Abstract
Background we intended to explore the functional implication of microRNA-183 (miR-183) in predicting clinical prognosis and regulating cancer proliferation and bufalin sensitivity in epithelial ovarian cancer (EOC). Methods In 75 EOC patients, miR-183 expression was examined, by quantitative RT-PCR (qRT-PCR), between paired EOC tumors and adjacent normal tissues, and between tumor samples from patients at early clinical stages and those at advanced clinical stages. The association of serum miR-183 and patients’ clinicopathological variables were examined. The overall survival (OS) was estimated by Kaplan-Meier model. And the possibility of miR-183 as a prognostic biomarker for EOC was examined by cox proportional hazard regression model. In EOC cell lines SKOV3 and ES-2 cells, lentiviral transduction was conducted to genetically suppress miR-183. The effect of miR-183 downregulation on EOC in vitro growth, bufalin sensitivity and in vivo tumorigenicity were examined. Results MiR-183 was highly expressed in EOC tumors, as well ass in patients at advanced clinical stages. Serum miR-183 was significantly associated with major clinicopathological variables in EOC patients, such as clinical stage and lymph node metastases. High level of serum miR-183 was associated with poor OS in EOC patients, and proved to be a potential biomarker for EOC. In EOC cell lines, functional assays demonstrated that miR-183 downregulation inhibited cancer proliferation, enhanced bufalin sensitivity and reduced tumorigenicity in vivo. Conclusion MiR-183 may be a prognostic biomarker for EOC, and inhibiting miR-183 may have therapeutic effect to inhibit tumor growth in EOC.
Keywords: Epithelial ovarian cancer, miR-183, biomarker, bufalin, proliferation
Introduction
Epithelial ovarian cancer (EOC) is the sixth most common and seventh most lethal gynecological cancer in the world [1-3]. Both genetic and environmental factors may contribute to the development of EOC, and clear cell carcinomas are the most common EOC sub-type among Asian patients [1,2]. Due to high metastasis rate and lack of diagnostic techniques to identify early symptoms, patients of EOC often experienced poor prognosis and high malignancy [3-5]. Therefore, much is needed to unravel the underlying mechanisms of EOC etiology, as well as identify efficient biomarker for early diagnosis of EOC.
MicroRNAs (miRNAs) are noncoding small RNAs (~18-25 nucleotides in length) that regulate various cellular or molecular mechanisms by binding the 3’-untranslated region (3’-UTR) of target genes to suppress gene expression or induce protein degradation [6,7]. In human EOC, aberrant expression patterns of miRNA, either downregulated or upregulated, were found to be closely associated with tumors in EOC patients [8]. Also, a study on 42 women with serous epithelial ovarian cancer found more than dozens of miRNA were aberrantly expressed in patients’ sera [9]. Most recently, several miRNAs, such as miR-25, miR-23b, miR-200, miR-335, were found to be independent prognostic factors in EOC [10-13]. Among the family of EOC-associated miRNAs, microRNA-183 (miR-183) was suggested to be highly expressed in EOC tumors [14,15]. However, the exact mechanism of miR-183, either as independent circulating biomarker or functional cancer regulator, is largely unknown.
In this study, we collected the clinical samples from 75 patients and applied qRT-PCR to definitively define the expression pattern of miR-183 in EOC. The correlation of miR-183 with clinicopathological variables of EOC patients, as well as the potential prognostic factors to independently predict patients’ overall survivals were analyzed. Furthermore, we applied lentivirus to genetically downregulate endogenous miR-183 in EOC cell lines, SKOV3 and ES-2 cells. The possible tumor-suppressive effects of miR-183 downregulation on EOC in vitro development, bufalin sensitivity and in vivo tumorigenicity were examined.
Materials and methods
Ethic statements
In this study, all experimental procedures were conducted in accordance with the Declaration of Helsinki, and the laws and medical practice regulations in People’s Republic of China. All experimental procedures were approved by the Human Research & Ethic Committees at the Liaocheng People’s Hospital in China. All patients signed consent forms before the enrollment of the study.
