Abstract
The objective of this study is to investigate the expression of microRNA (miR)-145 in human aortic vascular smooth muscle cells (VSMCs) and the effect of miR-145 in the biological behavior and expression of CD40 in VSMCs. Cells were treated with either miR-145 or miR-145 inhibitor. Cell proliferation was analyzed by a colony formation assay and a methyl thiazolyl tetrazolium assay. Cell migration and invasion were assessed using a transwell assay, an invasion assay, and a wound healing assay. A luciferase reporter assay was used to detect the interaction between miR-145 and CD40. Expression of α-SMA, calponin, osteopontin (OPN), epiregulin, activator protein-1 (AP-1) and CD40 was measured using real-time RT-PCR for mRNA levels and Western blotting for protein levels. Overexpression of miR-145 significantly inhibited VSMC proliferation, invasion and migration. Furthermore, OPN, epiregulin, AP-1 and CD40 expression at the mRNA and protein levels was down-regulated by overexpression of miR-145. However, α-SMA and calponin expression at the mRNA and protein levels was up-regulated by overexpression of miR-145. In addition, the luciferase reporter assay showed that CD40 may be a direct target gene of miR-145 in VSMC initiation and development. Moreover, these data demonstrate that the up-regulation of CD40 is critical for miR-145-mediated inhibitory effects on platelet-derived growth factor-induced cell proliferation and migration in human VSMCs. In summary, CD40, a direct target of miR-145, reverses the inhibitory effects of miR-145. These results suggest that the specific modulation of miR-145 in human VSMCs may be an attractive approach for the treatment of proliferative vascular diseases.
Keywords: microRNA-145, vascular smooth muscle cells, proliferation, migration, CD40, platelet-derived growth factor
Introduction
MicroRNAs (miRNAs) are short 18-24 nucleotide, single stranded, noncoding RNAs that bind to their complementary target sites in the 3-untranslated regions (3-UTRs) of specific mRNA targets to inhibit translation or cause mRNA degradation [1,2]. miRNAs are involved in complex processes related to diverse cellular functions, such as cell proliferation and differentiation, apoptosis, neuronal patterning, immunity, fat metabolism, and phenotypic switching of vascular smooth muscle cells (VSMCs) [3,4]. Recent studies indicated that many miRNAs are highly expressed in the vascular system and are involved in the control of proliferation and differentiation of VSMCs [5,6]. Moreover, overexpression of miR-145 up-regulates the expression of VSMC differentiation marker genes, such as alpha smooth muscle actin, calponin, and smooth muscle-myosin heavy chain (SM-MHC), thereby promoting the differentiation of VSMCs into the contractile phenotype [7]. In response to vascular injury or growth factor signaling, VSMCs dedifferentiate and adopt a synthetic phenotype, which is characterized by increased proliferation and migration, enhanced production of collagens and matrix metalloproteinases, and decreased expression of SMC specific contractile markers, such as OPN and epiregulin [8,9]. Recently, studies showed that the transcription factor AP-1 is critical and correlated with the initiation and progression of vascular dysfunction. Therefore, AP-1 inhibition has been proposed as an attractive target to prevent the progression of cardiovascular disease [10].
miRNA dysregulation is linked to the development of cardiovascular diseases, and restoration of dysregulated miRNAs to their normal levels can potentially reduce or even eliminate diseases, at least in animal models [11]. The dysregulation of several miRNAs is associated with many diseases, including cancer, cardiovascular diseases, diabetes and neurological disorders [12-15]. miRNA-based therapy has become a promising treatment for many human diseases, including cardiovascular diseases, cancer and diabetes.
Growth factor signaling pathways are involved in the phenotype modulation of VSMCs [16,17]. Platelet-derived growth factor (PDGF) is one of the most prominent inducers of smooth muscle cell (SMC) migration and proliferation. However, VSMC proliferation and migration are markedly increased in response to various growth factors and cytokines, such as platelet-derived growth factor-BB (PDGF-BB), fibroblast growth factor, insulin-like growth factor-1, tumor necrosis factor-alpha (TNF-a), and interleukin-1. PDGF-BB, which is released primarily by vascular endothelial cells and platelets at the sites of vascular injury, is one of the most potent stimulants of VSMC proliferation and migration via its modulation of several transcription factors and key molecular signaling pathways [18].
A recent report demonstrated that miR-145 is implicated in the modulation of VSMC differentiation [19]. miRNA expression can be transcriptionally induced by PDGF signaling to mediate its action on VSMC phenotypic switching [20]. Down-regulation of miR-15b decreased the expression of alpha-smooth muscle actin (α-SMA), a differentiation marker of VSMCs, suggesting that miR-15b possibly functions in the regulation of VSMC differentiation and proliferation [21]. miRNA-638 is highly expressed in human vascular smooth muscle cells and inhibits PDGF-BB-induced cell proliferation and migration by targeting the orphan nuclear receptor, NOR1 [22]. Moreover, miR-145 is important for the expression of the VSMC contractile phenotype [23,24] because it mediates the stretch-induced differentiation of VSMCs [25,26]. Functional CD40 is expressed not only by leukocytes but also by vascular VSMCs and endothelial cells (ECs) [27]. However, it is unclear whether miR-145 expression is modulated by PDGF, which is the essential signal for VSMC dedifferentiation. In this study, we assessed whether miR-145 is a key molecule for the regulation of VSMC proliferation and migration by targeting CD40.
Materials and methods
Cells
The human aortic smooth muscle cell line was purchased from Shanghai Cell Bank, Chinese Academy of Sciences (Shanghai, China). DMEM cell culture medium and 10% fetal bovine serum (FBS) were obtained from Gibco (Invitrogen Co, Carlsbad, CA, USA). The cell line was cultured in DMEM containing 10% FBS, 100 μg/ml streptomycin and 100 IU/ml penicillin in a humidified environment in the presence of 5% CO2. The miR-145 mimics and miR-145 inhibitor were synthesized by Tiangen Biochemical Technology Co., Ltd. (Beijing, China). For transfection, cells were grown to 90% confluence and transfected with miR-145 mimics and miR-145 inhibitor using Lipofectamine2000 (Invitrogen, Carlsbad, CA, USA) by incubation in DMEM for 4 h. The cells were then transferred into fresh DMEM containing 10% FBS.
