Abstract
Electrospun nanofibrous sheets get increasing attention in myocardial infarction (MI) treatment due to their good cytocompatibility to deliver transplanted stem cells to infarcted areas and due to mechanical characteristics to support damaged tissue. Cardiac extracellular matrix is essential for implanted cells since it provides the cardiac microenvironment. In this study, we hypothesized high concentrations of cardiac nature protein (NP), namely elastin and collagen, in hybrid polycaprolactone (PCL) electrospun nanofibrous sheets could be effective as cardiac-mimicking patch. Optimal ratio of elastin and collagen with PCL in electrospun sheets (80% NP/PCL) was selected based on cytocompatibility and mechanical characteristics. Bone-marrow (BM) c-kit+ cells anchoring onto NP/PCL sheets exhibited increased proliferative capacity compared with those seeded on PCL in vitro. Moreover, we examined the improvement of cardiac function in MI mice by cell-seeded cardiac patch. Green Fluorescent Protein (GFP)-labeled BM c-kit+ cells were loaded on 80% NP/PCL sheets which was transplanted into MI mice. Both 80% NP/PCL and c-kit+-seeded 80% NP/PCL effectively improved cardiac function after 4 weeks of transplantation, with reduced infarction area and restricted LV remodeling. C-kit+-seeded 80% NP/PCL was even superior to the 80% NP/PCL alone and both superior to PCL. GFP+ cells were identified both in the sheets and local infarcted area where transplanted cells underwent cardiac differentiation after 4 weeks. To the best of our knowledge, this is the first report that sheets with high concentrations of nature proteins loaded with BM c-kit+ cells might be a novel promising candidate for tissue-engineered cardiac patch to improve cardiac repair after MI.
Keywords: Hybrid electrospun nanofibers, polycaprolactone, cardiac nature protein, bone marrow c-kit positive cells, cardiac repair
Introduction
Myocardial infarction (MI), commonly known as a heart attack, is worldwide a major cause of morbidity and mortality. A typical MI causes a great loss of cardiomyocytes which result in left ventricular (LV) remodeling, progressive contractile dysfunction and eventually heart failure [1]. It is well-known that current clinical treatment, such as drug, percutaneous interventional (PCI) treatment and Coronary Artery Bypass Grafting (CABG), can only save residual cardiomyocytes of forming scar tissue, but not regenerate new myocardium. Therefore, accumulating experimental and clinical researches have been evaluating transplantation of stem cells to improve cardiac repair [2-4]. Abundant researches showed that c-kit+ cells are promising candidates in the field of cell therapy and can be found in the heart and bone marrow among others. Cardiac c-kit+ progenitor cells exhibit all characteristics of stem cells including self-renewal, multipotency and involvement in the endogenous regeneration of myocardium and endothelial cells [5,6]. BM-derived c-kit+ stem cells, of which the source are richer than that in cardiac tissue, can differentiate into cardiomyocytes and vascular endothelial cells [7-9]. They play a crucial role in mobilization of progenitor cells which is critical for recovery of heart function [10]. For these reasons BM c-kit+ cells were selected as therapeutic stem cells for MI repair in this study.
In most cases, transplantation of stem cells is performed by peripheral or intramyocardial injection [11-13], but low cell retention rates limit the application in experimental clinical research [14,15]. To overcome the problem of low cell retention, it was hypothesized that providing the cells with an environment similar to their original one, the cardiac extracellular matrix, could improve their survival and proliferation [16]. And so providing a cardiac ECM to the cells which does not only mimic the structural properties but also biochemical components of native myocardial ECM, seems to be necessary for a successful transplantation of stem cells. The cardiac ECM is composed of various proteins. With 28 subtypes of fibrillar collagen, it is one of the most common structural proteins of the cardiac ECM with a concentration varying from approximately 460 to 500 μg/mg; out of which Type I collagen is the most abundant one [17,18]. Elastin, which is another natural structural protein in the ECM, can provide elastic properties [19]. In cardiac ECM, the normal ratio of elastin to collagen is around 1:10 [20]. A study where they made an artificial ECM by seeding cells on plate which contained various concentrations of collagen and elastin reported that the scaffold was competent for delivering seeded cells from epicardium to endocardium in mice [21]. Therefore, taking into account the main components in cardiac ECM and elastic properties provided by elastin, collagen type I and elastin were selected in this study.
Recently, electrospinning has become more popular to provide a micron range network to mimic native ECM structure [22,23]. Furthermore, hybrid nanofibers fabricated by electrospinning technique, using various kinds of natural proteins, were favorable for adhesion and proliferation of cells on the scaffolds [24-28]. Similarly to our work, a study where they used collagen (10%)/elastin (5%)/PCL (10%) electrospun sheets, found that combination of natural proteins and synthetic polymers to create electrospun fibrous structures resulted in scaffolds with favorable mechanical and biological properties. However, the concentration of collagen and elastin that were mixed with PCL were low and the performance of electrospun sheets in vivo remain still unclear [29].
In this study, high concentrations of collagen and elastin were selected as cardiac NP for the composition of hybrid electrospun sheets. PCL, a US FDA approved polyester, was added to fabricate electrospun nanofibrous sheets [27]. To investigate the best materials to mimic cardiac ECM, we optimized the ratio of collagen/elastin/PCL. We also hypothesized that our hybrid electrospun sheets seeded with BM-derived c-kit+ cells would represent a promising treatment option for tissue repair in myocardial infarction therapy.
Materials and methods
Preparation and characterization of electrospun sheets
The electrospun sheets used in the present study were prepared according to the procedures previously described [30]. Briefly, PCL (Mw = 80 kDa, sigma, USA) NP were blended with certain mass ratios (w/w = 2/8, 5/5, 8/2). The natural proteins consisted of elastin (Herochem Biochemical reagent CO. LTD, China) and collagen type I (Sigma, US) with a 3/7 mass proportion according to our preliminary experiments (data not shown). The blends were dissolved in Hexafluoroisopropanol (HFIP)(Aladdin, China) and stirred for 12 h to obtain a homogeneous and stable solution with a mixing solvent concentration of 15% (w/v). The prepared solutions, 20% NP/80% PCL (20% NP), 50% NP/50% PCL (50% NP) and 80% NP/20% PCL (80% NP), were loaded into a 2 mL syringe. During electrospinning, the flow rate of the solution in the syringe was set to 0.02 mL min-1 using a syringe pump (LSP01-1A, Baoding Longer Precision Pump, China). The voltage applied to the needle was 15 kV and the distance between the tip of the needle and the collector was 12.5 cm. As control, pure PCL electrospun sheets were similarly prepared. All the experiments were conducted at room temperature and relative humidity between 40-60%. The prepared electrospun sheets were vacuum-dried for 24 h to completely remove residual solvent prior to further characterization.
