Abstract
As an autoimmune disease, myasthenia gravis is caused by the dysfunction of neural transmission. Acetylcholine is known to exert its function after entering into synaptic cleft through binding onto postsynaptic membrane. The role of acetylcholine in binding MuSK in myasthenia gravis, however, remains unknown. A total of 38 myasthenia gravis patients and 27 healthy controls were included in this study for the detection of the expression of MuSK using immunofluorescent method. Expression of both MuSK and interleukin-6 (IL-6) were measured by Western blot, followed by the correlation analysis between heat shock protein 90 (HSP90) and IL-6 which were measured by enzyme-linked immunosorbent assay (ELISA). In myasthenia gravis patients, MuSK was co-localized with acetylcholine at the postsynaptic membrane. Such accumulation of MuSK, however, did not occur in normal people. Meanwhile we also observed elevated expression of IL-6 in myasthenia gravis patients (p<0.05). ELISA assay showed higher expression of HSP90 in patients. Further signaling pathway screening revealed the activation of IL-6-mediated pathways including STAT3 and SPH2. In conclusion, MuSK was co-localized with acetylcholine in myasthenia gravis patients, with elevated expression. HSP90 in disease people can activate IL-6 mediated signaling pathways.
Keywords: Acetylcholine, MuSK, heat-shock protein 90, molecular chaperone
Introduction
As the basic unit for connecting neurons and their effector cells, the structure of synapse has been comprehensively studied [1]. Acetylcholine is one neurotransmitter that is synthesized by choline and acetyl co-enzyme A. It mainly participates in signaling transduction and physiological modulation inside our body. Therefore the abnormality of acetylcholine metabolism often leads to diseases such as Parkinson’s and myasthenia gravis. As an auto-immune disease, myasthenia gravis is caused by the abnormal function of neuromuscular junction, which is formed between spinal cord-derived neurons and skeletal muscle cells, and is critical for controlling body’s motor function. Similar to other auto-immune diseases, multiple factors are involved in the occurrence and progression of myasthenia [2,3]. Molecular study showed the binding of antibody and α-subunit of acetylcholine at the neuromuscular junction, leads to the blockage of the transmission of acetylcholine and consequent muscle constriction [4,5]. It is interesting that the content of acetylcholine in myasthenia gravis patients was even higher than that in healthy people [6].
Muscle-specific tyrosine kinase (MuSK) has been drawn lots of research interests recently. Some studies have found the effect of MuSK on the accumulation of acetylcholine receptor at the postsynaptic membrane. Epidemiology survey has shown the elevated expression of MuSK in myasthenia gravis patients compared to healthy people. Some studies have shown the facilitated synthesis of proteins at neuromuscular junction by MuSK via forming complex with acetylcholine for activating downstream signals [7-9]. The exact mechanism by how acetylcholine binds onto MuSK, however, remains unknown yet. We thus focus on this topic in our studies.
Interleukin-6 (IL-6) can facilitate the maturation of B lymphocytes and stimulate the differentiation of myeloid precursor cells in conjunction with colony stimulating factor (CSF), and potentiate the lytic function of natural killer cells. IL-6 was firstly discovered in leukocytes and later found to be produced and secreted in some bone marrow cells and tumor cells [10]. Previous study has suggested the correlation between IL-6 and the bone marrow development via modulating cell-to-cell adhesion and expression of surface antigen [11]. Therefore we hypothesize that IL-6 might be involved in the progression of myasthenia gravis. Molecular chaperon is one kind of HSP90/HSP70 binding protein for assisting the modulation of molecular function [12]. Heat shock protein 90 (HSP90) mainly maintains the signal transduction inside body and assists the programmed protein folding. Recent study has reported the role of HSP90α as a diagnostic marker for pulmonary carcinoma. Previous study agreed that HSP90 molecular chaperon provides energy for HSP90 through ATP hydrolysis [13]. Recent report indicted the requirement of HSP90 chaperon in the induction of telomerase activity by IL-2 [14]. The role of IL-6 in HSP90 molecular chaperon, however, remains unknown. We thus focused on the modulation of IL-6 in HSP90-mediated mechanisms in this study.
Materials and methods
Patients
A total of 38 myasthenia gravis patients (21 males and 17 females) were recruited in this study between 2010 and 2014 from The First Affiliated Hospital of Shantou University Medical College. Out-patient follow-ups were performed on all participants. Another cohort of 27 healthy volunteers (14 males and 13 females) were recruited, with no significant difference regarding age or sex ratio (p>0.05).
The experimental protocol has been pre-approved by the ethical committee of The First Affiliated Hospital of Shantou University Medical College and informed consents have been obtained from all patients and healthy volunteers.