Methyl thiazolyl tetrazolium (MTT) assay
Cell growth was determined using the methyl thiazolyl tetrazolium spectrophotometric dye assay according to published protocols [28]. At 24 h post-transfection with the miR-145 inhibitor, miR-145 mimic or negative control, VSMCs (2×103 cells/well) were plated into 96-well plates, and cell proliferation was documented at 24 h for 4 days. The number of viable cells was measured at 450 nm using a plate reader (Thermo Scientific, Watertown, USA). All data were expressed as mean ± standard deviations (SD) of three independent experiments. A two-tailed Student’s t-test was used for the comparison of differences between two independent groups.
Colony formation assay
VSMCs were transfected with 50 nM miR-145 inhibitor, miR-145 mimic or negative control, and cultured in DMEM containing 10% FBS. Cells were seeded at a density of 1×103 cells/well in a 6 cm Petri dish. After incubation for 15 days, cells were fixed with methanol and stained with 0.1% crystal violet. Visible colonies were manually counted. The experiment was repeated three times. All data were expressed as mean ± standard deviations (SD) of three independent experiments. A two-tailed Student’s t-test was used for the comparison of differences between two independent groups.
Wound healing assay
Cells were transfected with 50 nM miR-145 inhibitor, miR-145 mimic or negative control, which were plated into 12-well plates and incubated at room temperature with 5% CO2 until a complete monolayer was formed. A scratch was made in the cell monolayer, and then the medium was replaced with complete medium. The cells were washed and migration was assessed at 0 or 24 h. The level of migration in each group at 0 and 24 h was assessed using photographs. Migration was quantified by counting the total number of cells that migrated across the scratch towards the original wound field. The data were obtained from three independent wound healing experiments. All data were expressed as mean ± standard deviations (SD) of three independent experiments. A two-tailed Student’s t-test was used for the comparison of differences between two independent groups.
Migration and invasion assay
Cells were transfected with miR-145 inhibitor, miR-145 mimic or negative control. For transwell migration assay, cells were assessed using an 8-μm pore size transwell, and 5×104 cells were plated into each well of the migration chamber containing an uncoated membrane. For the transwell invasion assay, 3×105 cells were plated into the top chamber with a Matrigel-coated membrane. The transwell assay was also performed using a 24-well cell transwell assay kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions, with experiments performed in triplicate for each condition. All data were expressed as mean ± standard deviations (SD) of three independent experiments. A two-tailed Student’s t-test was used for the comparison of differences between two independent groups.
Luciferase reporter assay
Cells cultured in 6-well plates were transfected with 1 μg pMIR/activator CD40 vector or pMIR/CD40/mut vector containing firefly luciferase, or 0.05 μg pRLTK vector (Invitrogen, Carlsbad, CA, USA) containing Renilla luciferase and 30 nM miR-145 or negative control. Cells were treated in 24-well plates with miR-145 inhibitor or miR-145 mimic plasmids using Lipofectamine 2000. After transfection for 6 h, cells were transfected again with miR-145 inhibitor, miR-145 mimic or negative control. Luciferase activity was evaluated with the dual luciferase assay system (Promega, CA, USA) after 36 h incubation. All data were expressed as mean ± standard deviations (SD) of three independent experiments. A two-tailed Student’s t-test was used for the comparison of differences between two independent groups.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted and isolated from the cells using Trizol reagent (Invitrogen, Carlsbad, CA, USA) for both miR-145 and CD40 mRNA analyses. miR-145 expression was detected using qRT-PCR with SYBR Premix Ex Taq™ (Invitrogen, CA, USA) according to the manufacturer’s protocol. For the detection of CD40 mRNA expression, qRT-PCR was performed using Quantities SYBR Green PCR Kit (Invitrogen, CA, USA). β-actin was used to normalize the CD40 mRNA expression levels. All qRT-PCR experiments were performed in triplicate for each condition. All data were expressed as mean ± standard deviations (SD) of three independent experiments. A two-tailed Student’s t-test was used for the comparison of differences between two independent groups. One-way ANOVA was to compare the differences among multiple groups and post hoc Bonferroni correction was conducted for multiple comparisons.
Western blotting
Western blotting was performed as previously described [29]. Total protein was extracted from cells treated with miR-145 inhibitor, miR-145 mimic or negative control. The lysates were separated on 12% sodium dodecyl sulfate-polyacrylamide gels, and protein quantification was performed using a bicinchoninic acid kit (Invitrogen, CA, USA). The proteins were then transferred to polyvinylidene difluoride (PVDF) membranes (Invitrogen, CA, USA), which were blocked for 1 h with 5% non-fat milk at 37°C with agitation. The membranes were incubated with primary antibody at 4°C overnight. After blocking with 5% non-fat milk at room temperature for 1 h, the membranes were incubated with primary antibodies for CD40 (1:3000 dilution) and β-actin (1:3000), and β-actin was the internal reference. Next, the membranes were washed three times for 5 minutes each. After 3 washes, the PVDF membranes were incubated with secondary antibodies (Invitrogen, CA, USA) at 37°C for 1 h. The blots were visualized using the electrochemiluminescence detection system (Invitrogen, CA, USA). All data were expressed as mean ± standard deviations (SD) of three independent experiments. A two-tailed Student’s t-test was used for the comparison of differences between two independent groups. One-way ANOVA was to compare the differences among multiple groups and post hoc Bonferroni correction was conducted for multiple comparisons.
Statistical analysis
All data were analyzed and all graphs were plotted using SPSS 17.0 software (Chicago, IL, USA). All data were expressed as mean ± standard deviations (SD) of three independent experiments. A two-tailed Student’s t-test was used for the comparison of differences between two independent groups. One-way ANOVA was to compare the differences among multiple groups and post hoc Bonferroni correction was conducted for multiple comparisons. P < 0.05 was considered to indicate a statistically significant difference All experiments were performed in triplicate, and P < 0.05 was considered statistically significant.
Results
Up-regulation of miR-145 significantly inhibits VSMC growth
To determine whether overexpression of miR-145 in VSMCs affects cell growth, an MTT assay was used to determine cell proliferation. The data showed that VSMCs treated with the miR-145 inhibitor grew more rapidly than the control group, whereas cells overexpressing miR-145 grew more slowly than the control (Figure 1A). A colony formation assay showed that VSMCs transfected with the miR-145 inhibitor had a higher level of proliferation compared to cells transfected with control or overexpressing miR-145 (Figure 1B). These data indicate that overexpression of miR-145 inhibits VSMC proliferation in vitro. The wound healing assay results showed that higher levels of wound repair occurred in cells transfected with miR-145 inhibitor for 12 h or 24 h (Figure 2A, 2B). The results showed that cells transfected with the miR-145 inhibitor had higher levels of migration and invasion compared to those treated with control or miR-145 (Figure 3A, 3B). These data indicate that overexpression of miR-145 inhibits VSMC migration and invasion in vitro.
