Abstract
As a highly conserved protein of the Golgi apparatus, Golgi phosphoprotein 3 (GOLPH3) has been shown to be involved in tumorigenesis. This study aims to explore the expression and significance of GOLPH3 in non-small-cell lung cancer (NSCLC). We found that GOLPH3 expression was significantly elevated in NSCLC tissues when compared with adjacent lung tissues (p<0.01). Moreover, GOLPH3 expression was significantly associated with histological type (p<0.01), differentiation (p<0.01), and lymph node metastasis (p<0.05). Kaplan-Meier survival analysis showed that overall survival of patients with high expression of GOLPH3 was significantly shorter (n=100, p<0.05). In addition, GOLPH3 knock-down in two independent NSCLC cell lines inhibited cell viability through the induction of cell cycle arrest and apoptosis. In conclusion, GOLPH3 is closely related to the progression in NSCLC and could be served as a potential prognostic biomarker and therapeutic target for NSCLC.
Keywords: GOLPH3, non-small-cell lung cancer
Introduction
Lung cancer is the first most common malignancy and the leading cause of cancer-related death. With the deterioration of the environment and air pollution, the incidence of non-small cell lung cancer are still on the rise. Despite much progress has been made in the screening, diagnosis and clinical management of lung cancer, only 16.6% of patients with lung cancer are alive more than 5 years after diagnosis. Non-small-cell lung carcinoma (NSCLC) is the major class of lung cancer. NSCLCs including mainly lung adenocarcinoma and squamous cell lung carcinoma are relatively insensitive to chemotherapy when compared to small cell carcinoma. Understanding the molecular mechanisms underlying the pathogenesis of NSCLC could help to develop more effective regiments and improve the clinical outcome of NSCLC.
GOLPH3 is a highly conserved 34-kDa protein initially identified through proteomic characterization of the Golgi apparatus [1]. It is highly conserved from yeast to human. Recently, GOLPH3 has been shown to be involved in tumorigenesis [2]. For example, GOLPH3 overexpression was associated with the poor prognosis in variety of solid tumors such as glioma and rhabdomyosarcoma [3,4]. In addition, the overexpression of GOLPH3 can promote cell transformation via enhancing the activity of the mTOR signaling pathway [2]. However, the relevance of GOLPH3 to the pathogenesis of NSCLC remains unknown.
In this study, we found that GOLPH3 expression was associated with the clinical outcome of NSCLC. Knockdown of GOLPH3 expression led to the growth inhibition of NSCLC cells accompanied with the induction of cell apoptosis and cell cycle arrest. Therefore, GOLPH3 could be a novel biomarker and treatment target for NSCLC.
Materials and methods
Cell culture and reagents
A549 and SPC-A1 cell lines were purchased from Shanghai cell center China Academy of Medical Sciences (Shanghai, China). A549 cells were cultured in RPMI 1640 while SPC-A1 was cultured in DMEM. Both medium are supplemented with 10% fetal bovine serum (Sijiqin, Hangzhou CHINA). For GOLPH3 knockdown, A549 or SPC-A1 Cells cultured in 6-well culture plates (3,0000/well) for 24 hours were transfected with GOLPH3 SiRNA using Lipofectamine RNAiMAX transfection reagent (Invitrogen, USA). 72 hours later, the cells were collected for analysis as indicated. The target sequence of GOLPH3 siRNA was 5’-CAAGAAAGGUAAUCUGUAATT-3’ (Jima, Shanghai, China).
Real-time reverse transcriptase polymerase chain reaction (RT-PCR)
Total RNA was extracted from cultured cells using the TRIzol reagent (Invitrogen, USA). cDNAs were synthesized from 2 μg RNA using the High Capacity cDNA ReverseTranscription Kit (AppliedBiosystems, USA) and the quantitation of GOLPH3 mRNA was performed by quantitative PCR using Bestar SybrGreen qPCR Mastermix (DBI Bioscience, Germany). Primers used for GOLPH3 amplification were GOLPH3-F: 5’-GGATTACGTGGCTGTATGT-3’ and GOLPH3-R: 5’-AGTTCTGGACCGTTTCTG-3’ (Biosune, Shanghai, China). PCR was performed with initial denaturation at 95°C for 2 minutes followed by 40 cycles of 95°C for 15 s, 60°C for 20 s and 72°C for 30 s. GOLPH3 expression were normalized by GAPDH and all experiments were performed in triplicate.
