Abstract
Ginseng and its components exert various biological effects, including antioxidant, anti-carcinogenic, anti-mutagenic, and antitumor activity. Ginsenosides are the main biological components of ginseng. Protopanaxadiol (PPD) and protopanaxatriol (PPT) are two metabolites of ginsenosides. However, the difference between these compounds in anti-lung cancer is unclear. The present study aimed to evaluate the antitumor activity of PPD, PPT, Ginsenosides-Rg3 (G-Rg3) and Ginsenosides-Rh2 (G-Rh2) in lung cancer cell. After treatment with cisplatin, PPD, PPT, G-Rg3 or G-Rh2, the viability, apoptosis level and invasiveness of lung cell lines (A549 cell, a lung adenocarcinoma cell line and SK-MES-1 cell, a lung squamous cell line) in vitro were analyzed by Cell Counting Kit-8 (CCK8), Annexin V/PI apoptosis and Matrigel invasion assays, respectively. Here we found that all these compounds led to significant decreases of viability and invasiveness and an obvious increase of apoptosis of A549 and SK-MES-1 cells. Among these, the viability of SK-MES-1 cell treated with PPT was decreased to 66.8%, and this effect was closest to Cisplatin. G-Rg3 had the highest stimulatory effect on apoptosis, and PTT had the highest inhibitory effect on cell invasiveness in A549 and SK-MES-1 cells. These results indicate that both ginsenosides and two metabolites have antitumor activity on lung cancer cell in vitro. However, PPT is more powerful for inhibiting the viability and invasiveness of lung cancer cell, especially lung squamous cell. G-Rg3 has the best pro-apoptosis effects. This study provides a scientific basis for potential therapeutic strategies targeted to lung cancer by further structure modification.
Keywords: Ginsenosides, PPD, PPT, antitumor activity, lung cancer cell
Introduction
Lung cancer is one of the most common cancers and its prognosis is poor. Of all people with lung cancer, about 15% survive for five years after diagnosis [1]. Treatment of lung cancer depends on the cancer’s histological type, staging and the person’s performance status. Common treatments include surgery, radiation and chemotherapy, either alone or in combination. In these therapies, chemotherapy improves survival and is used as first-line treatment [2,3]. Among these, cisplatin is most commonly used [4]. However, chemotherapeutic agents are not only harmful to tumor cells, but also to normal cells, limiting their therapeutic use in the clinic. Thus, new natural anticancer compounds are urgently needed, especially those compounds derived from long-term used traditional Chinese medicinal herbs [5], such as ginseng.
Ginseng is a medicinal herb widely used in Asian countries and the North American. Ginsenosides are main components extracted from ginseng, and ginsenoside Rg3 is one of the most important parts. Ginsenosides have various pharmaceutical activities, such as antitumor, antioxidant, immunomodulatory and anti-inflammatory etc [6]. Most ginsenosides are composed by a dammarane skeleton (30 carbons in a four-ring structure) and sugar moieties which attached to the C-3 and C-20 positions [7]. Differences in saponin structure can influence bioactivity and bioavailability, especially for the antitumor activity. The antitumor activities of ginsenosides are related to the number of sugar molecules, number and position of hydroxyl groups and stereoselectivity [8].
Researches indicate that ginsenoside-Rg3 (G-Rg3) exhibits anticancer activity in vitro and in vivo models as a relatively safe medicine in several kinds of tumors [9-15]. Ginsenoside Rh2 (G-Rh2) is also one of the bioactive components extracted from ginseng [16]. Several health benefits of Rh2 have been reported due to its anti-inflammatory, anti-osteoclastogenic, anti-hyperglycemic and antitumor effects [17-21]. As two metabolites of ginsenoside, protopanaxadiol (PPD) and protopanaxatriol (PPT), also exhibit activity against a variety of cancer cells [22,23]. The chemical structures of PPD, PPT, G-Rg3 and G-Rh2 were shown in Figure 1. However, so far it still has not reported about a comparative study of antitumor activities of these four compounds.
Figure 1.
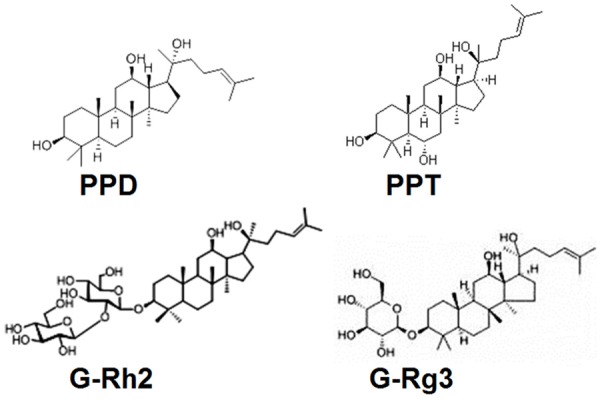
The Chemical structures of PPD, PPT, G-Rg3 and G-Rh2.
Therefore, the present study is undertaken to evaluate the antitumor activities of PPD, PPT, G-Rg3 and G-Rh2 on lung cancer cells in vitro, with cisplatin as a positive control. This project will provide a new direction for research on antitumor effects of ginsenosides. In addition, this study is expected for us to find a more effective antitumor compounds. On this basis, it will provide targeted compounds used to further chemical structural modification for finding new strategies to treat lung cancer.
