Abstract
This study aims to investigate the role of endocytoplasmic reticulum (ER) stress induced by spinal cord injury (SCI) in blood-spinal cord barrier (BSCB) disruption and the effect of phenylbutyrate (PBA) on BSCB disruption after SCI. After a moderate contusion injury at the T9 level of spinal cord with a vascular clip, PBA was immediately administered into injured rat via intraperitoneal injection (100 mg/kg) and then further treated once a day for 2 weeks for behavior test. Spinal cord was collected at 1 day post-injury for evaluation of the effects of ER stress and PBA on BSCB disruption after SCI. PBA significantly attenuated BSCB permeability and degradation of tight junction molecules such as P120, β-catenin, Occludin and Claudin5 at 1 day after injury and improved functional recovery in the rat model of trauma. The BSCB protective effect of PBA is related to the inhibition of ER stress induced by SCI. In addition, PBA significantly inhibited the increase of ER stress markers and prevents loss of tight junction and adherens junction proteins in TG-treated human brain microvascular endothelial cells (HBMEC). Taken together, our data demonstrate that therapeutic strategies targeting ER stress may be suitable for the therapy of preserving BSCB integrity after SCI. PBA may be a new candidate as a therapeutic agent for protecting SCI by a compromised BSCB.
Keywords: Blood-spinal cord barrier, phenylbutyrate, ER stress, spinal cord injury
Introduction
Blood-spinal cord barrier (BSCB) is composed of highly specialized brain endothelial cells (BMECs), which form a tight seal due to the presence of well-developed tight junction (TJ) that form an almost impenetrable barrier and play a critical role in maintaining the integrity of the BSCB [1,2]. BSCB plays a key role in central nervous system (CNS) homeostasis by limiting the entry of plasma components and blood cells into the spinal cord, thus the BSCB is necessary and especially important for the highly precise control of the microenvironment [3]. Impairment of the BSCB may induce spinal cord edema and allow inflammatory cells to enter the injury site, resulting in secondary injury in SCI, resulting the ‘programmed death’ of neurons and glia, leading to permanent neurological deficits [4]. Thus, by targeting the BSCB disruption should be considered as a potential approach for therapeutic interventions after SCI.
Activation of endoplasmic reticulum (ER) stress is involved in the injured spinal cord acutely following traumatic SCI [5]. Accumulation of unfolded proteins in the ER lumen, inhibition of protein synthesis depletion of Ca2+ from ER stores, activation of CHOP expression and caspase-12, eventually result in neural apoptosis [6,7]. A growing body of studies focusing on ER stress in various cells (neuron, astrocytes, oligodendrocytes, and microglia) [8-10] after SCI have been done, however, very little attention has been paid to the role of ER stress in the BSCB disruption after acute SCI and the cellular mechanisms involved. Thus, clarification of the effects of ER stress on the expression of TJ proteins and identification of the ER stress-related regulatory pathway might provide a potential target for SCI induced BSCB disruption and promote the locomotor function recovery of SCI.
The chemical chaperone, phenylbutyric acid (PBA), has been recently applied to various diseases that involve a regulatory mechanism of the ER stress response [11]. These small molecules, referred to as small molecular chaperones, are thought to decrease the protein folding load. In cells and in whole animals, PBA enhances the adaptive capacity of the ER and decreases ER stress response signaling [12]. It has been used for the clinical treatment of urea cycle disorders in children, and for the treatment of sickle cell disease, thalassemia, and cystic fibrosis [11,13,14]. Previous study suggests that intravenous administration of PBA is beneficial to protecting the spinal cord against ischemic damage in a rabbit model [12]. Based on these observations, PBA appears to have neuroprotective effects after SCI, but the mechanism of its action is not fully known, especially its role in BSCB. In the present study, we investigated the effect of PBA and the involvement of ER stress in BSCB disruption after SCI.
Materials and methods
Reagents and antibodies
Endothelial Cell Medium (ECM) was purchased from ScienCell Research Laboratories. Antibodies against Occludin, Claudin5, ATF4, CHOP, GRP78 and GAPDH were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-XBP-1, β-catenin, P120, Cleaved caspase-12 and PDI were purchased from Abcam. All chemicals including PBA were from Sigma Chemical Company.
Spinal cord injury model and PBA treatment
Adult female Sprague-Dawley rats (220-250 g) were used for surgical procedures. The protocol for animal care and use conformed to the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health and was approved by the Animal Care and Use Committee of Wenzhou Medical University. All the rats were fed under controlled temperature (23-25°C), 12 h light and 12 h dark cycles and free access to food and tap water. Rats were anesthetized with 10% chloralic hydras (3.6 ml/kg, IP.) and the model of a T9 half-cut spinal cord column was made. For each rat, with the back shaved and sterilized, an incision was made on the back posterior to the lower thoracic region and the vertebral column was exposed. A laminectomy was performed at the T9 level. The exposed spinal cord was subjected to crushed injury compressing with a vascular clip (30 g forces, Oscar, China) for 2 minutes. The incision sites were then closed in layers and a topical antibiotic (cefazolin sodium salt) was applied to the incision site. Preparation of PBA involved dilution to a stock solution of 100 mg/mL in 100% DMSO. PBA was immediately administered into injured rat via intraperitoneal injection (100 mg/kg) [15] after spinal cord injury and then further treated once a day for 2 weeks for behavior test, and equal DMSO was administered for vehicle control. For the sham-operated controls, the animals underwent a T9 laminectomy without contusion injury and received no pharmacological treatment. Animals either received vehicle solution (n = 14), PBA (n = 14). Postoperative care involved manually emptying the urinary bladder twice a day. All animals had no significant side effects (such as an increase in mortality or infectious diseases) after drugs treatment during our experiments.
Cell culture
Primary cultures of Human Brain Microvascular Endothelial Cells (HBMVEC) were purchased from ScienCell Research Laboratories. BMVEC cultures were expanded and maintained in Endothelial Cell Medium (ECM). They were then incubated in a humidified atmosphere containing 5% CO2 at 37°C. PBA involved dilution to a stock solution of 1 M in 100% DMSO, In order to prevent the drug acid stimulation on the cells, we titrated with NaOH to PH 7.4. Cells were treated with Thapsigargin (TG, 10 μM), TG compound with PBA (1 mM), PBA (1 mM) alone. All experiments were performed in triplicate.