Figure 1.
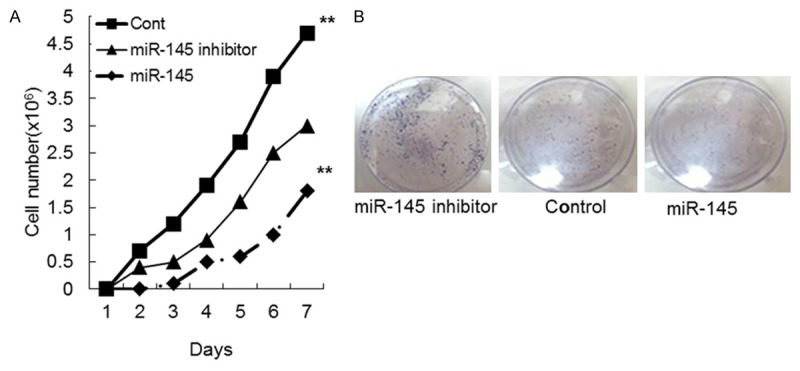
Vascular smooth muscle cell growth in miR-145 mimics, miR-145 inhibitor or control mimics groups. A. Proliferation curve of cells stably transfected with miR-145 mimics, miR-145 inhibitor or control mimics. B. Colony formation of cells transfected with miR-145 mimics, miR-145 inhibitor or control mimics.
Figure 2.
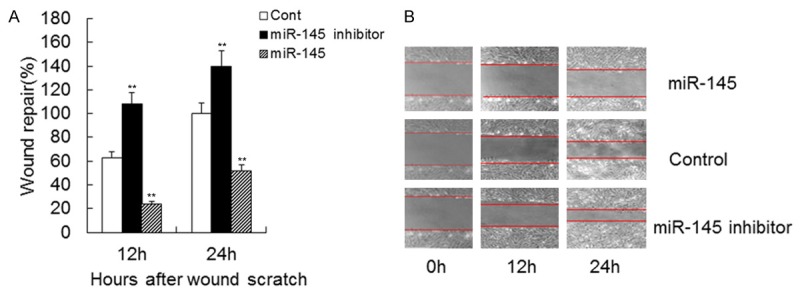
Effect of miR-145 on VSMC migration. Migration of VSMCs transfected with miR-145 mimics, miR-145 inhibitor or control mimics. Quantification of wound repair at 0, 12 and 24 h after wound scratch in wound-healing assay. Data are shown as means ± SD. **, P < 0.01 compared with control.
Figure 3.
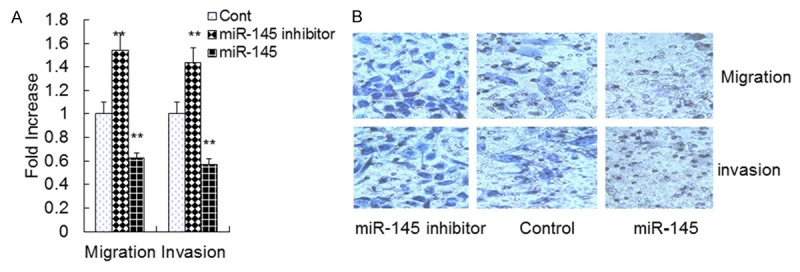
Effect of miR-145 on VSMC migration, invasion. A and B. Migration and invasion of VSMCs transfected with miR-145 mimics, miR-145 inhibitor or control mimics. Transwell assay was performed. Data are means ± SD. **, P <0.01 compared with control.
Up-regulated expression of miR-145 significantly decreases OPN and epiregulin mRNA and protein levels in VSMCs
To test the effect of miR-145 on OPN and epiregulin mRNA and protein expression, qRT-PCR and Western blotting were performed as previously described. The qRT-PCR results revealed that the miR-145 inhibitor increased OPN and epiregulin mRNA expression by 52.00% (1.52 ± 0.15 vs. 1.0 ± 0.09) and 43.00% (1.43 ± 0.14 vs. 1.0 ± 0.10) compared to the control (P < 0.01), respectively. By contrast, overexpression of miR-145 decreased OPN and epiregulin mRNA expression by 47.00% (0.53 ± 0.06 vs. 1.0 ± 0.09) and 58% (0.42 ± 0.04 vs. 1.0 ± 0.09) compared to the control (P < 0.01) (Figure 4A, 4C), respectively. Western blotting results revealed that the miR-145 inhibitor increased the level of OPN and epiregulin protein by 57.00% (1.57 ± 0.14 vs. 1.0 ± 0.09) and 65.00% (1.65 ± 0.15 vs. 1.0 ± 0.10) compared to the control (P < 0.01), respectively. By contrast, overexpression of miR-145 decreased OPN and epiregulin protein expression by 42.00% (0.58 ± 0.07 vs. 1.0 ± 0.09) and 43.00% (0.57 ± 0.06 vs. 1.0 ± 0.10) compared to the control (P < 0.01) (Figure 4B, 4D, 4E), respectively.
Figure 4.