Materials and methods
Reagents
All these compounds (PPD, PPT, G-Rg3, and G-Rh2) were bought from Sigma-Aldrich Co. LLC., (USA).
Cell culture
The human lung cancer cells (Human lung adenocarcinoma cell line, A549 cell, and human lung squamous cell line, SK-MES-1 cell) were obtained from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). These cells were grown were grown in RPMI-1640 medium (Gibco, USA) supplemented with 5% fetal bovine serum (FBS; Hyclone, Logan, UT, USA), and were incubated in a humidified incubator at 37°C and 5% CO2.
The cell-counting kit-8 (CCK-8) assay
A549 cells or SK-MES-1 cells were seeded in 96-well plates (3×103 cells/well), and then treated with different concentration of PPD, PPT, G-Rg3 or G-Rh2 (0, 0.4, 4 or 40 uM) for 48 h, with cisplatin (2 ug/ml) as a positive control and 0.1% DMSO as a blank control. Then these cells were collected and detected the viability by the cell-counting Kit-8 (CCK-8) assay (Dojindo, Japan). According to the manufacturer’s protocol, the CCK8 reagent was added to each well and cells were incubated at 37°C for 1-4 h. The absorbance (optical density) at 450 nm was measured and used to represent the viability of cells. In addition, A549 cells (3×104 cells/well) in 12-well flat-bottom microplates after treatment were counted at a magnification of 100x with microscope (Olympus 1X71, Olympus, Tokyo, Japan). Each experiment was performed in six parallel wells, and repeated three times.
Annexin V/PI apoptosis assay
A549 or SK-MES-1 cells were seeded at a density of 2 × 105 cells/well in 12-well flat-bottom microplates, and then treated with cisplatin (2 ug/ml), PPD (40 uM), PPT (40 uM), G-Rg3 (40 uM) or G-Rh2 (40 uM) for 48 h, with 0.1% DMSO as the control. Subsequently, these A549 or SK-MES-1 cells were digested by 0.25% trypsin without EDTA, and then centrifuged 1000 g for 5 min, resuspended with PBS, labeled the cells by Annexin V and PI according to the instruction manual, and detected the percentage of early apoptosis cells by flow cytometry. The apoptosis detection kit was obtained from Invitrogen Company (Invitrogen, USA). The experiments were carried out in triplicate, and repeated three times.
Matrigel invasion assay
The invasiveness of the A549 or SK-MES-1 cells across matrigel was evaluated objectively in an invasion chamber, based on our previous procedure [24]. Briefly, the cell inserts (8-um pore size, 6.5-mm diameter, Corning, USA) coated with 20 uL of matrigel (BD, USA) were placed in a 24-well plate. The A549 or SK-MES-1 cells of 2 ×104 were plated in the upper chamber (the media contained 1% charcoal stripped FCS). Cisplatin (2 ug/ml), PPD (40 uM), PPT (40 uM), G-Rg3 (40 uM), G-Rh2 (40 uM) or vehicle (0.1% DMSO) was added, respectively. The lower chamber (the media was contained 5% charcoal stripped FCS) was filled with 800 ul of medium. The cells were then incubated at 37°C for 48 hours. The inserts were removed, washed in PBS, and the non-invading cells together with the matrigel were removed from the upper surface of the filter by wiping with a cotton bud. The inserts were then fixed in methanol for 10 minutes at room temperature and stained with hematoxylin. The result was observed under Olympus BX51+DP70 microscope (Olympus, Tokyo, Japan). The cells migrating to the lower surfaces were counted at a magnification of X200. The cells migrating to the lower surfaces were counted in five predetermined fields. The invasion index of each group was calculated as the ratio of the cells number migrated to the lower surfaces to the vehicle control. Each experiment was carried out in triplicate, and repeated three times.
Statistics
All values are shown as the mean ± SEM. The data were analyzed with GraphPad Prism version 5 by one-way ANOVA with Bonferroni post hoc t tests. Differences were considered statistically significant at P<0.05.
Results
PPD, PPT, G-Rg3 and G-Rh2 suppress the viability of A549 cell
To evaluate whether PPD, PPT, G-Rg3 and G-Rh2 regulate viability of lung cancer cells, the CCK-8 assay was performed to analysis the effect of these four compounds on the viability of A549 or SK-MES-1 cells. For A549 cells, as shown in Figure 2, all these compounds (PPD, PPT, G-Rg3 and G-Rh2) could notably inhibit the viability in a dosage-dependent manner (P<0.05, P<0.01 or P<0.001) (Figure 2A), especially at the concentration of 40 uM. But these effect was weaker than that of cisplatin (P<0.001) (Figure 2B and 2C).
Figure 2.