Locomotion recovery assessment
Examination of functional deficits after injury was conducted as previously described [16]. Two blind independent examiners scored the locomotion recovery in an open field, according to the Basso, Beattie and Bresnahan (BBB) scale ranging from 0 (no limb movement or weight support) to 21 (normal locomotion) during the 14-day postoperative period. Animals were placed individually on open field and allowed to move freely for 5 min. The scale was developed using the natural progression of locomotion recovery in rats with thoracic SCI.
Evans Blue-BSCB disruption
The integrity of the BSCB was examined with Evans blue dye extravasation according to previous reports [17] with minor modifications. After the rats survived for 24 h, Evans blue (EB) content was used for quantitative and qualitative analysis of BSCB disruption after SCI, as described previously [18]. 1 ml of 2% Evan’s Blue dye solution in saline was injected intravenously into the tail vein. Two hours later, animals were anaesthetized and killed by intracardiac perfusion with saline. One centimeter of the T9 spinal cord surrounding the injury site was extracted, weighed, and snap-frozen in -80°C. Samples (400 mg) were then homogenized in 400 μL of N, N’-dimethylformamide (DMF) and incubated at 70°C for 72 h. Samples were centrifuged at 18,000 rpm for 20 min twice. The supernatant was collected, aliquoted (200 μL) into a 96-well glass plate, and its fluorescence was quantified using a spectrophotometer at an excitation wavelength of 620 nm and an emission wavelength of 680 nm. Samples were normalized to the original sample weight, and EB concentration was calculated based on a standard curve of EB in DMF (data reported as EB per spinal cord weight: μg/g).
Western blot analysis
For protein analysis in vivo, a spinal cord segment (0.5 cm length) at the contusion epicenter was dissected and rapidly stored at -80°C for western blotting. Briefly, frozen animal spinal cord tissues and cells were homogenized in ice-cold lysis buffer containing 50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS, 10 mM Na2P2O7, 10 mM NaF, 1 mg/ml aprotinin, 10 mg/ml leupeptin, 1 mM sodium vanadate and 1 mM PMSF. Tissue homogenates were incubated for 20 min at 4°C, and centrifuged at 12,000 rpm, for 15 min at 4°C. Protein samples (60 μg) were separated on SDS-PAGE and transferred to PVDF membrane (Bio-Rad). The membrane was blocked with 5% non-fat milk in TBS with 0.05% Tween 20 for 90 min, then incubated overnight at 4°C with following antibody solutions: Occludin (1:800), Claudin5 (1:800), P120 (1:1000), β-catenin (1:1000), CHOP (1:300), GRP78 (1:300), PDI (1:1000), XBP-1 (1:1000), ATF4 (1:1000), Cle-caspase12 (1:1000). Then the membranes were washed with TBS for 5 min three times and incubated with secondary antibodies for 1 h at room temperature. Signals were visualized using the ChemiDicTM XRS + Imaging System (Bio-Rad). Experiments were repeated three times.
Immunofluorescence staining
Spinal cord segments obtained from animals 1 day after surgery were cryoprotected in 4% paraformaldehyde for 6 h, then embedded in paraffin and sectioned into 5 μm slices. Sections were deparaffinized, rehydrated and washed twice for 10 minutes in PBS. Then sections were incubated with 5% Albumin from bovine serum in PBS containing 0.1% Triton X-100 in a 37°C oven for 30 min. They were then incubated with the appropriate primary antibodies overnight at 4°C in the same buffer. Sections were rinsed three times in PBS after primary antibody (Ab) incubation and incubated with either fluorescent secondary Ab for 1 h at room temperature. Sections were rinsed three times with PBS and cover slipped with Mowiol mounting medium containing 4’, 6-diamidino-2-pheny-lindole (DAPI) to counterstain the nuclei. All images were captured on a Nikon ECLIPSE Ti microscope (Nikon, Japan).
Cell viability assay
Cells were seeded on a 96-well plate at 7x103 cells/well for 24 h, followed by individual treatment for the indicated time periods. Before the end of treatment, 0.5 mg/ml MTT was added to each well for 4 h. The supernatants were carefully aspirated, and the formazan crystals were dissolved in DMSO. The absorbance was measured at 550 nm with a Thermo Varioskan Flash reader (Thermo Electron Corporation, France).
Statistical analysis
Data are presented as the Mean values ± SEM from three independent experiments. Statistical significance was examined using Student’s t-test when there were two experimental groups. When more than two groups were compared, statistical evaluation of the data was performed using one-way analysis of variance (ANOVA) and Dunnett’spost hoc test. P values < 0.05 were considered statistically significant.
Results
PBA inhibits the increase of BSCB permeability after SCI
To determine whether PBA attenuates the increases of permeability induced by compression injury, the content of EB in the injured spinal cord tissue was determined. We examined the effect of PBA on BSCB permeability at 24 h post-SCI by EB assay (n = 4). As shown in Figure 1A and 1B, BSCB permeability was changing significantly in response to spinal cord compression injury as compared with uninjured sham control, indicating that injury elicits BSCB disruption. After treatment with PBA, the content of EB in spinal cord tissue was significantly decreased compared with SCI group (9.58 ± 1.04 vs. 15.99 ± 1.267, μg/g, P < 0.01) (Figure 1C). In addition, the fluorescence intensity of EB in the injured spinal cord (at 1 day) was higher than sham controls, and PBA significantly reduced the fluorescence intensity (Figure 1D and 1E). Hindlimb locomotor functional recovery was then evaluated for 2 weeks after injury using the BBB rating scale. As a result, PBA treatment significantly increased the hindlimb locomotor function 6 to 14 days after injury, compared with that observed in vehicle-treated controls (n = 6/group, 14 days, BBB rating scale, PBA, 10.2±0.74 vs. 5.6±0.51; P <0.01) (Figure 1F). These data imply that PBA can effectively prevent BSCB disruption and promote functional improvement of locomotor activity after SCI.