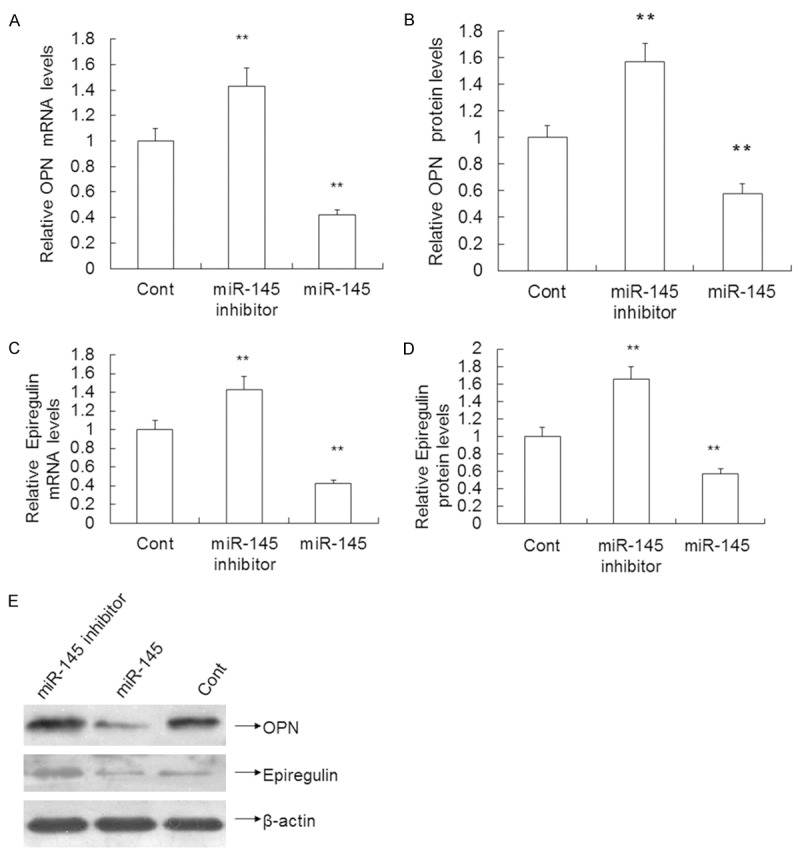
Effect of miR-145 on OPN, Epiregulin expression in VSMCs transfected with miR-145 mimics, miR-145 inhibitor or control mimics. A and C. The level of OPN, Epiregulin mRNA determined using qRT-PCR. B and D. OPN, Epiregulin protein expression measured using Western blotting. E. Quantification of OPN, Epiregulin protein expression. Data are means ± SD. **, P < 0.01 compared with control.
Up-regulated expression of miR-145 significantly increases α-SMA and calponin mRNA and protein levels in VSMCs
To test the effect of miR-145 on α-SMA and calponin mRNA and protein expression, qRT-PCR and Western blotting were performed as previously described. The qRT-PCR results revealed that the miR-145 inhibitor decreased α-SMA and calponin mRNA expression by 45.00% (0.55 ± 0.05 vs. 1.0 ± 0.08) and 37.00% (0.63 ± 0.06 vs. 1.0 ± 0.10) compared to the control (P < 0.01), respectively. By contrast, overexpression of miR-145 increased α-SMA and calponin mRNA expression by 53.00% (1.53 ± 0.13 vs. 1.0 ± 0.08) and 63.00% (1.63 ± 0.15 vs. 1.0 ± 0.10) compared to the control (P < 0.01) (Figure 5A, 5C), respectively. Western blotting results revealed that the miR-145 inhibitor decreased the level of α-SMA and calponin protein by 40.00% (0.60 ± 0.07 vs. 1.0 ± 0.09) and 55.00% (0.45 ± 0.05 vs. 1.0 ± 0.09) compared to the control (P < 0.01), respectively. By contrast, overexpression of miR-145 increased α-SMA protein levels by 58.00% (1.58 ± 0.16 vs. 1.0 ± 0.09) and 60.00% (1.60 ± 0.16 vs. 1.0 ± 0.09) compared to the control (P < 0.01) (Figure 5B, 5D, 5E).
Figure 5.
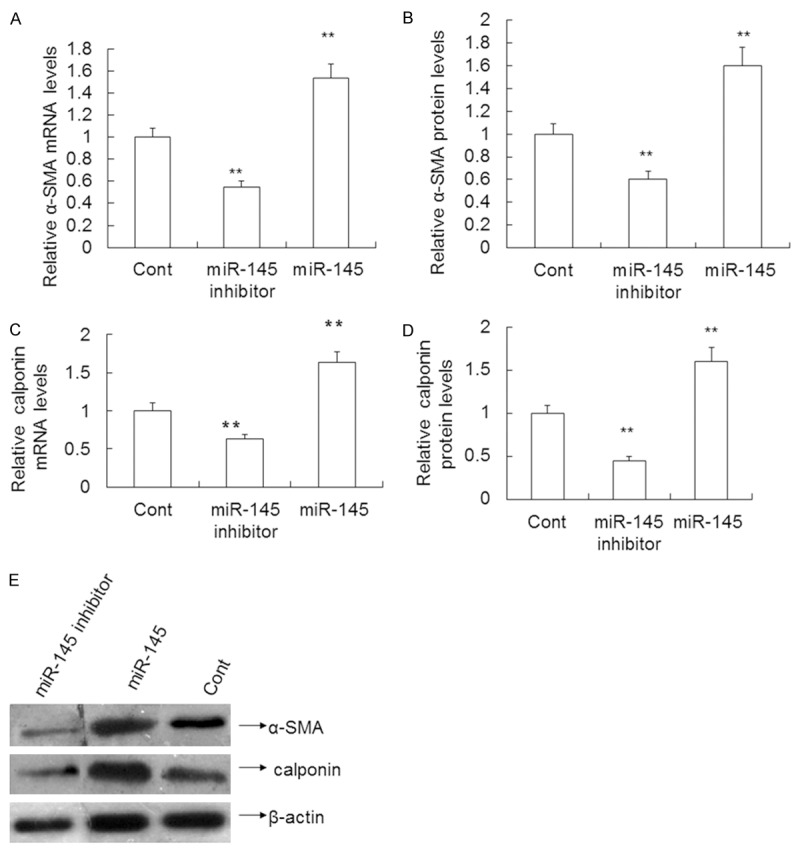
Effect of miR-145 on α-SMA, calponin expression in VSMCs transfected with miR-145 mimics, miR-145 inhibitor or control mimics. A and C. The level of α-SMA,calponin mRNA determined using qRT-PCR. B and D. α-SMA, calponin protein expression measured using Western blotting. E. Quantification of α-SMA, calponin protein expression. Data are means ± SD. **, P < 0.01 compared with control.
Up-regulated expression of miR-145 significantly reduces CD40 mRNA and protein levels in VSMCs
To test the effect of miR-145 on CD40 mRNA and protein expression, qRT PCR was performed and revealed that the miR-145 inhibitor increased the CD40 mRNA level by 64.00% (1.64 ± 0.12 vs. 1.0 ± 0.10) compared to the control (P < 0.01). By contrast, overexpression of miR-145 decreased the CD40 mRNA levels by 45.00% (0.55 ± 0.05 vs. 1.0 ± 0.10) compared to the control (P < 0.01) (Figure 6A). Western blotting results revealed that the miR-145 inhibitor increased the CD40 protein expression by 55.00% (1.55 ± 0.13 vs. 1.0 ± 0.10) compared to the control (P < 0.01). By contrast, overexpression of miR-145 decreased the CD40 protein levels by 28.00% (0.72 ± 0.07 vs. 1.0 ± 0.10) compared to the control (P < 0.01) (Figure 6B, 6C). These results indicate that overexpression of miR-145 decreases the expression of CD40 mRNA and CD40 protein levels.