Enzyme-linked immunosorbent assay (ELISA)
ELISA kit for human IL-6 (BD Biosciences, San Jose, CA, USA) was used to quantify IL-6 levels following manufacture’s instruction. In brief, 0.1 mL standards and samples were added into 96-well plate. After 90-min incubation at 37°C, 0.1 mL biotin-labelled antibody solution was added into each well for 1-hour incubation. The plate was then washed with 0.01 M PBS for 3 times, followed by the addition of 0.1 mL ABC reagents. The development persisted for 30 min, and was stopped by TMB buffer. A microplate reader was used for the measurement of optical density value at each well.
Western blotting
Total proteins were extracted from serum samples using protein extraction kit (Beyotime Institute of Biotechnology, Jiangsu, China). Purified proteins were diluted in PBS and BCA buffer with equal concentrations, with 20% bromophenol blue. Protein mixtures were loaded onto SDS-PAGE gel for separation and then transferred to PVDF membrane. The membrane was gently rinsed in TBST, and was blocked by 5% defatted milk powder for 1 hour. Primary antibody was added for overnight incubation, followed by TBST washing. Secondary antibody was then added for 1-hour incubation, followed by ECL development.
Immunofluorescent imaging
Tissue samples were fixed, dehydrated in gradient sucrose, and cryosectioned into 6-μm thickness slices. Tissues were firstly processed by heated antigen retrieval, and then were blocked in 10% BSA for 50 min. Primary antibody was added for overnight incubation. Fluorescent secondary antibody (Santa Cruz Biotechnology, Inc., Dallas, Texas, US) was applied for developing.
Statistical analysis
SPSS11.0 software package was used to process all collected data. Data were presented as Mean ± SD. Student t-test was used to compare means between groups. Pearson analysis was used to analyze the correlation. Non-parametric data were analyzed by chi-square test. A statistical significance was defined when p<0.05.
Results
Co-localization of acetylcholine and MuSK
Immunofluorescent examination of muscular tissues showed co-localization of acetylcholine and MuSK at post-synaptic membrane in myasthenia gravis patients (Figure 1A-C) but not in healthy individuals (Figure 1D, 1F).
Figure 1.
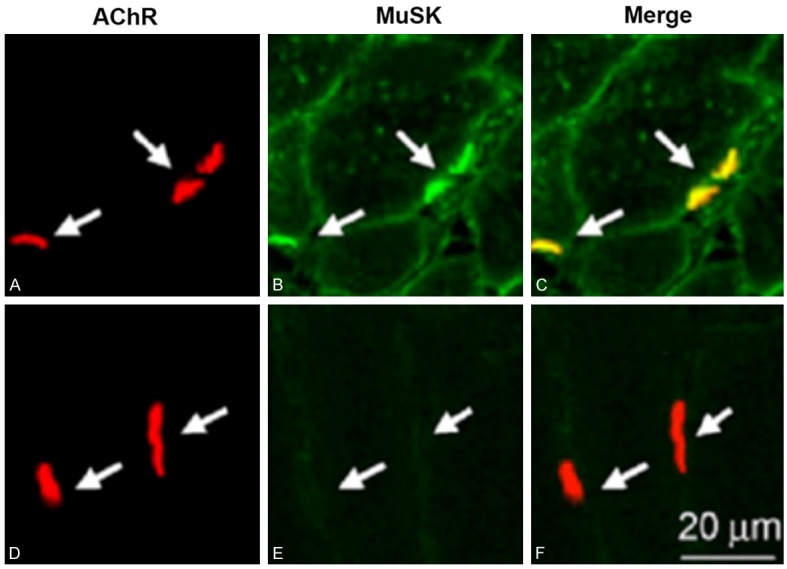
Co-localization of acetylcholine and MuSK at postsynaptic membrane. Tissue samples were fixed, dehydrated in gradient sucrose, and cryosectioned into 6-μm thickness slices followed by blockage with 10% BSA and subsequent incubation with primary and secondary antibody. A-C. Myasthenia gravis patients; D-F. Normal healthy people. A, D. Acetylcholine (red); B, E. MuSK (green); C, F. Merge images.
Serum level of MuSK and IL-6
Based on the co-localization of MuSK and acetylcholine in the previous result, we speculate the expression of MuSK might be elevated in myasthenia gravis patients. Western blot results confirmed our hypothesis as serum MuSK protein level was significantly potentiated in patients (Figure 2A, p<0.05). Meanwhile IL-6 was also elevated in myasthenia gravis patients compared to healthy people (Figure 2B, p<0.05).
Figure 2.