Figure 1.
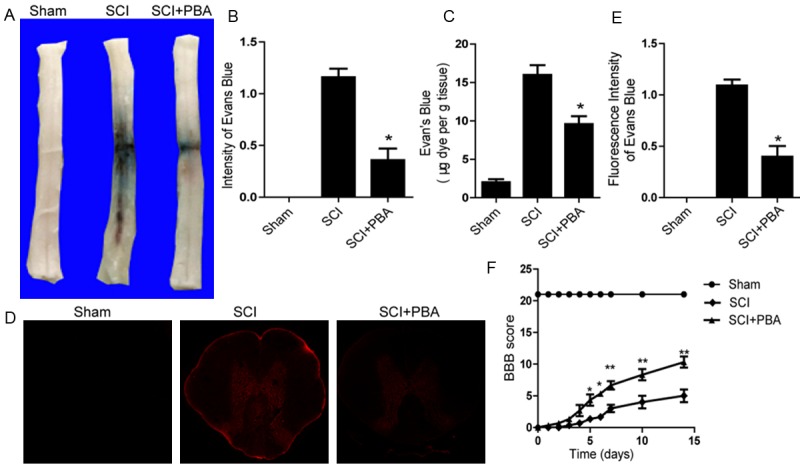
PBA inhibits the increase of BSCB permeability and improves locomotor functional recovery after SCI. After SCI, rat were treated with PBA and BSCB permeability was measured at 24 h post-SCI by using Evan’s Blue dye (n = 4/group). A. Representative whole spinal cords showing Evan’s Blue dye permeabilized into spinal cord at 24 h. B. Quantification of BSCB permeability data from A by software ImageJ. C. Quantification data of EB content of spinal cord (μg/g). D. Representative confocal images of an Evan’s Blue extravasation in each group at 24 h after SCI. E. Quantification of the fluorescence intensity of Evan’s Blue in each group. All data represent Mean values ± SEM, n =5. *P < 0.01 versus vehicle controls. F. The BBB scores of sham, SCI group and SCI rat treated with PBA group. *P < 0.05 versus the SCI group, and **P < 0.01 versus the SCI group, n = 6.
PBA prevents the loss of TJ and adherens junction (AJ) proteins after SCI
The TJ and AJ in the endothelial cells of blood vessel is involved in BSCB integrity [19]. To determine whether PBA attenuates the increased permeability by preventing the loss of TJ and AJ proteins after SCI, we examined the alterations of SCI-induced TJ and AJ proteins and the effect of PBA on these alterations at 24 h after SCI by western blot. Western blot results showed that (Figure 2A) the levels of AJ (β-catenin, P120) were decreased at early times after SCI, as well as the TJ (Occludin, Claudin5) (Figure 2C). Furthermore, these decreases were significantly attenuated in the PBA-treated group (Figure 2A-D). Double labelling immunofluorescence also showed that the fluorescence intensity of Claudin5 and CD31 immunoreactivity was decreased after injury as compared to sham controls, and PBA treatment attenuated the decrease in its intensity (Figure 2E). Immunofluorescence staining results of Occludin, Claudin5, and ZO-1 are consistent with the western blot results (Figure 2F). These data suggest that PBA preserves BSCB integrity by inhibiting degradation of TJ and AJ molecules after SCI.
Figure 2.
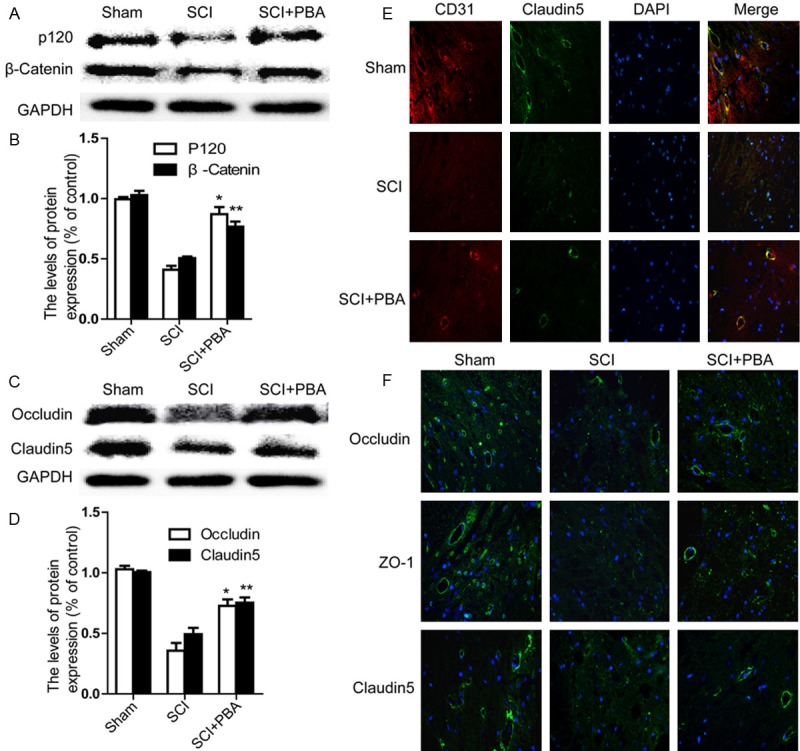
PBA prevents loss of tight junction and adheren junction proteins after SCI. A. Representative western blots of adherens junction proteins β-catenin, P120 in the sham, SCI model and SCI model treated PBA groups. B. Quantification of western blot data from A. *P < 0.01,**P < 0.01 versus the SCI group, Mean values ± SEM, n = 5. C. Representative western blots of tight junction proteins Occluding, Claudin5 in the sham, SCI model and SCI model treated PBA groups. D. Quantification of western blot data from C. *P < 0.01, **P < 0.01 versus the SCI group, Mean values ± SEM, n = 5. E. Representative micrographs showing (original magnification × 400) double immunofluorescence with Claudin5 (green) and CD31 (endothelial cell marker, red), nuclei are labeled with DAPI (blue) in each group. F. Occludin, Claudin5, and ZO-1 staining (green) results of the sham, SCI group and SCI rat treated with PBA group.