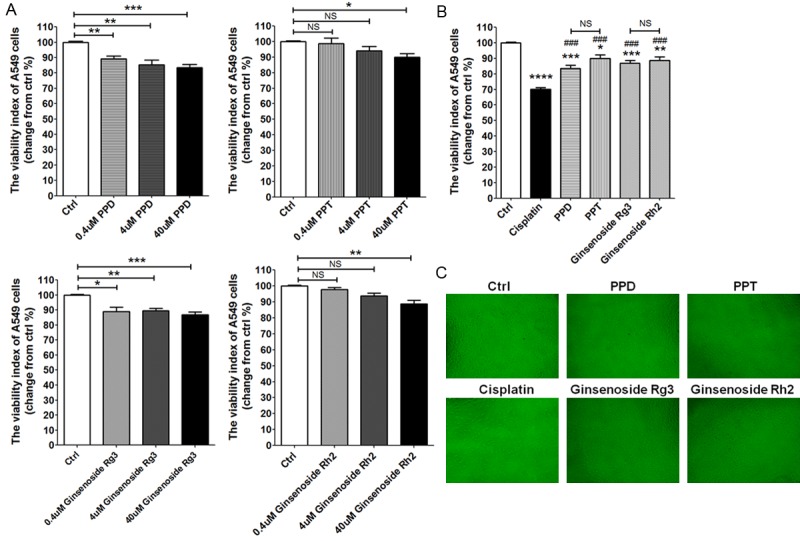
PPD, PPT, G-Rg3 and G-Rh2 suppresse the viability of A549 cell. (A) A549 cells were treated with different concentrations of PPD, PPT, G-Rg3 or G-Rh2 (0.4 uM, 4 uM, 40 uM) for 48 h, the media was added to some wells as a negative control, and then we performed CCK-8 assay to detect the viability of A549 cells. Meanwhile, we compared the antitumor effects between cisplatin (2 ug/ml), PPD (40 uM), PPT (40 uM), G-Rg3 (40 uM) and G-Rh2 (40 uM) (B, C). The data are expressed as the mean ± SEM. Original magnification: X100. *P<0.05, **P<0.01, ***P<0.001 or ***P<0.0001 to the vechile control (one-way ANOVA); ###P<0.001 compared to cisplatin (one-way ANOVA). NS: no statistically difference.
SK-MES-1 cell is more sensitive to PPT
To analysis the effect of these four compounds on lung squamous cell carcinoma, we treated SK-MES-1 cells with different concentration of PPD, PPT, G-Rg3 and G-Rh2 for 48 h. As shown, all these compounds also led to a marked decrease of viability of SK-MES-1 cells (P<0.05, P<0.01 or P<0.0001) (Figure 3A). Among these, PPT was more powerful (P<0.01) (Figure 3A and 3B). After treatment with PPT, the viability of SK-MES-1 cell was decreased to 66.8%, and this effect was closest to cisplatin (P<0.01) (Figure 3B). The antitumor effect on squamous cell carcinoma (SK-MES-1 cell) induced by PPT was more powerful than it on adenocarcinoma (A549 cell). These results suggest that lung squamous cell carcinoma may be more sensitive to PPT.
Figure 3.
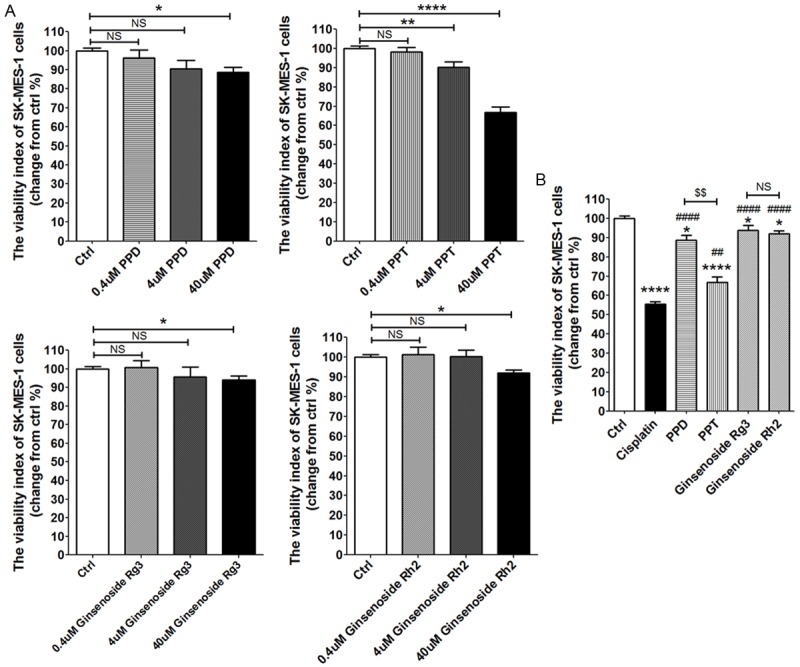
SK-MES-1 cell is more sensitive to PPT. (A, B) CKK-8 assay analysis of SK-MES-1 cells viability, which were treated as described in 2A and 2B. The data are expressed as the mean ± SEM. *P<0.05, **P<0.01 and ****P<0.0001 to the vechile control (one-way ANOVA); ##P<0.01 and ####P<0.0001 compared to cisplatin (one-way ANOVA). $$P<0.01 compared to PPD. NS: no statistically difference.