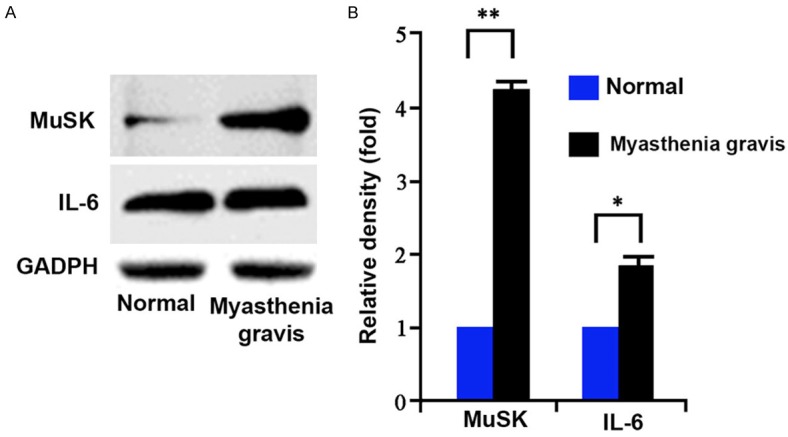
Serum MuSK and IL-6 levels. Total proteins were extracted from serum samples using protein extraction kit fo (A) western blot analysis of the expression of MuSK and IL-6 in serum samples from both normal and myasthenia gravis patients. (B) Both MuSK and IL-6 were up-regulated in patients. *, p<0.05, **, p<0.01 compared to normal group.
Regulation of IL-6 in myasthenia gravis by HSP90
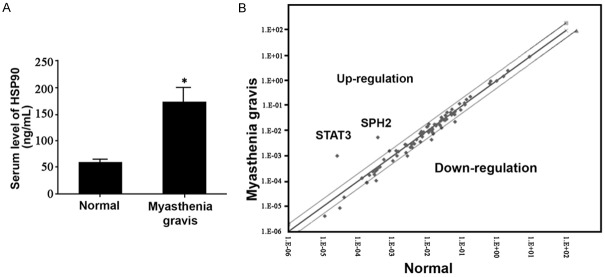
We detected the up-regulation of HSP90 in myasthenia gravis patients (Figure 3A). Using pathway finder kit for signal pathway screening, we found elevated expressions of STAT3 and SPH2 in myasthenia gravis patients (Figure 3B), suggesting the activation of IL-6 induced JAK/STAT signaling pathway.
Figure 3.
Participation of HSP90 in IL-6 in myasthenia gravis. A. Serum level of HSP90; B. The activation of IL-6 induced JAK/STAT signaling pathway.
Discussion
With the gradual elevation of incidence, myasthenia gravis has a complicated mechanism as multiple factors are involved, including the abnormality of cell proliferation, apoptosis, invasion and migration [15]. Acetylcholine has been studied as an important factor during the pathogenesis of myasthenia gravis. Recent study found the novel function of acetylcholine is to denature its receptor (AChR) [16], which directly affects the function of neuromuscular junction at the synapse between spinal cord motor neuron and muscle cells. The denature of AChR by acetylcholine is due to one calcium-dependent proteinase calpain, which can be activated by acetylcholine. In contrast, clusterin can stabilize the synaptic structure by inhibiting calpain [17].
How neurotransmitter can be localized at certain region at postsynaptic membrane is one critical step in the formation of synapse, with significant implication in understanding molecular mechanism of synaptic transmission [18]. Using neuromuscular junction as the model, we studied the accumulation of AChR and found the co-localization between MuSK and acetylcholine in myasthenia gravis patients. Moreover, serum MuSK level was potentiated in patients compared to control people, along with higher expression of IL-6 [19]. Molecular chaperon is one kind of protein providing energy for HSP90 through ATP hydrolysis. Recent study indicated the normal activity of telomerase mediated by IL-2 is relied ob the participation of HSP90 molecular chaperon [20]. Our study showed higher expression of serum HSP90 in myasthenia gravis patients as compared to normal group, in addition to enhanced expression of IL-6 signaling pathway-associated molecules including STAT3 and SPH2 in those patients. These results collectively suggested the correlation between HSP90 and IL-6 mediated signal pathway.
In summary, our study for the first time showed the co-localization between MuSK and acetylcholine in myasthenia gravis patients, with significantly elevated expression of IL-6 and MuSK in patients. Furthermore, we also showed the alternation molecular chaperon HSP90 mediated by IL-6. Future works should be focused on the interaction between IL-6 and HSP90. Our study provided evidences for the mechanism underlying the localization of neurotransmitter receptor molecule at the postsynaptic membrane, and suggested potential treatment strategy for neurodegenerative diseases.
Disclosure of conflict of interest
None.