Patients
Serum and clinical samples from a total of 75 EOC patients were included in this study between December 2006 and December 2011. All patients’ clinical stages and histological grades were evaluated by a group of independent pathologists and oncologists in accordance with criteria of International Federation of Gynecology and Obstetrics (FIGO). Paired clinical samples from each EOC patient included tumorous tissue, and adjacent normal tissue extracted at least 3 cm from the clear edge of tumor. Upon extraction, all samples were immediately snap-frozen in liquid nitrogen and stored at a bio-freezer at -80°C until processed.
RNA extraction and qRT-PCR
Total RNA was extracted from samples with a Trizol reagent (ThermoFisher Scientific, USA) according to the manufacturer’s protocol. The reverse transcription was conducted using a TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, USA) according to the manufacturer’s protocol. The reaction conditions were 16°C for 30 min, followed by 42°C for 30 min and 85°C for 5 min, then held at 4°C. The detection of hsa-miR-183 gene expression was conducted by quantitative reverse-transcription polymerase chain reaction (qRT-PCR), with a TaqMan Human MicroRNA assay kit (Applied Biosystems, USA) on an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, USA) according to the manufacturer’s protocol. U6 snRNA was used as internal standard. The reaction conditions for qRT-PCR were 95°C for 30 s, followed by 35 cycles of 95°C for 5 s, and 62°C for 40 s. Fold changes of miR-183 were quantified by 2-∆∆Ct method.
Cell lines
Two EOC cell lines, SKOV3 and ES-2 cells, were purchased from American Type Culture Collection (ATCC, USA). They were maintained in RPMI-1640 medium (ThermoFisher Scientific, USA) supplemented with 15% fetal calf serum (ThermoFisher Scientific, USA), 1% non-essential amino acid (NEAA, ThermoFisher Scientific, USA), 100 U/ml penicillin and 100 μg/ml streptomycin (ThermoFisher Scientific, USA) in a tissue incubator with 5% CO2 at 37°C.
MiR-183 downregulation
The lentivirus expressing human miR-183 inhibitor (L-miR183-inhibitor), and an empty lentivirus (L-Blank) were purchased from RiboBio (RiboBio Biotech, China). SKOV3 and ES-2 cells were transduced with 100 nM lentiviruses with 8 μg/ml polybrene with multiplicity of infection (MOI) of 15~30. 72 h after transduction, lentivirus-induced miR-183 downregulation was assessed by qRT-PCR.
Cancer proliferation assay
The in vitro proliferations of SKOV3 and ES-2 cells were measured by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (ThermoFisher Scientific, USA) according to the manufacturer’s protocol. After lentiviral transduction was stabilized, SKOV3 and ES-2 cells were suspended, re-plated in 96-well plates (5,000 cells per well), and maintained for 5 days. At every 24 h interval, cells were incubated with MTT solution (5 mg/mL) for 4 h. After medium aspiration, dimethyl sulfoxide was added into culture for 30 mins. Plates were then mounted and the optical density (O.D.) at 570 nm was measured using a Synergy 2 multi-mode microplate reader (BioTek, USA).
Bufalin assay
After lentiviral transduction, SKOV3 and ES-2 cells were treated with four concentrations of bufalin (0, 1, 10, 100 ng/mL) for 48 h. The relative viability of the cells was assessed by MTT assay. The measured O.D. values were then normalized to values with no bufalin treatment (0 ng/mL).
In vivo tumorigenicity assay
After lentiviral transduction, SKOV3 cells were suspended from culture plates, and subcutaneously inoculated into the left flanks of female athymic mice (6-week old). The in vivo growth of EOC transplant was monitored by weekly measurement of tumor volumes (mm3) using equation, length × width2/2, for five consecutive weeks.