Morphology of PCL and NP/PCL electrospun sheets were observed under the scanning electron microscopy (SEM) (Quanta 2000, FEI, USA). Average diameter of fibers was determined by measuring 100 fiber segments from different SEM images for each type of sample using Image J® software. Water contact angle (WCA) measurement of electrospun sheets was performed using sessile drop method by a video-enabled goniometer (FM40MK2 EasyDrop, KRÜSS GmbH, Germany). At least 3 mL distilled water was placed randomly at different locations of each sample. After settling the droplets on the fibrous membrane sheets with no noticeable changes in their shapes, the projected images of the droplets were analyzed for determining contact angle.
Mechanical properties of the PCL and NP/PCL electrospun sheets were determined using a material testing machine (H5K-S, Hounsfield, United Kingdom) equipped with a 10 N load cell. At least 3 rectangular-shaped specimens (10×10×0.10-0.15 mm) were stretched at a constant cross-head speed of 10 mm/min. According to the stress-strain curves, Young’s modulus, elongation at break, and tensile strength were calculated.
Induction of MI model in mice
All C57BL/6 mice used in this study were purchased from SLAC Laboratories Co. LTD (Shanghai, China). GFP+ transgenic mice were obtained from Shanghai Biomodel Organism Science & Technology Development Co. LTD (Shanghai, China). All procedures in this study including animal use, housing, and surgeries were approved by the Shanghai Jiao Tong University Institutional Animal Care and Use Committee (IACUC).
Myocardial infarction mice models were performed as previously described [31]. Briefly, animals were anesthetized utilizing an isoflurane (Jiupai, Hebei, China) inhalational chamber. Subsequently, a small skin cut, muscle dissection and heart externalization was performed. Once the pericardium was opened, a 7-0 prolene was utilized to ligate the left descending artery (LDA). Hereafter, possible gas inside the chest was eliminated and the wound cleaned, closed by suturing and disinfected.
Isolation and culture of c-kit+ cells
C-kit+ cells were all freshly isolated from bone marrow of MI mice. Briefly, the bone marrow of the femur and tibia of 6-8 weeks old heart-infarcted C57BL/6 or GFP+ mice was flushed using Hank’s balance salt solution (HBSS; Thermo, USA) with 40 μm cell strainer (FALCON, BD). Mononuclear cells were collected by gradient centrifugation. Finally, c-kit+ cells were isolated from the mononuclear cells using a CD117 MACS kit (MiltenyiBiotec, Germany). Anti-CD117 antibody labeled with PE (BD, Germany) was used for examining the purity by Flow Cytometer (ACCURI C6, BD, Germany). Collected cells were cultured in DMEM supplemented with 15% fetal bovine serum (Gibco, Thermo, USA) and 1% Penicillin-Streptomycin solution (P/S, Thermo, USA).
PCL and NP/PCL electrospun sheets were placed on the bottom of the 96-well plates. Sheets were washed 3-5 times using PBS (Medicago, Sweden) after sterilization of UV-light for 2 hours. C-kit+ cells were then seeded in 96-well plates at concentrations of 1×105/well and culture performed in humidity incubator at 37°C and 5% CO2.
Cell proliferation assay
Cell proliferation was tested on day 0, 1, 3, 5, and 7 using the colorimetric Cell Titer 96 Aqueous One solution assay (Promega, USA) and examined at 490 nm using a spectrophotometric plate reader (Biotech, USA). To examine proliferation marker expression, cells cultured for 5 days were fixed by 4% paraformaldehyde and permeabilized by 0.5% Triton X-100. Cells were then incubated with rabbit anti-ki67 antibody (1:200, Abcam, UK) at 4°C overnight, followed by incubation with donkey anti-rabbit IgG H&L AlexaFluor 594 (1:500, Invitrogen, USA) at room temperature for 1 hour and 4’,6-diamidino-2-phenylindole (DAPI) (Sigma, USA) for 1 min. 5 visual fields were randomly selected for all groups and ki67 expression was determined.
Cardiac differentiation
0.4 μm transwell plates (Corning, USA) were used for c-kit+-seeded electrospun sheets and infarcted cardiac tissue co-culture. The seeded electrospun sheets were cultured in the lower layer of 24-well plate. Small amount of ground infarcted cardiac tissue was added into the upper transwell chamber. Twenty days after co-culture, RNA from the cells located in the lower wells was obtained using Trizol. Complementary DNA (cDNA) was synthesized using the 1st synthesize cDNA kit (Vanzyme Biotech) according to the manufacturer’s instruction. Polymerase chain reaction (PCR) was performed with GoTaq Green Master mix (Promega, USA) with the following genes: Gata4, Nkx2.5, Cardiac Troponin T (cTnT) and alpha-sarcomeric actin (α-SMA). Primer information is specified in Table S1.
Transplantation of electrospun sheets
Fifty-four C57BL/6 mice were randomly assigned to 5 groups: 1) sham operation group (n = 6); 2) MI group (n = 12); 3) PCL group (cell-free PCL electrospun sheets, n = 12); 4) 80% NP (cell-free 80% NP electrospun sheets, n = 12); 5) c-kit+-seeded 80% NP group (c-kit+-seeded 80% NP electrospun sheets, n = 12). Transplanted c-kit+ cells were derived from bone marrow of GFP+ transgenic mice to identify conveniently cell survival and differentiation. The c-kit+-seeded 80% NP electrospun sheets were cultured for 3 days before transplantation.
Surgical anesthetization and method of exposure of mice hearts of transplanting cardiac patches were the same as MI surgery. Once the pericardium was opened, a 7-0 prolene, which was sutured in advance in the electrospun sheet, was utilized to ligate the LDA. Hereafter, the sheet was set to the left ventricle. The electrospun sheet attached to the epicardium without using glue. Finally, gas inside the chest was eliminated, and the wound closed by suturing. All mice received daily injection, starting 1 day before surgery until sacrifice, of cyclosporine A (25 mg/kg/day, Neocyspin, China) by intragastric administration.
Echocardiography
In vivo cardiac function for each groups were assessed at week one and four by transthoracic echocardiography (LG, South Korean). Briefly, mice were anesthetized with 5% chloralhydrate (Sigma, USA) by intraperitoneal injection. 2D mode and M-mode images were recorded by short axis view from the mid-LV between the 4th to 5th intercostal space. The LV end-diastolic diameter (LVEDD) and end-systolic diameter (LVESD) were measured from 3-5 consecutive cardiac cycles on the M-mode images. To assess cardiac function, the ejection fraction (EF) and fractional shortening (FS) were automatically generated by the machine. All measurements were performed by an experienced ultrasound doctor blinded to treatment groups.