PBA inhibits the activation of ER stress after SCI
Previous work demonstrated that ER stress markers are elevated in the injured spinal cord acutely following traumatic SCI [20]. To assess the ER dysfunction after SCI and the effect of PBA on ER stress, we determined ER stress associated proteins including PDI, GRP78 and CHOP by western blot. Our results showed that (Figure 3A) the levels of GRP78, PDI and CHOP were increased at early times (1 day) after SCI and these increases were significantly inhibited in the PBA-treated group (Figure 3A and 3B). The immunofluorescence staining results of PDI and CHOP are consistent with the western blot results (Figure 3C). The level of cleaved caspase-12 (38 kDa, active form), a downstream molecule of CHOP, was markedly increased in the injured spinal cord and this increase was significantly alleviated by PBA (Figure 3D and 3E). These results indicate that PBA effectively inhibit ER stress-associated proteins expression in the spinal cord after SCI.
Figure 3.
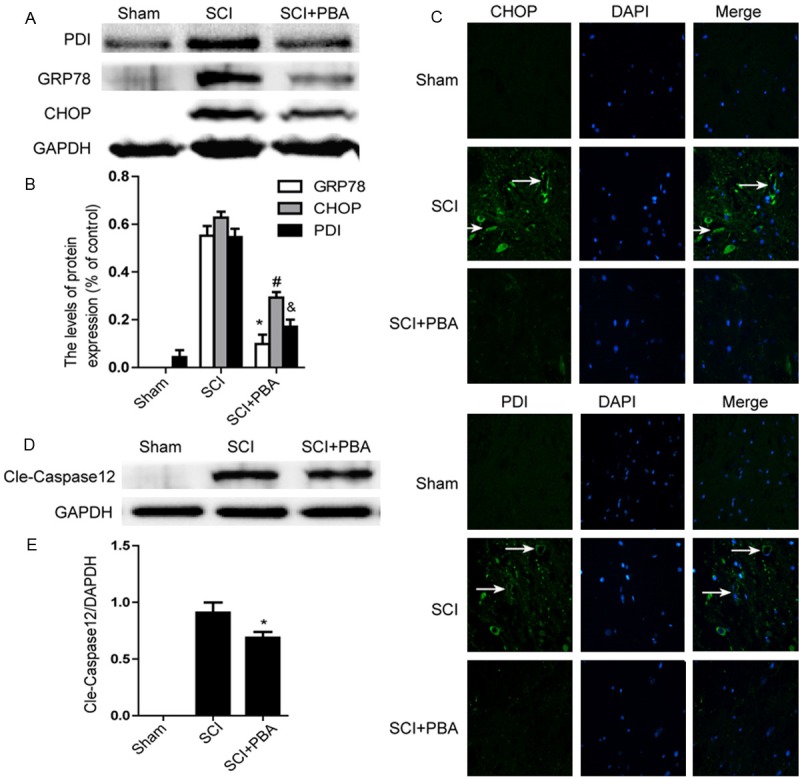
SCI leads to the activation of ER Stress markers 1 day after operation and PBA significantly altered them. Rat receiving spinal contusion injury was administrated immediately with PBA (100 mg/kg IP.). Spinal samples were prepared from injured spinal cords 1 day post the injury. A. Representative western blots of ER stress markers GRP78, PDI and CHOP in the sham, SCI model and SCI model treated PBA groups. B. Quantification of western blot data from A. *P < 0.01, #P < 0.01, &P < 0.01 versus the SCI group. Mean values ± SEM, n = 4. C. Immunofluorescence staining (original magnification × 400) results of CHOP, PDI (green), nuclei are labeled with DAPI (blue), and microvessel with obvious CHOP, PDI signals are identified using white arrowheads. D. Protein expression of cleaved caspase-12 in the sham, SCI model and SCI model treated PBA groups. E. The optical density analysis of cleaved caspase-12 protein. *P < 0.05 versus the SCI group, Mean values ± SEM, n = 4.
PBA attenuates the decreases of TJ and AJ proteins induced by TG in vitro
To further confirm that inhibition of ER stress is important for the protective effect of PBA in BSCB recovery in vitro, we detected the change of TJ and AJ in ECs. ECs were treated with TG (10 μM, 6 h) or combined with PBA (1 mM). Western blot results showed that (Figure 4A and 4C) the levels of P120, β-catenin, Occludin and Claudin5 were decreased after treated with TG, compared with the control group. At the same time, the decrease in P120, β-catenin, Occludin, Claudin5 were significantly attenuated in the PBA-treated group as compared with the TG-treated group (Figure 4B and 4D, P < 0.01). Except Claudin5, no effect of PBA alone on these proteins was observed. As shown, a clear increase in Claudin5 accumulation was observed (Figure 4D) in PBA alone group as compared with the control group (P < 0.05). Immunocytochemistry also revealed that the intensity of Occludin expression decreased after treated with TG. However, this decrease was significantly reversed by PBA treatment (Figure 4E). Collectively, these results suggest that the protective effect of PBA on BSCB permeability after SCI is due to the decrease of TJ and AJ loss after injury.
Figure 4.