Figure 6.
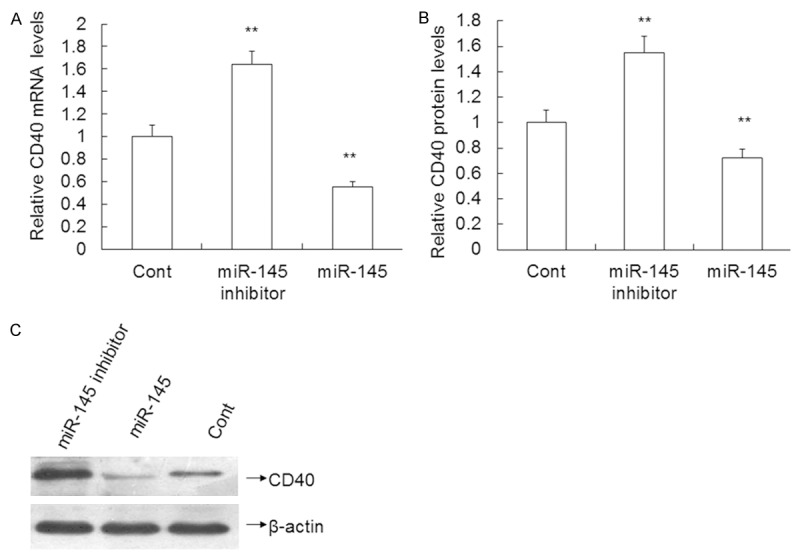
Effect of miR-145 on CD40 expression in VSMCs transfected with miR-145 mimics, miR-145 inhibitor or control mimics. A. The level of CD40 mRNA determined using qRT-PCR. B and C.CD40 protein expression measured using Western blotting. Data are means ± SD. **, P < 0.01 compared with control.
miR-145 regulates CD40 expression by directly targeting the 3’-UTR of CD40 mRNA
To determine the mechanism by which miR-145 effects VSMCs, we identified its potential target genes. Among these genes, CD40 plays a key role in the signaling pathway that modulates VSMC proliferation, migration and apoptosis. To evaluate CD40 as a target gene of miR-145 in VSMCs, wt or mutant reporter plasmids were cotransfected into VSMCs together with miR-145 or control. miR-145 significantly inhibited the activity when cotransfected with the wt reporter plasmid compared to the control (0.74 ± 0.08 vs. 1.83 ± 0.13, P < 0.01) (Figure 7). When miR-145 and control were treated with the mutant reporter plasmid, there was no significant difference in relative luciferase activity between the two groups (1.76 ± 0.16 vs. 1.83 ± 0.17, P > 0.05) (Figure 5). These data reveal that miR-145 regulates CD40 expression by targeting the 3’-UTR of the CD40 mRNA.
Figure 7.
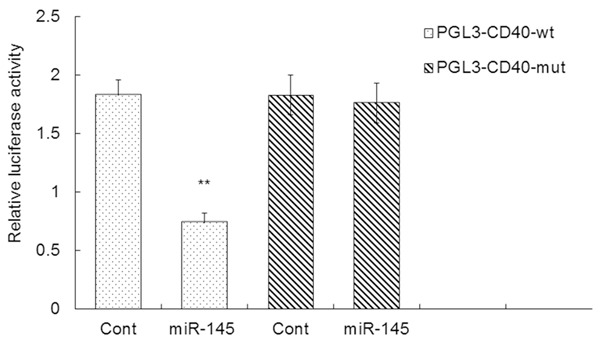
Relative luciferase activity in the detection of miR-145 binding to 3’-UTR of CD40 mRNA in VSMCs. The miR-145 reporter plasmid was transfected into VSMCs together with CD40 or negative control. **, P < 0.01 compared with control in PGL3-CD40-wt.
miR-145 inhibits cell proliferation induced by PDGF
To test the effect of miR-145 on cell proliferation induced by PDGF, VSMCs were treated with miR-145 mimics, miR-145 inhibitor or control and were analyzed using the MTT assay. Overexpression of miR-145 inhibited cell proliferation induced by PDGF by 22.11% (74 ± 6 vs. 95 ± 9, P < 0.01) compared to miR-145 alone. Co-transfection with miR-145 and CD40 inhibited cell proliferation induced by PDGF by 29.73% (52 ± 5 vs. 74 ± 6, P < 0.05) (Figure 8). These results indicate that miR-145 is responsible for cell proliferation stimulated by PDGF.
Figure 8.
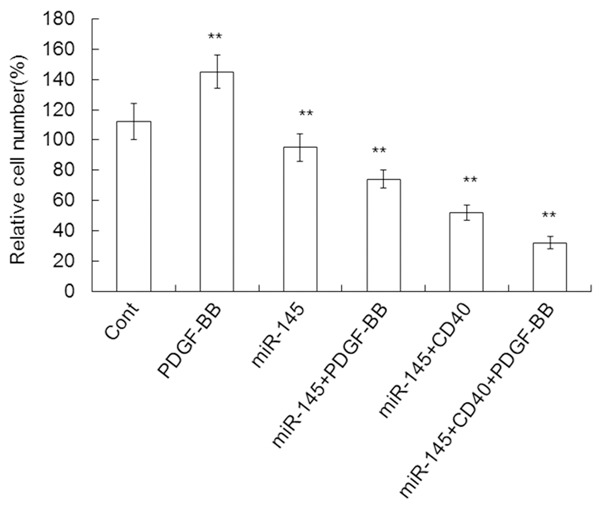
Proliferation of cells stably transfected with control mimics, PDGF, miR-145mimics, miR-145+PDGF-BB, miR-145+CD40, or miR-145+CD40+ PDGF-BB. Data are means ± SD. **, P < 0.01 compared with control.