Western blotting
Whole-cell lysates were subjected to SDS-PAGE and transferred to PVDF membrane (Bio-Rad, USA). The antibodies used for immunoblotting included anti-GOLPH3 (Abcam, USA), anti-tubulin (Sigma-aldrich, USA), anti-caspase 3 (Cell Signaling Technology, USA), anti-PARP (Cell Signaling Technology, USA), Goat anti-rabbit secondary antibody (Invitrogen, USA), and Rabbit anti-Mouse secondary antibody (DuKO, Japan).
Cell viability assay (MTS)
Cells were seeded into 96-well plates. After 24 hours, the quantity of formazan was measured at 490 nm after one hour incubation with CellTiter 96® AQueous One Solution Reagent (Promega, USA) following the instructions provided.
Flow cytometer analysis
For apoptosis detection, cells transfected with GOLPH3 siRNA for 72 hours were collected and stained with PI and Annexin V-FITC (BD, USA). For cell cycle analysis, cells transfected with GOLPH3 siRNA for 72 hours were collected and fixed with cold alcohol before stained with PI.
Tissue samples
100 NSCLC patients in Jinhua Guangfu Hospital from January 2, 2009 to September 26, 2013 were included in this study for the survival analysis. The age of patients ranged from 40 to 78 years with the median age as 59 years. Another 20 patients were recruited for the evaluation of differential GOLPH3 expression in tumor and non-tumor tissues. The age range was 46-68 years with the median of 57 years.
Immunohistochemical staining
Immunohistochemical staining was performed on formalin-fixed, paraffin-embedded tissues with the Immunohistochemical kit (Zhongshaijinqiao, Beijing China). The stained tissues were scored independently by two pathologists. Each section was observed in 5 fields at high magnification view (×400). The percentage of positively stained cells was scored as follows: 0, no staining or <5% of the entire malignant cell population; 1, 5-24% of the entire malignant cell population; 2, 25-49% of the entire malignant cell population; 3, 50-74% of the entire malignant cell population; 4, ≥75% of the entire malignant cell population. Staining intensity was graded from 0 to 3: 0, no immunostaining; 1, light-brown color; 2, medium-brown color; 3, dark-brown color. The final score was the sum of the intensity and percentage scores, being classified as strong (+++, final score=6-7), moderate (++, final score=4-5), weak (+, final score=1-3), or negative (-, final score=0). GOLPH3 expression was divided into “high” (++ and +++) and “low” (+ and -).
Statistical analysis
All in vitro experiments were done in triplicate and repeated three times. All statistical analysis of the in vitro data was determined using GraphPad prism software version 6.0 (GraphPad Software, USA). Associations of GOLPH3 expression with clinicopathological factors were analyzed using chi-square test. Survival curves were plotted using the Kaplan-Meier method and differences between survival curves were tested with Log-Rank test using SPSS software version 19.0 (SPSS, USA). A p value of less than 0.05 was considered statistically significant.
Results
GOLPH3 knockdown inhibited human NSCLC cell viability
To investigate the function of GOLPH3 in NSCLC, we used RNA interference to knock down GOLPH3 expression in in vitro cultured NSCLC cell lines. After the knockdown of GOLPH3 at both mRNA and protein level (Figure 1A and 1B), the viability of both A549 and SPC-A1 were significantly decreased (Figure 1C).
Figure 1.
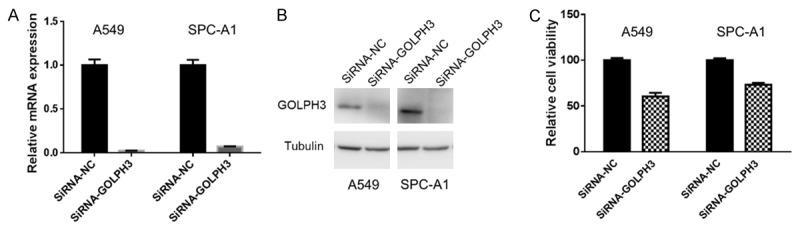
GOLPH3 knockdown inhibited viability of NSCLC cells. The expression of GOLPH3 in A549 and SPC-A1 cells with or without GOLPH3 knockdown were determined by real-time RT-PCR (A) or western blotting analysis (B), respectively. The viability of A549 and SPC-A1 cells with or without GOLPH3 knockdown were tested by MTS (p<0.05, Student’s t test) (C).