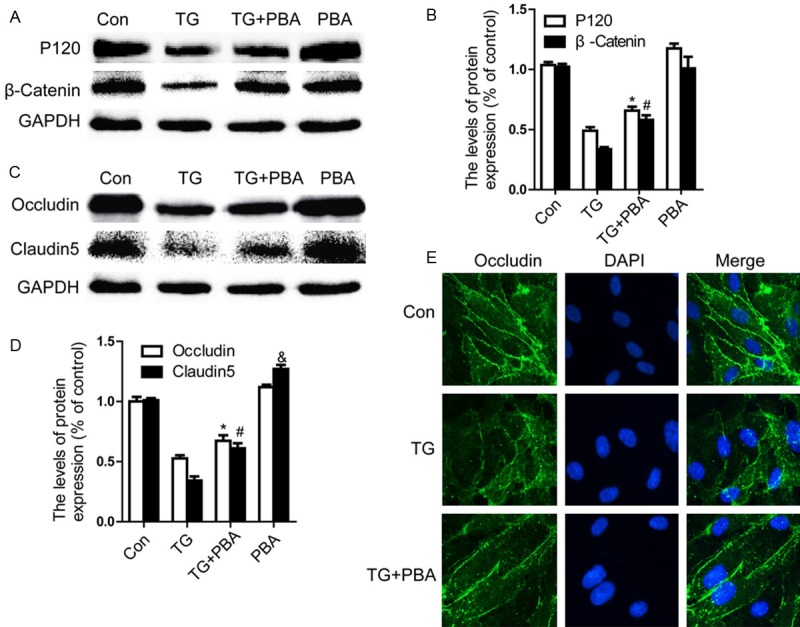
PBA prevents loss of TJ and AJ proteins in TG-treated endothelial cells. A, B. Representative western blot and quantification data of adherens junction proteins β-catenin, P120-catenin Occludin, and Claudin5 in each group of cells. C, D. Representative western blots and quantification data of tight junction proteins Occludin, Claudin5 in each group cells. All experiments were repeated three times. All data represent Mean values ± SEM, *P < 0.01, #P < 0.01 versus TG treated cells. &P < 0.05 versus control cells. E. Immunofluorescence staining of Occludin (green)in endothelial cells treated with TG for 6 h, nuclei are labeled with DAPI (blue).
PBA inhibits ER stress and apoptosis in TG-treated ECs
We next investigated the effect of PBA on protein levels of ER stress associated proteins in vitro. Our data showed that the protein levels of GRP78, PDI, ATF4, XBP-1, CHOP and caspase-12 were significantly increased, and PBA significantly prevented these increases at concentrations of 1 mM (Figure 5A-C; P < 0.01). No effect of PBA alone on these proteins was observed. Additionally, we studied the effect of TG on EC viability. In MTT assays, TG inhibited cell viability and this inhibition was partly restored after PBA addition. As shown in Figure 5D, treatment with 1 mM of PBA protected cell viability to 81.7 ± 4.6% compared to TG only groups 60.7 ± 5.4%. These results suggest that PBA acts as a chaperone in alleviating sustained ER stress in ECs.
Figure 5.
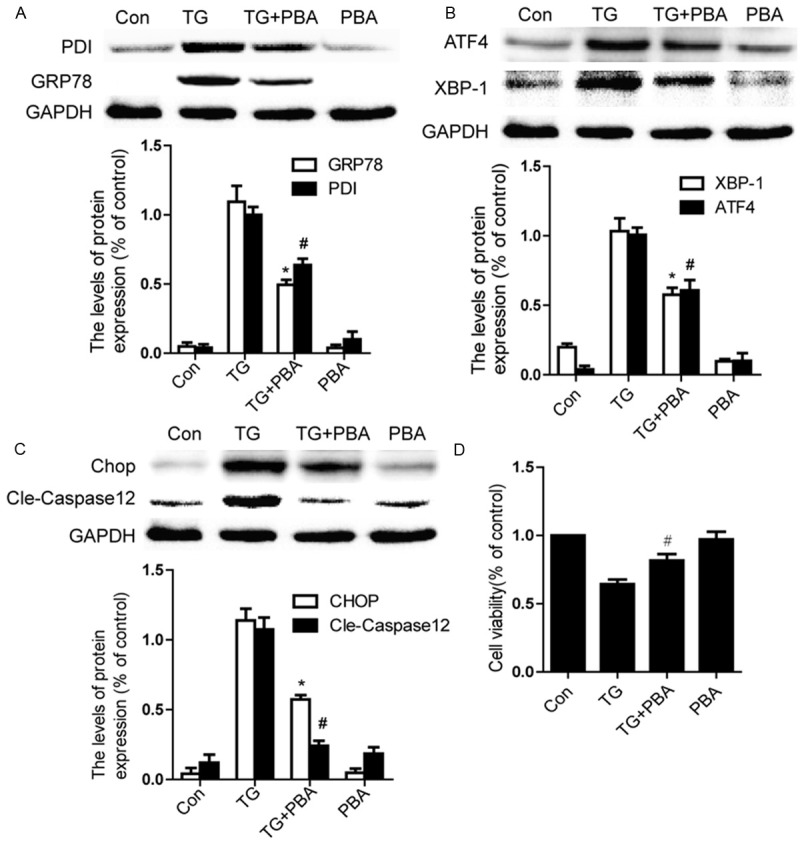
PBA inhibits ER stress and apoptosis in TG-treated endothelial cells. Endothelial cells were treated with TG (10 μM) or together with PBA (1 mM) or PBA (1 mM) alone for 6 h. A. Representative western blots and quantification data of ER stress markers GRP78, PDI in each group cells. B. Representative western blots and quantification data of ER stress markers ATF4 and XBP-1 in each group cells. C. Representative western blots and quantification data of apoptosis-regulating proteins CHOP, Caspase-12, in each group cells. D. MTT results of PBA-treated endothelial cells induced by TG. All experiments were repeated three times. All data represent Mean values ± SEM, *P < 0.01, #P < 0.01 versus TG treated cells.