In vivo cardiac hemodynamics
Four weeks after cardiac electrospun sheet transplantation, mice were monitored continuously to measure pressure-volume (P/V) using Millar Pressure-Volume system [32] (ULTRA-Miniature Pressure-Volume Catheter, model SPR-1049, Millar Instrument, USA). LabChart Pro v.7 software was used for analysis of left ventricle systolic and diastolic function. All hemodynamic parameters were automatically calculated and generated. Briefly, mice were anesthetized using isoflurane inhalational chamber as described earlier. After calibration, the probe was inserted from the left common carotid artery to the left ventricle, enabling to obtain the LV pressure and conductance in real time. This was followed by intravenous administration of 20% sodium chloride solution for 3 times. After correction of volume data by measurement of conductance, mice were sacrificed for tissue collection.
Examination of infarct size and histological staining
After cardiac hemodynamics measurements, mice were all sacrificed. Hearts (n = 6) from each group were cut into slices from about 2 mm for 2, 3, 5-Triphenyltetrazolium Chloride (TTC) staining. The remaining hearts (n = 6) from each group were fixed in 2% paraformaldehyde and embedded in Tissue-Tek OCT Compound (Sakura Finetek, CA). 8-10 μm sections were cut using cryostat and stained with H&E, Sirius Red [33]. Changes in thickness of LV wall was examined by comparing the average thickness of left ventricles. The scar size was evaluated by calculating the percentage of viable red areas to the whole area using Image J® software.
Immunofluorescence staining
Immunostaining was used for tracing transplanted GFP+ cells and analyzing myocardium regeneration. Briefly, frozen sections were incubated with chicken anti-GFP antibody (1:1000, Abcam, UK) and mouse anti-Cardiac Troponin T antibody (1:500, Abcam, UK) at 4°C overnight. Sections were then incubated with secondary antibody goat anti-chicken IgG AlexaFluor 488 (1:1000, Abcam, UK) and donkey anti-mouse IgG AlexaFluor 594 (1:1000, Invitrogen, USA) at room temperature for 1 h and DAPI for 1 min. Images were obtained using fluorescent microscopy (Nikon, Japan).
Statistical analyses
All data are expressed as mean ± standard deviation. SPSS software (v.18.0) was used for analysis with P<0.05 set as statistically significant. Comparisons among multiple groups were performed using one-way ANOVA with Tukey’s multiple post-test.
Results
Characteristics of PCL and NP/PCL electrospun sheets
All electrospun sheets presented porous and uniform structures; however, SEM observation demonstrated that mixing NPs with PCL changed the morphology of electrospun sheets (Figure 1A). Interestingly, diameter of fibers in electrospun sheets decreased with the increase of weight ratio of NP to PCL. The fiber diameter of PCL (925.25 ± 127 nm) was significantly higher than those of hybrid nanofibrous sheets (P<0.01). The fiber diameter of 20% NP group (684.65 ± 123.61 nm) was significantly higher than 50% NP group (526.7 ± 113.11 nm) and 80% NP group (497 ± 104.01 nm; Table 1). There was also significant difference between 50% NP and 80% NP (P<0.01; Figure 1A, 1C).
Figure 1.
Characteristics of PCL and NP/PCL electrospun sheets. 3:7 ratio of elastin to collagen in 20% NP/80% PCL (20% NP), 50% NP/50% PCL (50% NP), 80% NP/20% PCL (80% NP) groups are shown as well as PCL. A. Scanning electron microscope (SEM) analysis of PCL and NP/PCL electrospun sheets. Scale bar = 30 μm. B. Photographs of contact angle. C. Statistical analysis of fiber diameter of PCL, 20% NP, 50% NP and 80% NP electrospun sheets. D. Statistical analysis of static water contact angle for PCL, 20% NP, 50% NP and 80% NP electrospun sheets. *P<0.05; **P<0.01.
Table 1.
Mechanical characteristics of electrospun sheets
| Sample | PCL | 20% NP | 50% NP | 80% NP |
|---|---|---|---|---|
| Young’s modulus (Mpa) | 3.73 ± 0.59 | 10.32 ± 0.41# | 16.80 ± 1.33#,& | 23.14 ± 1.76#,&,† |
| Tensile strength (Mpa) | 1.742 ± 0.437 | 10.970 ± 2.116# | 3.683 ± 0.444#,& | 2.348 ± 0.346&,† |
| Elongation at break (%) | 118.970 ± 28.826 | 59.052 ± 16.226# | 32.490 ± 18.712# | 10.680 ± 4.685#,& |
compared with PCL electrospun sheets, P < 0.01;
compared with 20% NP electrospun sheets, P < 0.01;
compared with 50% NP electrospun sheets, P < 0.01;
Data are mean ± SEM of five experiments.
Wettability measurement of electrospun sheets in each group indicated that addition of proteins endowed electrospun sheets with better hydrophilicity (Figure 1B, 1D). The hybrid electrospun sheets were more hydrophilic as compared with pure PCL electrospun sheets (141.1 ± 4.6°; P<0.01). The static water contact angle decreased with the increase of NP content from 71.1 ± 1.1° of the 20% NP group to 50.0 ± 2.6° of 50% NP and 45.9 ± 3.3° of 80% NP (P<0.01).
Mechanical properties of electrospun sheets changed significantly with addition of NPs (Table 1). The Young’s modulus of high concentration protein hybrid electrospun sheets was higher than PCL (3.73 ± 0.59 MPa; P<0.01). Young’s modulus of 80% NP electrospun sheet (23.14 ± 1.76 MPa) was significantly higher compared to 20% NP (10.32 ± 0.41 MPa) and 50% NP (16.80 ± 1.33 MPa) sheets (P<0.01). In addition, with the increase of NP content in electrospun sheets, tensile strength of sheets first shows an increased and followed by a decrease. The tensile strength values of 20% NP (10.970 ± 2.116 MPa) and 50% NP (3.683 ± 0.444 MPa) electrospun sheets were higher than those of the PCL (1.742 ± 0.437 MPa; P<0.01) and 80% NP (2.348 ± 0.346 MPa; P<0.01) electrospun membranes. Moreover, the elongation at break of PCL (118.970 ± 28.826%) was significantly longer compared with that of hybrid electrospun sheets (P<0.01).