G-Rg3 significantly promote the apoptosis in A549 and SK-MES-1 cells
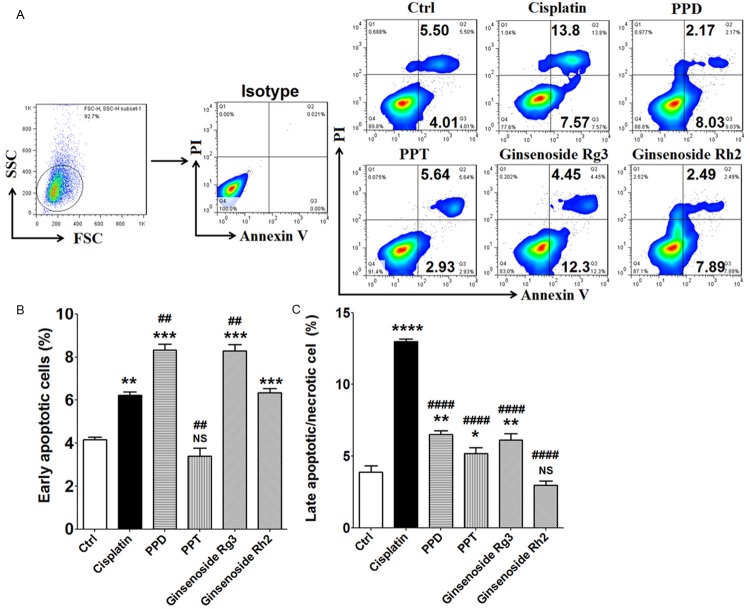
In order to evaluate whether these compounds modulate the apoptosis of lung cancer cells, A549 and SK-MES-1 cells were analyzed for the apoptosis level after treatment with cisplatin (2 ug/ml), PPD (40 uM), PPT(40 uM), G-Rg3 (40 uM) or G-Rh2 (40 uM) for 48 h, respectively. As shown, PPD and G-Rg3 and G-Rh2 not PPT could promote early apoptosis in A549 cells (P<0.01 and P<0.001) (Figure 4A and 4B), especially PPD and G-Rg3. The stimulatory effect on the early apoptosis of A549 cell mediated by PPD and G-Rg3 was more powerful compared to cisplatin (P<0.01) (Figure 4A and 4B). However, compared to PPD, G-Rg3 and G-Rh2, treatment with cisplatin led to an obvious increase of late apoptotic or necrotic A549 cells (P<0.0001) (Figure 4A and 4C).
Figure 4.
PPD and G-Rg3 significantly promote early apoptosis of A549 cell. (A-C) A549 cells (2×105 cells/well) were treated with cisplatin (2 ug/ml), PPD (40 uM), PPT (40 uM), G-Rg3 (40 uM) or G-Rh2 (40 uM) for 48 h. Subsequently, Annexin V-FITC apoptosis assay was used to analyze the apoptosis of A549 cells. Results were highly reproducible in three independent experiments. The early apoptotic cells were Annexin V+PI- cells; the late apoptotic/necrotic cells were Annexin V+PI+ cells. The data are expressed as the mean ± SEM. *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001 compared to the vechile control (one-way ANOVA); ##P<0.01 and ###P<0.001 compared to cisplatin (one-way ANOVA); NS: no statistically difference.
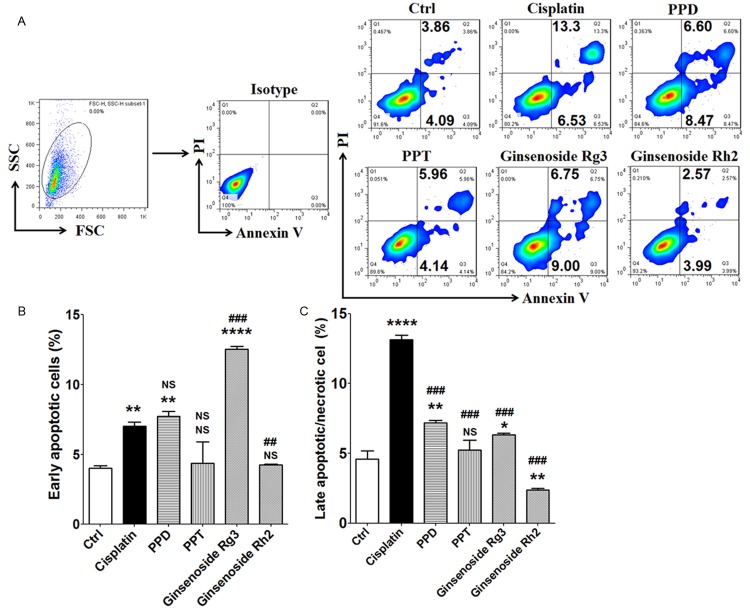
Meanwhile, PPD and G-Rg3 also had the stimulatory effect on early apoptosis of SK-MES-1 cells (P<0.01, P<0.001 or P<0.0001) (Figure 5A and 5B). Treatment with PPT or G-Rh2 did not change apoptosis level of SK-MES-1 cells (P>0.05) (Figure 5A and 5B). Similar with A549 cells, treatment with G-Rg3 led to higher level of early apoptotic SK-MES-1 cell compared to cisplatin (P<0.01) (Figure 5A and 5B). However, there was no significant difference between PPD and cisplatin (P>0.05) (Figure 5A and 5B). Further analysis showed that cisplatin resulted in the highest elevation of late apoptotic or necrotic SK-MES-1 cells (P<0.001) (Figure 5A and 5C), which echoed the results of A549 cells. Our current results suggest that G-Rg3 significantly stimulate the apoptosis of lung cancer cells (both adenocarcinoma and squamous cell carcinoma).
Figure 5.
G-Rg3 stimulates early apoptosis of SK-MES-1 cell. (A-C) Annexin V-FITC apoptosis detection assay were used to analyze the apoptosis of SK-MES-1 cells, which were treated as described in 4A and 4B. The data are expressed as the mean ± SEM. **P<0.01, ***P<0.001 and ****P<0.0001 compared to control; ##P<0.01 and ###P<0.001 compared to cisplatin; NS: no statistically difference.