Discussion
Under physiological conditions, an intact BSCB is essential for building and maintaining a microenvironment that allows neuronal circuits to function properly [1,21]. The mechanisms by which BSCB breakdown occurs and progresses and the consequences of a compromised barrier are complex [22]. Reactive oxygen species (ROS) provide a common trigger for many downstream pathways that directly mediate BBB compromise such as oxidative damage, TJ modification and matrix metalloproteinases (MMP) activation [23,24]. MMPs have been implicated in SCI in that plasma MMP-9 disrupted TJ proteins, rendering the BSCB leaky and allowing neurotoxic agents to enter the injured spinal cord [22]. Inhibiting MMP activation can prevent BSCB disruption after SCI [4,25]. Inflammation also plays a critical role in BSCB dysfunction during the bimodal stage after ischemia/reperfusion injury in rats [18,26,27]. Inhibition of inflammation by salvianolic acid B decreases BSCB disruption and eventually results in alleviation of pathological changes that were caused by SCI [28]. Our results showed that BSCB permeability increased significantly in response to spinal cord compression injury. However, PBA, a specific ER stress inhibitor, significantly attenuated BSCB permeability at 1 day post-injury (Figure 1). In vitro, TG as a typical ER stress activator, inhibited ECs viability and this inhibition was partly restored after PBA addition (Figure 5D). It is universally accepted that at the early phase of ER stress or in the case of mild ER stress, cells exhibit a self-protective signal-transcription pathway by upregulated expression of molecular chaperones [10,29,30]. However, at the late phase of ER stress or ER stress produced excessively, excessive ER stress may activate an apoptotic pathway by activating CHOP and caspase-12 that induce cell apoptosis [31-33], which may lead to secondary injury after SCI. Previous studies have indicated a role of ER stress in neurocyte and all major glial sub-types (astrocytes, oligodendrocytes, and microglia) [8-10] after SCI. Here, our results, for the first time, suggested that ER stress may be protective for BSCB integrity.
Adjacent ECs express continuous rows of transmembrane proteins that make homophilic contact in the intercellular space and form junctional complex (TJs) [34]. TJs are a key feature of the blood-CNS barrier and significantly reduce permeation of polar solutes through paracellular diffusional pathways between the ECs from the blood plasma to the CNS extracellular fluid [2]. The TJs between endothelial cells include AJ and TJ. Claudins and occludin are the most important membranous components of TJ, but the participation of AJ molecules which include P120 and β-catenin are important as well [34,35]. Emerging evidence shows that SCI is accompanied by the loss of the TJ and AJ proteins [4,25,28,36]. The current findings are consistent with previous results showing the disruption of BSCB and the loss of TJ and AJ proteins after SCI. In addition, numerous studies have demonstrated that increased expression of TJ proteins is correlated with an enhanced barrier function. A recent literature indicate that GRP78 plays a central role in human pulmonary artery EC inflammation and injury via its action on NF-kB activation and regulation of vascular permeability [37]. Preconditioning cells with SubAB-induced ER stress potentiated F-actin formation and abrogated endothelial permeability, indicating that preconditioning of ECs with ER stress is protective against ECs inflammation and injury. The proteins and mRNAs of occludin and claudin-1 were significantly increased in TG treated retinal pigment epithelial (RPE) cells in vitro [37,38]. Contrary to the above, other latter findings indicate that the expression of Claudin-5 but not Occludin was significantly decreased by the treatment with TG, the down-regulation of Claudin-5 by the treatment with TG was significantly suppressed by the pre-treatment with PBA in retinal microvascular ECs [39-41]. One possibility for this discrepancy is that the RPE cells used in Tadanobu’s study are different from primary human retinal microvascular ECs used in Tetsuo’s study, however, the precise role of ER stress in TJ proteins regulation remains unclear in various ECs especially in BSCB and warrants further investigation. In the present study, we demonstrated that ER stress was involved in the responses of SCI, the levels of related proteins including PDI, GRP78, CHOP and caspase-12 protein increased obviously and decreased by treatment of PBA at 1 day post-injury in vivo (Figure 3), which also been detected in primary cultures of human brain microvascular ECs injury models induced by TG (Figure 5). Furthermore, PBA administration attenuated BSCB permeability and degradation of TJ molecules such as P120, β-catenin, Occludin and Claudin5 both in vivo and in vitro. The current findings suggest that ER stress plays an important role in BSCB disruption, the loss of TJ proteins after SCI and that the regulation of the ER stress related pathway might provide a potential target for BSCB disruption in SCI treatments.
By targeting components of these ER signaling responses, to explore clinical treatment strategies or new drugs in CNS neurological diseases characterized by a compromised BSCB might become possible and valuable in the future. Sodium phenylbutyrate (PB) is approved by Food and Drug Administration for children and adults with hyperammonemia associated with urea cycle disorders (recommended dose of 13 g/m2/day) and has been used for adult patients with hyperammonemia secondary to high-dose chemo-therapy for leukemia and transplant therapies [14,42]. PB is also under investigation for cystic fibrosis and adrenal leukodystrophy [11]. It should be noted that in a phase I clinical and pharmacological evaluation of PB on an 120-h infusion schedule, patients experienced mild side effects including fatigue and nausea at the higher dose levels, in particular, the 410 mg/kg/day dose [14]. Early study has demonstrated that the neuroprotective effect of PBA promote neurologic function recovery after spinal cord ischemia [12,43]. In this study, we demonstrated that PBA effectively prevents BSCB disruption and attenuates the loss of TJ proteins after SCI. The present study lays the ground work for future translational confidence of PBA in CNS diseases, especially the relations to the BSCB disruption.
In conclusion, our research demonstrated that PBA significantly attenuated BSCB permeability and degradation of TJ molecules such as P120, β-catenin, Occludin and Claudin5 at 1 day after injury, thus, improved functional recovery in SCI. We first reported that the BSCB protective effect of PBA is related to the inhibition of ER stress induced by SCI. In addition, PBA significantly inhibited the increase of ER stress markers and prevents loss of TJ and AJ proteins in TG-treated cells. These data demonstrate that therapeutic strategies targeting on ER stress may be suitable for the therapy of preserving BSCB integrity following SCI, and PBA may be a new candidate as a therapeutic agent for protecting the CNS neurological diseases characterized by a compromised BSCB.