Cell proliferation stimulated by PDGF inhibits the levels of epiregulin and AP-1 mRNA and protein in VSMCs
To test whether the effects of miR-145 on epiregulin and AP-1 are related to cell proliferation induced by PDGF, miR-145 was cotransfected with CD40 into VSMCs. The data revealed that the epiregulin and AP-1 mRNA level in the presence of miR-145 and PDGF was decreased by 26.39% (0.53 ± 0.06 vs. 0.72 ± 0.16, P < 0.05), 47.00% and 15.38% (0.55 ± 0.05 vs. 0.65 ± 0.06, P < 0.05) compared to miR-145 alone or PDGF alone, with 63.45% (0.53 ± 0.06 vs. 1.45 ± 0.13, P < 0.05) and 75.86% (0.55 ± 0.05 vs. 1.65 ± 0.15, P < 0.05), respectively (Figure 8). The epiregulin and AP-1 protein levels gave similar results. The data revealed that the epiregulin and AP-1 protein levels in the presence of miR-145 and PDGF were significantly decreased by 15.58% (0.65 ± 0.05 vs. 0.77 ± 0.07, P < 0.05) and 31.03% (0.60 ± 0.05 vs. 0.87 ± 0.09, P < 0.05) compared to miR-145 alone or PDGF alone, with 58.06% (0.65 ± 0.05 vs. 1.55 ± 0.15, P < 0.05) and 59.18% (0.60 ± 0.05 vs. 1.47 ± 0.14, P < 0.05), respectively. The results also showed that the epiregulin and AP-1 mRNA levels in the presence of miR-145, CD40 and PDGF were decreased by 33.96% (0.35 ± 0.03 vs. 0.53 ± 0.06, P < 0.05) and 50.91% (0.27 ± 0.03 vs. 0.55 ± 0.05, P < 0.05) compared to miR-145 and PDGF (P < 0.05), respectively (Figure 9A, 9C). Similarly, the epiregulin and AP-1 protein levels in the presence of miR-145, CD40 and PDGF were significantly decreased by 30.77% (0.45 ± 0.03 vs. 0.65 ± 0.05, P < 0.05) and 58.33% (0.25 ± 0.03 vs. 0.60 ± 0.05, P < 0.05) compared to miR-145 and PDGF, respectively (Figure 9B, 9D, 9E). These data suggest that cell proliferation stimulated by PDGF decreases epiregulin and AP-1 mRNA and protein expression.
Figure 9.
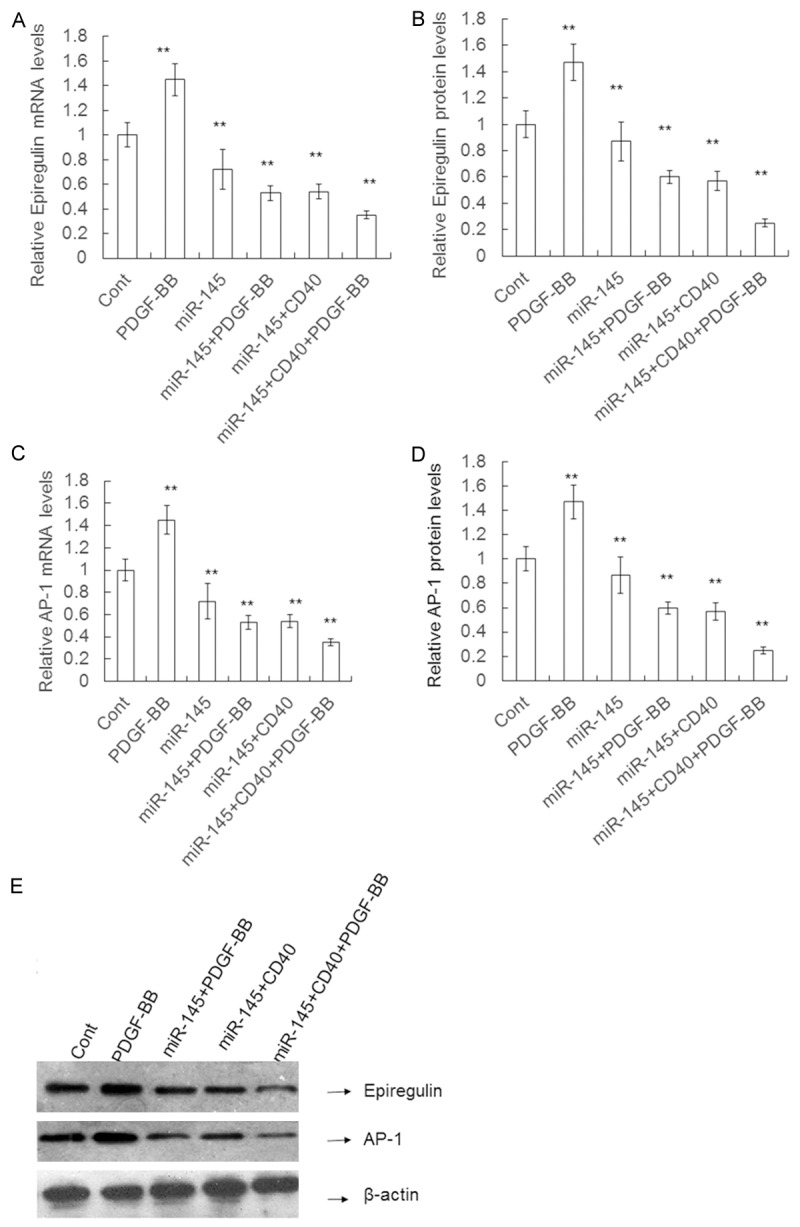
The Epiregulin, AP-1 expression in the presence of transient CD40 expression. VSMCs were transfected with the control mimics, PDGF, miR-145 mimics, miR-145+PDGF-BB, miR-145+CD40, or miR-145+CD40+PDGF-BB. A and C. The relative level of Epiregulin, AP-1 mRNA determined using qRT-PCR. B and D. The Epiregulin, AP-1 protein expression measured using Western blotting. E. Quantification of Epiregulin, AP-1 protein expression. Data are means ± SD. **, P < 0.01 compared with control.
