated cap-dependent mRNA translational activity of HIF1α which downregulated VEGF expression and tumor angiogenesis. Although a significant proportion of trastuzumab-treated patients are prone towards the development of drug-induced resistance, till date trastuzumab remains as a standard drug for the first-line of treatment in HER2 amplified breast cancers. Our data provide first evidence to show that BEZ235, by targeting two nodal points of the PI3K-AKT-mTOR pathway is beneficial in achieving an anti-tumor efficacy in both tumor and angiogenic compartments and this effect of BEZ235 is worth exploring as a potentially superior therapeutic strategy as compared to trastuzumab/trastuzumab plus pertuzumab in the context of trastuzumab-resistant condition.
One of the key advantages of BEZ235 over the isoform-specific PI3K inhibitor is its potential to treat tumors harboring oncogenic abnormalities that signal through all PI3K isoforms and mTOR (it’s downstream) which are activated by upstream PI3K-dependent and independent manners. The PI3K-AKT-mTOR pathway, a central regulator of diverse normal cellular functions is often subverted during neoplastic transformation [63]. Activation of this signaling pathway lying downstream of HER2 and its family members, which facilitates signaling independently of the HER2 kinase, plays an important role in the development of de novo and acquired resistance to trastuzumab therapy. Resistance to current anti-HER2 therapies including trastuzumab is a major hurdle in the eradication of HER2+ breast cancer. Amplification of HER2 and PIK3CA mutations (30-40%) often co-occur in breast cancer. Sarat Chandarlapaty and his group recently reported that the combined rate of PTEN loss and PIK3CA mutation in the trastuzumab-resistant tumors was 71% [64].
Current PI3K inhibitors under clinical development are grouped by their specificity, ranging from pure PI3K inhibitors to compounds that block both PI3K and its downstream effector, mTOR (dual inhibitor) to pure catalytic mTOR inhibitors, and to inhibitors that block AKT. However it remains uncertain which type of inhibitor would be more appropriate clinically; isoform-specific inhibitors, pan-PI3K inhibitors or PI3K/mTOR dual inhibitors. Selective PI3K p110a (PI3Ka) inhibitors are currently being tested in the clinic in patients with advanced malignancies, with promising results in patients with breast tumors harboring PIK3CA mutations [65,66]. However, not all the patients benefit equally from these agents and even those that initially respond typically relapse after months of therapy. A few studies have reported that a combined use of two different molecules targeting the same cellular effectors through independent inhibitory mechanisms can improve the ultimate outcome [67-69]. We have recently showed that a combination of a dual PI3K/mTOR inhibitor, GDC-0980 induced an efficient antitumor effect in the BRCA-competent triple-negative breast cancer (TNBC) model when combined with a PARP inhibitor [31].
BEZ235 treatment resulted in viability/survival changes measured by MTT, 2D, 3D-ON-TOP clonogenic growth, cell cycle and apoptotic markers, while apoptosis was not observed with RAD001 (an allosteric inhibitor of mTOR), indicating that either PI3K and/or mTORC2 is sufficient for cell viability/survival. Despite some success in selected tumor types, rapalogs generally showed very limited anticancer efficacy as a single agent [70]. At 2013 ASCO meeting Ruth O’Regan and her group reported that the addition of everolimus (RAD001) did not improve clinical benefits rate with HER2+/trastuzumab-resistant, taxane-pretreated advanced breast cancer in the BOLERO-3 trial [71]. Similarly, a frontline treatment with everolimus combined with trastuzumab and paclitaxel failed to delay the progression of the disease when compared with trastuzumab and paclitaxel in patients with HER2+ advanced breast cancers (according to the result from phase III BOLERO-I presented at 2014 SABCS, San Antonio). Negative feedback loops involving S6K have been described significant effects on drug responses for mTORC1 inhibitors [72]. Targeting tumors with rapalogs can result in increased PI3K-AKT activity leading to an enhanced proliferation rate of the tumor and decreased the efficacy of allosteric mTOR inhibitors [72]. ATP-competitive mTOR kinase inhibitors (e.g. OSI-027, AZD2014, and MLN0128) have been developed and are undergoing clinical trials. However resistance to these mTOR kinase inhibitors can still arise via the PI3K feedback mechanism, by increased p-AKT at threonine 308 or activation of AKT-independent PI3K targets [73]. Similarly, inhibition of PI3K leads to compensatory activation of upstream receptor tyrosine kinases that limit the effectiveness of those compounds [74,75]. We have shown here that BEZ235 with its dual PI3K/mTOR kinase activity overcame these feedback signaling mechanisms. Moreover, upregulation of the RAS-MAPK pathway that occurs following mTORC1 inhibition alone [41] as well as BEZ235 at 500 nM concentration [74], was not detected with BEZ235 at 50 nM or 200 nM concentration (except UACC893 cells, Figure 2H lower panel) as it simultaneously blocked mTORC1, mTORC2, and PI3K. It has been recently proposed that p70S6K mostly regulates by mTOR in controlling cell size whereas mTOR’s effects on proliferation are mainly attributable to 4EBPs [76]. Our data demonstrated that dual PI3K/mTOR inhibition caused sustained inhibition of both p70S6K and 4EBPs. Differing from the report by Choo and Blenis [77] and Choo et al. [78] who showed that with rapamycin, after an initial inhibitory effect on 4EBP1 phosphorylation, phosphorylation re-appeared, we observed (Figure 2E-G) a complete inhibition of phosphorylation of 4EBP1 and no reactivation of the pathway until after 24 hours of treatment. BEZ235 mediated progressive dephosphorylation of 4EBP1 led to a selective inhibition of translation of cap-dependent protein synthesis including HIF1α. We and others have previously shown that the PI3K-AKT-mTOR pathway plays an important role in the activity of HIF1α in solid tumors [56,57,79]. Like PI3K signaling, hypoxia and HIF1α are associated with aggressive disease, metastasis, and treatment resistance in many cancers [80]. Activation of HIF1α is normally observed under hypoxic condition but can be influenced by oncogenic expression [81,82]. Here our data showed that BEZ235 substantially blocked HIF1α synthesis probably via inhibition of mTOR-4EBP1-cap-dependent translational machinery in trastuzumab-sensitive, trastuzumab-resistant and PIK3CA mutated cells (Figure 6).