Acknowledgements
This study was partly supported by a research grant from the National Natural Science Funding of China (81302775, 81472165, 81200958, 81372112), Zhejiang Provincial Natural Science Foundation of China (LY14H090013, LY14H150010, LY14H170002), Zhejiang Provincial Program for the Cultivation of High-level Innovative Health talents (to J.X.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Bartanusz V, Jezova D, Alajajian B, Digicaylioglu M. The blood-spinal cord barrier: morphology and clinical implications. Ann Neurol. 2011;70:194–206. doi: 10.1002/ana.22421. [DOI] [PubMed] [Google Scholar]
- 2.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Cardoso FL, Brites D, Brito MA. Looking at the blood-brain barrier: molecular anatomy and possible investigation approaches. Brain Res Rev. 2010;64:328–363. doi: 10.1016/j.brainresrev.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Lee JY, Kim HS, Choi HY, Oh TH, Yune TY. Fluoxetine inhibits matrix metalloprotease activation and prevents disruption of blood-spinal cord barrier after spinal cord injury. Brain. 2012;135:2375–2389. doi: 10.1093/brain/aws171. [DOI] [PubMed] [Google Scholar]
- 5.Zhang H, Wu F, Kong X, Yang J, Chen H, Deng L, Cheng Y, Ye L, Zhu S, Zhang X, Wang Z, Shi H, Fu X, Li X, Xu H, Lin L, Xiao J. Nerve growth factor improves functional recovery by inhibiting endoplasmic reticulum stress-induced neuronal apoptosis in rats with spinal cord injury. J Transl Med. 2014;12:130. doi: 10.1186/1479-5876-12-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JY, Maeng S, Kang SR, Choi HY, Oh TH, Ju BG, Yune TY. Valproic acid protects motor neuron death by inhibiting oxidative stress and endoplasmic reticulum stress-mediated cytochrome C release after spinal cord injury. J Neurotrauma. 2014;31:582–594. doi: 10.1089/neu.2013.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang HY, Zhang X, Wang ZG, Shi HX, Wu FZ, Lin BB, Xu XL, Wang XJ, Fu XB, Li ZY, Shen CJ, Li XK, Xiao J. Exogenous basic fibroblast growth factor inhibits ER stress-induced apoptosis and improves recovery from spinal cord injury. CNS Neurosci Ther. 2013;19:20–29. doi: 10.1111/cns.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuang X, Hu W, Yan M, Wong PK. Phenylbutyric acid suppresses protein accumulationmediated ER stress in retrovirus-infected astrocytes and delays onset of paralysis in infected mice. Neurochem Int. 2010;57:738–748. doi: 10.1016/j.neuint.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mecha M, Torrao AS, Mestre L, Carrillo-Salinas FJ, Mechoulam R, Guaza C. Cannabidiol protects oligodendrocyte progenitor cells from inflammation-induced apoptosis by attenuating endoplasmic reticulum stress. Cell Death Dis. 2012;3:e331. doi: 10.1038/cddis.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valenzuela V, Collyer E, Armentano D, Parsons GB, Court FA, Hetz C. Activation of the unfolded protein response enhances motor recovery after spinal cord injury. Cell Death Dis. 2012;3:e272. doi: 10.1038/cddis.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortez L, Sim V. The therapeutic potential of chemical chaperones in protein folding diseases. Prion. 2014;8:197–202. doi: 10.4161/pri.28938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizukami T, Orihashi K, Herlambang B, Takahashi S, Hamaishi M, Okada K, Sueda T. Sodium 4-phenylbutyrate protects against spinal cord ischemia by inhibition of endoplasmic reticulum stress. J Vasc Surg. 2010;52:1580–1586. doi: 10.1016/j.jvs.2010.06.172. [DOI] [PubMed] [Google Scholar]
- 13.Qi X, Hosoi T, Okuma Y, Kaneko M, Nomura Y. Sodium 4-phenylbutyrate protects against cerebral ischemic injury. Mol Pharmacol. 2004;66:899–908. doi: 10.1124/mol.104.001339. [DOI] [PubMed] [Google Scholar]
- 14.Carducci MA, Gilbert J, Bowling MK, Noe D, Eisenberger MA, Sinibaldi V, Zabelina Y, Chen TL, Grochow LB, Donehower RC. A Phase I clinical and pharmacological evaluation of sodium phenylbutyrate on an 120-h infusion schedule. Clin Cancer Res. 2001;7:3047–3055. [PubMed] [Google Scholar]
- 15.Lupachyk S, Watcho P, Stavniichuk R, Shevalye H, Obrosova IG. Endoplasmic Reticulum Stress Plays a Key Role in the Pathogenesis of Diabetic Peripheral Neuropathy. Diabetes. 2013;62:944–952. doi: 10.2337/db12-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang HY, Wang ZG, Wu FZ, Kong XX, Yang J, Lin BB, Zhu SP, Lin L, Gan CS, Fu XB, Li XK, Xu HZ, Xiao J. Regulation of autophagy and ubiquitinated protein accumulation by bFGF promotes functional recovery and neural protection in a rat model of spinal cord injury. Mol Neurobiol. 2013;48:452–464. doi: 10.1007/s12035-013-8432-8. [DOI] [PubMed] [Google Scholar]
- 17.Wang HL, Lai TW. Optimization of Evans blue quantitation in limited rat tissue samples. Sci Rep. 2014;4:6588. doi: 10.1038/srep06588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xanthos DN, Pungel I, Wunderbaldinger G, Sandkuhler J. Effects of peripheral inflammation on the blood-spinal cord barrier. Mol Pain. 2012;8:44. doi: 10.1186/1744-8069-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernacki J, Dobrowolska A, Nierwińska K, Małecki A. Physiology and pharmacological role of the blood-brain barrier. Pharmacol Rep. 2008;60:600–622. [PubMed] [Google Scholar]
- 20.Ohri SS, Maddie MA, Zhao Y, Qiu MS, Hetman M, Whittemore SR. Attenuating the endoplasmic reticulum stress response improves functional recovery after spinal cord injury. Glia. 2011;59:1489–1502. doi: 10.1002/glia.21191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer AM. The role of the blood-CNS barrier in CNS disorders and their treatment. Neurobiol Dis. 2010;37:3–12. doi: 10.1016/j.nbd.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 22.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pun PB, Lu J, Moochhala S. Involvement of ROS in BBB dysfunction. Free Radic Res. 2009;43:348–364. doi: 10.1080/10715760902751902. [DOI] [PubMed] [Google Scholar]