Statistical analysis
All experiments were performed in biological triplicates and data were presented as mean +/- S.E.M.. Statistical analysis was performed using a SPSS software (SPSS, USA). For analysis on two groups of samples, Student’s t-test was used. For analysis on more than two groups of samples, one-way ANOVA with post hoc test was used. For analysis on association of serum miR-183 and clinicopathological variables of patients with EOC, Chi-squared test was used. Analysis on patients’ overall survival was performed using Kaplan-Meier model with log-rank test. Hazard ratios and confidence intervals at 95% was analyzed using cox proportional hazard regression model. Statistical significance was determined if P < 0.05.
Results
MicroRNA-183 is highly expressed in EOC
Previous microarray studies suggested that miR-183 was likely upregulated in EOC [14,15]. In this study, we used qRT-PCR to compare miR-183 expression level between paired tumors and adjacent normal tissues in 75 patients with EOC. It showed that miR-183 was generally upregulated in EOC tumors than in adjacent normal tissues (Figure 1A, *: P < 0.001). MiR-183 expression was also compared between EOC patients at different clinical stages. Among all the enrolled patients with EOC, 34 of them were at early stages (stage I & II). The remaining 41 patients were at advanced stages (stage III & IV). Analysis of qRT-PCR demonstrated that miR-183 was upregulated in patients at advanced stages than in patients at early stages (Figure 1B, *: P < 0.001).
Figure 1.
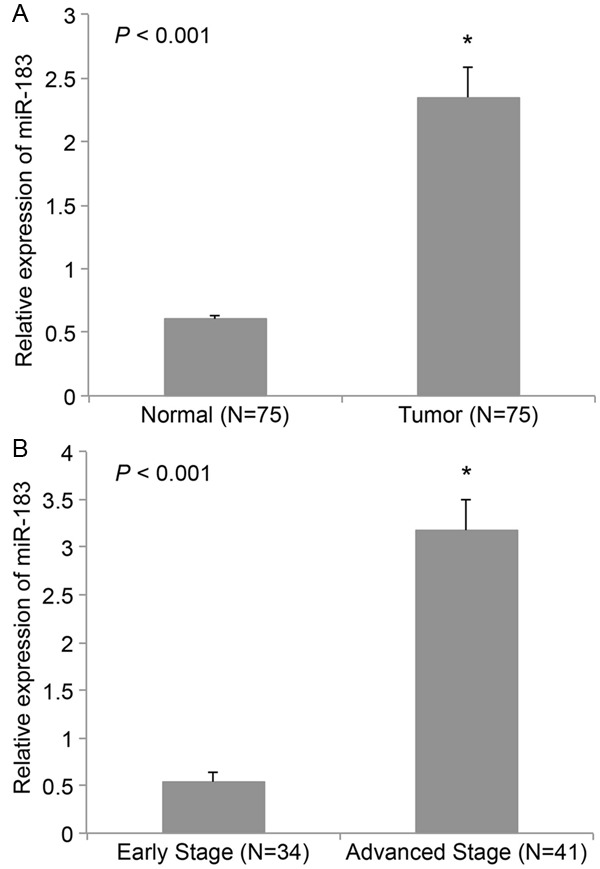
MiR-183 is upregulated in EOC. A. Tumor tissues and adjacent normal tissues were extracted from 75 EOC patients. The expression of miR-183 was compared by qRT-PCR (*: P < 0.001). B. The tumor tissues were divided into two groups. One was from the patients at early stages (I & II, N=34). The other was from the patients at advanced stages (III & IV, N=41). The expression of miR-183 was compared between two groups by qRT-PCR (*: P < 0.001).