Two recent clinical studies have demonstrated that HER2-positive breast cancer patients with PIK3CA mutations are less likely to achieve a pCR compared with those with wild-type, when the patients were treated with neoadjuvant chemotherapy plus anti-HER2 therapies in the GeparQuattro, GeparQuinto, and GeparSixto trials [83] and the NeoALTO trial [84]. Similarly, PIK3CA mutation is associated with a shorter progression-free survival in HER2-positive metastatic breast cancer treated with HER2-targeted therapies in CLEOPATRA trial [85]. However unlike the results observed in the neoadjuvant and metastatic settings, there is no association between the PIK3CA mutation and the degree of benefit from trastuzumab in the adjuvant setting in the NSABP B-31 trial [86]. Recently published studies and our current data may explain why mutational activation of the PI3K pathway (PIK3CA) promotes resistance to trastuzumab. HER2 blockade by treating the cells with trastuzumab did not affect p-AKT or its downstream effectors in HER2+/PIK3CA mutated HCC1954 (Figure 2F lane 2 and 3), suggesting that trastuzumab can no longer inhibit PI3K and its downstream signaling molecules in the presence of mutant PIK3CA. Elkabets and colleagues [87] recently reported that p110α-isoform specific inhibitor such as BYL719 was not effective in PIK3CA mutated breast tumors with persistent activation of S6K (a marker of mTORC1 signaling) and suggested that simultaneous inhibition of mTORC1 may enhance the clinical efficacy of the p110α-specific drug.
Progression of solid tumors (including HER2+ breast tumor) to the metastatic stage is accountable for the majority of cancer-related death. Additionally, patients with tumors having increased phosphorylation of p70S6K (an immediate downstream effector of mTOR) showed a trend for worse disease-free survival and increased metastasis [88]. Accumulating evidence is emerging that the PI3K-AKT-mTOR signaling axis also actively engages with the migratory process in motile cells including metastatic cancer cells [89]. Interference with PI3K-AKT-mediated cell motility impairs and attenuates malignant progression/metastasis of cancers. Because metastasis is responsible for 90% of mortality in cancer patients, the acceleration of cancer cell spreading observed in association with hyperactivation of the PI3K pathway has highlighted the awareness of the regulation of cancer cell motility to distant organs via inhibition of the PI3K pathway. It has been also reported that activation of the HER2 receptor tyrosine kinase stimulates breast cancer cell motility [90]. From these data, we hypothesized that the PI3K-AKT-mTOR pathway may be the key for HER2 overexpressing cell motility. Here our data showed that BEZ235 significantly abrogated HER2+/PIK3CA or HER2+/PTEN mutated cells migration on fibronectin (Figure 5A). Several reports suggest that activation of RAC-GTP is necessary for integrin-mediated cell migration [33,91,92]. Literature references suggest that PI3K is a possible upstream regulator of the RAC signaling pathway [93,94]. Evidence suggests that the RAC1’s immediate downstream effector PAK1 is implicated in breast tumor progression. Indeed, more than 50% of breast tumors show overexpression and/or hyperactivation of PAK1 [95]. In the same line, our data also showed that integrin-mediated RAC1 activation was substantially inhibited following the treatment of BEZ235 (Figure 5B). Taken together (migration and RAC1 activation data), we can suggest that a dual PI3K/mTOR inhibitor may control HER2+ breast tumor cells metastasis. It warrants further investigation.
In in vivo studies BEZ235 treatment did impact the tumor stromal compartment (Figure 8) as it appeared to either directly or indirectly inhibit angiogenesis. This aligns with extensive literature demonstrating that PI3K is a key signaling node for the induction of angiogenesis by regulating the production of VEGF [79,96,97] and VEGF is the transcriptional effector of HIF1α. In the present study CD31-positive microvessel density (MVD) in whole tumor sections of subcutaneous HER2+ xenografts in the BEZ235-treated group was strikingly diminished. This effect was much more pronounced in the combination arms in all three groups (trastuzumab-sensitive, trastuzumab-resistant and PIK3CA mutated models) (Figure 7Ab, 7Bb and 7Cb). While the exact mechanism is not known, the effect of BEZ235 on HIF1α synthesis and subsequent production of VEGF suggest a direct link between the PI3K-AKT-mTOR inhibition and the inhibition of angiogenesis, as demonstrated by decreased MVD in our mouse xenografts.
Figure 8.
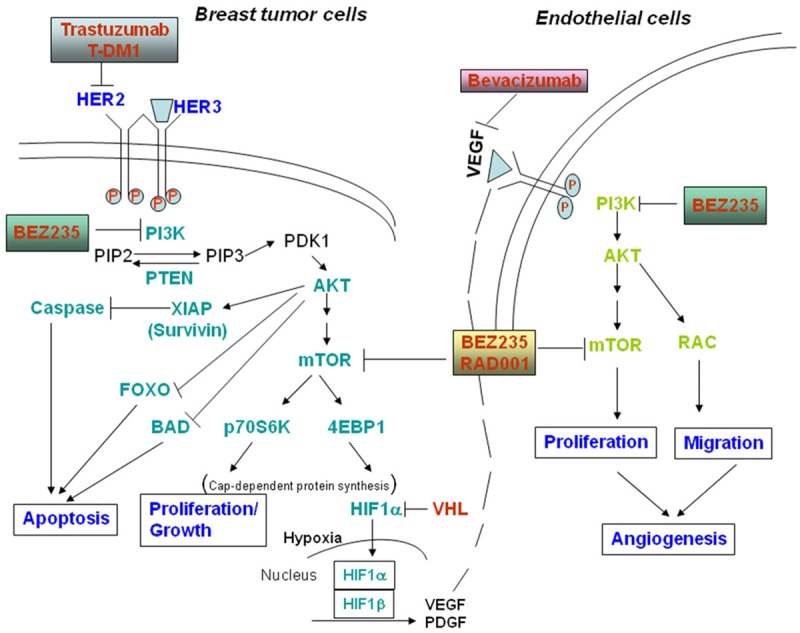
Schematic representation of the central theme of our study: PI3K-mTOR dual inhibitor BEZ235 blocks the transcriptional activity of HIF1alpha by decreasing its synthesis which in turn downregulates tumor-associated stromal endothelial cells in a paracrine manner and thereby inhibits tumor-angiogenesis.