GOLPH3 knockdown promoted cell apoptosis and cell cycle arrest in NSCLC cells
Next, we would like to know how GOLPH3 influenced cell viability. Flow cytometry analysis indicated that knocking down GOLPH3 significantly increased the apoptosis of both A549 and SPC-A1 cell lines (Figure 2A). In addition, silencing of GOLPH3 expression also decreased the percentage of cells in the G1-phase and increased the percentage of G2-M phase cells (Figure 2B). Taken together, these results suggest that the knockdown of GOLPH3 inhibited cell viability by inducing cell apoptosis and cell cycle arrest.
Figure 2.
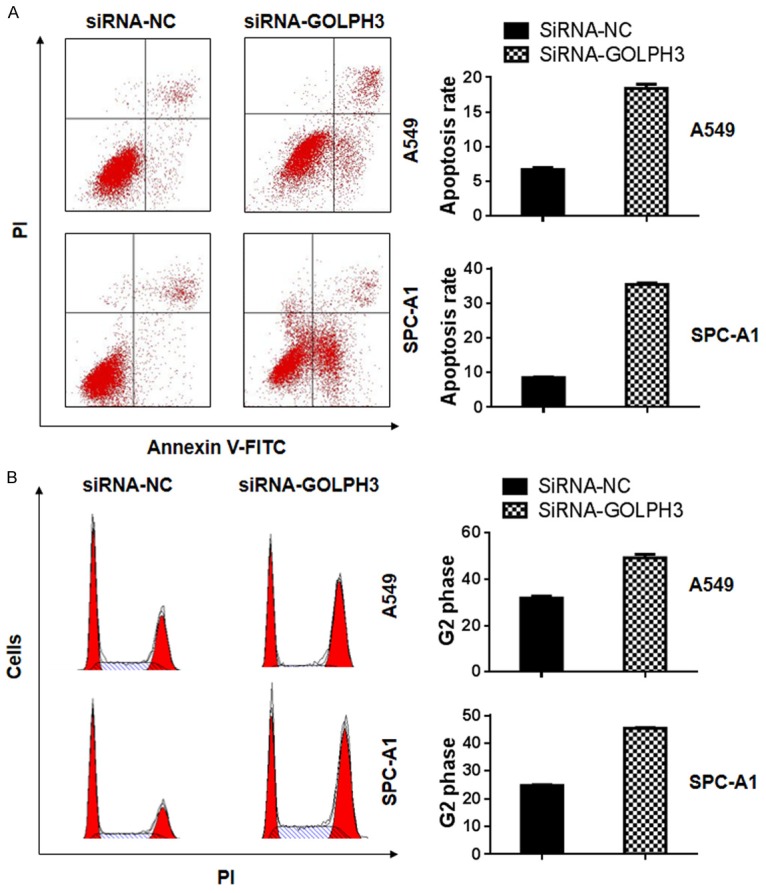
GOLPH3 knock-down induced cell apoptosis and cell cycle arrest in NSCLC cells. The apoptosis of A549 and SPC-A1 cells with or without GOPLH3 knockdown were double-stained by PI and Annexin-V-FITC and detected by flow cytometry analysis (p<0.05) (A). The cell cycle distribution of A549 and SPC-A1 cells before and after GOLPH3 knockdown were stained by PI and evaluated by flow cytometry analysis (p<0.05) (B).