Serum miR-183 is correlated with clinicopathological variables of EOC patients
The clinicopathological variables of 75 EOC patients were analyzed in correlation with differential expression level of miR-183 in serum samples. Based on mean serum miR-183 level, there were 36 patients with low serum miR-183 and 39 patients with high serum miR-183. Upon analysis, there were no statistically significant correlations between serum miR-183 and patients’ age (P=0.539), histology (P=0.533), or histological grade (P=0.178) (Table 1). However, we found there were strong correlations between serum miR-183 and some of the key clinicopathological variables of EOC patients, including clinical stage (P=0.008) and lymph node metastases (P=0.001) (Table 1).
Table 1.
Association of serum miR-183 with clinicopathological variables in 75 patients with epithelial ovarian cancer
| Variables | miR-183 expression | P-value | |
|---|---|---|---|
|
| |||
| Low miR-183 (N=36) N (%) | High miR-183 (N=39) N (%) | ||
| Age (years) | |||
| < 50 | 27 (75.0%) | 28 (71.8%) | 0.539 |
| ≥ 50 Clinical Stage | 9 (25.0%) | 11 (28.2%) | |
| Stage I/II | 25 (69.4%) | 9 (23.1%) | 0.008* |
| Stage III/IV | 11 (30.6%) | 30 (76.9%) | |
| Histology | |||
| Serous | 11 (30.6%) | 8 (20.5%) | 0.533 |
| Mucinous | 15 (41.7%) | 19 (48.7%) | |
| Other | 10 (27.7%) | 12 (30.8%) | |
| Histological Grade | |||
| 1 | 12 (34.4%) | 15 (38.5%) | 0.178 |
| 2 | 17 (47.2%) | 17 (43.6%) | |
| 3 | 7 (19.4%) | 7 (17.9%) | |
| Lymph node metastases | |||
| Negative | 24 (66.7%) | 14 (35.9%) | 0.011* |
| Positive | 12 (33.3%) | 25 (64.1%) | |
P < 0.05.
Serum miR-183 is correlated with overall survival in EOC patients
The overall survivals (OSs) of 75 EOC patients were assessed using Kaplan-Meier model, and then analyzed in correlation with differential expression level of serum miR-183 (Figure 2). Our data demonstrated that EOC patients with high serum miR-183 had significantly shorter OS than those patients with low serum miR-183 (Log-rank test, P < 0.05).
Figure 2.
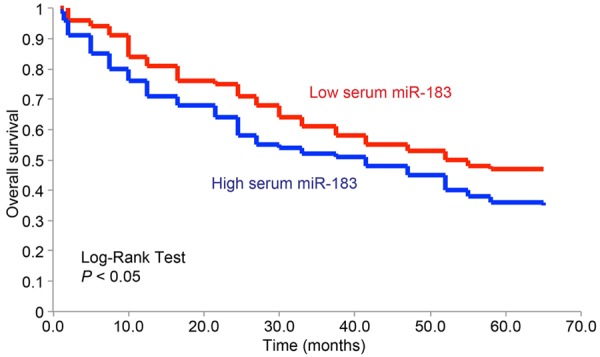
Serum miR-183 correlates overall survival in EOC patents. Kaplan-Meier model was used to analyze the overall survivals for EOC patients with low serum miR-183 (red), and the patients with high serum miR-183 (blue).
We then used Cox’s hazards analysis on the correlation of patients’ clinicopathological variables and overall survival to identify possible prognostic factors. We discovered, through univariate analysis, that clinical stage (P=0.003), histology type (P=0.008), lymph node metastasis (P=0.002) and serum miR-183 expression level (P=0.001), were significantly associated with prognosis (Table 2). Furthermore, multivariate Cox analysis identified that clinical stage (P=0.019, HR=2.841, 95% CI=1.346~4.529), lymph node metastasis (P=0.009, HR=2.893, 95% CI=1.563~5.897) and serum miR-183 expression level (P=0.003, HR=3.862, 95% CI=2.774~7.963) were independent prognostic factors of EOC (Table 2).
Table 2.