In all three xenograft models, antitumor responses to single agent BEZ235 was observed, however, a combination of BEZ235 plus trastuzumab showed more antitumor activity ranging from tumor growth inhibition to stasis but not tumor regression (Figure 7Aa, 7Ba and 7Ca). Unlike Violeta Serra and group (using PIK3CA mutant overexpressing cells) [98], in our hands, PIK3CA mutant tumor did not show better response compared to the other two xenograft models. It was previously reported by others that MYC a commonly deregulated breast cancer oncogene was responsible for the acquired BEZ235 resistance [99,100] and HCC1954 are also MYC-dependent cells [101]. HCC1954 cells have a high copy number of MYC (greater than 7). Additionally, very high level of amphiregulin (ligand for EGFR) mRNA is also expressed in HCC1954 cells [102]. It has been also reported that amphiregulin-induced EGFR-mediated breast cancer cell proliferation is independent of PI3K inhibition [103]. From our PD data, we could suggest that BEZ235-mediated (either alone or in combination with trastuzumab) antitumor response was due to suppression of p-AKT, p-S6 ribosomal protein, reduced proliferation (low Ki67) and reduced tumor-induced angiogenesis (CD31 and pVEGFR) in all treated samples. In our study the superiority of a combined HER-targeted agent (using trastuzumab) with an anti-PI3K/mTOR pathway approach was found effective in only one trastuzumab-resistant HER2-amplified breast cancer cell line and one PIK3CA mutated HER2-amplified breast cancer cell line and understandably cannot necessarily be extrapolated to other HER2+/PIK3CA mutated or HER2+/PTEN null cell lines. It has been also suggested by other that trastuzumab treatment increased PTEN phosphatase activity and PTEN was required for antitumor activity of trastuzumab [19]. Recently, it was also reported that dual blockade of the HER2 signaling network with HER2: HER3 anti-dimerization antibody or HER3 specific antibody (inhibits HER2: HER3 dimer) in combination with a PI3K pathway inhibitor may be an effective treatment approach to HER2 overexpressing breast cancers [104].
In summary, combination treatment also leads to a reduction of HER2-positive breast cancer cell proliferation and tumor-induced angiogenesis when compared to single agent therapy. Our results further substantiate the previously reported treatment strategy in HER2+/trastuzumab-resistant as well as HER2+/PIK3CA mutated breast cancers that could be used for the design of phase II/III clinical studies. It is generally assumed that targeting a single or subgroup of isoforms will achieve the goal of limiting systemic toxicities [105]. However, a central question has been whether anticancer efficacy can be achieved by just targeting a specific single target alone. In regards to simultaneously targeting HER2 and the PI3K-AKT-mTOR pathway, there are hypothetical concerns about the safety and tolerability of this approach. Clinical investigations will be needed to determine whether this is tolerable and is an effective method of dual PI3K/mTOR signaling blockade in combination with anti-HER2 therapy in HER2 amplified breast cancer patients. Considering the existence of an intricate feedback loop within the PI3K-AKT-mTOR pathway we argued that targeting two major nodal points of this pathway, PI3K and mTOR will be ideal in order to achieve a maximum inhibition of the signaling pathway. Inhibition of mTOR following BEZ235 treatment in our study provided three significant advantages over the use of pan-PI3K or isoform-specific PI3K inhibitors. It caused a blockade of the a) cap-dependent mRNA translation, b) HIF1α-VEGF angiogenic pathway and c) AKT-independent LKB-AMPK-mTOR mediated nutrient/energy signaling of the tumor cells. Thus, we successfully targeted the survival/proliferation component of the “oncogenic event” rather than a single gene (HER2)-mediated signals in HER2+ breast cancers (Figure 8). Our data indicate that further study is warranted to test the efficacy of the combination of BEZ235 plus trastuzumab in order to obtain a superior therapeutic strategy in trastuzumab-sensitive and trastuzumab-resistant HER2+ BC carrying upregulation of the PI3K-mTOR pathway.
Acknowledgements
We are grateful to Novartis Pharmaceuticals, Basel, Switzerland for providing us with BEZ235 and RAD001. Authors acknowledge Avera Research Institute, Sioux Falls, SD and Department of Internal Medicine, SSOM, USD, Sioux Falls, SD. We acknowledge the cBioPortal for Cancer Genomics site (http://cbioportal.org). The portal reduces molecular profiling data from cancer tissues and cell lines into readily understandable genetic, epigenetic, gene expression and proteomic events (Gao and group 2013, Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal, Sci. Signal., 2 April, Vol. 6, Issue 269, p. pl1). We acknowledge works of Cerami and group (The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discovery. May 2012 2; 401) and works of Gao and group (Integrative analysis of complex cancer genomics and clinical profiles using the c-BioPortal. Sci. Signal. 6, pl1, 2013). We acknowledge the TCGA Research Network for generating TCGA datasets. cBioPortal data is subjected to scheduled updates.
Authors’ contribution
The study was conceptualized and designed by PD and the article was written by PD. JHC, PD, ND, and YS were responsible for the in vitro experiments. YS was responsible for the in vivo studies. XL was responsible for the IHC staining. HW was responsible for the technical support. The MS was critically evaluated by ND and by JHC. The overall evaluation of the study was carried out by BLJ.
Disclosure of conflict of interest
None.