References
- 1.Dong XP, Li XM, Gao TM, Zhang EE, Feng GS, Xiong WC, Mei L. Shp2 is dispensable in the formation and maintenance of the neuromuscular junction. Neurosignals. 2006;15:53–63. doi: 10.1159/000094484. [DOI] [PubMed] [Google Scholar]
- 2.Elias GM, Funke L, Stein V, Grant SG, Bredt DS, Nicoll RA. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron. 2006;52:307–320. doi: 10.1016/j.neuron.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Ferns M, Deiner M, Hall Z. Agrin-induced acetylcholine receptor clustering in mammalian muscle requires tyrosine phosphorylation. J Cell Biol. 1996;132:937–944. doi: 10.1083/jcb.132.5.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finn AJ, Feng G, Pendergast AM. Postsynaptic requirement for Abl kinases in assembly of the neuromuscular junction. Nat Neurosci. 2003;6:717–723. doi: 10.1038/nn1071. [DOI] [PubMed] [Google Scholar]
- 5.Frail DE, Musil LS, Buonanno A, Merlie JP. Expression of RAPsyn (43K protein) and nicotinic acetylcholine receptor genes is not coordinately regulated in mouse muscle. Neuron. 1989;2:1077–1086. doi: 10.1016/0896-6273(89)90232-8. [DOI] [PubMed] [Google Scholar]
- 6.Fu AK, Cheung ZH, Ip NY. Beta-catenin in reverse action. Nat Neurosci. 2008;11:244–246. doi: 10.1038/nn0308-244. [DOI] [PubMed] [Google Scholar]
- 7.Fuhrer C, Gautam M, Sugiyama JE, Hall ZW. Roles of rapsyn and agrin in interaction of postsynaptic proteins with acetylcholine receptors. J Neurosci. 1999;19:6405–6416. doi: 10.1523/JNEUROSCI.19-15-06405.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gautam M, Noakes PG, Mudd J, Nichol M, Chu GC, Sanes JR, Merlie JP. Failure of postsynaptic specialization to develop at neuromuscular junctions of rapsyn-deficient mice. Nature. 1995;377:232–236. doi: 10.1038/377232a0. [DOI] [PubMed] [Google Scholar]
- 9.Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, Sanes JR. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85:525–535. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- 10.Gerges NZ, Tran IC, Backos DS, Harrell JM, Chinkers M, Pratt WB, Esteban JA. Independent functions of hsp90 in neurotransmitter release and in the continuous synaptic cycling of AMPA receptors. J Neurosci. 2004;24:4758–4766. doi: 10.1523/JNEUROSCI.0594-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gervasio OL, Phillips WD. Increased ratio of rapsyn to ACh receptor stabilizes postsynaptic receptors at the mouse neuromuscular synapse. J Physiol. 2005;562:673–685. doi: 10.1113/jphysiol.2004.077685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glass DJ, Bowen DC, Stitt TN, Radziejewski C, Bruno J, Ryan TE, Gies DR, Shah S, Mattsson K, Burden SJ, DiStefano PS, Valenzuela DM, DeChiara TM, Yancopoulos GD. Agrin acts via a MuSK receptor complex. Cell. 1996;85:513–523. doi: 10.1016/s0092-8674(00)81252-0. [DOI] [PubMed] [Google Scholar]
- 13.Grady RM, Zhou H, Cunningham JM, Henry MD, Campbell KP, Sanes JR. Maturation and maintenance of the neuromuscular synapse: genetic evidence for roles of the dystrophin--glycoprotein complex. Neuron. 2000;25:279–293. doi: 10.1016/s0896-6273(00)80894-6. [DOI] [PubMed] [Google Scholar]
- 14.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 15.Herbst R, Burden SJ. The juxtamembrane region of MuSK has a critical role in agrin-mediated signaling. EMBO J. 2000;19:67–77. doi: 10.1093/emboj/19.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim S, Nelson PG. Involvement of calpains in the destabilization of the acetylcholine receptor clusters in rat myotubes. J Neurobiol. 2000;42:22–32. [PubMed] [Google Scholar]
- 17.Kim N, Burden SJ. MuSK controls where motor axons grow and form synapses. Nat Neurosci. 2008;11:19–27. doi: 10.1038/nn2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li XM, Dong XP, Luo SW, Zhang B, Lee DH, Ting AK, Neiswender H, Kim CH, Carpenter-Hyland E, Gao TM, Xiong WC, Mei L. Retrograde regulation of motoneuron differentiation by muscle beta-catenin. Nat Neurosci. 2008;11:262–268. doi: 10.1038/nn2053. [DOI] [PubMed] [Google Scholar]
- 19.Lin W, Burgess RW, Dominguez B, Pfaff SL, Sanes JR, Lee KF. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature. 2001;410:1057–1064. doi: 10.1038/35074025. [DOI] [PubMed] [Google Scholar]
- 20.Lin W, Dominguez B, Yang J, Aryal P, Brandon EP, Gage FH, Lee KF. Neurotransmitter acetylcholine negatively regulates neuromuscular synapse formation by a Cdk5-dependent mechanism. Neuron. 2005;46:569–579. doi: 10.1016/j.neuron.2005.04.002. [DOI] [PubMed] [Google Scholar]