Univariate and multivariate Cox’s hazards analysis on possible prognostic factors for patients with epithelial ovarian cancer. HR, hazard ratio. CI, confidence interval
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
|
|
|||||
| Factors | P-value | HR (95% CI) | P-value | HR (95% CI) | |
| Age (years) | < 50 vs. ≥ 50 | 0.471 | 2.093 (1.451~2.699) | ||
| Clinical Stage | I & II vs. III & IV | 0.003* | 4.891 (3.991~7.902) | 0.019* | 2.841 (1.346~4.529) |
| Histology | Serous vs. Others | 0.008* | 3.766 (2.891~6.226) | 0.264 | 1.578 (0.783~2.582) |
| Histology grade | 1 & 2 vs. 3 | 0.079 | 1.482 (1.028~3.782) | ||
| Lymph node metastasis | Negative vs. Positive | 0.002* | 4.752 (2.672~8.921) | 0.009* | 2.893 (1.563~5.897) |
| Serum miR-183 | Low vs. High | 0.001* | 7.541 (5.885~11.329) | 0.003* | 3.862 (2.774~7.963) |
P < 0.05.
MiR-183 downregulation modulates EOC cell line proliferation and bufalin sensitivity
As we discovered miR-183 was highly expressed in EOC tumors, we wondered whether downregulating miR-183 would have tumor-suppressive effect on EOC development. We transduced two EOC cell lines, SKOV3 and ES-2 with lentiviral vector (L-miR183-inhibitor) to knock down the endogenous expression of miR-183. The control EOC cells were transduced with an empty lentivirus (L-Blank). By comparing the endogenous miR-183 expression using qRT-PCR, we confirmed that L-miR183-inhiibtor significantly downregulated miR-183 expressions in SKOV3 and ES-2 cells (Figure 3A, *, P < 0.05, student’s t-test). We then used a MTT assay to assess the proliferation in EOC cells with miR-183 downregulation. Five days after MTT assay, it showed that cell proliferations in both SKOV3 and ES-2 cells were significantly inhibited by miR-183 downregulation (Figure 3B, **, P < 0.05, one-way ANOVA). We also examined the effect of miR-183 downregulation on EOC sensitivity to bufalin. Using a gradient concentration assay, we discovered that in both SKOV3 and ES-2 cells, bufalin-induced apoptosis was significantly enhanced by miR-183 downregulation (Figure 3C, **, P < 0.05, one-way ANOVA).