Clinical relevance of GOLPH3 to NSCLC
When compared with adjacent non-tumor tissues, GOLPH3 expression was significantly increase in primary NSCLC tissues (n=20, p<0.05) (Figure 3A and 3B). In another cohort including 100 NSCLC patients, GOLPH3 was expressed at high lever in 52 (52/100, 52%) NSCLC patients. Interestingly, GOLPH3 expression was significantly higher in adenocarcinoma, poorly differentiated cancer tissues and lymph nodes with metastasis (Table 1, p<0.05). However, GOLPH3 expression was not significantly correlated with tumor size, the age of the patients and clinical stages (p>0.05, Table 1). Moreover, Kaplan-Meier survival Curves showed that high expression of GOLPH3 protein was significantly associated with poor overall survival of NSCLC patients (Figure 3C). To further explore the association of GOLPH3 with clinical outcomes of NSCLC patients, we performed both univariate and multivariate Cox regression analysis of overall survival. A shown in Table 2, high expression of GOLPH3 was significantly associated with the poor outcome of NSCLC patients.
Figure 3.
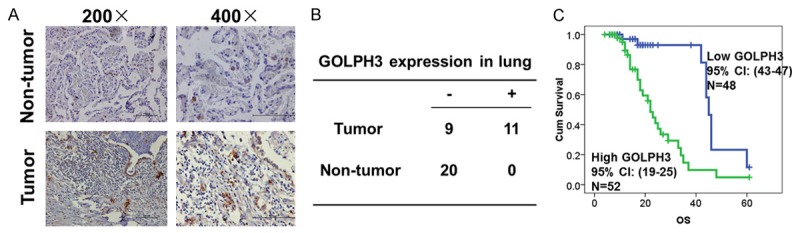
GOLPH3 expression was increased in primary NSCLC tissues. The expression of GOLPH3 in 20 primary NSCLC tissues and paired non-tumor lung tissues were evaluated by immunohistochemistry staining. The reprehensive staining were shown in A and the staining results were summarized in B (p<0.05). C. The association of GOLPH3 expression with overall survival of 100 NSCLC patients was analyzed by Kaplan–Meier survival Curves (Log Rand test, p<0.01).
Table 1.
Association of GOLPH3 with clinicopathological features in NSCLC
| Clinicopathological features | GOLPH3 expression | p | |
|---|---|---|---|
|
| |||
| Low | High | ||
| Age (years) | >0.05 | ||
| <60 | 17 | 22 | |
| ≥60 | 31 | 30 | |
| Gender | <0.01 | ||
| Male | 42 | 32 | |
| Female | 6 | 20 | |
| Tumor Size | >0.05 | ||
| ≥5 cm | 21 | 18 | |
| <5 cm | 27 | 34 | |
| Pathological type | <0.01 | ||
| Adenocarcinoma | 15 | 35 | |
| Squamous cell carcinoma | 33 | 17 | |
| Differentiation status | <0.01 | ||
| Well differentiated | 17 | 1 | |
| Moderate differentiated | 29 | 24 | |
| Poor differentiated | 2 | 27 | |
| Lymph node metastasis | <0.05 | ||
| Without | 39 | 32 | |
| With | 9 | 20 | |
| TNM stage | >0.05 | ||
| I-II | 42 | 42 | |
| III | 6 | 10 | |
Table 2.
Cox regression analysis of overall survival
| Factors | Univariate | Multivariate | ||
|---|---|---|---|---|
|
| ||||
| RR (95% CI) | p | RR (95% CI) | p | |
| Gender | ||||
| Female | 1 | 1 | ||
| Male | 0.71 (0.32-1.58) | 0.40 | 2.62 (1.02-6.75) | 0.045 |
| Tumor Size | ||||
| <5 cm | 1 | 1 | ||
| ≥5 cm | 1.05 (0.49-2.19) | 0.232 | 1.70 (0.71-4.08) | 0.232 |
| Pathological type | ||||
| SCC | 1 | 1 | ||
| AC | 2.62 (1.21-5.66) | 0.014 | 2.37 (0.99-5.68) | 0.052 |
| Differentiation | ||||
| Well | 1 | 1 | ||
| Moderate | 2.67 (0.99-7.24) | 0.053 | 0.51 (0.09-2.66) | 0.425 |
| Poor | 4.66 (1.61-13.48) | 0.005 | 1.79 (0.39-8.26) | 0.455 |
| LNM | ||||
| Without | 1 | 1 | ||
| With | 1.73 (0.86-3.48) | 0.125 | 0.66 (0.76-3.63) | 0.20 |
| GOLPH3 | ||||
| Low | 1 | 1 | ||
| High | 3.91 (1.79-8.50) | 0.001 | 5.67 (1.52-21.11) | 0.01 |
Discussion
GOLPH3 and other GOLPH family proteins might play an important role in maintaining the homeostasis of trans-Golgi network [5]. Emerging evidence shows that GOLPH3 moves between the trans-Golgi network and endosomal structures, and participates in many important cellular processes, such as trafficking, receptor recycling, and protein glycosylation [6,7]. In addition, Golgi-localized GOLPH3 exchanged dynamically with its cytosolic pool in endosomal compartments or plasma membrane [5]. Moreover, GOLPH3 could regulate receptor recycling possibly through its interaction with VPS35 [8]. Its yeast homologue VPS74 could interact with N-terminal of the glycosyltransferase, thus participating the regulation of protein glycosylation and the subsequent physiological processes such as cell migration and invasion [8,9].