References
- 1.Slamon DJ, Clark GM. Amplification of cerbB-2 and aggressive human breast tumors? Science. 1988;240:1795–1798. doi: 10.1126/science.3289120. [DOI] [PubMed] [Google Scholar]
- 2.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J. Clin. Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 3.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD Herceptin Adjuvant (HERA) Trial Study Team. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 4.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 5.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 6.Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G, Baselga J, Kaufmann M, Cameron D, Bell R, Bergh J, Coleman R, Wardley A, Harbeck N, Lopez RI, Mallmann P, Gelmon K, Wilcken N, Wist E, Sánchez Rovira P, Piccart-Gebhart MJ HERA study team. 2-year followup of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 7.Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CE Jr, Martino S, Rastogi P, Gralow J, Swain SM, Winer EP, Colon-Otero G, Davidson NE, Mamounas E, Zujewski JA, Wolmark N. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J. Clin. Oncol. 2014;32:3744–3752. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gianni L, Dafni U, Gelber RD, Azambuja E, Muehlbauer S, Goldhirsch A, Untch M, Smith I, Baselga J, Jackisch C, Cameron D, Mano M, Pedrini JL, Veronesi A, Mendiola C, Pluzanska A, Semiglazov V, Vrdoljak E, Eckart MJ, Shen Z, Skiadopoulos G, Procter M, Pritchard KI, Piccart-Gebhart MJ, Bell R Herceptin Adjuvant (HERA) Trial Study Team. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol. 2011;12:236–244. doi: 10.1016/S1470-2045(11)70033-X. [DOI] [PubMed] [Google Scholar]
- 9.Perez EA, Romond EH, Suman VJ, Jeong JH, Davidson NE, Geyer CE Jr, Martino S, Mamounas EP, Kaufman PA, Wolmark N. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J. Clin. Oncol. 2011;29:3366–3373. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, Shak S, Stewart SJ, Press M. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J. Clin. Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 11.Gajria D, Chandarlapaty S. HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies. Expert Rev Anticancer Ther. 2011;11:263–275. doi: 10.1586/era.10.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montemurro F, Donadio M, Clavarezza M, Redana S, Jacomuzzi ME, Valabrega G, Danese S, Vietti-Ramus G, Durando A, Venturini M, Aglietta M. Outcome of patients with HER2-positive advanced breast cancer progressing during trastuzumab-based therapy. Oncologist. 2006;11:318–324. doi: 10.1634/theoncologist.11-4-318. [DOI] [PubMed] [Google Scholar]
- 13.Pohlmann PR, Mayer IA, Mernaugh R. Resistance to Trastuzumab in Breast Cancer. Clin Cancer Res. 2009;15:7479–7491. doi: 10.1158/1078-0432.CCR-09-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kataoka Y, Mukohara T, Shimada H, Saijo N, Hirai M, Minami H. Association between gain-of-function mutations in PIK3CA and resistance to HER2-targeted agents in HER2-amplified breast cancer cell lines. Ann Oncol. 2010;21:255–262. doi: 10.1093/annonc/mdp304. [DOI] [PubMed] [Google Scholar]
- 15.Eichhorn PJ, Gili M, Scaltriti M, Serra V, Guzman M, Nijkamp W, Beijersbergen RL, Valero V, Seoane J, Bernards R, Baselga J. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 2008;68:9221–9230. doi: 10.1158/0008-5472.CAN-08-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzalez-Angulo AM, Stemke-Hale K, Hauptmann M, Beijersbergen RL, Mills GB, van de Vijver MJ, Bernards R. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 17.Razis E, Bobos M, Kotoula V, Eleftheraki AG, Kalofonos HP, Pavlakis K, Papakostas P, Aravantinos G, Rigakos G, Efstratiou I, Petraki K, Bafaloukos D, Kostopoulos I, Pectasides D, Kalogeras KT, Skarlos D, Fountzilas G. Evaluation of the association of PIK3CA mutations and PTEN loss with efficacy of trastuzumab therapy in metastatic breast cancer. Breast Cancer Res Treat. 2011;128:447–456. doi: 10.1007/s10549-011-1572-5. [DOI] [PubMed] [Google Scholar]
- 18.Fujita T, Doihara H, Kawasaki K, Takabatake D, Takahashi H, Washio K, Tsukuda K, Ogasawara Y, Shimizu N. PTEN activity could be a predictive marker of trastuzumab efficacy in the treatment of ErbB2-overexpressing breast cancer. Br J Cancer. 2006;94:247–252. doi: 10.1038/sj.bjc.6602926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT, Hortobagyi GN, Hung MC, Yu D. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 20.Dave B, Migliaccio I, Gutierrez MC, Wu MF, Chamness GC, Wong H, Narasanna A, Chakrabarty A, Hilsenbeck SG, Huang J, Rimawi M, Schiff R, Arteaga C, Osborne CK, Chang JC. Loss of phosphatase and tensin homolog or phosphoinositol-3 kinase activation and response to trastuzumab or lapatinib in human epidermal growth factor receptor 2-overexpressing locally advanced breast cancers. J. Clin. Oncol. 2011;29:166–173. doi: 10.1200/JCO.2009.27.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen JD, Knoop A, Laenkholm AV, Grauslund M, Jensen MB, Santoni-Rugiu E, Andersson M, Ewertz M. PIK3CA mutations, PTEN, and pHER2 expression and impact on outcome in HER2-positive early-stage breast cancer patients treated with adjuvant chemotherapy and trastuzumab. Ann Oncol. 2012;23:2034–2042. doi: 10.1093/annonc/mdr546. [DOI] [PubMed] [Google Scholar]