PPT obviously inhibits the invasiveness of A549 and SK-MES-1 cells
We next investigated whether PPD, PPT, G-Rg3 and G-Rh2 could regulate the invasion of lung cancer cell. The results of Matrigel invasion assays showed that four compounds could restrict the invasion of A549 (P<0.01 and P<0.0001) (Figure 6A and 6B) and SK-MES-1 cells (P<0.01, P<0.001 and P<0.0001) (Figure 6C and 6D). The invasiveness regulation of A549 induced by PPT was similar with cisplatin (P>0.05) (Figure 6A and 6B). For SK-MES-1 cells, the inhibitory effects on cell invasion mediated by PPD, PPT, G-Rg3 and cisplatin had no obviously differences (P>0.05) (Figure 6C and 6D). These data suggest that both ginsenosides and metabolites have a powerful role in suppressing the invasion and metastasis of lung cancer cell, especially PPT.
Figure 6.
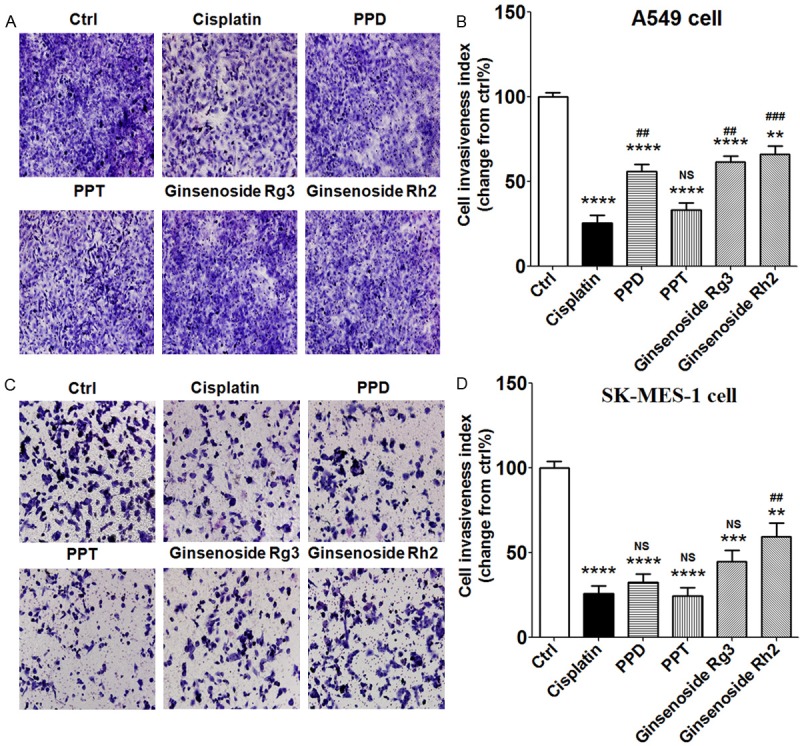
PPT obviously inhibits the invasiveness of A549 and SK-MES-1 cells. A549 or SK-MES-1 cells were treated with cisplatin (2 ug/ml), PPD (40 uM), PPT (40 uM), G-Rg3 (40 uM) or G-Rh2 (40 uM) for 48 h, with vehicle as controls. Then the Matrigel invasion assay was used to detect the invasiveness of A549 cells (A, B) and SK-MES-1 cells (C, D). Results were highly reproducible in three independent experiments. Original magnification: X200. The data are expressed as the mean ± SEM. **P<0.01, ***P<0.001 and ****P<0.0001 compared to control; ##P<0.01 and ###P<0.001 compared to cisplatin; NS: no statistically difference.
Discussion
Lung cancer is the leading cause of cancer-related deaths among both men and women [25], which accounts for about 27% of all cancer deaths [26]. Non-small cell lung cancer (NSCLC), is approximately 80% of all lung cancers. Surgery is to be considered the curative treatment of NSCLC. Overall, surgically-treated patient survival is only around 40% at 5 years. The majority of NSCLC patients present with advanced disease at diagnosis and a large part of those diagnosed with early stage disease eventually recur, experiencing metastatic disease. The advanced disease palliation and the patients’ quality of life are still the primary goals of therapy, although total cure is remaining elusive.
With overall disappointing results, the therapy of non-small cell lung cancer (NSCLC) has reached a plateau in improving patient survival. Therefore, clinical research for looking for new treatment strategies is warranted. The main clinical data currently available on targeted agents in the treatment of NSCLC, focusing on epidermal growth factor receptor family inhibitors, signal transduction inhibitors, angiogenesis inhibitors, eicosanoid pathway inhibitors, vaccines and gene therapy.