Discussion
MicroRNAs (miRNAs) are short 18-24 nucleotide, single stranded, noncoding RNAs that bind to their complementary target sites in the 3-untranslated regions (3-UTRs) of specific mRNA targets to inhibit translation or cause mRNA degradation [2,30]. miRNAs play important roles in regulating diverse cellular processes, including proliferation, apoptosis, migration and invasion [31,32]. Moreover, miR-145 is essential for VSMC differentiation [33,34]. However, miR-145 expression and function in VSMCs are unclear. To investigate miRNA function, functional targets are identified. This involves the analysis of changes in target proteins following the dysregulation of a function of the specific miRNA. In this study, we investigated overexpressed miR-145 in VSMCs and its effects. We found that overexpression of miR-145 significantly inhibited the growth, invasion and migration of VSMCs in vitro. Additionally, overexpression of miR-145 inhibited OPN, epiregulin and CD40 expression at both the mRNA and protein levels in VSMCs. Moreover, CD40 was a direct target of miR-145. Furthermore, the effects of miR-145 on VSMC proliferation and OPN and AP-1 expression were reversed by CD40.
Recently, the inhibitory role of miR-145 in VSMC proliferation and eNOS expression was demonstrated [35]. In this study, we found that miR-145 significantly inhibited VSMC proliferation. In addition, OPN and epiregulin expression were significantly decreased. However, α-SMA and calponin expression were significantly increased. These effects were partially reversed in VSMCs treated with miR-145. Therefore, our results indicate the inhibitory effects of miR-145 on VSMC proliferation and α-SMA, calponin, OPN, and epiregulin expression. Moreover, overexpression of miR-145 significantly inhibited the invasion and migration of VSMCs. These results suggest that miR-145 may be involved in VSMC processes. The mechanism underlying the effects of miR-145 on cell invasion and migration must be further elucidated, such as whether other CD40 plays in the role in miR-145-mediated regulation of its target gene (s).
miRNAs can regulate the expression of TFs [36,37]. Consistently in this study, we found that the mRNA and protein expression of CD40 was inhibited by the overexpression miR-145, and this inhibition was alleviated when cells were transfected with miR-145. Moreover, the luciferase reporter results showed that the CD40 mRNA was the direct target of miR-145. Our previous studies also provide further evidence that miR-21 regulates VSMC proliferation by inhibiting the transcription factor AP-1 [38]. Therefore, we hypothesize that CD40 may also be involved in one of the mechanisms underlying the effects of miR-145.
To further verify this hypothesis, we introduced CD40 into VSMCs overexpressing miR-145. To stimulate VSMC proliferation and OPN and AP-1 expression, cells were treated with PDGF. Then, VSMC proliferation and OPN and AP-1 expression were analyzed. The results showed that PDGF stimulated the proliferation of VSMCs and the expression of OPN and AP-1. However, these effects of PDGF were inhibited by miR-145 overexpression, which further confirmed the inhibitory effect of miR-145. Interestingly, we demonstrated that the effect of miR-145 on CD40 was related to VSMC proliferation stimulated by PDGF. These results suggest that CD40 reverses the inhibitory effects of miR-145 on VSMC proliferation and PDGF expression.
In summary, we demonstrated that the up-regulation of miR-145 plays a role in VSMC proliferation and migration by suppression of the miR-145 target, CD40 and that miR-145 directly bound to CD40. These results suggest that miR-145 may exert its effects through targeting CD40. Based on our findings, we propose that miR-145 regulation of CD40 will be a new therapy for vascular diseases.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 81260634), the Research Foundation of the Education Department of Guangxi Province, China (No. 2013GXNSFAA019204). and Guangxi Natural Science Foundation (No. GXNSFCA 053005).
Disclosure of conflict of interest
None.
References
- 1.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Hata A. Functions of microRNAs in cardiovascular biology and disease. Annu Rev Physiol. 2013;75:69–93. doi: 10.1146/annurev-physiol-030212-183737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 4.Rangrez AY, Massy ZA, Metzinger-Le Meuth V, Metzinger L. miR-143 and miR-145: molecular keys to switch the phenotype of vascular smooth muscle cells. Circ Cardiovasc Genet. 2011;4:197–205. doi: 10.1161/CIRCGENETICS.110.958702. [DOI] [PubMed] [Google Scholar]
- 5.Quiat D, Olson EN. MicroRNAs in cardiovascular disease: from pathogenesis to prevention and treatment. J Clin Invest. 2013;123:11–18. doi: 10.1172/JCI62876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albinsson S, Sward K. Targeting smooth muscle microRNAs for therapeutic benefit in vascular disease. Pharmacol Res. 2013;75:28–36. doi: 10.1016/j.phrs.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, Deitch EA, Huo Y, Delphin ES, Zhang C. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. 2009;105:158–166. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi M, Hayashi K, Yoshida K, Ohkawa Y, Komurasaki T, Kitabatake A, Ogawa A, Nishida W, Yano M, Monden M, Sobue K. Epiregulin as a major autocrine/paracrine factor released from ERK- and p38MAPK-activated vascular smooth muscle cells. Circulation. 2003;108:2524–2529. doi: 10.1161/01.CIR.0000096482.02567.8C. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Ren Y, Kang L, Zhang L. Oxidized lowdensity lipoprotein increases the proliferation and migration of human coronary artery smooth muscle cells through the upregulation of osteopontin. Int J Mol Med. 2014;33:1341–1347. doi: 10.3892/ijmm.2014.1681. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, An FS, Zhang W, Gong L, Wei SJ, Qin WD, Wang XP, Zhao YX, Zhang Y, Zhang C, Zhang MX. Inhibition of c-Jun N-terminal kinase attenuates low shear stress-induced atherogenesis in apolipoprotein E-deficient mice. Mol Med. 2011;17:990–999. doi: 10.2119/molmed.2011.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wahid F, Shehzad A, Khan T, Kim YY. MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochim Biophys Acta. 2010;1803:1231–1243. doi: 10.1016/j.bbamcr.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Saiselet M, Gacquer D, Spinette A, Craciun L, Decaussin-Petrucci M, Andry G, Detours V, Maenhaut C. New global analysis of the microRNA transcriptome of primary tumors and lymph node metastases of papillary thyroid cancer. BMC Genomics. 2015;16:828. doi: 10.1186/s12864-015-2082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milenkovic D. MicroRNAs as novel nutrigenomic targets for cardiovascular health. Free Radic Biol Med. 2014;75(Suppl 1):S11. doi: 10.1016/j.freeradbiomed.2014.10.856. [DOI] [PubMed] [Google Scholar]