- 22.Cizkova M, Dujaric ME, Lehmann-Che J, Scott V, Tembo O, Asselain B, Pierga JY, Marty M, de Cremoux P, Spyratos F, Bieche I. Outcome impact of PIK3CA mutations in HER2-positive breast cancer patients treated with trastuzumab. Br J Cancer. 2013;108:1807–1809. doi: 10.1038/bjc.2013.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaught DB, Stanford JC, Young C, Hicks DJ, Wheeler F, Rinehart C, Sanchez V, Koland J, Muller WJ, Arteaga CL, Cook RS. HER3 is required for HER2-induced preneoplastic changes to the breast epithelium and tumor formation. Cancer Res. 2012;72:2672–2682. doi: 10.1158/0008-5472.CAN-11-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dey N, Williams C, Leyland-Jones B, De P. A critical role for HER3 in HER2-amplified and non-amplified breast cancers: function of a kinase-dead RTK. Am J Transl Res. 2015;7:733–750. [PMC free article] [PubMed] [Google Scholar]
- 25.Gibbons JJ, Abraham RT, Yu K. Mammalian target of rapamycin: discovery of rapamycin reveals a signaling pathway important for normal and cancer cell growth. Semin Oncol. 2009;36(Suppl 3):S3–S17. doi: 10.1053/j.seminoncol.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y, Dey N, Brammer M, De P, Leyland-Jones B. Bevacizumab confers additional advantage to the combination of trastuzumab plus pertuzumab in trastuzumabrefractory breast cancer model. Cancer Chemother Pharmacol. 2013;72:733–745. doi: 10.1007/s00280-013-2233-7. [DOI] [PubMed] [Google Scholar]
- 27.Kim SH, Juhnn YS, Song YS. Akt involvement in paclitaxel chemoresistance of human ovarian cancer cells. Ann N Y Acad Sci. 2007;1095:82–89. doi: 10.1196/annals.1397.012. [DOI] [PubMed] [Google Scholar]
- 28.Mabuchi S, Altomare DA, Cheung M, Zhang L, Poulikakos PI, Hensley HH, Schilder RJ, Ozols RF, Testa JR. RAD001 inhibits human ovarian cancer cell proliferation, enhances cisplatin-induced apoptosis, and prolongs survival in an ovarian cancer model. Clin Cancer Res. 2007;13:4261–4270. doi: 10.1158/1078-0432.CCR-06-2770. [DOI] [PubMed] [Google Scholar]
- 29.Schlosshauer PW, Li W, Lin KT, Chan JL, Wang LH. Rapamycin by itself and additively in combination with carboplatin inhibits the growth of ovarian cancer cells. Gynecol Oncol. 2009;114:516–522. doi: 10.1016/j.ygyno.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods. 2007;4:359–365. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De P, Sun Y, Carlson JH, Friedman LS, Leyland-Jones BR, Dey N. Doubling down on the PI3K-AKT-mTOR pathway enhances the antitumor efficacy of PARP inhibitor in triple negative breast cancer model beyond BRCA-ness. Neoplasia. 2014;16:43–72. doi: 10.1593/neo.131694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dey N, Howell BW, De PK, Durden DL. CSK negatively regulates nerve growth factor induced neural differentiation and augments AKT kinase activity. Exp Cell Res. 2005;307:1–14. doi: 10.1016/j.yexcr.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 33.Pradip D, Bouzyk M, Dey N, Leyland-Jones B. Dissecting GRB7-mediated signals for proliferation and migration in HER2 overexpressing breast tumor cells: GTP-ase rules. Am J Cancer Res. 2013;3:173–195. [PMC free article] [PubMed] [Google Scholar]
- 34.De P, Carlson JH, Leyland-Jones B, Dey N. Role of “oncogenic nexus” of CIP2A in breast oncogenesis: how does it work? Am J Cancer Res. 2015;5:2872–2891. [PMC free article] [PubMed] [Google Scholar]
- 35.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pradip D, Peng X, Durden DL. Rac2 specificity in macrophage integrin signaling: potential role for Syk kinase. J Biol Chem. 2003;278:41661–41669. doi: 10.1074/jbc.M306491200. [DOI] [PubMed] [Google Scholar]
- 38.Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dey N, Barwick BG, Moreno CS, Ordanic-Kodani M, Chen Z, Oprea-Ilies G, Tang W, Catzavelos C, Kerstann KF, Sledge GW Jr, Abramovitz M, Bouzyk M, De P, Leyland-Jones BR. Wnt signaling in triple negative breast cancer is associated with metastasis. BMC Cancer. 2013;13:537. doi: 10.1186/1471-2407-13-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Brien NA, Browne BC, Chow L, Wang Y, Ginther C, Arboleda J, Duffy MJ, Crown J, O’Donovan N, Slamon DJ. Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Mol Cancer Ther. 2010;9:1489–1502. doi: 10.1158/1535-7163.MCT-09-1171. [DOI] [PubMed] [Google Scholar]
- 41.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, Papa A, Nardella C, Cantley LC, Baselga J, Pandolfi PP. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu CX, Li Y, Yue P, Owonikoko TK, Ramalingam SS, Khuri FR, Sun SY. The combination of RAD001 and NVP-BEZ235 exerts synergistic anticancer activity against non-small cell lung cancer in vitro and in vivo. PLoS One. 2011;6:e20899. doi: 10.1371/journal.pone.0020899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas HE, Mercer CA, Carnevalli LS, Park J, Andersen JB, Conner EA, Tanaka K, Matsutani T, Iwanami A, Aronow BJ, Manway L, Maira SM, Thorgeirsson SS, Mischel PS, Thomas G, Kozma SC. mTOR inhibitors synergize on regression, reversal of gene expression, and autophagy in hepatocellular carcinoma. Sci Transl Med. 2012;4:139ra184. doi: 10.1126/scitranslmed.3003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Y, Shang X, Sarkissyan M, Slamon D, Vadgama JV. FOXO1A is a target for HER2-overexpressing breast tumors. Cancer Res. 2010;70:5475–5485. doi: 10.1158/0008-5472.CAN-10-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–859. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 46.Gomes AR, Brosens JJ, Lam EW. Resist or die: FOXO transcription factors determine the cellular response to chemotherapy. Cell Cycle. 2008;7:3133–3136. doi: 10.4161/cc.7.20.6920. [DOI] [PubMed] [Google Scholar]