However, its function and relevance to cancer development were poorly defined. Through functional genomic approaches including array-based comparative genome hybridization analyses (array-CGH), GOLPH3 was identified to be a novel oncogene [2]. Subsequently, GOLPH3 was found to enhance mTOR signaling in various cancers [2,10-12]. Its overexpression could suppress autophagy and promote tumor growth in a mitochondria-dependent manner [13]. In prostate cancer, its overexpression promoted the transition from hormone-sensitive phase to hormone-refractory phase [14]. In addition, GOLPH3 overexpression was associated with poor prognosis in many cancers such as oral tongue cancer, stomach cancer, esophageal squamous cell carcinoma, glioma and rhabdomyosarcoma [3,4,15-17].
Herein we reported that GOLPH3 expression in lung cancer tissues was significantly higher than in adjacent lung tissues. Its expression was significantly associated with histological type, differentiation, and lymph node metastasis. Survival analysis showed that survival time of patients with high expression of GOLPH3 was significantly shorter than those with low expression. Mechanistically, GOLPH3 knockdown decreased cell proliferation probably through the induction of cells apoptosis and cell cycle arrest. However, whether mTOR or other oncogenic signaling was relevant to the growth inhibitory effect induced by GOLPH3 depletion remain further investigations. Nevertheless, our findings indicate that GOLPH3 could be used as a biomarker for prognosis prediction and molecular target for clinical intervention for NSCLC.
In conclusion, GOLPH3 expression was increased in NSCLC and its expression was significantly associated with prognosis of NSCLC patients. GOLPH3 knockdown inhibited cell growth through the induction of cell apoptosis and cell cycle arrest. Therefore, it is closely related to the progression in NSCLC and could be served as a potential prognostic biomarker and therapeutic target for NSCLC.
Acknowledgements
This work was supported by High Level Talents Program from the Department of Health, Zhejiang and Ministry of Healthcare (2015103070), Natural Science foundation of Zhejiang Province (LY12H16026) and 151 talents program in Zhejiang. We thank Dr. Ping Zhu for the assistance in experiment design, data analysis and manuscript preparation.