Figure 3.
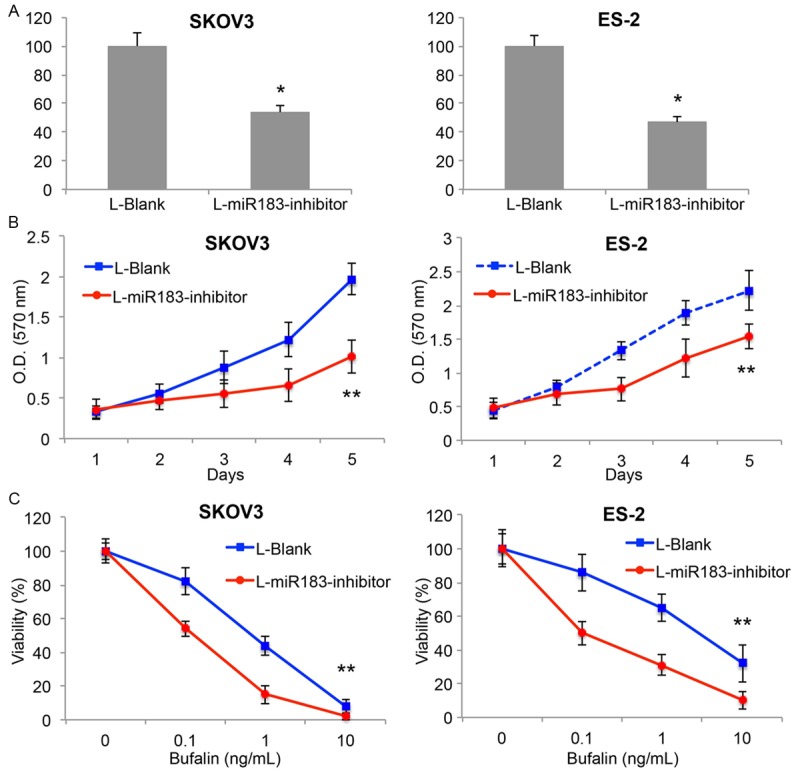
MiR-183 regulates EOC growth and bufalin sensitivity in vitro. A. SKOV3 and ES-2 cells were transduced with L-miR183-inhibitor or L-Blank. QRT-PCR demonstrated that lentiviral transduction was efficient to downregulate miR-183 in both cell lines (*P < 0.05). B. After lentiviral transduction, in vitro proliferation of SKOV3 and ES-2 cells were assessed by MTT assay for 5 days (**P < 0.05, one-way ANOVA). C. After lentiviral transduction, SKOV3 and ES-2 cells were treated with bufalin (0~10 ng/mL) for 48 h, followed by MTT assay to assess cancer cell viability (**P < 0.05, one-way ANOVA).
MiR-183 downregulation modulates in vivo EOC tumorigenicity
Finally, we wondered whether downregulation of miR-183 would also have tumor-suppressive effect on in vivo development of EOC tumor. We used lentiviral transduced SKOV3 cells, which were transduced with either L-miR183-inhibitor or L-Blank, and injected them into the subcutaneous flanks of female athymic mice. Volumes of EOC tumors were assessed every week, using fomula: length × width2/2, for 5 consecutive weeks. The growth curves in tumorigenicity assay showed that downregulation of miR-183 significantly inhibited the in vivo development of EOC tumors (Figure 4A, **P < 0.05, one-way ANOVA). The inhibitory effect of miR-183 downregulation on in vivo EOC development was further assessed by extracting the tumors at the end of tumorigenicity assay. It showed that tumors transduced with L-miR183-inhiibtor were significantly smaller than the tumors transduced with L-Blank (Figure 4B).
Figure 4.
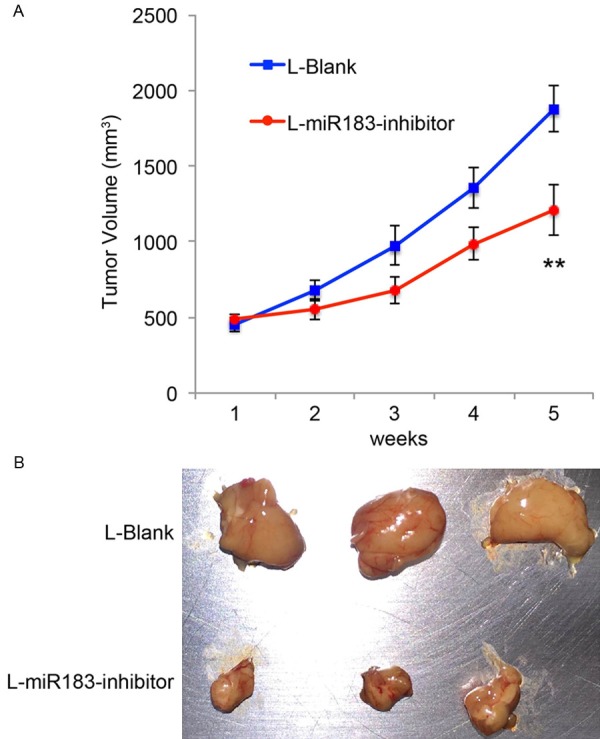
MiR-183 regulates EOC growth in vivo. A. After lentiviral transduction, SKOV3 cells were subcutaneously injected into 6-week-old female athymic mice. The growth curves were monitored every week by measuring tumor volumes (length × width2/2, mm3) (**P < 0.05, one-way ANOVA). B. 5 weeks after tumorigenicity assay, EOC tumors were extracted and compared.