- 47.Lloyd RV, Erickson LA, Jin L, Kulig E, Qian X, Cheville JC, Scheithauer BW. p27kip1: a multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol. 1999;154:313–323. doi: 10.1016/S0002-9440(10)65277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu S, Goldstein RH, Scepansky EM, Rosenblatt M. Inhibition of rho-associated kinase signaling prevents breast cancer metastasis to human bone. Cancer Res. 2009;69:8742–8751. doi: 10.1158/0008-5472.CAN-09-1541. [DOI] [PubMed] [Google Scholar]
- 49.Ong CC, Gierke S, Pitt C, Sagolla M, Cheng CK, Zhou W, Jubb AM, Strickland L, Schmidt M, Duron SG, Campbell DA, Zheng W, Dehdashti S, Shen M, Yang N, Behnke ML, Huang W, McKew JC, Chernoff J, Forrest WF, Haverty PM, Chin SF, Rakha EA, Green AR, Ellis IO, Caldas C, O’Brien T, Friedman LS, Koeppen H, Rudolph J, Hoeflich KP. Small molecule inhibition of group I p21-activated kinases in breast cancer induces apoptosis and potentiates the activity of microtubule stabilizing agents. Breast Cancer Res. 2015;17:59. doi: 10.1186/s13058-015-0564-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De P, Hasmann M, Leyland-Jones B. Molecular determinants of trastuzumab efficacy: What is their clinical relevance? Cancer Treat Rev. 2013;39:925–934. doi: 10.1016/j.ctrv.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 51.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanker AB, Pfefferle AD, Balko JM, Kuba MG, Young CD, Sanchez V, Sutton CR, Cheng H, Perou CM, Zhao JJ, Cook RS, Arteaga CL. Mutant PIK3CA accelerates HER2-driven transgenic mammary tumors and induces resistance to combinations of anti-HER2 therapies. Proc Natl Acad Sci U S A. 2013;110:14372–14377. doi: 10.1073/pnas.1303204110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Esteva FJ, Guo H, Zhang S, Santa-Maria C, Stone S, Lanchbury JS, Sahin AA, Hortobagyi GN, Yu D. PTEN, PIK3CA, p-AKT, and pp70S6K status: association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am J Pathol. 2010;177:1647–1656. doi: 10.2353/ajpath.2010.090885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whelan KA, Schwab LP, Karakashev SV, Franchetti L, Johannes GJ, Seagroves TN, Reginato MJ. The oncogene HER2/neu (ERBB2) requires the hypoxia-inducible factor HIF-1 for mammary tumor growth and anoikis resistance. J Biol Chem. 2013;288:15865–15877. doi: 10.1074/jbc.M112.426999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Su JD, Mayo LD, Donner DB, Durden DL. PTEN and phosphatidylinositol 3’-kinase inhibitors up-regulate p53 and block tumor-induced angiogenesis: evidence for an effect on the tumor and endothelial compartment. Cancer Res. 2003;63:3585–3592. [PubMed] [Google Scholar]
- 56.Garlich JR, De P, Dey N, Su JD, Peng X, Miller A, Murali R, Lu Y, Mills GB, Kundra V, Shu HK, Peng Q, Durden DL. A vascular targeted pan phosphoinositide 3-kinase inhibitor prodrug, SF1126, with antitumor and antiangiogenic activity. Cancer Res. 2008;68:206–215. doi: 10.1158/0008-5472.CAN-07-0669. [DOI] [PubMed] [Google Scholar]
- 57.Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wen S, Stolarov J, Myers MP, Su JD, Wigler MH, Tonks NK, Durden DL. PTEN controls tumor-induced angiogenesis. Proc Natl Acad Sci U S A. 2001;98:4622–4627. doi: 10.1073/pnas.081063798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blancher C, Moore JW, Robertson N, Harris AL. Effects of ras and von Hippel-Lindau (VHL) gene mutations on hypoxia-inducible factor (HIF)-1alpha, HIF-2alpha, and vascular endothelial growth factor expression and their regulation by the phosphatidylinositol 3’-kinase/Akt signaling pathway. Cancer Res. 2001;61:7349–7355. [PubMed] [Google Scholar]
- 60.Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 61.Kamel D, Brady B, Tabchy A, Mills GB, Hennessy B. Proteomic classification of breast cancer. Curr Drug Targets. 2012;13:1495–1509. doi: 10.2174/138945012803530080. [DOI] [PubMed] [Google Scholar]
- 62.Dey N, Young B, Abramovitz M, Bouzyk M, Barwick B, De P, Leyland-Jones B. Differential activation of Wnt-beta-catenin pathway in triple negative breast cancer increases MMP7 in a PTEN dependent manner. PLoS One. 2013;8:e77425. doi: 10.1371/journal.pone.0077425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 64.Chandarlapaty S, Sakr RA, Giri D, Patil S, Heguy A, Morrow M, Modi S, Norton L, Rosen N, Hudis C, King TA. Frequent mutational activation of the PI3K-AKT pathway in trastuzumab-resistant breast cancer. Clin Cancer Res. 2012;18:6784–6791. doi: 10.1158/1078-0432.CCR-12-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Juric D, Krop I, Ramanathan RK, Xiao J, Sanabria S, Wilson TR, Choi Y, Parmar H, Hsu J, Baselga J, Hoff DDV. GDC-0032, a beta isoform-sparing PI3K inhibitor: Results of a first-in-human phase Ia dose escalation study. Cancer Res. 2013;73 LB-64. [Google Scholar]
- 66.Juric D, Rodon J, Gonzalez-Angulo AM, Burris HA, Bendell J, Berlin JD, Middleton MR, Bootle D, Boehm M, Schmitt A, Rouyrre N, Quadt C, Baselga J. BYL719, a next generation PI3K alpha specific inhibitor: Preliminary safety, PK, and efficacy results from the first-inhuman study. Cancer Res. 2012;72 CT-01. [Google Scholar]
- 67.Gori S, Sidoni A, Colozza M, Ferri I, Mameli MG, Fenocchio D, Stocchi L, Foglietta J, Ludovini V, Minenza E, De Angelis V, Crino L. EGFR, pMAPK, pAkt and PTEN status by immunohistochemistry: correlation with clinical outcome in HER2-positive metastatic breast cancer patients treated with trastuzumab. Ann Oncol. 2009;20:648–654. doi: 10.1093/annonc/mdn681. [DOI] [PubMed] [Google Scholar]