NP/PCL electrospun sheets promote proliferation of BM c-kit+ cells in vitro
To evaluate possible cell-material adhesion, murine BM c-kit+ cells (1×105 cells/cm2; c-kit+ purity over 85%) were seeded on electrospun sheets and cultured for 7 days. C-kit+ cells adhered well on electrospun sheets (Figure 2A). After seeded onto the electrospun sheets and cultured for 7 days, cell number increased proportionally with NPs concentration, while cells in c-kit+ only group did not show obviously proliferation (Figure 2A). In addition, cell proliferation was also assessed by immunofluorescence staining. Ki67, a proliferation marker, was expressed significantly higher in c-kit+-seeded electrospun sheet groups compared to c-kit+ only group (Figure 2B, 2C). The percentage of ki67 positive cells in electrospun sheet groups were significantly increased compared with c-kit+ cells only group after cultured for 5 days (1.8 ± 0.4%; P<0.01). The hybrid electrospun sheet groups led to a significantly increased ki67-positivive rate compared with PCL (12.7 ± 3.0%) group in which the most effective group was 80% NP.
Figure 2.

NP/PCL electrospun sheets promote proliferation of c-kit positive cells in vitro. A. Optical microscope photographs of c-kit+ cell proliferation for different study groups. Scale bar = 25 μm. B. Cell proliferation assessed using Ki67 immunofluorescene staining. Positive cells stained red. Scale bar = 25 μm. C. Percentage of ki67-positive cells from immunofluorescence staining calculated by counting positive cells in 5 different random view fields. D. The proliferation tested by MTS assay on day 0, 1, 3, 5, and 7. *P<0.05; **P<0.01.
Proliferation was also investigated by MTS assay on day 0, 1, 3, 5 and 7. An increase in proliferation was observed from day 1 to day 7 in all groups, reaching the maximum on day 5. Hereafter, proliferation gradually decreased. However, the 80% NP nanofibers was superior in promoting cell proliferation in general, and significantly higher than 50% NP group from day 1 to day 7 (day 1, 3 and 7: P<0.05, day 5: P<0.01; Figure 2D). In general, we observed a positive correlation of NPs content and c-kit+ cell proliferation. In addition, proliferation tests were also performed in different ratios of elastin to collagen NP/PCL hybrid electrospun sheets, and the 3:7 ratio of elastin to collagen in the NP/PCL group significantly promoted proliferation of c-kit+ cells compared to other ratios (data not shown). Therefore, the 3:7 ratio of elastin to collagen was selected for following experiments.
In vitro experiments showed that c-kit+ cells underwent cardiac differentiation when co-cultured with infarcted cardiac tissue. Reverse transcription PCR showed that immature and mature cardiac marker genes including Gata4, Nkx2.5, cTnT and α-SMA were expressed (Figure S1). The expression of these cardiac differentiation makers was the same in cell seeded electrospun sheets or cell-only group. Our results indicate that collagen and elastin added into the electrospun sheets cannot further induce cardiac differentiation of c-kit+ cells in vitro.
80% NP and c-kit+-seeded 80% NP electrospun sheets improve cardiac repair in vivo
Based on obtained results in vitro, 80% NP was selected for transplantation due to the best ECM simulation and cytocompatibility. C-kit+-seeded electrospun sheets and cell-free electrospun sheets were sewed on the epicardium of left ventricles which at the same time occluded the left anterior descending coronary artery. Cell-free and cell-seeded 80% NP electrospun sheets are shown in Figure S2A and S2B. To ensure a complete cover and adhesion on the surface of left ventricle of the beating heart, forceps were used to gently place the patch and keep it flat (Figure S2C). Because 6 electrospun sheets folded and could not be unfolded in time during operation, 6 mice were excluded from this study (two mice in PCL, 80% NP, and 80% NP+c-kit+ groups dead during operation). All other mice survived until sacrifice. All sheets covered the epicardium of the left ventricles completely when hearts were collected (Figure 3A). In addition, formation of neovascular networks were observed in thin connective tissue membranes attached to the surface of electrospun sheets but no neovascularization in MI group was present. Moreover, left ventricular aneurysm formation was observed in MI group (short dash line Figure 3A).
Figure 3.

Holistic view and measurement of infarct size. Mice hearts were collected at day 28 after transplantation. A. All electrospun sheets covered well the epicardium of left ventricles when hearts were collected. Yellow short dash line indicates the area of ventricle aneurysms in MI model. Yellow arrows point to branches of new blood vessels. Scale bar = 1000 μm. B. TTC staining of the study groups 28 days after transplantation. Healthy myocardium is stained red and infarcted area stained white. Scale bar = 1000 μm. C. Statistical analysis of infarcted area ratio in whole tissue. *P<0.05; **P<0.01.
To quantify the area of myocardial infarction, TTC staining was performed 28 days after transplantation (Figure 3B). Healthy myocardium stained red while the infarcted area stained white. The infarcted area ratio in the whole tissue was acquired using Image J® software. The infarcted area for electrospun sheets was significantly smaller than that in MI group (24.3128 ± 3.814%; P<0.05). The infarcted area for hybrid electrospun sheet groups were significantly decreased compared to PCL group (19.1613 ± 2.4799%; P<0.05). Compared with cell-free group (18.6808 ± 2.6486%), the cell-seeded group (8.929 ± 1.5483%) was significantly smaller in infarcted area (P<0.01; Figure 3C). Moreover, obvious compensatory hypertrophy appeared in the survival healthy myocardium in MI, PCL and 80% NP groups.
M mode and 2-D mode echocardiography were performed (Figure 4A) to assess left ventricular function (LVF) after electrospun sheets transplantation for each group, which showed ejection fraction (EF%), fractional shortening (FS%), left ventricle end-systolic diameter (LVESDmm) and left ventricle end-diastolic diameter (LVEDDmm; Figure 4). Electrospun sheets dramatically improved cardiac function indices, as shown by the significantly increase in EF and FS on both day 7 and day 28 after transplantation, and by statistically decrease in LVESD on both day 7 and day 28 as well as LVEDD on day 28 after transplantation compared with MI group (Figure 4B-E). In addition, EF, FS and LVESD on day 7 and day 28 as well as LVEDD on day 28 after transplantation for hybrid electrospun sheet groups performed significantly better compared with PCL group. Moreover, c-kit+ seeded 80% NP group was better than the cell-free 80% NP group in EF, FS, LVESD on day 7 and day 28 as well as LVEDD on day 28 after transplantation (Figure 4B-E).
Figure 4.

Echocardiographic examination one and four weeks after MI/implantation. A. M mode echocardiography. The movement of LV wall was significantly improved in c-kit+ 80% NP group (arrows indicate the movement of LV free wall). B-E. EF, FS, LVEDD, and LVESD of the study groups, respectively. *P<0.05; **P<0.01.