Discussions
It was suggested in previous microarray studies that miR-183 may be upregulated in EOC tumors [14,15]. In the present study, we applied much accurate quantitative method (qRT-PCR in current study vs. microarray in previous studies), and used much bigger sample size (75 in current study vs. 28 in previous study) to clearly demonstrate that miR-183 was indeed aberrantly upregulated in EOC. In addition, we found that miR-183 was also upregulated in patients at advanced clinical stages than in patients at early clinical stages. This data is in line with another finding of our study, which shows miR-183 was significantly correlated with the clinical stage of EOC patients. The aberrant overexpression of miR-183 was also seen in other cancers, such as synovial sarcoma, rhabdomyosarcoma and colon cancer [16]. Thus, it seems like miR-183 upregulation may be the predominant aberrant expression pattern among various tumor types, which makes it an interesting candidate biomarker for general cancer detection or diagnosis.
Our data showed that high-level serum miR-183 was closely associated with poor overall survival of EOC patients. Further statistical analysis demonstrated that miR-183 was an independent prognostic factor for EOC. To date, there are only a few miRNA candidates that had been identified as non-invasive biomarker for EOC prediction [10-12,17-19]. The discovery of our study may help to develop more reliable combination of miRNA biomarkers for early diagnosis of EOC.
Also in this study, we reported for the first time that, miR-183 was a mechanistic regulator in EOC development. Functional assay on EOC cell lines, SKOV3 and ES-2 cells, demonstrated that down-regulating miR-183 had tumor-suppressive effects on EOC development (both in vitro and in vivo) and bufalin sensitivity. MiR-183 has been shown to functionally mediate cancer development through various signaling pathways, such as EGR1/PTEN [16] or PDCD4/TGF-b1 [20]. It would be interesting to find out, through future studies, which target genes or signaling pathways may be associated with miR-183 in regulating cancer development in EOC.
Overall, the data of current study demonstrated first-ever evidence that miR-183 was aberrantly overexpressed in EOC tumors, and miR-183 expression was higher in EOC patients at advanced clinical stages. Also, we demonstrated that serum miR-183 might be acting as an independent prognostic factor for EOC. Furthermore, we used functional experiments to show that down-regulating miR-183 may act as a tumor suppressor to regulate cancer development of EOC. These information may further our understanding on how miRNAs may be applied as cancer biomarker, as well as therapeutic targets for patients with EOC.
Disclosure of conflict of interest
None.
References
- 1.Haruta S, Furukawa N, Yoshizawa Y, Tsunemi T, Nagai A, Kawaguchi R, Tanase Y, Yoshida S, Kobayashi H. Molecular genetics and epidemiology of epithelial ovarian cancer (Review) Oncol Rep. 2011;26:1347–1356. doi: 10.3892/or.2011.1456. [DOI] [PubMed] [Google Scholar]
- 2.Al Bakir M, Gabra H. The molecular genetics of hereditary and sporadic ovarian cancer: implications for the future. Br Med Bull. 2014;112:57–69. doi: 10.1093/bmb/ldu034. [DOI] [PubMed] [Google Scholar]
- 3.Chornokur G, Amankwah EK, Schildkraut JM, Phelan CM. Global ovarian cancer health disparities. Gynecol Oncol. 2013;129:258–264. doi: 10.1016/j.ygyno.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon JS. Improving survival after endometrial cancer: the big picture. J Gynecol Oncol. 2015;26:227–231. doi: 10.3802/jgo.2015.26.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin. 2011;61:183–203. doi: 10.3322/caac.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miska EA. How microRNAs control cell division, differentiation and death. Curr Opin Genet Dev. 2005;15:563–568. doi: 10.1016/j.gde.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Liang TJ, Qin CY. The emerging role of microRNAs in immune cell development and differentiation. APMIS. 2009;117:635–643. doi: 10.1111/j.1600-0463.2009.02520.x. [DOI] [PubMed] [Google Scholar]