Natural products are of increasing interest and importance to cancer patients. Ginseng has been used in Asia for millennia, and is believed to have effective antitumor activity. As the major active chemical components of ginseng, the ginsenosides mainly consist of dammarane-type saponin derivatives. Ginsenosides are a special group of triterpenoid saponins that can be classified into two groups by the skeleton of their aglycones, namely dammarane- and oleanane-type. Ginsenosides are found nearly exclusively in Panax species (ginseng). Up to now more than 150 naturally occurring ginsenosides have been isolated from roots, fruits, leaves/stems, and/or flower heads of ginseng [27]. Ginsenosides have been the target of a lot of research, and they are believed to be the main active principles behind the claims of ginsengs efficacy. The potential health effects of ginsenosides include anticarcinogenic, immunomodulatory, anti-inflammatory, antiallergic, antiatherosclerotic, antihypertensive, and antidiabetic effects as well as antistress activity, regulation of blood pressure and metabolism [28-30]. Ginsensoides can be metabolized in the stomach (acid hydrolysis) and in the gastrointestinal tract (bacterial hydrolysis) or transformed to other ginsenosides by drying and steaming of ginseng to more bioavailable and bioactive ginsenosides. Ginsenosides within the dammarane-type consist mainly of three types classified according to their genuine aglycone moieties: PPD, PPT and ocotillol.
In our current study, we observed that these compounds (PPD, PPT, G-Rg3 and G-Rh2) could repress the viability and invasiveness, and promote the apoptosis in A549 and SK-MES-1 cells. Among these, PPT induced more powerfully antitumor activity (anti-viability and anti-invasiveness), especially for squamous cell carcinoma (SK-MES-1 cell). This effect may be dependent on the down-signaling pathways, such as mitogen-activated protein kinase (MAPK) and PI3K/Akt signaling pathways and downstream transcription factors such as NF-κB and AP-1 [31,32]. But the exact molecular mechanism need to research. Compared to cisplatin, treatment with G-Rg3 led to a higher level of early apoptotic lung cancer cells. These data above indicate that different compounds have different antitumor activities of lung cancer cell.
Most patients with lung cancer are diagnosed at the advanced stages of disease and approximately the half of lung cancer patients is inoperable due to metastasized diseases. Lung cancer frequently metastasizes to the brain, liver, bones, and adrenals et al [33-35]. Here we found that these four compounds could inhibit the invasiveness of A549 and SK-MES-1 cells. In addition, the invasiveness regulation of lung cancer cells induced by PPT was similar with cisplatin. Previous reports have paid more attention on the antitumor activities of Ginsensoides, such as G-Rg3, G-Rh2 and G-Rg1 [36-39]. However, our current study in the first time evaluates the antitumor activity difference between PPD, PPT, G-Rg3 and G-Rh2 in lung cancer cells, suggesting that PPT may be a potential compound for targeting lung cancer, especially metastasized lung cancer. Further research should be focused on whether the combination of PPT and cisplatin has a synergistic effect, and whether PPT also plays an effective anti-lung cancer effect in vivo, especially during distant metastasis.
A major barrier to restrict the clinical use of ginseng or ginsenosides now is their low oral bioavailability, usually <5% in rodent model [40,41]. The low oral bioavailability of ginsenosides was attributed previously to their poor oral absorption, which is caused by a large molecular weight and bulky sugar moieties [42]. Differences in saponin structure can fundamentally influence biological responses, especially for the antitumor activity, which is also estimated in the current study. Therefore, chemical synthesis and structure modification plays an important role in the acquirement of various ginsenosides, and finding out the better ideal drugs for treating cancer.
Therefore, based on our findings, it can be concluded that PPT is the best effective compound for anti-lung cancer, especially for lung squamous cell carcinoma or the advanced stages of metastasized lung cancer. Further study should be focused on the structure modification of PPT, which will be helpful for finding out the better ideal drugs for treating lung cancer.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (NSFC) 21372252, NSFC 21572081 and the Start-up Funds for High Level Talents of Jiangxi Normal University (all to J-S Sun), the Training Program for Young Talents of Shanghai Health System XYQ2013104, the Development Fund of Shanghai Talents (201557) and the Program for Zhuoxue of Fudan University (all to M-Q Li).
Disclosure of conflict of interest
None.