To determine the effect of electrospun sheets on cardiac hemodynamics improvement, mice hearts underwent catheter tests. Cardiac output (CO) for each study group was examined for comprehensive assessment for cardiac hemodynamics. CO increased significantly in hybrid electrospun sheet groups compared with MI (2481.1 ± 1144.7 μL) and PCL group (3128.0 ± 1777.6 μL). Cell-seeded hybrid electrospun sheet group (4610.0 ± 850.6 μL) performed better than the cell-free hybrid electrospun sheet group (3600.0 ± 1643.3 μL; P<0.05; Figure 5A). The 80% NP alone as well as the c-kit+ cell-seeded 80% NP electrospun sheets significantly improved cardiac systolic and diastolic indices, as shown by the statistically increased Stroke volume (SV), LV end-systolic pressure (LVESP), LV end-diastolic pressure (LVEDP), and decreased minimal peak rate of LV pressure (dp/dt min) compared to pure PCL group (Figure 5B, 5C, 5F, 5G). In addition, the c-kit+ cell-seeded 80% NP was superior to 80% NP alone to increase the SV, maximal peak rate of LV pressure (dp/dt max), and LV end-diastolic volume (LVEDV), as well as to improve dp/dt min and LV end-systolic volume (LVESV) (Figure 5B, 5D, 5E, 5G, 5H).
Figure 5.
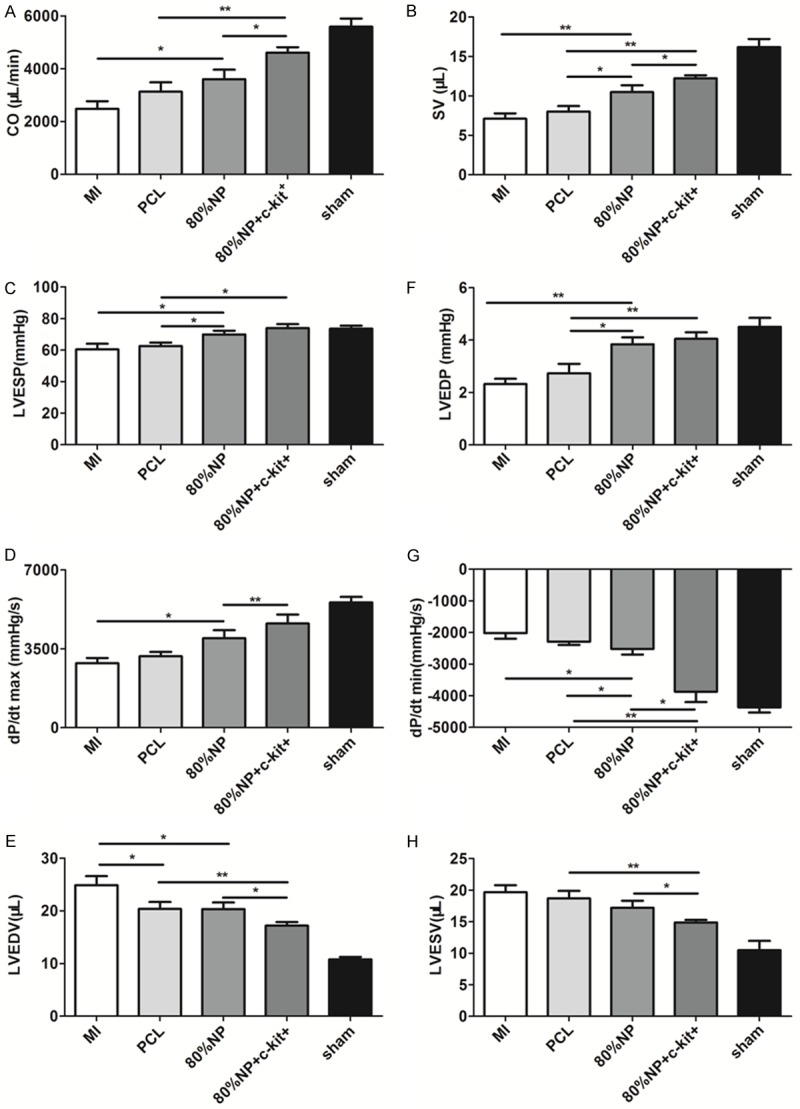
Hemodynamic measurements via a Millar pressure-volume catheter. A. CO for each study groups was examined for comprehensive assessment for cardiac hemodynamics. B-E. SV, LVESP, dp/dt max, LVESV was examined for systolic function parameter, respectively. F-H. LVEDP, dp/dt min, LVEDV was examined for systolic function parameter, respectively. *P<0.05; **P<0.01.
80% NP and c-kit+-seeded 80% NP electrospun sheets restrain LV remodeling
To assess the remodeling of left ventricles and histological changes after patch transplantation, H&E staining was performed 4 weeks after transplantation. The results indicate that a thin, expanded, inflammatory cell-laden anterior wall appeared in the left ventricles in the MI groups. The PCL, 80% NP and c-kit+-seeded 80% NP groups showed thicker left ventricular walls with surviving myocardium (Figure 6A). Average thickness of left ventricles of the MI group (0.7272 ± 0.1260 mm) was significantly lower than that of the electrospun sheet groups (P<0.05). The LV wall for hybrid electrospun sheets was significantly thicker when compared to the PCL group (0.8754 ± 0.0385 mm). The c-kit+-seeded hybrid electrospun sheet (1.3951 ± 0.0748 mm) group possessed significantly thicker LV wall than 80% NP electrospun sheet group (0.9996 ± 0.0571 mm; P<0.01; Figure 6C).
Figure 6.

Histological examination. A, B. H&E and Sirius Red staining for transverse frozen sections for each groups 4 weeks after electrospun sheet transplantation, respectively. Upper panel shows the microphotographs for each group (scale bar = 50 μm), the lower panel shows enlarged photographs of black box from the top panel (scale bar = 50 μm). For Sirius Red staining, the fibrous tissue is stained red, while myocardium is stained yellow. C. Statistical analysis of H&E staining for average thickness of left ventricles. *P<0.05; **P<0.01. D. Statistical analysis of Sirius Red staining of scar size which is the ratio of red to the whole area. *P<0.05; **P<0.01.
An MI is often followed by formation of a fibrotic area due to inflammatory cells and myofibroblast recruitment. Therefore, collagen hyperplasia and survival myocadium were evaluated for all groups 4 weeks after MI. As shown by Sirius Red staining, a large number of collagen was deposited in the local myocardial infarction area and survival myocardium was hardly detectable in the MI group (Figure 6B). On the contrary, in the c-kit+-seeded 80% NP group, collagen hyperplasia was clearly lower and a large proportion of the myocardium can be observed under the epicardium (Figure 6B). Collagen hyperplasia in MI group (31.2 ± 2.48%) was significantly greater than in electrospun sheet groups. There was also a significant lower collagen hyperplasia seen in the hybrid electrospun sheet groups compared to the PCL group (24.93 ± 3.13%). The c-kit+-seeded 80% NP group (12.91 ± 3.12%) showed a significantly reduced collagen hyperplasia compared to 80% NP group (P<0.01; Figure 6D).