- 8.Iorio MV, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, Calin GA, Menard S, Croce CM. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007;67:8699–8707. doi: 10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]
- 9.Shapira I, Oswald M, Lovecchio J, Khalili H, Menzin A, Whyte J, Dos Santos L, Liang S, Bhuiya T, Keogh M, Mason C, Sultan K, Budman D, Gregersen PK, Lee AT. Circulating biomarkers for detection of ovarian cancer and predicting cancer outcomes. Br J Cancer. 2014;110:976–983. doi: 10.1038/bjc.2013.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao J, Cai J, Huang D, Han Q, Chen Y, Yang Q, Yang C, Kuang Y, Li D, Wang Z. miR-335 represents an independent prognostic marker in epithelial ovarian cancer. Am J Clin Pathol. 2014;141:437–442. doi: 10.1309/AJCPLYTZGB54ISZC. [DOI] [PubMed] [Google Scholar]
- 11.Cao Q, Lu K, Dai S, Hu Y, Fan W. Clinicopathological and prognostic implications of the miR-200 family in patients with epithelial ovarian cancer. Int J Clin Exp Pathol. 2014;7:2392–2401. [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, Liu Z, Chen L, Zhou L, Yao Y. MicroRNA-23b is an independent prognostic marker and suppresses ovarian cancer progression by targeting runt-related transcription factor-2. FEBS Lett. 2014;588:1608–1615. doi: 10.1016/j.febslet.2014.02.055. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Meng X, Li H, Liu W, Shen S, Gao Z. MicroRNA-25 expression level is an independent prognostic factor in epithelial ovarian cancer. Clin Transl Oncol. 2014;16:954–958. doi: 10.1007/s12094-014-1178-6. [DOI] [PubMed] [Google Scholar]
- 14.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 15.Navon R, Wang H, Steinfeld I, Tsalenko A, Ben-Dor A, Yakhini Z. Novel rank-based statistical methods reveal microRNAs with differential expression in multiple cancer types. PLoS One. 2009;4:e8003. doi: 10.1371/journal.pone.0008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarver AL, Li L, Subramanian S. MicroRNA miR-183 functions as an oncogene by targeting the transcription factor EGR1 and promoting tumor cell migration. Cancer Res. 2010;70:9570–9580. doi: 10.1158/0008-5472.CAN-10-2074. [DOI] [PubMed] [Google Scholar]
- 17.Peng DX, Luo M, Qiu LW, He YL, Wang XF. Prognostic implications of microRNA-100 and its functional roles in human epithelial ovarian cancer. Oncol Rep. 2012;27:1238–1244. doi: 10.3892/or.2012.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin M, Yang Z, Ye W, Xu H, Hua X. MicroRNA-150 predicts a favorable prognosis in patients with epithelial ovarian cancer, and inhibits cell invasion and metastasis by suppressing transcriptional repressor ZEB1. PLoS One. 2014;9:e103965. doi: 10.1371/journal.pone.0103965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang W, Jiang Y, Mu X, Xu L, Cheng W, Wang X. MiR-135a functions as a tumor suppressor in epithelial ovarian cancer and regulates HOXA10 expression. Cell Signal. 2014;26:1420–1426. doi: 10.1016/j.cellsig.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Fu H, Xu C, Tie Y, Xing R, Zhu J, Qin Y, Sun Z, Zheng X. miR-183 inhibits TGF-beta1-induced apoptosis by downregulation of PDCD4 expression in human hepatocellular carcinoma cells. BMC Cancer. 2010;10:354. doi: 10.1186/1471-2407-10-354. [DOI] [PMC free article] [PubMed] [Google Scholar]