References
- 1.Nag SA, Qin JJ, Wan W, Wang MH, Wang H, Zhang R. Ginsenosides as anticancer agents: in vitro and in vivo activities, structure-activity relationships, and molecular mechanisms of action. Front Pharmacol. 2012;3:25. doi: 10.3389/fphar.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hann CL, Rudin CM. Management of smallcell lung cancer: incremental changes but hope for the future. Oncology (Williston Park) 2008;22:1486–1492. [PMC free article] [PubMed] [Google Scholar]
- 3.Burdett S, Stephens R, Stewart L, Tierney J, Auperin A, Le Chevalier T, Le Pechoux C, Pignon JP, Arriagada R, Higgins J, Johnson D, van Meerbeeck J, Parmar M, Souhami R, Bell D, Cartei G, Cormier Y, Cullen M, Ganz P, Gridelli C, Kaasa S, Quoix E, Rapp E, Seymour L, Spiro S, Thatcher N, Tummarello D, Williams C, Williamson I. Chemotherapy in Addition to Supportive Care Improves Survival in Advanced Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis of Individual Patient Data From 16 Randomized Controlled Trials. J. Clin. Oncol. 2008;26:4617–4625. doi: 10.1200/JCO.2008.17.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray N, Turrisi AT. A review of first-line treatment for small-cell lung cancer. J Thorac Oncol. 2006;1:270–278. doi: 10.1016/s1556-0864(15)31579-3. [DOI] [PubMed] [Google Scholar]
- 5.Johnson TE, Hermanson D, Wang L, Kassie F, Upadhyaya P, O’Sullivan MG, Hecht SS, Lu J, Xing C. Lung tumorigenesis suppressing effects of a commercial kava extract and its selected compounds in A/J mice. Am J Chin Med. 2011;39:727–742. doi: 10.1142/S0192415X11009202. [DOI] [PubMed] [Google Scholar]
- 6.Christensen LP. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 7.De Smet PA. Herbal remedies. N Engl J Med. 2002;347:2046–2056. doi: 10.1056/NEJMra020398. [DOI] [PubMed] [Google Scholar]
- 8.Qi LW, Wang CZ, Yuan CS. American ginseng: potential structure-function relationship in cancer chemoprevention. Biochem Pharmacol. 2010;80:947–954. doi: 10.1016/j.bcp.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 9.Iishi H, Tatsuta M, Baba M, Uehara H, Nakaizumi A, Shinkai K, Akedo H, Funai H, Ishiguro S, Kitagawa I. Inhibition by ginsenoside Rg3 of bombesin-enhanced peritoneal metastasis of intestinal adenocarcinomas induced by azoxymethane in Wistar rats. Clin Exp Metastasis. 1997;15:603–611. doi: 10.1023/a:1018491314066. [DOI] [PubMed] [Google Scholar]
- 10.Shinkai K, Akedo H, Mukai M, Imamura F, Isoai A, Kobayashi M, Kitagawa I. Inhibition of in vitro tumor cell invasion by ginsenoside Rg3. Jpn J Cancer Res. 1996;87:357–362. doi: 10.1111/j.1349-7006.1996.tb00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu TM, Xin Y, Cui MH, Jang X, Gu LP. Inhibitory effect of ginsenoside Rg3 combined with cyclophosphamide on growth and angiogenesis of ovarian cancer. Chin Med J (Engl) 2007;120:584–588. [PubMed] [Google Scholar]
- 12.Zhang Q, Kang X, Zhao W. Antiangiogenic effect of low-dose cyclophosphamide combined with ginsenoside Rg3 on Lewis lung carcinoma. Biochem Biophys Res Commun. 2006;342:824–828. doi: 10.1016/j.bbrc.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 13.Yue PY, Wong DY, Wu PK, Leung PY, Mak NK, Yeung HW, Liu L, Cai Z, Jiang ZH, Fan TP, Wong RN. The angiosuppressive effects of 20(R)- ginsenoside Rg3. Biochem Pharmacol. 2006;72:437–445. doi: 10.1016/j.bcp.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Peng H, Ou-Yang X, He X. Research on the antitumor effect of ginsenoside Rg3 in B16 melanoma cells. Melanoma Res. 2008;18:322–329. doi: 10.1097/CMR.0b013e32830b3536. [DOI] [PubMed] [Google Scholar]
- 15.Qian T, Cai Z, Wong RN, Mak NK, Jiang ZH. In vivo rat metabolism and pharmacokinetic studies of ginsenoside Rg3. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;816:223–232. doi: 10.1016/j.jchromb.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 16.Shibata S. Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J Korean Med Sci. 2001;16(Suppl):S28–S37. doi: 10.3346/jkms.2001.16.S.S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bi WY, Fu BD, Shen HQ, Wei Q, Zhang C, Song Z, Qin QQ, Li HP, Lv S, Wu SC, Yi PF, Wei XB. Sulfated derivative of 20(S)-ginsenoside Rh2 inhibits inflammatory cytokines through MAPKs and NF-kappa B pathways in LPS-induced RAW264.7 macrophages. Inflammation. 2012;35:1659–1668. doi: 10.1007/s10753-012-9482-1. [DOI] [PubMed] [Google Scholar]
- 18.He L, Lee J, Jang JH, Lee SH, Nan MH, Oh BC, Lee SG, Kim HH, Soung NK, Ahn JS, Kim BY. Ginsenoside Rh2 inhibits osteoclastogenesis through down-regulation of NF-kappaB, NFATc1 and c-Fos. Bone. 2012;50:1207–1213. doi: 10.1016/j.bone.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Luo JZ, Luo L. Ginseng on hyperglycemia: effects and mechanisms. Evid Based Complement Alternat Med. 2009;6:423–427. doi: 10.1093/ecam/nem178. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Wu N, Wu GC, Hu R, Li M, Feng H. Ginsenoside Rh2 inhibits glioma cell proliferation by targeting microRNA-128. Acta Pharmacol Sin. 2011;32:345–353. doi: 10.1038/aps.2010.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li B, Zhao J, Wang CZ, Searle J, He TC, Yuan CS, Du W. Ginsenoside Rh2 induces apoptosis and paraptosis-like cell death in colorectal cancer cells through activation of p53. Cancer Lett. 2011;301:185–192. doi: 10.1016/j.canlet.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu GY, Li YW, Tse AK, Hau DK, Leung CH, Yu ZL, Fong WF. 20(S)-Protopanaxadiol, a metabolite of ginsenosides, induced cell apoptosis through endoplasmic reticulum stress in human hepatocarcinoma HepG2 cells. Eur J Pharmacol. 2011;668:88–98. doi: 10.1016/j.ejphar.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Wang W, Rayburn ER, Hao M, Zhao Y, Hill DL, Zhang R, Wang H. Experimental therapy of prostate cancer with novel natural product anti-cancer ginsenosides. Prostate. 2008;68:809–819. doi: 10.1002/pros.20742. [DOI] [PubMed] [Google Scholar]