Survival of seeded GFP+ cells in vivo
In order to detect cardiomyogenesis and trace grafted GFP+ cells in the local myocardial infarcted area of host C57 mice, immunofluorescence staining was performed. cTnT staining showed that infarcted myocardium co-existed with some injured myocardium in the para-infarcted area(PIA)(upper two panels in Figure 6A). Some surviving myocardium still maintained the normal morphology, while some other morphological characteristics were lost. In the infarcted area (IA), the typical myocardium disappeared but cTnT-positive cells were present which may indicate cardiomyogenesis (lower two panels in Figure 7A). The survival of transplanted cells was identified by immunofluorescent staining with GFP (Figure 7B). GFP+ cells were detected within patches and local infarcted tissues of c-kit+ seeded 80% NP patches, suggesting that some transplanted cells migrated into the infarcted myocardial wall (Figure 7B). Moreover, some GFP+ cells expressed the cardiomyocyte-specific marker cTnT, indicating the possibility that transplanted stem cells underwent cardiac differentiation which could be one of the reasons for improved cardiac function.
Figure 7.

Increase in myocardium in PIA (para-infarcted area) and IA (infarcted area) after 4 weeks transplantation. A. Visualization of myocardium in the region of PIA and IA. Scale bar = 25 μm. The regenerated or remaining myocardium was identified by immunofluorescence staining of c-TnT. For PIA in the upper two panels, IA in the lower two panels. The second and the fourth row of photos in A showed the merged photos in which the box represents magnified photos (400×). B. The survival of transplanted cells was identified by immunofluorenscent staining of GFP. In merged photographs the box represents magnified photos (400×). ***Represents the electrospun sheets. Scale bar = 25 μm.
Discussion
In this study, we have developed high concentration cardiac NP hybrid PCL electrospun nanofibrous sheets, containing elastin and collagen. These sheets showed to be effective as cardiac-mimicking microenvironments to promote cell survival and proliferation of seeded cells, as well as in improving cardiac function and attenuating LV remodeling within 4 weeks after MI. Based on the cytocompatibility and mechanical characteristics, the optimal ratio of elastin and collagen with PCL in our electrospun sheets was selected. After tracing GFP+ cells, we found that transplanted cells could be identified both in the electrospun sheets and into the local infarcted area. The transplanted cells showed cTnT expression after 4 weeks which can partly explain the improved cardiac function since cTnT is considered as myocardial marker.
Via the composition and structure of the ECM, it contains various cytokines which are secreted by cells and which guarantee good-functioning cells in the heart [34,35]. Moreover, the ECM, acting as a scaffold for tissue, provides the right microenvironment for therapeutic stem cells by positively influencing the differentiation of cells when transplanted into myocardium[35,36]. Fibrillar collagen, of which type I is the most abundant in the cardiac ECM, and elastin, which mediate the elastic properties of the ECM, are two important proteins present in cardiac ECM [19,37]. Therefore, collagen and elastin were selected as components in our hybrid electrospun sheets.
In this study we found that c-kit+ cells showed improved proliferation on PCL electrospun sheets compared with c-kit+ only group, and performed even better in hybrid NP/PCL groups, especially the 80% NP group. Similar to our observation, a study reported that collagen added to electrospun sheets can increase the ability to induce proliferation of seeded cells [38]. Moreover, another study reported that vascular smooth muscle cell proliferation on hybrid electrospun sheets composed of urinary bladder matrix and polyester-urethane urea with higher matrix concentrations was increased compared with that of lower matrix concentration hybrids or pure synthetic electrospun sheets. This suggest a beneficial effect of high density protein electrospun sheets on proliferation [39]. In line with our work, a study where they used collagen (10%)/elastin (5%)/PCL (10%) electrospun sheets, found that combination of natural proteins and synthetic polymers to create electrospun fibrous structures resulted in scaffolds with favorable mechanical and biological properties. However, the concentration of collagen and elastin they mixed with PCL were lower and the performance of electrospun sheets in vivo remain still unclear. Being the first in this field, we developed a novel high concentration NP/PCL hybrid electrospun sheets which showed improved proliferation of seeded cells. In fact, proliferation of c-kit+ cells on hybrid NP/PCL electrospun sheets increased proportionally with increasing concentrations of NPs. However, the underlying mechanism still remains largely unknown. A possible explanation is that it is due to the hydrophilic character, morphology and composition of cell-seeded substrates but the details need to be illustrated further.
The newly-developed high concentration hybrid electrospun sheets did not induce cardiac differentiation of c-kit+ cells. Taking into consideration that there are various cytokines present in ECM, we assume that collagen/elastin addition is not sufficient for inducing cardiac differentiation of c-kit+ cells. Consequently, further work needs to be performed in the future, e.g. addition of cytokines or growth factors to the matrices.
Providing mechanical support to bridge the LV scar area is another superiority of electrospun cardiac patches. Synchronizing ventricle movement can reduce the burden of myocardial compensation outside of aneurysms and prevent heart failure progress [27]. In this treatment strategy, the resistance of materials to repeated contraction force in vivo is required. Concerning the tensile strength, the 80% NP hybrid electrospun sheet was superior to the PCL. PCL has been shown to be a much stiffer material than the myocardial tissue (200-500 KPa at the beginning of the systole) [17,18]. Increasing concentrations NP mixed with PCL did not improve the Young’s modulus for the hybrid electrospun sheets. However, all the electrospun sheets were competent for perfectly covering the epicardium without any fracture. Although the used nanofibrous sheeds showed imperior mechanical characteristics, 80% NP was selected for in vivo experiments since those mixed scaffolds presented better proliferation capabilities for possible future cell-based applications.