- 14.Al-Kafaji G, Al-Mahroos G, Alsayed NA, Hasan ZA, Nawaz S, Bakhiet M. Peripheral blood microRNA-15a is a potential biomarker for type 2 diabetes mellitus and pre-diabetes. Mol Med Rep. 2015;12:7485–7490. doi: 10.3892/mmr.2015.4416. [DOI] [PubMed] [Google Scholar]
- 15.Gupta S, Verma S, Mantri S, Berman NE, Sandhir R. Targeting MicroRNAs in Prevention and Treatment of Neurodegenerative Disorders. Drug Dev Res. 2015;76:397–418. doi: 10.1002/ddr.21277. [DOI] [PubMed] [Google Scholar]
- 16.Lagna G, Ku MM, Nguyen PH, Neuman NA, Davis BN, Hata A. Control of phenotypic plasticity of smooth muscle cells by bone morphogenetic protein signaling through the myocardin-related transcription factors. J Biol Chem. 2007;282:37244–37255. doi: 10.1074/jbc.M708137200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Putta S, Lanting L, Sun G, Lawson G, Kato M, Natarajan R. Inhibiting microRNA-192 ameliorates renal fibrosis in diabetic nephropathy. J Am Soc Nephrol. 2012;23:458–469. doi: 10.1681/ASN.2011050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millette E, Rauch BH, Kenagy RD, Daum G, Clowes AW. Platelet-derived growth factor-BB transactivates the fibroblast growth factor receptor to induce proliferation in human smooth muscle cells. Trends Cardiovasc Med. 2006;16:25–28. doi: 10.1016/j.tcm.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Riches K, Alshanwani AR, Warburton P, O’Regan DJ, Ball SG, Wood IC, Turner NA, Porter KE. Elevated expression levels of miR-143/5 in saphenous vein smooth muscle cells from patients with Type 2 diabetes drive persistent changes in phenotype and function. J Mol Cell Cardiol. 2014;74:240–250. doi: 10.1016/j.yjmcc.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quintavalle M, Elia L, Condorelli G, Courtneidge SA. MicroRNA control of podosome formation in vascular smooth muscle cells in vivo and in vitro. J Cell Biol. 2010;189:13–22. doi: 10.1083/jcb.200912096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S, Kang H. miR-15b induced by platelet-derived growth factor signaling is required for vascular smooth muscle cell proliferation. BMB Rep. 2013;46:550–554. doi: 10.5483/BMBRep.2013.46.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li P, Liu Y, Yi B, Wang G, You X, Zhao X, Summer R, Qin Y, Sun J. MicroRNA-638 is highly expressed in human vascular smooth muscle cells and inhibits PDGF-BB-induced cell proliferation and migration through targeting orphan nuclear receptor NOR1. Cardiovasc Res. 2013;99:185–193. doi: 10.1093/cvr/cvt082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, Braun T. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. 2009;119:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu B, Song JT, Qu HY, Bi CL, Huang XZ, Liu XX, Zhang M. Mechanical stretch suppresses microRNA-145 expression by activating extracellular signal-regulated kinase 1/2 and upregulating angiotensin-converting enzyme to alter vascular smooth muscle cell phenotype. PLoS One. 2014;9:e96338. doi: 10.1371/journal.pone.0096338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turczynska KM, Sadegh MK, Hellstrand P, Sward K, Albinsson S. MicroRNAs are essential for stretch-induced vascular smooth muscle contractile differentiation via microRNA (miR)-145-dependent expression of L-type calcium channels. J Biol Chem. 2012;287:19199–19206. doi: 10.1074/jbc.M112.341073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song Z, Jin R, Yu S, Nanda A, Granger DN, Li G. Crucial role of CD40 signaling in vascular wall cells in neointimal formation and vascular remodeling after vascular interventions. Arterioscler Thromb Vasc Biol. 2012;32:50–64. doi: 10.1161/ATVBAHA.111.238329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao Q, Xu H, Zhang QQ, Zhou H, Qu LH. MicroRNA-21 promotes cell proliferation and down-regulates the expression of programmed cell death 4 (PDCD4) in HeLa cervical carcinoma cells. Biochem Biophys Res Commun. 2009;388:539–542. doi: 10.1016/j.bbrc.2009.08.044. [DOI] [PubMed] [Google Scholar]
- 29.Ou H, Shen YH, Utama B, Wang J, Wang X, Coselli J, Wang XL. Effect of nuclear actin on endothelial nitric oxide synthase expression. Arterioscler Thromb Vasc Biol. 2005;25:2509–2514. doi: 10.1161/01.ATV.0000189306.99112.4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang H, Hata A. MicroRNA regulation of smooth muscle gene expression and phenotype. Curr Opin Hematol. 2012;19:224–231. doi: 10.1097/MOH.0b013e3283523e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizuguchi Y, Takizawa T, Yoshida H, Uchida E. Dysregulated microRNAs in progression of hepatocellular carcinoma: A systematic review. Hepatol Res. 2015 doi: 10.1111/hepr.12606. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Choi YC, Yoon S, Byun Y, Lee G, Kee H, Jeong Y, Yoon J, Baek K. MicroRNA library screening identifies growth-suppressive microRNAs that regulate genes involved in cell cycle progression and apoptosis. Exp Cell Res. 2015;339:320–332. doi: 10.1016/j.yexcr.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Xin M, Small EM, Sutherland LB, Qi X, McAnally J, Plato CF, Richardson JA, Bassel-Duby R, Olson EN. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009;23:2166–2178. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elia L, Quintavalle M, Zhang J, Contu R, Cossu L, Latronico MV, Peterson KL, Indolfi C, Catalucci D, Chen J, Courtneidge SA, Condorelli G. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ. 2009;16:1590–1598. doi: 10.1038/cdd.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hobert O. Gene regulation by transcription factors and microRNAs. Science. 2008;319:1785–1786. doi: 10.1126/science.1151651. [DOI] [PubMed] [Google Scholar]
- 36.Makeyev EV, Maniatis T. Multilevel regulation of gene expression by microRNAs. Science. 2008;319:1789–1790. doi: 10.1126/science.1152326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, Zhao F, Yu X, Lu X, Zheng G. MicroRNA-155 modulates the proliferation of vascular smooth muscle cells by targeting endothelial nitric oxide synthase. Int J Mol Med. 2015;35:1708–1714. doi: 10.3892/ijmm.2015.2181. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Yan L, Zhang W, Hu N, Chen W, Wang H, Kang M, Ou H. MicroRNA-21 inhibits platelet-derived growth factor-induced human aortic vascular smooth muscle cell proliferation and migration through targeting activator protein-1. Am J Transl Res. 2014;6:507–516. [PMC free article] [PubMed] [Google Scholar]