- 68.Regales L, Gong Y, Shen R, de Stanchina E, Vivanco I, Goel A, Koutcher JA, Spassova M, Ouerfelli O, Mellinghoff IK, Zakowski MF, Politi KA, Pao W. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J Clin Invest. 2009;119:3000–3010. doi: 10.1172/JCI38746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wainberg ZA, Anghel A, Desai AJ, Ayala R, Luo T, Safran B, Fejzo MS, Hecht JR, Slamon DJ, Finn RS. Lapatinib, a dual EGFR and HER2 kinase inhibitor, selectively inhibits HER2-amplified human gastric cancer cells and is synergistic with trastuzumab in vitro and in vivo. Clin Cancer Res. 2010;16:1509–1519. doi: 10.1158/1078-0432.CCR-09-1112. [DOI] [PubMed] [Google Scholar]
- 70.Willems L, Tamburini J, Chapuis N, Lacombe C, Mayeux P, Bouscary D. PI3K and mTOR signaling pathways in cancer: new data on targeted therapies. Curr Oncol Rep. 2012;14:129–138. doi: 10.1007/s11912-012-0227-y. [DOI] [PubMed] [Google Scholar]
- 71.O’Regan R, Ozguroglu M, F A. Phase III, randomized, double-blind, placebo-controlled multicenter trial of daily everolimus plus weekly trastuzumab and vinorelbine in trastuzumab-resistant, advanced breast cancer (BOLERO-3) ASCO Annual meeting. Abstract 505. 2013 [Google Scholar]
- 72.O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, Shokat KM. Activesite inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Serra V, Scaltriti M, Prudkin L, Eichhorn PJ, Ibrahim YH, Chandarlapaty S, Markman B, Rodriguez O, Guzman M, Rodriguez S, Gili M, Russillo M, Parra JL, Singh S, Arribas J, Rosen N, Baselga J. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene. 2011;30:2547–2557. doi: 10.1038/onc.2010.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK, Baselga J, Rosen N. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dowling RJ, Topisirovic I, Alain T, Bidinosti M, Fonseca BD, Petroulakis E, Wang X, Larsson O, Selvaraj A, Liu Y, Kozma SC, Thomas G, Sonenberg N. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4EBPs. Science. 2010;328:1172–1176. doi: 10.1126/science.1187532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choo AY, Blenis J. Not all substrates are treated equally: implications for mTOR, rapamycin-resistance and cancer therapy. Cell Cycle. 2009;8:567–572. doi: 10.4161/cc.8.4.7659. [DOI] [PubMed] [Google Scholar]
- 78.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci U S A. 2008;105:17414–17419. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.De P, Dey N, Terakedis B, Bergsagel PL, Li ZH, Mahadevan D, Garlich JR, Trudel S, Makale MT, Durden DL. An integrin-targeted, pan-isoform, phosphoinositide-3 kinase inhibitor, SF1126, has activity against multiple myeloma in vivo. Cancer Chemother Pharmacol. 2013;71:867–881. doi: 10.1007/s00280-013-2078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Semenza G. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol. 2002;64:993–998. doi: 10.1016/s0006-2952(02)01168-1. [DOI] [PubMed] [Google Scholar]
- 82.Kasuno K, Takabuchi S, Fukuda K, Kizaka-Kondoh S, Yodoi J, Adachi T, Semenza GL, Hirota K. Nitric oxide induces hypoxia-inducible factor 1 activation that is dependent on MAPK and phosphatidylinositol 3-kinase signaling. J Biol Chem. 2004;279:2550–2558. doi: 10.1074/jbc.M308197200. [DOI] [PubMed] [Google Scholar]
- 83.Loibl S, von Minckwitz G, Schneeweiss A, Paepke S, Lehmann A, Rezai M, Zahm DM, Sinn P, Khandan F, Eidtmann H, Dohnal K, Heinrichs C, Huober J, Pfitzner B, Fasching PA, Andre F, Lindner JL, Sotiriou C, Dykgers A, Guo S, Gade S, Nekljudova V, Loi S, Untch M, Denkert C. PIK3CA mutations are associated with lower rates of pathologic complete response to anti-human epidermal growth factor receptor 2 (her2) therapy in primary HER2-overexpressing breast cancer. J. Clin. Oncol. 2014;32:3212–3220. doi: 10.1200/JCO.2014.55.7876. [DOI] [PubMed] [Google Scholar]
- 84.Majewski IJ, Nuciforo P, Mittempergher L, Bosma AJ, Eidtmann H, Holmes E, Sotiriou C, Fumagalli D, Jimenez J, Aura C, Prudkin L, Diaz-Delgado MC, de la Pena L, Loi S, Ellis C, Schultz N, de Azambuja E, Harbeck N, Piccart-Gebhart M, Bernards R, Baselga J. PIK3CA Mutations Are Associated With Decreased Benefit to Neoadjuvant Human Epidermal Growth Factor Receptor 2-Targeted Therapies in Breast Cancer. J. Clin. Oncol. 2015;33:1334–1339. doi: 10.1200/JCO.2014.55.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baselga J, Cortes J, Im SA, Clark E, Ross G, Kiermaier A, Swain SM. Biomarker analyses in CLEOPATRA: a phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J. Clin. Oncol. 2014;32:3753–3761. doi: 10.1200/JCO.2013.54.5384. [DOI] [PubMed] [Google Scholar]
- 86.Pogue-Geile KL, Song N, Jeong JH, Gavin PG, Kim SR, Blackmon NL, Finnigan M, Rastogi P, Fehrenbacher L, Mamounas EP, Swain SM, Wickerham DL, Geyer CE Jr, Costantino JP, Wolmark N, Paik S. Intrinsic Subtypes, PIK3CA Mutation, and the Degree of Benefit From Adjuvant Trastuzumab in the NSABP B-31 Trial. J. Clin. Oncol. 2015;33:1340–1347. doi: 10.1200/JCO.2014.56.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Elkabets M, Vora S, Juric D, Morse N, Mino-Kenudson M, Muranen T, Tao J, Campos AB, Rodon J, Ibrahim YH, Serra V, Rodrik-Outmezguine V, Hazra S, Singh S, Kim P, Quadt C, Liu M, Huang A, Rosen N, Engelman JA, Scaltriti M, Baselga J. mTORC1 inhibition is required for sensitivity to PI3K p110alpha inhibitors in PIK3CA-mutant breast cancer. Sci Transl Med. 2013;5:196ra199. doi: 10.1126/scitranslmed.3005747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Klos KS, Wyszomierski SL, Sun M, Tan M, Zhou X, Li P, Yang W, Yin G, Hittelman WN, Yu D. ErbB2 increases vascular endothelial growth factor protein synthesis via activation of mammalian target of rapamycin/p70S6K leading to increased angiogenesis and spontaneous metastasis of human breast cancer cells. Cancer Res. 2006;66:2028–2037. doi: 10.1158/0008-5472.CAN-04-4559. [DOI] [PubMed] [Google Scholar]