Bone marrow stem cells (c-kit+ cells) have been proven to contribute to myocardium regeneration in vitro and in vivo [8,9,40]. Since our 80% NP hybrid sheets showed sufficient mechanical and bioactive characteristics acting as cardiac patch, we seeded c-kit+ cells on them before transplantation. We observed significantly restrained LV remodeling, improved cardiac function and possible improved myocardium survival compared to cell-free patch after MI. Thus, the combination of ECM-mimicking hybrid electrospun sheets and seeded c-kit+ cells could be considered as a possible treatment option. The electrospun sheets (PCL and 80% NP) are hypothesized to provide mechanical support for the brittle and thinning LV wall, partially preventing the expansion of LV, which is important to improve cardiac function. Moreover, the c-kit+ cell-seeded 80% NP/PCL was even superior to the 80% NP alone and both superior to the pure PCL for improvement of cardiac function. We identified large numbers of implanted GFP+ cells expressing cTnT in the local infarcted area. Interestingly, the GFP+ cells located within the patch were not cTnT positive. This suggests that only grafted GFP+ cells which migrated deep into local tissue might have undergone cardiogenic differentiation, most probably based on the microenvironment. Another possible explanation is that the GFP+ cells participated in myogenesis through a fusion phenomenon. Alvarez-Dolado el al. [41] reported that bone-marrow-derived cells underwent fusion with cardiac muscle cells in the heart when they were transplanted in vivo, suggesting that cell fusion may also be an important mechanism for injured tissue repair. Whether or not the cardiac function improvement was based on cell fusion needs to be further investigated. Besides, as shown recently, bone marrow-derived c-kit+ cells might activate endogenous cardiac progenitors to regenerate myocardium [42]. This study cannot rule out with certainty that cardiomyogenesis might also be performed by endogenous cardiac progenitors, activated by exogenous c-kit+ cells. Moreover, it is suggested [43] that paracrine and trophic effects may also have a prominent contribution in inducing endogenous cardiogenesis. Although we found promising results, questions still remain that need to be answered. For example, further work should be performed to investigate possible regeneration of myocardium tissue in response to application of our c-kit+ cell-seeded patch, as well as evaluating the differentiation potential of seeded cells into cardiomyocytes.
In summary, we successfully developed a new ECM-mimicking hybrid electrospun sheet, containing high concentrations of NPs (collagen and elastin). The sheets were evaluated as treatment option in MI mice models. The 80% NP hybrid electrospun sheet possessed a high capability for inducing c-kit+ cell proliferation. C-kit+-seeded 80% NP electrospun sheets also performed significantly better to restrain LV remodeling, improve cardiac function and regenerate myocardium compared with cell-free PCL and 80% NP sheets.
Acknowledgements
This study was supported by grants from the National Science Foundation of China (81300088), Scientific Research Innovation Foundation of Shanghai Education Commission (14YZ031), Projects of Shanghai Science and Technology Commission (124119a2100) and Medicine-Engineering Cross-Research Foundation of Shanghai Jiao Tong University (YG2013MS38, YG2014ZD02).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 2.Dixit P, Katare R. Challenges in identifying the best source of stem cells for cardiac regeneration therapy. Stem Cell Res Ther. 2015;6:26. doi: 10.1186/s13287-015-0010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xue T, Cho HC, Akar FG, Tsang SY, Jones SP, Marban E, Tomaselli GF, Li RA. Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: insights into the development of cell-based pacemakers. Circulation. 2005;111:11–20. doi: 10.1161/01.CIR.0000151313.18547.A2. [DOI] [PubMed] [Google Scholar]
- 5.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 6.Kawaguchi N, Smith AJ, Waring CD, Hasan MK, Miyamoto S, Matsuoka R, Ellison GM. ckitpos GATA-4 high rat cardiac stem cells foster adult cardiomyocyte survival through IGF-1 paracrine signalling. PLoS One. 2010;5:e14297. doi: 10.1371/journal.pone.0014297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsui J, Wakabayashi T, Asada M, Yoshimatsu K, Okada M. Stem cell factor/c-kit signaling promotes the survival, migration, and capillary tube formation of human umbilical vein endothelial cells. J Biol Chem. 2004;279:18600–18607. doi: 10.1074/jbc.M311643200. [DOI] [PubMed] [Google Scholar]
- 8.Lagostena L, Avitabile D, De Falco E, Orlandi A, Grassi F, Iachininoto MG, Ragone G, Fucile S, Pompilio G, Eusebi F, Pesce M, Capogrossi MC. Electrophysiological properties of mouse bone marrow c-kit+ cells co-cultured onto neonatal cardiac myocytes. Cardiovasc Res. 2005;66:482–492. doi: 10.1016/j.cardiores.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Guo HD, Wang HJ, Tan YZ, Wu JH. Transplantation of marrow-derived cardiac stem cells carried in fibrin improves cardiac function after myocardial infarction. Tissue Eng Part A. 2011;17:45–58. doi: 10.1089/ten.TEA.2010.0124. [DOI] [PubMed] [Google Scholar]
- 10.Fransioli J, Bailey B, Gude NA, Cottage CT, Muraski JA, Emmanuel G, Wu W, Alvarez R, Rubio M, Ottolenghi S, Schaefer E, Sussman MA. Evolution of the c-kit-positive cell response to pathological challenge in the myocardium. Stem Cells. 2008;26:1315–1324. doi: 10.1634/stemcells.2007-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lichtenauer M, Mildner M, Baumgartner A, Hasun M, Werba G, Beer L, Altmann P, Roth G, Gyongyosi M, Podesser BK, Ankersmit HJ. Intravenous and intramyocardial injection of apoptotic white blood cell suspensions prevents ventricular remodelling by increasing elastin expression in cardiac scar tissue after myocardial infarction. Basic Res Cardiol. 2011;106:645–655. doi: 10.1007/s00395-011-0173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hale SL, Dai W, Dow JS, Kloner RA. Mesenchymal stem cell administration at coronary artery reperfusion in the rat by two delivery routes: a quantitative assessment. Life Sci. 2008;83:511–515. doi: 10.1016/j.lfs.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao T, Zhang D, Millard RW, Ashraf M, Wang Y. Stem cell homing and angiomyogenesis in transplanted hearts are enhanced by combined intramyocardial SDF-1alpha delivery and endogenous cytokine signaling. Am J Physiol Heart Circ Physiol. 2009;296:H976–986. doi: 10.1152/ajpheart.01134.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halkos ME, Zhao ZQ, Kerendi F, Wang NP, Jiang R, Schmarkey LS, Martin BJ, Quyyumi AA, Few WL, Kin H, Guyton RA, Vinten-Johansen J. Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic Res Cardiol. 2008;103:525–536. doi: 10.1007/s00395-008-0741-0. [DOI] [PubMed] [Google Scholar]