References
- 1.Wu CC, Taylor RS, Lane DR, Ladinsky MS, Weisz JA, Howell KE. GMx33: a novel family of trans-Golgi proteins identified by proteomics. Traffic. 2000;1:963–975. [PubMed] [Google Scholar]
- 2.Scott KL, Kabbarah O, Liang MC, Ivanova E, Anagnostou V, Wu J, Dhakal S, Wu M, Chen S, Feinberg T, Huang J, Saci A, Widlund HR, Fisher DE, Xiao Y, Rimm DL, Protopopov A, Wong KK, Chin L. GOLPH3 modulates mTOR signalling and rapamycin sensitivity in cancer. Nature. 2009;459:1085–1090. doi: 10.1038/nature08109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li XY, Liu W, Chen SF, Zhang LQ, Li XG, Wang LX. Expression of the Golgi phosphoprotein-3 gene in human gliomas: a pilot study. J Neurooncol. 2011;105:159–163. doi: 10.1007/s11060-011-0573-x. [DOI] [PubMed] [Google Scholar]
- 4.Kunigou O, Nagao H, Kawabata N, Ishidou Y, Nagano S, Maeda S, Komiya S, Setoguchi T. Role of GOLPH3 and GOLPH3L in the proliferation of human rhabdomyosarcoma. Oncol Rep. 2011;26:1337–1342. doi: 10.3892/or.2011.1413. [DOI] [PubMed] [Google Scholar]
- 5.Snyder CM, Mardones GA, Ladinsky MS, Howell KE. GMx33 associates with the trans-Golgi matrix in a dynamic manner and sorts within tubules exiting the Golgi. Mol Biol Cell. 2006;17:511–524. doi: 10.1091/mbc.E05-07-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dippold HC, Ng MM, Farber-Katz SE, Lee SK, Kerr ML, Peterman MC, Sim R, Wiharto PA, Galbraith KA, Madhavarapu S, Fuchs GJ, Meerloo T, Farquhar MG, Zhou H, Field SJ. GOLPH3 bridges phosphatidylinositol-4- phosphate and actomyosin to stretch and shape the Golgi to promote budding. Cell. 2009;139:337–351. doi: 10.1016/j.cell.2009.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Korolchuk VI, Schutz MM, Gomez-Llorente C, Rocha J, Lansu NR, Collins SM, Wairkar YP, Robinson IM, O’Kane CJ. Drosophila Vps35 function is necessary for normal endocytic trafficking and actin cytoskeleton organisation. J Cell Sci. 2007;120:4367–4376. doi: 10.1242/jcs.012336. [DOI] [PubMed] [Google Scholar]
- 9.Schmitz KR, Liu J, Li S, Setty TG, Wood CS, Burd CG, Ferguson KM. Golgi localization of glycosyltransferases requires a Vps74p oligomer. Dev Cell. 2008;14:523–534. doi: 10.1016/j.devcel.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott KL, Chin L. Signaling from the Golgi: mechanisms and models for Golgi phosphoprotein 3-mediated oncogenesis. Clin Cancer Res. 2010;16:2229–2234. doi: 10.1158/1078-0432.CCR-09-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 12.Abraham RT. GOLPH3 links the Golgi network to mTOR signaling and human cancer. Pigment Cell Melanoma Res. 2009;22:378–379. doi: 10.1111/j.1755-148X.2009.00596.x. [DOI] [PubMed] [Google Scholar]
- 13.Sotgia F, Whitaker-Menezes D, Martinez-Outschoorn UE, Salem AF, Tsirigos A, Lamb R, Sneddon S, Hulit J, Howell A, Lisanti MP. Mitochondria “fuel” breast cancer metabolism: fifteen markers of mitochondrial biogenesis label epithelial cancer cells, but are excluded from adjacent stromal cells. Cell Cycle. 2012;11:4390–4401. doi: 10.4161/cc.22777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hua X, Yu L, Pan W, Huang X, Liao Z, Xian Q, Fang L, Shen H. Increased expression of Golgi phosphoprotein-3 is associated with tumor aggressiveness and poor prognosis of prostate cancer. Diagn Pathol. 2012;7:127. doi: 10.1186/1746-1596-7-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Guo L, Chen SW, Zhao XH, Zhuang SM, Wang LP, Song LB, Song M. GOLPH3 overexpression correlates with tumor progression and poor prognosis in patients with clinically N0 oral tongue cancer. J Transl Med. 2012;10:168. doi: 10.1186/1479-5876-10-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu BS, Hu H, Zhu CY, Gu YL, Li JP. Overexpression of GOLPH3 is associated with poor clinical outcome in gastric cancer. Tumour Biol. 2013;34:515–520. doi: 10.1007/s13277-012-0576-z. [DOI] [PubMed] [Google Scholar]
- 17.Wang JH, Chen XT, Wen ZS, Zheng M, Deng JM, Wang MZ, Lin HX, Chen K, Li J, Yun JP, Luo RZ, Song LB. High expression of GOLPH3 in esophageal squamous cell carcinoma correlates with poor prognosis. PLoS One. 2012;7:e45622. doi: 10.1371/journal.pone.0045622. [DOI] [PMC free article] [PubMed] [Google Scholar]