- 89.Xue G, Hemmings BA. PKB/Akt-dependent regulation of cell motility. J Natl Cancer Inst. 2013;105:393–404. doi: 10.1093/jnci/djs648. [DOI] [PubMed] [Google Scholar]
- 90.Benseddik K, Sen Nkwe N, Daou P, Verdier-Pinard P, Badache A. ErbB2-dependent chemotaxis requires microtubule capture and stabilization coordinated by distinct signaling pathways. PLoS One. 2013;8:e55211. doi: 10.1371/journal.pone.0055211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dey N, Crosswell HE, De P, Parsons R, Peng Q, Su JD, Durden DL. The protein phosphatase activity of PTEN regulates SRC family kinases and controls glioma migration. Cancer Res. 2008;68:1862–1871. doi: 10.1158/0008-5472.CAN-07-1182. [DOI] [PubMed] [Google Scholar]
- 92.Hage B, Meinel K, Baum I, Giehl K, Menke A. Rac1 activation inhibits E-cadherin-mediated adherens junctions via binding to IQGAP1 in pancreatic carcinoma cells. Cell Commun Signal. 2009;7:23. doi: 10.1186/1478-811X-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eriksson A, Cao R, Roy J, Tritsaris K, Wahlestedt C, Dissing S, Thyberg J, Cao Y. Small GTP-binding protein Rac is an essential mediator of vascular endothelial growth factor-induced endothelial fenestrations and vascular permeability. Circulation. 2003;107:1532–1538. doi: 10.1161/01.cir.0000055324.34758.32. [DOI] [PubMed] [Google Scholar]
- 94.Paruchuri S, Broom O, Dib K, Sjolander A. The pro-inflammatory mediator leukotriene D4 induces phosphatidylinositol 3-kinase and Rac-dependent migration of intestinal epithelial cells. J Biol Chem. 2005;280:13538–13544. doi: 10.1074/jbc.M409811200. [DOI] [PubMed] [Google Scholar]
- 95.Balasenthil S, Sahin AA, Barnes CJ, Wang RA, Pestell RG, Vadlamudi RK, Kumar R. p21-activated kinase-1 signaling mediates cyclin D1 expression in mammary epithelial and cancer cells. J Biol Chem. 2004;279:1422–1428. doi: 10.1074/jbc.M309937200. [DOI] [PubMed] [Google Scholar]
- 96.Graupera M, Guillermet-Guibert J, Foukas LC, Phng LK, Cain RJ, Salpekar A, Pearce W, Meek S, Millan J, Cutillas PR, Smith AJ, Ridley AJ, Ruhrberg C, Gerhardt H, Vanhaesebroeck B. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature. 2008;453:662–666. doi: 10.1038/nature06892. [DOI] [PubMed] [Google Scholar]
- 97.Schnell CR, Stauffer F, Allegrini PR, O’Reilly T, McSheehy PM, Dartois C, Stumm M, Cozens R, Littlewood-Evans A, Garcia-Echeverria C, Maira SM. Effects of the dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 on the tumor vasculature: implications for clinical imaging. Cancer Res. 2008;68:6598–6607. doi: 10.1158/0008-5472.CAN-08-1044. [DOI] [PubMed] [Google Scholar]
- 98.Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, Guzman M, Botero ML, Llonch E, Atzori F, Di Cosimo S, Maira M, Garcia-Echeverria C, Parra JL, Arribas J, Baselga J. NVPBEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 99.Ilic N, Utermark T, Widlund HR, Roberts TM. PI3K-targeted therapy can be evaded by gene amplification along the MYC-eukaryotic translation initiation factor 4E (eIF4E) axis. Proc Natl Acad Sci U S A. 2011;108:E699–708. doi: 10.1073/pnas.1108237108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dey N, Leyland-Jones B, De P. MYC-xing it up with PIK3CA mutation and resistance to PI3K inhibitors: summit of two giants in breast cancers. Am J Cancer Res. 2015;5:1–19. [PMC free article] [PubMed] [Google Scholar]
- 101.Kang J, Sergio CM, Sutherland RL, Musgrove EA. Targeting cyclin-dependent kinase 1 (CDK1) but not CDK4/6 or CDK2 is selectively lethal to MYC-dependent human breast cancer cells. BMC Cancer. 2014;14:32. doi: 10.1186/1471-2407-14-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, Speed T, Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo WL, Stilwell JL, Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB, Johnson MD, Lippman M, Ethier S, Gazdar A, Gray JW. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Young CD, Zimmerman LJ, Hoshino D, Formisano L, Hanker AB, Gatza ML, Morrison MM, Moore PD, Whitwell CA, Dave B, Stricker T, Bhola NE, Silva GO, Patel P, Brantley-Sieders DM, Levin M, Horiates M, Palma NA, Wang K, Stephens PJ, Perou CM, Weaver AM, O’Shaughnessy JA, Chang JC, Park BH, Liebler DC, Cook RS, Arteaga CL. Activating PIK3CA mutations induce an EGFR/ERK paracrine signaling axis in basal-like breast cancer. Mol Cell Proteomics. 2015;14:1959–76. doi: 10.1074/mcp.M115.049783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dey N, Williams C, Leyland-Jones B, De P. A Critical Role for HER3 in HER2-Amplified and non-Amplified Breast Cancers: Function of a Kinase-dead RTK Am. Am J Transl Res. 2015;7:733–50. [PMC free article] [PubMed] [Google Scholar]
- 105.Ruckle T, Schwarz MK, Rommel C. PI3Kgamma inhibition: towards an ‘aspirin of the 21st century’? Nat Rev Drug Discov. 2006;5:903–918. doi: 10.1038/nrd2145. [DOI] [PubMed] [Google Scholar]