- 24.Li MQ, Hou XF, Shao J, Tang CL, Li DJ. The DSCs-expressed CD82 controls the invasiveness of trophoblast cells via integrinbeta1/MAPK/MAPK3/1 signaling pathway in human first-trimester pregnancy. Biol Reprod. 2010;82:968–979. doi: 10.1095/biolreprod.109.080739. [DOI] [PubMed] [Google Scholar]
- 25.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer Statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 26.American Cancer Society. Cancer Facts and Figures. 2015 [Google Scholar]
- 27.Christensen LP. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 28.Chang YS, Seo EK, Gyllenhaal C, Block KI. Panax ginseng: a role in cancer therapy? Integr Cancer Ther. 2003;2:13–33. doi: 10.1177/1534735403251167. [DOI] [PubMed] [Google Scholar]
- 29.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 30.Kitts DD, Hu C. Efficacy and safety of ginseng. Pub Health Nut. 2000;3:473–485. doi: 10.1017/s1368980000000550. [DOI] [PubMed] [Google Scholar]
- 31.Jung JS, Ahn JH, Le TK, Kim DH, Kim HS. Protopanaxatriol ginsenoside Rh1 inhibits the expression of matrix metalloproteinases and the in vitro invasion/migration of human astroglioma cells. Neurochem Int. 2013;63:80–86. doi: 10.1016/j.neuint.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Jung SH, Woo MS, Kim SY, Kim WK, Hyun JW, Kim EJ, Kim DH, Kim HS. Ginseng saponin metabolite suppresses phorbol ester-induced matrix metalloproteinase-9 expression through inhibition of activator protein-1 and mitogenactivated protein kinase signaling pathways in human astroglioma cells. Int J Cancer. 2006;118:490–497. doi: 10.1002/ijc.21356. [DOI] [PubMed] [Google Scholar]
- 33.Antler AS, Ough Y, Pitchumoni CS, Davidian M, Thelmo W. Gastrointestinal metastases from malignant tumors of the lung. Cancer. 1982;49:170–172. doi: 10.1002/1097-0142(19820101)49:1<170::aid-cncr2820490134>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 34.McNeill PM, Wagman LD, Neifeld JP. Small bowel metastases from primary carcinoma of the lung. Cancer. 1987;59:1486–1489. doi: 10.1002/1097-0142(19870415)59:8<1486::aid-cncr2820590815>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 35.Stenbygaard LE, Sorensen JB. Small bowel metastases in non-small cell lung cancer. Lung Cancer. 1999;26:95–101. doi: 10.1016/s0169-5002(99)00075-6. [DOI] [PubMed] [Google Scholar]
- 36.Liu T, Zhao L, Zhang Y, Chen W, Liu D, Hou H, Ding L, Li X. Ginsenoside 20(S)-Rg3 targets HIF-1α to block hypoxia-induced epithelialmesenchymal transition in ovarian cancer cells. PLoS One. 2014;9:e103887. doi: 10.1371/journal.pone.0103887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee YJ, Lee S, Ho JN, Byun SS, Hong SK, Lee SE, Lee E. Synergistic antitumor effect of ginsenoside Rg3 and cisplatin in cisplatinresistant bladder tumor cell line. Oncol Rep. 2014;32:1803–1808. doi: 10.3892/or.2014.3452. [DOI] [PubMed] [Google Scholar]
- 38.Li L, Wang Y, Qi B, Yuan D, Dong S, Guo D, Zhang C, Yu M. Suppression of PMA-induced tumor cell invasion and migration by ginsenoside Rg1 via the inhibition of NF-κBdependent MMP-9 expression. Oncol Rep. 2014;32:1779–1786. doi: 10.3892/or.2014.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi Q, Li J, Feng Z, Zhao L, Luo L, You Z, Li D, Xia J, Zuo G, Chen D. Effect of ginsenoside Rh2 on the migratory ability of HepG2 liver carcinoma cells: recruiting histone deacetylase and inhibiting activator protein 1 transcription factors. Mol Med Rep. 2014;10:1779–1785. doi: 10.3892/mmr.2014.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu QF, Fang XL, Chen DF. Pharmacokinetics and bioavailability of ginsenoside Rb1 and Rg1 from Panax notoginseng in rats. J Ethnopharmacol. 2003;84:187–192. doi: 10.1016/s0378-8741(02)00317-3. [DOI] [PubMed] [Google Scholar]
- 41.Joo KM, Lee JH, Jeon HY, Park CW, Hong DK, Jeong HJ, Lee SJ, Lee SY, Lim KM. Pharmacokinetic study of ginsenoside Re with pure ginsenoside Re and ginseng berry extracts in mouse using ultra performance liquid chromatography/mass spectrometric method. J Pharm Biomed Anal. 2010;51:278–283. doi: 10.1016/j.jpba.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 42.Liu H, Yang J, Du F, Gao X, Ma X, Huang Y, Xu F, Niu W, Wang F, Mao Y, Sun Y, Lu T, Liu C, Zhang B, Li C. Absorption and disposition of ginsenosides after oral administration of Panax notoginseng extract to rats. Drug Metab Dispos. 2009;37:2290–2298. doi: 10.1124/dmd.109.029819. [DOI] [PubMed] [Google Scholar]