- 24.Olmez I, Ozyurt H. Reactive oxygen species and ischemic cerebrovascular disease. Neurochem Int. 2012;60:208–212. doi: 10.1016/j.neuint.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Lee JY, Kim HS, Choi HY, Oh TH, Ju BG, Yune TY. Valproic acid attenuates blood-spinal cord barrier disruption by inhibiting matrix metalloprotease-9 activity and improves functional recovery after spinal cord injury. J Neurochem. 2012;121:818–829. doi: 10.1111/j.1471-4159.2012.07731.x. [DOI] [PubMed] [Google Scholar]
- 26.Li XQ, Lv HW, Tan WF, Fang B, Wang H, Ma H. Role of the TLR4 pathway in blood spinal cord barrier dysfunction during the bimodal stage after ischemiareperfusion injury in rats. J Neuroinflammation. 2014;11:62. doi: 10.1186/1742-2094-11-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li XQ, Wang J, Fang B, Tan WF, Ma H. Intrathecal antagonism of microglial TLR4 reduces inflammatory damage to blood-spinal cord barrier following ischemia/reperfusion injury in rats. Mol Brain. 2014;7:28. doi: 10.1186/1756-6606-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan ZK, Lv G, Wang YF, Li G, Yu DS, Wang YS, Zhang YQ, Mei XF, Cao Y. The protective effect of salvianolic acid B on blood-spinal cord barrier after compression spinal cord injury in rats. J Mol Neurosci. 2013;51:986–993. doi: 10.1007/s12031-013-0083-8. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Yuan Y, Jiang L, Zhang J, Gao J, Shen Z, Zheng Y, Deng T, Yan H, Li W, Hou WW, Lu J, Shen Y, Dai H, Hu WW, Zhang Z, Chen Z. Endoplasmic reticulum stress induced by tunicamycin and thapsigargin protects against transient ischemic brain injury: Involvement of PARK2-dependent mitophagy. Autophagy. 2014;10:1801–1813. doi: 10.4161/auto.32136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang HY, Wang ZG, Lu XH, Kong XX, Wu FZ, Lin L, Tan X, Ye LB, Xiao J. Endoplasmic Reticulum Stress: relevance and therapeutics in central nervous system diseases. Mol Neurobiol. 2015;51:1343–52. doi: 10.1007/s12035-014-8813-7. [DOI] [PubMed] [Google Scholar]
- 31.Sheng R, Liu XQ, Zhang LS, Gao B, Han R, Wu YQ, Zhang XY, Qin ZH. Autophagy regulates endoplasmic reticulum stress in ischemic preconditioning. Autophagy. 2012;8:310–325. doi: 10.4161/auto.18673. [DOI] [PubMed] [Google Scholar]
- 32.Ohri SS, Hetman M, Whittemore SR. Restoring endoplasmic reticulum homeostasis improves functional recovery after spinal cord injury. Neurobiol Dis. 2013;58:29–37. doi: 10.1016/j.nbd.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohri SS, Maddie MA, Zhang Y, Shields CB, Hetman M, Whittemore SR. Deletion of the pro-apoptotic endoplasmic reticulum stress response effector CHOP does not result in improved locomotor function after severe contusive spinal cord injury. J Neurotrauma. 2012;29:579–588. doi: 10.1089/neu.2011.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizee MR, de Vries HE. Blood-brain barrier regulation. Tissue Barriers. 2013;1:e26882. doi: 10.4161/tisb.26882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 36.Fang B, Li XM, Sun XJ, Bao NR, Ren XY, Lv HW, Ma H. Ischemic Preconditioning Protects against Spinal Cord Ischemia-Reperfusion Injury in Rabbits by Attenuating Blood Spinal Cord Barrier Disruption. Int J Mol Sci. 2013;14:10343–10354. doi: 10.3390/ijms140510343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leonard A, Paton AW, El-Quadi M, Paton JC, Fazal F. Preconditioning with Endoplasmic Reticulum Stress Ameliorates Endothelial Cell Inflammation. PLoS One. 2014;9:e110949. doi: 10.1371/journal.pone.0110949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshikawa T, Ogata N, Izuta H, Shimazawa M, Hara H, Takahashi K. Increased expression of tight junctions in ARPE-19 cells under endoplasmic reticulum stress. Curr Eye Res. 2011;36:1153–1163. doi: 10.3109/02713683.2011.606592. [DOI] [PubMed] [Google Scholar]
- 39.Adachi T, Teramachi M, Yasuda H, Kamiya T, Hara H. Contribution of p38 MAPK, NFkappaB and glucocorticoid signaling pathways to ER stress-induced increase in retinal endothelial permeability. Arch Biochem Biophys. 2012;520:30–35. doi: 10.1016/j.abb.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Adachi T, Yasuda H, Nakamura S, Kamiya T, Hara H, Hara H, Ikeda T. Endoplasmic reticulum stress induces retinal endothelial permeability of extracellular-superoxide dismutase. Free Radic Res. 2011;45:1083–1092. doi: 10.3109/10715762.2011.595408. [DOI] [PubMed] [Google Scholar]
- 41.Galan M, Kassan M, Kadowitz PJ, Trebak M, Belmadani S, Matrougui K. Mechanism of endoplasmic reticulum stress-induced vascular endothelial dysfunction. Biochim Biophys Acta. 2014;1843:1063–1075. doi: 10.1016/j.bbamcr.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim DS, Li B, Rhew KY, Oh HW, Lim HD, Lee W, Chae HJ, Kim HR. The regulatory mechanism of 4-phenylbutyric acid against ER stressinduced autophagy in human gingival fibroblasts. Arch Pharm Res. 2012;35:1269–1278. doi: 10.1007/s12272-012-0718-2. [DOI] [PubMed] [Google Scholar]
- 43.Mimori S, Ohtaka H, Koshikawa Y, Kawada K, Kaneko M, Okuma Y, Nomura Y, Murakami Y, Hamana H. 4-Phenylbutyric acid protects against neuronal cell death by primarily acting as a chemical chaperone rather than histone deacetylase inhibitor. Bioorg Med Chem Lett. 2013;23:6015–6018. doi: 10.1016/j.bmcl.2013.08.001. [DOI] [PubMed] [Google Scholar]