- 15.Britten MB, Abolmaali ND, Assmus B, Lehmann R, Honold J, Schmitt J, Vogl TJ, Martin H, Schachinger V, Dimmeler S, Zeiher AM. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003;108:2212–2218. doi: 10.1161/01.CIR.0000095788.78169.AF. [DOI] [PubMed] [Google Scholar]
- 16.de Haas HJ, Arbustini E, Fuster V, Kramer CM, Narula J. Molecular imaging of the cardiac extracellular matrix. Circ Res. 2014;114:903–915. doi: 10.1161/CIRCRESAHA.113.302680. [DOI] [PubMed] [Google Scholar]
- 17.Kai D, Prabhakaran MP, Jin G, Ramakrishna S. Guided orientation of cardiomyocytes on electrospun aligned nanofibers for cardiac tissue engineering. J Biomed Mater Res B Appl Biomater. 2011;98:379–386. doi: 10.1002/jbm.b.31862. [DOI] [PubMed] [Google Scholar]
- 18.Chen QZ, Bismarck A, Hansen U, Junaid S, Tran MQ, Harding SE, Ali NN, Boccaccini AR. Characterisation of a soft elastomer poly (glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials. 2008;29:47–57. doi: 10.1016/j.biomaterials.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Cheng JK, Wagenseil JE. Extracellular matrix and the mechanics of large artery development. Biomech Model Mechanobiol. 2012;11:1169–1186. doi: 10.1007/s10237-012-0405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merna N, Robertson C, La A, George SC. Optical imaging predicts mechanical properties during decellularization of cardiac tissue. Tissue Eng Part C Methods. 2013;19:802–809. doi: 10.1089/ten.tec.2012.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmuck EG, Mulligan JD, Ertel RL, Kouris NA, Ogle BM, Raval AN, Saupe KW. Cardiac fibroblast-derived 3D extracellular matrix seeded with mesenchymal stem cells as a novel device to transfer cells to the ischemic myocardium. Cardiovasc Eng Technol. 2014;5:119–131. doi: 10.1007/s13239-013-0167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12:1197–1211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 23.Prabhakaran MP, Ghasemi-Mobarakeh L, Ramakrishna S. Electrospun composite nanofibers for tissue regeneration. J Nanosci Nanotechnol. 2011;11:3039–3057. doi: 10.1166/jnn.2011.3753. [DOI] [PubMed] [Google Scholar]
- 24.Ashammakhi N, Ndreu A, Nikkola L, Wimpenny I, Yang Y. Advancing tissue engineering by using electrospun nanofibers. Regen Med. 2008;3:547–574. doi: 10.2217/17460751.3.4.547. [DOI] [PubMed] [Google Scholar]
- 25.Ashammakhi N, Ndreu A, Piras AM, Nikkola L, Sindelar T, Ylikauppila H, Harlin A, Gomes ME, Neves NM, Chiellini E, Chiellini F, Hasirci V, Redl H, Reis RL. Biodegradable nanomats produced by electrospinning: expanding multifunctionality and potential for tissue engineering. J Nanosci Nanotechnol. 2007;7:862–882. doi: 10.1166/jnn.2007.485. [DOI] [PubMed] [Google Scholar]
- 26.Zhang YZ, Su B, Venugopal J, Ramakrishna S, Lim CT. Biomimetic and bioactive nanofibrous scaffolds from electrospun composite nanofibers. Int J Nanomedicine. 2007;2:623–638. [PMC free article] [PubMed] [Google Scholar]
- 27.Kai D, Wang QL, Wang HJ, Prabhakaran MP, Zhang Y, Tan YZ, Ramakrishna S. Stem cellloaded nanofibrous patch promotes the regeneration of infarcted myocardium with functional improvement in rat model. Acta Biomater. 2014;10:2727–2738. doi: 10.1016/j.actbio.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 28.Feng B, Duan H, Fu W, Cao Y, Jie Zhang W, Zhang Y. Effect of inhomogeneity of the electrospun fibrous scaffolds of gelatin/polycaprolactone hybrid on cell proliferation. J Biomed Mater Res A. 2015;103:431–438. doi: 10.1002/jbm.a.35184. [DOI] [PubMed] [Google Scholar]
- 29.Heydarkhan-Hagvall S, Schenke-Layland K, Dhanasopon AP, Rofail F, Smith H, Wu BM, Shemin R, Beygui RE, MacLellan WR. Threedimensional electrospun ECM-based hybrid scaffolds for cardiovascular tissue engineering. Biomaterials. 2008;29:2907–2914. doi: 10.1016/j.biomaterials.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He X, Wenguo C, Jiang C. Fabrication of patterned PDLLA-PCL composite scaffold. J Appl Polym Sci. 2013;127:1550–1554. [Google Scholar]
- 31.Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, Fan Q, Chuprun JK, Ma XL, Koch WJ. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res. 2010;107:1445–1453. doi: 10.1161/CIRCRESAHA.110.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc. 2008;3:1422–1434. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teng F, Yin Y, Cui Y, Deng Y, Li D, Cho K, Zhang G, Lu A, Wu W, Yang M, Liu X, Guo DA, Yin J, Jiang B. Salvianolic acid A inhibits endothelial dysfunction and vascular remodeling in spontaneously hypertensive rats. Life Sci. 2016;144:86–93. doi: 10.1016/j.lfs.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Curtis MW, Russell B. Micromechanical regulation in cardiac myocytes and fibroblasts: implications for tissue remodeling. Pflugers Arch. 2011;462:105–117. doi: 10.1007/s00424-011-0931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakayama KH, Hou L, Huang NF. Role of extracellular matrix signaling cues in modulating cell fate commitment for cardiovascular tissue engineering. Adv Healthc Mater. 2014;3:628–641. doi: 10.1002/adhm.201300620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravi S, Caves JM, Martinez AW, Xiao J, Wen J, Haller CA, Davis ME, Chaikof EL. Effect of bone marrow-derived extracellular matrix on cardiac function after ischemic injury. Biomaterials. 2012;33:7736–7745. doi: 10.1016/j.biomaterials.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eghbali M, Weber KT. Collagen and the myocardium: fibrillar structure, biosynthesis and degradation in relation to hypertrophy and its regression. Mol Cell Biochem. 1990;96:1–14. doi: 10.1007/BF00228448. [DOI] [PubMed] [Google Scholar]
- 38.Shin YC, Lee JH, Jin L, Kim MJ, Kim YJ, Hyun JK, Jung TG, Hong SW, Han DW. Stimulated myoblast differentiation on graphene oxideimpregnated PLGA-collagen hybrid fibre matrices. J Nanobiotechnology. 2015;13:21. doi: 10.1186/s12951-015-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stankus JJ, Freytes DO, Badylak SF, Wagner WR. Hybrid nanofibrous scaffolds from electrospinning of a synthetic biodegradable elastomer and urinary bladder matrix. J Biomater Sci Polym Ed. 2008;19:635–652. doi: 10.1163/156856208784089599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Transplanted adult bone marrow cells repair myocardial infarcts in mice. Ann N Y Acad Sci. 2001;938:221–229. doi: 10.1111/j.1749-6632.2001.tb03592.x. discussion 229-230. [DOI] [PubMed] [Google Scholar]
- 41.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 42.Loffredo FS, Steinhauser ML, Gannon J, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389–398. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duran JM, Makarewich CA, Sharp TE, Starosta T, Zhu F, Hoffman NE, Chiba Y, Madesh M, Berretta RM, Kubo H, Houser SR. Bone-derived stem cells repair the heart after myocardial infarction through transdifferentiation and paracrine signaling mechanisms. Circ Res. 2013;113:539–552. doi: 10.1161/CIRCRESAHA.113.301202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



